Abstract
Most children with biallelic SMN1 deletions and three SMN2 copies develop spinal muscular atrophy (SMA) type 2. SPR1NT (NCT03505099), a Phase III, multicenter, single-arm trial, investigated the efficacy and safety of onasemnogene abeparvovec for presymptomatic children with biallelic SMN1 mutations treated within six postnatal weeks. Of 15 children with three SMN2 copies treated before symptom onset, all stood independently before 24 months (P < 0.0001; 14 within normal developmental window), and 14 walked independently (P < 0.0001; 11 within normal developmental window). All survived without permanent ventilation at 14 months; ten (67%) maintained body weight (≥3rd WHO percentile) without feeding support through 24 months; and none required nutritional or respiratory support. No serious adverse events were considered treatment-related by the investigator. Onasemnogene abeparvovec was effective and well-tolerated for presymptomatic infants at risk of SMA type 2, underscoring the urgency of early identification and intervention.
Subject terms: Gene therapy, Development
For infants with three copies of SMN1 at risk for spinal muscular atrophy (SMA) type 1, onasemnogene abeparvovec improves ventilator-free survival and nutritional/respiratory independence and allows motor development indistinguishable from healthy children without SMA.
Main
Spinal muscular atrophy (SMA) is an autosomal recessive neuromuscular disease caused by deficiency of survival motor neuron (SMN) protein resulting from biallelic deletions or pathogenic variants of the SMN1 (survival motor neuron 1) gene. SMN protein is essential for the development and survival of motor neurons in the ventral spinal cord1. SMN2, a homologous gene to SMN1, partially compensates for SMN1 loss by producing low amounts of SMN protein2. SMN2 copy number correlates with SMA onset and severity. Patients with three copies of SMN2 may develop SMA types 1, 2 or 3, but the presence of three copies is 54% predictive of intermediate-severity SMA type 2 with onset between 7 months and 18 months of age, 15% predictive of type 1 and 31% predictive of the milder type 3 phenotype3.
Untreated children with SMA type 2 experience relatively rapid neuromuscular decline before 13 years of age, followed by more gradual debilitation through adulthood4,5. Patients with SMA type 2 achieve the ability to sit independently, but few stand and none walk independently. With advancing age, nearly all patients with SMA type 2 develop dysphagia, joint contractures, scoliosis, and restrictive lung disease, and some may lose the ability to sit independently6–9. SMA type 3 causes less severe disability than SMA type 2, with patients being able to stand and walk independently, although with increasing difficulty as they age. Patients with SMA type 3 have later symptom onset and develop fewer and less severe musculoskeletal, respiratory, and feeding problems7,8,10–12. Considerable heterogeneity within this clinical framework exists10,11. Observational studies of SMA type 2 and type 3 use continuous variables such as measures of motor performance, upper limb strength and activity, pulmonary function, and compound motor action potential (CMAP)7,8,12–15. However, the slow pace of clinical deterioration can obscure changes in these measures over intervals typical of clinical trials8,12, and periods of 24 months or longer may be needed to assess the natural progression of SMA type 2 or type 3.
Two approved therapies for SMA (nusinersen and risdiplam) increase SMN protein production via modified splicing of SMN2 and require serial dosing16. A third approved disease-modifying treatment, onasemnogene abeparvovec, is a gene replacement therapy that delivers SMN cDNA using an adeno-associated virus-9 (AAV9) vector designed for one-time intravenous infusion17. Because of early successes with SMA treatments, the United States and several other countries have implemented widespread neonatal screening for SMN1 deletions, enabling identification of children at risk for SMA before symptom onset. This has critical implications for therapeutic interventions18–20.
Single-center case series21–23, an observational cohort study24, post-marketing data25,26 and the RESTORE patient registry27–29 demonstrate the safety and efficacy of onasemnogene abeparvovec for symptomatic patients with SMA with three SMN2 copies. However, few interventional studies have targeted children with three SMN2 copies who are at risk for SMA but have yet to demonstrate signs of disease. This group is largely underrepresented in clinical trials.
Results from a Phase II study of nusinersen (NURTURE) indicate the potential of disease-modifying therapy for presymptomatic children at risk for SMA type 2. Ten children with three copies of SMN2 started nusinersen between 3 days and 42 days of age, before symptom onset30. All children achieved independent sitting and walking (most within the World Health Organization (WHO) normal reference interval), and none required respiratory intervention.
SPR1NT was the first Phase III study of onasemnogene abeparvovec for the treatment of presymptomatic infants at risk for SMA types 1, 2 or 3. The SPR1NT trial focused on efficacy measures, such as motor milestones, as they compared with normal developmental benchmarks31 and the ability to survive and thrive without mechanical interventions. We also compared efficacy and exploratory measures with the Pediatric Neuromuscular Clinical Research (PNCR) natural history population, which enrolled infants with SMA and two or three copies of SMN214. A total of 29 SPR1NT participants comprised 14 children with two copies of SMN2 and 15 with three copies of SMN2. The former cohort is the subject of a companion paper32. Here we focus on the 15 SPR1NT participants with three copies of SMN2 (hereafter referred to as the three-copy cohort) and provide important new efficacy and safety data about neonatal AAV9 vector infusion in this population. Safety and efficacy data from both cohorts have critical implications for newborn screening programs and the clinical timing of therapeutic intervention.
Results
Screening and demographics
Of 44 newborns screened for SPR1NT who had biallelic SMN1 deletions and two or three copies of SMN2, 14 were excluded. The most common reasons for exclusion were clinical signs at screening or immediately before dosing that were, in the opinion of the investigator, strongly suggestive of SMA (n = 4) and peroneal nerve to tibialis anterior CMAP <2 mV (n = 4) (Supplementary Table 1 and Supplementary Fig. 1). A total of 15 infants (female, n = 9; 60%) with three SMN2 copies were enrolled in SPR1NT. Children in the three-copy cohort were born between 35 and 41 gestational weeks (median, 39.0) at a median weight of 3.4 kg (range, 2.55–3.81) (Table 1). Ten children were born at a gestational age less than 40 weeks, and one was born at a gestational age less than 37 weeks. None of the 15 infants had a c.859 G>C modifier variant, which is associated with a milder disease course. Most children (n = 13; 87%) were diagnosed by newborn screening. The 14 infants diagnosed after birth had a confirmed molecular diagnosis at a median age of 8.0 days (range, 2–26).
Table 1.
Baseline characteristics of children with three copies of SMN2
| Baseline characteristics | All patients (n = 15)a |
|---|---|
| Age at dosing, daysb | |
| Mean (s.d.) | 28.7 (11.68) |
| Median (range) | 32.0 (9–43) |
| Gestational age at birth, weeks | |
| Mean (s.d.) | 38.8 (1.47) |
| Median (range) | 39.0 (35–41) |
| Weight at baseline, kg | |
| Mean (s.d.) | 4.1 (0.53) |
| Median (range) | 4.1 (3.10–5.20) |
| Sex, n (%) | |
| Male | 6 (40) |
| Female | 9 (60) |
| Race, n (%) | |
| White | 10 (67) |
| Asian | 2 (13) |
| Other | 2 (13) |
| American Indian or Alaska Native | 1 (7) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 13 (87) |
| Hispanic or Latino | 2 (13) |
| Modality of SMA diagnosis, n (%) | |
| Prenatal testing | 1 (7) |
| Newborn screening | 13 (87) |
| Other | 1 (7) |
| Age at SMA diagnosis, daysc | |
| n (number of children diagnosed after birth) | 14 |
| Mean (s.d.) | 9.9 (7.69) |
| Median (range) | 8.0 (2–26) |
aITT population, n = 6 males and n = 9 females; mean (s.d.) age at dosing, 28.7 (11.68) days.
bAge at dosing = (dose date − date of birth + 1).
cAge at SMA diagnosis = (SMA diagnosis date − date of birth + 1). Only calculated for patients who were diagnosed after birth.
At screening, all included infants demonstrated normal neuromuscular function and were able to swallow and breathe normally. Median baseline peroneal CMAP was 4.10 mV (range, 2.7–7.0). All 15 infants enrolled in the three-copy cohort received the onasemnogene abeparvovec infusion at a median age of 32 days (range, 9–43), with a median baseline weight of 4.1 kg (range, 3.1–5.2). Infusion interruption occurred in one child because of a pump malfunction, but this child still received all of the intended dose. All children completed the study and were included in the intention-to-treat (ITT) population.
Primary and secondary motor milestone endpoints
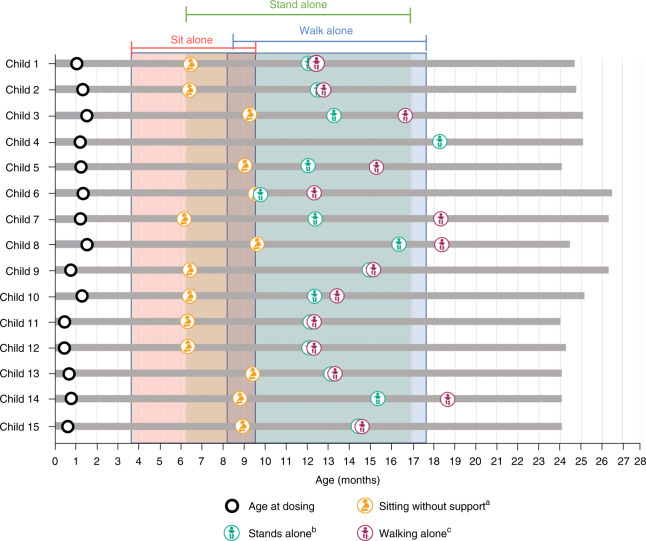
All 15 (100%) children achieved the primary endpoint of independent standing, confirmed by independent video review, for at least 3 seconds at any visit up to 24 months of age. Children achieved this motor milestone at a median age of 377 days (range, 284–549), and 14 of 15 (93%) did so within the normal WHO developmental window of ≤514 days (99th percentile) (Fig. 1). All children maintained this motor milestone at the 24-month study visit. For comparison, only 19 of 81 (24%) patients with SMA in the PNCR natural history population achieved independent standing (P < 0.0001)14.
Fig. 1. Video-confirmed developmental motor milestones for children with three copies of SMN2.
Months calculated as days / 30. Only the first observed instance of a milestone is included in this figure. Shaded areas indicate the World Health Organization Multicentre Growth Reference Study (WHO-MGRS) windows for normal development; the 99th percentile (that is, upper bound of normal development) of sits without support is 279 days, stands alone is 514 days, and walks alone is 534 days. aBayley Scales gross motor subtest item #26: child sits alone without support for at least 30 seconds. bBayley Scales gross motor subtest item #40: child stands alone. Child stands alone for at least 3 seconds after you release his or her hands. cBayley Scales gross motor subtest item #43: child walks alone. Child takes at least five steps independently, displaying coordination and balance. n = 6 males and n = 9 females; mean (s.d.) age at dosing, 28.7 (11.68) days.
Fourteen (93%) children in the three-copy cohort walked independently for at least five steps at any visit up to 24 months of age, compared to 17 of 81 patients (21%) in the PNCR population (P < 0.0001)14. The median age of independent walking was 422.0 days (range, 362–563), and 11 (73%) children achieved this motor milestone within the WHO normal developmental window of ≤534 days of age. Notably, one additional child in the three-copy cohort was observed walking during the 24-month study visit (conducted via video call) by the clinical evaluator, but video was not recorded. Therefore, per the study protocol, the child was judged not to have achieved this motor milestone in the absence of an independent video review. A detailed summary of motor milestones is included in Supplementary Table 2.
Exploratory functional endpoints
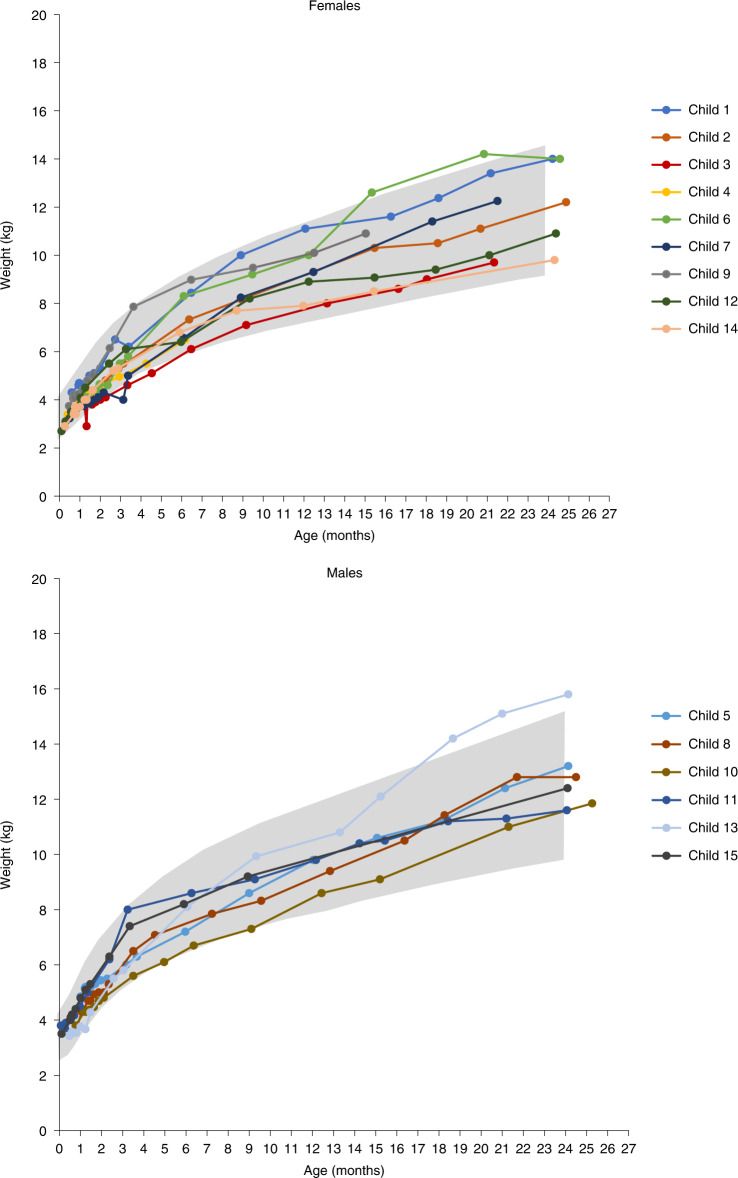
All 15 (100%) children in the three-copy cohort were alive and free from permanent ventilation at 14 months of age, and ventilator-free survival remained 100% at the end of the study. In fact, no child required mechanical respiratory support of any kind (for example, cough-assist, bilevel positive airway pressure, or invasive ventilatory support) throughout the duration of the trial. Ten of 15 (67%) children were at or above the 3rd reference percentile for weight at all study visits, and all children were at or above this percentile at the end of the study (Fig. 2). In addition, no child required a feeding tube at any point during the study.
Fig. 2. Growth charts for children with three copies of SMN2.
Ten (67%) children achieved the ability to maintain weight at or above the WHO 3rd percentile without the need for non-oral/mechanical feeding support at all visits up to 24 months of age. The ability to maintain weight at or above the 3rd percentile without the need for non-oral/mechanical feeding support was defined by meeting both of the following criteria at all visits: (1) does not receive nutrition through mechanical support (that is, feeding tube) and (2) maintains weight (≥3rd percentile for age and sex as defined by WHO guidelines) consistent with the patient’s age at the assessment. The gray shading represents WHO growth standards for the 3rd through 97th percentiles. n = 6 males and n = 9 females; mean (s.d.) age at dosing, 28.7 (11.68) days.
Exploratory motor endpoints
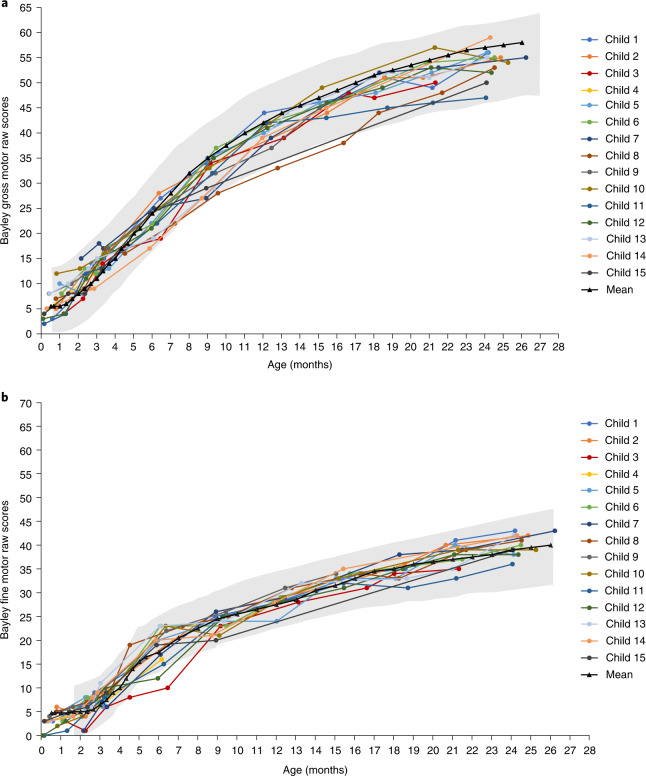
The Bayley Scales of Infant and Toddler Development (BSID) provide a more granular appraisal of development compared with an age-matched reference population33. All 15 children were adequately assessed using the BSID, although one (7%) child missed the baseline assessment, precluding calculation of a change from baseline. Incremental gains in gross and fine motor raw scores generally tracked with the normal reference population (Fig. 3 and Supplementary Table 3). Raw scores were converted to scaled scores with a normative mean of 10 and a standard deviation (s.d.) of 3, such that scaled scores of 4–16 capture the 3rd to 97th percentile range for normal motor development. All 15 (100%) children in the three-copy cohort achieved a scaled score of ≥4 (within 2 s.d. of the reference mean) on both the gross motor and fine motor subtests during at least one post-baseline visit. For each scheduled visit, most assessed children (78–100%) met the criteria at that visit. At the 24-month visit, all ten (100%) children who were assessed achieved a scaled score of ≥4, with the median gross motor scaled score of 9 (range, 5–12), close to the normative population mean (Supplementary Table 4). Gains in motor function were paralleled by electrophysiologic evidence of preserved motor nerve integrity. The median maximum peroneal CMAP recorded at any post-infusion visit was 6.00 mV (range, 4.2–8.5), representing a median increase from baseline of 1.80 mV (range, −0.6 to 5.0) (Supplementary Table 5).
Fig. 3. Bayley scales fine motor and gross motor raw scores.
Improvements were observed in all children for both gross (a) and fine (b) subtests of the Bayley Scales of Infant and Toddler Development after onasemnogene abeparvovec infusion and up to 24 months of age. The gray shading represents Bayley-III gross and fine motor normal ranges (±2 s.d.). n = 6 males and n = 9 females; mean (s.d.) age at dosing, 28.7 (11.68) days.
Safety observations
To attenuate the inflammatory response to AAV9, all 15 children commenced oral prednisolone 1 day before onasemnogene abeparvovec infusion and completed a median of 63 days (range, 49–321) of therapy. A total of 166 treatment-emergent adverse events (TEAEs) were reported (Supplementary Tables 6 and 7). Each child experienced at least one TEAE, and three (20%) had a TEAE reported as serious. Eight of 15 (53%) children had a TEAE considered by the investigator to be related to the study treatment, but none was serious.
Transient decreases in platelets have been observed after administration of onasemnogene abeparvovec34. Preclinical studies in animal models have reported cardiac thrombi and dorsal root ganglia toxicities, but these have not been observed clinically25. On the basis of these data, the study sponsor (Novartis Gene Therapies) identified five specific categories of adverse events (AEs) of special interest (AESI): hepatotoxicity, thrombocytopenia, cardiac toxicity, thrombotic microangiopathy (TMA), and sensory abnormalities suggestive of dorsal root ganglionopathy (Table 2). AESIs were identified using specific terms in standardized Medical Dictionary for Regulatory Activities (MedDRA) coding dictionary queries related to these categories (see Methods for further details).
Table 2.
TEAEs of special interest in children with three copies of SMN2
| Category of AESI | n = 15a |
|---|---|
| Preferred term | n (%) |
| Hepatotoxicity | |
| Any TEAE | 4 (27) |
| Aspartate aminotransferase increased | 4 (27) |
| Alanine aminotransferase increased | 3 (20) |
| Blood alkaline phosphatase increased | 1 (7) |
| Gamma-glutamyltransferase increased | 1 (7) |
| Thrombocytopenia | |
| Any TEAE | 2 (13) |
| Hematemesis | 1 (7) |
| Hematochezia | 1 (7) |
| Contusion | 1 (7) |
| Cardiac adverse events | |
| Any TEAE | 3 (20) |
| Blood creatine phosphokinase MB increased | 2 (13) |
| Troponin increased | 2 (13) |
| Thrombotic microangiopathy | |
| Any TEAE | 0 (0) |
| Thrombocytopenia | 0 (0) |
| Sensory abnormalities suggestive of dorsal root ganglionopathy | |
| Any TEAE | 1 (7) |
| Areflexia | 1 (7) |
aSafety population: n = 6 males and n = 9 females; mean (s.d.) age at dosing, 28.7 (11.68) days.
Thirteen hepatotoxicity AESIs occurred in four of 15 (27%) children. All events were mild or moderate, except for a single Grade 3 event of increased alanine aminotransferase (five or more times the upper limit of normal). The investigator considered all events as related to treatment. All hepatotoxicity events resolved, including the Grade 3 event that resolved with augmented prednisolone (Supplementary Table 8). No clinically observed events of jaundice or hepatic encephalopathy were reported. Three thrombocytopenia-related events occurred in two of 15 (13%) children. None of the events was associated with decreased platelet counts. These events were mild or moderate, considered unrelated to treatment, and resolved without sequelae (Supplementary Table 9). To assess cardiac toxicity, creatinine phosphokinase (CK)-MB was initially measured but was changed, mid-study, to the more reliable cardiac tissue marker troponin I. CK-MB was not assessed after this change. Eight children had both baseline and post-baseline CK-MB values, and five children had both baseline and post-baseline troponin I values. Four AEs of elevated cardiac enzymes were reported in three children: one had elevated CK-MB and troponin I; one had an isolated elevation of CK-MB; and one had an isolated elevation of troponin I (Table 2). All events were mild or moderate and considered possibly or probably related to treatment. At the end of the study, serum CK-MB remained elevated in one child, and the two cardiac AESIs resolved without sequelae in the other two children (Supplementary Table 10). No TMA events were reported during the study. One of 15 (7%) children had two AESIs (areflexia), which could potentially be related to dorsal root ganglionopathy. However, these events were mild and not related to treatment. One event resolved and one was ongoing at the last study visit (Supplementary Table 11).
Discussion
SPR1NT demonstrates that a single intravenous dose of onasemnogene abeparvovec promotes motor development for presymptomatic neonates with biallelic deletions of SMN1 and three copies of SMN2 who are primarily at risk for SMA type 2. Without treatment, most of these children would achieve motor milestones no more advanced than independent sitting, whereas those treated with onasemnogene abeparvovec displayed patterns of motor development indistinguishable from healthy children without SMA. Specifically, all but one of the 15 children achieved independent walking (the remaining child stood independently), and all had BSID gross and fine motor scores similar to neurologically normal peers. Exceptional motor and functional outcomes were also observed for children in the two-copy cohort of SPR1NT32.
Remarkably, no child in SPR1NT required mechanical feeding or respiratory support, indicating that presymptomatic gene therapy has the potential to prevent some musculoskeletal, pulmonary, and growth complications characteristic of classic SMA type 2. This represents a profound shift in the early course of illness to a much milder SMA phenotype or possibly even to normal motor development. Given the durability of benefit observed in the follow-up study of the Phase I START trial35, and the fact that motor neurons are non-dividing cells, we are optimistic that one-time treatment with onasemnogene abeparvovec will add years of independent mobility, intact bulbar function, and good health-related quality of life for children in the three-copy cohort.
Table 3 places SPR1NT in the context of three other clinical trials: STR1VE-US36, STR1VE-EU37 and a Phase II study of infants with two or three copies of SMN2 treated with nusinersen before symptom onset (NURTURE)29. A presymptomatic study with risdiplam (RAINBOWFISH) is still in progress38. Overall, Table 3 highlights the importance of treatment timing (that is, before the onset of clinical symptoms) as a potentially important determinant of outcome, but noteworthy differences in the designs of these trials prevent direct comparisons between them. Primary endpoints of the percentage of children who achieved the independent standing (BSID item #40) and independent walking (BSID item #43) motor milestones were included for the SPR1NT three-copy cohort versus only independent sitting (using WHO and Hammersmith Infant Neurological Examination section 2 criteria) in NURTURE. Eligibility criteria also differed, including ability to tolerate thin liquids, peroneal CMAP ≥2 mV, and presymptomatic SMA in SPR1NT versus ulnar CMAP ≥1 mV, absence of hypoxia, and no clinical signs or symptoms suggestive of SMA in NURTURE. Motor milestone achievement in both the two-copy and three-copy cohorts of SPR1NT is also distinguished from other studies by its stringency, requiring video confirmation by an independent observer in both the two-copy and three-copy cohorts. In NURTURE, however, parents or caregivers reported motor milestone achievement, and confirmation followed at the next site visit, and age at milestone achievement was reported by parents, caregivers, or site investigators. Regardless of these differences, children with few or no clinical signs of SMA who receive treatment appear to achieve more advanced developmental milestones than children who receive treatment after the clinical onset of disease.
Table 3.
Summary of SPR1NT results and other SMA studies and cohortsa
| Onasemnogene abeparvovec | Nusinersen | ||||||
|---|---|---|---|---|---|---|---|
| Symptomatic patients | Presymptomatic children | Presymptomatic children | |||||
| PNCR14 | STR1VE-US36 | STR1VE-EU37 | SPR1NT, two-copy cohort | SPR1NT, three-copy cohort | NURTURE,b two-copy cohort30 | NURTURE,b three-copy cohort30 | |
| Intention-to-treat population, n | 23 | 22 | 32 | 14 | 15 | 15 | 10 |
| SMN2 copies | 2 | 2 | 2 | 2 | 3 | 2 | 3 |
| Median (range) age at diagnosis, days | N/A | 67 (56–126)c | 76 (26–156) | 8 (1–14) | 8 (2–26) | N/A | N/A |
| Median (range) age at infusion, days | N/A | 105 (15–177) | 123 (54–180) | 21 (8–34) | 32 (9–43) | 19 (8–41) | 23 (3–42) |
| Baseline median (range) CHOP INTEND | 32.5 (31–33)d | 33.5 (18–52) | 28.0 (14–55) | 48.5 (28–57) | N/A | 45.0 (25–60) | 53.5 (40–60) |
| Baseline median (range) CMAP amplitude, mVe | 0.3 (0.04–1.1) | N/A | N/A | 3.9 (2.1–6.1) | 4.1 (2.7–7.0) | 3.2 (1.1–9.7) | 4.0 (0.2–7.0) |
| Sitting independently by 18 months, n (%)f | 0 | 14 (64) | 14 (44) | 14 (100) | N/A | N/A | N/A |
| Sitting independently by 24 months of age, n (%)f | 0 | N/A | N/A | N/A | 14 (93) | 15 (100) | 10 (100) |
| Standing independently by 18 months of age, n (%)f | 0 | 1 (5) | 1 (3) | 11 (79) | N/A | N/A | N/A |
| Standing independently by 24 months of age, n (%)f | 0 | N/A | N/A | N/A | 15 (100) | 9 (60) | 10 (100) |
| Walking independently by 18 months of age, n (%)f | 0 | 1 (5) | 1 (3) | 9 (64) | N/A | N/A | N/A |
| Walking independently by 24 months of age, n (%)f | 0 | N/A | N/A | N/A | 14 (93) | 9 (60) | 10 (100) |
| Alive without permanent ventilation at 18 months of age, n (%)f | 6 (26)g | 20 (91) | 31 (97) | 14 (100) | 15 (100) | 15 (100) | 10 (100) |
HINE-2, Hammersmith Infant Neurological Examination section 2; N/A, not available.
aThere are no published head-to-head studies of onasemnogene abeparvovec and nusinersen. Differences in trial design, including primary endpoints, how endpoints were measured, and eligibility criteria, make direct comparison of results from these studies infeasible. The PNCR measured CHOP INTEND; NURTURE measured WHO and HINE-2 criteria; and STR1VE-US and STR1VE-EU measured WHO criteria and CHOP INTEND.
bNURTURE results represent interim analysis at data cut of 29 March 2019. At the time of this analysis, the median age of the infants was 34.8 months (range, 25.7–45.4)26.
cMedian (range) is reported as the interquartile range.
dValue obtained for patients with symptom onset <3 months of age, including seven patients with two SMN2 copies and one patient with three SMN2 copies
eUlnar CMAP amplitude recorded from the abductor digiti minimi muscle at baseline for the PNCR and NURTURE studies and peroneal CMAP amplitude recorded from the tibialis anterior muscle for SPR1NT.
fMilestones were evaluated over different observation periods between studies and included 18 months for STR1VE-US, STR1VE-EU, and SPR1NT two-copy cohort; 24 months for the SPR1NT three-copy cohort; and a median follow-up time of 35 months for NURTURE.
gSurvival without permanent ventilation at 14 months.
We also observed that presymptomatic neonatal treatment with intravenous onasemnogene abeparvovec demonstrated a favorable safety profile, and no new or unexpected safety concerns were identified with treatment administration between 9 days and 43 days of age. Although all children had at least one AE, few experienced serious AEs, and no treatment-related serious AEs or deaths related to treatment were reported. Furthermore, AESIs were generally mild or moderate and resolved. Transient elevations of liver enzymes were asymptomatic and generally mild. Transient changes in platelet counts were observed, but no child had a platelet count below 75,000 cells per µl. All thrombocytopenia-related AESIs were mild to moderate, and all resolved. Asymptomatic and mild elevations of cardiac enzymes occurred in a minority of children but were not associated with signs of ventricular dysfunction or thrombosis. TMA, which presents clinically as hemolytic anemia, thrombocytopenia, and acute kidney injury, has been identified as a risk for onasemnogene abeparvovec based on post-marketing safety surveillance39, but no cases of TMA occurred in SPR1NT. A single case of areflexia persisted at the time of study conclusion, and, although areflexia is a component of the clinical picture of sensory ganglionopathy, other clinical symptoms of this condition were absent, increasing the likelihood that this AESI was a complication of one child’s underlying SMA diagnosis. The possibility that the favorable safety profile of onasemnogene abeparvovec observed in SPR1NT relates to maturational differences in the immune response is discussed briefly in the companion manuscript32. Limitations of SPR1NT are the relatively small number of participants, the use of an external comparator group, and the exclusion of participants with CMAP <2 mV at screening.
Two decades ago, the Human Genome Project promised new diagnostics and therapeutics based on the identification of underlying genetic mechanisms of disease40. Ultimately, genomics research aimed to change medical practice from a reactive stance, in which presenting signs and symptoms of disease prompt treatment, to a proactive one, in which deep understanding of underlying vulnerabilities within the genome allows providers to anticipate future health risks and apply precise interventions that keep people healthy. The goal was to find the right treatment for the right patient at the right time and, thereby, prevent disease and disability41,42. This goal may soon be realized for children with SMA, with the discovery of its molecular basis, effective therapies, and the optimal timing for intervention.
For all forms of SMA, genomic medicine appears to be entering the realms of public health and preventive pediatrics. Children at risk for SMA types 2 or 3 who were treated once with onasemnogene abeparvovec before 6 weeks of age, before the onset of symptoms, demonstrated normal or nearly normal patterns of growth and neuromuscular development in this study. Our findings underscore the urgency of early identification of children at risk for SMA by newborn screening, followed by timely treatment to prevent death and disability. This has critical implications for the implementation of universal newborn screening for SMN1 deletions, discussed more fully in the companion two-copy manuscript32.
In the past 2 decades, advances in medical genetics have propelled the development of new therapies for monogenic disorders such as SMA, increased understanding of their underlying pathophysiology, and permitted development of new genetic diagnostic tools42–44. However, treating individuals who demonstrate no symptoms of disease remains controversial. SMA offers an example of what can be achieved when newborn screening identifies at-risk infants who can potentially be spared the consequences of severe, debilitating weakness. Children with three copies of SMN2 have a greater likelihood of developing SMA type 2 or type 3, but SPR1NT demonstrates that treating three-copy children before the appearance of SMA symptoms essentially allows them to grow and develop as normal children. This represents a remarkable evolution in the standard of care for SMA: from a reactive to a proactive paradigm, from a focus on patients who survive to children who thrive.
Methods
Study design
SPR1NT was an open-label, single-arm, Phase III study conducted at 16 sites in six countries (Australia, Belgium, Canada, Japan, the United Kingdom and the United States). The study was conducted in accordance with the Declaration of Helsinki, International Council for Harmonisation/Good Clinical Practice guidelines and applicable regulatory requirements (for example, those relating to informed consent and the protection of human patients in biomedical research). The study was approved by institutional review boards (IRBs) at all participating institutions (Advarra Center for IRB Intelligence, Nationwide Children’s Hospital; UCLA Medical Center IRB #3, David Geffen School of Medicine at the University of California, Los Angeles; Nemours Office of Human Subjects Protection, Nemours Children’s Clinic; Columbia University Medical Center IRB, Columbia University Medical Center; Advarra Center for IRB Intelligence, Massachusetts General Hospital; Children’s Hospital of Eastern Ontario Research Ethics Board, Children’s Hospital of Eastern Ontario; Sydney Children’s Hospitals Network Human Research Ethics Committee, Sydney Children’s Hospital; University of Pennsylvania IRB, Clinic for Special Children; Tokyo Women’s Medical University IRB, Tokyo Women’s Medical University Hospital; The Dubowitz Neuromuscular Centre IRB, University College London; and The Neuromuscular Center of Liège, CHU & University of Liège), and written informed consent was obtained from parents or legal guardians of enrolled patients.
Patients
The study included presymptomatic children who had SMA genetically defined by biallelic deletions of SMN1 with either two or three copies of SMN2 expected to develop SMA type 1 or SMA types 2 or 3, respectively. Children were enrolled in two separate cohorts according to the number of SMN2 copies present. Children with SMN1 point mutations (that is, pathogenic variants) or the SMN2 gene modifier variant (c.859 G>C) could enroll, but those with the SMN2 gene modifier variant would not be included in the ITT population. Efficacy and safety findings for children with three SMN2 copies are reported. The study planned to enroll at least 12 children with three copies of SMN2 who met the ITT criteria and were ≤6 weeks of age at the time of gene replacement therapy (Day 1). Full eligibility criteria are described in the Supplementary Material.
The Coronavirus Disease 2019 (COVID-19) pandemic did not affect retention. No participant withdrew from SPR1NT or was lost to follow-up because of the COVID-19 pandemic. However, some scheduled study visits and assessments were delayed or cancelled because of restrictions caused by the COVID-19 pandemic.
Procedures
All children were admitted into the hospital for pretreatment baseline procedures 1 day before infusion. For all assessments, baseline was defined as the last assessment conducted before dosing. Onasemnogene abeparvovec (1.1 × 1014 vector genomes per kilogram (vg kg−1)) was administered as a single intravenous infusion (given over approximately 60 minutes) between 18 September 2018 and 9 July 2019. Safety monitoring was conducted while the children remained in the hospital for a minimum of 24 hours after infusion. All children received prophylactic prednisolone (initially 1 mg/kg/day, increased to 2 mg/kg/day following a protocol amendment in May 2019) beginning 24 hours before infusion and for 48 hours after infusion, after which the dosage was 1 mg/kg/day during a minimum of 30 days. Thereafter, prednisolone was tapered according to a standard algorithm and based on a requirement that gamma-glutamyltransferase, alanine aminotransferase, and aspartate aminotransferase values were below the threshold of twice the upper limit of normal. Investigators were permitted to use other glucocorticosteroids in place of prednisolone, change the daily prednisolone dosage, and alter the taper schedule according to their clinical judgment.
Outpatient follow-up assessments were conducted on Days 7, 14, 21, 30, 44, 51 (in Japan only), 60, and 72 post-dose, and then at 3 months of age and every 3 months thereafter through 24 months of age (that is, the end-of-study visit). All eligible children were invited to enroll in an ongoing long-term follow-up study (LT-002, NCT04042025).
Outcomes
The primary efficacy endpoint was the ability to stand independently for ≥3 seconds at any visit up to 24 months of age, as stipulated by item #40 from the gross motor subtest of the BSID. The secondary efficacy endpoint was the ability to walk alone for at least five steps at any visit up to 24 months of age, as stipulated by item #43 of the BSID33. Exploratory endpoints were survival at 14 months of age, defined as the avoidance of death or requirement of permanent ventilation (tracheostomy or ≥16 hours of daily respiratory assistance for ≥14 consecutive days in the absence of an acute reversible illness, excluding perioperative ventilation) and the ability to maintain body weight at or above the 3rd percentile without the need for feeding support at any visit up to 24 months of age. Other exploratory endpoints included achievement of motor milestones and changes from baseline as assessed by World Health Organization Multicentre Growth Reference Study (WHO-MGRS) and BSID version 3 gross motor criteria, Children’s Hospital of Pennsylvania Infant Test of Neuromuscular Disorders (CHOP INTEND) scores, and scores on the BSID gross and fine motor subtests. Videos demonstrating developmental milestones meeting WHO and BSID criteria (as part of clinical evaluation at study visits or submitted by parent(s)/legal guardian(s) at any time during the study) were reviewed by an independent, central reviewer for unbiased assessment and confirmation of developmental milestone achievement.
Safety monitoring
Safety was assessed by monitoring for AEs, physical examinations, pulmonary examinations, vital signs, weight and length measurements, 12-lead electrocardiograms, 24-hour Holter monitoring, echocardiograms, swallowing tests, laboratory assessments, and photographs of the infusion site. Pulmonary examinations were performed by a pulmonologist or appropriate individual according to standard institutional practice. All AEs were recorded and classified in accordance with the Common Terminology Criteria for Adverse Events (version 4.03) (https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5×7.pdf).
Statistical analysis
Data were analyzed using SAS version 9.4 software (SAS Institute). Primary and secondary efficacy analyses were performed for participants with biallelic SMN1 deletions and three copies of SMN2 without the SMN2 gene modifier variant (c.859 G>C), which is associated with a less severe clinical course45, who were included in the ITT population. Primary and secondary outcomes were compared with a cohort of population-matched patients from the PNCR natural history data set (all patients with any type of SMA and three copies of SMN2; the SMN2 modifier mutation (c.859 G>C) was not assessed in the PNCR study cohort)14. This study was designed to have >90% power with α = 0.05 to detect a significant difference in independent standing using a two-sided Fisher exact test on a sample size of ≥12 children into the ITT population as well as assumptions based on a matched PNCR dataset14 and START study data17. Formal testing for the primary and secondary efficacy endpoints was performed using a hierarchical approach to protect against Type I error as follows. First, the primary motor endpoint of independent standing for ≥3 seconds was assessed. If the analysis of the primary endpoint was determined to be statistically significant (P < 0.05), then formal testing of the secondary motor endpoint, walking independently, was conducted.
The safety population included all children who received onasemnogene abeparvovec. Safety was evaluated through reported AEs as well as objective data variables, including vital signs, physical examinations, and laboratory studies. These data are presented in a descriptive fashion. AEs were coded using an industry standardized MedDRA coding dictionary (version 23.0), and AESIs were classified through specific predefined MedDRA terms (Supplementary Table 12).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41591-022-01867-3.
Supplementary information
SPR1NT Study Group, Supplementary Methods, Supplementary Fig. 1 and Supplementary Tables 1–12
Acknowledgements
The authors wish to thank the investigators, site coordinators, and study teams, administrators of newborn screening programs and, most importantly, the patients, families, and caregivers for their participation in these studies. The authors also thank M. Milton of Novartis Institutes for Biomedical Research, who provided critical review and input on content related to biodistribution, immune response and safety; S. P. Reyna of Novartis Gene Therapies, Inc., who provided critical review and input on SMA patient care; and M. Wolf of Novartis Gene Therapies, Inc., who provided functional outcome measure expertise and contributions to study conduct and oversight. Medical writing assistance and editorial support was provided by J. Gibson from Kay Square Scientific and M. Nissen of Novartis Gene Therapies, Inc. This support was funded by Novartis Gene Therapies, Inc. The NSW Pilot NBS study was funded by Luminesce Alliance, a not-for-profit cooperative joint venture across the Sydney Children’s Hospital Network, Children’s Medical Research Institute, and Children’s Cancer Institute, established with the support of the New South Wales Government. Luminesce Alliance is also affiliated with UNSW Sydney and The University of Sydney. The SPR1NT study was designed and funded by Novartis Gene Therapies, Inc., which was involved in the study design, data collection, data analysis, data interpretation, and writing of all related reports and publications.
Author contributions
Conceived or designed the study: B.E.M. and T.A.M. Collected data: K.A.S., M.A.F., F.M., K.S., J.R.M., L.S., H.J.M., R.S.F., K.J.S., J.M.K., C.M.Z., C.A.C., S.T.I., J.M.K., J.A.P., and P.B.S. Accessed and verified data: K.A.S., T.A.M., B.E.M., S.K., and M.W. Interpreted data: all authors. Wrote or contributed to the writing of the manuscript: all authors.
Peer review
Peer review information
Nature Medicine thanks Susan Matesanz, Charlotte Sumner, Victor Volovici, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Jerome Staal, in collaboration with the Nature Medicine team.
Data availability
A redacted version of the SPR1NT study protocol and a redacted version of the statistical analysis plan are available at ClinicalTrials.gov (NCT03505099). Novartis is committed to sharing clinical trial data with external researchers and has been doing so voluntarily since 2014.
Novartis is committed to sharing, upon requests from qualified external researchers and subsequent approval by an independent review panel based upon scientific merit, anonymized patient-level and study-level clinical trial data and redacted clinical study reports for medicines and indications approved in the United States and Europe after the respective study is accepted for publication. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
Competing interests
Novartis Gene Therapies, Inc. sponsored this clinical trial. The authors declare the following competing interests. K.A.S. has received personal compensation from Novartis Gene Therapies, Inc. (formerly AveXis, Inc.) for serving as an advisory board member and from Biogen for serving as a visiting professor, and has received research support from clinical trials sponsored by Novartis Gene Therapies, Inc., and Biogen. M.A.F. has received honoraria for scientific advisory boards from Novartis Gene Therapies, Inc., Biogen, and Roche and research grants from Biogen. F.M. reports grants and personal fees from Novartis Gene Therapies, Inc., including participation in the STR1VE-EU grant and the SPR1NT study; grants, personal fees, and other (participation in the SHINE clinical trial and principal investigator of the investigator-initiated UK SMA REACH UK registry) from Biogen; and grants and personal fees from Roche, including participation in the JEWELFISH clinical trial of risdiplam and participation in the olesoxime clinical trial, during the conduct of the study. F.M. has also received honoraria for scientific advisory boards from Biogen, Novartis, Novartis Gene Therapies, Inc., PTC, Roche, and Sarepta Therapeutics. K.S. has served on advisory boards for Biogen, Novartis Gene Therapies, Inc./Novartis, and Chugai (Roche) and has received search funding from Biogen. J.R.M. has received personal compensation for clinical trial consulting and for serving on scientific advisory boards, as well as research support, from Novartis Gene Therapies, Inc. L.S. has received personal compensation as an advisory committee board member/consultant from Novartis Gene Therapies, Inc., Biogen, Biophytis, Cytokinetics, Dynacure, Roche, Santhera, and Sarepta Therapeutics, and has received research support from Novartis Gene Therapies, Inc., Biogen, Dynacure, and Roche. H.J.M. has received honoraria for scientific advisory boards from Novartis Gene Therapies, Inc., and has received research funding from Roche. R.S.F. has received personal compensation for advisory board participation from Novartis Gene Therapies, Inc., Biogen, Roche, and Scholar Rock, and for consulting from Novartis; editorial fees from Elsevier for co-editing a neurology textbook; license fees from the Children’s Hospital of Philadelphia; and research funding from Novartis Gene Therapies, Inc., Biogen, Roche/Genentech, and Scholar Rock. K.J.S. has received personal compensation for a speaking engagement from Biogen, and research funding as a principal investigator for clinical trials sponsored by Novartis Gene Therapies, Inc. and Biogen. J.M.K. was site principal investigator for clinical trials sponsored by Novartis Gene Therapies, Inc., Biogen, and Scholar Rock. C.M.Z. has received research support from Biogen. C.A.C. has served on advisory boards for Novartis Gene Therapies, Inc., and Roche/Genentech; has served as an educational speaker for Biogen; and has received research funding from Novartis Gene Therapies, Inc., Biogen, and Roche. S.T.I. has received personal compensation for service on advisory boards or consulting from Novartis Gene Therapies, Inc., Biogen, Roche/Genentech, and Sarepta Therapeutics; and research support from Novartis Gene Therapies, Inc., Biogen, Capricor, PTC, Scholar Rock, and Sarepta Therapeutics. J.M.K. is a principal investigator for clinical trials sponsored by Novartis Gene Therapies, Inc., Scholar Rock, and Roche/Genentech. J.A.P. has served as an investigator on clinical trials for Biogen, Novartis, PTC Therapeutics, and Scholar Rock, and has served in an advisory capacity for Biogen, Scholar Rock, Roche/Genentech, and Novartis. P.B.S. has served as a consultant for Alexion, Biogen, Genentech, Novartis Gene Therapies, Inc., and Sarepta and has served as a speaker for Alexion, Biogen, Genentech, Grifols, Novartis Gene Therapies, Inc., and PTC Therapeutics. S.K. is an employee of Novartis Gene Therapies, Inc., and receives consulting fees from UCB Pharma, Karuna Therapeutics, Worldwide Clinical Trials, CPC Clinical Research, Zosano Pharmaceuticals, PharPoint Research, and Nesos, Inc. M.W. is an employee of Novartis Gene Therapies, Inc., and owns Novartis stock or other equities. S.T.-W. is an employee of Novartis Gene Therapies, Inc., and owns Novartis stock or other equities. B.E.M. is an employee of Translational Medicine, Novartis Institutes for BioMedical Research (Cambridge), and owns Novartis stock or other equities. T.A.M. is an employee of Novartis Gene Therapies, Inc., and owns Novartis stock or other equities.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41591-022-01867-3.
References
- 1.Coovert DD, et al. The survival motor neuron protein in spinal muscular atrophy. Hum. Mol. Genet. 1997;6:1205–1214. doi: 10.1093/hmg/6.8.1205. [DOI] [PubMed] [Google Scholar]
- 2.Mailman MD, et al. Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet. Med. 2002;4:20–26. doi: 10.1097/00125817-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Calucho M, et al. Correlation between SMA type and SMN2 copy number revisited: an analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul. Disord. 2018;28:208–215. doi: 10.1016/j.nmd.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Carson VJ, et al. Nusinersen by subcutaneous intrathecal catheter for symptomatic spinal muscular atrophy patients with complex spine anatomy. Muscle Nerve. 2022;65:51–59. doi: 10.1002/mus.27425. [DOI] [PubMed] [Google Scholar]
- 5.Muntoni F, et al. Long-term follow-up of patients with type 2 and non-ambulant type 3 spinal muscular atrophy (SMA) treated with olesoxime in the OLEOS trial. Neuromuscul. Disord. 2020;30:959–969. doi: 10.1016/j.nmd.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 6.D’Amico A, Mercuri E, Tiziano FD, Bertini E. Spinal muscular atrophy. Orphanet J. Rare Dis. 2011;6:71. doi: 10.1186/1750-1172-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chabanon A, et al. Prospective and longitudinal natural history study of patients with type 2 and 3 spinal muscular atrophy: baseline data NatHis-SMA study. PLoS ONE. 2018;13:e0201004. doi: 10.1371/journal.pone.0201004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufmann P, et al. Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurology. 2012;79:1889–1897. doi: 10.1212/WNL.0b013e318271f7e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trucco F, et al. Respiratory trajectories in type 2 and 3 spinal muscular atrophy in the iSMAC cohort study. Neurology. 2021;96:e587–e599. doi: 10.1212/WNL.0000000000011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coratti G, et al. Motor function in type 2 and 3 SMA patients treated with nusinersen: a critical review and meta-analysis. Orphanet J. Rare Dis. 2021;16:430. doi: 10.1186/s13023-021-02065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercuri E, et al. Patterns of disease progression in type 2 and 3 SMA: implications for clinical trials. Neuromuscul. Disord. 2016;26:126–131. doi: 10.1016/j.nmd.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annoussamy M, et al. Natural history of type 2 and 3 spinal muscular atrophy: 2-year NatHis-SMA study. Ann. Clin. Transl. Neurol. 2021;8:359–373. doi: 10.1002/acn3.51281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrar MA, et al. Pathophysiological insights derived by natural history and motor function of spinal muscular atrophy. J. Pediatr. 2013;162:155–159. doi: 10.1016/j.jpeds.2012.05.067. [DOI] [PubMed] [Google Scholar]
- 14.Finkel RS, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83:810–817. doi: 10.1212/WNL.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swoboda KJ, et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann. Neurol. 2005;57:704–712. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramdas S, Servais L. New treatments in spinal muscular atrophy: an overview of currently available data. Expert Opin. Pharmacother. 2020;21:307–315. doi: 10.1080/14656566.2019.1704732. [DOI] [PubMed] [Google Scholar]
- 17.Mendell JR, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 18.Hale K, et al. Landscape of spinal muscular atrophy newborn screening in the United States: 2018–2021. Int. J. Neonatal Screen. 2021;7:33. doi: 10.3390/ijns7030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kariyawasam DST, et al. The implementation of newborn screening for spinal muscular atrophy: the Australian experience. Genet. Med. 2020;22:557–565. doi: 10.1038/s41436-019-0673-0. [DOI] [PubMed] [Google Scholar]
- 20.Jedrzejowska M. Advances in newborn screening and presymptomatic diagnosis of spinal muscular atrophy. Degener. Neurol. Neuromuscul. Dis. 2020;10:39–47. doi: 10.2147/DNND.S246907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friese J, et al. Safety monitoring of gene therapy for spinal muscular atrophy with onasemnogene abeparvovec—a single centre experience. J. Neuromuscul. Dis. 2021;8:209–216. doi: 10.3233/JND-200593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaber Ali H, et al. Gene therapy for spinal muscular atrophy: the Qatari experience. Gene Ther. 2021;28:676–680. doi: 10.1038/s41434-021-00273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldrop MA, et al. Gene therapy for spinal muscular atrophy: safety and early outcomes. Pediatrics. 2020;146:e2022729. doi: 10.1542/peds.2020-0729. [DOI] [PubMed] [Google Scholar]
- 24.Weiβ C, et al. Gene replacement therapy with onasemnogene abeparvovec in children with spinal muscular atrophy aged 24 months or younger and bodyweight up to 15 kg: an observational cohort study. Lancet Child Adolesc. Health. 2022;6:P17–P27. doi: 10.1016/S2352-4642(21)00287-X. [DOI] [PubMed] [Google Scholar]
- 25.Day JW, et al. Clinical trial and postmarketing safety of onasemnogene abeparvovec therapy. Drug Saf. 2021;44:1109–1119. doi: 10.1007/s40264-021-01107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Silva AM, et al. Onasemnogene abeparvovec in spinal muscular atrophy: an Australian experience of safety and efficacy. Ann. Clin. Transl. Neurol. 2022;9:339–350. doi: 10.1002/acn3.51519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Servais, L. et al. Real-world treatment patterns and outcomes in patients with spinal muscular atrophy: updated findings from the RESTORE registry. Presented at: World Muscle Society 2021 Congress, 20–24 September 2021; Virtual.
- 28.Servais, L. et al. The RESTORE Registry: real-world assessments of interventions and long-term outcomes in patients with spinal muscular atrophy. Presented at: British Paediatric Neurology Association 2022 Annual Conference, 19–21 January 2022; Virtual.
- 29.Servais, L. et al. Effectiveness and safety of onasemnogene abeparvovec in older patients with spinal muscular atrophy (SMA): real-world outcomes from the RESTORE Registry. Presented at: British Paediatric Neurology Association 2022 Annual Conference, 19–21 January 2022; Virtual.
- 30.De Vivo DC, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul. Disord. 2019;29:842–856. doi: 10.1016/j.nmd.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO Multicentre Growth Reference Study Group. WHO Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatr. Suppl. 2006;450:86–95. doi: 10.1111/j.1651-2227.2006.tb02379.x. [DOI] [PubMed] [Google Scholar]
- 32.Strauss, K. A. et al. Onasemnogene abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy: The Phase III SPR1NT trial. Nat. Med.10.1038/s41591-022-01866-4 (2022). [DOI] [PMC free article] [PubMed]
- 33.Bayley, N. Bayley Scales of Infant and Toddler Development: Administration Manual 3rd edn (Pearson PsychCorp, 2006).
- 34.Mendell JR, et al. Current clinical applications of in vivo gene therapy with AAVs. Mol. Ther. 2021;29:464–488. doi: 10.1016/j.ymthe.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendell JR, et al. Five-year extension results of the phase 1 START trial of onasemnogene abeparvovec in spinal muscular atrophy. JAMA Neurol. 2021;78:834–841. doi: 10.1001/jamaneurol.2021.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Day JW, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20:284–293. doi: 10.1016/S1474-4422(21)00001-6. [DOI] [PubMed] [Google Scholar]
- 37.Mercuri E, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20:832–841. doi: 10.1016/S1474-4422(21)00251-9. [DOI] [PubMed] [Google Scholar]
- 38.Finkel RS, et al. RAINBOWFISH: a study of risdiplam in newborns with presymptomatic spinal muscular atrophy (SMA) Neurology. 2021;96:4281. [Google Scholar]
- 39.Chand D, et al. Thrombotic microangiopathy following onasemnogene abeparvovec for spinal muscular atrophy: a case series. J. Pediatr. 2021;231:265–268. doi: 10.1016/j.jpeds.2020.11.054. [DOI] [PubMed] [Google Scholar]
- 40.International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 41.Guttmacher AE, Jenkins J, Uhlmann WR. Genomic medicine: who will practice it? A call to open arms. Am. J. Med. Genet. 2001;106:216–222. doi: 10.1002/ajmg.10008. [DOI] [PubMed] [Google Scholar]
- 42.Hall WD, Mathews R, Morley KI. Being more realistic about the public health impact of genomic medicine. PLoS Med. 2010;7:e1000347. doi: 10.1371/journal.pmed.1000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lander ES. Initial impact of the sequencing of the human genome. Nature. 2011;470:187–197. doi: 10.1038/nature09792. [DOI] [PubMed] [Google Scholar]
- 44.Green ED, Guyer MS. National Human Genome Research Institute. Charting a course for genomic medicine from base pairs to bedside. Nature. 2011;470:204–213. doi: 10.1038/nature09764. [DOI] [PubMed] [Google Scholar]
- 45.Prior TW, et al. A positive modifier of spinal muscular atrophy in the SMN2 gene. Am. J. Hum. Genet. 2009;85:408–413. doi: 10.1016/j.ajhg.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SPR1NT Study Group, Supplementary Methods, Supplementary Fig. 1 and Supplementary Tables 1–12
Data Availability Statement
A redacted version of the SPR1NT study protocol and a redacted version of the statistical analysis plan are available at ClinicalTrials.gov (NCT03505099). Novartis is committed to sharing clinical trial data with external researchers and has been doing so voluntarily since 2014.
Novartis is committed to sharing, upon requests from qualified external researchers and subsequent approval by an independent review panel based upon scientific merit, anonymized patient-level and study-level clinical trial data and redacted clinical study reports for medicines and indications approved in the United States and Europe after the respective study is accepted for publication. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.