Abstract
The thermotolerance of E. coli O157:H7 cells (strain 380-94) heated in pepperoni is reported. Information on the pattern of thermal inactivation of E. coli O157:H7 in pepperoni was applied in the development of heating processes designed to reduce E. coli O157:H7 numbers therein by 5 log10 units.
The identification of fermented meat as a vehicle of infection for Escherichia coli O157:H7 (4) has led to the development of heat treatments for such products (3, 8, 10) such that a 5-log10-unit reduction in E. coli O157:H7 numbers can be achieved during processing. Fermented meat had traditionally relied on its low pH status as its primary preservative mechanism; however, E. coli O157:H7 has been shown to be unusually resistant to inactivation at low pHs (9, 12, 13). Thus, it is imperative that this factor be borne in mind when heat treatments are designed. Previous studies involving E. coli O157:H7 (5, 16) have shown that cross-protection, i.e., preadaptation to one stress encountered in the environment, e.g., acid, can lead to enhanced resistance to a different, and possibly previously unencountered, stress, e.g., heat. Therefore, the possibility that cross-protection could occur must be considered in the development of thermal inactivation protocols. This study aimed to elucidate the effects of prior acid adaptation and final product pH on the thermotolerance of E. coli O157:H7 in pepperoni. The information derived was used to develop a commercially applicable postfermentation heating process capable of inducing a 5-log10-unit reduction in E. coli O157:H7 numbers.
E. coli O157:H7 (strain 380-94) was obtained from the Centers for Disease Control and Prevention, Atlanta, Ga., maintained on tryptone soya agar (TSA; Oxoid, Basingstoke, England) at 4°C, and recultured monthly. Inocula were prepared by adding one loopful of culture from TSA into (i) 50 ml of brain heart infusion broth (BHI; Oxoid) (0.2% [wt/vol] glucose) or (ii) 50 ml of BHI supplemented with 1% (wt/vol) glucose (1.2% [wt/vol] glucose). Cultures were grown at 37°C (18 h) to provide non-acid-adapted organisms and acid-adapted organisms, respectively (2). pH was estimated using a model 210 pH meter (Orion Research Corp., Boston, Mass.). Pepperoni batters were prepared and fermented by the following modifications of the method previously described (18). Meat mixtures were inoculated with acid-adapted and non-acid-adapted E. coli O157:H7, as previously outlined, and stored at 4°C overnight. The meat mixtures were then incorporated into standard- and low-pH batter mixes. The standard-final-pH product was formulated to ferment to a final pH of 4.8 by the addition of 0.625% sodium nitrite (0.01%), Pediococcus spp. starter culture (0.028%), sodium ascorbate (0.048%), spice mix (0.302%), dextrose (0.625%), mustard flour (1.5%), and salt (2.5%) (all wt/wt) with other ingredients previously described (18). The low-final-pH product was formulated to ferment to a final pH of 4.4 by the addition of dextrose at 1% (wt/wt). Approximately 12 sausages per batch (150 by 30 mm) (three batches per combination of bacterial inoculum and final product pH) were produced and fermented as described previously (18). The pH of the pepperoni was determined at the end of fermentation.
Thermal inactivation trials were performed on acid-adapted and non-acid-adapted E. coli O157:H7 cells in pepperoni pre- and postfermentation made from standard- and low-pH formulation batters. Samples of pepperoni (2 g) were placed into sterile sampling bags (15 by 22.9 cm), and the samples were compressed into a layer approximately 2 mm thick by pressing them against a flat surface to exclude air. The bags were sealed with a vacuum bag sealer (Multivac, Kansas City, Mo.). Batches of sealed bags (10 to 15 bags per batch), including one bag per batch fitted with a thermocouple (Ellab A/S, Copenhagen, Denmark), were prepared. The bags were submerged in water baths preequilibrated to 55, 58, 60, or 62°C (±0.2°C). Process timing commenced when the thermocouples indicated that the samples had attained the target internal temperature (<20 s). Bags were then sequentially removed from each water bath at regular intervals and cooled to 0°C within 5 min in an ice bath. Each heating trial at each temperature for each combination of bacterial inoculum and final product pH was repeated three times.
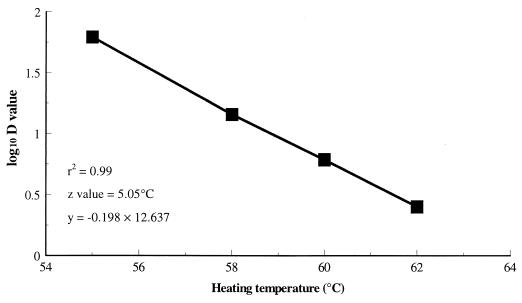
In the second part of the study, heat treatments were derived using data from non-acid-adapted E. coli O157:H7 heated in pepperoni fermented to pH 4.8, as this combination is closest to that which occurs in the processing environment. These data were compiled into a thermal-death time curve (Fig. 1) and used to derive an equation relating decimal reduction time (i.e., D value, the time required to achieve a 1-log10-unit [90%] reduction in bacterial numbers) to heating temperature; thus, the heating time required to achieve a 1 log10 reduction in E. coli O157:H7 numbers is −0.198 times the heating temperature plus 12.637.
FIG. 1.
Thermal-death time curve and associated equation for non-acid-adapted cells heated in pepperoni postfermentation to pH 4.8.
The heating temperatures chosen (58.3 and 61°C, to give relatively long and short heating times, respectively) were inserted into this equation, and the D values were calculated as follows: heating time for a 1-log reduction at 58.3°C is −0.198 times 58.3 plus 12.637, which yields a D value of 12.26 min, and heating time for a 1-log reduction at 61°C is −0.198 times 61 plus 12.637, which yields a D value of 3.58 min.
Therefore, a 5-log10-unit reduction in numbers should be achieved by heating fermented pepperoni sausages to internal temperatures of 58.3°C for 61.3 min or 61°C for 17.9 min (i.e., five times the D value at each heating temperature). To test if these heat treatments would achieve the desired reduction in E. coli O157:H7 numbers, batches of sausages (standard-pH formulation), inoculated with non-acid-adapted E. coli O157:H7, were produced as described above. Two sausages in each batch of 12 were fitted with thermocouples (Barnant Co., Barrington, Ill.). The entire batch of 12 sausages was fermented as described above and heated to the target heating temperature immediately following fermentation. The relative humidity of the controlled humidity cabinet was adjusted to 55%. This procedure ensured that the target internal temperatures were uniformly attained within 1 h, whereupon timing of the heat treatment commenced and were maintained (±0.2°C) during the heat treatment period. Sausages were sampled every 5 to 10 min during the heat treatment. Heating trials were repeated three times at each heating temperature.
Pepperoni was produced to the specifications previously outlined by a commercial manufacturer (International Meat Ingredients, Naas, County Kildare, Ireland) and subjected to the heat treatments described above. The resultant products were subjected to sensory analysis in collaboration with the product development team at this company, using directional triangle tests (11). Trials were repeated three times.
E. coli O157:H7 numbers were estimated by plating bacteria onto TSA, incubating the plates at 37°C for 2 h to allow recovery of injured E. coli O157:H7 cells, and overlaying the plates with Sorbitol MacConkey agar (SMAC; Oxoid) (6). Samples heated in pepperoni were also plated on SMAC only. All samples were incubated overnight at 37°C. E. coli O157:H7 colonies were identified by a commercial latex test which identifies the O157 antigen (Oxoid).
The D value was calculated by plotting log10-unit numbers of survivors, as enumerated on TSA-SMAC after being heated in pepperoni, against time and obtaining the reciprocal of the slope of the line (14), using Lotus spreadsheet software. Analyses of variance were performed to compare D values obtained from the heating trials and counts obtained by plating bacteria on TSA-SMAC and SMAC alone during the heating trials in the second part of the study.
E. coli O157:H7 numbers were reduced by 0.23 to 1.03 log10 CFU g−1 during pepperoni fermentation. Overall, a significantly greater decline was observed in acid-adapted E. coli O157:H7 cell numbers (0.95 log10 CFU g−1) than in non-acid-adapted cell numbers (0.42 log10 CFU g−1) (P < 0.01). There was no significant difference between the reduction in E. coli O157:H7 numbers during fermentation to pH 4.8 (0.62 log10 CFU g−1) and the reduction observed during fermentation to pH 4.5 (0.78 log10 CFU g−1).
Survivor curves for E. coli O157:H7 following heating at 55, 58, 60, and 62°C in pepperoni demonstrated a linear decrease in organism numbers. Figure 1 shows the thermal-death time curve, z value, and the associated equation from which the heat treatment for non-acid-adapted cells in standard-pH pepperoni postfermentation was developed. Table 1 presents the D values derived from the survivor curves and indicates the associated least significant difference for each treatment at each heating temperature. There was a significant three-way interaction (P < 0.001) among the factors (i.e., acid-adapted cells/non-acid-adapted cells × prefermentation/postfermentation × standard-pH product/low-pH product); consequently, it was not possible to accurately determine the individual significance of any one factor on the D values obtained. However, general trends could be deduced.
TABLE 1.
Effect of cell status and final product pH on the thermotolerance at 55, 58, 60, and 62°C of E. coli O157:H7, heated in pepperoni pre- and postfermentation
| Heating temp (°C) | Cell status | D value (min)a
|
Least-significant difference (7 df)b | |||
|---|---|---|---|---|---|---|
| Final product pH of 4.8 (standard)
|
Final product pH of 4.5 (low)
|
|||||
| Prefermentation | Postfermentation | Prefermentation | Postfermentation | |||
| 55 | Acid adapted | 35.09 (7.87) | 22.10 (0.72) | 39.25 (12.10) | 17.62 (5.06) | |
| Non-acid adapted | 39.52 (10.03) | 62.02 (16.09) | 46.21 (1.75) | 16.01 (2.89) | 14.05 | |
| 58 | Acid adapted | 10.97 (1.43) | 5.22 (0.71) | 14.81 (2.68) | 4.51 (1.34) | |
| Non-acid adapted | 17.21 (2.08) | 14.35 (2.31) | 18.39 (1.47) | 4.10 (0.63) | 2.77 | |
| 60 | Acid adapted | 3.62 (0.19) | 4.37 (1.44) | 5.36 (0.47) | 2.90 (0.56) | |
| Non-acid adapted | 5.87 (0.78) | 6.12 (0.82) | 6.73 (1.11) | 2.35 (0.45) | 1.39 | |
| 62 | Acid adapted | 1.33 (0.22) | 1.64 (0.25) | 1.89 (0.05) | 1.25 (0.15) | |
| Non-acid adapted | 2.30 (0.41) | 2.53 (0.24) | 2.48 (0.47) | 1.16 (0.21) | 0.52 | |
Standard deviations are in parentheses.
95% confidence limit.
Non-acid-adapted cells from pepperoni fermented to pH 4.8 had higher D values than non-acid-adapted cells from pepperoni fermented to pH 4.5. Also, the D values obtained for non-acid-adapted cells heated in pepperoni fermented to pH 4.8 were generally higher than the D values obtained for acid-adapted cells heated in this medium.
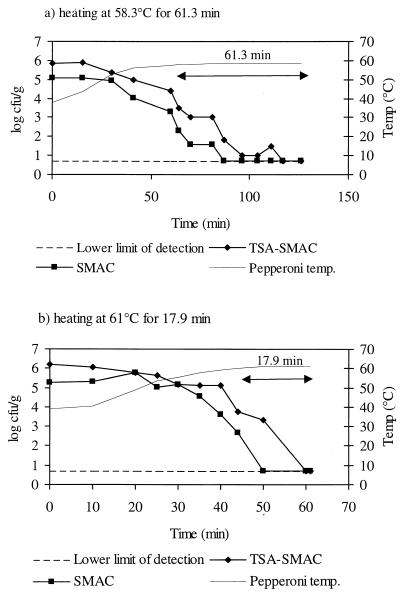
E. coli O157:H7 numbers declined steadily during the heat treatments applied (Fig. 2). Counts were significantly lower on SMAC than on TSA-SMAC (P < 0.05). E. coli O157:H7 numbers enumerated on TSA-SMAC declined by >5 log10 units following the heat treatments described. Sensory trials indicated that pepperoni heated at 58.3°C for 61.3 min was readily discernible from non-heat-treated pepperoni, while pepperoni heated at 61°C for 17.9 min was undistinguishable from the non-heat-treated product.
FIG. 2.
Survivor curves for E. coli O157:H7 (non-acid-adapted cells) heated in pepperoni postfermentation to pH 4.8. Growth on TSA-SMAC represents total viable count; growth on SMAC represents uninjured cells only.
This study has shown that the physiological status of the cells and the final product pH can affect the thermotolerance of E. coli O157:H7 in pepperoni. Non-acid-adapted cells were generally more resistant to heat than acid-adapted cells; hence, no cross-protection was observed. It is possible that a large proportion of the acid-adapted cells (as much as 50 to 90%) were in fact stressed during growth to stationary phase, as found by Buchanan and Edelson (2). Growth in BHI supplemented with 1% (wt/vol) glucose, intended to enhance the acid tolerance of these cells, may actually have sublethally injured them and increased their sensitivity to the stresses encountered during pepperoni production. For future studies of this nature, it is advisable to investigate alternative methods, such as those described by Lin et al. (12), of acid adapting E. coli O157:H7.
While the net reductions in E. coli O157:H7 numbers during the fermentation process were small, the process was shown to contribute to a reduction in the risks posed by this organism, as cells present in pepperoni fermented to the lower final pH had lower D values than cells in pepperoni heated to the standard pH. Studies have shown that E. coli O157:H7 has less resistance to heat in lower-final-pH fermented meat products than in higher-final-pH products (1, 3, 8). This finding has a commercial application, as it suggests that in meat products fermented to lower pH values (i.e., pH 4.5 rather than pH 4.8), desirable reductions in E. coli O157:H7 numbers may be achieved with milder heat treatments, entailing less thermal damage to the organoleptic quality of a product.
Both of the heating protocols applied in this study achieve reductions in line with those required by the U.S. Department of Agriculture Food Safety and Inspection Service directive to fermented meat processors (15). Other heating protocols have also been designed for this purpose (3, 8, 10). The heat treatments reported by these researchers as effective in these products are milder than the treatments identified as necessary in this study. This may be a consequence of the specific natures of different fermented meat products, suggesting that heating protocols for different products must be calculated individually. Additionally, observed differences may be due to differences in enumeration protocols, rather than real differences in survival rates. Hinkens et al. (10) enumerated E. coli O157:H7 survivors by plating heated samples directly onto SMAC, with no resuscitation interval. A previous study (7) has shown that significant numbers of heat-injured cells fail to grow on this medium, which can lead to considerable underestimation of the numbers of E. coli O157:H7 surviving in heat-treated products. The procedure used in the present study, i.e., the use of TSA-SMAC, allowed the recovery and enumeration of sublethally injured cells, in addition to uninjured cells. Silk and Donnelly (18) found that plating on TSA, with no selective agent, resulted in the highest recovery of acid-injured E. coli O157:H7 in autoclaved apple cider. However, it was not feasible to use this nonselective medium in the present study due to the presence of competing microfloras in the fermented meat system.
The thermal inactivation data supplied in this study, specifically the thermal-death time equation, can be used to calculate and select equivalent alternative times and temperatures for heating steps within the range 55 to 62°C. The data obtained in this study has, perhaps, greater utility than that from other studies (3, 8, 10), as it can be used by a manufacturer to identify an optimum heat treatment step which meets relevant product requirements, in terms of product safety, while maintaining other important sensory characteristics.
Acknowledgments
We acknowledge the assistance of Damien O'Connor, Sara Kane, and John Donnelly (International Meat Ingredients) for the gift of ingredients used and their assistance with the sensory analysis. Thanks go to Jim Bacus (Diversitech, Gainesville, Fla.) for the gift of the starter culture used and to Donald DiBello (Heller Seasonings and Ingredients, Bethlehem, Pa.) for the gift of the mustard flour. Thanks also go to Marsha Golden (USDA, Wyndmoor, Pa.) for excellent technical assistance and to Tony Hegarty (Teagasc) for assistance with the statistical analyses.
REFERENCES
- 1.Bacus J. Processing procedures to control Salmonella and E. coli in fermented sausage products. Food Aust. 1997;49:543–547. [Google Scholar]
- 2.Buchanan R L, Edelson S G. Culturing enterohemorrhagic Escherichia coli in the presence and absence of glucose as a simple means of evaluating the acid tolerance of stationary-phase cells. Appl Environ Microbiol. 1996;62:4009–4013. doi: 10.1128/aem.62.11.4009-4013.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calicioglu M, Faith N G, Buege D R, Luchansky J B. Viability of Escherichia coli O157:H7 in fermented semidry low-temperature-cooked beef summer sausage. J Food Prot. 1997;60:1158–1162. doi: 10.4315/0362-028X-60.10.1158. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Escherichia coli O157:H7 outbreak linked to commercially distributed dry-cured salami—Washington and California, 1994. Morbid Mortal Weekly Rep. 1995;44:157–160. [PubMed] [Google Scholar]
- 5.Cheville A M, Arnold K W, Buchreiser C, Cheng C-M, Kaspar C W. rpoS regulation of acid, heat and salt tolerance in Escherichia coli O157:H7. Appl Environ Microbiol. 1996;62:1822–1824. doi: 10.1128/aem.62.5.1822-1824.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle M P, Schoeni J L. Survival and growth characteristics of Escherichia coli associated with hemorrhagic colitis. Appl Environ Microbiol. 1984;48:855–856. doi: 10.1128/aem.48.4.855-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffy G, Riordan D C R, Sheridan J J, Eblen B S, Whiting R C, Blair I S, McDowell D A. Differences in thermotolerance of various Escherichia coli O157:H7 strains in a salami matrix. Food Microbiol. 1999;16:83–91. [Google Scholar]
- 8.Ellajosyula K R, Doores S, Mills E W, Wilson R A, Anantheswaran R C, Knabel S J. Destruction of Escherichia coli O157:H7 and Salmonella typhimurium in Lebanon bologna by interaction of fermentation pH, heating temperature, and time. J Food Prot. 1998;61:152–157. doi: 10.4315/0362-028x-61.2.152. [DOI] [PubMed] [Google Scholar]
- 9.Gorden J, Small P L C. Acid resistance in enteric bacteria. Infect Immun. 1993;61:364–367. doi: 10.1128/iai.61.1.364-367.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinkens J C, Faith N G, Lorang T D, Bailey P, Buege D, Kaspar C W, Luchansky J B. Validation of pepperoni processes for control of Escherichia coli O157:H7. J Food Prot. 1996;59:1260–1266. doi: 10.4315/0362-028X-59.12.1260. [DOI] [PubMed] [Google Scholar]
- 11.Lawless H T, Heymann H. Sensory evaluation of food—principles and practices. New York, N.Y: Chapman and Hall; 1998. pp. 121–122. [Google Scholar]
- 12.Lin J, Lee I S, Fey J, Slonczewski J L, Foster J W. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J Bacteriol. 1995;177:4097–4104. doi: 10.1128/jb.177.14.4097-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin J, Smith M P, Chapin K C, Baik H S, Bennett G N, Foster J W. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl Environ Microbiol. 1996;62:3094–3100. doi: 10.1128/aem.62.9.3094-3100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miles C A, Mackey B M. A mathematical analysis of microbial inactivation at linearly rising temperatures: calculation of the temperature rise needed to kill Listeria monocytogenes in different foods and methods for dynamic measurements of D and z values. J Appl Bacteriol. 1994;77:14–20. doi: 10.1111/j.1365-2672.1994.tb03038.x. [DOI] [PubMed] [Google Scholar]
- 15.Reed C A. Approaches for ensuring safety of dry and semi-dry fermented sausage products. 21 August 1995 letter to plant managers. Washington, D.C.: Food Safety and Inspection Service, U.S. Department of Agriculture; 1995. [Google Scholar]
- 16.Riordan D C R. A study on the survival of Escherichia coli O157:H7 in fermented meat. Ph.D. thesis. Newtownabbey, County Antrim, Northern Ireland: University of Ulster, Jordanstown; 1998. [Google Scholar]
- 17.Riordan D C R, Duffy G, Sheridan J J, Eblen B S, Whiting R C, Blair I S, McDowell D A. Survival of Escherichia coli O157:H7 during the manufacture of pepperoni. J Food Prot. 1998;61:146–151. doi: 10.4315/0362-028x-61.2.146. [DOI] [PubMed] [Google Scholar]
- 18.Silk T M, Donnelly C W. Increased detection of acid-injured Escherichia coli O157:H7 in autoclaved apple cider by using non selective repair on Trypticase soy agar. J Food Prot. 1997;60:1463–1465. doi: 10.4315/0362-028X-60.12.1483. [DOI] [PubMed] [Google Scholar]