Abstract
Purpose
Infected pancreatic necrosis (IPN) is a highly morbid complication of acute necrotising pancreatitis (ANP). Since there is evidence of early-onset immunosuppression in acute pancreatitis, immune enhancement may be a therapeutic option. This trial aimed to evaluate whether early immune-enhancing Thymosin alpha 1 (Tα1) treatment reduces the incidence of IPN in patients with predicted severe ANP.
Methods
We conducted a multicentre, double-blind, randomised, placebo-controlled trial involving ANP patients with an Acute Physiology and Chronic Health Evaluation II (APACHE II) score ≥ 8 and a computed tomography (CT) severity score ≥ 5 admitted within 7 days of the advent of symptoms. Enrolled patients were assigned to receive a subcutaneous injection of Tα1 1.6 mg every 12 h for the first 7 days and 1.6 mg once a day for the subsequent 7 days or matching placebos (normal saline). The primary outcome was the development of IPN during the index admission.
Results
A total of 508 patients were randomised, of whom 254 were assigned to receive Tα1 and 254 placebo. The vast majority of the participants required admission to the intensive care unit (ICU) (479/508, 94.3%). During the index admission, 40/254(15.7%) patients in the Tα1 group developed IPN compared with 46/254 patients (18.1%) in the placebo group (difference -2.4% [95% CI − 7.4 to 5.1%]; p = 0.48). The results were similar across four predefined subgroups. There was no difference in other major complications, including new-onset organ failure (10.6% vs. 15%), bleeding (6.3% vs. 3.5%), and gastrointestinal fistula (2% vs. 2.4%).
Conclusion
The immune-enhancing Tα1 treatment of patients with predicted severe ANP did not reduce the incidence of IPN during the index admission.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-022-06745-7.
Keywords: Acute pancreatitis, Immunosuppression, Thymosin, Pancreatic necrosis, Infection
Take-home message
| Compared with placebo, immune-enhancing Thymosin α1 treatment did not reduce the incidence of infected pancreatic necrosis in patients with predicted severe acute necrotising pancreatitis. However, it might be effective in specific subgroups of acute necrotising pancreatitis, like patients with extended pancreatic necrosis. |
Introduction
The annual global incidence of acute pancreatitis (AP) is estimated to be 34 per 100,000 individuals [1]. A smaller subgroup of patients with AP (5–10%) develop acute necrotising pancreatitis (ANP) [2] and can experience a more prolonged disease course that commonly requires intensive care unit (ICU) admission, especially if infected pancreatic necrosis (IPN) develops [3, 4]. The bacteria responsible for IPN are often translocated from the gastrointestinal tract and reach the pancreas through several different transmission routes, including haematogenous, lymphatic, and transcoelomic [5, 6].
Attempts to reduce the risk of infection in ANP have included the use of prophylactic antibiotics [7] and enteral probiotics [8]. The former is no longer recommended because of issues like antibiotic resistance, methodological quality in previous studies, and fungal superinfection [9, 10]. The latter is controversial, as a prominent randomised controlled trial found an increased risk of gastrointestinal necrosis associated with probiotic treatment [8]. Given that there is evidence of immunosuppression in the early phase of AP and it is associated with infectious complications [11–14], a theoretical strategy to reduce the risk of IPN is to boost the host defence (immune enhancement) against bacterial infection [15].
Thymosin alpha 1 (Tα1), a polypeptide hormone isolated from the thymus, stimulates both innate and adaptive immunity [16]. In a pilot study of patients with AP, Tα1 was effective in reducing the risk of developing IPN [17]. Based on this preliminary data, we conducted a multicentre randomised clinical trial to determine the effect of Tα1 treatment. We hypothesised that early immune enhancement with Tα1 may reduce the incidence of IPN in predicted severe ANP. The main results of this trial were presented at American Pancreatic Association (APA) 2021 Annual Meeting and published as an abstract [18].
Methods
Trial design and oversight
This is a multicentre, double-blind, randomised, placebo-controlled, parallel-group trial to assess the efficacy of Tα1 in addition to standard care on the development of IPN in patients with predicted severe ANP. The trial was approved by the local hospital ethics committees of all the participating sites and registered on the ClinicalTrials.gov Registry (NCT02473406) before enrolment commenced. The trial protocol was published in 2020 [19], and the full protocol and statistical analysis plan are available in the Supplementary Protocol. This study was funded by the Science and Technology Project of Jiangsu Province of China (no. SBE2016750187) and partly supported by SciClone Pharmaceuticals Holding Limited, which provided trial drugs and support for meetings during the study period. The funders were not involved in the trial's design, data collection, interpretation, or manuscript preparation.
Study population
Patients diagnosed with AP aged 18 to 70 years and with an Acute Physiology and Chronic Health Evaluation II (APACHE II) score ≥ 8 and computed tomography (CT) severity score [20] ≥ 5 admitted to any of the participating sites within seven days of the onset of abdominal pain were eligible for inclusion. The diagnosis of AP was based on the Revised Atlanta Classification (RAC) criteria [2]. Patients were excluded if they were pregnant, had a history of chronic pancreatitis, had underlying malignancy, received intervention for pancreatic necrosis prior to enrolment, had a known history of severe cardiovascular, respiratory, renal, or hepatic diseases, or had pre-existing immune disorders such as acquired immune deficiency syndrome (AIDS). Detailed inclusion and exclusion criteria are provided in the Supplementary Protocol.
At each site, informed consent was obtained from the patients or their next of kin before randomisation. Patients were enrolled from March 18, 2017, to December 10, 2020. Follow-up was completed on March 10, 2021.
Randomisation, masking and interventions
Each eligible participant was assigned randomly from a computer-generated sequence to either the Tα1 or placebo group in a 1:1 ratio, using a block size of 4 stratified by site. The random allocation sequence was generated by an independent statistician at the Jinling Hospital. Allocation concealment was achieved using blinded medication packs. Patients were assigned to receive a subcutaneous injection of Tα1 (SciClone Pharmaceutical Co., Ltd, Hong Kong) 1.6 mg every 12 h for the first 7 days and 1.6 mg once a day for the following 7 days or a matching placebo (normal saline, Chengdu Tongde Pharmaceutical Co., Ltd, Chengdu) during the same period. The trial drug was administered for a maximum of 14 days, or until hospital discharge or death, whichever occurred first.
Participants, treating physicians, and investigators were blinded to the treatment allocation to minimise potential bias. The trial statistician was also blinded when developing the statistical programmes. Tα1 and placebo were supplied in identically labelled individual vials. All other aspects of the patients' care were provided based on the international guidelines [21]. Prophylactic antibiotics were not recommended accordingly. For the management of IPN, intervention was indicated when the collection was suspected or confirmed to be infected, and preferably after four weeks from the onset of the disease, per the guidelines [21]. Technically, either a surgical or endoscopic step-up approach was applied based on the location and morphology of the collection and the technical expertise available at each participating site. The decision for a fine-needle aspiration or invasive therapeutic intervention was made by the senior treating clinician. The details for the management of AP are described in the published protocol [19] and in the Supplementary Protocol.
Data collection
A web-based database (Unimed Scientific Inc., Wuxi, China) was developed for data collection (accessed at capctg.medbit.cn). Before enrolment, a start-up meeting for data entry and storage training was organised at each participating site to ensure high-quality data collection.
Trial outcomes
The primary outcome was the development of IPN during the index admission. We define the term "index admission" as the first admission in a series of hospital admissions. The diagnosis of IPN was made when one or more of the following criteria were present: gas bubbles within pancreatic and/or peripancreatic necrosis on CT; a positive culture from pancreatic and/or peripancreatic necrosis obtained by fine-needle aspiration, drainage, or necrosectomy [2]. All the reported positive cases were reviewed by a remote adjudication committee. Decisions of the remote adjudicating committee took precedence over the treating clinicians. Secondary clinical outcomes include IPN at 90 days after randomisation and new-onset organ failure as defined by the Revised Atlanta Classification [2], mortality, bleeding requiring intervention, gastrointestinal fistula requiring intervention, positive blood culture, and pancreatic fistula during the index admission. Secondary laboratory outcomes include C-reactive protein (CRP), monocyte human leukocyte antigen-DR (mHLA-DR), and lymphocyte count on day 7 and day 14 after randomisation and positive blood cultures. The details and definitions of all outcomes are provided in the Supplementary Protocol.
Statistical analysis
The incidence of IPN during the index admission in our study population was approximately 25%, according to our previous studies [22, 23]. A sample size of 520 patients was estimated to provide 80% power at a 2-sided alpha of 5% to demonstrate an absolute risk reduction of 10% in IPN during the index admission (25% in the placebo group vs 15% in the Tα1 group) after accounting for 4% dropouts (PASS V.11, NCSS software, Kaysville, USA). The treatment effect was estimated based on our pilot study, which demonstrated an 80% relative reduction in the incidence of IPN (42 to 8%) [17].
Primary analyses were based on the intention-to-treat (ITT) population, and secondary sensitivity analyses were done on the per-protocol (PP) population for the primary outcome and key secondary outcomes. Continuous data are reported as means and standard deviations or as medians and interquartile ranges as appropriate, depending on their normality. Categorical data are expressed as numbers and percentages.
The generalised linear model (GLM) was employed to compare group differences and calculate the risk difference for the primary outcome, together with its 95% confidence intervals. The GLM was also employed to analyse secondary outcomes with treatment as the predictor. Kaplan–Meier curves were used to compare the cumulative incidence of IPN to 90 days after randomisation tested by log-rank test. Detailed descriptions for the analyses could be found in the Supplementary statistical analysis plan. Four subgroups were predefined for the evaluation of the incidence of IPN during the index admission and 90 days after randomisation: the severity of AP (severe and non-severe [2]), age (> 60 and ≤ 60 years old), etiologies of AP (biliary and non-biliary) and extent of pancreatic necrosis (> 50% and ≤ 50%).
Analyses were conducted using SAS 9.4®. Statistical tests will be two sided, and p values < 0.05 will be deemed as significant. All authors had access to the study data and had reviewed and approved the final manuscript.
Results
Results of recruitment and baseline characteristics
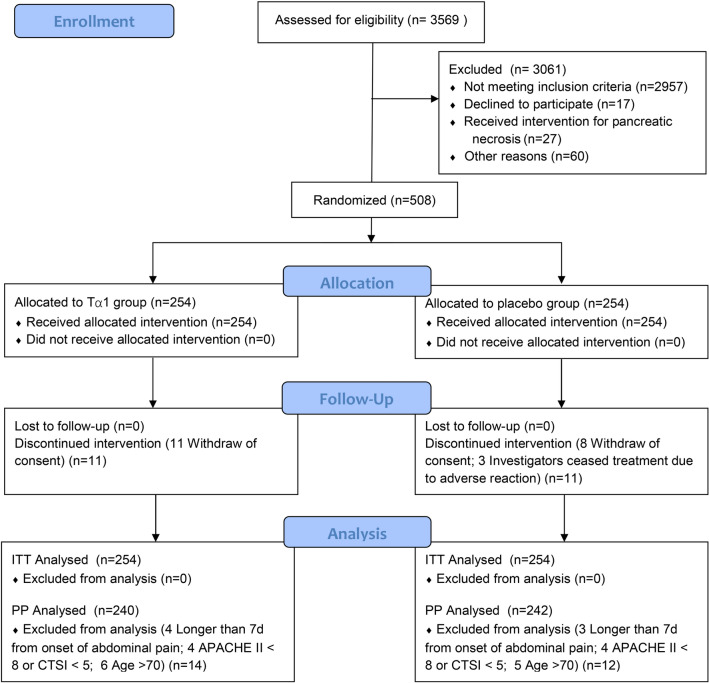
During the study period, 3569 AP patients were assessed for eligibility, of whom 508 were enrolled in the trial at 16 hospitals across China. The numbers of cases from each site are shown in online supplemental Table S1. Among those 508 randomised patients, 254 were assigned to receive Tα1 and 254 placebo. The most common reasons for exclusion were admission > 7 days before evaluation and APACHE score < 8. Ten patients (2%) received the study drug on the randomisation day, while the others did so on the following day. Eleven patients in the Tα1 group and eight patients in the placebo group withdrew consent during treatment but did not refuse follow-up and data usage (Fig. 1). Three patients in the placebo group stopped research intervention midway due to adverse reactions.
Fig. 1.
Enrolment, randomisation, and follow-up of patients in the TRACE trial. TRACE denotes thymosin α1 in prevention of infected pancreatic necrosis following acute necrotising pancreatitis. APACHE II denotes acute physiology and chronic health evaluation II. CTSI denotes computed tomography severity index. ITT denotes intention to treat. Tα1 denotes Thymosin α1
Baseline demographics and characteristics were not significantly different between the Tα1 and placebo groups (Table 1). Hypertriglyceridemia was the leading cause of AP in both groups, accounting for approximately half of the cases (48.8% vs. 50%). The vast majority of the trial participants required ICU admission(479/508, 94.3%). The numbers of patients who received the trial agent on each trial day are shown in online supplemental Table S2.
Table 1.
Baseline characteristics of the study subjects
| Characteristics | Tα1 group (N = 254) | Placebo group (N = 254) |
|---|---|---|
| Age, mean (SD), y | 44.3 (13.2) | 45.4 (13.4) |
| Gender | ||
| Women, (n, %) | 92 (36.2) | 97 (38.2) |
| Men, (n, %) | 162 (63.8) | 157 (61.8) |
| BMI, mean (SD), kg/m2 | 26.4 (3.9) | 26.8 (4.2) |
| Etiologies | ||
| Alcoholic, (n, %) | 17 (6.7) | 15 (5.9) |
| Biliary, (n, %) | 101 (39.8) | 100 (39.4) |
| Idiopathic, (n, %) | 12 (4.7) | 12 (4.7) |
| Hypertriglyceridemia, (n, %) | 124 (48.8) | 127 (50) |
| Charlson score, mean (SD) | 0.6 (0.7) | 0.5 (0.8) |
| Interval between onset and hospital admission, mean (SD),d | 2.9 (2.5) | 3.0 (1.9) |
| Interval between onset and ICU admission, mean (SD),d | 3.2 (2.7) | 3.2 (1.9) |
| Interval between onset and randomisation, mean (SD),d | 4.2 (2.6) | 4.1 (2) |
| Extent of pancreatic necrosis | ||
| < 30%, (n, %) | 165 (65) | 151 (59.4) |
| 30–50%, (n, %) | 55 (21.7) | 72 (28.3) |
| > 50%, (n, %) | 34 (13.4) | 31 (12.2) |
| Disease severity | ||
| CTSI score, median (IQR) | 6 (5–8) | 6 (5–8) |
| APACHE II score, median (IQR) | 10 (8–13) | 10 (8–13) |
| BISAP score, median (IQR) | 2 (1–2) | 2 (1–2) |
| PASS score, median (IQR) | 235 (190–523) | 235 (190–550) |
| CRP, median (IQR), mg/L | 172.1 (95.4–236.2) | 160.6 (105.5–236.4) |
| Lymphocyte count, median (IQR), × 109/L | 0.9 (0.6–1.2) | 0.9 (0.7–1.2) |
| SOFA score, median (IQR) | 4 (2–6) | 4 (2–6) |
| Respiration, median (IQR) | 2 (1–2) | 2 (1–2) |
| Cardiovascular, median (IQR) | 0 (0–0) | 0 (0–0) |
| Renal, mean median (IQR) | 0 (0–1) | 0 (0–1) |
| Requirement of organ support at randomisation | ||
| Mechanical ventilation, (n, %) | 63 (24.8) | 69 (27.2) |
| Renal replacement therapy, (n, %) | 63 (24.8) | 63 (24.8) |
| Vasoactive agents, (n, %) | 30 (11.8) | 26 (10.2) |
p > 0.05 for the comparison between the groups for all characteristics. SD denotes standard deviation. BMI denotes body mass index. ICU denotes intensive care unit. IQR denotes interquartile range. CTSI denotes computed tomography severity index. APACHE II denotes acute physiology and chronic health evaluation II, which ranges from 0 to 71, with higher scores indicating more severe disease. BISAP denotes bedside index for severity in acute pancreatitis. PASS denotes pancreatitis activity scoring system. CRP denotes C-reactive protein. SOFA denotes sequential organ failure assessment, which ranges from 0 to 24, with higher scores indicating more severe organ failure. Tα1 denotes thymosin α1
Primary outcome and secondary outcomes
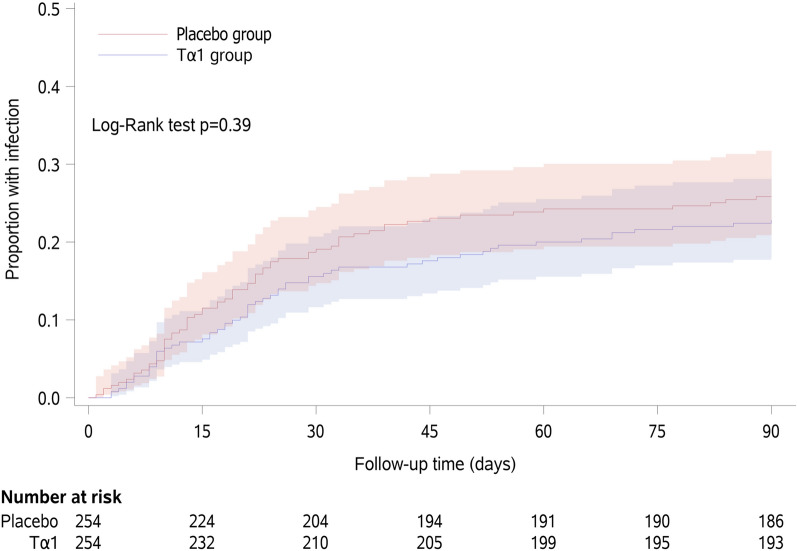
During the index admission, 40/254 (15.7%) patients in the Tα1 group developed IPN compared with 46/254 patients (18.1%) patients in the placebo group (difference − 2.4% [95% CI − 7.4 to 5.1%]; p = 0.48). Of the 86 IPN patients, 74 were diagnosed according to microbiological results(66 obtained during catheter drainage procedures, 8 during open surgery), and 12 were based on radiological findings alone. No fine-needle aspiration procedure was performed during the TRACE trial. At 90 days after randomisation, 57/254 (22.4%) patients in the Tα1 group developed IPN compared with 65/254 patients (25.6%) in the placebo group (difference − 3.3% [95% CI − 9.2 to 4.8%]; p = 0.39). For the 74 microbiologically confirmed IPN patients, the microorganisms we found are shown in online supplemental Table S3. There was no difference in mortality between groups either within the index admission or at 90 days after randomisation (Table 2). The Kaplan–Meier curves for the cumulative incidence of IPN until 90 days after randomisation are shown in Fig. 2. There was no significant difference in the probability of developing IPN between the Tα1 and placebo groups (Log-Rank p = 0.39). The results of the per-protocol analysis of the primary outcome and key secondary outcomes are shown in online supplemental Table S4.
Table 2.
Primary and secondary endpoints
| Tα1 group (N = 254) |
Placebo group (N = 254) |
Risk difference (95% CI) |
p value | |
|---|---|---|---|---|
| Primary endpoint | ||||
| IPN during the index admission, (n, %) | 40 (15.7) | 46 (18.1) | − 2.36 (− 7.41, 5.07) | 0.48 |
| Secondary endpoints* | ||||
| New-onset organ failure | 27 (10.6) | 38 (15) | − 4.26 (− 8.18, 1.93) | 0.15 |
| Respiratory, (n, %) | 9 (3.5) | 17 (6.7) | − 3.15 (− 5.08, 1.08) | 0.11 |
| Renal, (n, %) | 10 (3.9) | 7 (2.8) | 1.25 (− 1.19, 7.48) | 0.43 |
| Cardiovascular, (n, %) | 14 (5.5) | 20 (7.9) | − 2.33 (− 5.00, 2.82) | 0.30 |
| Mortality (n, %) | 18 (7.1) | 22 (8.7) | − 1.55 (− 4.73, 4.21) | 0.52 |
| 90-Day mortality (n, %) | 23 (9.1) | 23 (9.1) | 0.03 (− 3.79, 6.63) | 0.99 |
| IPN within 90 days after randomisation (n, %) | 57 (22.4) | 65 (25.6) | − 3.25 (− 9.18, 4.83) | 0.39 |
| Bleeding, (n, %) | 16 (6.3) | 9 (3.5) | 2.80 (− 0.69, 10.53) | 0.15 |
| Positive blood culture (n, %) | 18 (7.1) | 25 (9.8) | − 2.73 (− 5.85, 2.83) | 0.27 |
| Gastrointestinal fistula, (n, %) | 5 (2) | 6 (2.4) | − 0.41 (− 1.76, 3.91) | 0.75 |
| Length of ICU stay, mean (SD), d | 14.4 (16.2) | 13.6 (16.4) | 0.75 (− 2.07, 3.57) | 0.60 |
| Length of hospital stay, mean (SD), d | 21.0 (21.3) | 20.5 (20) | 0.46 (− 3.12, 4.03) | 0.80 |
| In-hospital cost, mean (SD), Kyuan | 143 (177) | 138 (206) | 5 (− 28, 38) | 0.77 |
| Use of invasive interventions | ||||
| Requiring catheter drainage (n, %)# | 36 (14.2) | 39 (15.4) | − 1.27 (− 6.08, 6.02) | 0.69 |
| Requiring MI debridement (n, %)** | 17 (6.7) | 12 (4.7) | 1.96 (− 1.44, 8.91) | 0.34 |
| Requiring open surgery (n, %) | 8 (3.1) | 5 (2) | 1.14 (− 0.93, 7.37) | 0.41 |
| Number of procedures in patients requiring invasive interventions during the index admission | ||||
| Number of catheter drainage procedures, median (IQR)+ | 2.5 (1–4) | 2 (1–3) | 0.14 (− 0.58, 0.85) | 0.71 |
| Number of MI debridement procedures, median (IQR)++ | 3 (2–6) | 4 (3–5) | − 0.52 (− 1.95, 0.91) | 0.48 |
CI denotes confidential interval. IPN denotes infected pancreatic necrosis. ICU denotes intensive care unit. MI denotes minimally invasive. *All secondary endpoints were registered during the index admission unless otherwise specified; # both percutaneous and transluminal drainage included; **27 patients underwent exclusive percutaneous surgical MI debridement, one patient underwent exclusive endoscopic transluminal debridement, and one combined; + only patients undergoing catheter drainage procedures are included; ++ only patients undergoing MI debridement procedures are included
Fig. 2.
Time- to-infection by day 90. The Kaplan–Meier curves for the cumulative incidence of infected pancreatic necrosis from randomisation to day 90 in the intention-to-treat population
There was no difference in other major complications, including new-onset organ failure (10.6% vs. 15%; difference − 4.3% [95% CI − 8.2 to 1.9%]; p = 0.15), bleeding (6.3% vs. 3.5%; difference 2.8 [95% CI − 0.7 to 10.5]; p = 0.15), and gastrointestinal fistula (2% vs. 2.4%; difference − 0.4% [95% CI − 1.8 to 3.9%]; p = 0.75) during the index admission. Moreover, there were no significant differences in length of ICU or hospital stay, the requirement for catheter drainage, minimally invasive debridement, or open surgery (Table 2). For mHLA-DR, no difference was detected on day7 and day14 between groups (online supplemental Table S5). The additional secondary endpoints regarding organ failure and laboratory parameters are shown in online supplemental Tables S5–6.
Subgroup analyses
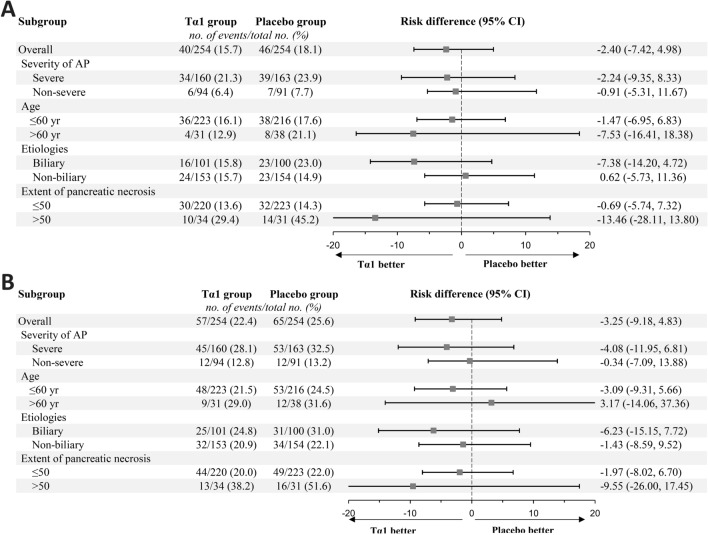
There was no significant heterogeneity in the effect of Tα1 on the incidence of IPN during the index admission and 90 days after randomisation in any of the four predefined subgroups (Fig. 3, online supplemental Tables S7–8). In a post hoc subgroup analysis, the effect of Tα1 did not differ in patients caused by hypertriglyceridemia or other etiologies (online supplemental Tables S7–8).
Fig. 3.
Subgroup analysis of the risk of infected pancreatic necrosis by the index hospital discharge and day 90. Panel A shows the risk difference of infected pancreatic necrosis during the index admission between the two treatment groups. Panel B shows the risk difference of infected pancreatic necrosis up to 90 days after randomisation. A risk difference of less than 0 indicates better results for the Tα1 group
Adverse events
Adverse events occurred in 21 patients in the Tα1 group and 19 in the placebo group (8.3% vs. 7.5%, p = 0.742) (online supplemental Table S9). The most common adverse event was venous thrombosis which occurred in 6 patients (2.4%) in the Tα1 group vs. 5 (2%) in the placebo group. All adverse events are listed in online supplemental Table S9.
Discussion
In this multicentre, double-blind, randomised, placebo-controlled trial, immune enhancement using Tα1 did not significantly reduce the incidence of IPN during the index admission or within 90 days of randomisation in patients with ANP. Given the varied range of severity of AP [2], this study was designed to select more severe patients based on the APACHE II score at enrolment [24]. However, we failed to show a difference in the primary outcome.
Our results are not consistent with the results from an experimental animal study [17] and the pilot clinical study [25]. There are several possible explanations. First, current animal models cannot recapitulate all aspects of human AP, especially for a complication like IPN, which often occurs several weeks after admission [26, 27]. Second, the pilot study recruited only 24 patients from a single centre, making its findings vulnerable to type I error. Third, the dose regimen in this trial is different from the pilot one with a longer duration of drug administration (one week in the pilot vs. two weeks in the present) and a lower initial dose (6.4 mg per day in the pilot vs. 3.2 mg per day in the present). There were two time-course considerations in designing the dose regimen: (1) infection mainly occurs beyond the second week after disease onset [3, 28], and a 2-week regimen should be able to cover the period interval better when prevention is possible; (2) immunosuppression typically develops early in the first week and usually slowly recovers during the second week [12], which is the reason for prescribing half the dose during the second week of treatment. A similar step-wise dose reduction was used in a previous study testing Tα1 in sepsis [29], showing that Tα1 could reduce 28-day mortality. Moreover, since Tα1 has a short elimination half-life ranging from 1.7 to 2.1 h [30], an expanded drug administration period may exert better effects. Besides, the incidence of IPN during the index admission is lower than expected in the placebo group (18.1% vs. 25% for sample size estimation), which might make our trial underpowered. Before initiation of recruitment, we changed the time interval of the primary outcome from “28 days” to time until “index hospital discharge” due to concerns regarding loss of follow-ups and the possibility of incomplete data. During the trial, we followed up with all the participants for 90 days after randomisation, and the incidence of IPN was 25.6% in the placebo group by then. Still, the Tα1 treatment did not result in a reduction of IPN by 90 days after randomisation. Last, since we chose either microbiologically or radiologically confirmed IPN as our primary outcome, underestimating the number of infections is highly likely. Patients with IPN that resolved without invasive intervention and did not present with CT evidence of air within the collection were not diagnosed as IPN in the TRACE trial. Moreover, fine-needle aspiration was not used in the TRACE trial, since the guidelines do not recommend this procedure considering substantial false-negative results [21, 31]. However, this may also contribute to an underestimation of IPN patients.
There is evidence to support a shifting balance between the systemic pro-inflammatory response and the compensatory anti-inflammatory response over the early course of AP [13, 32]. It was considered that the pro-inflammatory response occurs in the first few days to weeks and that the compensatory anti-inflammatory response occurs later. However, analyses in patients with sepsis and AP suggest that these responses can also run in parallel and that there is an association between early-onset immunosuppression and poor outcomes in AP, including increased risk of infectious complications [33, 34]. In sepsis patients, immune-enhancing therapies had been evaluated with agents like Tα1 [29] and nivolumab [35]. In AP patients, previous trials investigating immunomodulatory therapy to block the early pro-inflammatory response have not been convincing[36], and this includes drugs like lexipafant [37, 38] and octreotide [39]. In patients with severe COVID-19, observational studies showed that Tα1 attenuated lung injury and decreased mortality [40, 41]. Despite the theoretical benefits of immune enhancement with Tα1 and the encouraging results from the pilot study [17], Tα1 did not reduce the incidence of IPN or improve any of the clinical outcomes in this trial.
In the subgroup analyses, larger treatment effects were seen in patients with a greater extent of pancreatic necrosis (> 50%) and those aged more than 60 years old, although not statistically significant. We should interpret all the subgroup results with caution. First, the power was not enough to detect the differences among treatments. Second, the definition of necrosis is relatively subject based on a single CT scan. Third, we excluded patients with advanced age because age ≥ 70 years is an independent risk factor for mortality in severe AP [42], and the immune system becomes slower and less responsive over age [43], thereby limiting the chance to observe the treatment effect. However, excluding these patients makes the study subgroup for elderly patients even smaller.
In line with the excellent safety profile reported in previous studies, Tα1 showed satisfactory safety performance in this trial. Three patients discontinued treatment due to adverse reactions (one erythema and two unexplainable fever) but received the placebo. For the other secondary outcomes, although the incidence of bleeding did not differ from previous reports [44] and was not significantly higher in the intervention group, we strongly recommend future studies regarding necrotising pancreatitis monitor this potentially lethal complication.
The study has several limitations. The first is that the incidence of IPN may have been affected by the use of antibiotics and the criterion for repeating a CT scan because they were not mandatory standardised but left to the clinical team to decide. The second is that there were problems (failed multisite lab standardisation) with the measurement of mHLA-DR, a validated cell-surface signature for risk stratification in critically ill patients [45]. We obtained mHLA-DR data from less than half of the study subjects, which may be underpowered to detect the difference between groups. The third is that APACHE II misclassifies the severity of AP in almost a third of patients, which could also have contributed to the negative results [46]. Moreover, the timing of treatment might have been too late. The current trial included patients up to one week after the advent of symptoms, which may increase the heterogeneity of the study population. Apart from the timing, the appropriate duration of therapy is unclear. Last, nearly 50% of the study patients had hypertriglyceridemia as etiology, significantly higher than results from an international registry [47]. The increase of hypertriglyceridemia-induced AP in Chinese cohorts might be attributed to changes in dietary habits [48] and genetic factors [49]. Although the effect of Tα1 did not vary across patients caused by hypertriglyceridemia or other etiologies, the distinct etiological distribution leaves the generalisability of the observed results in doubt.
In conclusion, the immune-enhancing Tα1 treatment of patients with predicted severe ANP (APACHE II ≥ 8 at enrolment) did not significantly reduce the incidence of IPN during the index admission compared with placebo. Future trials seeking to investigate this approach will need to determine the best way to select patients and decide on the most effective dose and duration of Tα1 treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the contribution of Mengjie Lu, Gang Li, Bo Ye, Yan Chen, Zhenping Chen, Youdong Wan, Miao Chen, Qingbo Zeng, Wei Zhao, Xiaofei Huang, Lening Ren, Dahuan Li, Qingcheng Xu, Keke Xin, Bing Xue, Hongguo Yang, Dongsheng Zhao, Feng Zhou, and Zigui Zhu in the development and execution of this study.
Members of the Chinese Acute Pancreatitis Clinical Trials Group (CAPCTG): Lu Ke, Jing Zhou, Wenjian Mao, Wendi Jiang, He Zhang, Jiajia Lin, Mingfeng Huang, Longxiang Cao, Mengjie Lu, Yan Chen, Gang Li, Bo Ye, Baiqiang Li, Zhihui Tong, Yuxiu Liu, Weiqin Li, Jinling hospital. Tao Chen, Liverpool University. Fang Shao, Nanjing Medical University. Nonghua Lv, Yin Zhu, Liang Xia, Wenhua He, Zhenping Chen, The First Affiliated Hospital of Nanchang University. Xinting Pan, Qingyun Zhu, Youdong Wan, The Affiliated Hospital of Qingdao University. Hong Mei, Kang Li, Miao Chen, The Affiliated Hospital of Zunyi Medical University. Chengjian He, Hongyi Yao, Zigui Zhu, Nanhua Hospital. Weili Gu, Affiliated Hospital 2 of Nantong University. Weihua Lu, Jingyi Wu, Feng Zhou, The First Affiliated Hospital of Wannan Medical College. Shumin Tu, Long Fu, Bing Xue, Shangqiu First People's Hospital. Haibin Ni, Xiaofei Huang, Dandan Zhou, Jiangsu Provincial Hospital of Integrated Chinese and Western Medicine. Guoxiu Zhang, Lening Ren, Dahuan Li, The First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology. Xiangyang Zhao, Wei Zhao, Xiaomei Chen, Qilu Hospital of Shandong University. Junli Sun, Keke Xin, Luoyang Central Hospital. Weiwei Chen, Qingcheng Xu, Clinical Medical College of Yangzhou University. Jingchun Song, Qingbo Zeng, 94th Hospital of PLA. Min Shao, Dongsheng Zhao, The First Affiliated Hospital of Anhui Medical University. Jianfeng Tu, Hongguo Yang, Zhejiang Provincial People's Hospital. Bin Wu, Huaguang Ye, The Third Hospital of Xiamen City. Mingzhi Chen, Yingjie Chen, Jinjiang Hospital of Traditional Chinese Medicine. Mei Yang, Hong Gao, The Qujing NO.1 People’s Hospital. Qiang Li, The First Affiliated Hospital of Nanjing Medical University. Lijuan Zhao, Guobing Chen, Yafei Li, First People’s Hospital of Yunnan Province. Honghai Xia, Dongliang Yang, Shusheng Zhou, The First Affiliated Hospital of the University of Science and Technology of China. Jiyan Lin, Siyao Liu, The First Affiliated Hospital of Xiamen University. Donghuang Hong, Songjing Shi, Fujian Provincial Hospital. Zuozheng Wang, Weijie Yao, General Hospital of Ningxia Medical University. Yi Sun, Suining Central Hospital. Kaixiu Qin, Shan Xu, Lei Yu, The Second Affiliated Hospital of Chongqing Medical University. Feng Guo, Yongjun Lin, Sir Run Run Shaw Hospital of Zhejiang University. Yun Zhou, Pingxiang People’s Hospital. Qinghai Jiao, The First Hospital of HanDan. Quanxing Feng, The Fourth Military Medical University. Zhiyong Liu, Xiangya Hospital.
Author contributions
Study concept and design: WL, JW, YL, ZT, and LK. Acquisition of data: WL, ZT, LK, JZ, WM, WJ, HZ, YZ, XP, HM, CH, WG, WL, ST, HN, GZ, XZ, JS, WC, JS, MS, JT, LX, WH, QZ, KL, HY, JW, LF. Obtained funding: WL, ZT. Technical support: YL, WM. Methodology support: YL, TC, GD. Study supervision: WL, ZT, LK. Drafting and revision of manuscript: All. Statistical analysis: TC, GD. TC, GD, WL, ZT, LK, JZ, WM accessed and verified the data underlying this article. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
The TRACE trial was funded by the Science and technology project of Jiangsu Province (No. SBE2016750187) and SciClone Pharmaceuticals Holding Limited. The funders were not involved in the trial’s design, data collection, interpretation, or manuscript preparation.
Availability of data and material
Deidentified individual participant data are available indefinitely in the electronic database. Data can be accessed through capctg.medbit.cn with the approval of the authors. Request for data can be made to the corresponding author (ctgchina@medbit.cn) and will be discussed during a meeting of the Chinese Acute Pancreatitis Clinical Trials Group (CAPCTG).
Declarations
Conflict of interest
WL reports consultancy fees and grants from SciClone Pharmaceuticals. ZT reports speaker fees from SciClone Pharmaceuticals. LK reports speaker fees from SciClone Pharmaceuticals. VS reports consultant fees and grants Abbvie, medical advisory board participant for Envara, and grants from Theraly and Orgenesis. The other authors have no relevant conflict of interest to declare.
Ethical approval
The protocol was prospectively approved by the human research ethics committee of all participating institutions and reported prior to completion of the study. This study was approved by the ethics committee of Jinling Hospital. The ethical approval document ID is 2015NZKY-004-02. Site ethical approvals were obtained from ethics committees of the participating sites before commencement of recruitment.
Consent to participate
Consent to participate was provided prospectively from all participants or their next of kin. The signed consent forms for all participants included consent to publication of aggregate data. The authors all consent to publication of the manuscript.
Footnotes
The members of the Chinese Acute Pancreatitis Clinical Trials Group (CAPCTG) are listed in the acknowledgement section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lu Ke, Jing Zhou and Wenjian Mao contributed equally to this work.
Contributor Information
Zhihui Tong, Email: njzyantol@aliyun.com.
Weiqin Li, Email: ctgchina@medbit.cn.
the Chinese Acute Pancreatitis Clinical Trials Group (CAPCTG):
Lu Ke, Jing Zhou, Wenjian Mao, Wendi Jiang, He Zhang, Jiajia Lin, Mingfeng Huang, Longxiang Cao, Mengjie Lu, Yan Chen, Gang Li, Bo Ye, Baiqiang Li, Zhihui Tong, Yuxiu Liu, Weiqin Li, Tao Chen, Fang Shao, Nonghua Lv, Yin Zhu, Liang Xia, Wenhua He, Zhenping Chen, Xinting Pan, Qingyun Zhu, Youdong Wan, Hong Mei, Kang Li, Miao Chen, Chengjian He, Hongyi Yao, Zigui Zhu, Weili Gu, Weihua Lu, Jingyi Wu, Feng Zhou, Shumin Tu, Long Fu, Bing Xue, Haibin Ni, Xiaofei Huang, Dandan Zhou, Guoxiu Zhang, Lening Ren, Dahuan Li, Xiangyang Zhao, Wei Zhao, Xiaomei Chen, Junli Sun, Keke Xin, Weiwei Chen, Qingcheng Xu, Jingchun Song, Qingbo Zeng, Min Shao, Dongsheng Zhao, Jianfeng Tu, Hongguo Yang, Bin Wu, Huaguang Ye, Mingzhi Chen, Yingjie Chen, Mei Yang, Hong Gao, Qiang Li, Lijuan Zhao, Guobing Chen, Yafei Li, Honghai Xia, Dongliang Yang, Shusheng Zhou, Jiyan Lin, Siyao Liu, Donghuang Hong, Songjing Shi, Zuozheng Wang, Weijie Yao, Yi Sun, Kaixiu Qin, Shan Xu, Lei Yu, Feng Guo, Yongjun Lin, Yun Zhou, Qinghai Jiao, Quanxing Feng, and Zhiyong Li
References
- 1.Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55. doi: 10.1016/S2468-1253(16)30004-8. [DOI] [PubMed] [Google Scholar]
- 2.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS, Acute Pancreatitis Classification Working G Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 3.Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. Current concepts in severe acute and necrotizing pancreatitis: an evidence-based approach. Gastroenterology. 2019;156(1994–2007):e1993. doi: 10.1053/j.gastro.2019.01.269. [DOI] [PubMed] [Google Scholar]
- 4.Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, van Santvoort HC, Bruno MJ, Dutch Pancreatitis Study G Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68:1044–1051. doi: 10.1136/gutjnl-2017-314657. [DOI] [PubMed] [Google Scholar]
- 5.Mowbray NG, Ben-Ismaeil B, Hammoda M, Shingler G, Al-Sarireh B. The microbiology of infected pancreatic necrosis. Hepatobiliary Pancreat Dis Int. 2018;17:456–460. doi: 10.1016/j.hbpd.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Mittal A, Phillips AR, Middleditch M, Ruggiero K, Loveday B, Delahunt B, Cooper GJ, Windsor JA. The proteome of mesenteric lymph during acute pancreatitis and implications for treatment. JOP. 2009;10:130–142. [PubMed] [Google Scholar]
- 7.Wittau M, Mayer B, Scheele J, Henne-Bruns D, Dellinger EP, Isenmann R. Systematic review and meta-analysis of antibiotic prophylaxis in severe acute pancreatitis. Scand J Gastroenterol. 2011;46:261–270. doi: 10.3109/00365521.2010.531486. [DOI] [PubMed] [Google Scholar]
- 8.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, Rosman C, Ploeg RJ, Brink MA, Schaapherder AF, Dejong CH, Wahab PJ, van Laarhoven CJ, van der Harst E, van Eijck CH, Cuesta MA, Akkermans LM, Gooszen HG. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 9.Lim CL, Lee W, Liew YX, Tang SS, Chlebicki MP, Kwa AL. Role of antibiotic prophylaxis in necrotizing pancreatitis: a meta-analysis. J Gastrointest Surg. 2015;19:480–491. doi: 10.1007/s11605-014-2662-6. [DOI] [PubMed] [Google Scholar]
- 10.de Vries AC, Besselink MG, Buskens E, Ridwan BU, Schipper M, van Erpecum KJ, Gooszen HG. Randomized controlled trials of antibiotic prophylaxis in severe acute pancreatitis: relationship between methodological quality and outcome. Pancreatology. 2007;7:531–538. doi: 10.1159/000108971. [DOI] [PubMed] [Google Scholar]
- 11.Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Immunosuppression in patients with severe acute pancreatitis. J Gastroenterol. 2006;41:779–784. doi: 10.1007/s00535-006-1852-8. [DOI] [PubMed] [Google Scholar]
- 12.Yu WK, Li WQ, Li N, Li JS. Mononuclear histocompatibility leukocyte antigen-DR expression in the early phase of acute pancreatitis. Pancreatology. 2004;4:233–243. doi: 10.1159/000078748. [DOI] [PubMed] [Google Scholar]
- 13.Pan T, Zhou T, Li L, Liu Z, Chen Y, Mao E, Li M, Qu H, Liu J. Monocyte programmed death ligand-1 expression is an early marker for predicting infectious complications in acute pancreatitis. Crit Care. 2017;21:186. doi: 10.1186/s13054-017-1781-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Yang WJ, Huang LM, Tang CW. Immunomodulatory therapies for acute pancreatitis. World J Gastroenterol. 2014;20:16935–16947. doi: 10.3748/wjg.v20.i45.16935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garaci E. Thymosin alpha1: a historical overview. Ann N Y Acad Sci. 2007;1112:14–20. doi: 10.1196/annals.1415.039. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Li W, Niu C, Pan L, Li N, Li J. Thymosin alpha 1 is associated with improved cellular immunity and reduced infection rate in severe acute pancreatitis patients in a double-blind randomized control study. Inflammation. 2011;34:198–202. doi: 10.1007/s10753-010-9224-1. [DOI] [PubMed] [Google Scholar]
- 18.(2021) Abstracts of Papers Submitted to the 52nd Meeting of the American Pancreatic Association, November 3–6, 2021, Miami Beach, Florida. Pancreas 50: 1044–1115 [DOI] [PubMed]
- 19.Zhou J, Mao W, Ke L, Chen T, He W, Pan X, Chen M, He C, Gu W, Wu J, Song J, Ni H, Tu J, Sun J, Zhang G, Chen W, Xue B, Zhao X, Shao M, Liu Y, Tong Z, Li W, Chinese Acute Pancreatitis Clinical Trials G Thymosin alpha 1 in the prevention of infected pancreatic necrosis following acute necrotising pancreatitis (TRACE trial): protocol of a multicentre, randomised, double-blind, placebo-controlled, parallel-group trial. BMJ Open. 2020;10:e037231. doi: 10.1136/bmjopen-2020-037231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology. 2002;223:603–613. doi: 10.1148/radiol.2233010680. [DOI] [PubMed] [Google Scholar]
- 21.Working Group IAPAPAAPG IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1–15. doi: 10.1016/j.pan.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 22.Sun JK, Li WQ, Ni HB, Ke L, Tong ZH, Li N, Li JS. A modified gastrointestinal failure score for patients with severe acute pancreatitis. Surg Today. 2013;43:506–513. doi: 10.1007/s00595-013-0496-6. [DOI] [PubMed] [Google Scholar]
- 23.Ke L, Ni HB, Tong ZH, Li WQ, Li N, Li JS. D-dimer as a marker of severity in patients with severe acute pancreatitis. J Hepatobiliary Pancreat Sci. 2012;19:259–265. doi: 10.1007/s00534-011-0414-5. [DOI] [PubMed] [Google Scholar]
- 24.Bradley EL., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Zeng X, Yang B, Zhao S, Chen W, Guo X. Efficacy of thymosin alpha1 and interferon alpha for the treatment of severe acute pancreatitis in a rat model. Mol Med Rep. 2015;12:6775–6781. doi: 10.3892/mmr.2015.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue J, Sharma V, Habtezion A. Immune cells and immune-based therapy in pancreatitis. Immunol Res. 2014;58:378–386. doi: 10.1007/s12026-014-8504-5. [DOI] [PubMed] [Google Scholar]
- 27.Gorelick FS, Lerch MM. Do animal models of acute pancreatitis reproduce human disease? Cell Mol Gastroenterol Hepatol. 2017;4:251–262. doi: 10.1016/j.jcmgh.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg PK, Madan K, Pande GK, Khanna S, Sathyanarayan G, Bohidar NP, Tandon RK. Association of extent and infection of pancreatic necrosis with organ failure and death in acute necrotizing pancreatitis. Clin Gastroenterol Hepatol. 2005;3:159–166. doi: 10.1016/S1542-3565(04)00665-2. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Zhou L, Liu J, Ma G, Kou Q, He Z, Chen J, Ou-Yang B, Chen M, Li Y, Wu X, Gu B, Chen L, Zou Z, Qiang X, Chen Y, Lin A, Zhang G, Guan X. The efficacy of thymosin alpha 1 for severe sepsis (ETASS): a multicenter, single-blind, randomized and controlled trial. Crit Care. 2013;17:R8. doi: 10.1186/cc11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ancell CD, Phipps J, Young L. Thymosin alpha-1. Am J Health Syst Pharm. 2001;58:879–885. doi: 10.1093/ajhp/58.10.879. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez JR, Razo AO, Targarona J, Thayer SP, Rattner DW, Warshaw AL, Fernandez-del Castillo C. Debridement and closed packing for sterile or infected necrotizing pancreatitis: insights into indications and outcomes in 167 patients. Ann Surg. 2008;247:294–299. doi: 10.1097/SLA.0b013e31815b6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minkov GA, Yovtchev YP, Halacheva KS. Increased circulating CD4+CD25+CD127low/neg regulatory T-cells as a prognostic biomarker in acute pancreatitis. Pancreas. 2017;46:1003–1010. doi: 10.1097/MPA.0000000000000894. [DOI] [PubMed] [Google Scholar]
- 33.Novotny AR, Reim D, Assfalg V, Altmayr F, Friess HM, Emmanuel K, Holzmann B. Mixed antagonist response and sepsis severity-dependent dysbalance of pro- and anti-inflammatory responses at the onset of postoperative sepsis. Immunobiology. 2012;217:616–621. doi: 10.1016/j.imbio.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Simovic MO, Bonham MJ, Abu-Zidan FM, Windsor JA. Anti-inflammatory cytokine response and clinical outcome in acute pancreatitis. Crit Care Med. 1999;27:2662–2665. doi: 10.1097/00003246-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Hotchkiss RS, Colston E, Yende S, Crouser ED, Martin GS, Albertson T, Bartz RR, Brakenridge SC, Delano MJ, Park PK, Donnino MW, Tidswell M, Mayr FB, Angus DC, Coopersmith CM, Moldawer LL, Catlett IM, Girgis IG, Ye J, Grasela DM. Immune checkpoint inhibition in sepsis: a Phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensive Care Med. 2019;45:1360–1371. doi: 10.1007/s00134-019-05704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munir F, Jamshed MB, Shahid N, Hussain HM, Muhammad SA, Mamun AA, Zhang Q. Advances in immunomodulatory therapy for severe acute pancreatitis. Immunol Lett. 2020;217:72–76. doi: 10.1016/j.imlet.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Johnson CD, Kingsnorth AN, Imrie CW, McMahon MJ, Neoptolemos JP, McKay C, Toh SK, Skaife P, Leeder PC, Wilson P, Larvin M, Curtis LD. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62–69. doi: 10.1136/gut.48.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abu-Zidan FM, Windsor JA. Lexipafant and acute pancreatitis: a critical appraisal of the clinical trials. Eur J Surg. 2002;168:215–219. doi: 10.1080/11024150260102816. [DOI] [PubMed] [Google Scholar]
- 39.Wang R, Yang F, Wu H, Wang Y, Huang Z, Hu B, Zhang M, Tang C. High-dose versus low-dose octreotide in the treatment of acute pancreatitis: a randomized controlled trial. Peptides. 2013;40:57–64. doi: 10.1016/j.peptides.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Pan Y, Hu Z, Wu M, Wang C, Feng Z, Mao C, Tan Y, Liu Y, Chen L, Li M, Wang G, Yuan Z, Diao B, Wu Y, Chen Y. Thymosin alpha 1 reduces the mortality of severe coronavirus disease 2019 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin Infect Dis. 2020;71:2150–2157. doi: 10.1093/cid/ciaa630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu M, Ji JJ, Zhong L, Shao ZY, Xie QF, Liu ZY, Wang CL, Su L, Feng YW, Liu ZF, Yao YM. Thymosin alpha1 therapy in critically ill patients with COVID-19: a multicenter retrospective cohort study. Int Immunopharmacol. 2020;88:106873. doi: 10.1016/j.intimp.2020.106873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardner TB, Vege SS, Chari ST, Pearson RK, Clain JE, Topazian MD, Levy MJ, Petersen BT. The effect of age on hospital outcomes in severe acute pancreatitis. Pancreatology. 2008;8:265–270. doi: 10.1159/000134274. [DOI] [PubMed] [Google Scholar]
- 43.Weyand CM, Goronzy JJ. Aging of the immune system. Mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13(Suppl 5):S422–S428. doi: 10.1513/AnnalsATS.201602-095AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans RP, Mourad MM, Pall G, Fisher SG, Bramhall SR. Pancreatitis: Preventing catastrophic haemorrhage. World J Gastroenterol. 2017;23:5460–5468. doi: 10.3748/wjg.v23.i30.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conway Morris A, Datta D, Shankar-Hari M, Stephen J, Weir CJ, Rennie J, Antonelli J, Bateman A, Warner N, Judge K, Keenan J, Wang A, Burpee T, Brown KA, Lewis SM, Mare T, Roy AI, Hulme G, Dimmick I, Rossi AG, Simpson AJ, Walsh TS. Cell-surface signatures of immune dysfunction risk-stratify critically ill patients: INFECT study. Intensive Care Med. 2018;44:627–635. doi: 10.1007/s00134-018-5247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomatos IP, Xiaodong X, Ghaneh P, Halloran C, Raraty M, Lane B, Sutton R, Neoptolemos JP. Prognostic markers in acute pancreatitis. Expert Rev Mol Diagn. 2014;14:333–346. doi: 10.1586/14737159.2014.897608. [DOI] [PubMed] [Google Scholar]
- 47.Matta B, Gougol A, Gao X, Reddy N, Talukdar R, Kochhar R, Goenka MK, Gulla A, Gonzalez JA, Singh VK, Ferreira M, Stevens T, Barbu ST, Nawaz H, Gutierrez SC, Zarnescu NO, Capurso G, Easler J, Triantafyllou K, Pelaez-Luna M, Thakkar S, Ocampo C, de Madaria E, Cote GA, Wu BU, Paragomi P, Pothoulakis I, Tang G, Papachristou GI. Worldwide Variations in demographics, management, and outcomes of acute pancreatitis. Clin Gastroenterol Hepatol. 2020;18:1567–1575 e1562. doi: 10.1016/j.cgh.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian X, Huang Y, Wang H (2017) Deviation of Chinese Adults' Diet from the Chinese Food Pagoda 2016 and Its Association with Adiposity. Nutrients 9 [DOI] [PMC free article] [PubMed]
- 49.Li XY, Pu N, Chen WW, Shi XL, Zhang GF, Ke L, Ye B, Tong ZH, Wang YH, Liu G, Chen JM, Yang Q, Li WQ, Li JS. Identification of a novel LPL nonsense variant and further insights into the complex etiology and expression of hypertriglyceridemia-induced acute pancreatitis. Lipids Health Dis. 2020;19:63. doi: 10.1186/s12944-020-01249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified individual participant data are available indefinitely in the electronic database. Data can be accessed through capctg.medbit.cn with the approval of the authors. Request for data can be made to the corresponding author (ctgchina@medbit.cn) and will be discussed during a meeting of the Chinese Acute Pancreatitis Clinical Trials Group (CAPCTG).