The proportion of pregnant persons screened for hepatitis C virus increased after updated universal screening recommendations, yet rates remain low overall.
Abstract
The study evaluates the effect of the 2020 Centers for Disease Control and Prevention and U.S. Preventive Services Task Force recommendations on hepatitis C virus (HCV) screening among pregnant persons nationally and by health insurance type. The study included 5,048,428 pregnant persons aged 15–44 years with either Medicaid or commercial health insurance who had obstetric panel testing performed by Quest Diagnostics, January 2011–June 2021. Antibody screening for HCV infection increased before and accelerated after the updated recommendations in early 2020. Disparities in HCV testing by health insurance status were substantial over the entire study period. Despite substantial progress in the proportion of pregnant persons screened for HCV infection, current testing rates fall short of universal recommendations.
INTRODUCTION
The U.S. Preventive Services Task Force (USPSTF) recommends hepatitis C virus (HCV) screening for adults, including pregnant persons,1 and the Centers for Disease Control and Prevention (CDC) recommends screening all pregnant persons during each pregnancy, except in settings in which the prevalence of HCV infection is less than 0.1%.2 The American College of Obstetricians and Gynecologists issued a Practice Advisory (May 2021) consistent with the CDC recommendations.3 Our objective was to evaluate the effects of the CDC and USPSTF 2020 updated recommendations on HCV screening among pregnant persons nationally and by health insurance type.
METHODS
This was a retrospective cohort study of pregnant individuals aged 15–44 years with Medicaid or commercial health insurance with obstetric panel testing performed by Quest Diagnostics from January 2011 through June 2021. The first HCV antibody test within a rolling 365-day period was used for analysis. The CDC and USPSTF updated recommendations were issued on March 2, 2020, and April 10, 2020, respectively (approximately the end of the second quarter of 2020). We defined the pre-2020 HCV recommendation period as the first quarter of 2011 through the first quarter of 2020 and the post-2020 HCV recommendation period as the second quarter of 2020 through the second quarter of 2021. Models included linear and quadratic terms for time (ie, quarter tested) and a dichotomous variable corresponding to the period after which the HCV 2020 recommendations were in effect (second quarter of 2020 onward). Rate ratios (RR) and 95% CIs were determined. Analyses were conducted in SAS 9.4. This activity was research not involving identifiable human subjects; institutional review board review was not required.
RESULTS
The study included 5,048,428 pregnant persons; 3,765,931 (74.6%) had commercial health insurance, and 1,282,497 (25.4%) had Medicaid health insurance. Overall, 23.3% had an HCV screening test. The percentage with an HCV screening test increased 145% (ie, mean 3.5%/quarter), from 16.6% in quarter 1 of 2011 to 40.6% in quarter 2 of 2021 (Fig. 1).
Fig. 1. Pregnant persons with an obstetric panel test combined with hepatitis C virus antibody screens (percentage): quarter (Q) 1 2011 through Q2 2021.
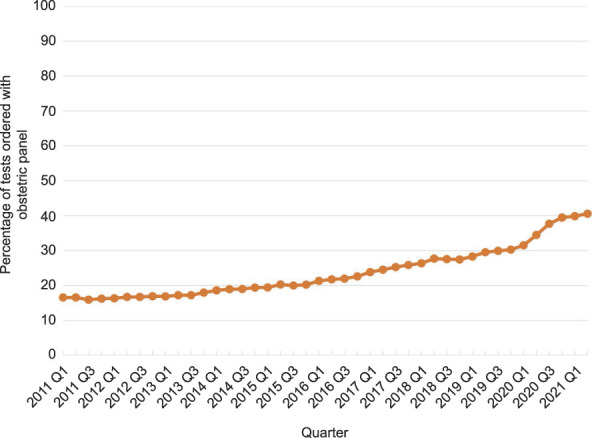
Kaufman. Hepatitis C Virus Screening in Pregnancy. Obstet Gynecol 2022.
During the post-2020 HCV recommendation period, the rate of HCV screening significantly increased relative to the preupdated recommendation period (RR 1.084, 95% CI 1.075–1.093). The percentage of individuals with HCV screening was higher for persons with commercial insurance (25.0%, 941,160 HCV screens/3,524,837 obstetric panels) than for those with Medicaid insurance (18.4%, 236,458 HCV screens/1,219,961 obstetric panels) (P<.001). During the postupdated recommendation period, there was a significant increase in the rate of testing for both individuals with commercial insurance and those with Medicaid insurance (RR 1.088, 95% CI 1.078–1.099; and RR 1.138, 95% CI 1.116–1.161, respectively) (Fig. 2).
Fig. 2. Pregnant persons with an obstetric panel test combined with hepatitis C virus antibody screens (percentage), by commercial insurance (blue line) and Medicaid insurance (brown line): quarter (Q) 1 2011 through Q2 2021.
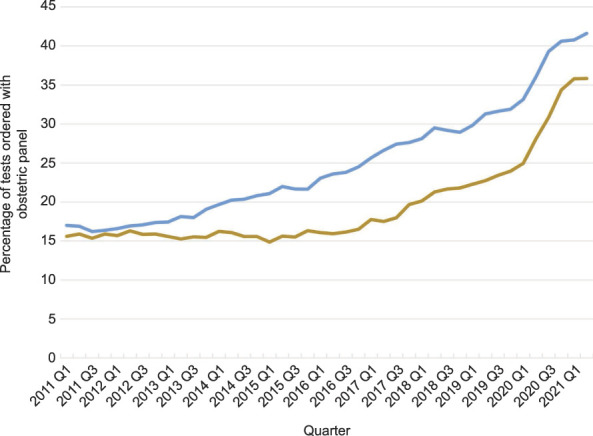
Kaufman. Hepatitis C Virus Screening in Pregnancy. Obstet Gynecol 2022.
DISCUSSION
Antibody screening for HCV infection increased throughout the prior decade in pregnant persons and accelerated after the 2020 CDC and USPSTF updated recommendations.1,2 Pregnant persons with Medicaid insurance had lower HCV screening rates compared with those with commercial insurance. The more recent May 2021 American College of Obstetricians and Gynecologists’ Practice Advisory may further increase adoption of universal screening.3
The study is limited by including only patients tested by Quest Diagnostics. Nevertheless, the substantial number of individuals included (more than 5 million) are from all 50 U.S. states and the District of Columbia and are generally representative of pregnant persons. In closing, despite progress overall and by insurance payer, wider adoption of HCV screening in pregnancy is needed.1–3
Footnotes
Financial Disclosure Harvey W. Kaufman, William A. Meyer, III, and Xiaohua Huang are employees of and own stock of Quest Diagnostics. All other authors are Federal Government employees (Centers for Disease Control and Prevention).
The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the CDC, or the authors' affiliated institutions. The study was performed as a collaborative research study, and no specific funding was provided.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C735.
Figure.
No available caption
REFERENCES
- 1.U.S Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Hepatitis C virus infection in adolescents and adults: screening: US Preventive Services Task Force statement. JAMA 2020;323:970–75. doi: 10.1001/jama.2020.1123 [DOI] [PubMed] [Google Scholar]
- 2.Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC Recommendations for hepatitis C screening among adults—United States, 2020. MMWR Recomm Rep 2020;69:1–17. doi: 10.15585/mmwr.rr6902a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists. Routine hepatitis C virus screening in pregnant individuals. Accessed June 4, 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/05/routine-hepatitis-c-virus-screening-in-pregnant-individuals [Google Scholar]



