Abstract
BACKGROUND
Aplastic or twiglike middle cerebral artery (Ap/T-MCA) is a rare anomaly characterized by a unilateral MCA occlusion with plexiform vessels that causes hemorrhagic and (less commonly) ischemic strokes. The reasons for this are rarely discussed, and thus optimal treatment for ischemic Ap/T-MCA remains controversial. Here, the authors report a case of Ap/T-MCA with transient ischemic attacks treated by bypass surgery and discuss the mechanism of ischemic development and treatment methods.
OBSERVATIONS
A 62-year-old hypertensive man with transient, recurrent left hemiparesis visited the authors’ hospital. Magnetic resonance angiography showed proximal occlusion of the right MCA and stenosis in the left MCA. Digital subtraction angiography revealed occlusion of the right MCA and abnormal vascular networks, leading to a diagnosis of Ap/T-MCA with contralateral MCA stenosis. Antiplatelet therapy with aspirin was insufficient, and a superficial temporal artery–MCA bypass was performed. There were no ischemic or hemorrhagic events postoperatively.
LESSONS
Atherosclerosis seems to have a significant impact on the development of ischemic stroke in patients with Ap/T-MCA, and the presence of coexisting atherosclerotic stenotic vascular lesions outside the Ap/T-MCA site is substantial in its development. Bypass surgery is a promising treatment option for ischemic Ap/T-MCA.
Keywords: atherosclerosis, bypass surgery, ischemia, aplastic or twiglike middle cerebral artery
ABBREVIATIONS : Ap/T-MCA = aplastic or twiglike middle cerebral artery, CBF = cerebral blood flow, DSA = digital subtraction angiography, EMS = encephalomyosynangiosis, ICA = internal carotid artery, MMD = moyamoya disease, MRI = magnetic resonance imaging, SPECT = single-photon emission computed tomography, STA = superficial temporal artery, TIA = transient ischemic attack
Aplastic or twiglike middle cerebral artery (Ap/T-MCA) is a rare anomaly characterized by a unilateral MCA occlusion with plexiform vessels. This anomaly, with an angiographic incidence rate of 0.088%–1.17%, is considered congenital and related to embryonic MCA development interference.1–5 Moyamoya disease (MMD) is an embryologically different condition from Ap/T-MCA but similar in that it causes hemorrhagic or ischemic strokes. Previous reports have shown that approximately 70% of patients with Ap/T-MCA develop hemorrhagic stroke, whereas 20% develop ischemic stroke.1,3,4,6–22 Reasons for why ischemic stroke is common in MMD23 but less common in Ap/T-MCA are rarely discussed.
Bypass surgery is the standard treatment for both ischemic and hemorrhagic MMD.24,25 On the one hand, in hemorrhagic cases of Ap/T-MCA, aneurysm clipping is usually performed if an aneurysm is involved, as previously reported.26 On the other hand, patients with ischemic Ap/T-MCA are often treated conservatively, and bypass surgery is rarely performed. Therefore, the optimal treatment for ischemic cases of Ap/T-MCA remains unclear.
This article reports a case of Ap/T-MCA with repeated transient ischemic attack (TIA) treated by bypass surgery. In addition, relevant literature is reviewed to delineate the clinical features of ischemic Ap/T-MCA.
Illustrative Case
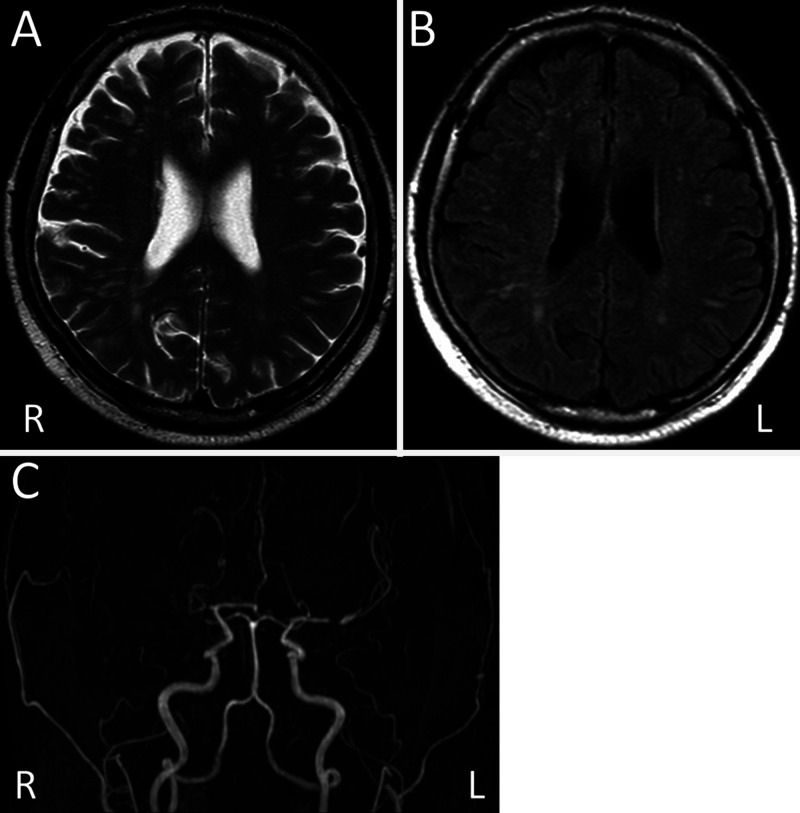
A 62-year-old man with hypertension and diabetes mellitus visited our hospital because of repetitive, transient left hemiparesis episodes lasting about 5 minutes each. On admission, although he had no neurological deficits, magnetic resonance imaging (MRI) showed mild ischemic changes in the white matter but no acute cerebral infarction. Magnetic resonance angiography showed proximal occlusion of the right MCA and stenosis in the proximal portion of the left MCA (Fig. 1).
FIG. 1.
T2-weighted (A) and fluid-attenuated inversion recovery (B) MRI showing mild ischemic changes in the white matter. Magnetic resonance angiography (C) showing proximal occlusion of the right MCA and stenosis in the proximal portion of the left MCA.
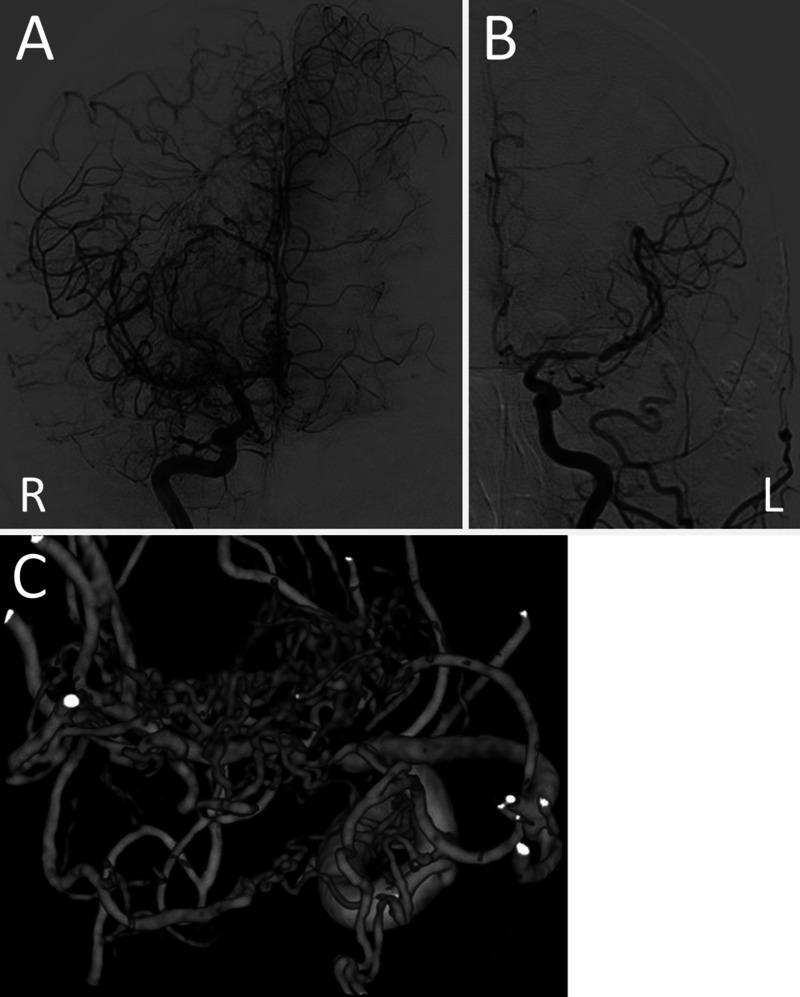
Digital subtraction angiography (DSA) revealed occlusion of the right M1 segment and abnormal vascular networks around the occluded segment. The vessel diameter of the M2 portion was almost normal. There was no stenosis in the supraclinoid segments of the internal carotid artery (ICA) and no transdural anastomosis through the external carotid artery. DSA also demonstrated antegrade blood flow via the abnormal vascular network to the distal portion of the occlusion. These findings suggested Ap/T-MCA (Fig. 2). The proximal portion of the left MCA was stenotic, and part of the left cerebral hemisphere was perfused with blood flow from the right side.
FIG. 2.
DSA showing occlusion of the right M1 segment associated with plexiform collateral vessels (A) and stenosis of the left MCA (B). Blood flow from the right side also perfusing part of the left cerebral hemisphere (A). Three-dimensional rotational angiography showing abnormal vascular network around the occluded segment (C) of the right MCA.
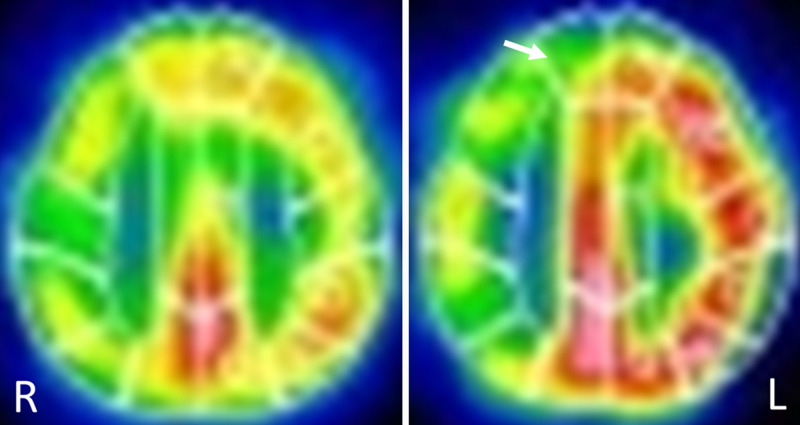
A 99mTc-ethyl cysteinate dimer single-photon emission computed tomography (SPECT) scan demonstrated right-dominant decreases in the cerebral blood flow (CBF) and reactivity to acetazolamide. Furthermore, the steal phenomenon was observed in the right cerebral hemisphere (Fig. 3). Due to the contralateral left MCA stenosis, assessing the actual CBF reduction in the right cerebral hemisphere was difficult; however, progression of left MCA stenosis was thought to have caused a decrease in CBF on the right side, resulting in repetitive TIA.
FIG. 3.
99mTc-ethyl cysteinate dimer SPECT showing right-dominant decreases in the CBF and reactivity to acetazolamide (ACZ). Baseline (left panel) and after ACZ administration (right panel). Blood flow in part of the right frontal cortex decreasing after ACZ administration (arrow).
Antiplatelet therapy with aspirin was initiated, but ischemic episodes persisted. In addition, the patient also experienced one episode of transient right hemiparesis, putatively due to left MCA stenosis. Therefore, additional cilostazol was administered, and this dual-antiplatelet therapy prevented further ischemic events. However, because patients with Ap/T-MCA are known to be at increased risk of hemorrhage, we avoided a long-term continuation of dual-antiplatelet therapy.
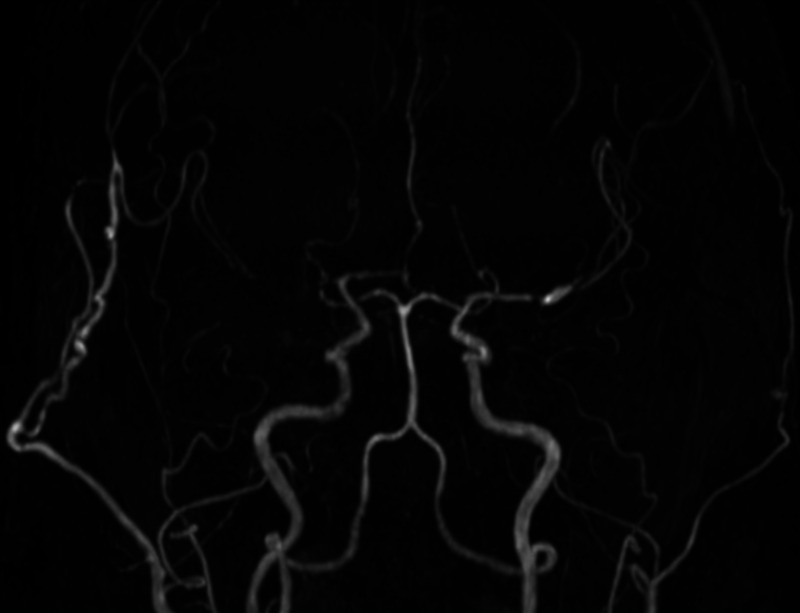
Therefore, a right superficial temporal artery (STA)-MCA bypass with encephalomyosynangiosis (EMS) was performed 3 months after onset. Cilostazol was discontinued 5 days before surgery, and, instead, intravenous administration of heparin was performed until the day of surgery. The frontal and parietal branches of the STA were bypassed to the M4 portion of the right MCA, and EMS using the inner layer of the temporal muscle was also performed. There were no intraoperative complications, and the postoperative course was uneventful. SPECT at 3 months after surgery showed improvement in CBF and cerebrovascular reactivity. MRI at 10 months after surgery showed no new cerebral infarcts and persistent bypass patency (Fig. 4).
FIG. 4.
Postoperative MRA performed at 10 months after surgery, showing bypass patency. The right cerebral hemisphere is well perfused through the bypass.
Because the left MCA stenosis was also symptomatic, all antiplatelet drugs could not be discontinued. Single-antiplatelet therapy with aspirin was continued, but there were no ischemic or hemorrhagic events during the 2-year postoperative follow-up period.
Discussion
Ap/T-MCA is a vascular malformation with the following characteristics: (1) a plexiform vascular network with unilateral M1 steno-occlusive lesion, (2) antegrade flow and nearly normal vessel caliber of the MCA branches distal to the steno-occlusive lesion, (3) no steno-occlusive lesions in adjacent major arteries (with no transdural collaterals), and (4) no progression of the steno-occlusive lesion.1,2,27
Here, the main differential diagnosis was MMD. MMD is an idiopathic, progressive disease characterized by stenosis or occlusion of the bilateral supraclinoid ICA with secondary collateral formation in the basal brain. MMD causes ischemic strokes if the collateral perfusion is insufficient and hemorrhagic strokes if the hemodynamic stress in the collaterals is excessive. Bypass surgery has been shown to reduce the risk of future ischemic and hemorrhagic strokes by improving cerebral perfusion and decreasing hemodynamic stress in patients with MMD.24,25 Because MMD and Ap/T-MCA are similar in their propensity to induce ischemic or hemorrhagic strokes involving vulnerable collaterals, bypass surgery should also be effective for Ap/T-MCA.
Our literature review identified 22 ischemic Ap/T-MCA cases, including our present case (Table 1).1,6–8,28–33 Ten patients were treated conservatively or with antiplatelets; two (20%) of these experienced hemorrhagic strokes during antiplatelet therapy.11,21 Six patients, including ours, underwent direct or indirect bypass surgery, preventing subsequent hemorrhagic and ischemic events. In our case, bypass surgery effectively prevented ischemic stroke and allowed discontinuation of dual-antiplatelet therapy, resulting in a reduced hemorrhage risk. These findings suggest that bypass surgery is a promising treatment option to resolve Ap/T-MCA, but further long-term follow-up clinical studies with large patient numbers (with and without bypass surgery) are required to determine the optimal treatment for Ap/T-MCA.
TABLE 1.
Summary of cases with aplastic or twiglike middle cerebral artery presenting with ischemia
| Case No. | Authors & Year | Age (yrs)/Sex | Side | Clinical Presentation | Vascular Risk | Coexisting Atherosclerotic Vascular Change | Treatment | Outcome (GOS) |
|---|---|---|---|---|---|---|---|---|
| 1 |
Fukawa et al., 19816 |
57/F |
L |
CI |
ND |
ND |
ND |
GR |
| 2 |
|
52/M |
R |
TIA, CI |
(−) |
Unilateral proximal ICA stenosis |
ND |
|
| 3 |
Takahashi et al., 199728 |
54/F |
R |
TIA, CI |
(−) |
Contralateral MCA occlusion |
STA-MCA bypass |
|
| 4 |
Edgell et al., 20107 |
42/M |
R |
CI |
HL |
ND |
ND |
|
| 5 |
|
42/F |
R |
TIA |
Smoking |
Contralateral MCA stenosis |
ND |
|
| 6 |
Seo et al., 20121 |
10/F |
L |
CI |
ND |
(−) |
EDAS |
|
| 7 |
|
56/F |
R |
CI |
ND |
(−) |
ND |
|
| 8 |
|
51/F |
L |
CI |
ND |
(−) |
ND |
|
| 9 |
|
58/F |
R |
CI |
ND |
(−) |
ND |
|
| 10 |
|
56/F |
R |
CI |
ND |
Atherosclerotic generation |
ND |
|
| 11 |
Uchiyama et al., 201629 |
52/F |
L |
TIA→ICH |
HT, HL |
ND |
Cilostazol→discontinue |
GR |
| 12 |
Hirai et al., 201830 |
52/M |
L |
TIA |
ND |
ND |
Cilostazol |
GR |
| 13 |
Lutz et al., 201831 |
40/F |
L |
TIA |
ND |
ND |
Antiplatelet |
GR |
| 14 |
Matsunaga et al., 201832 |
19/F |
R |
TIA |
ND |
ND |
STA-MCA bypass & EMS |
GR |
| 15 |
Cho et al., 201933 |
61/M |
R |
CI |
ND |
ND |
STA-MCA bypass |
|
| 16 |
|
73/M |
R |
CI |
ND |
ND |
Antiplatelet |
|
| 17 |
|
63/F |
L |
TIA |
ND |
ND |
Antiplatelet |
|
| 18 |
|
26/F |
R |
TIA |
ND |
ND |
Antiplatelet |
|
| 19 |
|
29/F |
L |
TIA |
ND |
ND |
Antiplatelet |
|
| 20 |
|
37/M |
R |
CI |
ND |
ND |
Antiplatelet |
|
| 21 |
Yamada et al., 20208 |
88/M |
R |
CI→ICH |
HT |
ND |
Aspirin/STA-MCA bypass |
MD |
| 22 | Present case | 62/M | R | TIA | HT, DM | Contralateral MCA stenosis | Aspirin + cilostazol/STA-MCA bypass & EMS | GR |
CI = cerebral infarction; DM = diabetes mellitus; EDAS = encephaloduoarteriosynangiosis; EMS = encephalomyosynangiosis; GOS = Glasgow Outcome Scale; GR = good recovery; HL = hyperlipidemia; HT = hypertension; ICA = internal carotid artery; ICH = intracerebral hemorrhage; MCA = middle cerebral artery; MD = moderate disability; ND = not described; STA = superficial temporal artery; TIA = transient ischemic attack.
Ischemic stroke is common in MMD23 but not in Ap/T-MCA. The reason for this is not currently reported, but it may be because Ap/T-MCA is a congenital anomaly that does not progress, unlike MMD. Our literature review demonstrates that ischemic cases of Ap/T-MCA tend to be more common in females and on the right side (Table 1). Importantly, the mean reported onset age was 48.2 years, which trends younger than most ischemic stroke cases. A younger age of onset suggests that patients with Ap/T-MCA may be less tolerant to ischemia due to vascular anomalies, and thus CBF on the Ap/T-MCA side may be insufficient even in asymptomatic patients. Also, these reports did not mention risk factors for atherosclerosis or coexisting arteriosclerotic vascular lesions. Among those described, patients with Ap/T-MCA with an ischemic onset often had risk factors for atherosclerosis and also a high incidence of other atherosclerotic vascular lesions that could have affected local blood flow on the Ap/T-MCA side through collateral vessels. Cases of MCA occlusion or stenosis at the contralateral side of the Ap/T-MCA showing ischemic symptoms have been reported.7,28 In our case, the right Ap/T-MCA was initially asymptomatic but most likely became symptomatic due to reduced right-side blood flow as atherosclerotic stenosis developed in the left MCA. Because atherosclerotic degeneration may occur not only in normal arteries but also in congenitally abnormal arteries, atherosclerosis may have a more significant impact on the development of ischemic symptoms in patients with Ap/T-MCA than in the general population. It therefore seems essential to control risk factors for atherosclerosis in patients with Ap/T-MCA.
Observations
We reported a case of Ap/T-MCA with repetitive TIA that was successfully treated by bypass surgery.
Lessons
Atherosclerosis is thought to strongly influence the development of ischemia in Ap/T-MCA patients, and the presence of coexisting atherosclerotic stenotic vascular lesions outside the Ap/T-MCA site is substantial in its development. Bypass surgery is also a promising option for both hemorrhagic and ischemic Ap/T-MCA.
Acknowledgments
We thank Dr. Ayako Takarada of the University of Tsukuba Faculty of Medicine, Department of Hematology, for data collection. We also thank Dr. Alexander Zaboronok of the University of Tsukuba Faculty of Medicine, Department of Neurosurgery, and Dr. Bryan J. Mathis of the University of Tsukuba Hospital International Medical Center for professional and language revision.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Takeda, Yanaka, Nakamura. Acquisition of data: Takeda, Yanaka, Nakamura. Analysis and interpretation of data: Takeda, Yanaka, Nakamura, Ishii. Drafting the article: Takeda, Yanaka, Ishii. Critically revising the article: Ishikawa, Ishii. Reviewed submitted version of manuscript: Ishikawa, Onuma, Nakamura, Ishii. Approved the final version of the manuscript on behalf of all authors: Ishikawa. Administrative/technical/material support: Nakamura. Study supervision: Ishikawa, Nakamura.
References
- 1. Seo BS, Lee YS, Lee HG, Lee JH, Ryu KY, Kang DG. Clinical and radiological features of patients with aplastic or twiglike middle cerebral arteries. Neurosurgery. 2012;70(6):1472–1480. doi: 10.1227/NEU.0b013e318246a510. [DOI] [PubMed] [Google Scholar]
- 2. Viso R, Lylyk I, Albiña P, Lundquist J, Scrivano E, Lylyk P. Hemorrhagic events associated with unfused or twig-like configuration of the middle cerebral artery: a rare vascular anomaly with clinical relevance. Interv Neuroradiol. 2021;27(2):285–290. doi: 10.1177/1591019920970430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu HM, Lai DM, Tu YK, Wang YH. Aneurysms in twig-like middle cerebral artery. Cerebrovasc Dis. 2005;20(1):1–5. doi: 10.1159/000086119. [DOI] [PubMed] [Google Scholar]
- 4. Akkan K, Ucar M, Kilic K, Celtikci E, Ilgit E, Onal B. Unfused or twig-like middle cerebral artery. Eur J Radiol. 2015;84(10):2013–2018. doi: 10.1016/j.ejrad.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 5. Onoue K, Nguyen TN, Mian A, Dasenbrock H, Bedi H, Abdalkader M. Twig-like middle cerebral arteries: clinical and radiological findings. Clin Imaging. 2021;73:31–37. doi: 10.1016/j.clinimag.2020.11.049. [DOI] [PubMed] [Google Scholar]
- 6.Fukawa O, Aihara H, Ishii K, et al. Middle cerebral artery occlusion with moyamoya phenomenon. First Report: Clinical Course and Angiographical Findings; 10th Conference of Surgery for Cerebral Stroke; Tokyo, Japan. 1981. pp. 36–41. [Google Scholar]
- 7. Edgell RC, Boulos AS, Borhani Haghighi A, Bernardini GL, Yavagal DR. Middle cerebral artery stenosis associated with moyamoya pattern collateralization. Front Neurol. 2010;1:119. doi: 10.3389/fneur.2010.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamada D, Ishibashi R, Kinosada M, et al. Aplastic or twig-like cerebral artery with short-term ischemia and bleeding. Jpn J Stroke. 2020;42:190–195. [Google Scholar]
- 9. Cekirge HS, Peynircioglu B, Saatci I. Endovascular treatment of an “anterior cerebral artery” aneurysm in a patient with “embryonic unfused middle cerebral artery” anomaly: a case report. Neuroradiology. 2005;47(9):690–694. doi: 10.1007/s00234-005-1407-3. [DOI] [PubMed] [Google Scholar]
- 10. Shin HS, Lee SH, Ryu CW, Koh JS. Flow-related intracranial aneurysms associated with unfused arterial twigs relevant to different vascular anomalies: embryologic and hemodynamic considerations. Acta Neurochir (Wien) 2014;156(9):1637–1646. doi: 10.1007/s00701-014-2165-y. [DOI] [PubMed] [Google Scholar]
- 11. Tashiro R, Inoue T, Shibahara I, et al. Nonaneurysmal subarachnoid hemorrhage due to unfused or twiglike middle cerebral artery rupture: two case reports. J Stroke Cerebrovasc Dis. 2016;25(6):e77–e78. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 12. Uchiyama N. Anomalies of the middle cerebral artery. Neurol Med Chir (Tokyo) 2017;57(6):261–266. doi: 10.2176/nmc.ra.2017-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodríguez-Hernández A, Lu DC, Miric S, Lawton MT. Aneurysms associated with non-moyamoya collateral arterial networks: report of three cases and review of literature. Neurosurg Rev. 2011;34(4):517–522. doi: 10.1007/s10143-011-0342-5. [DOI] [PubMed] [Google Scholar]
- 14. Park J, Hwang JH, Hamm IS. Aneurysm rupture at an anomalous collateral artery that extended from the proximal A2 segment to the middle of the M1 segment, bypassing atresia of the internal carotid artery bifurcation. Case report. J Neurosurg. 2004;100(2):332–334. doi: 10.3171/jns.2004.100.2.0332. [DOI] [PubMed] [Google Scholar]
- 15. Fukuda Y, Matsunaga Y, Hirayama K, et al. A case of aplastic or twig-like middle cerebral artery associated with a ruptured A1 aneurysm at the origin of the anomalous collateral artery. Jpn J Stroke. 2018;40(2):75–80. [Google Scholar]
- 16. Kim MS, Oh CW, Hur JW, Lee JW, Lee HK. Aneurysms located at the proximal anterior cerebral artery and anterior communicating artery associated with middle cerebral artery aplasia: case report. Surg Neurol. 2005;64(6):534–537. doi: 10.1016/j.surneu.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 17. Lang M, Moore NZ, Witek AM, Kshettry VR, Bain MD. Microsurgical repair of ruptured aneurysms associated with moyamoya-pattern collateral vessels of the middle cerebral artery: a report of two cases. World Neurosurg. 2017;105:1042.e5–1042.e10. doi: 10.1016/j.wneu.2017.06.166. [DOI] [PubMed] [Google Scholar]
- 18. Rivera R, Sordo J, Badilla L, Bravo E, Riveros R, Giacaman P. Middle cerebral artery occlusion with moyamoya-like vessels and aneurysms. A report of two cases. Interv Neuroradiol. 2014;20(1):96–99. doi: 10.15274/INR-2014-10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inoue R, Katayama S, Kasai N, Hori S. Middle cerebral artery occlusion with unilateral moyamoya like vessels and with ruptured anterior cerebral artery aneurysm – its relation to the antiphospholipid antibody syndrome. Article in Japanese. No To Shinkei. 1994;46(10):995–998. [PubMed] [Google Scholar]
- 20. Sakai K, Mizumatsu S, Terasaka K, Sugatani H, Higashi T. Surgical treatment of a lenticulostriate artery aneurysm. Case report. Neurol Med Chir (Tokyo) 2005;45(11):574–577. doi: 10.2176/nmc.45.574. [DOI] [PubMed] [Google Scholar]
- 21. Fukuyama R, Yamamura K, Murata H, Miyatake S, Matsumoto N, Abe H. Ruptured aneurysm of an aplastic or twig-like middle cerebral artery with ring finger protein 213 mutation: a case report. Article in Japanese. No Shinkei Geka. 2020;48(6):533–540. doi: 10.11477/mf.1436204224. [DOI] [PubMed] [Google Scholar]
- 22. Seno T, Kohno K, Tanaka H, et al. A case of ruptured distal anterior choroidal artery aneurysm associated with a twig-like middle cerebral artery, treated with single-stage aneurysm clipping and STA-MCA double anastomoses in the acute phase. Article in Japanese. No Shinkei Geka. 2017;45(8):691–697. doi: 10.11477/mf.1436203576. [DOI] [PubMed] [Google Scholar]
- 23. Konoeda F, Ito Y, Yamada T, Suzuki N. Moyamoya disease (occlusion of the circle of Willis) research group database aggregation of 2012. Diagnoses and treatments of Occlusion of the Circle of Willis research of 2012. 2012:20–24. [Google Scholar]
- 24. Arias EJ, Derdeyn CP, Dacey RG, Jr, Zipfel GJ. Advances and surgical considerations in the treatment of moyamoya disease. Neurosurgery. 2014;74(Suppl 1):S116–S125. doi: 10.1227/NEU.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 25. Miyamoto S, Yoshimoto T, Hashimoto N, et al. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan Adult Moyamoya Trial. Stroke. 2014;45(5):1415–1421. doi: 10.1161/STROKEAHA.113.004386. [DOI] [PubMed] [Google Scholar]
- 26. Takarada A, Yanaka K, Onuma K, Nakamura K, Takahashi N, Ishikawa E. Aplastic or twig-like middle cerebral artery harboring unruptured cerebral aneurysms treated by clipping and bypass surgery: illustrative case. J Neurosurg Case Lessons. 2021;2(9) doi: 10.3171/CASE21360. CASE21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fuse Y, Takasu S, Seki Y. Preventive effect of bypass surgery on rebleeding in patients with hemorrhagic twiglike middle cerebral artery. World Neurosurg. 2021;148:e495–e501. doi: 10.1016/j.wneu.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi M, Fujimoto T, Suzuki R, Asai J, Miyo T, Hokaku H. A case of spontaneous middle cerebral artery occlusion associated with a cerebral aneurysm angiographically disappearing after STA-MCA anastomosis. Article in Japanese. No Shinkei Geka. 1997;25(8):727–732. [PubMed] [Google Scholar]
- 29. Uchiyama T, Okamoto H, Koguchi M, Tajima Y, Suzuyama K. A case of aplastic or twig-like middle cerebral artery presenting with an intracranial hemorrhage two years after a transient ischemic attack. Article in Japanese. No Shinkei Geka. 2016;44(2):143–148. doi: 10.11477/mf.1436203246. [DOI] [PubMed] [Google Scholar]
- 30. Hirai S, Ogawa Y, Takai Y, et al. Report of 3 cases of twig-like middle cerebral artery and review of the literature. No Shinkei Geka Sokuho. 2018;28(4):410–416. [Google Scholar]
- 31. Lutz T, Mönnings P, Ayzenberg I, Lukas C. Twig-like middle cerebral artery: a seldom vessel anomaly of important relevance. Clin Neuroradiol. 2018;28(3):441–443. doi: 10.1007/s00062-017-0613-9. [DOI] [PubMed] [Google Scholar]
- 32. Matsunaga Y, Izumo T, Morofuji Y, Horie N, Hayashi K, Matsuo T. Revascularization for aplastic or twiglike middle cerebral artery: a case report. J Stroke Cerebrovasc Dis. 2018;27(5):e78–e79. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 33. Cho KC, Kim JJ, Jang CK, Hong CK, Joo JY, Kim YB. Rete middle cerebral artery anomalies: a unifying name, case series, and literature review. J Neurosurg. 2018;131(2):453–461. doi: 10.3171/2018.2.JNS1832. [DOI] [PubMed] [Google Scholar]