Abstract
Soluble methane monooxygenase (sMMO) activity in Methylosinus trichosporium OB3b was found to be more strongly affected as copper-to-biomass ratios changed in a newly developed medium, M2M, which uses pyrophosphate for metal chelation, than in nitrate mineral salts (NMS), which uses EDTA. When M2M medium was amended with EDTA, sMMO activity was similar to that in NMS medium, indicating that EDTA-bound copper had lower bioavailability than pyrophosphate-bound copper. EDTA did not limit the association of copper with the cells; rather, copper was sequestered in a form which did not affect sMMO activity.
Methanotrophs, a group of gram-negative bacteria that utilize methane as their sole source of carbon and energy, can express two forms of methane monooxygenase (MMO), depending on the amount of copper available. At low copper-to-biomass ratios, a cytoplasmic or soluble methane monooxygenase (sMMO) is synthesized by some methanotrophs, while a membrane-associated or particulate methane monooxygenase (pMMO) is found at high copper-to-biomass ratios (5). Both forms of the MMO can degrade priority pollutants, such as trichloroethylene (TCE), but at much different rates (7, 9). Although the mechanism(s) of copper uptake by methanotrophs is less well characterized than the effect of changing copper on methanotrophic activity, recent studies indicate the existence of specific copper uptake systems in methanotrophs (1, 2, 4, 12, 14).
It is unknown which forms of copper are bioavailable to methanotrophs, however, and such information is needed to optimize methanotroph-mediated bioremediation. A system in which metal speciation (i.e., its distribution in chelated, free, and precipitated forms) can be easily controlled must be used to address this issue. Typical microbial media, however, are designed to optimize growth and often have metal precipitates, poorly understood metal complexation, and ill-defined equilibrium conditions that prevent the assessment of metal bioavailability. To better understand what forms of copper are bioavailable to methanotrophs, a new growth medium, M2M medium, was developed that had few to no copper precipitates and rapid chemical equilibrium of copper species and that allowed for changes in copper speciation that did not affect the speciation of other metals, particularly iron. The bioavailability of different copper species was assessed using this well-defined growth medium by monitoring whole-cell sMMO activity in Methylosinus trichosporium OB3b with different copper concentrations and different chelating agents as well as through comparisons to whole-cell activity in the commonly used growth medium nitrate mineral salts (NMS).
Medium preparation and analysis.
NMS medium was prepared as described previously (13). M2M medium was prepared by adding, per liter of deionized distilled water, the following: 11 ml of pyrophosphate stock solution (44.6 g of Na4P2O7 · 10H2O/liter at pH 6.0), 1.4 ml of 0.5-g/liter FeSO4 · 7H2O stock adjusted to pH 7.1 to 7.5, 10 ml of a background electrolyte stock (2.46 g of MgSO4 · 7H2O/liter, 101 g of KNO3/liter, 1.47 g of CaCl2 · 2H2O/liter), 2 ml of an acidified (pH 3) trace element stock (0.025 g of CoCl2 · 6H2O/liter, 9.9 × 10−3 g of MnCl2 · 4H2O/liter, 0.2 g of ZnSO4 · 7H2O/liter, 5.1 × 10−3 g of NiCl2 · 6H2O/liter, 7.4 × 10−3 g of H3BO3/liter, 0.25 g of Na2MoO4 · 2H2O/liter), 2.5 ml of phosphate stock solution (26 g of KH2PO4 · H2O/liter, 33 g of Na2HPO4 · 7H2O/liter), and various amounts of copper from an acidified Cu(NO3)2 stock. The pH of the medium was adjusted to 6.8 using NaOH. To avoid copper contamination, all glassware was carefully acid washed and medium components of American Chemical Society grade or better were used. Based on the manufacturer's specifications, total heavy metal contamination was less than 5 × 10−8 and 10−7 M for M2M and NMS media, respectively. When prepared without added copper, however, all media contained no detectable copper (less than 5 × 10−9 M) as measured by atomic absorption spectrophotometry (AAS).
The growth media were characterized with regard to the time required for copper speciation to reach an apparent equilibrium as well as the amounts of precipitated copper and iron. To measure the equilibration time in each medium, the free copper (Cu2+) concentration was monitored using a cupric ion-selective electrode in stirred polycarbonate reactors at 30°C until the change in output voltage was less than 0.02 mV · min−1. Free copper concentrations at equilibrium are reported as pCu, or −log([Cu2+]). The amounts of copper and iron precipitated in each medium were determined by centrifuging the media at 206,000 × g for 40 min at 30°C and measuring the copper and iron in the supernatant using AAS. The amounts of precipitated copper and iron were calculated as the differences between the total and supernatant metal concentrations.
The major differences between the two media were in the buffers and metal chelating agents used. Buffering and metal chelation in M2M were provided by pyrophosphate, while in NMS, phosphate and EDTA were used for buffering and metal chelation, respectively. The substitution of pyrophosphate for phosphate to provide buffering capacity in M2M facilitated medium preparation by preventing phosphate precipitation. As a result, the buffer could be autoclaved with the contents of M2M medium, and copper speciation immediately reached equilibrium, as the free copper concentration did not change within 25 h after autoclaving. Free copper concentrations in NMS medium, however, decreased by more than 2 orders of magnitude in the same time period. The likely cause for this slower equilibration was the exchange of copper for iron in EDTA and the subsequent precipitation of iron. Such precipitation increased with increasing copper concentrations in NMS, as shown in Table 1. Therefore, it was not possible to vary copper concentrations over this range without also altering the speciation of iron in NMS. In M2M, changing copper concentrations changed the amount of free copper, as shown in Table 1, but the concentration of soluble iron did not vary greatly with changing copper concentrations. These data underscore the advantage of using a medium such as M2M, with easily controlled metal speciation, when studying the effect of copper on methanotrophs.
TABLE 1.
Equilibrium metal speciation in M2M and NMS media at different total copper concentrationsa
| Medium | Metal speciation | Result obtained with total copper (μM)
|
||||
|---|---|---|---|---|---|---|
| 0.02 | 0.11 | 5 | 10 | 20 | ||
| M2M | Chelated Cu (%) | 100 | 100 | 99 | 96 | 94 |
| Precipitated Cu (%) | 0 | 0 | 0.5 | 3.6 | 5.6 | |
| Free Cu (%) | 0.089 | 0.048 | 0.013 | 0.014 | 0.015 | |
| pCub | 10.7 | 10.3 | 9.2 | 8.8 | 8.5 | |
| Soluble Fe (%) | 16 | 9 | 14 | 11 | 11 | |
| Precipitated Fe (%) | 84 | 91 | 86 | 89 | 89 | |
| NMS | Chelated Cu (%) | 100 | 94 | 93 | 70 | 59 |
| Precipitated Cu (%) | 0 | 5.9 | 7.2 | 30 | 38 | |
| Free Cu (%) | 0.16 | 0.14 | 0.08 | 0.21 | 2.8 | |
| pCu | 10.3 | 9.8 | 8.4 | 7.7 | 6.3 | |
| Soluble Fe (%) | 99 | 99 | 68 | 28 | 0 | |
| Precipitated Fe (%) | 1 | 1 | 32 | 62 | 100 | |
M2M and NMS have total iron concentrations of 0.9 and 9 μM, respectively.
Free copper concentrations at equilibrium, reported as the negative log of [Cu2+].
sMMO activity assays.
sMMO activity in M. trichosporium OB3b grown in either NMS, M2M, or M2M with EDTA was assayed using a modified version of the naphthalene assay (3). The media were inoculated with cells grown on NMS agar plates with no added copper. Over the tested copper concentrations, M. trichosporium OB3b had average growth rates of 0.075 h−1 in NMS and 0.045 h−1 in M2M with and without EDTA, although the extent of growth was the same in all three media. The cells were grown to an optical density at 600 nm of 0.2 to 0.5, and triplicate samples of 2 ml each were put in 6-ml serum vials with naphthalene, which were capped and sealed. The cells were incubated for 1 h at 270 rpm and 30°C, after which 4 mM acetylene was added to inhibit sMMO activity. The cell suspension was then centrifuged for 5 min at 12,000 × g. One hundred thirty microliters of 4.21 mM tetrazotized o-dianisidine was placed in a 1.5-ml cuvette with 1.3 ml of the culture supernatant, and the absorbance at 528 nm was monitored immediately to assay for the production of naphthol. As the complex that formed between the naphthol and the tetrazotized o-dianisidine was unstable, the absorbance increased to a maximum level and then decreased over a period of time ranging from a few seconds to several minutes, depending on the sample. By taking the maximum value, a measurement was obtained that produced consistent standard curves with standard naphthol solutions ranging from 0 to 7.5 mg/liter. With this procedure, the triplicate samples had an average standard deviation of ±3.6%. The amount of cell-associated copper was determined by AAS as the difference between the total amount of copper in the growth medium and that remaining in the spent medium.
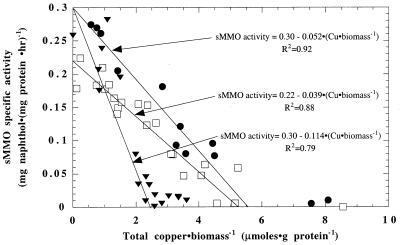
The trends of sMMO activity measured after growth in NMS and M2M media are shown in Fig. 1. Generally, maximal activity decreased linearly with increasing total copper-to-biomass ratios in both media. sMMO activity was apparent at levels of up to 5.64 μmol of copper · g of protein−1 in NMS that had EDTA as the chelating agent. In M2M with pyrophosphate as the chelating agent, however, no detectable sMMO activity was observed when >2.63 μmol of copper · g of protein−1 was present. Evidently, the amount of bioavailable copper in NMS medium is half of that in M2M medium, as it took twice as much copper per unit of biomass to reduce sMMO activity to below measurable levels in NMS as in M2M.
FIG. 1.
sMMO whole-cell activity in M. trichosporium OB3b in NMS medium (□), M2M medium (▾), and M2M with EDTA (●).
The changing sMMO activity trends in the two media are likely due to changes in copper speciation. If free copper were controlling copper bioavailability, it would be expected that the media with smaller amounts of free copper would have smaller amounts of bioavailable copper and thus would cause less repression of sMMO activity. As shown in Table 2, with the copper concentrations used to monitor sMMO activity, M2M had slightly smaller amounts of free copper than NMS; therefore, sMMO activity would be expected to be less strongly affected by copper in M2M than in NMS. Whole-cell sMMO activity, however, was lower in M2M medium than in NMS, indicating that the difference in the amounts of free copper in the two media cannot account for the observed changes in sMMO activity. The small difference between the amounts of precipitated copper in the two media (2%) cannot account for the differences in the amounts of available copper; therefore, it is likely that differences in amounts of chelated copper affected sMMO activity. The amounts of chelated copper in the two media were similar, suggesting that the differences are related to the type of chelating agent.
TABLE 2.
Equilibrium metal speciation of media during measurement of whole-cell sMMO activity
| Metal speciation | Result in medium (chelating agent)
|
||
|---|---|---|---|
| NMS (EDTA) | M2M (pyrophosphate) | M2M (EDTA) | |
| Total Cu (μM) | 0–0.13 | 0–0.08 | 0.01–0.2 |
| Chelated Cu (%) | 97.7 | 99.9 | 100 |
| Precipitated Cu (%) | 2.2 | 0 | 0 |
| Free Cu (%) | 0.15 | 0.06 | <10−4 |
| pCua | 10.3–9.8 | 10.8–10.3 | >11.6 |
| Total Fe (μM) | 9.4 | 1.2 | 1.0 |
| Soluble Fe (%) | 95 | 8 | 20 |
| Precipitated Fe (%) | 5 | 92 | 80 |
Free copper concentrations at equilibrium, reported as the negative log of [Cu2+].
To test this hypothesis, M2M medium was amended with the same concentration of EDTA used in NMS. As shown in Fig. 1, the whole-cell sMMO activity of M. trichosporium OB3b as a function of changing copper-to-biomass ratios in M2M with EDTA was similar to that in NMS. The copper-to-biomass ratio at which sMMO activity was no longer measurable was 5.77 μmol of copper · g of protein−1 for M2M with EDTA, similar to that for NMS and twice as great as that for M2M without EDTA, indicating that EDTA reduced the amount of bioavailable copper.
It is possible that the changes in whole-cell sMMO activity were due in part to changes in iron speciation. In addition to the established data on the effect of copper on expression of sMMO, some evidence has also indicated that iron may affect sMMO activity. For example, M. trichosporium OB3b grown in NMS with iron concentrations of <10 μM had low sMMO activity, but sMMO activity could be increased if the iron concentration was increased above 30 μM (11). Similar results were found for M. trichosporium OB3b grown in Higgins medium with various iron concentrations (10). In the sMMO activity experiments reported here, however, the soluble iron concentration was 10-fold higher in NMS medium than in M2M with EDTA (Table 2). As the measured whole-cell sMMO activities were similar in the two media regardless of the copper-to-biomass ratio, it appears that in this study iron bioavailability did not affect sMMO activity. Rather, since whole-cell sMMO activities were markedly different at the same copper-to-biomass ratios in M2M with and without EDTA, it appears that EDTA limited the bioavailability of copper to M. trichosporium OB3b compared to pyrophosphate. Although it is difficult to quantitatively compare these results with those of earlier studies, some interesting trends with relevance to copper bioavailability can be discerned. One study found that adding 84 μM Na2EDTA to NMS medium with 1.6 μM copper enhanced the rates of measured TCE degradation by a mixed methanotrophic culture (6). As TCE degradation rates are known to be significantly higher for sMMO than for pMMO, the increased rates may be due to decreased copper bioavailability and enhanced expression of sMMO in the presence of high EDTA concentrations. The concomitant expression of sMMO and pMMO by Methylococcus capsulatus Bath observed upon the addition of 2 μM FeEDTA may also be due to enhanced expression of sMMO as a result of lower copper bioavailability (8).
Copper sorption on inactive cells.
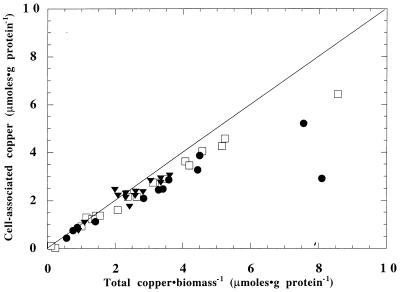
After growth, close to 100% of the copper in all three growth media was cell associated, as shown in Fig. 2. This is surprising, as at low total copper-to-biomass ratios, M. trichosporium OB3b had markedly different sMMO activities, depending on the type of chelating agent present. Apparently, copper in the presence of EDTA was cell associated but was not in a form that could affect whole-cell sMMO activity. It is unclear what prevents such cell-associated copper from being bioavailable. The larger binding constant of CuEDTA (log k = 18.8) than of copper pyrophosphate (log k = 10.4) may limit the cells' ability to accumulate copper in a form necessary to affect sMMO activity.
FIG. 2.
Copper accumulation in M. trichosporium OB3b in NMS medium (□), M2M medium (▾), and M2M with EDTA (●). The line indicates 100% copper accumulation by the cells.
To test this hypothesis, the sorption of copper onto acetylene-inactivated M. trichosporium OB3b, which was incapable of actively sequestering copper, was measured in the presence and absence of EDTA. M. trichosporium OB3b was grown in M2M with 0.12 μM copper, inactivated with the addition of acetylene, and collected by centrifugation. The cells were then washed with phosphate buffer (3.1 mM PO43−, 20 mM NaNO3 [pH 7]) and resuspended in M2M with 0.12 μM Cu and either 0 or 11 μM EDTA along with acetylene for 24 h at 30°C. In M2M, nearly 100% of the added copper was cell associated after 24 h. If 11 μM EDTA was added to M2M, however, only 25% of the total copper was cell associated, indicating that copper uptake in the presence of EDTA requires an energy-dependent extracellular uptake mechanism, possibly the recently discovered copper binding cofactor (CBC) (4, 12). It is possible that these biogenic metal ligands bind copper in CuEDTA complexes but cannot effectively transport these copper species into the cell, thus reducing their bioavailability. It is interesting that in solutions of EDTA, copper, and the purified CBC, 68% of the copper was associated with EDTA. In the presence of ethylenediamine, a chelating agent with a copper binding constant similar to that of pyrophosphate, only 3.7% of the copper was associated with ethylenediamine (12). These trends, which are similar to the data presented here, point to the possibility that the copper binding sites on the cell surface have the same affinity for copper as the CBC and may be cell-bound CBC.
Acknowledgments
Research support from the National Science Foundation (MCB-9708552) is gratefully acknowledged, as are the assistance of Candice Winful and helpful discussions with A. A. DiSpirito (Iowa State University).
REFERENCES
- 1.Berson O, Lidstrom M E. Study of copper accumulation by the type I methanotroph Methylomicrobium albus BG8. Environ Sci Technol. 1996;30:802–809. [Google Scholar]
- 2.Berson O, Lidstrom M E. Cloning and characterization of corA, a gene encoding a copper-repressible polypeptide in the type I methanotroph Methylomicrobium albus BG8. FEMS Microbiol Lett. 1997;148:169–174. doi: 10.1111/j.1574-6968.1997.tb10284.x. [DOI] [PubMed] [Google Scholar]
- 3.Brusseau G A, Tsien H C, Hanson R S, Wackett L P. Optimization of trichloroethylene oxidation by methanotrophs and the use of a colorimetric assay to detect soluble methane monooxygenase activity. Biodegradation. 1990;1:19–29. doi: 10.1007/BF00117048. [DOI] [PubMed] [Google Scholar]
- 4.DiSpirito A A, Zahn J A, Graham D W, Kim H J, Larive C K, Derrick T S, Cox C D, Taylor A. Copper-binding compounds from Methylosinus trichosporium OB3b. J Bacteriol. 1998;180:3606–3613. doi: 10.1128/jb.180.14.3606-3613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanson R S, Hanson T E. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry S, Grbic-Galic D. Effect of mineral media on trichloroethylene oxidation by aquifer methanotrophs. Microb Ecol. 1990;20:151–169. doi: 10.1007/BF02543874. [DOI] [PubMed] [Google Scholar]
- 7.Lontoh S, Semrau J D. Methane and trichloroethylene degradation by Methylosinus trichosporium OB3b expressing particulate methane monooxygenase. Appl Environ Microbiol. 1998;64:1106–1114. doi: 10.1128/aem.64.3.1106-1114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen H T, Elliott S J, Yi J H, Chan S I. The particulate methane monooxygenase from Methylococcus capsulatus (Bath) is a novel copper-containing three subunit enzyme. J Biol Chem. 1998;273:7957–7966. doi: 10.1074/jbc.273.14.7957. [DOI] [PubMed] [Google Scholar]
- 9.Oldenhuis R, Oedzes J Y, van der Waarde J J, Janssen D B. Kinetics of chlorinated hydrocarbon degradation by Methylosinus trichosporium OB3b and toxicity of trichloroethylene. Appl Environ Microbiol. 1991;57:7–14. doi: 10.1128/aem.57.1.7-14.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S, Hanna M L, Taylor R T, Droege M W. Batch cultivation of Methylosinus trichosporium OB3b. I. Production of soluble methane monooxygenase. Biotechnol Bioeng. 1991;38:423–433. doi: 10.1002/bit.260380412. [DOI] [PubMed] [Google Scholar]
- 11.Sayler G S, Bowman J P. Optimization and maintenance of soluble methane monooxygenase activity in Methylosinus trichosporium OB3b. Biodegradation. 1994;5:1–11. doi: 10.1007/BF00695208. [DOI] [PubMed] [Google Scholar]
- 12.Téllez C M, Gaus K P, Graham D W, Arnold R G, Guzman R Z. Isolation of copper biochelates from Methylosinus trichosporium OB3b and soluble methane monooxygenase mutants. Appl Environ Microbiol. 1998;64:1115–1122. doi: 10.1128/aem.64.3.1115-1122.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whittenbury R, Phillips K C, Wilkinson J F. Enrichment, isolation, and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970;59:2771–2776. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- 14.Zahn J A, DiSpirito A A. Membrane-associated methane monooxygenase from Methylococcus capsulatus (Bath) J Bacteriol. 1996;178:1018–1029. doi: 10.1128/jb.178.4.1018-1029.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]