Abstract
Patients with congenitally determined aortic root aneurysms are at risk of aortic valve regurgitation, aortic dissection, rupture and death. Personalised external aortic root support (PEARS) may provide an alternative to aortic root replacement.
This was a multi-centre, prospective cohort of all consecutive patients who received ExoVasc mesh implants for a dilated aortic root between 2004 and 2017. Baseline and peri-operative characteristics, as well as early postoperative outcomes are described, and time-related survival and re-operation free survival are estimated using the Kaplan-Meier method.
From 2004 through 2017, 117 consecutive patients have received ExoVasc mesh implants for aortic root aneurysm. The inclusion criteria were an aortic root/sinus of Valsalva and ascending aorta with asymptomatic dilatation of between 40 and 50 mm in diameter in patients aged 16 years or more. Patients with more than mild aortic regurgitation were excluded. There was one early death. The length of stay was within seven days in 75% of patients.
In conclusion, the operation achieves the objectives of valve-sparing root replacement. PEARS may be seen as a low-risk conservative operation, which can be applied earlier on in the disease process, and which is complementary to more invasive procedures, such as valve-sparing root replacement or total root replacement.
Keywords: aortic root aneurysm, Marfan syndrome, personalised medicine, prophylactic surgery
Introduction
Prophylactic replacement of the aortic root aims to resect the aortic aneurysm and so mitigate the risk of dissection or rupture. There are two established surgical techniques: total root replacement (TRR), which involves replacement of the aortic root and valve with a composite valve conduit, or valve-sparing root replacement (VSRR). TRR is now a straightforward operation with a low operative risk, but it involves replacement of the aortic valve, which is normal in many patients. This then commits the patient either to life-long anticoagulation with a mechanical valve, or structural valve degeneration if a tissue valve is used. Alternatively, VSRR has the advantage of preserving the native aortic valve, but the disadvantage of being a more challenging operation with a long learning curve. Even in the most experienced centres, there is a risk of serious aortic regurgitation, with the possibility of re-operation after VSRR.1
Is there a third possibility? The use of computer-aided design (CAD) in the manufacture of a personalised external aortic root support (PEARS) was proposed in 2000 and implemented clinically in 2004.2 The ExoVasc mesh support is made from the same polymer (polyethylene terephthalate) as standard vascular prostheses (figure 1). The fabric of the ExoVasc is knitted and has an open-mesh structure with 0.7 mm pores, compared with the familiar woven, low porosity, corrugated vascular grafts. There has been step-by-step investigation of the operation and the implant: operative factors (operative times and blood product usage) were reported in the first 20 patients,3 technical efficacy up to six years was reported in the first 24 patients,4 and clinical results up to nine years in the first 30 patients.5 Technical details of the methods of manufacture have remained consistent throughout the series.6 The primary indication remains prophylactic treatment of root aneurysms to prevent further expansion with the intention of averting the risk of dissection and rupture. This report concerns all operated patients on an intention-to-treat basis, within a consecutive log of all patients in whom ExoVasc mesh implants were considered. It is known that this form of mesh is consistently incorporated to form a neo-aorta with conservation of the endothelium/blood interface from experimental implantation in sheep,7-9 clinical experience at re-operation10 and autopsy examination four years after implantation (figure 2).11 In the first 24 patients, all were operated in the lead hospital and with high-quality imaging available, the three commissure-to-cusp diameters were measured after an average of 50 months.12 Based on 72 (24 × 3) measurements, there was a small but significant reduction of the mean of the diameters from 4.4 cm to 4.3 cm.4 The cross-sectional area was also reduced (not significant [NS]) from 16.3 ±1.9 cm2 to 15.7 ± 2.7 cm2. In none of the patients was there an increase in the severity of aortic regurgitation or more than mild aortic regurgitation at follow-up. In the 24 patients studied, measurable enlargement was seen in the descending aortic dimensions during a median period of less than two years, while the aortic root was held at a smaller size than that prior to surgery.
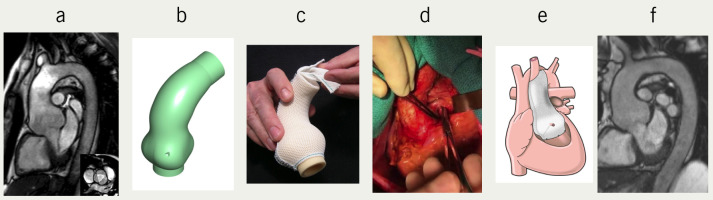
Figure 1. From left to right the figure illustrates the design, manufacture and implantation of the ExoVasc personalised mesh support. Digital image (a) is used to make a 3D replica (b) of the patient’s aortic root and ascending aorta. On this a customised sleeve of an open mesh fabric is manufactured (c). Each stage requires expertise and time is measured in hours. The aorta is dissected down to the aortoventricular junction (d). The dissection extends proximal to both coronary ostia. The longitudinal seam in the mesh is opened and incisions made to the point where the coronary arteries must pass through fashioning asterisk shaped incisions to conserve the mesh support. It extends to the aorto-ventricular junction and distally to just beyond the brachiocephalic artery (e). The final picture (f) is of the image of the first recipient (TG) 15 years after the operation.
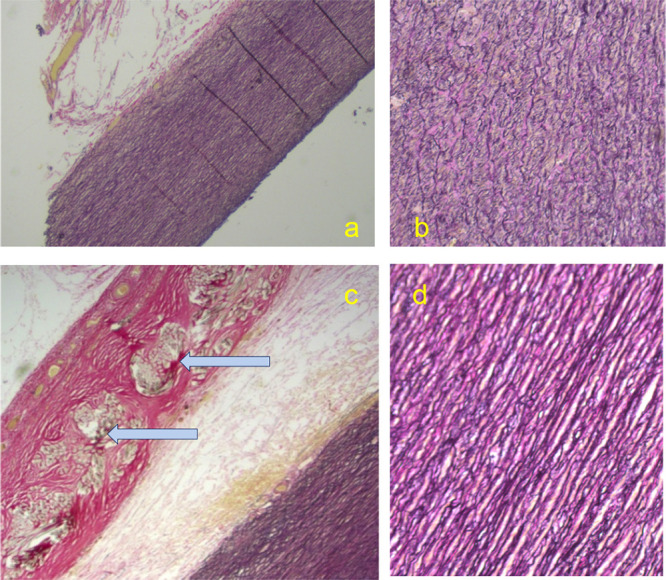
Figure 2. Patient XN16 who is the only case to have died with the mesh in place. This occurred 4.5 years after operation. The upper two panels are aorta from the arch beyond the mesh at low power (a) and high power (b) where the loss of the structure of the media characteristic of Marfan syndrome can be seen. Below (c) the mesh (filaments cut in cross-section, marked) is surrounded by healthy collagen fully incorporating it. At high power (d) the media has a normal appearance. The pathological interpretation is that the support has allowed the collagen to be restored to a normal appearance.
The purpose of the present paper is to provide clinical data on all 117 consecutive patients who have had at least one-year follow-up after this operation, performed for its primary indication of an aortic root aneurysm.
Method
The concept was first proposed in 2000, and this was followed by a period of pre-clinical development, mainly at Imperial College, London. In 2003, an application was made to the Local Research and Ethics committee and subsequently approved by the Clinical Practice Committee of the Royal Brompton Hospital. Twenty operations were to be performed on patients with Marfan syndrome and the results reported to the Committee. The inclusion criteria agreed were an aortic root/sinus of Valsalva and ascending aorta asymptomatic dilatation of between 40 mm and 50 mm in diameter in patients aged over 16 years. The first operation was performed in 2004.2 In 2010, after 23 patients had received this implant, approval was given to continue and to recruit surgeons at other centres. Patients eligible for inclusion in this report all had surgery for the primary indication: prophylactic treatment of life-threatening aortic root aneurysm, usually with a recognised and diagnosed syndrome. In accordance with the Clinical Governance Committee all patients received an information leaflet about PEARS and were asked to sign a procedure-specific consent form.
This was a multi-centre prospective cohort study. The full manufacturing records and CAD models were available from the secure file server at Exstent Ltd. The surgery case record forms (CRFs) were available from the operating surgeons and kept securely at Exstent Ltd. The Exstent records, the Royal Brompton Hospital database, and correspondence from the operating teams, were used to compile a full dataset of demography, aetiology, aortic dimensions, the operation performed, operating time, cardiopulmonary bypass time (if used), and hospital stay. Intra-operative adverse events, and any adjunctive surgery were recorded, and any later cardiac, aortic, neurological or infective events were tabulated. All patients underwent transoesophageal echocardiography at the time of operation. This was used to assess laminar flow in the coronary ostia by colour Doppler and to check on regional wall motion at the end of the procedure. Electrocardiogram (ECG) monitoring was routine, but we have found that laminar flow is a more sensitive indicator of the normality of coronary flow. In the postoperative phase, all patients received a transthoracic echocardiogram before discharge and a magnetic resonance imaging (MRI) scan on an annual basis. The assessment of aortic regurgitation was made by transthoracic echocardiogram, reported by a consultant echo-cardiologist. Two patients who had ferro-magnetic material (Harrington rods in the spine) had a computed tomography (CT) scan for follow-up.
The follow-up interval was from the date of operation to the date on which the patient was last clinically assessed and/or had cardiac investigations. The Kaplan-Meier method was used to obtain estimates of patient survival and re-operation.
This retrospective study using data that had been collected prospectively, was registered as an audit with our Trust institution. Patient informed consent was, therefore, waived.
For the statistical methods, descriptives were analysed as continuous variables: median (interquartile range [IQR]) and range. The following statistical software was used for the Kaplan-Meier analysis: SPSS V23.
Results
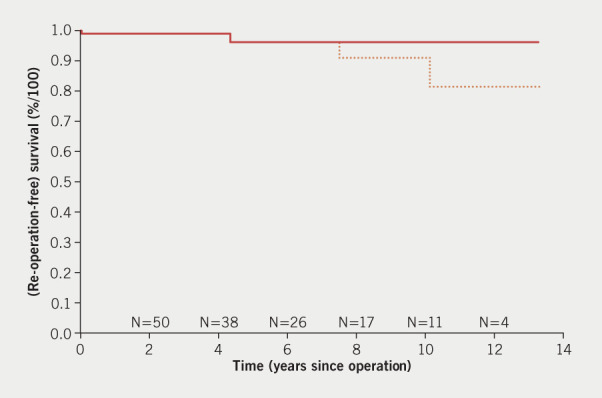
Between May 2004 and December 2018, 182 consecutive patients had a PEARS operation. The full dataset used for the purposes of the current study includes a consecutive series of 143 patients who had a PEARS operation up to and including December 2017 when we closed the analysis reported in this paper. At the end of 2017, of 132 patients who had surgery, 15 had operations for pathology other than aortic root aneurysm: eight were complex revision operations for congenital heart disease, some already reported,13 and seven had PEARS-supported pulmonary autografts in Ross operations. The Ross-PEARS experience will be reported separately. After induction, and with proctoring in each instance, 14 surgical teams have contributed to this surgical case series. The accrual of patient numbers is shown in figure 3. A flow chart of the entire cohort is shown in figure 4. Surgeons altered the plan from PEARS during the operation (termed a conversion) in four patients as indicated in the flow chart. There was one peri-operative death on postoperative day 5 due to damage to the left main stem at the operation. Seven patients had significant postoperative complications but made a complete recovery. There were no major bleeding events and only one superficial wound infection. Two patients had intraoperative ischaemic events resulting in 19- and 25-day hospital stays. The survival and re-operation-free survival for all 117 operated patients are shown in the Kaplan-Meier analysis (figure 5). This includes the one peri-operative death at day 5, a late death at 4.5 years related to arrhythmia,11 and two re-operations (one after nine years and the other after six years). These re-operations were necessary because of the development of significant aortic regurgitation. The right and non-coronary sinuses were not completely covered by the mesh due to deviations from the protocol for intra-operative reasons.
Figure 3. Cumulative plot of patients operated from May 2004 to December 2017.
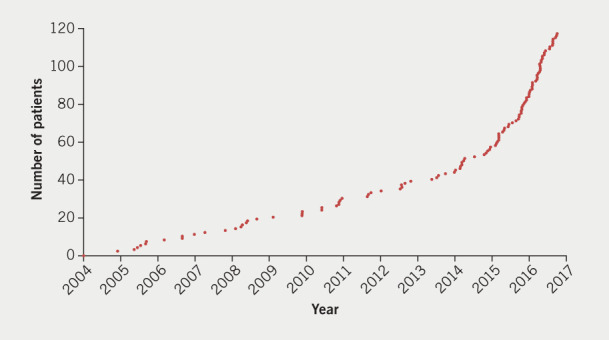
Figure 4. This flow chart includes all 117 consecutive patients for whom there was an intention to treat and who had surgery before the end of December 2017. All perioperative adverse events, conversions and adverse outcomes are described in the Appendix of Clinical Events. There is 100% follow-up and all patients are traceable.
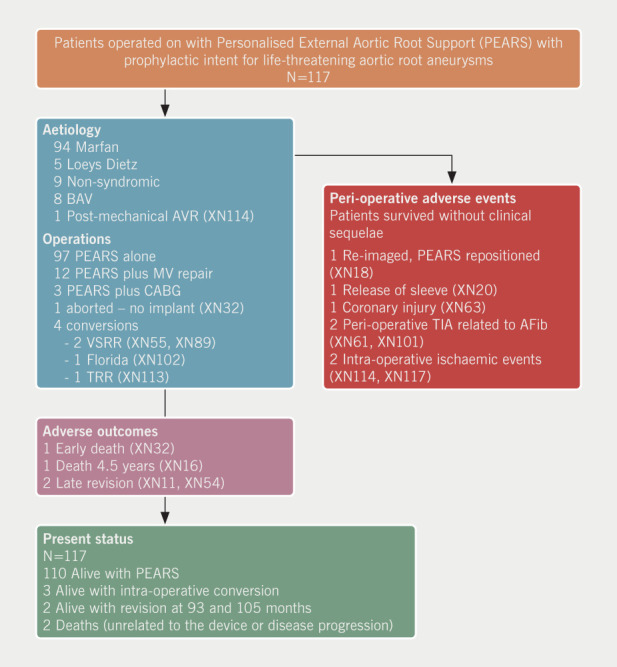
Key: AFib = atrial fibrillation; AVR = aortic valve replacement; BAV = bicuspid aortic valve; CABG = coronary artery bypass graft; MV = mitral valve; TIA = transient ischaemic attack; TRR = total root replacement; VSRR = valve-sparing root replacement
Figure 5. Kaplan-Meier analysis prepared by Professor J J M Takkenberg. Time-to-event analysis shows two deaths at 5 days and at 4.5 years and two re-operations at 6 and 9 years. The small numbers of patients ‘at risk’ with more than 2 years of follow-up affects the appearance of the chart. The single event at 9 years has a large impact on the overall analysis of survival because of the few patients operated on that long ago.
Discussion
In patients with a genetically driven aortic pathology the disease affects the entire aorta and its major branches. If it is possible to strengthen the aortic wall at the position where it is most exposed to risk, rather than resect the tissue, a similar result to the established procedures, TRR and VSRR, could be achieved at lower risk, and could, therefore, be offered to patients at an earlier time in the natural history of their disease. When we embarked on the development and evaluation of PEARS we ensured at an early stage that the innovation was evaluated by the National Institute for Health and Care Excellence (NICE).14 We recognised that an early challenge could be the placement of the mesh around the aortic root down to the aorto-ventricular junction. But we reasoned that some of this difficulty would be mitigated by the relative displacement of the coronary ostia from the level of the annulus. In the first 20 patients we ensured that there was at least 10 mm space, as measured on the CT scan. We also reasoned that in order to see the tissue planes clearly in these thin aortic roots, haemostasis was critical, and this would be optimal if heparinisation was avoided. Of course, we had the ability to place the patient on cardiopulmonary bypass without delay if required, and in the first patient a period of such bypass was used for 21 minutes. In the whole series, 73% of patients received their PEARS implant without cardiopulmonary bypass.
At the beginning of this project we thoroughly explored the possibility of a randomised-controlled trial (RCT). The project development team worked with clinical researchers, established research agencies and grant giving bodies, including the British National Institute for Health Research (NIHR), the British Heart Foundation, and the Medical Research Council to devise a controlled study to compare our novel approach with the established operations. The NIHR Research Design Service helped us identify two decision-making nodes that might be amenable to testing. One was the timing of the intervention: put bluntly to ‘go for it’ or to procrastinate, or to put it more gently, the ‘early/defer’ dilemma. The other was whether to have the more predictable mechanical solution and accept life-long anticoagulation or accept the less durable but more attractive valve-sparing operation, the ‘conserve/replace’ dilemma. We published these considerations in an attempt to organise a trial.15,16 There was no prospect of professional equipoise, as has been illustrated by arguments made in opposition to this conservative approach.17-19 It should be noted that neither total root nor valve-sparing root replacements were evaluated with animal experiments or controlled trials.
We sought to establish an informed patients’ perspective. The decision nodes were explored, along with other factors, using the Ottawa Decision Support Framework. We found that people have cogently weighted and strongly expressed preferences on both the ‘early/defer’ question and the ‘conserve/replace’ choice.20 Evidence concerning thromboembolism and bleeding with mechanical valves is plentiful and is well reviewed in a recent meta-analysis,21 which demonstrated that outcome after mechanical aortic valve replacement (AVR) in non-elderly adults is characterised by suboptimal survival and considerable lifetime risk of anticoagulation-related complications, as well as re-operation. There has also been a meta-analysis of the two approaches for aortic root replacement.22 The decision is amenable to evidence-based balancing of the pros and cons and is, thus, realistically not a matter for random assignment. The absence of randomly derived control data is, therefore, a limitation we must live with. What we can do is to ensure that patients who are to have a prophylactic operation face peri-operative risks that are as low as are achievable. They should also be given evidence-based estimates of the durability of the operations available, and reliable estimates of future failure and complications, from the best available observational data.
In an observational study of 148 patients seen in one Marfan centre between 1980 and 1990 we had usable aortic dimensions on 11 patients who dissected and 95 whose aorta remained intact. The latest (pre-dissection) aortic dimensions differed significantly (5.1 ± 1.3 cm versus 3.7 ± 0.9 cm, p<0.005) but they overlapped to such an extent that no value had sufficient sensitivity or specificity to be of reliable clinical value.23 We then developed personalised nomograms on which we depicted the rate of change in a patient’s own aorta to aid shared decision-making.24 While we respect the guidance given,25 our philosophy has been that if the aorta is progressively enlarging, particularly in a young patient where operation will inevitably be advised at some point, and we have a low-risk, conservative operation, the patient should be offered that option. This seems to us a reasonable way to handle the ‘early/defer’ dilemma.
Further comparisons can be made, but are difficult because of the variations in the nature and severity of aortic disease, comorbidity, use of cardiopulmonary bypass, myocardial ischaemia and circulatory arrest. Because CAD modelling and rapid prototyping (3D printing) are inherent requirements, PEARS is an elective operation. Of note, one quarter of PEARS patients had some aortic regurgitation, and this was deliberately corrected in some by using an undersized (95%) ExoVasc mesh. This possibility is being further explored and there are reasons to believe this may be feasible in a wider group of patients.26 In two patients there has been progression of aortic regurgitation. The point of note is that parts of the right and non-coronary sinuses were not completely covered by the mesh. Over years these areas expanded, resulting in aortic regurgitation due to single leaflet prolapse, thus, providing an accidental experiment comparing supported and unsupported sinuses in the same patient. This is in line with old evidence where only the more accessible part of the aortic root was covered with mesh. This part was stabilised while the uncovered part continued to dilate.10 Stabilising the dimensions of the aortic root and its architecture has preserved aortic leaflet function well. There has been freedom from valve- and aortic-related events in longer-term follow-up. There have been no episodes of bleeding, embolic events or endocarditis.
Limitations
The major limitation of this report, as with all other reports on aortic root surgery, is that there are no contemporary control data. For data on the natural history of aortic root aneurysms we have to rely, as do others, on estimates from 25 years ago.27 They, in turn, cited sources 20 years before that.28 In Murdoch’s landmark study of men aged 25 with Marfan syndrome, 27% had died before they were 35 years old and 48% before they were 45. For women, 13% had died before they were 35 and 24% before they reached 45. The steep fall in the numbers surviving continued for men to 55, 65 and 70 years.
A further limitation of this study was that because of the skewed accrual of patients with a recent upsurge (figure 3) there are relatively few patients with long-term follow-up. This makes the best comparable data on VSRR, of which we are aware, that in the early and one-year report of the Aortic Valve Operative Outcomes in the Marfan Patients Study (AVOOMPS).29 A strength shared by PEARS and VSRR is that both kept the record on ‘intention to treat’. All patients scheduled for PEARS (n=117) and VSRR (n=239) have been reported. The age, sex and aneurysm diameters for PEARS are similar to those for VSRR. There was one early death in each group.
Clinical perspective
Participating surgeons have taken varying views on the application of PEARS in their practice. Early in the experience we used conservative criteria. A relatively early departure was in patients in whom mitral valve regurgitation was determining the need for surgery. PEARS was used rather than leave the aortic root unprotected and presenting trouble at a later date.30 In the present report, 12 patients had combined surgery. Some surgeons have used PEARS to spare marginal patients, with comorbidities, the deleterious effects of cardiopulmonary bypass. We are not suggesting that a short period of cardiopulmonary bypass in the current era is very damaging, but the ability to do the precise dissection required around the base of the aortic root is enhanced by the absence of anticoagulation. The overall results, seem to us, to be competitive with the results of VSRR, as reported prospectively, in an intention-to-treat analysis, which allows for full reporting of clinical practice. In this study, we have presented the results in all patients where there was an intention-to-treat an aortic root aneurysm that might have been operated on by VSRR with the more conservative alternative of PEARS.
We think PEARS may also have a role in complex congenital heart disease where the aortic root is expanding, for example late changes 20 or more years after the arterial switch operation for transposition, and in preventing expansion of the autograft root after a Ross operation.
Key messages
A custom-made implant personalised to the patient's anatomy
A tissue/textile neo-adventitial format
A low-risk prophylactic operation for a genetically driven aortopathy
Conflicts of interest
The only author with a potential conflict of interest is TJG who is the inventor of the device. He was the first patient to have this operation in 2004, and is a shareholder in Exstent Ltd., the company that manufactures the Personalised External Aortic Root Support (PEARS) ExoVasc device.
Study approval
This retrospective study using data that had been collected prospectively, was registered as an audit with our Trust institution. Patient informed consent was therefore waived.
Consent
In accordance with the Clinical Governance Committee all patients received an information leaflet about PEARS and were asked to sign a procedure-specific consent form.
Funding Statement
Funding None.
Contributor Information
John Pepper, Consultant Cardiac Surgeon, Royal Brompton and Harefield NHS Foundation Trust, Sydney Street, London, SW3 6NP.
Cemil Izgi, Consultant Cardiac Radiologist, Royal Brompton and Harefield NHS Foundation Trust, Sydney Street, London, SW3 6NP.
Tal J Golesworthy, Chartered Energy Engineer, Extent Ltd., Theocsbury House, 18–20 Barton Street, Tewkesbury Gloucestershire.
Johanna J M Takkenberg, Cardiac Epidemiologist, Erasmus University Medical Centre, PO Box 2040, 3000 CA Rotterdam The Netherlands.
Tom Treasure, Consultant Cardiothoracic Surgeon, Clinical Operational Research Unit, University College London, 4 Taviton Street, London WC1H 0BT.
References
- 1.David TE. Aortic valve repair and aortic valve-sparing operations. J Thorac Cardiovasc Surg. 2015;149:9–11. doi: 10.1016/j.jtcvs.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Golesworthy T, Lamperth M, Mohiaddin R, Pepper J, Thornton W, Treasure T. A jacket for the Marfan’s aorta. Lancet. 2004;364:1582. doi: 10.1016/S0140-6736(04)17308-X. [DOI] [PubMed] [Google Scholar]
- 3.Pepper J, Golesworthy T, Utley M, et al. Manufacturing and placing a bespoke support for the Marfan aortic root: description of the method and technical results and status at one year for the first ten patients. Interact Cardiovasc Thorac Surg. 2010;10:360–365. doi: 10.1510/icvts.2009.220319. [DOI] [PubMed] [Google Scholar]
- 4.Treasure T, Crowe S, Chan KM, et al. A method for early evaluation of a recently introduced technology by deriving a comparative group from existing clinical data: a case study in external support of the Marfan aortic root. BMJ Open. 2012;2:e000725. doi: 10.1136/bmjopen-2011-000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treasure T, Takkenberg JJ, Golesworthy T, et al. Personalised external aortic root support (PEARS) in Marfan syndrome: analysis of 1-9 year outcomes by intention-to-treat in a cohort of the first 30 consecutive patients to receive a novel tissue and valve-conserving procedure, compared with the published results of aortic root replacement. Heart. 2014;100:969–975. doi: 10.1136/heartjnl-2013-304913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pepper J, Petrou M, Rega F, Rosendahl U, Golesworthy T, Treasure T. Multimedia Manual of Cardio-Thoracic Surgery. 2013. Implantation of an individually computer-designed and manufactured external support for the Marfan aortic root. [DOI] [PubMed] [Google Scholar]
- 7.Cohen O, Odim J, de la Zerda D, et al. Long-term experience of girdling the ascending aorta with Dacron mesh as definitive treatment for aneurysmal dilation. Ann Thorac Surg. 2007;83:S780–S784. doi: 10.1016/j.athoracsur.2006.10.086. [DOI] [PubMed] [Google Scholar]
- 8.Verbrugghe P, Verbeken E, Pepper J, et al. External aortic root support: a histological and mechanical study in sheep. Interact Cardiovasc Thorac Surg. 2013;17:334–339. doi: 10.1093/icvts/ivt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Hoof L, Verbrugghe P, Verbeken E, et al. Support of the aortic wall: a histological study in sheep comparing a macroporous mesh with low-porosity vascular graft of the same polyethylene terephthalate material. Interact Cardiovasc Thorac Surg. 2017;25:89–95. doi: 10.1093/icvts/ivx009. [DOI] [PubMed] [Google Scholar]
- 10.Vastmans J, Fehervary H, Verbrugghe P, et al. Biomechanical evaluation of a personalized external aortic root support applied in the Ross procedure. J Mech Behav Biomed Mater. 2018;78:164–174. doi: 10.1016/j.jmbbm.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Pepper J, Goddard M, Mohiaddin R, Treasure T. Histology of a Marfan aorta 4.5 years after personalized external aortic root support. Eur J Cardiothorac Surg. 2015;48:502–505. doi: 10.1093/ejcts/ezu415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izgi C, Nyktari E, Mohiaddin R. Cardiovascular magnetic resonance follow-up of the Marfan’s thoracic aorta after personalized external aortic root support surgery. J Cardiovasc Mag Res. 2014;16((suppl 1)):116. doi: 10.1186/1532-429X-16-S1-P116. [DOI] [Google Scholar]
- 13.Austin C, Mathur SK, Pepper J. Personalised External Aortic Root Support (PEARS): utilisation in dilatational aortopathies after the arterial switch operation V4. Int J Cardiol. 2016;220:772–774. doi: 10.1016/j.ijcard.2016.06.248. [DOI] [PubMed] [Google Scholar]
- 14.Treasure T, Pepper J, Golesworthy T, Mohiaddin R, Anderson RH. External aortic root support: NICE guidance. Heart. 2012;98:65–68. doi: 10.1136/heartjnl-2011-301017. [DOI] [PubMed] [Google Scholar]
- 15.Treasure T. Options for preemptive aortic root surgery for people with Marfan syndrome. Eur Heart J. 2013;34:1947–1949. doi: 10.1093/eurheartj/eht191. [DOI] [PubMed] [Google Scholar]
- 16.Treasure T, Pepper J. A call for expressions of interest in a comparative study of the options for pre-emptive aortic root surgery for people with Marfan syndrome. Eur J Cardiothorac Surg. 2013;44:588. doi: 10.1093/ejcts/ezt335. [DOI] [PubMed] [Google Scholar]
- 17.Cameron D. External support of the dilated aorta: back to the future? Heart. 2014;100:908. doi: 10.1136/heartjnl-2013-305279. [DOI] [PubMed] [Google Scholar]
- 18.Jahangiri M, Leigh B, Cameron D. External aortic root support: a viable alternative treatment option? Consent and duty of candour. Eur J Cardiothorac Surg. 2017;51:1020. doi: 10.1093/ejcts/ezw440. [DOI] [PubMed] [Google Scholar]
- 19.Treasure T, Pepper J, Jahangiri, et al. Eur J Cardiothorac Surg. 2017;51:1021. doi: 10.1093/ejcts/ezx051. [DOI] [PubMed] [Google Scholar]
- 20.Treasure T, King A, Hidalgo LL, Golesworthy T, Pepper J, Takkenberg JJ. Developing a shared decision support framework for aortic root surgery in Marfan syndrome. Heart. 2018;104:480–486. doi: 10.1136/heartjnl-2017-311598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korteland NM, Etnel JRG, Arabkhani B, et al. Mechanical aortic valve replacement in nonelderly adults: meta-analysis and microsimulation. Eur Heart J. 2017;38:3370–3377. doi: 10.1093/eurheartj/ehx199. [DOI] [PubMed] [Google Scholar]
- 22.Benedetto U, Melina G, Takkenberg JJ, Roscitano A, Angeloni E, Sinatra R. Surgical management of aortic root disease in Marfan syndrome: a systematic review and meta-analysis. Heart. 2011;97:955–958. doi: 10.1136/hrt.2010.210286. [DOI] [PubMed] [Google Scholar]
- 23.Murgatroyd F, Child A, Poloniecki J, Treasure T, Pumphrey C. Does routine echocardiographic measurement of the aortic root diameter predict the risk of aortic dissection in the Marfan syndrome. Eur Heart J. 1991;(12(suppl)):410. doi: 10.1093/eurheartj/12.Abstract_Supplement.1. [DOI] [Google Scholar]
- 24.Treasure T, Reynolds C, Valencia O, Child A, Gallivan S. Cardiac Surgery and Concomitant Disease. Darmstadt: Springer; 1999. The timing of aortic root replacement in the Marfan syndrome: computer aided decision support. In: Enker J, editor; pp. 91–98. [Google Scholar]
- 25.Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33:2451–2496. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 26.Plonek T, Dumanski A, Obremska M, Kustrzycki W. First beatingheart valve-sparing aortic root repair: a “corset” technique. Ann Thorac Surg. 2015;99:1464–1466. doi: 10.1016/j.athoracsur.2014.11.062. [DOI] [PubMed] [Google Scholar]
- 27.Roman MJ, Rosen SE, Kramer-Fox R, Devereux RB. Prognostic significance of the pattern of aortic root dilation in the Marfan syndrome. J Am Coll Cardiol. 1993;22:1470–1476. doi: 10.1016/0735-1097(93)90559-J. [DOI] [PubMed] [Google Scholar]
- 28.Murdoch JL, Walker BA, Halpern BL, Kuzma JW, McKusick VA. Life expectancy and causes of death in the Marfan syndrome. N Engl J Med. 1972;286:804–808. doi: 10.1056/NEJM197204132861502. [DOI] [PubMed] [Google Scholar]
- 29.Coselli JS, Volguina IV, Lemaire SA, et al. Early and 1-year outcomes of aortic root surgery in patients with Marfan syndrome: a prospective, multicenter, comparative study. J Thorac Cardiovasc Surg. 2014;147:1758–1767. doi: 10.1016/j.jtcvs.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Benedetto U, Jin XY, Hill E, Treasure T, Petrou M. An option for concomitant management of moderate Marfan root aneurysm at the time of mitral valve repair: a role for Personalized External Aortic Root Support. Ann Thorac Surg. 2016;102:e499–e501. doi: 10.1016/j.athoracsur.2016.05.031. [DOI] [PubMed] [Google Scholar]