Abstract
Atrial fibrillation (AF), the most prevalent cardiac arrhythmia encountered in clinical practice, is linked with substantial morbidity and mortality due to accompanying risk of stroke and thromboembolism. Patients with AF are at a five-fold higher risk of suffering from a stroke. Anticoagulation therapy, with either vitamin K antagonists or novel oral anticoagulants (NOACs), is a standard approach to reduce the risk. Consultant physicians (CPs) in India are the primary point of contact for the majority of patients before they approach a specialist. The CPs may face challenges in screening and diagnosing AF patients. The apprehensions associated with managing AF patients with anticoagulants, further add to the challenges of a CP. This review aimed to identify the key decision points for the CPs to diagnose AF and initiate anticoagulation in patients with non-valvular AF (NVAF) and bring to the table a simplified recommendation supported by expert opinion and guidelines for stroke prevention in NVAF patients.
Keywords: frail elderly, renal insufficiency, hemorrhage, stroke, atrial fibrillation
Introduction and background
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia encountered in clinical practice [1]. The estimated global prevalence of AF in 2010 was reported to be nearly 33.5 million individuals [2]. The World Heart Federation (WHF) in its 2020 update of the "Roadmap" initiative reported the prevalence of AF in India to be between 0.1% and 0.5% in the general population for the period of 2001-2010. The prevalence for the general population between 2011 and 2020 was estimated to be 1.6% and 5.6%, respectively, in the population aged ≥75 years [3]. The Indian Heart Rhythm Society (IHRS) AF registry revealed that Indian patients with AF are more than a decade younger than those in the Western world. The underlying cause in Indian patients was determined to be rheumatic valvular heart disease (RHD), followed by hypertension, diabetes, and coronary artery disease [4].
AF is associated with substantial mortality and morbidity from stroke and thromboembolism [5]. AF patients pose a five-fold higher risk of stroke [6]. The mortality associated with ischemic stroke can be nearly twice in patients with AF as compared to those without AF [7]. Traditionally, vitamin K antagonists (VKA), especially warfarin were the preferred anticoagulants. But due to their many limitations including narrow therapeutic window, need for regular monitoring, slow onset of action, and numerous drug and food interactions, novel oral anticoagulants (NOACs) have evolved as the preferred agents [8]. The time in therapeutic range (TTR) is a quality measure commonly used for the assessment of anticoagulation therapy with warfarin and it co-relates with improved patient outcomes for patients with AF treated with warfarin [9]. Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) study and Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) study, which compared apixaban and dabigatran, respectively, with warfarin for stroke prevention in AF (SPAF), had the lowest TTR recorded from India [10].
Consultant physicians (CPs) are the primary point of contact for patients. In the opinion of the experts, the major issues faced in India are the lack of adequate training for screening and diagnosis of AF. Additionally, due to apprehensions related to the risk of bleeding, the CPs are hesitant to initiate oral anticoagulation. Available evidence suggests that patients with AF, stroke, or transient ischemic attack were found to be undertreated with oral anticoagulation therapy in the majority of studies [5]. The authors in this review aim to identify the main points for the CPs to identify AF and initiate anticoagulation in patients with non-valvular AF (NVAF) and bring to the table a simplified recommendation supported by expert opinion and guidelines for stroke prevention in NVAF patients.
All the experts who were invited for the preceding advisory board are of ~20 to 30 years of clinical experience in the management of NVAF and are very well aware of the evolving landscape of anticoagulants. Their expert opinion was weighed along with the evidence from the literature including the recommendations from the guidelines to propose the protocol for the management of stroke prevention in AF suitable for the primary care setting in India.
Review
Recent guidelines
Various clinical practice guidelines have provided recommendations for the prevention of stroke in patients with AF. The recommendations made by the 2019 American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society (AHA/ACC/HRS Focused Update of the latest AHA/ACC/HRS Guideline) [11], the 2020 European Society of Cardiology (ESC) [12], and the 2021 European Heart Rhythm Association Practical Guide [13] are summarized in Table 1.
Table 1. Summary of guidelines.
AF: atrial fibrillation; CHA2DS2-VASc: congestive heart failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65-74, and sex (female); INR: international normalized ratio; NOAC: novel oral anticoagulants; OAC: oral anticoagulation; VKA: vitamin K antagonists
The table is adapted from the 2019 American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society (AHA/ACC/HRS Focused Update of the latest AHA/ACC/HRS Guideline) [11], the 2020 European Society of Cardiology (ESC) [12], and the 2021 European Heart Rhythm Association Practical Guide [13].
| Guideline recommendations | |
| Anticoagulants are recommended | In AF patients, oral anticoagulants are recommended when a CHA2DS2-VASc score of ≥2 in men or ≥3 in women. (Level of evidence: I; strength of recommendation: A) |
| NOACs are preferred over warfarin in NOAC-eligible patients with AF (exception: patients with “moderate-to-severe mitral stenosis or a mechanical heart valve”) | |
| Pointers to be noted before initiating anticoagulants | Choice of OACs should be based on thromboembolism risk, irrespective of the AF pattern |
| Evaluate renal function and hepatic function before starting NOAC therapy and should be reevaluated at least once a year | |
| Reevaluate the need for and choice of anticoagulant therapy periodically | |
| Reassess stroke and bleeding risks | |
| Use of antiplatelet monotherapy or aspirin-clopidogrel combination is discouraged | |
| Contraindications of NOACs | Patients with mechanical prosthetic valve and moderate to severe mitral stenosis |
| Patients with bioprosthetic valve can be anticoagulated with NOACs only if it is degenerative mitral regurgitation or if the valve is in the aortic position | |
| Patients with hypertrophic cardiomyopathy can be considered for NOAC treatment but the data is limited | |
Protocol for consulting physicians
Screening
In 20-45% of stroke cases, the underlying AF is detected at the time of stroke. The reason for under-diagnosis of AF can be because of a significant proportion of cryptogenic strokes attributed to undetected AF. AF remains asymptomatic in almost one-third of the cases and most of the symptomatic patients have atypical symptoms [14]. Paroxysmal AF can progress to sustained forms of AF within the first year and the progression rate ranges from 8.6% to 15% [15]. Unattended sustained AF has a high risk of stroke and heart failure (HF). Hence, for many patients, the steps required to prevent sustained AF should begin early. Stroke is preventable with a 66% reduction in risk when appropriate anticoagulation therapy is given to eligible patients with AF. Hence screening of patients for AF is vital to prevent stroke in patients [14].
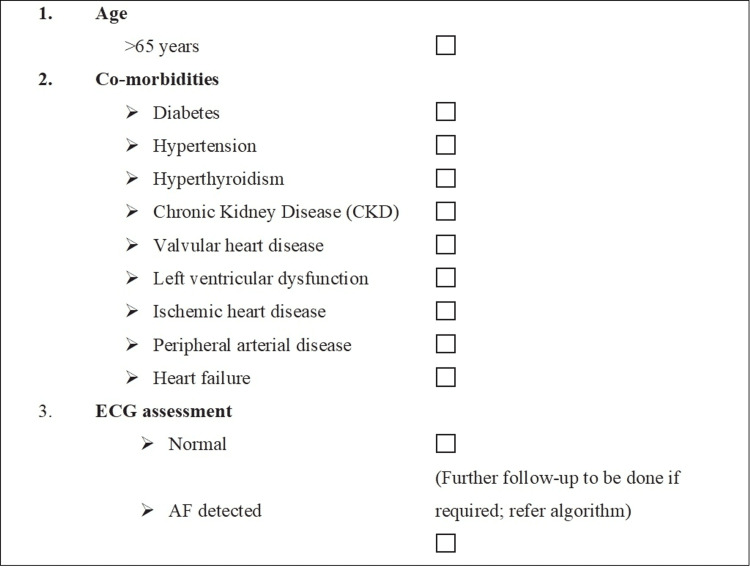
The conventional risk factors for AF include coronary heart disease, hypertension, heart failure, left ventricular diastolic dysfunction, diabetes, hyperthyroidism, obesity, and valvular heart disease [16]. The experts in the advisory board opined that CPs should assess the high-risk patients for AF and can follow a checklist to identify patients (Figure 1). The panelists suggested that if the electrocardiogram (ECG) is normal, the patients should be followed up for their primary condition for which they visited. The CPs can consider doing echocardiography if the patient has cardiac co-morbidities.
Figure 1. Panel recommendations for screening of high-risk patients for AF.
AF: atrial fibrillation; CKD: chronic kidney disease; ECG: electrocardiogram
Diagnosis
AF is suspected when an irregular pulse is observed in a patient and must be confirmed using a 12-lead ECG [17]. The diagnosis of AF requires documentation of ECG recorded cardiac rhythm showing the typical pattern of AF where one episode lasts for at least 30 seconds. When clinical suspicion of atrial fibrillation persists despite normal ECG, a Holter monitor (24-hour recording) or event monitor (seven to 30-day recording) may be warranted [17]. There are five types of AF identified based on the presentation, duration, and spontaneous termination of AF episodes (Table 2) [12]. The anticoagulation strategy however does not depend upon the type of AF.
Table 2. Types of atrial fibrillation.
AF: atrial fibrillation
The table is adapted from Hindricks et al., (2021; ESC 2020) [12].
| Sr. no. | Type of AF | Presentation |
| 1. | First diagnosed AF | Previous diagnosis not established regardless of AF duration or severity |
| 2. | Paroxysmal AF | AF episodes that terminate within 7 days (spontaneously or with cardioversion) |
| 3. | Persistent AF | AF episode lasting for more than 7 days. This definition is inclusive of episodes terminated by cardioversion (with drugs or by direct current cardioversion) after 7 days or more |
| 4. | Long-standing persistent AF | AF which continues for >12 months before rhythm control is implemented |
| 5. | Permanent AF | AF accepted by both: the patient and the physician; rhythm control not implemented. |
Management
The congestive heart failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65-74, and sex (female) (CHA2DS2-VASc) score is used to identify the risk of thromboembolism in AF patients and hypertension, abnormal renal and liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol (HAS-BLED) score assesses the risk of bleeding in anticoagulation (Table 3) [18,19].
Table 3. Calculation of CHA2DS2-VASc score and HAS-BLED score .
*HAS-BLED score of ≥3 warrants regular clinical review and follow-up.
CHA2DS2-VASc: congestive heart failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65-74, and sex (female); HAS-BLED: hypertension, abnormal renal and liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol; PAD: peripheral arterial disease; MI: myocardial infarction; TIA: transient ischemic attack; TE: transesophageal echocardiography; LV: left ventricular; BP: blood pressure; INR: international normalized ratio: NSAIDs: non-steroidal antiinflammatory drugs
The table is adapted from CHA2DS2-VASc 1 [18] and HAS-BLED Tool (2012) [19].
| Stroke risk factors | Score |
| CHA2DS2-VASc score | |
| Congestive heart failure/LV dysfunction | 1 |
| Hypertension | 1 |
| Aged ≥ 75 years | 2 |
| Diabetes mellitus | 1 |
| Stroke/ TIA/ TE | 2 |
| Vascular disease (prior MI, PAD, or aortic plaque) | 1 |
| Aged 65-75 years | 1 |
| Sex category (i.e., female gender) | 1 |
| Maximum score | 9 |
| HAS-BLED score * | |
| Hypertension, i.e., uncontrolled BP | 1 |
| Abnormal renal/liver function | 1 or 2 |
| Stroke | 1 |
| Bleeding tendency or predisposition | 1 |
| Labile INR | 1 |
| Age (e.g., > 65) | 1 |
| Drugs (e.g., concomitant aspirin or NSAIDs) or alcohol | 1 |
| Maximum score | 9 |
| HAS-BLED score of ≥3 warrants for regular clinical review and follow-up | |
Evolution of treatment
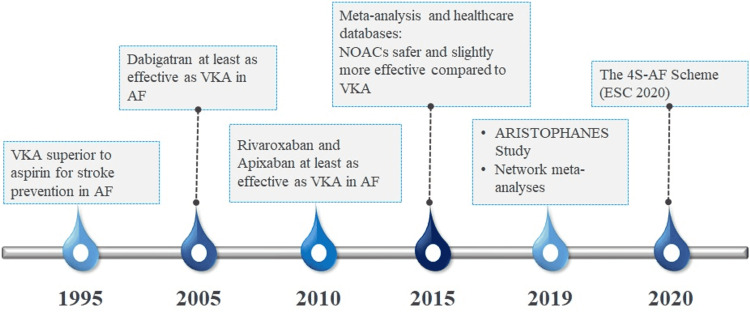
The management strategies for stroke prevention have evolved substantially in the past three decades. Novel drugs have been developed and robust clinical studies and meta-analyses have supported the use of anticoagulants for stroke prevention. Some of the landmark events in the evolution of stroke prevention management have been presented in Figure 2 [12,20].
Figure 2. Evolution of treatment for stroke prevention in AF.
VAK: vitamin K antagonists; NOACs: novel oral anticoagulants; 4S-AF: stroke risk, symptoms, severity of AF burden, and substrate; AF: atrial fibrillation; ESC: European Society of Cardiology; ARISTOPHANES: Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients
Issues with warfarin
Warfarin, a vitamin K antagonist is the most widely used anticoagulant. However, in the recent past, its use for the same has decreased due to many challenges including unreliable INR values, unpredictable outcomes, and need for continuous monitoring and availability of NOACs [21]. Warfarin loading doses may result in a hypercoagulable state and potential clot formation because of significant reductions in protein C and protein S levels (Table 4) [22].
Table 4. Advantages of NOACs over VKA in the management of stroke prevention in patients with AF.
The table mentions the limitations of VKA in stroke prevention in AF patients and its implications on clinical practice which are addressed by the NOACs [23,24].
*As per the expert opinion.
AF: atrial fibrillation; NOAC: novel oral anticoagulant; VKA: vitamin K antagonists
| Limitations of VKA | Implications on clinical practice* | Advantages of NOACs |
| Risk of bleeding complications, including intracranial hemorrhage | Increased hospital cost, increased hospitalization rate, increased mortality | Lower incidence of major bleeding |
| Routine monitoring required | Increased laboratory cost, increased hospital visits | Convenience of use, no need for laboratory monitoring |
| Dose adjustments frequently needed | Increased hospital visits | NOACs are administered in fixed doses, except when a patient has a disorder of the liver or kidney |
| Slow onset of action | Increased risk of stroke | Rapid onset and offset of action, a short half-life |
| Narrow therapeutic window | Increased risk of stroke and bleeding | Wide therapeutic window |
| Dietary restrictions Numerous drug interaction | Reduced adherence, increased risk of stroke and bleeding | Fewer drug and food interactions |
| Variability in patient response | Difficulty in standardized approach and frequent follow-ups | Less variability |
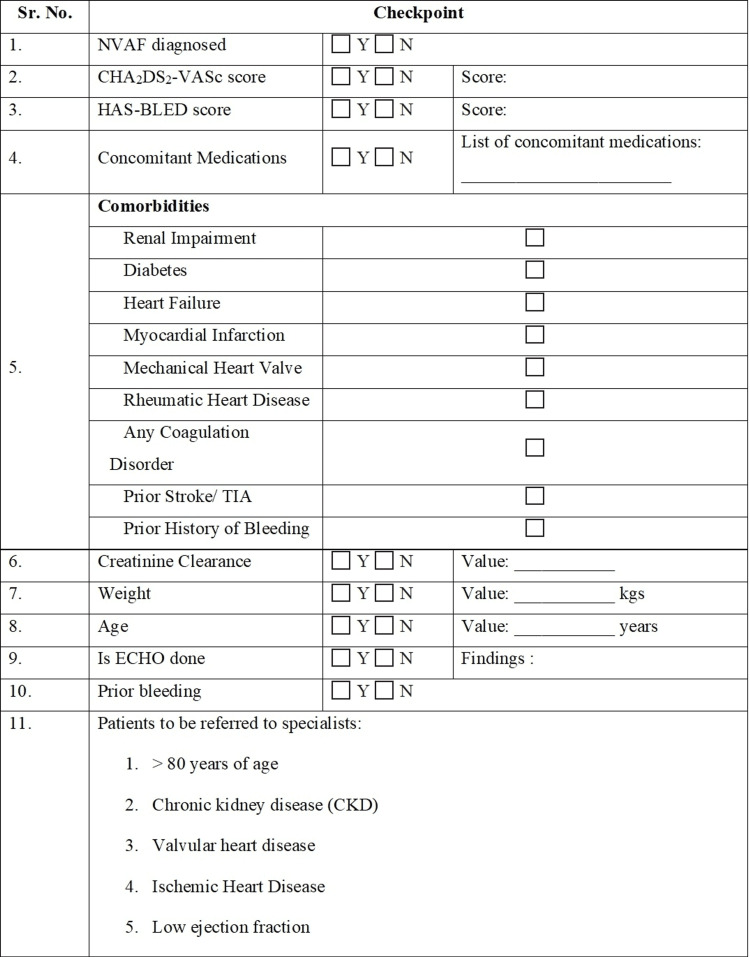
In a meta-analysis by Ruff et al., the safety and efficacy of NOACs were compared to warfarin in patients with atrial fibrillation. NOACs were associated with a reduced composite of stroke or systemic embolic events by 19% as compared with warfarin. There was also a reduction in major bleeding by 14% with NOACs [25]. In the Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients (ARISTOPHANES) study, warfarin was reported to have a higher stroke/systemic embolism rate as compared to apixaban (1.92 vs. 1.33 per 100 person-years), dabigatran (1.74 vs. 1.44 per 100 person-years), and rivaroxaban (1.90 vs. 1.51 per 100 person-years). Apixaban (hazard ratio {HR}, 0.60; 95% confidence interval {CI}, 0.56-0.63) and dabigatran (HR, 0.71; 95% CI, 0.65-0.78) were associated with lower rates of major bleeding as compared to warfarin, while rivaroxaban (HR, 1.06; 95% CI, 1.02-1.10) had a higher rate of major bleeding compared with warfarin [26]. Apixaban was associated with fewer major bleeds in comparison to all other NOACs. Figure 3 depicts the checklist for CPs designed based on the expert opinion of the advisors.
Figure 3. Checklist for CPs before initiating OAC therapy or referring to a specialist.
CKD: chronic kidney disease; ECHO: echocardiogram; MI: myocardial infarction; NVAF: non-valvular atrial fibrillation; OAC: oral anticoagulant; PCI: percutaneous coronary intervention; TIA: transient ischemic attack
Tailor-Made Therapy
Elderly and very elderly: Elderly patients (age above 75 years) [27] are more prone to thromboembolic events as well as have an increased risk of bleeding [28]. The factors apart from age that are responsible for venous thromboembolism (VTE) include the presence of comorbid conditions, increased risk of falls, renal insufficiency, potential drug interactions, and dementia [27]. The Fit fOR The Aged (FORTA) list is a drug classification combining positive and negative labeling of drugs frequently prescribed to elderly patients [29]. As per the consensus, apixaban has been rated as FORTA “A” (i.e., A-bsolutely = indispensable drug, clear-cut benefit in terms of efficacy/safety ratio proven in elderly patients for a given indication), while warfarin, dabigatran, and rivaroxaban are given FORTA B label (i.e., B-eneficial = drugs with proven or obvious efficacy in the elderly, but limited extent of effect or safety concerns) [30].
Renal impairment
Patients with chronic kidney disease (CKD) are at two to three times higher risk of developing AF as compared to the general population. The risk of thromboembolism due to AF in CKD patients is independently higher as compared to those without CKD [31-33].
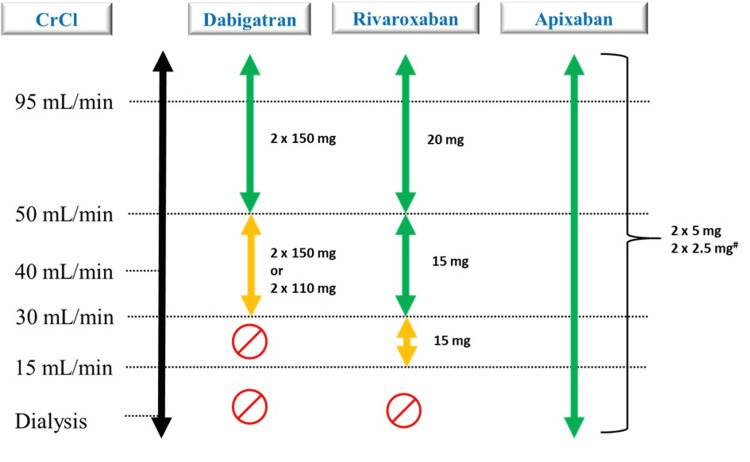
The NOACs are eliminated renally to varying extent and hence dose adjustment is warranted to manage the risk of bleeding. The calculation of dose reduction is critical as either efficacy or safety is affected with inappropriate doses [34]. Warfarin has been reported to cause renal damage in patients with CKD and is also associated with the progression of renal disease [35]. Apixaban is approved for patients with creatinine clearance <15 mL/min including dialysis for all indications in India [36]. The dosing of NOACs based on stages of CKD has been depicted in Figure 4 [36-40].
Figure 4. Dose adjustment for CKD patients.
CrCl: creatinine clearance; EHRA: European Heart Rhythm Association; CKD: chronic kidney disease
The figure is adapted from Shroff (2017) [37], Heine et al. (2018) [38], Aursulesei and Costache (2019) [39], Xarelto [40], Eliquis (apixaban) (2012) [36]; Steffel et al. (2021; EHRA Practical Guide) [13].
Impaired hepatic function
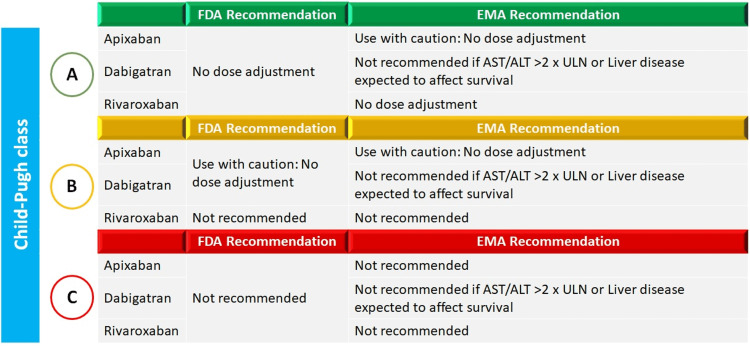
NOACs undergo significant metabolism in the liver. Hepatic impairment thus may lead to an increase in drug levels and decrease in coagulation factors and may result in consequent bleeding. Some NOACs are dependent on cytochrome P450 enzymes for metabolism and in case of hepatic impairment, the activity of these enzymes may be altered. Hence, dose adjustment in patients with hepatic impairment is warranted (Figure 5) [41].
Figure 5. FDA and EMA recommendations for use of NOACs in patients with hepatic impairment .
ALT: alanine transaminase; AST: aspartate transaminase; EMA: European Medical Agency; FDA: Food and Drug Administration; NOACs: novel oral anticoagulants; ULN: upper limit of normal
The figure is adapted from Qamar et al. (2018) [41].
Patients with increased risk of GI bleeding
Dabigatran and rivaroxaban are associated with more than 50% and more than a two-fold increased risk of gastrointestinal (GI) bleeding, respectively, when compared to warfarin [42]. Apixaban has been shown to have comparable major GI bleeds to warfarin. For patients at high risk of GI bleeding, ESC 2016 recommends the use of VKA or NOAC other than dabigatran 150 mg or rivaroxaban 20 mg (class IIa, level B) [20].
Extreme low body weight
The efficacy of NOACs is directly correlated to their plasma concentrations. Since the distribution volume is linked to body weight, extreme body weight can affect their efficacy or safety. Recommendations for dose adjustment based on body weight are represented in Table 5 [43].
Table 5. Dose adjustment for NOACs based on body weight .
NOACs: novel oral anticoagulants
The table is adapted from De Caterina and Lip (2017) [43].
| NOAC | Lower body weight allowed | Upper body weight allowed | Recommended dose adjustment |
| Dabigatran etexilate | 50 kg | 110 kg | No dose adjustment necessary |
| Rivaroxaban | None | None | No dose adjustment necessary |
| Apixaban | None | None | No dose adjustments required unless ABC criteria are met. |
Minor procedures
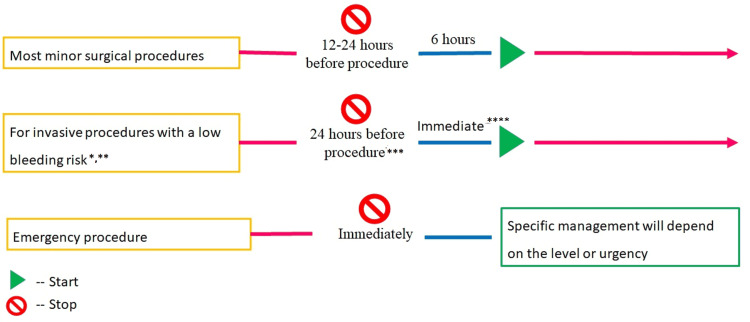
The EHRA has put together recommendations for temporarily discontinuing anticoagulants for patients undergoing minor invasive procedures (Figure 6) [13,44]. Due to the increased risk of bleeding, anticoagulant therapy may require temporary cessation in some patients. The conditions and timings for cessation and re-initiating anticoagulants depend on the type of procedure and patient characteristics.
Figure 6. Use of anticoagulants in case of minor procedures .
NOACs can be resumed six to eight hours after the end of the intervention.
*Including cardiac device implantations.
**A graded interruption should be considered for patients on dabigatran and a CrCl <80 mL/min.
***Twenty-four hours before in patients with normal kidney function.
****With complete hemostasis.
NOACs: novel oral anticoagulants
The figure is adapted from Steffel et al. (2021; EHRA Practical Guide) [13].
The decision of when to stop the NOACs therapy before the invasive procedure is dependent on renal function, type of surgery, and the risk of bleeding. In case a minor surgical intervention which is associated with minimal bleeding risk and/or adequate local hemostasis can be practiced, NOAC therapy can be restarted after six hours. Recommendations regarding restarting NOACs after invasive procedures with low-risk or high-risk bleeding are presented in Table 6 [45].
Table 6. Last intake of NOACs before an invasive procedure.
CrCl: creatinine clearance; NOACs: novel oral anticoagulants
The table is adapted from Chiang et al. (2017) [45].
| CrCl | Dabigatran | Apixaban, rivaroxaban | ||
| Low risk | High risk | Low risk | High risk | |
| ≥80 mL/min | ≥24 h | ≥48 h | ≥24 h | ≥48 h |
| 50−79 mL/min | ≥24 h | ≥48 h | ≥24 h | ≥48 h |
| 30−49 mL/min | ≥48 h | ≥96 h | ≥24 h | ≥48 h |
Caution should be exercised while using NOACs, especially in patients with co-morbidities. The recommendations for the tailor-made for individual patients with NVAF are summarized in Table 7 [27,36,46].
Table 7. Selection of NOACs for stroke prevention.
AF: atrial fibrillation; CKD: chronic kidney disease; GI: gastrointestinal; NOAC: novel oral anticoagulant; NSAID: non-steroidal antiinflammatory drug
The table is adapted from Kundu et al. (2016) [27], Eliquis (apixaban) (2012) [36], and Diener et al. (2017) [46].
| Recommendation | |
| Please consider | Prior to beginning NOAC therapy, patient’s cognitive function, level of dependence, mobility, possible issues with drug compliance, and risk of falls must be assessed |
| Avoid multiple medications wherever possible | |
| If a patient is taking non-steroidal antiinflammatory drugs (NSAIDs) switch to another analgesic | |
| Renal function should be checked before initiating NOAC therapy and thereafter at least once every 8-12 months | |
| Dose adjustments for specific NOACs should be made depending on the patient’s age, body weight, and renal function | |
| Patients with high risk of gastrointestinal (GI) bleeding | First choice: for patients with a high risk of gastrointestinal bleeding, apixaban 5 mg twice daily or dabigatran 110 mg twice daily may be used; second choice: dabigatran 150 mg twice daily, or rivaroxaban 20 mg once daily |
| Patients with renal impairment | First choice: patients with AF and stage III CKD (creatinine clearance 30–49 mL/min) may be treated with apixaban 5 mg twice daily (apixaban 2.5 mg twice a day if ≥1 additional criteria: age ≥80 years, body weight ≤60 kg, serum creatinine ≥ 1.5 mg/dL (133 mmol/L are present), rivaroxaban 15 mg daily; second choice: dabigatran 110 mg twice daily; not recommended: dabigatran 150 mg twice daily, rivaroxaban 20 mg once daily |
| Elderly patients | First choice: in patients older than 75 years, we suggest apixaban 5 mg twice daily (2.5 mg if ≥2 of the following: age ≥80 years, body weight ≤60 kg, or creatinine ≥1.5 mg/dL {133 mmol/L}); second choice: dabigatran 110 mg twice daily, or rivaroxaban 20 mg once daily |
| Previous history of stroke | First choice: NOACs are preferred over warfarin for secondary stroke prevention in patients with AF |
Management of bleeding
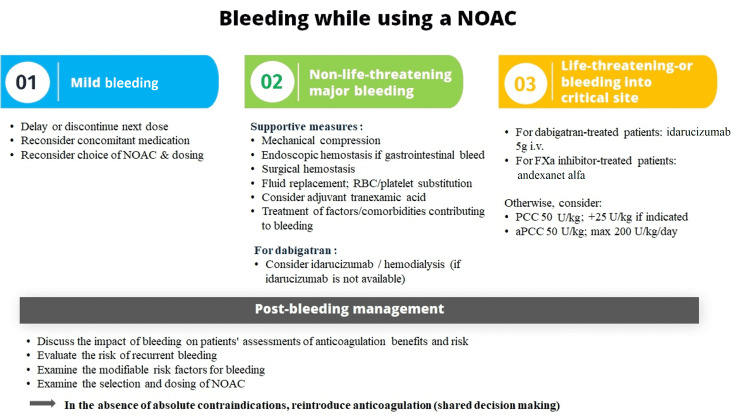
The panelists of the advisory board also opined that CPs who are treating AF patients with oral anticoagulants (OACs) in case of a bleeding event should immediately stop the anticoagulant, look for potential drug-drug interactions, and refer the patients to a specialist.
The factor concentrates recommended for the management of bleeding due to NOACs include prothrombin complex concentrate (PCC) and activated PCC (aPCC) [13]. For the reversal of direct NOACs, the DCGI has approved one specific antidote: idarucizumab for dabigatran (Figure 7).
Figure 7. Management of bleeding in patients taking NOAC .
aPCC: activated prothrombin complex concentrates; NOAC: novel oral anticoagulant; PCC: prothrombin complex concentrates; RBC: red blood cell; WBC: white blood cell
The figure is adapted from Steffel et al. (2021; EHRA Practical Guide) [13].
Evaluation of renal function should be considered annually or on a more frequent basis depending on medical history or age as per following EHRA recommendations (Table 8) [13].
Table 8. Follow-up of AF patients on NOACs.
AF: atrial fibrillation; NOAC: novel oral anticoagulant
The table is adapted from Steffel et al. (2021; EHRA Practical Guide 2021) [13].
| Patient type | Interval |
| Patients other than those specified below | Yearly |
| ≥ 75 years (especially if on dabigatran) or frail | 4-monthly |
| If renal function CrCl ≤60mL/min: recheck interval = CrCl/10 months | x-monthly |
| If intercurrent condition may impact renal or hepatic function | If needed |
For patients with prior unprovoked bleeding, or experienced warfarin-associated bleeding, or are at a high risk of bleeding, the use of apixaban or dabigatran 110 mg has been recommended since less major bleeding events are associated with them as compared to warfarin [47].
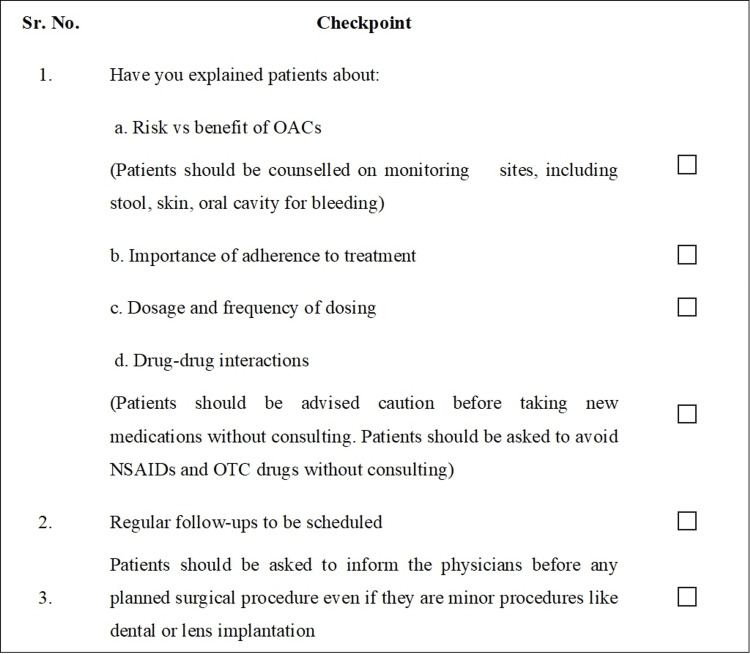
Counseling
Patients starting on any OACs should be counseled and properly educated on compliance and precautions related to the therapy [48]. Patient counseling helps in ensuring adherence to the dosing schedule and regular follow-up. The panel on the advisory board discussed the importance of counseling and reached a consensus to develop a checklist that CPs can follow as counseling checkpoints (Figure 8).
Figure 8. Checklist for patient counseling.
NSAID: non-steroidal antiinflammatory drug; OAC: oral anticoagulant; OTC: over-the-counter
Switching
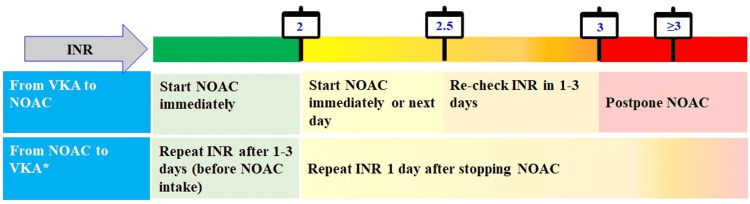
Switching from one oral anticoagulant therapy to another is decided by the treating physician and is dependent on the patient’s eligibility [49]. The major reason for switching from a VKA to a NOAC was occurrence of stroke. Occurrence of myocardial infarction or gastrointestinal bleeding after NOAC initiation significantly increased the chances of switching to VKA. The likelihood of switching from one NOAC to another increased in case of stroke, myocardial infarction, and gastrointestinal bleeding [50]. Ensuring continuous anticoagulant therapy with minimal bleeding risk is paramount while switching between therapies (Figure 9) [13,44].
Figure 9. Switching between therapies .
*Continue NOAC if INR <2 (half dose if on edoxaban), start VKA (loading dose usually used for phenprocoumon). Continue intensive INR sampling for one month, goal: ≥3 consecutive INR values between 2.0 and 3.0. Edoxaban is not approved in India.
INR: international normalized ratio; NOAC: novel oral anticoagulant; VKA: vitamin K antagonists
The figure is adapted from Steffel et al. (2021; EHRA Practical Guide) [13].
Algorithm
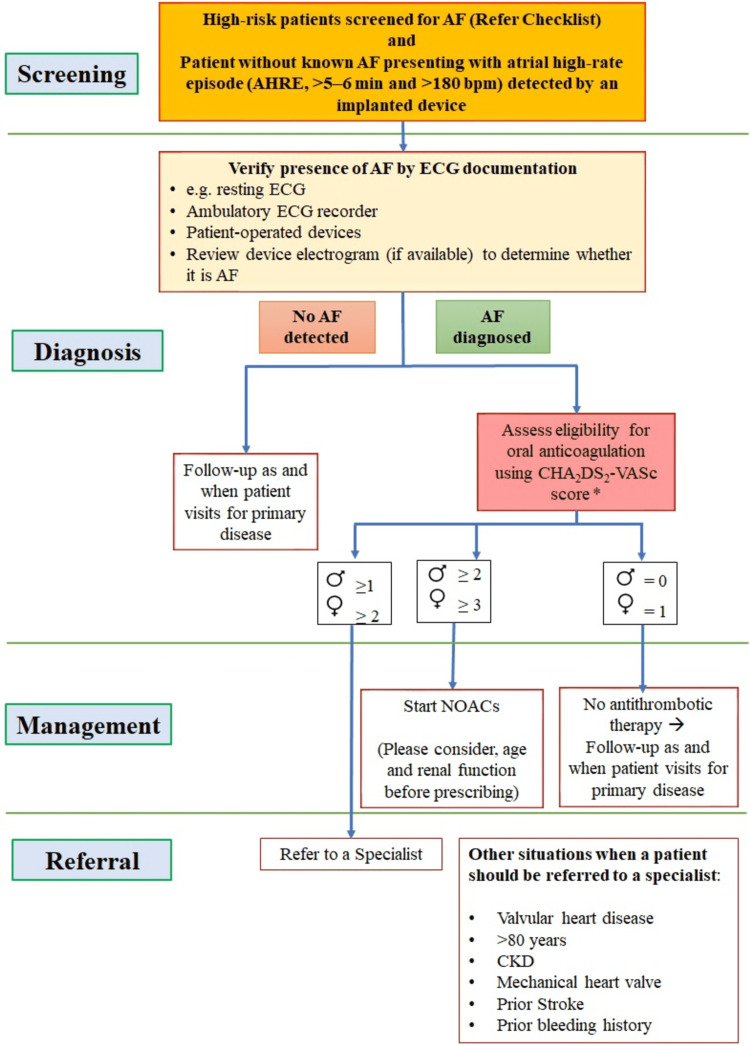
The experts who participated in the advisory board meeting suggested the development of a simplified algorithm that CPs can prevent stroke in AF patients. The algorithm provides a comprehensive summary of how to screen, diagnose, manage, and refer patients. The algorithm has been developed based on treatment guidelines, scientific literature, and clinical experience of the experts (Figure 10).
Figure 10. Algorithm for stroke prevention in AF patients.
*Additionally assess HAS-BLED score
AF: atrial fibrillation; AHRE: atrial high-rate episodes; bpm: beats per minute; CHA2DS2-VASc: congestive heart failure, hypertension, age ≥ 75 (doubled), diabetes, stroke (doubled), vascular disease, age 65-74, and sex (female); CKD: chronic kidney disease; ECG: electrocardiogram; HAS-BLED: hypertension, abnormal renal and liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol
Conclusions
The CPs being the primary point of contact for patients, need to be equipped for taking quick decisions regarding the need, choice, and dose of oral anticoagulants to reduce the risk of stroke/systemic embolism in patients with NVAF. In the advisory board convened, the experts came up with a simplified protocol based on the current guidelines of stroke prevention completed with simple checklists as ready reckoner for CPs to refer to. The ultimate goal is to empower the CPs to prescribe NOACs to patients with NVAF.
Acknowledgments
The authors would like to acknowledge Dr. Santosh Taur and Dr. Swetha ES, both paid employees of Pfizer, for their editorial support in developing the manuscript. The authors would also like to acknowledge Ms. Vaidehi Wadhwa (Medical Excellence, Emerging Markets, Pfizer) for her support in medical writing (not funded) in preparing this manuscript.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Funding Statement
All the authors declare that they received an honorarium as per the fair market value (FMV) from Pfizer for being the expert advisor in the advisory board before the development of the manuscript. None of the authors intend to unduly influence or promote any product through this publication.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Atrial fibrillation. Zimetbaum P. Ann Intern Med. 2017;166:33–48. doi: 10.7326/AITC201703070. [DOI] [PubMed] [Google Scholar]
- 2.Global rising trends of atrial fibrillation: a major public health concern. Patel NJ, Atti V, Mitrani RD, Viles-Gonzalez JF, Goldberger JJ. Heart. 2018;104:1989–1990. doi: 10.1136/heartjnl-2018-313350. [DOI] [PubMed] [Google Scholar]
- 3.World Heart Federation Roadmap on Atrial Fibrillation - a 2020 update. Freedman B, Hindricks G, Banerjee A, et al. Glob Heart. 2021;16 doi: 10.5334/gh.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical presentation, management, and outcomes in the Indian Heart Rhythm Society-Atrial Fibrillation (IHRS-AF) registry. Vora A, Kapoor A, Nair M, et al. http://dx.doi.org/10.1016/j.ihj.2016.06.006. Indian Heart J. 2017;69:43–47. doi: 10.1016/j.ihj.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Am J Med. 2010;123:638–645. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Camm AJ, Kirchhof P, Lip GY, et al. Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 7.Stroke severity in atrial fibrillation. The Framingham Study. Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, D'Agostino RB. Stroke. 1996;27:1760–1764. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 8.Anticoagulation in atrial fibrillation: is the paradigm really shifting? Deedwania P, Acharya T. J Am Coll Cardiol. 2017;69:786–788. doi: 10.1016/j.jacc.2016.11.062. [DOI] [PubMed] [Google Scholar]
- 9.Time in the therapeutic range for patients taking warfarin in clinical trials: useful, but also misleading, misused, and overinterpreted. Reiffel JA. Circulation. 2017;135:1475–1477. doi: 10.1161/CIRCULATIONAHA.116.026854. [DOI] [PubMed] [Google Scholar]
- 10.Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Oldgren J, Healey JS, Ezekowitz M, et al. Circulation. 2014;129:1568–1576. doi: 10.1161/CIRCULATIONAHA.113.005451. [DOI] [PubMed] [Google Scholar]
- 11.2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. January CT, Wann LS, Calkins H, et al. J Am Coll Cardiol. 2019;74:104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 12.2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Hindricks G, Potpara T, Dagres N, et al. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 13.2021 European Heart Rhythm Association Practical Guide on the use of non-vitamin k antagonist oral anticoagulants in patients with atrial fibrillation. Steffel J, Collins R, Antz M, et al. Europace. 2021;23:1612–1676. doi: 10.1093/europace/euab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Screening to identify unknown atrial fibrillation. A systematic review. Lowres N, Neubeck L, Redfern J, Freedman SB. Thromb Haemost. 2013;110:213–222. doi: 10.1160/TH13-02-0165. [DOI] [PubMed] [Google Scholar]
- 15.Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. Sandhu RK, Conen D, Tedrow UB, et al. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Risk factor management in atrial fibrillation. Brandes A, Smit MD, Nguyen BO, Rienstra M, Van Gelder IC. Arrhythm Electrophysiol Rev. 2018;7:118–127. doi: 10.15420/aer.2018.18.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diagnosis and treatment of atrial fibrillation. Gutierrez C, Blanchard DG. https://pubmed.ncbi.nlm.nih.gov/27637120/ Am Fam Physician. 2016;94:442–452. [PubMed] [Google Scholar]
- 18.CHA2DS2-VASc 1: to anticoagulate or not? [ Jan; 2020 ];https://www.escardio.org/static_file/Escardio/Subspecialty/EHRA/Documents/summit-2015/Lip%20EHRA%20summit%20CHA2DS2VASc%201.pptx%20(Read-Only).pdf
- 19.HAS-BLED tool - what is the real risk of bleeding in anticoagulation? Lip GYH. https://www.acc.org/latest-in-cardiology/articles/2014/07/18/15/13/has-bled-tool-what-is-the-real-risk-of-bleeding-in-anticoagulation 2012
- 20.2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Kirchhof P, Benussi S, Kotecha D, et al. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 21.Non-vitamin K antagonist oral anticoagulants (NOACs): clinical evidence and therapeutic considerations. Saraf K, Morris PD, Garg P, Sheridan P, Storey R. Postgrad Med J. 2014;90:520–528. doi: 10.1136/postgradmedj-2014-132605. [DOI] [PubMed] [Google Scholar]
- 22.Warfarin-induced deep vein thrombosis. Binymin KA, Nasher M, Patel D. Int Med Case Rep J. 2014;7:123–125. doi: 10.2147/IMCRJ.S62100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.New oral Xa and IIa inhibitors: updates on clinical trial results. Haas S. J Thromb Thrombolysis. 2008;25:52–60. doi: 10.1007/s11239-007-0108-7. [DOI] [PubMed] [Google Scholar]
- 24.New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Mekaj YH, Mekaj AY, Duci SB, Miftari EI. Ther Clin Risk Manag. 2015;11:967–977. doi: 10.2147/TCRM.S84210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comparison of the effi cacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Ruff CT, Giugliano RP, Hoffman EB, et al. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 26.Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Lip GY, Keshishian A, Li X, et al. Stroke. 2018;49:2933–2944. doi: 10.1161/STROKEAHA.118.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minimizing the risk of bleeding with NOACs in the elderly. Kundu A, Sardar P, Chatterjee S, Aronow WS, Owan T, Ryan JJ. Drugs Aging. 2016;33:491–500. doi: 10.1007/s40266-016-0376-z. [DOI] [PubMed] [Google Scholar]
- 28.Personalizing oral anticoagulant treatment in patients with atrial fibrillation. Capranzano P, Miccichè E, D'Urso L, Privitera F, Tamburino C. Expert Rev Cardiovasc Ther. 2013;11:959–973. doi: 10.1586/14779072.2013.818819. [DOI] [PubMed] [Google Scholar]
- 29.Consensus validation of the FORTA (Fit fOR The Aged) list: a clinical tool for increasing the appropriateness of pharmacotherapy in the elderly. Kuhn-Thiel AM, Weiß C, Wehling M. Drugs Aging. 2014;31:131–140. doi: 10.1007/s40266-013-0146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.A structured literature review and international consensus validation of FORTA labels of oral anticoagulants for long-term treatment of atrial fibrillation in older patients (OAC-FORTA 2019) Pazan F, Collins R, Gil VM, et al. Drugs Aging. 2020;37:539–548. doi: 10.1007/s40266-020-00771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stroke prophylaxis in patients with atrial fibrillation and end-stage renal disease. van Zyl M, Abdullah HM, Noseworthy PA, Siontis KC. J Clin Med. 2020;9 doi: 10.3390/jcm9010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Go AS, Fang MC, Udaltsova N, Chang Y, Pomernacki NK, Borowsky L, Singer DE. Circulation. 2009;119:1363–1369. doi: 10.1161/CIRCULATIONAHA.108.816082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Alonso A, Lopez FL, Matsushita K, et al. Circulation. 2011;123:2946–2953. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. J Am Coll Cardiol. 2017;69:2779–2790. doi: 10.1016/j.jacc.2017.03.600. [DOI] [PubMed] [Google Scholar]
- 35.Warfarin related acute kidney injury: a case report. Mendonca S, Gupta D, Valsan A, Tewari R. Indian J Nephrol. 2017;27:78–80. doi: 10.4103/0971-4065.177142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eliquis (apixaban) https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202155s006lbl.pdf Pfizer Ltd. 2014
- 37.NOAC dosing in atrial fibrillation and renal dysfunction: what measure are you using? Shroff GR. J Am Coll Cardiol. 2017;70:2733–2734. doi: 10.1016/j.jacc.2017.09.1091. [DOI] [PubMed] [Google Scholar]
- 38.Oral anticoagulation in chronic kidney disease and atrial fibrillation. Heine GH, Brandenburg V, Schirmer SH. Dtsch Arztebl Int. 2018;115:287–294. doi: 10.3238/arztebl.2018.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anticoagulation in chronic kidney disease: from guidelines to clinical practice. Aursulesei V, Costache II. Clin Cardiol. 2019;42:774–782. doi: 10.1002/clc.23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xarelto® - highlights of prescribing information. [ Jan; 2021 ];https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022406s028lbl.pdf 2018
- 41.Oral anticoagulation in patients with liver disease. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. J Am Coll Cardiol. 2018;71:2162–2175. doi: 10.1016/j.jacc.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 42.Risk of gastrointestinal bleeding associated with oral anticoagulants: population based retrospective cohort study. Chang HY, Zhou M, Tang W, Alexander GC, Singh S. BMJ. 2015;350 doi: 10.1136/bmj.h1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The non-vitamin K antagonist oral anticoagulants (NOACs) and extremes of body weight-a systematic literature review. De Caterina R, Lip GY. Clin Res Cardiol. 2017;106:565–572. doi: 10.1007/s00392-017-1102-5. [DOI] [PubMed] [Google Scholar]
- 44.The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Steffel J, Verhamme P, Potpara TS, et al. Eur Heart J. 2018;39:1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 45.2017 consensus of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation. Chiang CE, Okumura K, Zhang S, et al. J Arrhythm. 2017;33:345–367. doi: 10.1016/j.joa.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: part 2. Diener HC, Aisenberg J, Ansell J, et al. Eur Heart J. 2017;38:860–868. doi: 10.1093/eurheartj/ehw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Lip GY, Banerjee A, Boriani G, et al. Chest. 2018;154:1121–1201. doi: 10.1016/j.chest.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 48.My approach to the use of NOACs for stroke prevention in patients with atrial fibrillation. Lip GY. Trends Cardiovasc Med. 2014;24:265–266. doi: 10.1016/j.tcm.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Oral anticoagulants for stroke prevention in atrial fi brillation: current status, special situations, and unmet needs. Verheugt F, Granger C. Lancet. 2015;386:303–310. doi: 10.1016/S0140-6736(15)60245-8. [DOI] [PubMed] [Google Scholar]
- 50.Changes in oral anticoagulation therapy over one year in 51,000 atrial fibrillation patients at risk for stroke: a practice-derived study. Hohnloser SH, Basic E, Nabauer M. Thromb Haemost. 2019;119:882–893. doi: 10.1055/s-0039-1683428. [DOI] [PubMed] [Google Scholar]