Abstract
Corneal disease remains a leading cause of impaired vision world-wide, and advancements in gene therapy continue to develop with promising success to prevent, treat and cure blindness. Ideally, gene therapy requires a vector and gene delivery method that targets treatment of specific cells or tissues and results in a safe and nonimmunogenic response. The cornea is a model tissue for gene therapy due to its ease of clinician access and immuneprivileged state. Improvements in the past 5–10 years have begun to revolutionize the approach to gene therapy in the cornea with a focus on adeno-associated virus and nanoparticle delivery of single and combination gene therapies. In addition, the potential applications of gene editing (zinc finger nucleases [ZNFs], transcription activator-like effector nucleases [TALENs], Clustered Regularly Interspaced Short Palindromic Repeats/Associated Systems [CRISPR/Cas9]) are rapidly expanding. This review focuses on recent developments in gene therapy for corneal diseases, including promising multiple gene therapy, while outlining a practical approach to the development of such therapies and potential impediments to successful delivery of genes to the cornea.
Keywords: Cornea, Keratocyte, Fibrosis, Neovascularization, Viral vector, Non-viral vector, Combination gene therapy, Gene editing
1. Introduction
The last two decades of research have advanced eye gene therapy from a conceptual validation to a clinical reality as the first gene therapy treatment for a rare retinal disease, Leber congenital amaurosis, caused by mutations in RPE65 gene was approved by the U.S. Food and Drug Administration in 2017 (Prado et al., 2020). As of 2017, ocular diseases accounted for only 1.3% of all gene therapy clinical trials, whereas cancer accounted for 65% (Table 1) (Ginn et al., 2017). Investigations specifically related to the cornea are considerably less in number than the retina (Table 2) (Ginn et al., 2017), however, progress in the corneal gene therapy arena has been astounding despite remaining at the preclinical level. Accumulating literature reveals validation of proof-of-concept, identification of many therapeutic genes, optimization of critical tools and techniques for cell-targeted gene delivery, establishment of novel molecular targets, and demonstration of gene therapy success in treating corneal defects and diseases in vivo employing standard animal models (Di Iorio et al., 2019; Mohan et al., 2013, 2018). According to the World Health Organization, corneal blindness is the fourth leading cause of blindness globally with over 39 million people having complete blindness and 246 million experiencing compromised vision. Corneal disorders are responsible for vision loss in nearly 4% of the population of the United States; and are the second leading cause of blindness in most developing countries around the world (Oliva et al., 2012; Pascolini and Mariotti, 2012). Even more astonishing is the increasing number of young people developing corneal disease and living the rest of their lives with a disability (Oliva et al., 2012). The National Eye Institute reports that an annual estimate of $70 billion is spent for ophthalmic care at present and projects this amount to reach to $717 billion by 2050; therefore, clinicians/scientists must find preventives, treatments, and cures for corneal blindness. Principle causes of blindness include infection (trachoma, onchocerciasis) and injury (trauma, combat wounds), but iatrogenic etiologies are also a consideration due to routine medical practices such as photorefractive keratectomy (PRK) (World Health Organization, 2020).
Table 1.
Gene therapy clinical trials based on study classification as of 2017 (Ginn et al., 2017).
| Classification | Number of trials | Percentage (%) |
|---|---|---|
|
| ||
| Cancer diseases | 1688 | 65 |
| Monogenic diseases | 287 | 11.1 |
| Infectious diseases | 182 | 7 |
| Cardiovascular diseases | 180 | 6.9 |
| Other diseases | 58 | 2.2 |
| Healthy volunteers | 56 | 2.2 |
| Gene marking | 50 | 1.9 |
| Neurological diseases | 47 | 1.8 |
| Ocular diseases | 34 | 1.3 |
| Inflammatory diseases | 15 | 0.6 |
Table 2.
Ocular diseases for which human gene transfer clinical trials have been approved as of 2017 (Ginn et al., 2017).
| Cornea | Retina |
|---|---|
|
| |
| Superficial corneal opacity | Achromatopsia |
| Age-related macular degeneration | |
| Choroideraemia | |
| Diabetic macular edema | |
| Glaucoma | |
| Leber congenital amaurosis | |
| Macular telangiectasia type 2 | |
| Retinitis pigmentosa | |
Ideal gene therapy involves safe and non-immunogenic introduction of genetic material, by an able vector, into targeted cells rendering them capable of repairing, replacing, and/or overpowering the functions of defective/non-functional genes for therapeutic purposes. The cornea is a model tissue for gene therapy due to its easy accessibility, immuneprivileged status, and location allowing visualization of gene therapy response by the naked eyes. All possible methods such as topical, mechanical, surgical, electrical, or chemical can be utilized to administer gene therapy vector in the cornea, and employment of simple vector-delivery techniques have yielded tissue-specific gene delivery into desired cells in the rabbit and rodent cornea in vivo and human cornea ex vivo. In addition, the cornea can be maintained in an artificial physiological environment for several weeks for experimental purposes but also for the application of treatments prior to corneal transplantation, aiming to minimize deleterious effects of graft rejection (Mohan et al., 2013; Torrecilla et al., 2018).
Recently, gene therapy has embraced a new dynamic called gene editing, which essentially is a collection of state-of-the art technological advances enabling researchers to expedite editing of desired genetic material in the organism’s genome through insertion, deletion, modification, or replacement of genetic material. Facilitation of gene editing includes zinc finger nucleases (ZNFs), transcription activator-like effector nucleases (TALENs), and Clustered Regularly Interspaced Short Palindromic Repeats/Associated Systems (CRISPR/Cas9) with efficient vectors for systemic delivery to attain highly specific gene editing with minimal adverse effects (Chang et al., 2018; Raikwar et al., 2016). Gene editing has incredible potential to provide long-term resolution of corneal disease, especially those of congenital or inherited origin (Yin et al., 2014).
This review discusses recent developments in gene therapy for corneal diseases, including promising single and dual gene therapy approaches and gene editing promises, while outlining a practical approach for the further development of such molecular therapies and deliberating limitations to successful delivery of genes into the cornea.
2. Gene therapy delivery apparatuses
The success of gene therapy for corneal diseases is largely dependent on the introduction of adequate levels of therapeutic genes into sought cell populations while utilizing the least invasive methods (patient safety) and minimal immune reaction. Broadly, there are two major categories of vectors, viral and non-viral, that are used for this purpose and are described and shown here within.
2.1. Viral vectors
Viruses have emerged as one of the most effective vehicles to deliver therapeutic recombinant nucleic acids into cells for corneal gene therapy purposes. Viruses have a unique ability to invade living cells quickly and with minimal resistance; however, viral vectors used in gene delivery are replication-deficient. They utilize different mechanisms and start replicating and producing more copies by hijacking host’s cellular machinery. These characteristics have allowed researchers to rely on viruses to cultivate physiological gene function resulting in the treatment of various corneal ailments. Viruses are modulated through recombinant DNA technology for gene therapy purposes and are naturally occurring but not wild-type. The commonly tested recombinant viral vectors for corneal gene therapy include adenovirus (rAV), retrovirus (rRV), lentivirus (rLV), herpes simplex virus (rHSV), and adeno-associated virus (rAAV) (Mohan et al., 2013). Table 3, Table 4, and Fig. 1 describe properties, size, duration of delivered-gene expression, advantages, limitations, and mode of cellular entry of different vectors for various corneal disorders.
Table 3.
Characteristics, advantages, and limitations of commonly investigated vectors used in corneal gene therapy.
| Vectors Properties | Adenovirus | Retrovirus | Lentivirus | HSV | AAV | Nanoparticle | Non-viral |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Virus family | Adenoviridae | Retrovirdae | Retrovirdae | Herpesviridae | Parvoviridae | – | – |
| Size of vector | 70–90 nm | 80–130 nm | 80–130 nm | 120–300 nm | 18–26 nm | 1–100 nm | ≥500 nm |
| Size of genome | 38–39 kb | 3–9 kb | 3–9 kb | 152 kb | 4.7 kb | – | – |
| Type | dsDNA | ssRNA | ssRNA | dsDNA | ssDNA | Chemicals | Chemicals |
| Size of virion | 70–90 nm | 80–130 nm | 80–130 nm | 120–300 nm | 18–26 nm | – | – |
| Integration in host genome | No | Yes | No, Yes (if impaired) | No | No | No | No |
| Transduction efficiency | Low - High | Moderate - High | High | Low - High | Moderate - High | Moderate - High | Low - Moderate |
| Delivered-gene time duration | Days - Weeks | Months -Years | Months -Years | Days - Weeks | Weeks -Months | Hours -Months | Hours - Days |
| Immunogenicity | High | Moderate -High | Moderate -High | Low - Moderate | Low -Moderate | Low - High | Low |
| Ability to deliver genes into cells | Dividing | Dividing | Dividing and Non-dividing | Dividing | Dividing and Non-dividing | Dividing and Non-dividing | Dividing and Non-dividing |
| Therapeutic load carrying capacity | ≤7.5 kb | ≤8 kb | ≤8 kb | ≤30 kb | ≤1.8 kb | No limit | No limit |
| Advantages | Highly efficacious for proliferating corneal cells in vivo, in vitro and ex vivo, easily available, high success rate, non-mutagenic | Highly efficient for gene delivery into all major corneal cells in vivo, and ex vivo, provide high and long-term delivered-gene expression, high success rate, low immune reaction in host | High efficiency for delivering genes into all 3 major corneal cells and stem cells, sustained delivered-gene expression, new generation LVs are efficacious and safe, widely useful for in vitro gene therapy concept testing and expression profiling | Good for delivering genes in most of corneal cells, long-term high levels in vivo transgene expression | Highly efficient for delivering genes in dividing and non-dividing corneal cells in vivo, in vitro, and ex vivo, stable transgene expression, high and extended-time delivered-gene expression, low/no immune reaction, many serotypes, low safety risk, site-specific integration | Highly potent and safe for corneal cells, low immune reaction, multi-encapsulation ability, any size therapeutic gene transportation capacity, allows tracking while in cell/tissue, ability to dictate release of therapeutics | Mostly harmless and safe, induce mild local immune reaction, deliver large therapeutic genes, cost effective, easy commercial production |
| Limitations | High immune reaction, repeat doses ineffective, high safety risk, can harm caregiver, short-term gene expression | Variable in vivo gene transfer due to dependence on target cell mitosis, requires dividing cells, low titer, safety concerns, random integration | HIV origin, high immunogenicity, safety concerns, titer production pleads technical skills | Difficult to produce in large quantities | Small insert size, production needs technical skills, concerns of AV contamination | Not all NP show same response, often optimization required, variable gene transfer efficiency, short-term transgene expression | Poor efficiency, low-high immune reaction, short-term transgene expression |
Table 4.
Target therapeutic genes, study models, species, and delivery techniques are outlined by various vector platforms and corneal disorders.
| rAdenovirus Vector | |||||
|---|---|---|---|---|---|
|
| |||||
| Corneal Disorder | Therapeutic Gene | Model | Species | Delivery | References |
|
| |||||
| Corneal Graft Rejection | Interleukin 4, 12 | In Vivo | Rat | IP | Ritter et al. (2007) |
| Interleukin 10 | Ex Vivo | Sheep | IP | Yuan et al. (2013) | |
| Interleukin 12 | In Vivo | Rat | IP | Yuan et al. (2013) | |
| Inducible T-cell Co-stimulator (ICOS) | Ex Vivo | Rat | Topical | Gong et al. (2006) | |
| T-Lymphocyte-Associated Antigen 4 | In Vivo | Rat | IV | Nguyen et al. (2013); Yuan et al. (2013) | |
| NGF | In Vivo | Rat | IP | Nguyen et al. (2013) | |
| Corneal Scarring, Haze, and Wound Healing | Type II TGFβ Receptor | In Vivo, Ex Vivo | Mice, Rabbit, Human | IM, Topical | Sharma et al. (2012) |
| BMP7 | In Vivo | Mice | Topical | Gupta et al. (2018); Saika et al. (2005a) | |
| Smad7 | In Vivo | Mice | Topical | Saika et al. (2005b) | |
| Corneal Alkali Burn Injury | Smad7 | In Vivo | Mice | Topical | Saika et al. (2005b) |
| BMP7 | In Vivo, In Vitro | Mice, Human | Topical | Gupta et al. (2018); Saika et al. (2005a) | |
| PPAR γ | In Vivo | Mice | IV | Li et al. (2014) | |
| Corneal Neovascularization | VEGFR FLT-1 | In Vivo | Mice | IV | Lai et al. (2005) |
| Decorin | In Vivo | Rabbit | Topical | Chaudhary et al. (2014) | |
| Chloramphenicol acetyltransferase | In Vivo | Rabbit | Lamellar Flap | Mohan et al. (2003) | |
| β-Galactosidase | In Vivo | Mice, Rabbit | IS | Larkin et al. (1996); Selvam et al. (2006) | |
| sFlt-1 | In Vivo | Rat | IC | Lai et al. (2005) | |
| Vasohibin-1 | In Vivo | Mice | Subconj. | Zhou et al. (2010) | |
| Herpes Simplex Virus Keratitis | HGF c-met | In Vitro | Human | Topical | Gupta et al. (2018) |
| HSV Glycoproteins (gB, gC, gD, gE, gI) | In Vivo | Mice, Rabbit | IP, IM, Subconj | Elbadawy et al. (2012) | |
| Lacrimal Gland, Dry Eye, | TNF α Inhibitor | In Vivo | Rabbit | Lacrimal Gland | Selvam et al. (2006) |
| Sjogren syndrome | Interleukin 10 | In Vivo | Rabbit | Lacrimal Gland | Selvam et al. (2006) |
| Conjunctival Scarring | Smad7 | In Vivo | Mice | Topical | Saika et al. (2005b) |
| PPAR γ | In Vivo | Mice | IV | Li et al. (2014) | |
| rRetrovirus Vector | |||||
| Corneal Disorder | Therapeutic Gene | Model | Species | Delivery | References |
| Corneal Scarring, Haze, and Wound Healing | HSV Thymidine Kinase | In Vivo | Rabbit | Topical | Selvam et al. (2006) |
| Dom-Neg. Cyclin G1 | In Vivo | Rabbit | Topical | Behrens et al. (2002) | |
| Dom-Neg. p38MAPK | In Vivo | Mice | Topical | Behrens et al. (2002) | |
| rLentivirus Vector | |||||
| Corneal Disorder | Therapeutic Gene | Model | Species | Delivery | References |
| Corneal Graft Rejection | Bcl-xL | In Vitro | Human | Liu et al. (2005) | |
| Corneal Neovascularization | Endostatin/Kringle 5 Plasmin | In Vivo | Rabbit | Subconj. | Murthy et al. (2003) |
| rHerpes Simplex Virus Vector | |||||
| Corneal Disorder | Therapeutic Gene | Model | Species | Delivery | References |
| Corneal Endothelial Cell Wound Healing | LacZ | Ex Vivo | Rabbit, Human | Topical | Hudde et al. (2000) |
| LacZ | In Vivo | Monkey | IC | Liu et al. (1999) | |
| LacZ | In Vivo | Mice | IV, IC | Spencer et al. (2000) | |
| rAdeno-associated Virus Vector | |||||
| Corneal Disorder | Therapeutic Gene | Model | Species | Delivery | References |
| Corneal Scarring, Haze, and Wound Healing | Decorin | In Vivo, Ex Vivo | Mice, Rabbit, Human | Topical | Mohan et al. (2019); Mohan et al. (2011b) |
| Smad7 | Ex Vivo | Rabbit, Horse | Topical | Saika et al. (2005b); Marlo et al. (2018); Gupta et al. (2017) | |
| Type II TGFβ Receptor | In Vivo, In Vitro | Mice, Rabbit, Human | IM, Topical | Sharma et al. (2012) | |
| Corneal Neovascularization | sFlt-1 | In Vivo, Ex Vivo | Mice, Monkey | IC | Lai et al. (2005) |
| Decorin | In Vivo, Ex Vivo | Rabbit, Human | Topical | Mohan et al. (2011b), 2011c; Chaudhary et al. (2014) | |
| PEDF | In Vivo, Ex Vivo | Rabbit, Human | Topical | Yu et al. (2010) | |
| VEGFR FLT-1 | In Vivo | Mice | IS | Lai et al. (2005); Li et al. (2014); Mohan et al. (2011a) | |
| EGFP | In Vivo | Topical | Bosiak et al. (2012); Buss et al. (2010) | ||
| Corneal Alkali Burn Injury | Canine, Horse | ||||
| Angiostatin | In Vivo, Ex Vivo | Rat, Human | Subconj. | Cheng et al. (2007) | |
| Other Non-Viral Vectors/Methods | |||||
| Corneal Disorder | Therapeutic Gene | Model | Species | Delivery | References |
| Corneal Graft Rejection | Tissue Plasminogen Activator | In Vivo | Rat | Electroporation | Murthy et al. (2003) |
| Corneal Neovascularization | Kringle 5 Plasmin | In Vivo | Rabbit | Electroporation | Murthy et al. (2003) |
| Interleukin 10 | In Vivo | Mice | Topical | Nguyen et al. (2013) | |
| Interleukin 12 | In Vivo | Mice | Topical | Nguyen et al. (2013) | |
| VEGFR FLT-1 | In Vivo | Mice | IS | Lai et al. (2005) | |
| sFlt-1 | In Vivo | Mice | IS | Lai et al. (2005); Li et al. (2014) | |
| FLT23K, FLT24K | In Vivo | Mice | IS | Lai et al. (2005); Li et al. (2014) | |
| Herpes Simplex Virus Keratitis | HSV Glycoproteins (gB, gC, gD, gE, gI) | In Vivo | Mice, Rabbit | IP, IM, SubConj | Elbadawy et al. (2012) |
| Interleukin 2 | In Vivo | Mice | IV | Elbadawy et al. (2012) | |
| Interleukin 4 | In Vivo | Mice | IV | Elbadawy et al. (2012) | |
| Interleukin 10 | In Vivo | Mice | IV | Elbadawy et al. (2012) | |
| Nanoparticle Vector | |||||
| Corneal Disorder | Therapeutic Gene | Model | Species | Delivery | References |
| Corneal Scarring, Haze, and Wound Healing | BMP7 | In Vivo | Rabbit, Human | Topical | Gupta et al. (2018); Chaurasia et al. (2015) |
| Type II TGFβ Receptor | In Vivo, Ex Vivo | Mice, Rabbit, Human | IM, Topical | Sharma et al. (2012) | |
| PGLA | In Vivo | Rabbit | Topical | Chaurasia et al. (2015) | |
| Smad7 | In Vivo | Rabbit | Topical | Saika et al. (2005b) | |
| Corneal Neovascularization | FLT23K | In Vivo | Mice | IS | Lai et al. (2005) |
| Gene Editing by CRISPR/Cas9 | |||||
| Corneal Disorder | Therapeutic Gene | Model | Species | Delivery | References |
| Corneal Endothelial Cell Wound Healing | SOX2 | In Vivo | Rats | Electroporation | Chang et al. (2018) |
| Fungal Keratitis | Ura3 repair template | Ex Vivo | Human | Transformation | Lightfoot and Fuller (2019) |
| Congenital Aniridia | PAX6 of LSC | In Vitro | Human | Transformation | Roux et al. (2018) |
(BMP7: Bone Morphogenic Protein 7; HGF: Hepatocyte Growth Factor; HSV: Herpes Simplex Virus; PPAR γ: Peroxisome Proliferator-Activated Receptor gamma; TGFβ: Transforming Growth Factor Beta; TNFα: Tumor Necrosis Factor alpha; VEGF: Vascular Endothelial Growth Factor Receptor; IC: Intracameral; IM: Intramuscular; IP: Interpleural; IS: Intrastromal: IV: Intravenous; Subconj: Subconjunctival).
Fig. 1.
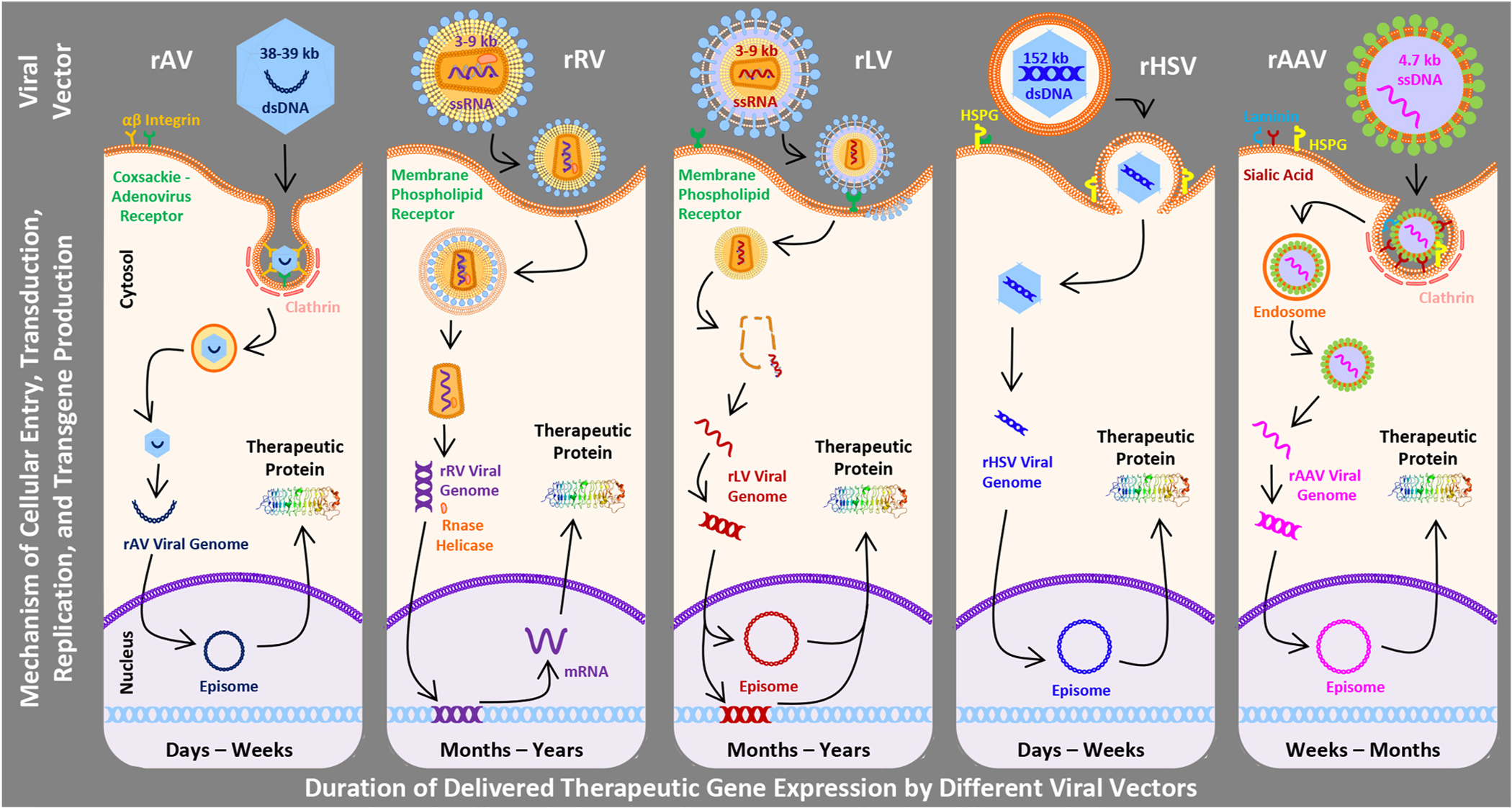
Schematic diagram showing vectors size, mechanisms of cellular entry, and gene transduction, replication, and production processes. Also shown are the respective durations of transgene expression for each viral vector.
2.1.1. Adenovirus
Adenovirus (AV) is a double-stranded DNA virus of the Adenoviridae family with a genome between 30 and 40 thousand base pairs. Out of 50 known human AV serotypes, serotypes 2 and 5 are commonly used for creating rAV by erasing the E1 gene required for viral gene expression and replication and producing them using a clever system (human kidney embryonic cells) affording erased E1 gene products (Sharma et al., 2010b). AV vectors transport therapeutic genes into the host cells via receptor-mediated endocytosis utilizing cell surface coxsackie-AV receptor, αvβ3 integrin, and clathrin-coated pit. After reaching the cytoplasm, the viral genome is released and transported to the nucleus for episomal replication to produce therapeutic DNA (Fig. 1) (Mohan et al., 2005). Typically, recombinant adenovirus vector (AV) is known to transfect corneal epithelial and endothelial cells and not keratocytes (Liu et al., 2008). Advantages of this vector include lack of integration into the host cell with no risk of insertional mutagenesis, transduction of many cell types (dividing and non-dividing), and ability to transport large genes as therapeutics (~30 kb). Disadvantages include a short duration of therapeutic gene expression (typically up to 2 weeks) and excessive inflammatory and immunogenic reactions due to the patient’s prior exposure to AVs (Table 3) (Mohan et al., 2013). Multiple generations of AV vectors have been designed to reduce the adverse reactions, and the most successful is the third/last-generation vector called the gutless AV (devoid of all AV genes) or helper-dependent AV. The production of gutless AV in the absence of a helper AV but rather the presence of a helper plasmid proved to be a new platform for gene therapy targeting inherited disorders. This method led to a significant reduction of contaminant helper AV during production resulting in less toxicity to cells infected with the desirable transgene (Lee et al., 2019).
2.1.2. Retrovirus
Retroviruses (RV), enveloped single-stranded RNA viruses of the Retrovirdae family, were one of the first viruses that were tested for gene therapy. RVs are composed of two alike, linear single-stranded RNA located in a cylindrical nuclear core with a genome size of ~7–11 kb. They consist of three subfamilies including oncovirinae, lentivirinae, and spumavirinae, with onco-RVs being the first viruses used in gene therapy (Mohan et al., 2013). The RVs contain three essential genes, gag, pol, and env that encode for capsid proteins, replication enzymes (reverse transcriptase or integrase), and envelope proteins, respectively. The retroviral envelope proteins bind to surface receptors of the host cell, and the viral core enters the cell through membrane fusion. Once inside the cell, the viral genome is released, converted to double-stranded proviral DNA by viral reverse transcriptase, and the newly synthesized genome enters the nucleus and integrates into the host cell genome to form therapeutic gene proteins (Fig. 1) (Sharma et al., 2010b). The major advantages of RV use include moderate immunogenicity and long-term therapeutic gene expression (several months to years) due to stable expression of the delivered-gene. Disadvantages include integration of RVs into the host cell genome with risk of insertional mutagenesis. Also, RVs can be oncogenic, highly immunogenic, and only transduce actively dividing cells. These properties limit their use for delivering gene therapy into mitotically incompetent corneal endothelial cells and other such cells in the eye (Table 3) (Mohan et al., 2013).
2.1.3. Lentivirus
Lentivirus (LV) is an enveloped single-stranded RNA virus of the Retrovirdae family that binds host cell receptors and results in ejection of transgene material into the cytoplasm. By viral reverse transcriptase, DNA is generated and moves into the nucleus to generate viral proteins. The mode of therapeutic gene introduction by LV is very similar to the RV except that the LV genome is expressed episomally (Fig. 1). LV has been shown to transfect epithelial and endothelial cells and keratocytes by various methods (Bainbridge et al., 2001; Bemelmans et al., 2009; Oliveira et al., 2010). The major advantages of LVs are improved safety over RVs, long-term transgene expression, broad tropism, and ability to transduce both dividing and non-dividing cells. Compared to RVs, the risk of insertional mutagenesis from LVs is notably lower and only occurs when impaired. Disadvantages include high immunogenicity, HIV origin, and random integration potential (Table 3). LV vectors have become a powerful tool for proof-of-concept gene therapy and gene expression profiling studies in a laboratory setting (Mohan et al., 2013).
2.1.4. Herpes simplex virus
Herpes simplex virus (HSV) is an enveloped, double-stranded DNA virus of the Herpesviridae family. The mature herpes simplex virion contains an external envelope of glycoproteins allowing entry into the host cell, an amorphous layer (tegument) of structural and regulatory proteins, and an icosadeltahedral capsid containing a toroidal dsDNA (Fig. 1). HSV-1, a human pathogen that causes HSV keratitis, genome has been manipulated for the purpose of corneal gene therapy purposes. The packaging capacity of rHSV vector is large up to 150 kb (Manservigi et al., 2010; Mohan et al., 2005). HSV vectors successfully deliver transgene into human and rabbit corneal endothelial cells ex vivo (Hudde et al., 2000) and mouse cornea in vivo (Spencer et al., 2000). Early generation HSV vectors showed high immune reaction, severe intraocular inflammation in primates but low in rodents, and short-term transgene expression (Liu et al., 1999; Spencer et al., 2000; Zhang et al., 2012). Subsequently developed HSV vectors show less immune reaction and sustained expression of delivered-gene in eye cells (Miyagawa et al., 2015). Nearly ninety percent of humans have antibodies to HSV-1 or HSV-2, which raises fears for their clinical utility (Wald and Corey, 2007).
2.1.5. Adeno-associated virus
Adeno-associated virus (AAV) is a single-stranded DNA virus of the Parvoviridae family that has been successfully used in humans for ocular gene therapy. Over 100 serotypes of AAV are known but only serotypes 1–10 are tested for gene therapy. AAV is an icosahedral capsid with 4.7 kb genome containing two open reading frames (ORFs) that encode for rep and cap. The rep ORF encodes for 4 distinct proteins, rep40, rep52, rep68, and rep78. Rep68 and rep78 are required for the replication and translation of viral genome and rep40 and rep52 for packaging the genome within the capsid (Naso et al., 2017). The AAV entry into the host cell is serotype specific and dictated by the sequence of the viral capsid which also accounts for variance in tissue trophism. Serotypes 1–3 bind to heparan sulphate proteoglycans, serotypes 4–6 bind to 2, 3-linked Sialic acid, whereas serotypes 8 and 9 bind to laminin. AAV after binding to its primary receptor and co-receptors, integrin αvβ5 or basic fibroblast growth factor, endocytoses via receptor-mediated formation of a clathrin-coated pit (Mohan et al., 2013). Once inside the cell, AAV genome is released into the cytosol through endosomal lysis and anneal to a complementary strand provided by another infecting virus or hijacking endogenous host machinery (Fig. 1). The AAV genome is either integrated on chromosome 19 or remains episomal. Recombinant AAVs (AAV) are produced by co-transfection of the packaging cell line with two parts: the viral vector and the helper plasmid. AAV vectors can infect all three major corneal cells, epithelium, keratocytes, and endothelium (Liu et al., 2008). Nevertheless, AAV vectors show unique cellular tropism and transduction efficiency for different corneal cells due to the distinctive capsid protein. A study comparing tropism of AV and AAV to different corneal cells using rabbit and human cornea organ cultures found more transduction to epithelium and endothelium compared to stroma (Liu et al., 2008). AAV1 and AAV8 showed higher transduction to epithelium compared to the AAV2, AAV5, or AAV7. These serotypes transduced stroma and endothelium in rabbit corneas to a different level (Liu et al., 2008). Cellular tropism and relative transduction efficiency of hybrid AAV2/6, AAV2/8 and AAV2/9 vectors was found in the order of AAV2/6 > AAV2/9 > AAV2/8 for primary human corneal fibroblasts (Sharma et al., 2010a). On the other hand, comparative analysis of cellular tropism and relative transduction efficiency of three hybrid AAV serotypes, AAV2/6, AAV2/8, and AAV2/9 for mouse cornea in vivo and donor human cornea ex vivo found larger with AAV9 followed by AAV8 and AAV6 (Sharma et al., 2010d). Recombinant AAV also stays episomal and expression is not carried over into daughter cells following cell division (O’Donnell et al., 2009; Colella et al., 2017). Advantages of AAV include high efficiency for both, dividing and non-dividing, corneal cells, stable transduction, long duration of delivered-gene expression, and site-specific integration due to the use of specific serotypes. Disadvantages include a small packing size and concerns of pre-existing immunity (Table 3).
AAV2 is the first serotype tested for corneal gene therapy and successfully delivered transgene in rabbit stroma in vivo only when administered topically onto bare stromal bed created with a microkeratome (Mohan et al., 2003a). A natural occurrence of AAV2 serotype in humans may negatively impact its efficiency and safety. To conquer this, hybrid rAAV vectors using genome of AAV2 and capsid of other serotypes are generated and tested for corneal gene therapy. Accruing literature reveals that transduction efficiency, timing of initial transgene appearance, levels, and lengths of delivered-gene expression are specific to each hybrid vector (Mohan et al., 2013). Comparative analysis of hybrid vectors finds high transgene delivery by AAV2/5 followed by AAV2/6, AAV2/8, and AAV2/9 into human corneal fibroblasts in vitro without significant cytotoxicity (Buss et al., 2010; Sharma et al., 2010a, d). On the other hand, single AAV2/5, AAV2/8, or AAV2/9 topical application on de-epithelialized cornea delivers significant transgene into rabbit/rodent stromal keratocytes in vivo without significant side effects for up to 12 months (Mohan et al., 2011a, b, c; Sharma et al., 2010b).
Subsequently, double stranded self-complimentary AAV (scAAV) vectors are produced to bypass the requirement for complementary strand generation and gain initial delivered-gene expression quickly. The scAAV vectors supposedly show higher efficiency at lower titers in a tissue-specific and serotype-dependent manner (Buie et al., 2010). These vectors show noticeable gene transfer into various targeted corneal cells in vivo (Gruenert et al., 2016; Kong et al., 2010; Wang et al., 2017; Yokoi et al., 2007). Since intracellular trafficking of the viral capsid through ubiquitin-proteasome pathway plays a major role in dictating transduction efficiency of AAV, recombinant tyrosine mutant AAV vectors are generated through modifications in tyrosine residues on the surface of the capsid. A mutation to surface-exposed tyrosine residues on AAV capsids circumvents the ubiquitination step and inhibits proteasome-mediated degradation, and consequently enhances transduction efficiency of AAV significantly. Single or multiple tyrosine-phenylalanine mutations in the tyrosine residues within the AAV capsid (Y252, Y272, Y444, Y500, Y700, Y704, and Y730) enhanced multiplicity of infections by 100 to 10,000 (Petrs-Silva et al., 2009; Ryals et al., 2011; Zhong et al., 2008a). Tyrosine mutant AAV2, AAV8, and AAV9 vectors efficiently deliver genes into keratocytes and endothelium of rabbit and mouse corneas in vivo and human corneal fibroblasts and endothelial cells in vitro (Mohan et al., unpublished data; Sharma et al., 2010c). Higher expression is achieved with use of tyrosine mutant AAVs due to absence of capsid ubiquitination and enhanced intracellular trafficking to the nucleus (Zhong et al., 2008b). Inability of rAAV to transport large therapeutic load (≤1.8 kb) and technical challenges in packaging and producing high titers narrow their scope ().
2.2. Non-viral vectors
Non-viral vectors do not have the same limitations as viral vectors including immunogenicity issues, vector production challenges, small DNA packaging capacity, and wide tropism. They do however, have their own set of challenges such as low transfection efficiency, aggregation with blood or other fluids, colloidal instability in other physiological fluids due to salt contents, and possible breakdown of the therapeutic gene by endonucleases (Yin et al., 2014). Non-viral vectors are usually simple, safe, easy to produce commercially, nonhazardous, low immunogenic, and cost effective. Various physical techniques, chemicals, and nanoparticles (NPs) have been used as a vector developing non-viral corneal gene therapy approaches (Mohan et al., 2013).
2.2.1. Physical systems
Several mechanical, electrical, and surgical techniques such as gene gun, hydrodynamics, electroporation, iontophoresis, ultrasonography, lasers, microinjection, stromal hydration, and more have been used to develop physical methods for corneal gene therapy. All these methods have introduced plasmid DNA into corneal cells at varying success. Gene gun utilizes adjustable low-pressure helium pulses to dispense plasmid DNA gelled with metallic microparticles into target corneal tissues (Lu et al., 2003). This method has successfully administered marker genes into corneal epithelium in vivo but with significant damage to corneal epithelium and stroma including concurrent inflammation (Hao et al., 2010; Klebe et al., 2000; Tanelian et al., 1997). Electroporation and iontophoresis exploit electrical current and electro-osmotic power to inject plasmid DNA carrying therapeutic gene into corneal cells with mild/moderate toxicity and side effects (Cassagne et al., 2016; Oshima et al., 2002). Ultrasonography employs ultrasound, microbubbles, and acoustic parameters settings to fire therapeutic DNA into corneal cells without apparent tissue damage (Nabili et al., 2014; Sonoda et al., 2006). Development of computational model to predict porosity changes in the cornea following ultrasound exposures has potential to tailor individualized patient-specific treatment plans (Hariharan et al., 2017). Expanding on a report showing that direct contact of vector to stroma is crucial for delivering genes into keratocytes (Mohan et al., 2003), gene transfer in the pig cornea was accomplished by creating a pocket with femtosecond laser and applying vector onto a delineated area (Bemelmans et al., 2009). Application of various common surgical techniques such as topical, microinjection, filtration bleb surgery, stromal hydration, LASIK, and others have been useful in enhancing as well as achieving tissue-targeted transgene delivery in preclinical rabbit and rodent corneas (Atencio et al., 2004; Bauer et al., 2006; Mohan et al., 2010; Yin et al., 2019). The above described physical methods offer many benefits including high safety profile, choice of delivering a large size therapeutic gene, low immunogenicity, and target selectivity, but unreliable non-specific transgene delivery and low transfection efficiency restrict their wider application.
2.2.2. Nanoparticles
Particles of 1–100 nm in size, popularly called “nanoparticles” (NPs), are easily endocytosed into cells without any compartment entry at first. Small size extending a large surface area to volume ratio makes NPs an ideal vehicle to transport a myriad of ligands (e.g. antibodies, peptides, DNA, and probes) into the cell for gene therapy treatment options. Multiplexing properties of NPs allow researchers to tag large therapeutic loads, molecular sensors, fluorescent markers, and cell-specific factors, which make these NPs exceedingly suitable for gene therapy. NPs employ diverse mechanisms such as phagocytosis, macro-pinocytosis, clathrin- or caveolae-dependent or -independent pathways to invade cells (Mohan et al., 2012).
NPs are widely investigated for corneal gene therapy development. Polymeric NPs are comprised of natural and synthetic polymers such as chitosan, albumin, dendrimers, polyethylene glycol (PEG), and polyethyleneimine (PEI). Chitosan is present in the skeleton of shellfish and is a linear polysaccharide consisting of randomly arranged acetylated and deacetylated sugar units (D-glucosamine). Various formulations of chitosan-DNA NPs can deliver genes successfully into rodent corneal cells (Klausner et al., 2010). A deacetylated chitin is biocompatible, biodegradable, and non-toxic to cells and viewed as a promising vector due to its electrostatic interaction with negative charges in the mucus layer (Klausner et al., 2012). Likewise, albumin NPs effectively deliver therapeutic genes encoding for a soluble vascular endothelium growth factor into the cornea and have been found to decrease neovascularization without major adverse effects (Jani et al., 2007). PEG is the most commonly studied polymer in ocular application due to low immunogenicity and its ability to successfully deliver plasmids to the rodent cornea and other ocular tissues in vivo (Cai et al., 2008). Other polymers and copolymers like poly (lactic acid) PLA and poly (D,L-lactide-co-glycolide) PLGA, which are also biocompatible and biodegradable, are used as building blocks for nanocarriers. PLGA NPs ability to deliver drug/genes in rodent cornea and periocular tissues in vitro and in vivo has been demonstrated (Amrite and Kompella, 2005; Li et al., 2019). Also, PLGA NPs loaded with shRNA (short hairpin RNA) of the VEGF gene are found effective and safe for the regression of murine corneal neovascularization in vivo (Qazi et al., 2012). Using plasmid transfection in an equine ex vivo experiment, Suppressor of mothers against decapentaplegic (Smad) 2, 3, and 4 profibrotic genes were individually silenced and Smad5 antifibrotic gene was overexpressed resulting in up to 83% reduction in corneal fibroblast transdifferentiation to myofibroblasts (Marlo et al., 2018). Torrecilla et al. (2018) used lipid NPs as a gene delivery system for the treatment of corneal neovascularization-associated inflammation by demonstrating downregulation of matrix-metalloproteinase-9 (MMP-9). Polyethylenimine (PEI) linear NPs, 22 kDa, have been optimized using various nitrogen-to-phosphate ratios for attaining maximal therapeutic response of gene therapy in human and equine corneal fibroblasts in vitro and rodent cornea in vivo (Bosiack et al., 2012b; Donnelly et al., 2014; Rodier et al., 2019). Dendrimers, a branched sphere-shaped molecule, made of a core, an inner shell and an outer shell encapsulating plasmid DNA, is used to deliver gene therapy into the corneal endothelium (Hudde et al., 1999; Souza et al., 2015). This vector-based gene therapy has shown promise in reducing corneal allograft rejection and corneal endothelial defects. Another non-viral vector, cationic liposome, when used in various formulations delivered plasmid DNA in corneal endothelial cells successfully with low cytotoxicity (Klebe et al., 2001; Pleyer et al., 2001).
Gold, a noble metal, has been used for controlling many ailments in various forms throughout the history of civilization. Its first use in medicine can be traced back to 2500 BC in Chinese medicine (Pricker, 1996). Because of an inert nature, biocompatibility, malleability to any size, and affinity to DNA, gold is used to synthesize scores of metallic and hybrid (metaling-polymeric) NPs for gene therapy delivery. Our laboratory has optimized polyethylenimine-conjugated gold NPs (PEI2-GNPs), 2–12 nM in size, for delivering genes in the human cornea in vitro and rabbit cornea in vivo with low toxicity (Sharma et al., 2011). Subsequently, we studied the response of various gene therapies facilitated via the PEI2-GNP vector and genes (BMP7, HGF, decorin, Smads, and others) using a well-established preclinical in vivo rabbit model and in vitro human, equine, and canine corneal disease models (Bosiack et al., 2012b; Buss et al., 2010; Chaudhary et al., 2014; Donnelly et al., 2014; Gupta et al., 2018; Mohan et al., 2011; Sharma et al., 2011; Tandon et al., 2013). The effects of tested mono and dual gene therapies in managing corneal wound healing, fibrosis, neovascularization, and transparency are discussed in later sections.
2.2.3. DNA transposons
Transposable elements are mobile genetic components that can be used for experimental and therapeutic purposes. The Sleeping Beauty (SB) transposon, originated from fish genomes, is well characterized and used as a vector system (Kebriaei et al., 2017). The SB vector has not been investigated in corneal research but is routinely used to transfect chimeric antigen receptors (CARs) for the treatment of various leukemias and lymphomas by targeting CD19, which is readily expressed in these conditions (Riviere and Sadelain, 2017). SB transposon vectors combat limitations associated with viral gene therapy vectors and short-lived non-viral gene delivery methods. Gene transfer is highly stable with sustained transgene expression. Presently, there is a Phase Ia/IIb clinical trial for the treatment of neovascular age-related macular degeneration (AMD) underway. In this study, autologous, genetically modified retinal pigment epithelial or iris pigment epithelial cells have been transfected with an SB transposon to promote overexpression of pigment epithelial-derived factor (PEDF) for surgical subretinal placement. If this therapeutic modality is successful, it would replace the need for life-long intravitreal injection, which is the present mainstay of treatment (Hudecek et al., 2017).
2.2.4. Gene editing
Inception of gene editing to precisely manipulate the genome and achieve expected results has potential to revolutionize healthcare including corneal diseases/disorders. Advances in the clustered regularly interspaced short palindromic repeats (CRISPRs), CRISPR-associated systems (Cas), zinc finger nucleases (ZFNs), and transcription activator-like effector nucleases (TALENs) systems crafted a new dynamic to genetic engineering. Gene therapy involving genome editing can be used to recognize complex molecular mechanisms responsible for corneal diseases and develop next generation personalized medicine for corneal care; however, gene editing in the cornea is in its infancy (Raikwar et al., 2016). Schematics in Figs. 2–5 show structure, design, components, and steps involving gene editing and gene therapy implementation.
Fig. 2.
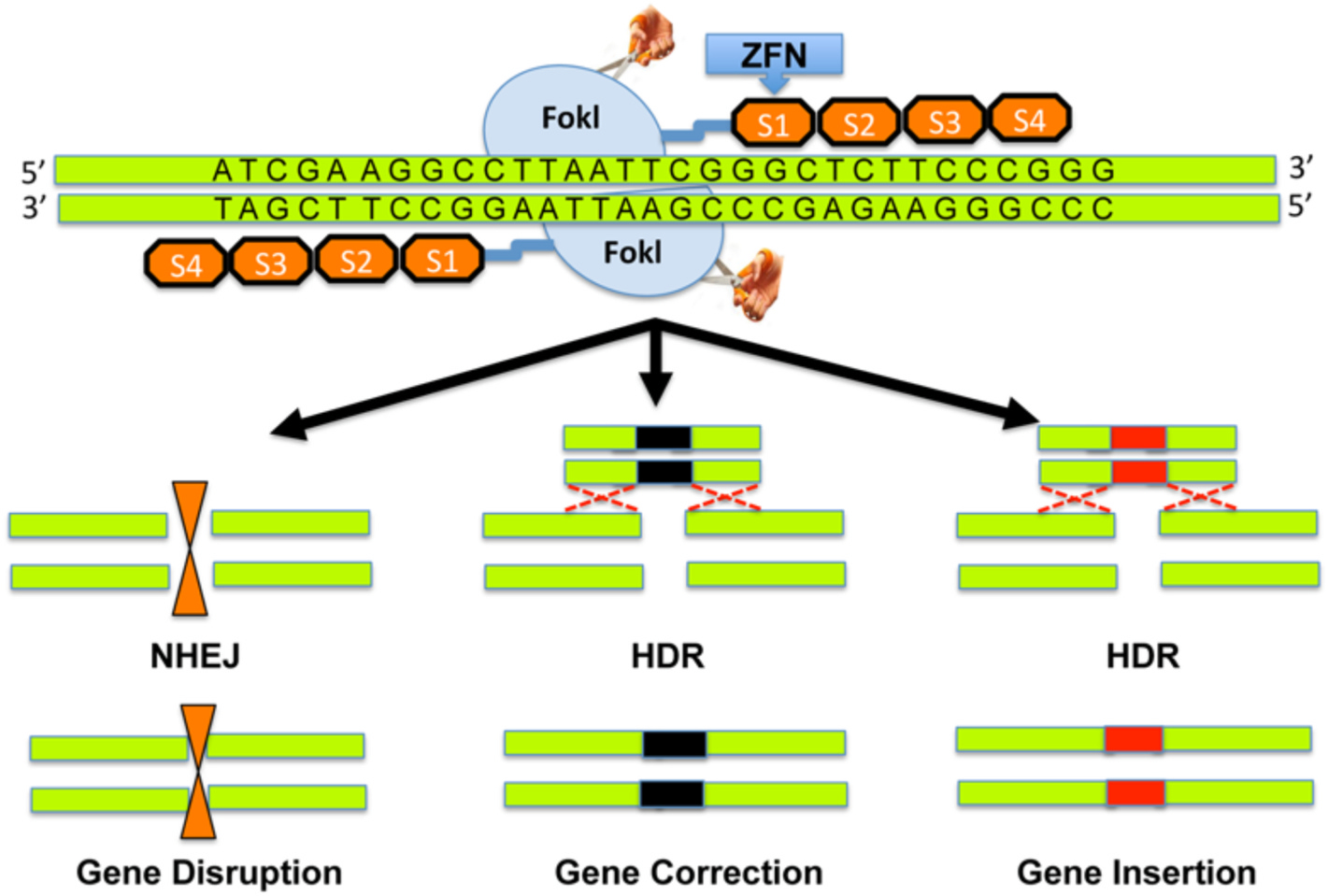
Zinc finger nucleases. ZFNs are specifically designed to target and bind a region of DNA with FokI. Cleavage by FokI promotes double strand breaks and either non-homologous end joining pathway (NHEJ) or homology directed repair (HDR) ensues. NHEJ results in insertions or deletions that lead to a frameshift or gene disruption, while HDR leads to genome repair or insertion of a desirable gene to improve function. Reprinted with permission from Raikwar SP et al. World J. of Transl. Med. 2016; 5(1):1–13.
Fig. 5.

Clustered Regularly Interspaced Short Palindromic Repeats/Associated Systems for corneal disorders. The CRISPR/Cas9 system is revolutionizing the way in which scientists investigate disease models and therapeutic modalities. The combined use of CRISPR/Cas9 technology with nanocarriers, recombinant adeno-associated virus (rAAV), or lentivirus vectors will afford multiple gene therapy that is particularly beneficial to inherited corneal disorders. Reprinted with permission from Raikwar SP et al. World J. of Transl. Med. 2016; 5(1):1–13.
ZNFs are DNA-binding proteins made of tandem arrays of zinc-fingers. Designer zinc-fingers recognize three specific DNA base pair sequences and can be built in tandem to recognize targeted nucleic acid sequences (Yin et al., 2014). ZNFs are well known for their use in knocking out CCR5 gene leaving HIV patient derived autologous CD4 T cells resistant to the virus (Tebas et al., 2014). Each ZFN has two functional domains: (a) a DNA-binding domain made of a chain of two-finger modules recognizing a unique hexamer sequence of DNA (two-finger modules are stitched together to form a zinc finger protein with specificity of ≥24 bp), and (b) a DNA-cleaving domain made of the nuclease domain of FokI. Fusion of the DNA-binding and DNA-cleaving domains creates a highly-specific pair of “genomic scissors” (Fig. 2). ZNFs enable very-targeted editing of the genome by producing double-strand breaks in DNA at user-defined locations (Raikwar et al., 2016). The literature is very sparse regarding the use of ZNFs for corneal disease. Although the benefits of ZFNs have been demonstrated, this modality of gene editing is expensive and technically challenging. There are also risks for unintended chromosomal rearrangements (Gupta and Musunuru, 2014).
TALENs have emerged as an alternative to ZFNs. Like ZFNs, TALENs consist of a non-specific FokI DNA-cleaving domain fused to a customizable DNA-binding domain (Fig. 3). It has highly conserved repeats derived from TALEs (Raikwar et al., 2016). TALEs are proteins secreted by Xanthomonas bacteria that hijack a host plant to initiate an infection. TALEs do this by binding to promoter DNA sequences and activating expression of host genes. TALE proteins contain a repeat amino acid domain in which two critical amino acids in each repeat allow identification of specific DNA bases. This allows rapid de novo synthesis, and TALENs can be engineered more easily than ZNFs (Moore et al., 2014; Yin et al., 2014). Similarly, to ZFNs, no investigations using TALEN-based genome editing have been reported specific to corneal disorders.
Fig. 3.
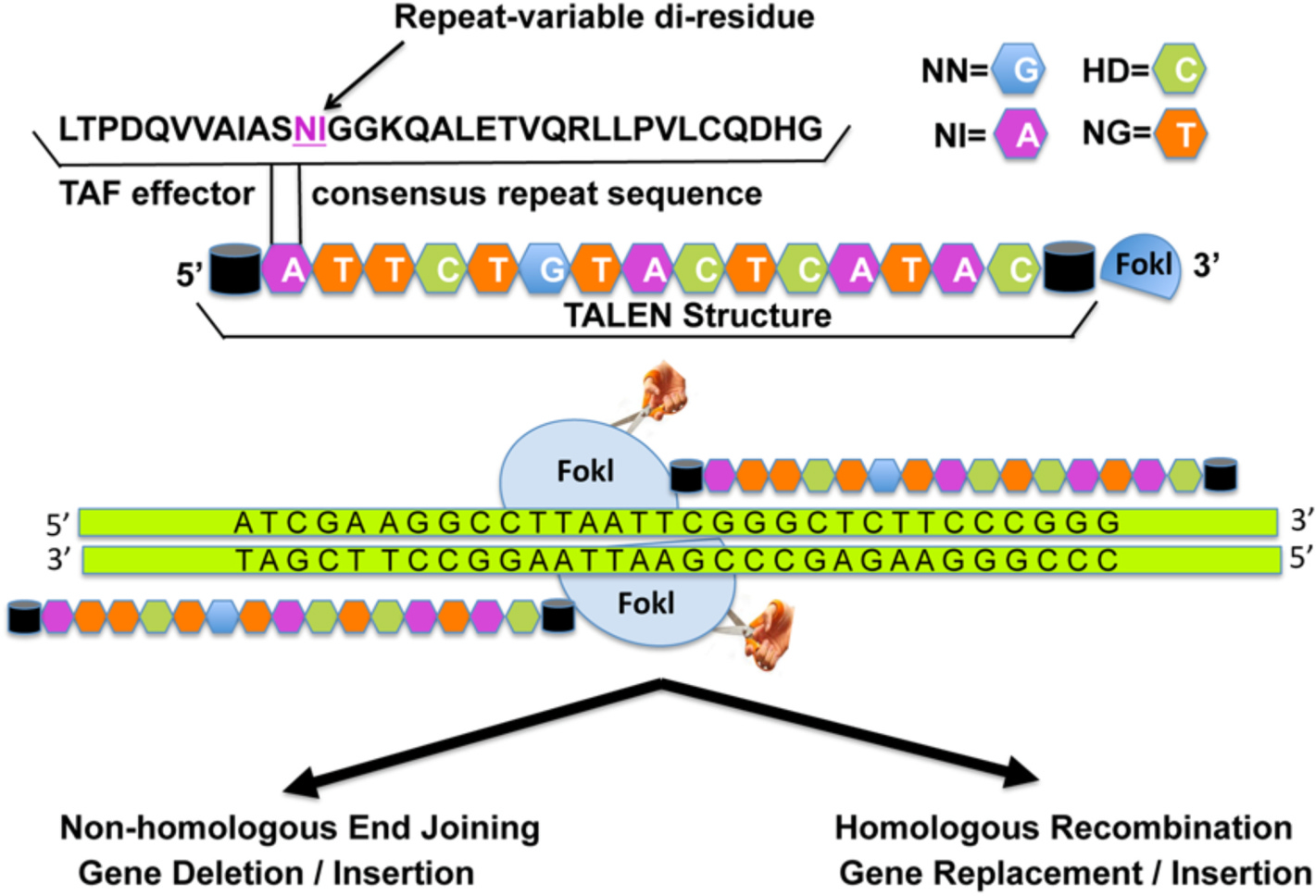
Transcription activator-like effector nucleases. TALENs are engineered arrays of amino acid modules built to target and bind any DNA sequence. TALEN-mediated cleavage of the target by FokI results in double strand breaks. Then, similarly to ZFNs, either insertions or deletions lead to gene disruption or repair leads to desirable gene function. TALENs are larger molecules than ZFNs, which makes them more difficult to deliver; however, TALENs are cheaper, easier to produce, and yield greater flexibility to the clinician-scientist. Reprinted with permission from Raikwar SP et al. World J. of Transl. Med. 2016; 5(1):1–13.
The CRISPR gene-editing system, traditionally known as the bacterial immune system, is made of an endonuclease protein whose DNA-targeting specificity and cutting activity can be programmed by a guide RNA (Fig. 4). The guide RNA is made of two components, the CRISPR-RNA (crRNA) and the trans-activating CRISPR RNA (tracrRNA). The crRNA has ability to slice the double stranded DNA and has a short region of homology letting it join tracrRNA, which affords a stem loop structure that associates with Cas9 protein. The Cas9 nuclease and gRNA (crRNA:tracrRNA duplex) form a Cas9 ribonucleoprotein (RNP) that creates a user-dictated cut in the DNA (Fig. 4) (Raikwar et al., 2016; Kantor et al., 2020). The CRISPR/Cas9 system was recently used to investigate a model of aniridia-related keratopthay (ARK) caused by a heterozygous PAX6 gene mutation causing congenital aniridia, a rare blinding congenital disorder involving corneal changes, employing a limbal epithelial stem cell (LSC) model (Roux et al., 2018). The addition of recombinant PAX6 protein improved the phenotypic appearance of the mutant LSCs, signifying the promise of this therapeutic modality for congenital aniridia (Roux et al., 2018). Additionally, Chang et al. (2018) demonstrated the promise of the CRISPR/Cas9 based gene therapy in promoting wound healing and corneal endothelial regeneration by manipulating sex-determining region Y-box 2 (SOX2) using in vivo and in vitro models. Considering endothelial cells do not regenerate, this study is exciting and opens doors to novel therapeutic drugs for endothelial diseases.
Fig. 4.
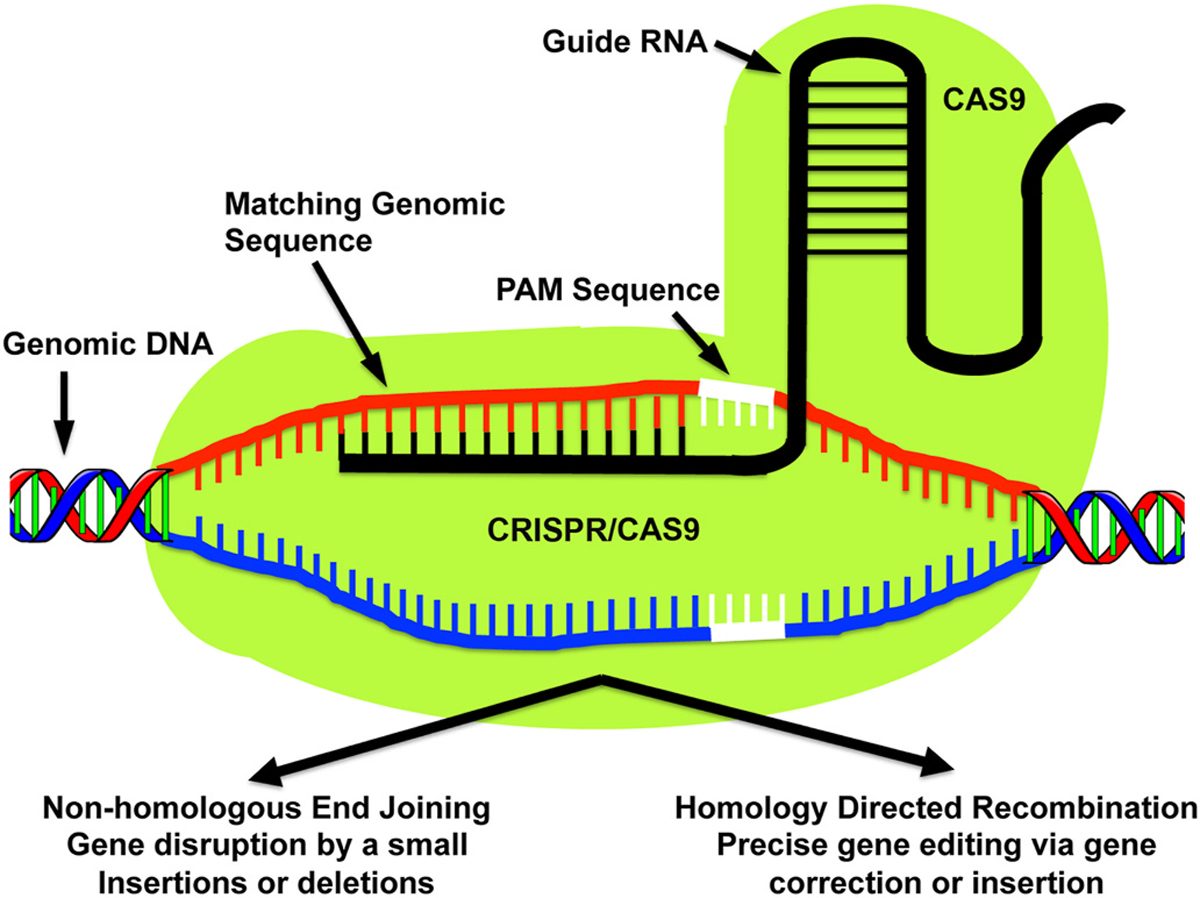
Clustered Regularly Interspaced Short Palindromic Repeats/Associated Systems. The CRISPR/Cas9 system begins with the Cas9 nuclease causing double strand breaks in DNA at a highly specific site identified by a short single guide RNA (sgRNA). Directly 3′ to the sgRNA, a protospacer-adjacent motif (PAM) sequence is present that aids in Cas9 target specificity. Ensuing repair follows either NHEJ or HDR methods. The CRISPR/Cas9 system stands apart from ZFN and TALEN systems because of its simplicity, very low cost, ease of production, and high efficiency. Reprinted with permission from Raikwar SP et al. World J. of Transl. Med. 2016; 5(1):1–13.
3. Approaches to design gene therapy for corneal diseases and disorders
Cornea is a unique transparent tissue and offers 2/3rd refraction to the eye. The refractive power and transparency of the cornea hinge on its peculiar shape and trilaminar structure comprising of a stratified epithelium, stroma, and metabolically active but mitotically inactive endothelium monolayer. The epithelium is covered with a tear film that ensures smoothness to corneal surface while the endothelium bathed to a nutrient-rich aqueous humor. Sandwiched between the epithelium and endothelium exists the stroma, which makes ~90% of the cornea. It is made of lamellae of collagen bundles and contains extracellular matrix, proteoglycans, and keratocytes. Keratocytes are quiescent and transparent cells residing between the collagen lamellae and express high levels of crystallins in their cytoplasm and are primarily responsible for maintaining corneal transparency, homeostasis, and wound repair. As scientists and physicians working together closely, the aims of our investigations are centered around better patient outcomes and maintaining quality of life by preventing, treating, or curing corneal disorders. Patient safety is the first and foremost consideration when developing a novel gene therapy. Depending on the corneal disorder, the selected new gene will either be used to silence of replace the mutated gene. Here within, we discuss a basic approach and considerations to gene therapy development.
3.1. Identify a vector
Once a specific gene has been identified to have a beneficial therapeutic effect for the corneal disease at hand, a means to transport the gene to specific cells within the cornea is needed. This transporter is known as the vector. As previously discussed, vectors are typically considered viral or non-viral. In order to select the best vector, we need to know if the corneal disorder at hand is congenital or not. If congenital, then having long-term or permanent transgene expression would be ideal. For this situation, one might consider gene editing with a CRISPR/Cas9 system. Alternatively, viral vectors AAV, LV, and RV generally have transgene expression for months to years. Vectors LV and RV integrate into the host DNA and might result in mutagenesis. For these reasons, AAV has become the most popular means for viral transduction in our laboratory. In an effort to carry a larger gene, NP vectors are more beneficial and have the ability to pass through the cell membrane quite easily. The SB transposon is also a consideration for long-term transgene expression, low cost, and ease of production. Once a vector is selected, then the delivery method is determined.
3.2. Identify delivery technique
The delivery technique should be simple and minimally invasive while selectively targeting the location of needed transgene delivery. Simple, topical administration of gene therapy in the cornea would be ideal, but that is not always feasible particularly for the corneal stroma and endothelium unless the epithelial barrier is evaded and vector gets direct access to these tissues. Normal epithelial anatomy and pathophysiology is highly protective of the deeper corneal layers and inner ocular structures. Invading pathogens are held at bay by epithelial cells tight junctions and hydrophobic properties. In addition, the high metabolic activity and constant turnover of epithelial cells are key for rapid healing (DelMonte and Kim, 2011; Kamil and Mohan, 2020). Trauma/injury to the cornea is known to compromise epithelial barrier function, and it is found to induce keratocyte apoptosis just beneath the corneal epithelium (Mohan et al., 2003b, c). This has been our enduring hypothesis that keratocyte apoptosis occurring post-injury creates a sheath of dead-cells beneath epithelium, which forms a transient barrier and is a secondary defense mechanism to prevent entry of pathogens in the eye and protect its tissues. To overcome natural defense barriers like these, we developed an innovative minimally invasive surgical technique to deliver gene therapy topically in the rabbit stroma in vivo. Our technique involves skillful removal of corneal epithelium in a user-specified area with a #64 surgical blade (Beaver-Visitec International, Inc., Waltham, MA) by applying and advancing the blade gently at 45° from the surface of the cornea in one-direction (right-to-left). This procedure is performed while the subject is under general and local anesthesia with a wire-speculum positioned within the interpalpebral fissure. The corneal section shown in Fig. 6 has been collected from a rabbit which had three distinct regions: (i) corneal epithelium removed following optimized parameters, (ii) corneal epithelium removed by random application of surgical blade, and (iii) an intact corneal epithelium, and humane euthanasia 30 min post-procedure. As evident from Fig. 6, our optimized technique barely induced keratocyte apoptosis in rabbit cornea in vivo whereas random-removal method produced significant keratocyte apoptosis. As anticipated, cornea with an intact epithelium showed no keratocyte apoptosis. More such vector-delivery techniques are needed for the success and advancement of corneal gene therapy from bench to bedside transformation. Various other vector-delivery techniques, methods, and protocols have been developed previously and published for AAV corneal gene delivery (Mohan et al., 2012; Song et al., 2020).
Fig. 6.
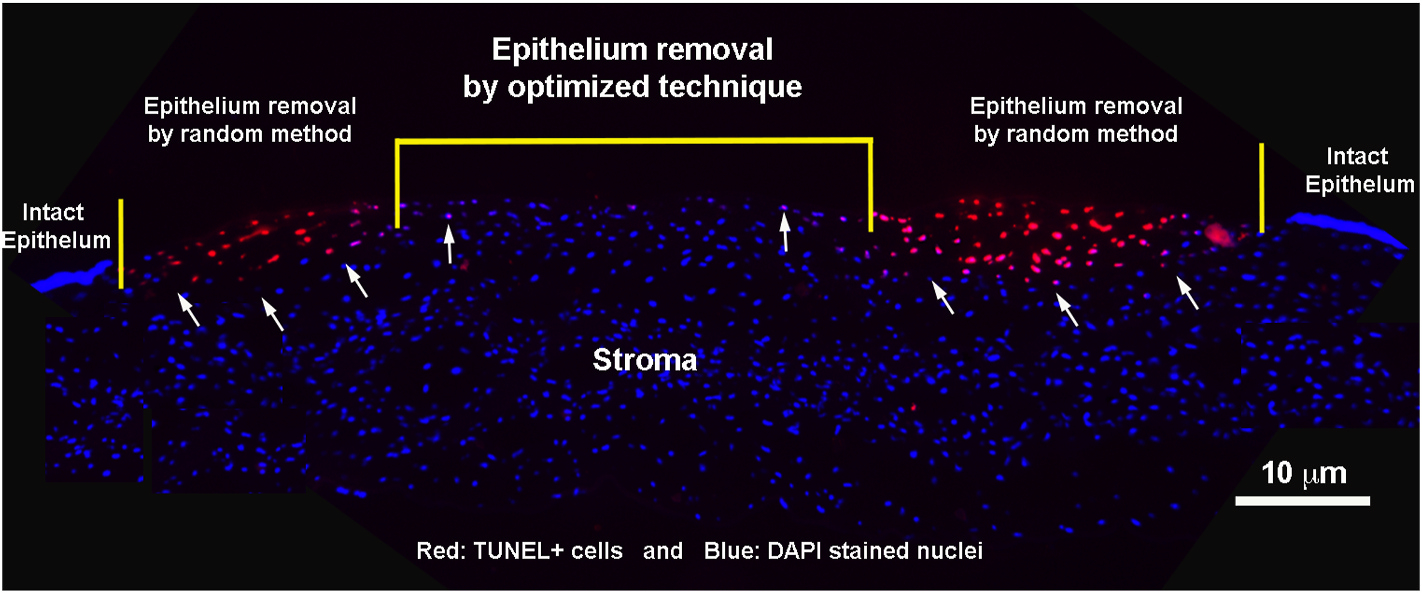
A customized vector-delivery technique to facilitate gene delivery into rabbit stroma in vivo by passing epithelial and keratocyte apoptosis barriers. Image is taken at the 100× magnification. Scale bar = 10 μm.
3.3. Identify interventional target genes
The fundamental understanding of various biological factors (cytokines, chemokines, growth factors, cell receptors, etc.), their role/function, and complex interwoven and overlapping signaling mechanisms managing corneal healing responses and events is required for the identification of interventional targets to treat corneal diseases and disorders. A comprehensive review of corneal wound healing is out of the scope of this review, however, wide-ranging pertinent information on this topic can be found in recent publications (Kamil and Mohan, 2020; Wilson, 2020). How a particular gene can prevent pathological/excessive corneal wound healing and promote physiological repair is shown in Fig. 7. The portrayed five genes that when applied to the corneal stroma have proven effective for attenuating conditions such as corneal haze, fibrosis/scars, neovascularization, fibroblast differentiation to myofibroblasts, and reviving corneal transparency. How these genes controlled excessive wound healing and led to control of corneal damage are discussed in later sections.
Fig. 7.
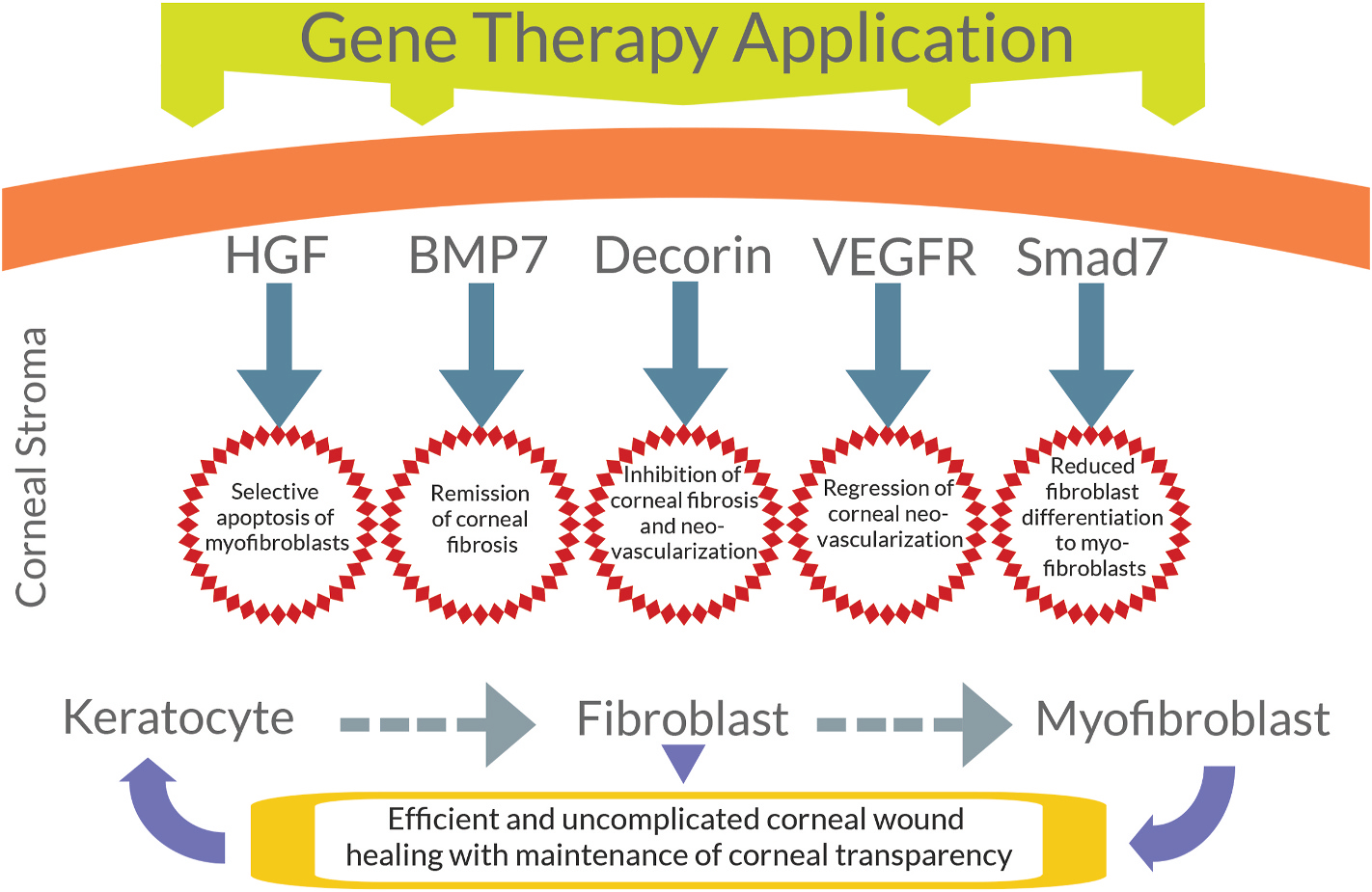
Application of specific genes (HGF, BPM7, decorin, VEGF-A, Smad7) delivered to the corneal stroma attenuates conditions such as corneal fibrosis, neovascularization and fibroblast differentiation to myofibroblasts.
4. Target applications for corneal healing
As discussed, identification of a specific target for treatment of corneal disorders is necessary to be efficient and effective. In our laboratory, we have classified corneal injury into three broad categories: mild, moderate, and severe based on the severity and extent of corneal damage. It is our postulate that wound healing responses and mechanisms in the classified three conditions differ considerably to control pathologic steps and facilitate physiologic repair. In general, mild to moderate corneal wounds will heal by targeting one factor/pathway with the application of a mono gene therapy while treatment of many moderate and severe corneal wounds will require simultaneous and/or serial targeting of multiple factors/pathways and a more robust dual gene therapy approach. Thus, it is prudent to develop efficient and safe gene therapy approaches to cure vision-impairing clinical conditions and reduce heavy reliance on corneal transplantation. At present, corneal transplant surgery is used to cure established fibrosis, neovascularization, etc. and restore vision in patients. Globally, greater than 12 million people need corneal tissues (Young et al., 2012) but the availability of donor cornea is sharply declining due to laser surgeries, hepatitis, HIV, etc. (Gain et al., 2016). Our tested mono gene therapies effectively and safely treated moderate corneal conditions such as thick haze and inflammation produced by photorefractive keratectomy (PRK) surgery with excimer laser in rabbits in vivo (Gupta et al., 2018; Mohan et al., 2011b). Likewise, a dual BMP7 and HGF gene therapy given topically post-injury effectively treated a severe corneal condition produced by the chemical injury (alkali burn) characterized by significant inflammation, edema, and thick scar/fibrosis in rabbits in vivo (Gupta et al., 2018). Although combination/dual gene therapy might result in better outcomes for severe corneal wounds, the application does not come without its own set of limitations including challenges in effective delivery, low potency, disruption of endogenous beneficial genes, and etc. Yang et al. (2016) utilized an intravenous dual AAV system to introduce multiple components in adult mouse liver in vivo. Transgene expression was less than 10% and large deletions resulted in reduced protein tolerance and deadly hyperammonemia due to the loss of normal beneficial genes. Specific areas of corneal disease are discussed below. In comparison to corneal wounds in which there is not a defined underlying inherited disorder and permanent therapeutic effects are not required, new insights to gene therapy and gene editing for inherited disorders are expanding and described as well.
4.1. Corneal wound healing and fibrosis
Trauma, infection, chemical or surgical injury to the cornea can promote fibrosis resulting in impaired vision. To date, corneal scarring is major leading cause of blindness in the world and no proven therapy to mitigate or cure fibrosis is available. Our laboratory has been investigating this field of study for several years, and there are favorable long-term and safe options through gene therapy on the horizon.
Recombinant AAV (rAAV) vectors have become widely used for delivering gene therapy to treat corneal neovascularization and fibrosis due to the previously mentioned advantages. Mohan et al. (2011b, c) found that a single topical AAV serotype 5 treatment effectively delivered decorin gene therapy to rabbit stroma in vivo and resulted in significant amelioration of corneal neovascularization and fibrosis in rabbit eyes in vivo with no alarming or clinically-relevant adverse effects. Decorin gene is a potent inhibitor of TGFβ and VEGF growth factors, which have been known to play an important role in promoting fibrosis and neovascularization in the cornea in vivo. (Chaudhary et al., 2014; Donnelly et al., 2014; Mohan et al., 2011b, c, 2019). Smad7 is another gene that has been investigated, and AAV5-Smad7 gene transduction to rabbit stroma in vivo post-PRK also inhibited corneal fibrosis (Gupta et al., 2017). AAV was used to deliver decorin gene therapy to successfully reduce peritoneal fibrosis in rats in vivo (Chaudhary et al., 2014). Additionally, subconjuctival injection of rAAV-angiostatin eases corneal neovascularization in a rat in vivo alkali-burn model (Cheng et al., 2007). Safety of AAV5 and/or efficacy of some of discussed genes have been evaluated in other animal species as well. For example, AAV5 vector is a safe and effective means to facilitate gene therapy in canine and equine cornea in vitro (Buss et al., 2010; Bosiack et al., 2012a).
Bone morphogenetic proteins (BMPs) are multifunctional cytokines that influence a multitude of processes during corneal wound healing by modulating keratocyte proliferation, differentiation, and apoptosis (Kim et al., 1999). BMP7 gene therapy has been applied in ocular diseases, and Tandon et al. (2013) demonstrated that a single administration of this gene with PEI2-GNP nanoparticles prevents exuberant wound healing and inhibits corneal fibrosis in a rabbit in vivo disease model. Taking this information, Gupta et al. (2018) combined BMP7 with hepatocyte growth factor (HGF) and the PEI2-GNP-mediated BMP7 and HGF gene therapy significantly reduced corneal haze/scarring and initiated selective apoptosis of myofibroblasts and not fibroblasts. These remarkable findings were also accompanied by no adverse effects. The selection of HGF in developing dual gene therapy was derived from one of our previous basic-science corneal wound healing studies that identified the expression HGF and its receptor (c-Met) in the cornea (Li et al., 1996) and investigated their role in normal and healing corneal epithelium and keratocytes in the anterior stroma in vivo (Wilson et al., 1999).
4.2. Neovascularization
Although the cornea is normally avascular, neovascularization often occurs after corneal injuries or prolonged wound healing, and is characterized by vascular intrusion from the limbal areas towards central cornea and vessels that have relatively poor structural integrity. These vessels might have leakage of blood components into the local tissue, and long-term effects can result in vision impairment (Stevenson et al., 2012). Antiangiogenic gene therapy applications have been investigated in experimentally induced in vivo models by several investigators. Vascular endothelial growth factor (VEGF) is a major cytokine that stimulates endothelial cells and promotes neovascularization. Investigations have shown that inhibition of VEGF can inhibit vessel formation and is a reasonable therapeutic target. Inhibitory antibodies targeting VEGF-A signaling pathways, such as bevacizumab and ranibizumab, are currently used in clinical settings (Stevenson et al., 2012). Mohan et al. (2011c) demonstrated favorable results with AAV5-mediated decorin gene therapy after only a single application in a rabbit model. Decorin, a small leucine-rich proteoglycan, has been shown to modulate angiogenesis in by suppressing synthesis of VEGF (Mohan et al., 2011d). Several other targets have been investigated including Flt-1, Flt23k, PEDF, VEGFR Flt-1, MMP-9, vasohibin-1 (Cho et al., 2012; Lai et al., 2005; Li et al., 2014; Qazi et al., 2012; Torrecilla et al., 2019; Yu et al., 2010; Zhou et al., 2010). Investigations targeting inhibition of VEGF to inhibit vessel formation in the cornea appears a very reasonable therapeutic strategy. Inhibitory antibodies targeting VEGF-A signaling pathways, such as bevacizumab and ranibizumab, are currently used in clinical settings and are standard of care (Stevenson et al., 2012). Thus, gene therapy sequestering VEGF might be better alternatives to longer term therapy by not requiring multiple dosing, frequent clinic visits, or patients’ compliance.
4.3. Corneal grafts
Corneal transplantation is commonly performed as a salvage procedure to preserve ocular vision. The health of the donor cornea and the recipient bed are critical for successful allogenic graft transplantation, to prevent graft rejection and failure. Low-risk avascular beds have a two-year graft survival rate greater than 90%, but this success greatly diminishes over time (Qazi and Hamrah, 2013). The prospect of using the ex vivo “down time” between donor cornea collection and recipient corneal transplant would be a perfect time to apply a pre-treatment to the cornea. What specific treatment is still unknown, but recently, rabbit and human donor corneal buttons were incubated ex vivo with an AAV vector-mediated human leukocyte antigen G (HLA-G). Allotransplantation (rabbit donor to rabbit recipient) in the treated group remained free of edema and neovascularization over 2.5 months. Xenotransplantation (human donor to rabbit recipient) in the treated group had a delayed rejection time from 18 days to 29 days (Gilger et al., 2018). Using a rat model, Qin et al. (2016) delivered CD25 siRNA with Entranster transfection reagent to recently transplanted corneas. The treatment group had a reduction in neovascularization, inflammatory cell infiltration, and expression of IL-10 and TGFβ. Grafts in treated corneas had a significantly increased graft survival time of 14.8 days compared to controls at 7.6 days. These beneficial effects were attributed to upregulation of T regulatory cytokine expression and downregulation of Th1 cytokine expression (Qin et al., 2016). Although gene therapy is aiming to improve survival of corneal grafts, gene therapy could also overcome the need for transplantation in the first place with continued investigations for resolution of inherited corneal disorders and/or treatment for transient corneal conditions with gene editing and gene therapy, respectively.
4.4. Corneal dystrophies
Corneal dystrophies are slowly progressive bilateral inherited disorders that can result in decreased corneal transparency, altered corneal shape, and loss of vision. Specifically, autosomal dominant transforming growth factor beta induced (TGFβI)-associated corneal dystrophies result in deposition of TGFβI protein (TGFβIp) aggregates in the stroma or Bowman layer and have been attributed to 63 mutants with positions 124 and 555 being the most common (Lakshminarayanan et al., 2014). At least five different mutations in TGFβI have been discovered, result in Reis-Bucklers corneal dystrophy, Thiel-Behnke corneal dystrophy, lattice corneal dystrophy type 1, granular corneal dystrophy type 1 and granular corneal dystrophy type 2 (Han et al., 2016). Recently, a mouse model was developed with CRISPR/Cas9 technology for TGFβI-R124C corneal dystrophy (lattice corneal dystrophy type 1) that resembled human disease histologically. In this study, corneal wound healing was delayed in homozygous TGFβI-R124C mice indicating the mutation is detrimental to corneal homeostasis (Kitamoto et al., 2020). Fuchs endothelial corneal dystrophy (FECD) is an endothelial disorder and most common cause for corneal transplantation procedures globally (Matthaei et al., 2019). Identification of an association between an intronic CTG trinucleotide repeat expansion sequence of the transcription factor 4 (TCF4) gene and FECD is a target for gene therapy or editing (Kocaba et al., 2018; Fautsch et al., 2020). Positive progression for the use of gene therapy for management of corneal dystrophies is inhibited by a lack of scientific models, but recent advancements with gene editing using the CRISPR/Cas9 system are beginning to revolutionize our ability to study and treat corneal dystrophies.
The phenotype and severity of clinical signs associated with corneal dystrophies is wide, and therefore, a spectrum of therapeutic options exist but do not offer individualized interventions. Keratoplasty and corneal transplantation is a routinely performed procedure today, there are drawbacks including graft rejection, procedural complications, and a limited supply of healthy donor corneas (Moore et al., 2018).
5. Mono versus dual gene therapy
With many corneal diseases, there are multiple clinical signs that manifest. For example, a corneal injury, trauma, or infection might result in a corneal ulcer with concurrent corneal edema, neovascularization, and fibrosis. Physicians ideally need therapeutic tools that target multiple issues, are long-lasting, and are safe for the patient with a lower frequency of administration to promote therapy compliance. Dual gene therapy is a promising option to address all of those considerations. Very few cornea studies have investigated combination gene therapies for disease management. Marlo et al. (2018) used various gene silencing and overexpression combinations comprising of pro-Smads (Smad2, Smad3, and Smad4) and anti-fibrotic (Smad7) Smads. Silencing of individual Smad2, 3, or 4 genes or overexpression of Smad7 demonstrated significant inhibition of equine corneal fibroblasts to myofibroblasts and corneal fibrosis in vitro but no additional significant anti-fibrotic response was yielded by a combination gene therapy (Marlo et al., 2018). However, combination of two genes attacking two different factors or pathways demonstrated dramatically different results. For example, the combination of BMP7 and HGF gene therapy in rabbit in vivo and human in vitro cornea models demonstrated significant additive response characterized by decreased corneal fibrosis, inflammation, edema, and restoration of corneal transparency (Gupta et al., 2018). These are fascinating discoveries that certainly encourage more research in this direction, and will hopefully enter clinical trials sooner rather than later.
6. Promising approaches that need further investigation
Within the field of corneal gene therapy, more research is needed regarding combination gene therapies. Marlo et al. (2018) aimed to silence a pathologic gene (Smad2) while bolstering a physiologically beneficial gene (Smad7), and while the combination therapy was not superior to simply overexpressing Smad7 alone, this allows investigators to move forward and combine Smad7 overexpression with another gene that potentially targets a problem separate to fibrosis such as inhibition of corneal neovascularization. Smad-mediated gene expression is affected by histone acetylase, one of two enzymes playing a critical role in epigenetic regulation of gene transcription (Struhl, 1998; Glenisson et al., 2007; Mottet and Castronovo, 2008). Epigenetic modulation might be another avenue to explore for the development of single and combination gene therapy. The Sleeping Beauty transposons are quite promising in other areas of research and need to be investigated in corneal disorders. The overwhelming benefits to the vector and the long-term transgene expression are favorable (Hudecek et al., 2017).
7. Potential challenges and limitations
Despite significant improvements in the development of corneal gene therapies more research is needed to address challenges for advancing molecular therapy from the preclinical setting to clinical setting. First, patient safety is paramount and the development of nonimmunogenic vectors and vector-delivery techniques introducing genes into desired cells will result in fewer adverse reactions in patients and challenges of preexisting viral antibodies in humans. Second, the advancement of vectors that accommodate larger gene packages or multiple genes for combination therapy with AAV are needed. Third, highly tissue specific vector development will reduce unintended damage to normal tissues and unwanted gene expression while improving efficiency of therapeutic gene delivery. Last but not least, conceived gene therapy and gene editing strategies need strict oversight and regulations because of the potential to risk patients and potential exploitation for bioterrorism.
8. Future directions and conclusion
Overall, gene therapy for patients with corneal disease is behind the progress that has been made for retinal diseases and other organ systems, but recent advancements from the benchtop are much closer to clinical trials and application in human patients. The colossal improvements in last decade have begun to revolutionize the approach to gene therapy in the cornea with a focus on AAV and NP delivery of single and combination gene therapies. In addition, the potential applications of gene editing (ZNFs, TALENs, CRISPR/Cas9) are rapidly expanding. With more clinical trials, gene therapy and gene editing may likely soon be an effective long-term treatment option (in some cases a cure) to patients for conditions such as corneal neovascularization, corneal fibrosis, corneal graft rejection, and corneal dystrophies.
Acknowledgments
This work was supported by the United States Veterans Health Affairs, Washington DC, USA 1I01BX000357 Merit and Research Career Scientist awards (RRM); the NEI/NIH Bethesda, Maryland, USA 5R01EY017294, 1R01EY030774, and 1U01EY031650 grants (RRM); and by the Ruth M. Kraeuchi Missouri Endowed Chair Ophthalmology Fund (RRM) of the University of Missouri, Columbia, Missouri, USA. This article is dedicated to James D. Zieke, Ph.D., Senior Scientist, Schepens Eye Research Institute and Associate Professor, Harvard Medical School, Boston, MA, USA and James L. Funderburgh, Ph.D., Professor of Ophthalmology, University of Pittsburgh, Pittsburgh, PA, USA for their contributions in the fields of corneal wound healing and corneal regenerative medicine, respectively. Author (RRM) has a privilege of learning many things from these remarkable corneal researchers.
Footnotes
Declaration of competing interest
The authors have no competing interests to declare.
References
- Amrite AC, Kompella UB, 2005. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J. Pharm. Pharmacol. 57, 1555–1563. [DOI] [PubMed] [Google Scholar]
- Atencio IA, Chen Z, Nguyen QH, Faha B, Maneval DC, 2004. p21WAF-1/Cip-1 gene therapy as an adjunct to glaucoma filtration surgery. Curr. Opin. Mol. Therapeut. 6, 624–628. [PubMed] [Google Scholar]
- Bainbridge JW, Stephens C, Parsley K, Demaison C, Halfyard A, Thrasher AJ, Ali RR, 2001. In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Ther. 8, 1665–1668. [DOI] [PubMed] [Google Scholar]
- Bauer D, Lu M, Wasmuth S, Li H, Yang Y, Roggendorf M, Steuhl KP, Heiligenhaus A, 2006. Immunomodulation by topical particle-mediated administration of cytokine plasmid DNA suppresses herpetic stromal keratitis without impairment of antiviral defense. Graefes Arch. Clin. Exp. Ophthalmol. 244, 216–225. [DOI] [PubMed] [Google Scholar]
- Behrens A, Gordon EM, Li L, Liu PX, Chen Z, Peng H, La Bree L, Anderson WF, Hall FL, McDonnell PJ, 2002. Retroviral gene therapy vectors for prevention of excimer laser-induced corneal haze. Invest. Ophthalmol. Vis. Sci. 43, 968–977. [PubMed] [Google Scholar]
- Bemelmans AP, Arsenijevic Y, Majo F, 2009. Efficient lentiviral gene transfer into corneal stroma cells using a femtosecond laser. Gene Ther. 16, 933–938. [DOI] [PubMed] [Google Scholar]
- Bosiack AP, Giuliano EA, Gupta R, Mohan RR, 2012a. Canine corneal fibroblast and myofibroblast transduction with AAV5. Vet. Ophthalmol. 15, 291–298. [DOI] [PubMed] [Google Scholar]
- Bosiack AP, Giuliano EA, Gupta R, Mohan RR, 2012b. Corneal Gene Therapy in Veterinary Medicine: A Review, pp. 1–8. [Google Scholar]
- Buie LK, Rasmussen CA, Porterfield EC, Ramgolam VS, Choi VW, Markovic-Plese S, Samulski RJ, Kaufman PL, Borras T, 2010. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest. Ophthalmol. Vis. Sci. 51, 236–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss DG, Giuliano E, Sharma A, Mohan RR, 2010. Gene delivery in the equine cornea: a novel therapeutic strategy. Vet. Ophthalmol. 13, 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Conley S, Naash M, 2008. Nanoparticle applications in ocular gene therapy. Vis. Res. 48, 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassagne M, Laurent C, Rodrigues M, Galinier A, Spoerl E, Galiacy SD, Soler V, Fournie P, Malecaze F, 2016. Iontophoresis transcorneal delivery technique for transepithelial corneal collagen crosslinking with riboflavin in a rabbit model. Invest. Ophthalmol. Vis. Sci. 57, 594–603. [DOI] [PubMed] [Google Scholar]
- Chang YK, Hwang JS, Chung TY, Shin YJ, 2018. SOX2 activation using CRISPR/dCas9 promotes wound healing in corneal endothelial cells. Stem Cell. 36, 1851–1862. [DOI] [PubMed] [Google Scholar]
- Chaudhary K, Moore H, Tandon A, Gupta S, Khanna R, Mohan RR, 2014. Nanotechnology and adeno-associated virus-based decorin gene therapy ameliorates peritoneal fibrosis. Am. J. Physiol. Ren. Physiol 307, F777–F782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Lim RR, Lakshminarayanan R, Mohan RR, 2015. Nanomedicine approaches for corneal diseases. J. Funct. Biomater. 6, 277–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HC, Yeh SI, Tsao YP, Kuo PC, 2007. Subconjunctival injection of recombinant AAV-angiostatin ameliorates alkali burn induced corneal angiogenesis. Mol. Vis. 13, 2344–2352. [PubMed] [Google Scholar]
- Cho YK, Uehara H, Young JR, Tyagi P, Kompella UB, Zhang X, Luo L, Singh N, Archer B, Ambati BK, 2012. Flt23k nanoparticles offer additive benefit in graft survival and anti- angiogenic effects when combined with triamcinolone. Invest. Ophthalmol. Vis. Sci. 53, 2328–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella P, Ronzitti G, Mingozzi F, 2017. Emerging issues in AAV-mediated in vivo gene therapy. Mol Ther Methods Clin Dev 8, 87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelMonte DW, Kim T, 2011. Anatomy and physiology of the cornea. J. Cataract Refract. Surg. 37, 588–598. [DOI] [PubMed] [Google Scholar]
- Di Iorio E, Barbaro V, Alvisi G, Trevisan M, Ferrari S, Masi G, Nespeca P, Ghassabian H, Ponzin D, Palu G, 2019. New frontiers of corneal gene therapy. Hum. Gene Ther. 30, 923–945. [DOI] [PubMed] [Google Scholar]
- Donnelly KS, Giuliano EA, Sharma A, Tandon A, Rodier JT, Mohan RR, 2014. Decorin-PEI nanoconstruct attenuates equine corneal fibroblast differentiation. Vet. Ophthalmol. 17, 162–169. [DOI] [PubMed] [Google Scholar]
- Elbadawy HM, Gailledrat M, Desseaux C, Ponzin D, Ferrari S, 2012. Targeting herpetic keratitis by gene therapy. Journal of ophthalmology 2012, 594869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fautsch MP, Wieben ED, Baratz KH, Bhattacharyya N, Sadan AN, Hafford-Tear NJ, Tuft SJ, Davidson AE, 2020. TCF4-mediated Fuchs endothelial corneal dystrophy: insights into a common trinucleotide repeat-associated disease. Prog. Retin. Eye Res 100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, Thuret G, 2016. Global survey of corneal transplantation and eye banking. JAMA ophthalmology 134, 167–173. [DOI] [PubMed] [Google Scholar]
- Gilger BC, Conatser L, Salmon JH, Davis R, Hirsch M, 2018. AAV vector-mediated HLA-G expression to prevent corneal transplant rejection. Invest. Ophthalmol. Vis. Sci. 59, 3317–3317. [Google Scholar]
- Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR, 2017. Gene therapy clinical trials worldwide to 2017: an update. J. Gene Med 20, e3015. [DOI] [PubMed] [Google Scholar]
- Glenisson W, Castronovo V, Waltregny D, 2007. Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim. Biophys. Acta 1773, 1572–1582. [DOI] [PubMed] [Google Scholar]
- Gong N, Pleyer U, Yang J, Vogt K, Hill M, Anegon I, Volk HD, Ritter T, 2006. Influence of local and systemic CTLA4Ig gene transfer on corneal allograft survival. J. Gene Med. 8, 459–467. [DOI] [PubMed] [Google Scholar]
- Gruenert AK, Czugala M, Mueller C, Schmeer M, Schleef M, Kruse FE, Fuchsluger TA, 2016. Self-complementary adeno-associated virus vectors improve transduction efficiency of corneal endothelial cells. PloS One 11, e0152589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RM, Musunuru K, 2014. Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J. Clin. Invest. 124, 4154–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Rodier JT, Sharma A, Giuliano EA, Sinha PR, Hesemann NP, Ghosh A, Mohan RR, 2017. Targeted AAV5-Smad7 gene therapy inhibits corneal scarring in vivo. PloS One 12, e0172928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Fink MK, Ghosh A, Tripathi R, Sinha PR, Sharma A, Hesemann NP, Chaurasia SS, Giuliano EA, Mohan RR, 2018. Novel combination BMP7 and HGF gene therapy instigates selective myofibroblast apoptosis and reduces corneal haze in vivo. Invest. Ophthalmol. Vis. Sci. 59, 1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KE, Choi SI, Kim TI, Maeng YS, Stulting RD, Ji YW, Kim EK, 2016. Pathogenesis and treatments of TGFBI corneal dystrophies. Prog. Retin. Eye Res. 50, 67–88. [DOI] [PubMed] [Google Scholar]
- Hao J, Li SK, Kao WW, Liu CY, 2010. Gene delivery to cornea. Brain Res. Bull. 81, 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan P, Nabili M, Guan A, Zderic V, Myers M, 2017. Model for porosity changes occurring during ultrasound-enhanced transcorneal drug delivery. Ultrasound Med. Biol. 43, 1223–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudde T, Rayner SA, Comer RM, Weber M, Isaacs JD, Waldmann H, Larkin DFP, George AJT, 1999. Activated polyamidoamine dendrimers, a non-viral vector for gene transfer to the corneal endothelium. Gene Ther. 6, 939–943. [DOI] [PubMed] [Google Scholar]
- Hudde T, Rayner SA, De Alwis M, Thrasher AJ, Smith J, Coffin RS, George AJ, Larkin DF, 2000. Adeno-associated and herpes simplex viruses as vectors for gene transfer to the corneal endothelium. Cornea 19, 369–373. [DOI] [PubMed] [Google Scholar]
- Hudecek M, Izsvak Z, Johnen S, Renner M, Thumann G, Ivics Z, 2017. Going non-viral: the Sleeping Beauty transposon system breaks on through to the clinical side. Crit. Rev. Biochem. Mol. Biol. 52, 355–380. [DOI] [PubMed] [Google Scholar]
- Jani PD, Singh N, Jenkins C, Raghava S, Mo Y, Amin S, Kompella UB, Ambati BK, 2007. Nanoparticles sustain expression of Flt intraceptors in the cornea and inhibit injury-induced corneal angiogenesis. Invest. Ophthalmol. Vis. Sci. 48, 2030–2036. [DOI] [PubMed] [Google Scholar]
- Kamil S, Mohan RR, 2020. Corneal stromal wound healing: major regulators and therapeutic targets. Ocul. Surf 28 10.1016/j.jtos.2020.10.006.S1542-0124(20)30164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor A, McClements ME, MacLaren RE, 2020. CRISPR-Cas9 DNA base-editing and prime-editing. Int. J. Mol. Sci. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebriaei P, Izsvak Z, Narayanavari SA, Singh H, Ivics Z, 2017. Gene therapy with the sleeping beauty transposon system. Trends Genet. 33, 852–870. [DOI] [PubMed] [Google Scholar]
- Kim W-J, Mohan RR, Mohan RR, Wilson SE, 1999. Effect of PDGF, IL-1 alpha and BMP2/4 on corneal fibroblasts chemotaxis: expression of platelet-derived growth factor system. Invest. Ophthalmol. Vis. Sci. 40, 1364–1372. [PubMed] [Google Scholar]
- Kitamoto K, Taketani Y, Fujii W, Inamochi A, Toyono T, Miyai T, Yamagami S, Kuroda M, Usui T, Ouchi Y, 2020. Generation of mouse model of TGFBI-R124C corneal dystrophy using CRISPR/Cas9-mediated homology-directed repair. Sci. Rep. 10, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner EA, Zhang Z, Chapman RL, Multack RF, Volin MV, 2010. Ultrapure chitosan oligomers as carriers for corneal gene transfer. Biomaterials 31, 1814–1820. [DOI] [PubMed] [Google Scholar]
- Klausner EA, Zhang Z, Wong SP, Chapman RL, Volin MV, Harbottle RP, 2012. Corneal gene delivery: chitosan oligomer as a carrier of CpG rich, CpG free or S/MAR plasmid DNA. J. Gene Med 14, 100–108. [DOI] [PubMed] [Google Scholar]
- Klebe S, Stirling JW, Williams KA, 2000. Corneal endothelial cell nuclei are damaged after DNA transfer using a gene gun. Clin. Exp. Ophthalmol. 28, 58–59. [DOI] [PubMed] [Google Scholar]
- Klebe S, Sykes PJ, Coster DJ, Krishnan R, Williams KA, 2001. Prolongation of sheep corneal allograft survival by ex vivo transfer of the gene encoding interleukin-10. Transplantation 71, 1214–1220. [DOI] [PubMed] [Google Scholar]
- Kocaba V, Soh YQ, Mehta JS, 2018. Evolving therapies for fuchs endothelial dystrophy. Eur. Ophthalmic Rev 12. [DOI] [PubMed] [Google Scholar]
- Kong F, Li W, Li X, Zheng Q, Dai X, Zhou X, Boye SL, Hauswirth WW, Qu J, Pang JJ, 2010. Self-complementary AAV5 vector facilitates quicker transgene expression in photoreceptor and retinal pigment epithelial cells of normal mouse. Exp. Eye Res. 90, 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CM, Shen WY, Brankov M, Lai YK, Barnett NL, Lee SY, Yeo IY, Mathur R, Ho JE, Pineda P, Barathi A, Ang CL, Constable IJ, Rakoczy EP, 2005. Long-term evaluation of AAV-mediated sFlt-1 gene therapy for ocular neovascularization in mice and monkeys. Mol. Ther. 12, 659–668. [DOI] [PubMed] [Google Scholar]
- Lakshminarayanan R, Chaurasia SS, Anandalakshmi V, Chai SM, Murugan E, Vithana EN, Beuerman RW, Mehta JS, 2014. Clinical and genetic aspects of the TGFBI-associated corneal dystrophies. Ocul. Surf. 12, 234–251. [DOI] [PubMed] [Google Scholar]
- Larkin DF, Oral HB, Ring CJ, Lemoine NR, George AJ, 1996. Adenovirus-mediated gene delivery to the corneal endothelium. Transplantation 61, 363–370. [DOI] [PubMed] [Google Scholar]
- Lee D, Liu J, Junn HJ, Lee EJ, Jeong KS, Seol DW, 2019. No more helper adenovirus: production of gutless adenovirus (GLAd) free of adenovirus and replication-competent adenovirus (RCA) contaminants. Exp. Mol. Med. 51, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Weng J, Mohan RR, Bennett GL, Schwall R, Wang Z, Tabor K, Kim J, Hargrave S, Cuevas KH, Wilson SE, 1996. Hepatocyte growth factor and HGF receptor in lacrimal gland, tears, and cornea. Invest. Ophthalmol. Vis. Sci. 37, 727–739. [PubMed] [Google Scholar]
- Li Z, He T, Du K, Xing YQ, Run YM, Yan Y, Shen Y, 2014. Inhibition of oxygen-induced ischemic retinal neovascularization with adenoviral 15-lipoxygenase-1 gene transfer via up-regulation of PPAR-gamma and down-regulation of VEGFR-2 expression. PloS One 9, e85824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wen Y, Zhang Y, Zheng K, Ban J, Xie Q, Wen Y, Liu Q, Chen F, Mo Z, Liu L, Chen Y, Lu Z, 2019. Characterisation of 2-HP-β-cyclodextrin-PLGA nanoparticle complexes for potential use as ocular drug delivery vehicles. Artif Cells Nanomed Biotechnol 47, 4097–4108. [DOI] [PubMed] [Google Scholar]
- Lightfoot JD, Fuller KK, 2019. CRISPR/Cas9-Mediated gene replacement in the fungal keratitis pathogen Fusarium solani var. petroliphilum. Microorganisms 7, 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Brandt CR, Gabelt BT, Bryar PJ, Smith ME, Kaufman PL, 1999. Herpes simplex virus mediated gene transfer to primate ocular tissues. Exp. Eye Res. 69, 385–395. [DOI] [PubMed] [Google Scholar]
- Liu L, Li X, Zhu X, Li G, 2005. Cationic liposome-mediated bcl-xl gene transfection into human keratocytes. J Huazhong Univ Sci Technolog Med Sci 25, 365–367. [DOI] [PubMed] [Google Scholar]
- Liu J, Saghizadeh M, Tuli SS, Kramerov AA, Lewin AS, Bloom DC, Hauswirth WW, Castro MG, Schultz GS, Ljubimov AV, 2008. Different tropism of adenoviruses and adeno-associated viruses to corneal cells: implications for corneal gene therapy. Mol. Vis. 14, 2087–2096. [PMC free article] [PubMed] [Google Scholar]
- Lu WN, Ebihara N, Fujiki K, Sakuma H, Nakamura S, Watanabe Y, Kanai A, 2003. Gene transfer into corneal endothelial cells by Helios gene gun. Nippon. Ganka Gakkai Zasshi 107, 189–195. [PubMed] [Google Scholar]
- Manservigi R, Argnani R, Marconi P, 2010. HSV recombinant vectors for gene therapy. Open Virol. J. 4, 123–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlo TL, Giuliano EA, Tripathi R, Sharma A, Mohan RR, 2018. Altering equine corneal fibroblast differentiation through Smad gene transfer. Vet. Ophthalmol. 21, 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthaei M, Hribek A, Clahsen T, Bachmann B, Cursiefen C, Jun AS, 2019. Fuchs endothelial corneal dystrophy: clinical, genetic, pathophysiologic, and therapeutic aspects. Annu Rev Vis Sci 5, 151–175. [DOI] [PubMed] [Google Scholar]
- Miyagawa Y, Marino P, Verlengia G, Uchida H, Goins WF, Yokota S, Geller DA, Yoshida O, Mester J, Cohen JB, Glorioso JC, 2015. Herpes simplex viral-vector design for efficient transduction of nonneuronal cells without cytotoxicity. Proc. Natl. Acad. Sci. U. S. A. 112, E1632–E1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AEK, Choi R, Hong J-W, Lee J-S, Mohan RR, Ambrósio R, Zieske JD, Wilson SE, 2003. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp. Eye Res. 76, 71–87. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Schultz GS, Hong J-W, Mohan RR, Wilson SE, 2003a. Gene transfer into rabbit keratocytes using AAV and lipid-mediated plasmid DNA vectors with a lamellar flap for stromal access. Exp. Eye Res. 76, 373–383. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Sharma A, Netto MV, Sinha S, Wilson SE, 2005. Gene therapy in the cornea. Prog. Retin. Eye Res. 24, 537–559. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Sharma A, Cebulko TC, Tandon A, 2010. Vector delivery technique affects gene transfer in the cornea in vivo. Mol. Vis. 16, 2494–2501. [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Sinha S, Tandon A, Gupta R, Tovey JC, Sharma A, 2011a. Efficacious and safe tissue-selective controlled gene therapy approaches for the cornea. PloS One 6, e18771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tandon A, Sharma A, Cowden JW, Tovey JC, 2011b. Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Invest. Ophthalmol. Vis. Sci. 52, 4833–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tovey JC, Sharma A, Schultz GS, Cowden JW, Tandon A, 2011c. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PloS One 6, e26432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tovey JCK, Gupta R, Tandon A, Sharma A, 2011d. Decorin biology, expression, function and therapy in the cornea. Curr. Mol. Med. 11, 110–128. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Tovey JC, Sharma A, Tandon A, 2012. Gene therapy in the cornea: 2005–present. Prog. Retin. Eye Res 31, 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Rodier JT, Sharma A, 2013. Corneal gene therapy: basic science and translational perspective. Ocul. Surf. 11, 150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tripathi R, Sharma A, Sinha PR, Giuliano EA, Hesemann NP, Chaurasia SS, 2019. Decorin antagonizes corneal fibroblast migration via caveolae-mediated endocytosis of epidermal growth factor receptor. Exp. Eye Res. 180, 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R, Chandrahas A, Bleris L, 2014. Transcription activator-like effectors: a toolkit for synthetic biology. ACS Synth. Biol. 3, 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CBT, Christie KA, Marshall J, Nesbit MA, 2018. Personalised genome editing - the future for corneal dystrophies. Prog. Retin. Eye Res. 65, 147–165. [DOI] [PubMed] [Google Scholar]
- Mottet D, Castronovo V, 2008. Histone deacetylases: target enzymes for cancer therapy. Clin. Exp. Metastasis 25, 183–189. [DOI] [PubMed] [Google Scholar]
- Murthy RC, McFarland TJ, Yoken J, Chen S, Barone C, Burke D, Zhang Y, Appukuttan B, Stout JT, 2003. Corneal transduction to inhibit angiogenesis and graft failure. Invest. Ophthalmol. Vis. Sci. 44, 1837–1842. [DOI] [PubMed] [Google Scholar]
- Nabili M, Shenoy A, Chawla S, Mahesh S, Liu J, Geist C, Zderic V, 2014. Ultrasound-enhanced ocular delivery of dexamethasone sodium phosphate: an in vivo study. J Ther Ultrasound 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naso MF, Tomkowicz B, Perry WL 3rd, Strohl WR, 2017. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs 31, 317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P, Yiu SC, 2013. Strategies for local gene therapy of corneal allograft rejection. Middle East Afr. J. Ophthalmol. 20, 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell J, Taylor KA, Chapman MS, 2009. Adeno-associated virus-2 and its primary cellular receptor–Cryo-EM structure of a heparin complex. Virology 385, 434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva MS, Schottman T, Gulati M, 2012. Turning the tide of corneal blindness. Indian J. Ophthalmol. 60, 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira LA, Kim C, Sousa LB, Schwab IR, Rosenblatt MI, 2010. Gene transfer to primary corneal epithelial cells with an integrating lentiviral vector. Arq. Bras. Oftalmol. 73, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y, Sakamoto T, Hisatomi T, Tsutsumi C, Sassa Y, Ishibashi T, Inomata H, 2002. Targeted gene transfer to corneal stroma in vivo by electric pulses. Exp. Eye Res. 74, 191–198. [DOI] [PubMed] [Google Scholar]
- Pascolini D, Mariotti SP, 2012. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 96, 614–618. [DOI] [PubMed] [Google Scholar]
- Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ, Zhong L, Zolotukhin S, Srivastava A, Lewin AS, Hauswirth WW, 2009. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol. Ther. 17, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleyer U, Groth D, Hinz B, Keil O, Bertelmann E, Rieck P, Reszka R, 2001. Efficiency and toxicity of liposome-mediated gene transfer to corneal endothelial cells. Exp. Eye Res. 73, 1–7. [DOI] [PubMed] [Google Scholar]
- Prado DA, Acosta-Acero M, Maldonado RS, 2020. Gene therapy beyond luxturna: a new horizon of the treatment for inherited retinal disease. Curr. Opin. Ophthalmol. 31, 147–154. [DOI] [PubMed] [Google Scholar]
- Pricker SP, 1996. Medical uses of gold compounds: past, present and future. Gold Bull. 29, 53–60. [Google Scholar]
- Qazi Y, Hamrah P, 2013. Gene therapy in corneal transplantation. Semin. Ophthalmol. 28, 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi Y, Stagg B, Singh N, Singh S, Zhang X, Luo L, Simonis J, Kompella UB, Ambati BK, 2012. Nanoparticle-mediated delivery of shRNA.VEGF-a plasmids regresses corneal neovascularization. Invest. Ophthalmol. Vis. Sci. 53, 2837–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q, Luo D, Shi Y, Zhao Q, Chen Y, Wu J, Zhao M, 2016. CD25 siRNA induces Treg/Th1 cytokine expression in rat corneal transplantation models. Exp. Eye Res. 151, 134–141. [DOI] [PubMed] [Google Scholar]
- Raikwar SP, Raikwar AS, Chaurasia SS, Mohan RR, 2016. Gene editing for corneal disease management. World J. Transl. Med. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter T, Yang J, Dannowski H, Vogt K, Volk HD, Pleyer U, 2007. Effects of interleukin-12p40 gene transfer on rat corneal allograft survival. Transpl. Immunol. 18, 101–107. [DOI] [PubMed] [Google Scholar]
- Riviere I, Sadelain M, 2017. Chimeric antigen receptors: a cell and gene therapy perspective. Mol. Ther. 25, 1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier JT, Tripathi R, Fink MK, Sharma A, Korampally M, Gangopadhyay S, Giuliano EA, Sinha PR, Mohan RR, 2019. Linear polyethylenimine-DNA nanoconstruct for corneal gene delivery. J. Ocul. Pharmacol. Therapeut. 35, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux LN, Petit I, Domart R, Concordet JP, Qu J, Zhou H, Joliot A, Ferrigno O, Aberdam D, 2018. Modeling of aniridia-related keratopathy by CRISPR/Cas9 genome editing of human limbal epithelial cells and rescue by recombinant PAX6 protein. Stem Cell. 36, 1421–1429. [DOI] [PubMed] [Google Scholar]
- Ryals RC, Boye SL, Dinculescu A, Hauswirth WW, Boye SE, 2011. Quantifying transduction efficiencies of unmodified and tyrosine capsid mutant AAV vectors in vitro using two ocular cell lines. Mol. Vis. 17, 1090–1102. [PMC free article] [PubMed] [Google Scholar]
- Saika S, Ikeda K, Yamanaka O, Flanders KC, Nakajima Y, Miyamoto T, Ohnishi Y, Kao WW, Muragaki Y, Ooshima A, 2005a. Therapeutic effects of adenoviral gene transfer of bone morphogenic protein-7 on a corneal alkali injury model in mice. Lab. Invest. 85, 474–486. [DOI] [PubMed] [Google Scholar]
- Saika S, Ikeda K, Yamanaka O, Miyamoto T, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Nakajima Y, Kao WWY, Flanders KC, Roberts AB, 2005b. Expression of Smad7 in mouse eyes accelerates healing of corneal tissue after exposure to alkali. Am. J. Pathol. 166, 1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvam S, Thomas PB, Hamm-Alvarez SF, Schechter JE, Stevenson D, Mircheff AK, Trousdale MD, 2006. Current status of gene delivery and gene therapy in lacrimal gland using viral vectors. Adv. Drug Deliv. Rev. 58, 1243–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Ghosh A, Hansen ET, Newman JM, Mohan RR, 2010a. Transduction efficiency of AAV 2/6, 2/8 and 2/9 vectors for delivering genes in human corneal fibroblasts. Brain Res. Bull. 81, 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Ghosh A, Siddappa C, Mohan RR, 2010b. Gene therapy for the cornea, conjunctiva, and lacrimal gland. In: Dartt DA (Ed.), Encyclopedia of the Eye. Academic Press, Oxford, pp. 185–194. [Google Scholar]
- Sharma A, Tovey JCK, Cowden JW, Chiodo V, Hauswirth WW, Mohan RR, 2010c. Remarkably high and selective transgene delivery into mouse corneal endothelium in vivo with tyrosine-mutant AAV8. Invest. Ophthalmol. Vis. Sci. 51, 2839–2839. [Google Scholar]
- Sharma A, Tovey JC, Ghosh A, Mohan RR, 2010d. AAV serotype influences gene transfer in corneal stroma in vivo. Exp. Eye Res. 91, 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Tandon A, Tovey JC, Gupta R, Robertson JD, Fortune JA, Klibanov AM, Cowden JW, Rieger FG, Mohan RR, 2011. Polyethylenimine-conjugated gold nanoparticles: gene transfer potential and low toxicity in the cornea. Nanomedicine 7, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Rodier JT, Tandon A, Klibanov AM, Mohan RR, 2012. Attenuation of corneal myofibroblast development through nanoparticle-mediated soluble transforming growth factor-b type II receptor (sTGFbRII) gene transfer. Mol. Vis. 18, 2598–2607. [PMC free article] [PubMed] [Google Scholar]
- Song L, Bower JJ, Hirsch ML, 2020. Preparation and administration of adeno-associated virus vectors for corneal gene delivery. Methods Mol. Biol. 2145, 77–102. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Tachibana K, Uchino E, Okubo A, Yamamoto M, Sakoda K, Hisatomi T, Sonoda KH, Negishi Y, Izumi Y, Takao S, Sakamoto T, 2006. Gene transfer to corneal epithelium and keratocytes mediated by ultrasound with microbubbles. Invest. Ophthalmol. Vis. Sci. 47, 558–564. [DOI] [PubMed] [Google Scholar]
- Souza JG, Dias K, Silva SA, de Rezende LC, Rocha EM, Emery FS, Lopez RF, 2015. Transcorneal iontophoresis of dendrimers: PAMAM corneal penetration and dexamethasone delivery. J. Contr. Release 200, 115–124. [DOI] [PubMed] [Google Scholar]
- Spencer B, Agarwala S, Miskulin M, Smith M, Brandt CR, 2000. Herpes simplex virus-mediated gene delivery to the rodent visual system. Invest. Ophthalmol. Vis. Sci. 41, 1392–1401. [PubMed] [Google Scholar]
- Stevenson W, Cheng SF, Dastjerdi MH, Ferrari G, Dana R, 2012. Corneal neovascularization and the utility of topical VEGF inhibition: ranibizumab (Lucentis) vs bevacizumab (Avastin). Ocul. Surf. 10, 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K, 1998. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 12, 599–606. [DOI] [PubMed] [Google Scholar]
- Tandon A, Sharma A, Rodier JT, Klibanov AM, Rieger FG, Mohan RR, 2013. BMP7 gene transfer via gold nanoparticles into stroma inhibits corneal fibrosis in vivo. PloS One 8, e66434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanelian DL, Barry MA, Johnston SA, Le T, Smith G, 1997. Controlled gene gun delivery and expression of DNA within the cornea. Biotechniques 23, 484–488. [DOI] [PubMed] [Google Scholar]
- Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G, Holmes MC, Gregory PD, Ando DG, Kalos M, Collman RG, Binder-Scholl G, Plesa G, Hwang WT, Levine BL, June CH, 2014. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 370, 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilla J, Del Pozo-Rodriguez A, Vicente-Pascual M, Solinis MA, Rodriguez-Gascon A, 2018. Targeting corneal inflammation by gene therapy: emerging strategies for keratitis. Exp. Eye Res. 176, 130–140. [DOI] [PubMed] [Google Scholar]
- Torrecilla J, Gomez-Aguado I, Vicente-Pascual M, Del Pozo-Rodriguez A, Solinis MA, Rodriguez-Gascon A, 2019. MMP-9 downregulation with lipid nanoparticles for inhibiting corneal neovascularization by gene silencing. Nanomaterials 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A, Corey L, 2007. Persistence in the population: epidemiology, transmission. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K (Eds.), Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press, Cambridge. [PubMed] [Google Scholar]
- Wang L, Xiao R, Andres-Mateos E, Vandenberghe LH, 2017. Single stranded adeno-associated virus achieves efficient gene transfer to anterior segment in the mouse eye. PloS One 12, e0182473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2020. Biology of keratorefractive surgery PRK, PTK, LASIK, SMILE, inlays and other refractive procedures. Exp. Eye Res. 198, 108136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Chen L, Mohan RR, Liang Q, Liu J, 1999. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp. Eye Res. 68, 377–397. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Blindness and vision impairment prevention. https://www.who.int/blindness/causes/priority/en/index8.html. (Accessed 4 February 2020).
- Yang Y, Wang L, Bell P, McMenamin D, He Z, White J, Yu H, Xu C, Morizono H, Musunuru K, Batshaw ML, Wilson JM, 2016. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat. Biotechnol. 34, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG, 2014. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 15, 541–555. [DOI] [PubMed] [Google Scholar]
- Yin H, Lu Q, Wang X, Majumdar S, Jun AS, Stark WJ, Grant MP, Elisseeff JH, 2019. Tissue-derived microparticles reduce inflammation and fibrosis in cornea wounds. Acta Biomater. 85, 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi K, Kachi S, Zhang HS, Gregory PD, Spratt SK, Samulski RJ, Campochiaro PA, 2007. Ocular gene transfer with self-complementary AAV vectors. Invest. Ophthalmol. Vis. Sci. 48, 3324–3328. [DOI] [PubMed] [Google Scholar]
- Young AL, Kam KW, Jhanji V, Cheng LL, Rao SK, 2012. A new era in corneal transplantation: paradigm shift and evolution of techniques. Hong Kong Med. J. 18, 509–516. [PubMed] [Google Scholar]
- Yu H, Chen L, Jiang J, 2010. Administration of pigment epithelium-derived factor delivered by adeno-associated virus inhibits blood-retinal barrier breakdown in diabetic rats. Mol. Vis. 16, 2384–2394. [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Liu Y, Huang W, Zhou S, Ling S, Chen J, 2013. The experimental treatment of corneal graft rejection with the interleukin-1 receptor antagonist (IL-1ra) gene. PloS One 8, e60714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GR, Zhao H, Cao H, Geller AI, 2012. Overexpression of either lysine-specific demethylase-1 or CLOCK, but not Co-Rest, improves long-term expression from a modified neurofilament promoter, in a helper virus-free HSV-1 vector system. Brain Res. 1436, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, Agbandje-McKenna M, Herzog RW, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A, 2008a. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology 381, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I, Warrington KH Jr., Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A, 2008b. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl. Acad. Sci. U. S. A. 105, 7827–7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SY, Xie ZL, Xiao O, Yang XR, Heng BC, Sato Y, 2010. Inhibition of mouse alkali burn induced-corneal neovascularization by recombinant adenovirus encoding human vasohibin-1. Mol. Vis. 16, 1389–1398. [PMC free article] [PubMed] [Google Scholar]


