Abstract
Existing therapies for epilepsy are primarily symptomatic and target mechanisms of neuronal transmission in order to restore the excitatory-inhibitory imbalance in the brain after seizures.However, approximately one third of individuals with epilepsy have medically refractory epilepsy and do not respond to available treatments. There is a critical need for the development of therapeutics that extend beyond manipulation of excitatory neurotransmission and target pathological changes underlying the cause of the disease. Epilepsy is a multifaceted condition, and it could be that effective treatment involves the targeting of several mechanisms. There is evidence for both dysregulated PI3K/Akt/mTOR (mTOR) signaling and heightened neuroinflammatory processes following seizures in the brain. Signaling via mTOR has been implicated in several epileptogenic processes, including synaptic plasticity mechanisms and changes in ion channel expression following seizures. Inflammatory signaling, such as increased synthesis of cytokines and other immune molecules, has also shown to play a significant role in the development of chronic epilepsy. mTOR pathway activation and immune signaling are known to interact in normal physiological states, as well as influence one another following seizures. Simultaneous inhibition of both processes could be a promising therapeutic avenue to prevent the development of chronic epilepsy by targeting two key pathological mechanisms implicated in epileptogenesis.
Keywords: seizures, epileptogenesis, inflammation, cytokines, mTOR, rapamycin
1. Introduction
Epilepsy is one of the most common neurological disorders, affecting approximately 65 million people in the world (Devinsky et al., 2018). It is characterized by recurrent, spontaneous seizures and is often associated with comorbidities, including cognitive, psychiatric, and other neurobiological consequences, which can significantly affect quality of life. Several causes of epilepsy have been identified, including structural causes (brain injury, stroke, tumors), gene mutations, infections, metabolic disorders, autoimmune conditions, as well as unknown etiologies (Stafstrom and Carmant, 2015). Despite the variety of pharmaceutical therapies available, approximately one third of those with epilepsy do not respond to anti-epileptic drugs (AEDs), and have medically refractory epilepsy (Dalic and Cook, 2016).
Due to seizures resulting in uncontrolled neural excitation in the brain, many AEDs primarily target ion channels or other components of neuronal transmission in order to restore the excitatory/inhibitory (E/I) imbalance (Cook and Bensalem-Owen, 2011). However, many of these pharmaceuticals are symptomatic, and function to reduce seizures but do not influence the underlying cause of the disorder. In addition, while AEDs suppress seizure frequency and ictal discharges, they often do not abolish interictal epileptiform discharges which is of concern (D’Antuono et al., 2010). Abnormal electroencephalogram activity is associated with neurological damage, such as impairments in the blood brain barrier (BBB) and also in cognitive abilities, including declarative memory (Milikovsky et al., 2019; Reed et al., 2019). Recent efforts have been aimed at identifying disease-modifying targets, that go beyond modulation of the E/I imbalance and focus on other mechanistic underpinnings of the disorder. The pathophysiological mechanisms that underlie the development of chronic epilepsy, or epileptogenesis, are for the most part still unknown. Epilepsy is a multifaceted disorder, and the progression of the disease most likely involves a combination of neurological changes in the brain (Curia et al., 2014).
Neuroinflammatory signaling has been demonstrated to play a significant role in the development of epilepsy, both in animal models of acquired and genetic epilepsies, and in clinical populations (Vezzani et al., 2019). The heightened neuroinflammatory responses following seizures can impact hyperexcitability, thus contributing to epileptogenesis (Iori et al., 2016). Therapeutics that target inflammatory mechanisms and attenuate seizure-induced neuroinflammation have shown significant promise in both pre-clinical rodent models and clinical studies. In addition, cell signaling cascades that are disrupted following seizures have been another alternative target for anti-epileptogenic therapies (Hodges and Lugo, 2018; Wong, 2013b). Specifically, phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) (PI3K/Akt/mTOR) signaling activity has been found to be upregulated in brain tissue of individuals with epilepsy, as well as in several genetic and acquired models of epilepsy in vivo (Ostendorf and Wong, 2015). Activation and signaling via the mTOR pathway regulates several seizure-induced processes, including synaptic plasticity mechanisms, cellular proliferation, and changes in ion channel protein expression that could potentially underlie epileptogenesis (Citraro et al., 2016). Inhibition of mTOR signaling has shown to be effective in reducing seizure frequency in patients with Tuberous sclerosis complex (TSC) caused by mutations in TSC1 or TSC2, suggesting that mTOR inhibition could be a promising target for other mTOR-related epilepsies (Cardamone et al., 2014).
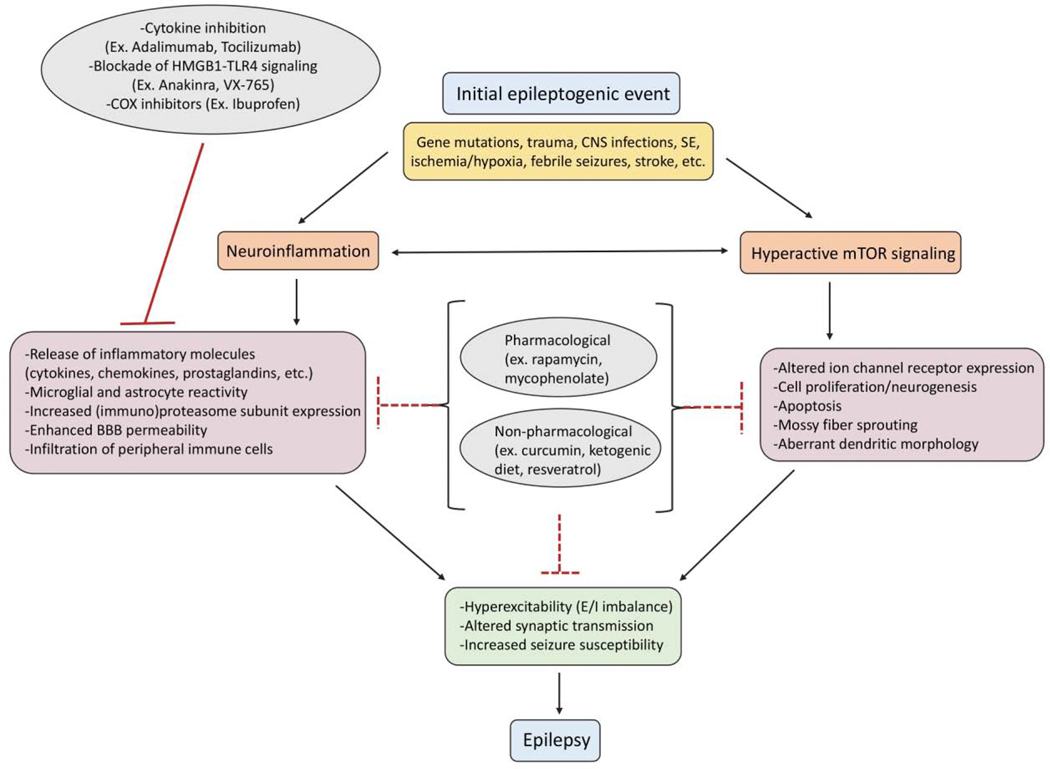
Epilepsy is a complex neurological condition, and effective treatments that target the underlying cause of the disorder potentially involves a combinatorial approach targeting multiple mechanisms (Curia et al., 2014). Pre-clinical studies that use therapeutics aimed at alternative targets beyond manipulation of the E/I imbalance often only focus on changes pertaining to the specific target and mechanism of interest of the therapeutic in use. For example, treatments that inhibit seizure-induced neuroinflammation identify key mechanisms related to the immune system that have been impacted by the treatment but may ignore other molecular changes such as cell signaling pathways that are also affected (Marchi et al., 2011). Signaling via the mTOR pathway and inflammatory processes interact in normal physiological states, and additionally there is evidence that both processes influence each other following seizures (Thomson et al., 2009; Weichhart et al., 2015). This review will highlight the role both mTOR signaling and neuroinflammation play in epilepsy, as well as review evidence of how both of these processes interact in epileptic states in a variety of models (Fig. 1).
Figure 1. Relationship between epilepsy, neuroinflammation, and hyperactive Mtor signaling.
Epileptic events in the brain can be caused by several etiologies, including gene mutations, trauma, CNS infections, SE, ischemia and hypoxia, febrile seizures, and stroke. Seizures are associated with both neuroinflammation and hyperactive mTOR signaling, which can lead to a cascade of downstream pathophysiological effects. These changes can result in hyperexcitability, altered synaptic transmission, and increased seizure susceptibility, which may underlie the development of chronic epilepsy. Many pharmacological (ex. rapamycin, mycophenolate) and non-pharmacological (ex. curcumin, ketogenic diet, resveratrol) compounds have effects on both neuroinflammatory signaling and mTOR pathway activity. These therapies could be beneficial in inhibiting two key processes that potentially underlie epileptogenesis. In addition, there are several anti-inflammatory treatments that have shown efficacy in reducing seizures and associated pathological damage, such as cytokine inhibitors, HMGB1-TLR4 signaling cascade inhibitors, and COX inhibitors. CNS, central nervous system; SE, status epilepticus; mTOR, mammalian target of rapamycin; HMGB1, high- mobility group box protein 1; TLR4, toll-like receptor 4; COX, cyclooxygenase; BBB, blood brain barrier; E/I, excitatory/inhibitory.
2. Role of mTOR signaling in epilepsy
2.1. The mTOR pathway in normal physiological conditions
The mTOR signaling pathway is critical for proper functioning of several physiological processes throughout the lifespan. Activation of the pathway has shown to modulate cell growth, proliferation, protein synthesis, neuronal morphology, cortical development, and immune responses (Laplante and Sabatini, 2012). Beyond these classical roles of mTOR, activation of the pathway in the central nervous system (CNS) can influence neuronal signaling and excitability, such as axonal and dendritic morphology, neurotransmitter expression, synaptic plasticity, and cognition and behavior (Bekinschtein et al., 2007; Jaworski et al., 2005; Tang et al., 2002).
Central to the pathway is the highly conserved serine/threonine protein kinase mTOR, which is part of two larger complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (Jhanwar-Uniyal et al., 2019). The mTORC1 is comprised of a group of binding proteins including the regulatory-associated protein of mTOR (raptor) and stimulates cell growth and proliferation via modulating protein synthesis. It is also highly sensitive to rapamycin, an inhibitor of the mTOR pathway, and by which TOR received its name following identification of the kinase being the target of rapamycin’s antiproliferative effects (Wong, 2013a). In contrast, mTORC2 complexes with a separate group of accessory proteins including rapamycinin-sensitive companion of mTOR (rictor), modulates processes related to cell structure and metabolism, and is relatively insensitive to rapamycin without prolonged exposure of the inhibitor (Sarbassov et al., 2006). Dysregulation of signaling mechanisms via both mTORC1 and mTORC2 have been shown to be involved in the pathogenesis of several disorders, including cancer, diabetes, cardiovascular disease, as well several neurodevelopmental and neurodegenerative disorders (Costa-Mattioli and Monteggia, 2013; Lee et al., 2017; Perluigi et al., 2015; Pópulo et al., 2012; Rosina et al., 2019; Saxton and Sabatini, 2017; Zhu et al., 2019). This review will primarily focus on the impacts of mTORC1 disruption, as this complex has been more heavily implicated in mechanisms underlying epilepsy pathogenesis (Citraro et al., 2016; Ostendorf and Wong, 2015).
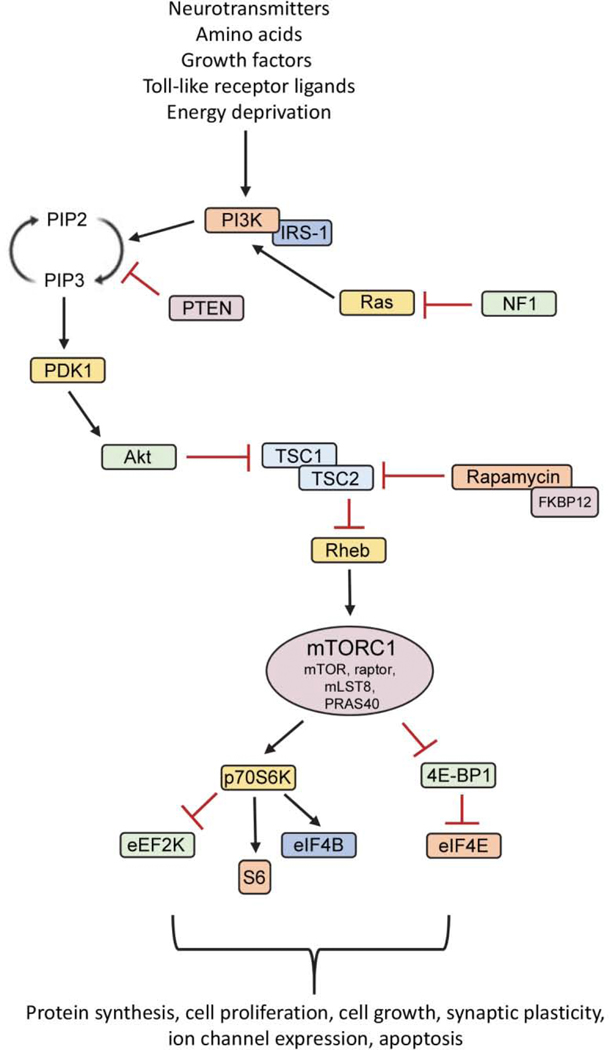
In normal physiological conditions, PI3K will be activated by receptor tyrosine kinases following activation from a variety of extracellular and intracellular stimuli, including amino acids, growth factors, neurotransmitters, intracellular stress signals, and immune molecules (Costa-Mattioli and Monteggia, 2013; Saxton and Sabatini, 2017). PI3K functions to convert phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3). Through the kinase phosphoinositide-dependent kinase-1 (PDK1), phosphorylation and activation of Akt leads to inactivation of the tuberous sclerosis complex (TSC1/TSC2), which functions to antagonize pathway activity. Phosphorylation of the TSC1/TSC2 dimer leads to inhibition of its’ GTPase-activating protein activity, allowing the active form of Rheb to activate mTORC1. mTOR functions in a complex with other regulatory and binding proteins including raptor, mLST8, and inhibitory protein proline-rich Akt substrate of 40 kDa (PRAS40) to create mTORC1. mTORC1 directly phosphorylates the p70S6 kinase (p70S6K), which itself phosphorylates many downstream targets (S6, eEF2K, eIF4B) to promote ribosomal protein recruitment and initiation of translation. In addition, mTOR phosphorylates and inhibits the activity of 4E - binding protein 1 (4E-BP1), causing it to dissociate from eukaryotic initiation factor 4E (eIF4E) and complex with other initiation factors to further stimulate the downstream translation of proteins (Fig. 2).
Figure 2. The PI3K/Akt/mTOR signaling pathway.
The mammalian target of rapamycin (mTOR) is a serine-threonine protein kinase, which forms two complexes, rapamycin-sensitive mTORC1 and relatively rapamycin-insensitive mTORC2 (not shown). The mTOR pathway can be activated by several extracellular (neurotransmitters, amino acids, growth factors, toll-like receptor ligands) and intracellular stimuli (energy deprivation), resulting in activation of a series of downstream effectors. Signaling via the mTOR pathway is initiated with activation of PI3K by receptor tyrosine kinases, which leads to conversion of PIP2 to PIP3. Akt is phosphorylated by PDK1, which leads to phosphorylation and inhibition of the TSC1/TSC2 complex. This allows the active form of GTP-binding protein Rheb to activate mTORC1. mTORC1 is comprised of mTOR, in addition to regulatory protein raptor, mLST8, and inhibitory protein PRAS40. mTORC1 activation leads to phosphorylation of several targets involved in ribosomal biogenesis (p70S6K, S6) and protein translation (eEF2K, eIF4B, 4E-BP, eIF4E). Beyond protein translation, mTOR pathway activity regulates cell proliferation, cell growth, synaptic plasticity, ion channel expression, and apoptosis. The mTOR pathway can be negatively regulated by stimuli, such as PTEN, which antagonizes pathway activity by dephosphorylating PIP3 to PIP2. In addition, rapamycin binds FKBP12 to inhibit mTORC1 activity. PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PDK1, phosphatidylinositol-dependent kinase 1; TSC, tuberous sclerosis complex; mLST8, mammalian lethal with SEC13 protein 8; PRAS40, proline-rich Akt substrate of 40 kDa; eEF2K, eukaryotic elongation factor-2 kinase; eIF4B, eukaryotic translation initiation factor 4B; eIF4E, eukaryotic translation initiation factor 4E; 4E-BP1, (eIF4E)-binding protein 1; PTEN; phosphatase and tensin homolog on chromosome 10; FKBP12, 12-kDa FK506 and rapamycin-binding protein; IRS-1, insulin receptor substrate 1; NF1, neurofibromatosis type I.
2.2. mTOR hyperactivation in epilepsy and impacts of pathway inhibition
Many physiological functions of the mTOR pathway, including synaptic plasticity mechanisms, cellular proliferation, and ion channel expression related to neuronal excitability, could underlie the development of epilepsy in pathological conditions (Wong, 2013a). There is evidence for hyperactive mTOR signaling in both genetic and acquired epilepsies, as demonstrated in animal models of epilepsy and in human tissue samples resected from individuals with epilepsy. For instance, several neurodevelopmental disorders with epileptic phenotypes stem from mutations in components of the mTOR pathway, the most notable of these being TSC1/TSC2 and phosphatase and tensin homolog (PTEN) mutations. A heterozygous mutation in either TSC1 or TSC2 results in the autosomal dominant disorder TSC, characterized by cortical malformations known as tubers and subependymal giant cell astrocytomas (SEGAs) in the brain (Jülich and Sahin, 2014). The respective protein products of TSC1 (hamartin) and TSC2 (tuberin) form a complex that antagonizes mTOR activity, such that loss of these proteins results in hyperactive mTOR signaling, along with often refractory epilepsy, intellectual impairment, and an autistic-like phenotype (Chu-Shore et al., 2010; Vignoli et al., 2015). PTEN is a tumor suppressor gene that also negatively controls mTOR activity, along with regulating cellular proliferation and survival (Bonneau and Longy, 2000). Similar to mutations in TSC1/TSC2, loss of PTEN results in hyperactive mTOR signaling accompanied by seizures and significant intellectual and behavioral impairments (Cupolillo et al., 2016). Other conditions associated with disrupted mTOR activity that have epileptic phenotypes include polyhydramnios, megalencephaly, and symptomatic epilepsy (PMSE), neurofibromatosis type I, Fragile X syndrome, focal cortical dysplasia, and hemimegaloencephaly associated with mutations in various elements of the mTOR pathway (Berry-Kravis, 2002; Crino, 2015; D’Gama et al., 2015; Hsieh et al., 2011; Nguyen et al., 2019; Orlova et al., 2010; Santoro et al., 2018).
Several genetic animal models of epilepsy exhibit mTOR hyperactivation and have been useful in exploring the potential of mTOR inhibitors as a rational anti-epileptogenic therapy. Various treatment paradigms with rapamycin has shown to suppress seizures, as well as attenuate many of the molecular and pathological changes that underlie epileptogenesis. For example, rapamycin administration has shown to reduce epileptiform activity, decrease the duration and severity of seizures, and prolong survival in both models of Tsc1/2 and Pten deletion (Way et al., 2012; Zeng et al., 2008). Additionally, rapamycin reduces mTOR hyperactivity and is protective against seizure-induced molecular changes such as ameliorating neuronal disorganization, dendritic abnormalities, aberrant mossy fiber sprouting, cellular hypertrophy, macrocephaly, and astrogliosis (Brewster et al., 2013; Ljungberg et al., 2009; Nguyen et al., 2015; Sunnen et al., 2011; Zhou et al., 2009). Unlike with the mechanism of traditional anti-seizure medications, rapamycin has minimal impact on neuronal excitability (Hartman et al., 2012). Instead, rapamycin most likely exerts its effects on the electrical activity of neurons indirectly, by modulating the expression of voltage-gated ion channels and neurotransmitter expression (Huang et al., 2012b; Niere and Raab-Graham, 2017; Raab-Graham et al., 2006; Tyan et al., 2010). Despite the beneficial effects of rapamycin on both seizure-induced molecular and behavioral changes, seizures often return with cessation of treatment and continuous intermittent rapamycin treatment may be necessary to sustain the anti-seizure effects (Sunnen et al., 2011).
Hyperactivity of the mTOR pathway has also been demonstrated in animal models of acquired epilepsy, following various insults that induce status epilepticus (SE) in the brain. Specifically, pre-clinical models of temporal lobe epilepsy (TLE) utilizing pilocarpine and kainate chemoconvulsants, as well as models of infantile spasms and hypoxic seizures results in mTOR hyperactivation (Buckmaster et al., 2009; Chachua et al., 2012; Huang et al., 2010; Raffo et al., 2011; Talos et al., 2012; van Vliet et al., 2012; Zeng et al., 2009). Unlike with genetic models of mTOR hyperactivation, treatment with rapamycin in acquired models of epilepsy has demonstrated significant variability. In some cases, mTOR inhibition has attenuated chronic seizures, and in other models rapamycin treatment has had minimal effect on epilepsy development (Buckmaster and Lew, 2011; Hartman et al., 2012; Zeng et al., 2009). Some studies have shown beneficial effects of mTOR inhibition on attenuating seizure-induced cognitive and behavioral impairments. For example, treatment with rapamycin following pilocarpine seizures has shown to protect against hippocampal-dependent spatial learning and memory deficits in a Morris water maze task and with recognition memory in a novel object recognition task (Brewster et al., 2013). Furthermore, rapamycin treatment immediately prior and following acute hypoxia-induced seizures attenuated seizure-induced impairments in sociability in adulthood (Talos et al., 2012). The efficacy of rapamycin has shown be to time, age, and model dependent, suggesting that mTOR activation in acquired models is most likely associated with specific pathological changes that vary across models (Chachua et al., 2012; Ostendorf and Wong, 2015).
Inhibition of mTOR with rapamycin and its analogs has shown therapeutic potential in reducing seizure frequency in clinical populations. The rapamycin analog, everolimus, is approved by the FDA for the treatment of SEGAs, kidney tumors, and partial epilepsy in individuals with TSC (Kim and Lee, 2019). There are several ongoing phase I, II, and III clinical trials investigating the effects everolimus has across multiple age groups and with varying treatment durations (Krueger et al., 2010; Krueger et al., 2016). One open-label, phase I/II clinical trial found that everolimus treatment for 12 weeks resulted in significant seizure reduction in 17 of the 20 TSC patients, along with improvements in patient-reported behavior and quality of life (Krueger et al., 2013). In the initial core phase of a prospective, multi-center phase 3 trial, adjunctive everolimus therapy for 18 weeks in individuals with treatment-resistant focal-onset seizures in TSC resulted in 28.2% of individuals experiencing at least a 50% reduction in seizure frequency when on the low-exposure regimen (3–7ng/ml), and 40% of individuals on the high-exposure regimen (9–15ng/ml) (French et al., 2016). Following the core phase, the efficacy of everolimus in reducing seizure frequency in patients persisted throughout the extension phase of the trial, in which individuals received everolimus (3–15ng/ml) for up to 48 weeks (EXIST-3 trial) (Curatolo et al., 2018). While there is accumulating evidence of the benefits of mTOR inhibition for seizure control in individuals with TSC, additional controlled studies are necessary to determine the efficacy of inhibition as a definitive treatment for epilepsy.
3. Role of neuroinflammation in epilepsy
3.1. Inflammation in the central nervous system
An increasing body of clinical and experimental evidence suggests that the immune system plays an important role in epilepsy. Seizures induce a cascade of biological events, characterized by the release of several inflammatory molecules, microglial and astrocyte activation, and disrupted BBB permeability leading to the infiltration of peripheral immune cells (Aronica et al., 2017; Rana and Musto, 2018). It is possible the upregulation in neuroinflammatory processes following seizures contributes to the hyper-excitable neuronal network and dysregulated neural connectivity that underlies epileptogenesis (Musto et al., 2016; Vezzani et al., 2019).
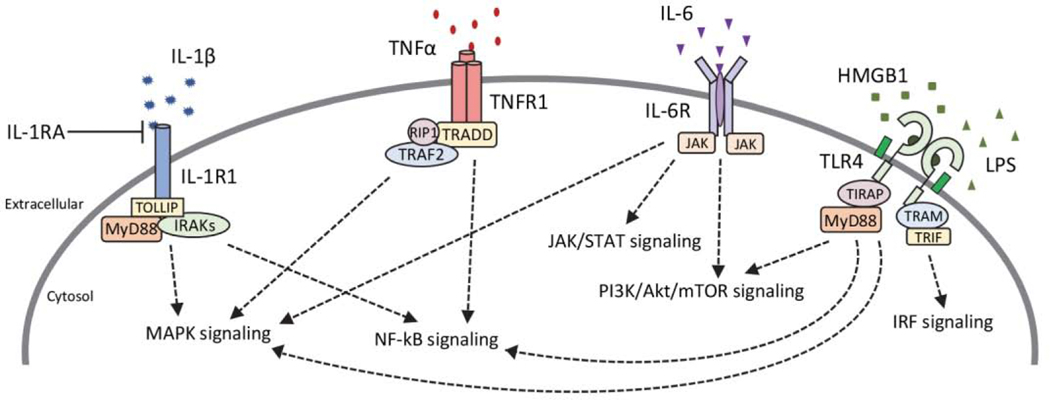
In normal physiological conditions, cells of the innate immune system will activate receptors following injury in order to stimulate a variety of pathways that function to repair damage to the CNS and provide protection from any additional injury. Cytokines are small, secreted and membrane-bound proteins that play an important role in mediating inflammatory signals in the CNS (Fig. 3). In addition to acting as chemical messengers, they regulate several other cellular activities including the survival and differentiation of cells, and mechanisms involved in neuronal development such as the pruning and refining of synapses (Deverman and Patterson, 2009; Paolicelli et al., 2011; Schafer et al., 2012). Cytokines include interleukins (IL), interferons (IFN), and tumor necrosis factors (TNF), and are typically considered to be primarily pro-inflammatory (IL-1β, IL-6, TNFα, IFNγ) or anti-inflammatory (IL-10, IL-4), although some can also have dual functions dependent on the physiological state (Cavaillon, 2001; Tanaka et al., 2014). They can be produced by a number of cells in the CNS, including glial cells such as microglia and astrocytes, as well as neurons and endothelial cells that make up the BBB (Galic et al., 2012). Cells of the innate immune system detect conserved pathogen-associated molecular patterns (PAMPs) in pathogens and damage-associated molecular patterns (DAMPs) released from damaged cells by the expression of germ-line encoded receptors known as pattern recognition receptors (PRRs) (Mogensen, 2009). In normal physiological states, these processes help to recruit cells to fight infection and aid in tissue repair and recovery, however, following an insult these signaling mechanisms can be disrupted and lead to a chronic neuroinflammatory state associated with cellular toxicity.
Figure 3. Cytokine signaling cascades.
Following seizures there is an upregulation in the synthesis of pro-inflammatory cytokines (IL-1β, TNFα, IL-6, i.e.) in the brain which can lead to activation of several downstream signaling cascades. Toll-like receptor 4 (TLR4) is also activated following seizures by its endogenous ligand high-mobility group box 1 (HMGB1) protein or can be exogenously stimulated via administration of lipopolysaccharide (LPS). Activation of cytokine receptors and their respective signaling cascades can initiate downstream transcription of cytokines and other inflammatory molecules, further enhancing the inflammatory environment after seizures. Together these signaling cascades can interact to enhance neuronal excitability and contribute to mechanisms underlying epileptogenesis. IL-1β, interleukin-1 beta; IL-1R1, IL-1 receptor 1; IL-1RA, IL-1R antagonist; IRAK, IL-1 receptor-associated kinase; MAPK, mitogen-activated protein kinases; TNFα, tumor necrosis factor alpha; TNFR1, TNF receptor 1; TRAF2, TNF receptor-associated factor 2; TRADD, TNFR1-associated death domain; RIP1, receptor-interacting protein 1; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; IL-6, interleukin 6; IL-6R, IL-6 receptor; JAK, janus kinase; STAT, signal transducer and activator of transcription; TIRAP, Toll/IL-1 receptor (TIR) domain containing adaptor protein; TRIF, TIR-domain-containing adaptor-inducing interferon-β; TRAM, translocating chain-associating membrane protein; IRF, interferon regulatory factor.
3.2. Neuroinflammatory targets and potential treatments in epilepsy
Cytokines are typically expressed at low concentrations in the brain, however, following an insult such as with seizures, a variety of cytokines (IL-1, IL-6, TNFα, IFNγ) and chemokines (MCP-1/CCL2, CCL3, CCL5) are rapidly produced and released in various brain regions (Arisi et al., 2015; Kosonowska et al., 2015; Uludag et al., 2015). The upregulation in cytokines can induce several downstream signaling events post-seizures, ultimately leading to neuronal hyperexcitability and a reduced threshold for future seizure events. For example, proinflammatory cytokine IL-1β has been shown to stimulate the release of glutamate from astrocytes, reduce glutamate re-uptake leading to elevated glutamate availability, and reduce inhibitory GABA-mediated neurotransmission (Alyu and Dikmen, 2017; Roseti et al., 2015). In addition, cytokine IL-1β has found to be upregulated in the cerebrospinal fluid of children with idiopathic epilepsy compared to individuals without epilepsy (Shi et al., 2017). Pro-inflammatory cytokine TNFα has similar effects, by increasing microglial glutamate release via upregulation of glutaminase, upregulating AMPA receptor expression, and inducing GABA receptor endocytosis, resulting in changes in excitability networks (Galic et al., 2012; Stellwagen et al., 2005; Takeuchi et al., 2006). Another pro-inflammatory cytokine often elevated post-seizures is IL-6, in which upregulations have shown to reduce long-term potentiation and hippocampal neurogenesis, as well as enhance gliosis (Levin and Godukhin, 2017; Vezzani et al., 2002).
One mechanism in which cytokines are produced following seizures is through activation of Toll-like receptors (TLRs). Toll-like receptors are a well-defined class of PRRs that induce inflammatory signaling events by activating a diverse set of molecules, including transcription factors (NF-kβ, AP-1) to stimulate production of immune molecules, and other receptors that activate additional downstream kinases (Kawai and Akira, 2007; Kawasaki and Kawai, 2014). Specifically, signaling via TLR4 and its endogenous ligand, high mobility group box 1 protein (HMGB1), play a significant role in initiating neuroinflammatory responses following seizures (Aronica et al., 2017). Both TLR4 and HMGB1 expression is upregulated in neurons, astrocytes, microglia, and endothelial cells compromising the BBB in both human and experimental epilepsies (Henneberger and Steinhauser, 2016; Hiragi et al., 2018; Maroso et al., 2010; Matin et al., 2015). Stimulation of TLR4 via exogenous activators, such as with agonist lipopolysaccharide (LPS) derived from the cell wall of gram negative bacteria, can also influence seizure progression by enhancing the excitability of neurons and decreasing seizure threshold in experimental models (Eun et al., 2015; Galic et al., 2008; Rodgers et al., 2009). Toll-like receptor 4 activation is interconnected with IL-1 receptor 1 (IL-1R1) signaling, as synthesis and release of their respective ligands, HMGB1 and IL-1β, both involve NLR family pyrin domain containing 3 (NLRP3) inflammasome and caspase 1 activation (Lopez-Castejon and Brough, 2011; Lu et al., 2012). Elevated IL-1R1-TLR4 signaling in both human epilepsies and experimental models of epilepsy has shown to contribute to mechanisms underlying epileptogenesis, along with increased neuronal hyperexcitability (Maroso et al., 2010; Ravizza et al., 2006; Ravizza et al., 2008).
Given the involvement of the immune system in epilepsy, targeting neuroinflammatory processes has become a potential therapeutic strategy as demonstrated in both animal models and recent clinical trials. Compounds that function to attenuate seizure-induced neuroinflammation have shown therapeutic potential in diverse seizure models. For example, direct inhibition of components of IL-1β biosynthesis, along with the HMGB1-TLR4 signaling cascade, have demonstrated beneficial effects. Pharmacological blockade or genetic inactivation in knockout (KO) models of IL-1R1 or TLR4 can significantly reduce seizure susceptibility and prevent seizure-induced damage (Bertani et al., 2017; Maroso et al., 2010; Semple et al., 2017). This effect can be partially attributed to the reduction in neuronal excitability due to the blockade of IL-1β-induced glutamate NMDA receptor-mediated neuronal Ca++ influx via signaling of downstream Src kinases (Balosso et al., 2014; Viviani et al., 2003).
Several anti-inflammatory treatments are currently in clinical trials to treat drug-resistant epilepsies. For example, oral compound VX-765 (Belnacasan) inhibits IL-1β biosynthesis and subsequent HMGB1 release by inhibiting the inflammasome via blockade of interleukin converting enzyme (ICE)/caspase-1 (Maroso et al., 2011). In a phase II clinical trial, VX-765 was found to be safe, well tolerated, and efficacious in individuals with drug-resistant partial epilepsy (Kaur et al., 2016). The human recombinant form of IL-1R1 antagonist, Anakinra, has also been shown to be clinically effective in controlling seizures in pediatric and adolescent patients with refractory epilepsy, as well as in children with febrile infection-related epilepsy syndromes (FIRES) (DeSena et al., 2018; Dilena et al., 2019; Jyonouchi and Geng, 2016; Kenney-Jung et al., 2016). The TNFα monoclonal antibody, Adalimumab, has found to be well tolerated and to reduce seizure frequency in an open label pilot study in a small cohort of adults with Rasmussen’s encephalitis, a severe inflammatory neurological disorder that leads to pharmacoresistant epilepsy (Lagarde et al., 2016). In addition, inhibition of IL-6 biosynthesis with an IL-6 receptor inhibitor (Tocilizumab) terminated seizures in 6 of 7 adults with new onset refractory SE (NORSE). However, 2 individuals in the study experienced severe adverse events that were related to infections during treatment (Jun et al., 2018). While heightened proinflammatory cytokine expression is consistently observed in preclinical models, findings have been variable across different human epilepsies (Bauer et al., 2009; Li et al., 2011; Peltola et al., 2000). Certain conditions, such as in FIRES and NORSE, in which there is significant upregulation in inflammatory cytokines, have shown to be responsive to monoclonal antibody treatments, but further research is needed to ascertain whether these treatments can be utilized in other human epilepsies (Choi et al., 2011; Jun et al., 2018; Sakuma et al., 2015).
Beyond inhibition of the HMGB1-TLR4 signaling cascade and direct antagonism of proinflammatory cytokine synthesis, several other targets that reduce neuroinflammation have been investigated. For example, targeting of cyclooxygenase enzymes (COX1, COX2) to interfere with prostaglandin synthesis has been examined extensively in epilepsy (Rojas et al., 2019). Several animal studies have shown that blockade of COX2 significantly reduces seizure frequency and severity, as well as is neuroprotective against seizure-induced mossy fiber sprouting and neurogenesis (Jung et al., 2006; Ma et al., 2012). However, some studies have shown that inhibition of COX2 worsened chronic seizures and produced adverse effects, suggesting the effects of inhibition are time and model dependent, and could also depend on the type of prostaglandins that are generated following seizures (Holtman et al., 2010; Holtman et al., 2009; Kim et al., 2008; Polascheck et al., 2010; Yoshikawa et al., 2006). Clinical trials with Ibuprofen and Aspirin, both COX inhibitors, have shown variable effects. For example, in pediatric populations with Sturge-Weber syndrome and adults with focal-onset epilepsy, COX inhibition reduced seizure frequency, while in a randomized placebo-controlled trial in children with febrile seizures COX inhibition had no preventative effect on seizure reoccurrence (Bay et al., 2011; Godfred et al., 2013; Lance et al., 2013; van Stuijvenberg et al., 1998).
4. Interaction between mTOR and neuroinflammatory signaling in epilepsy
4.1. Link between the mTOR pathway and inflammation in normal physiological conditions
Immune signaling and PI3K/Akt/mTOR pathway activity are known to interact both in normal physiological conditions and in diseased states. Activation of mTOR has shown to be critical for the development of immune cells in the CNS, as well as many cellular processes of immune cells are regulated by mTOR signaling. There is evidence for both immune-stimulatory and immunosuppressive functions mediated by mTOR activation and the exact mechanisms underlying the complex interplay have yet to be fully elucidated. Inhibition of pathway activity has been shown to impair the maturation and function of dendritic cells (DCs) and inhibit T cell proliferation, demonstrating the importance of mTOR signaling in early immune system development (Thomson et al., 2009). Activation of mTOR also influences cytokine production and release, especially in DCs. For example, mTOR activation suppresses caspase-1 activation and therefore IL-1β production, as well as upstream PI3K negatively regulates TLR-mediated IL-12 biosynthesis from DCs (Fukao et al., 2002; Schmitz et al., 2008). The mTOR pathway can also be activated by several TLR ligands and cytokines, including in both mouse and human monocytes, DCs, and neutrophils (Haidinger et al., 2010; Lehman et al., 2003; Weichhart et al., 2015).
While rapamycin is known for its immunosuppressive effects, particularly in organ transplantations, inhibition via rapamycin or its derivative everolimus have also shown to have immunostimulatory effects in individuals (Touzot et al., 2012; Weichhart et al., 2015). For example, transplant recipients administered mTOR inhibitors have presented with proinflammatory side effects, including elevated NFkB-mediated inflammatory responses in blood leukocytes and increased IL-12-induced signaling (Gallon et al., 2015). Despite the role both neuroinflammatory signaling and mTOR pathway activity have in seizure development in epilepsy, how these two processes influence one another during the development of epilepsy has only recently begun to be investigated. The rest of the review summarizes the evidence to date demonstrating how the two influential processes interact in epilepsy, and potential therapeutics that target both mTOR signaling and seizure-induced neuroinflammation.
4.2. Therapeutic potential of pharmacological mTOR inhibitors to reduce seizure-induced neuroinflammation
Several studies have demonstrated that the anti-epileptogenic effects of rapamycin could potentially be due to its immunosuppressant and anti-inflammatory properties. In one of the first studies to demonstrate the beneficial effects of rapamycin, treatment was examined in a mouse model of TSC (Tsc1GFAPCKO), a common genetic cause of epilepsy (Zeng et al., 2008). Zeng et al. (2008) found that rapamycin treatment starting on postnatal 14 prevented the development of epilepsy and premature death in Tsc1GFAPCKO mice compared to vehicle-treated mice. In addition, treatment starting at a later developmental timepoint (6 weeks of age), was able to suppress seizures and prolong survival in KO mice. The prevention and suppression of seizures was associated with a reduction in astrogliosis in the neocortex and hippocampus of Tsc1GFAPCKO mice, along with rapamycin-treatment prevented the enlarged brain size in KO mice (Zeng et al., 2008). Van Vliet et al. (2012) examined the effects of rapamycin treatment given daily for 7 days, beginning 4 h following SE onset induced by angular bundle stimulation, followed by administration every other day for 6 weeks after SE. In addition to reducing seizure frequency, treatment with rapamycin exhibited neuroprotective properties by reducing SE-induced hilar cell loss and mossy fiber sprouting but had no effect on microglial or astrocyte activation. Blood-brain barrier leakage following SE was minimally detected in rapamycin-treated rats, suggesting rapamycin may exert its anti-epileptogenic effects via restoration of BBB integrity (van Vliet et al., 2012). Van Vliet et al. (2016) further investigated the role of BBB leakage in the chronic phase after SE by examining glial and vascular markers both in vitro and in vivo. Six weeks following kainic acid (KA)-induced SE, rapamycin-treated rats had significantly reduced BBB leakage specifically in the piriform cortex and amygdala, which was associated with a milder form of epilepsy. In this study, rapamycin-treatment was also associated with a reduction in microglia and astrocyte activation, brain blood vessel density, and mossy fiber sprouting in the chronic phase of epilepsy in rats (van Vliet et al., 2016).
Astrocytes are implicated in promoting epileptogenesis by diverse mechanisms, including contributing to BBB disruption, the release of inflammatory molecules, and dysregulated astrocyte-neuronal synaptic signaling in epilepsy (Michinaga and Koyama, 2019; Seifert et al., 2010). During the chronic phase of epileptogenesis in an intrahippocampal mouse model of KA seizures and in individuals with drug-resistant TLE, mTOR activation has been found to be upregulated in reactive astrocytes (Sha et al., 2012). In addition, deletion of mTOR in reactive astrocytes prevented increases in seizure frequency and astrogliosis in mice with chronic epilepsy following KA-induced SE, however, did not have an effect on associated mossy fiber sprouting (Wang et al., 2017). In neuronal-specific Depdc5 KO mice that exhibit seizures and mTOR hyperactivation, rapamycin treatment normalized the increases in mTOR activity, along with the astrogliosis present in the brains of Depdc5 KO mice (Yuskaitis et al., 2019). Guo et al. (2017) utilized two-photon imaging to directly monitor in vivo the structural effects of KA seizures on cortical astrocytes. With both pre-treatment and post-treatment regimens, rapamycin (6 mg/kg) was able to prevent seizure-induced astrocyte vacuolization, astrogliosis, and decreases in astrocyte size (Guo et al., 2017). However, in both treatment regimens, rapamycin did not have an effect on astrocyte number following KA seizures.
The timing of rapamycin treatment has found to be critical in elucidating the seizure-suppressing effects of administration, and many studies have found the suppressant effects to be lost following cessation of treatment. A study by Drion et al. (2016) demonstrates the importance of timing when administering rapamycin in an electrogenic post-SE rat model of TLE. Rapamycin treatment (6 mg/kg) given to rats for 3 weeks following seizures significantly suppressed seizures, however, there was a gradual reappearance of seizures during the following 5 weeks once treatment was terminated and rapamycin blood levels decreased. When rapamycin (3 mg/kg) was given for 5 days in the chronic phase of epilepsy, treatment significantly decreased seizures, but as rapamycin washed out, seizure frequency began to increase again, although at still lower levels than baseline seizure frequency (Drion et al., 2016). In addition, pre-treatment with rapamycin (3 mg/kg) for three days prior to seizure induction did not reduce seizure frequency, concluding that rapamycin treatment was not able to prevent the development of epilepsy. In the same electrogenic rat model of TLE, rapamycin (6 mg/kg) administration starting 4 hours post-SE and continued only for 7 days did not reduce SE-induced increases in IL-1β, IL-6, TGFβ, Hmox-1, or HMGB1 one week following SE (Drion et al., 2018).
Other pharmacological therapies besides rapamycin that influence both inflammatory processes and mTOR signaling have begun to be investigated. One therapeutic commonly used as an immunosuppressant in organ transplantation surgeries, mycophenolate mofetil (MMF), has recently been examined in a rat model of lithium pilocarpine-induced TLE. The active form of MMF, mycophenolic acid (MPA), functions in nucleic acid biosynthesis pathways and is a rate-limiting enzyme in the synthesis of guanosine nucleotides which can result in inhibition of T-cell lymphocyte proliferation (Allison and Eugui, 2000). Following a latent phase (28 days) post-seizures in a mouse model, mice that were treated with MMF had reduced severity of spontaneous seizures, however treatment had no effect on seizure incidence compared to controls (Mazumder et al., 2019). Mycophenolate mofetil also had beneficial effects on seizure-induced behavioral impairments, as treatment reduced aggression and depressive-like behavior, and increased spatial and recognition memory in seizure rats. These behavioral changes could potentially be mediated by both inflammatory and cell signaling processes, as MMF was able to attenuate seizure-induced increases in glial fibrillary acidic protein (GFAP), mTOR, pS6, and S6 protein levels, as well as attenuate gene expression of many pro-inflammatory cytokines and mTOR pathway components (Mazumder et al., 2019).
4.3. Non-pharmacological agents that impact mTOR and neuroinflammatory signaling in epilepsy
Rapamycin treatment has shown to only suppress epileptogenesis with continual treatment, and prolonged treatment can also lead to many adverse side effects. For instance, mTOR inhibition with rapamycin or its analogs can lead to immunosuppression, mucositis, skin reactions, and dose-dependent increases in cholesterol and triglycerides (McDaniel and Wong, 2011; Tsang et al., 2007). As a result, other non-pharmacological compounds that have anti-epileptogenic properties by inhibiting mTOR activity and neuroinflammatory processes have been examined. One potential anti-epileptogenic therapeutic is curcumin, the primary compound present in turmeric from the Curcuma longa plant (Dhir, 2018; Rahmani et al., 2018).
The anti-inflammatory effects of curcumin can partially be attributed to both inhibition of the mTOR pathway and mitogen-activated kinase pathways (Beevers et al., 2013; Drion et al., 2018). In a study using a hippocampal-entorhinal cortex culture model of epileptogenesis, curcumin treatment reduced spontaneous seizure-like events, which was associated with a trending decrease in IL-1β and IL-6 and reduced mTOR activity, exemplified by low pS6 expression (Drion et al., 2019). In human astrocyte cultures, Drion et al. (2018) found that pre-treatment and simultaneous treatment with curcumin along with IL-1β stimulation, significantly reduced pro-inflammatory IL-6 expression, as well as simultaneous treatment significantly reduced COX-2 levels in vitro. However, curcumin administration for 7 days post-SE in a rat model of TLE did not reduce SE-induced elevations in IL-1β, IL-6, TGFβ, Hmox-1, or HMGB1 (Drion et al., 2018). While evidence suggests curcumin treatment could be a promising alternative to rapamycin that has similar anti-epileptogenic actions, additional studies are critical to determine the mechanisms underlying these effects.
Another non-pharmacological option that influences inflammation and mTOR activity could be resveratrol, a natural polyphenol compound found in a variety of plant species that has broad biological activity (Castro et al., 2017; Lu and Wang, 2015). It has been shown to have anti-epileptogenic effects in several seizure models, with administration of the compound prior to seizure induction preventing SE-induced neuroinflammation, neurodegeneration, and aberrant neurogenesis (Castro et al., 2017; Gupta et al., 2002; Lu and Wang, 2015; Shetty, 2011; Virgili and Contestabile, 2000; Wu et al., 2009). Its beneficial effects may partially involve suppression of NFkB and pro-inflammatory cytokine synthesis, which can be influenced by upstream mTOR signaling (Lu et al., 2010; Ren et al., 2013). Resveratrol administered 30 min prior to pilocarpine seizures in a rat model suppressed IL-1β, COX-2, and inducible nitric oxide synthase 3 hours after SE (Wang et al., 2013). Pre-seizure resveratrol treatment also increased expression of AMP-activated protein kinase (AMPK), which was associated with a reduction in phosphor-mTOR and phospho-S6, suggesting the anti-inflammatory properties of resveratrol following seizures involve regulation of AMPK/mTOR signaling (Wang et al., 2013).
Dietary therapies have also shown promise in controlling seizures in those with drug-refractory epilepsy (Bough and Rho, 2007; D’Andrea Meira et al., 2019; Lima et al., 2014; Marsh et al., 2006; Patel et al., 2010). The ketogenic diet (KD) consists of high-fat, low-carbohydrate, and adequate protein levels, which leads to the production of ketone bodies that function as the primary source of metabolic energy. The KD has both anti-inflammatory activity and may partially inhibit mTOR activity, contributing to its anticonvulsant effects. The diet’s anti-inflammatory and anti-convulsant effects may involve activation of peroxisome proliferator activated receptors (PPAR), which are involved in the regulation of anti-inflammatory and antioxidant genes (Boison, 2017; Lucchi et al., 2017). Activation of PPARγ is dependent on endogenous fatty acids, such as decanoic acid, that are increased in the KD diet. In a Kv1.1 KO mouse model that exhibits spontaneous epileptic activity, a PPARγ antagonist prevented the seizure-reducing effects of the KD diet in Kv1.1 KO mice (Simeone et al., 2017). In addition, the KD increased the latency to seizures, but was ineffective in PPARγ KO mice, further supporting the role of PPARs in the anticonvulsant mechanisms of the KD (Simeone et al., 2017).
Beyond the anti-inflammatory effects of the KD, it may also partially exert its effects through inhibition of mTOR signaling. Treatment with the KD following a single KA seizure has shown to reduce the frequency of spontaneous seizures, which was associated with less mossy fiber sprouting (Muller-Schwarze et al., 1999; Su et al., 2000). These anti-epileptogenic effects could be associated with a reduction in mTOR activity, as mTOR is known to be elevated following KA seizures and regulates pathological changes associated with epileptogenesis (Blazejczyk et al., 2017; Buckmaster et al., 2009; Zeng et al., 2009). The KD reduces insulin levels, which is an activator of PI3K/Akt/mTOR signaling and may indirectly reduce mTOR activity (Westman et al., 2008; Yoon, 2017). In addition, pro-inflammatory cytokine expression is reduced with KD therapy, and cytokines can activate the PI3K/Akt/mTOR pathway via TLR stimulation, thus serving as another potential link between the two mechanisms (Dupuis et al., 2015; Kim et al., 2012; Troutman et al., 2012). Mechanisms underlying the anti-seizure and anti-epileptogenic effects of the KD in epilepsy still requires additional investigation, especially regarding whether its effects on inflammation and mTOR could be associated.
4.4. In vitro evidence for link between mTOR signaling and neuroinflammation in epilepsy
The effect of rapamycin on components of immune function in epilepsy, specifically BBB permeability, has also been examined in vitro. To mimic inflammatory conditions observed during seizures, pro-inflammatory cytokines can be added to the medium of in vitro models which will result in disruption and reduced integrity of the BBB (Marchi et al., 2012; Pan et al., 2011; van Vliet et al., 2016). In the presence of pro-inflammatory cytokine TNFα, van Vliet et al. (2016) found that rapamycin treatment improved overall BBB function and demonstrated a beneficial role of rapamycin in restoration of the BBB following injury (van Vliet et al., 2016). However, this beneficial effect on the BBB could potentially be due to mechanisms other than a reduction in pro-inflammatory cytokine expression, as other studies have found contrasting results following rapamycin treatment. Drion et al. (2018) used astrocyte cultures to examine the effects of rapamycin on pro-inflammatory cytokine expression following an immune stimulus. Following both in vitro pre-treatment and simultaneous treatment of rapamycin along IL-1β stimulation, IL-1β-induced increases in pro-inflammatory IL-6 levels were not affected by treatment. Further, COX-2 mRNA expression was found to be significantly enhanced following rapamycin treatment compared to IL-1β-stimulated expression levels (Drion et al., 2018). In a hippocampal-entorhinal cortex slice culture model, Drion et al. (2019) examined the effects of rapamycin in slices obtained from 6-day old rat pups and found that in vitro administration of rapamycin from day 2 and onward, did not reduce spontaneous seizure-like events in cornus ammonis (CA) 1 neurons (Drion et al., 2019).
5. Perspectives and conclusions
Epilepsy is a complex neurological disorder that can be the result of several acquired and genetic etiologies. Current treatments are primarily symptomatic, and there is an unmet need for therapies that target the pathological mechanisms that underlie epileptogenesis. Both signaling via the mTOR pathway and neuroinflammatory processes have been shown to play a significant role in the development of chronic epilepsy (Aronica et al., 2017; Citraro et al., 2016; Wong, 2013a). In order to gain a better understanding of the intersection between mTOR signaling and the immune system in epilepsy, it is critical that studies examine the impact on both components when utilizing solely an mTOR inhibitor or anti-inflammatory agent. There is the possibility that several previously found benefits of one inhibitor, could also have been efficacious in controlling aspects of the other of the two non-targeted mechanisms. In regard to both inhibition of mTOR signaling and neuroinflammation, future studies are needed to determine the optimal timing of inhibition. For instance, many pre-clinical studies have found discontinuation of rapamycin treatment to be associated with reoccurrence of seizures, and for the timing of treatment to have differential effects on seizure-induced damage in the brain (Drion et al., 2016; Huang et al., 2010). In addition, neuroinflammation can be triggered at varying timepoints post-seizures dependent on the type of injury, so determining the optimal timing of intervention will most likely vary across diverse clinical populations with epilepsy. Understanding the temporal dynamics of epileptogenesis will clarify when the most beneficial time window of intervention will be to inhibit mTOR and neuroinflammatory processes simultaneously.
As a result of many current AEDs being primarily symptomatic, many individuals with epilepsy suffer from several comorbid cognitive, behavioral, and psychiatric conditions (Keezer et al., 2016; Srinivas and Shah, 2017). Targeting the pathological mechanisms that underlie epilepsy could potentially alleviate some of these comorbid impairments. Several studies have begun to elucidate the molecular impact of inhibition of mTOR or inflammatory mechanisms in epilepsy, however, there are a lack of studies examining the behavioral impact of these therapeutics in pre-clinical models. Due to these therapeutics targeting mechanisms underlying epilepsy, one would suspect a beneficial effect on associated seizure-induced cognitive and behavioral impairments, but without inclusion of behavioral components in studies this is difficult to determine. For example, studies have examined the impact of mTOR inhibition with rapamycin on learning and memory, activity levels, and aggression, yet there are few studies examining the effects of inhibition on autistic-like behaviors (Huang et al., 2012a; Raffo et al., 2011; Schneider et al., 2017; Talos et al., 2012; Yuskaitis et al., 2019). Many epilepsies are comorbid with other neurodevelopmental disorders, especially Autism spectrum disorder, and beneficial effects of these treatments in pre-clinical models may give rise to novel targets to attenuate impairments observed in autism as well (Bernard and Benke, 2015; Besag, 2017). In conclusion, the intersection between mTOR signaling and neuroinflammatory mechanisms in epilepsy serves as a promising avenue to pursue in the search for alternative therapeutic treatments that are anti-epileptogenic and target the pathological causes of the disorder.
Highlights.
Hyperactive mTOR signaling is implicated in several seizure-induced brain changes
Neuroinflammation can impact hyperexcitability and promote epileptogenesis
mTOR and inflammatory signaling interact in both physiological and diseased states
Dual inhibition of both processes could be a potential anti-epileptogenic therapy
Acknowledgments
Funding: This work was supported by the National Institutes of Health [NS088776] to JNL. Declarations of interest: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison AC, Eugui EM, 2000. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47, 85–118. [DOI] [PubMed] [Google Scholar]
- Alyu F, Dikmen M, 2017. Inflammatory aspects of epileptogenesis: contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr 29, 1–16. [DOI] [PubMed] [Google Scholar]
- Arisi GM, Foresti ML, Katki K, Shapiro LA, 2015. Increased CCL2, CCL3, CCL5, and IL-1β cytokine concentration in piriform cortex, hippocampus, and neocortex after pilocarpineinduced seizures. J Neuroinflammation 12, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Bauer S, Bozzi Y, Caleo M, Dingledine R, Gorter JA, Henshall DC, Kaufer D, Koh S, Loscher W, Louboutin JP, Mishto M, Norwood BA, Palma E, Poulter MO, Terrone G, Vezzani A, Kaminski RM, 2017. Neuroinflammatory targets and treatments for epilepsy validated in experimental models. Epilepsia 58 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balosso S., Liu J., Bianchi ME., Vezzani A., 2014. Disulfide-containing high mobility group box-1 promotes N-methyl-D-aspartate receptor function and excitotoxicity by activating Tolllike receptor 4-dependent signaling in hippocampal neurons. Antioxid Redox Signal 21, 1726–1740. [DOI] [PubMed] [Google Scholar]
- Bauer S, Cepok S, Todorova-Rudolph A, Nowak M, Koller M, Lorenz R, Oertel WH, Rosenow F, Hemmer B, Hamer HM, 2009. Etiology and site of temporal lobe epilepsy influence postictal cytokine release. Epilepsy Res 86, 82–88. [DOI] [PubMed] [Google Scholar]
- Bay MJ, Kossoff EH, Lehmann CU, Zabel TA, Comi AM, 2011. Survey of aspirin use in Sturge-Weber syndrome. J Child Neurol 26, 692–702. [DOI] [PubMed] [Google Scholar]
- Beevers CS, Zhou H, Huang S, 2013. Hitting the golden TORget: curcumin’s effects on mTOR signaling. Anticancer Agents Med Chem 13, 988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Katche C, Slipczuk LN, Igaz LM, Cammarota M, Izquierdo I, Medina JH, 2007. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiology of learning and memory 87, 303–307. [DOI] [PubMed] [Google Scholar]
- Bernard PB, Benke TA, 2015. Early life seizures: evidence for chronic deficits linked to autism and intellectual disability across species and models. Exp Neurol 263, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, 2002. Epilepsy in fragile X syndrome. Dev Med Child Neurol 44, 724–728. [DOI] [PubMed] [Google Scholar]
- Bertani I, Iori V, Trusel M, Maroso M, Foray C, Mantovani S, Tonini R, Vezzani A, Chiesa R, 2017. Inhibition of IL-1β Signaling Normalizes NMDA-Dependent Neurotransmission and Reduces Seizure Susceptibility in a Mouse Model of Creutzfeldt–Jakob Disease. J Neurosci 37, 10278–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besag FM, 2017. Epilepsy in patients with autism: links, risks and treatment challenges. Neuropsychiatr Dis Treat 14, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazejczyk M, Macias M, Korostynski M, Firkowska M, Piechota M, Skalecka A, Tempes A, Koscielny A, Urbanska M, Przewlocki R, Jaworski J, 2017. Kainic Acid Induces mTORC1-Dependent Expression of Elmo1 in Hippocampal Neurons. Mol Neurobiol 54, 2562–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, 2017. New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol 30, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau D, Longy M, 2000. Mutations of the human PTEN gene. Hum Mutat 16, 109–122. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Rho JM, 2007. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia 48, 43–58. [DOI] [PubMed] [Google Scholar]
- Brewster AL, Lugo JN, Patil VV, Lee WL, Qian Y, Vanegas F, Anderson AE, 2013. Rapamycin Reverses Status Epilepticus-Induced Memory Deficits and Dendritic Damage. PloS one 8, e57808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Ingram EA, Wen X, 2009. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci 29, 8259–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Lew FH, 2011. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci 31, 2337–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardamone M, Flanagan D, Mowat D, Kennedy SE, Chopra M, Lawson JA, 2014. Mammalian target of rapamycin inhibitors for intractable epilepsy and subependymal giant cell astrocytomas in tuberous sclerosis complex. J Pediatr 164, 1195–1200. [DOI] [PubMed] [Google Scholar]
- Castro OW, Upadhya D, Kodali M, Shetty AK, 2017. Resveratrol for Easing Status Epilepticus Induced Brain Injury, Inflammation, Epileptogenesis, and Cognitive and Memory Dysfunction-Are We There Yet? Front Neurol 8, 603–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon JM, 2001. Pro- versus anti-inflammatory cytokines: myth or reality. Cellular and molecular biology (Noisy-le-Grand, France) 47, 695–702. [PubMed] [Google Scholar]
- Chachua T., Poon KL., Yum MS., Nesheiwat L., DeSantis K., Veliskova J., Velisek L., 2012. Rapamycin has age-, treatment paradigm-, and model-specific anticonvulsant effects and modulates neuropeptide Y expression in rats. Epilepsia 53, 2015–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Min HJ, Shin J-S, 2011. Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. Journal of neuroinflammation 8, 135–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA, 2010. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia 51, 1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citraro R, Leo A, Constanti A, Russo E, De Sarro G, 2016. mTOR pathway inhibition as a new therapeutic strategy in epilepsy and epileptogenesis. Pharmacol Res 107, 333–343. [DOI] [PubMed] [Google Scholar]
- Cook AM, Bensalem-Owen MK, 2011. Mechanisms of action of antiepileptic drugs. Therapy 8, 307+. [Google Scholar]
- Costa-Mattioli M, Monteggia LM, 2013. mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat Neurosci 16, 1537–1543. [DOI] [PubMed] [Google Scholar]
- Crino PB, 2015. mTOR signaling in epilepsy: insights from malformations of cortical development. Cold Spring Harb Perspect Med 5, a022442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupolillo D, Hoxha E, Faralli A, De Luca A, Rossi F, Tempia F, Carulli D, 2016. Autistic-Like Traits and Cerebellar Dysfunction in Purkinje Cell PTEN Knock-Out Mice. Neuropsychopharmacology 41, 1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curatolo P., Franz DN., Lawson JA., Yapici Z., Ikeda H., Polster T., Nabbout R., de Vries PJ., Dlugos DJ., Fan J., 2018. Adjunctive everolimus for children and adolescents with treatment-refractory seizures associated with tuberous sclerosis complex: post-hoc analysis of the phase 3 EXIST-3 trial. Lancet Child Adolesc Health 2, 495–504. [DOI] [PubMed] [Google Scholar]
- Curia G, Lucchi C, Vinet J, Gualtieri F, Marinelli C, Torsello A, Costantino L, Biagini G, 2014. Pathophysiogenesis of mesial temporal lobe epilepsy: is prevention of damage antiepileptogenic? Current medicinal chemistry 21, 663–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea Meira I, Romão TT, Pires do Prado HJ, Krüger LT, Pires MEP, da Conceição PO, 2019. Ketogenic Diet and Epilepsy: What We Know So Far. Front Neurol 13, 5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antuono M, Köhling R, Ricalzone S, Gotman J, Biagini G, Avoli M, 2010. Antiepileptic drugs abolish ictal but not interictal epileptiform discharges in vitro. Epilepsia 51, 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Gama AM, Geng Y, Couto JA, Martin B, Boyle EA, LaCoursiere CM, Hossain A, Hatem NE, Barry BJ, Kwiatkowski DJ, Vinters HV, Barkovich AJ, Shendure J, Mathern GW, Walsh CA, Poduri A, 2015. Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann Neurol 77, 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalic L, Cook MJ, 2016. Managing drug-resistant epilepsy: challenges and solutions. Neuropsychiatr Dis Treat 12, 2605–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSena AD, Do T, Schulert GS, 2018. Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. J Neuroinflammation 15, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH, 2009. Cytokines and CNS development. Neuron 64, 61–78. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Vezzani A, O’Brien TJ, Jette N, Scheffer IE, de Curtis M, Perucca P, 2018. Epilepsy. Nat Rev Dis Primers 4, 18024. [DOI] [PubMed] [Google Scholar]
- Dhir A, 2018. Curcumin in epilepsy disorders. Phytother Res 32, 1865–1875. [DOI] [PubMed] [Google Scholar]
- Dilena R, Mauri E, Aronica E, Bernasconi P, Bana C, Cappelletti C, Carrabba G, Ferrero S, Giorda R, Guez S, Scalia Catenacci S, Triulzi F, Barbieri S, Calderini E, Vezzani A, 2019. Therapeutic effect of Anakinra in the relapsing chronic phase of febrile infection-related epilepsy syndrome. Epilepsia open 4, 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drion CM, Borm LE, Kooijman L, Aronica E, Wadman WJ, Hartog AF, van Vliet EA, Gorter JA, 2016. Effects of rapamycin and curcumin treatment on the development of epilepsy after electrically induced status epilepticus in rats. Epilepsia 57, 688–697. [DOI] [PubMed] [Google Scholar]
- Drion CM, Kooijman L, Aronica E, van Vliet EA, Wadman WJ, Chameau P, Gorter JA, 2019. Curcumin reduces development of seizurelike events in the hippocampal-entorhinal cortex slice culture model for epileptogenesis. Epilepsia 60, 605–614. [DOI] [PubMed] [Google Scholar]
- Drion CM., van Scheppingen J., Arena A., Geijtenbeek KW., Kooijman L., van Vliet EA., Aronica E., Gorter JA., 2018. Effects of rapamycin and curcumin on inflammation and oxidative stress in vitro and in vivo - in search of potential anti-epileptogenic strategies for temporal lobe epilepsy. J Neuroinflammation 15, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis N, Curatolo N, Benoist J-F, Auvin S, 2015. Ketogenic diet exhibits antiinflammatory properties. Epilepsia 56, e95–e98. [DOI] [PubMed] [Google Scholar]
- Eun B-L, Abraham J, Mlsna L, Kim MJ, Koh S, 2015. Lipopolysaccharide potentiates hyperthermia-induced seizures. Brain Behav 5, e00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Lawson JA, Yapici Z, Ikeda H, Polster T, Nabbout R, Curatolo P, de Vries PJ, Dlugos DJ, Berkowitz N, 2016. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. The Lancet 388, 2153–2163. [DOI] [PubMed] [Google Scholar]
- Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S, 2002. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol 3, 875–881. [DOI] [PubMed] [Google Scholar]
- Galic MA, Riazi K, Heida JG, Mouihate A, Fournier NM, Spencer SJ, Kalynchuk LE, Teskey GC, Pittman QJ, 2008. Postnatal inflammation increases seizure susceptibility in adult rats. J Neurosci 28, 6904–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic MA, Riazi K, Pittman QJ, 2012. Cytokines and brain excitability. Front Neuroendocrinol 33, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallon L, Traitanon O, Sustento-Reodica N, Leventhal J, Ansari MJ, Gehrau RC, Ariyamuthu V, De Serres SA, Alvarado A, Chhabra D, Mathew JM, Najafian N, Mas V, 2015. Cellular and molecular immune profiles in renal transplant recipients after conversion from tacrolimus to sirolimus. Kidney Int 87, 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfred RM, Parikh MS, Haltiner AM, Caylor LM, Sepkuty JP, Doherty MJ, 2013. Does aspirin use make it harder to collect seizures during elective video-EEG telemetry? Epilepsy Behav 27, 115–117. [DOI] [PubMed] [Google Scholar]
- Guo D, Zou J, Wong M, 2017. Rapamycin attenuates acute seizure-induced astrocyte injury in mice in vivo. Sci Rep 7, 2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta Y, Briyal S, Chaudhary G, 2002. Protective effect of trans-resveratrol against kainic acid-induced seizures and oxidative stress in rats. Pharmacology, biochemistry, and behavior 71, 245–249. [DOI] [PubMed] [Google Scholar]
- Haidinger M., Poglitsch M., Geyeregger R., Kasturi S., Zeyda M., Zlabinger GJ., Pulendran B., Hörl WH., Säemann MD., Weichhart T., 2010. A Versatile Role of Mammalian Target of Rapamycin in Human Dendritic Cell Function and Differentiation. J Immunol 185, 3919–3931. [DOI] [PubMed] [Google Scholar]
- Hartman AL, Santos P, Dolce A, Hardwick JM, 2012. The mTOR inhibitor rapamycin has limited acute anticonvulsant effects in mice. PloS one 7, e45156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Steinhauser C, 2016. Astrocytic TLR4 at the crossroads of inflammation and seizure susceptibility. J Cell Biol 215, 607–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiragi T, Ikegaya Y, Koyama R, 2018. Microglia after Seizures and in Epilepsy. Cells 7, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges SL, Lugo JN, 2018. Wnt/β-catenin signaling as a potential target for novel epilepsy therapies. Epilepsy Res 146, 9–16. [DOI] [PubMed] [Google Scholar]
- Holtman L, van Vliet EA, Edelbroek PM, Aronica E, Gorter JA, 2010. Cox-2 inhibition can lead to adverse effects in a rat model for temporal lobe epilepsy. Epilepsy Res 91, 49–56. [DOI] [PubMed] [Google Scholar]
- Holtman L, van Vliet EA, van Schaik R, Queiroz CM, Aronica E, Gorter JA, 2009. Effects of SC58236, a selective COX-2 inhibitor, on epileptogenesis and spontaneous seizures in a rat model for temporal lobe epilepsy. Epilepsy Res 84, 56–66. [DOI] [PubMed] [Google Scholar]
- Hsieh HY, Fung HC, Wang CJ, Chin SC, Wu T, 2011. Epileptic seizures in neurofibromatosis type 1 are related to intracranial tumors but not to neurofibromatosis bright objects. Seizure 20, 606–611. [DOI] [PubMed] [Google Scholar]
- Huang X, McMahon J, Huang Y, 2012a. Rapamycin attenuates aggressive behavior in a rat model of pilocarpine-induced epilepsy. Neuroscience 215, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, McMahon J, Yang J, Shin D, Huang Y, 2012b. Rapamycin down-regulates KCC2 expression and increases seizure susceptibility to convulsants in immature rats. Neuroscience 219, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y, 2010. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiology of disease 40, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iori V, Frigerio F, Vezzani A, 2016. Modulation of neuronal excitability by immune mediators in epilepsy. Curr Opin Pharmacol 26, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M, 2005. Control of dendritic arborization by the phosphoinositide-3’-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci 25, 11300–11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhanwar-Uniyal M, Wainwright JV, Mohan AL, Tobias ME, Murali R, Gandhi CD, Schmidt MH, 2019. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv Biol Regul 72, 51–62. [DOI] [PubMed] [Google Scholar]
- Jülich K, Sahin M, 2014. Mechanism-based treatment in tuberous sclerosis complex. Pediatr Neurol 50, 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JS, Lee ST, Kim R, Chu K, Lee SK, 2018. Tocilizumab treatment for new onset refractory status epilepticus. Ann Neurol 84, 940–945. [DOI] [PubMed] [Google Scholar]
- Jung KH., Chu K., Lee ST., Kim J., Sinn DI., Kim JM., Park DK., Lee JJ., Kim SU., Kim M., Lee SK., Roh JK., 2006. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiology of disease 23, 237–246. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Geng L, 2016. Intractable Epilepsy (IE) and Responses to Anakinra, a Human Recombinant IL-1 Receptor Agonist. J Clin Cell Immunol 7, 456. [Google Scholar]
- Kaur H, Kumar B, Medhi B, 2016. Antiepileptic drugs in development pipeline: A recent update. eNeurologicalSci 4, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S, 2007. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med 13, 460–469. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kawai T, 2014. Toll-like receptor signaling pathways. Front Immunol 5, 461–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keezer MR, Sisodiya SM, Sander JW, 2016. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol 15, 106–115. [DOI] [PubMed] [Google Scholar]
- Kenney-Jung DL, Vezzani A, Kahoud RJ, LaFrance-Corey RG, Ho M-L, Muskardin TW, Wirrell EC, Howe CL, Payne ET, 2016. Febrile infection-related epilepsy syndrome treated with anakinra. Ann Neurol 80, 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Hao J, Liu R, Turner G, Shi FD, Rho JM, 2012. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PloS one 7, e35476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Chung JI, Lee SH, Jung YS, Moon CH, Baik EJ, 2008. Involvement of endogenous prostaglandin F2alpha on kainic acid-induced seizure activity through FP receptor: the mechanism of proconvulsant effects of COX-2 inhibitors. Brain Res 1193, 153–161. [DOI] [PubMed] [Google Scholar]
- Kim JK, Lee JH, 2019. Mechanistic Target of Rapamycin Pathway in Epileptic Disorders. J Korean Neurosurg Soc 62, 272–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosonowska E, Janeczko K, Setkowicz Z, 2015. Inflammation induced at different developmental stages affects differently the range of microglial reactivity and the course of seizures evoked in the adult rat. Epilepsy Behav 49, 66–70. [DOI] [PubMed] [Google Scholar]
- Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T, Franz DN, 2010. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med 363, 1801–1811. [DOI] [PubMed] [Google Scholar]
- Krueger DA, Wilfong AA, Holland-Bouley K, Anderson AE, Agricola K, Tudor C, Mays M, Lopez CM, Kim MO, Franz DN, 2013. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol 74, 679–687. [DOI] [PubMed] [Google Scholar]
- Krueger DA, Wilfong AA, Mays M, Talley CM, Agricola K, Tudor C, Capal J, Holland-Bouley K, Franz DN, 2016. Long-term treatment of epilepsy with everolimus in tuberous sclerosis. Neurology 87, 2408–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde S, Villeneuve N, Trebuchon A, Kaphan E, Lepine A, McGonigal A, Roubertie A., Barthez MA., Trommsdorff V., Lefranc J., Wehbi S., des Portes V., Laguitton V., Quartier P., Scavarda D., Giusiano B., Milh M., Bulteau C., Bartolomei F., 2016. Anti-tumor necrosis factor alpha therapy (adalimumab) in Rasmussen’s encephalitis: An open pilot study. Epilepsia 57, 956–966. [DOI] [PubMed] [Google Scholar]
- Lance EI, Sreenivasan AK, Zabel TA, Kossoff EH, Comi AM, 2013. Aspirin use in Sturge-Weber syndrome: side effects and clinical outcomes. J Child Neurol 28, 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM, 2012. mTOR signaling in growth control and disease. Cell 149, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Kwon B, Lemere CA, de la Monte S, Itamura K, Ha AY, Querfurth HW, 2017. mTORC2 (Rictor) in Alzheimer’s Disease and Reversal of Amyloid-beta ExpressionInduced Insulin Resistance and Toxicity in Rat Primary Cortical Neurons. J Alzheimers Dis 56, 1015–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JA, Calvo V, Gomez-Cambronero J, 2003. Mechanism of ribosomal p70S6 kinase activation by granulocyte macrophage colony-stimulating factor in neutrophils: cooperation of a MEK-related, THR421/SER424 kinase and a rapamycin-sensitive, m-TOR-related THR389 kinase. J Biol Chem 278, 28130–28138. [DOI] [PubMed] [Google Scholar]
- Levin SG, Godukhin OV, 2017. Modulating Effect of Cytokines on Mechanisms of Synaptic Plasticity in the Brain. Biochemistry (Mosc) 82, 264–274. [DOI] [PubMed] [Google Scholar]
- Li G, Bauer S, Nowak M, Norwood B, Tackenberg B, Rosenow F, Knake S, Oertel WH, Hamer HM, 2011. Cytokines and epilepsy. Seizure 20, 249–256. [DOI] [PubMed] [Google Scholar]
- Lima P.A.d., Sampaio L.P.d.B., Damasceno NRT, 2014. Neurobiochemical mechanisms of a ketogenic diet in refractory epilepsy. Clinics (Sao Paulo) 69, 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D’Arcangelo G, 2009. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech 2, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Castejon G, Brough D, 2011. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev 22, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JP, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ, 2012. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488, 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Wang X, 2015. The role and potential mechanism of resveratrol in the prevention and control of epilepsy. Future Med Chem 7, 2005–2018. [DOI] [PubMed] [Google Scholar]
- Lu X, Ma L, Ruan L, Kong Y, Mou H, Zhang Z, Wang Z, Wang JM, Le Y, 2010. Resveratrol differentially modulates inflammatory responses of microglia and astrocytes. J Neuroinflammation 7, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchi C, Costa AM, Giordano C, Curia G, Piat M, Leo G, Vinet J, Brunel L, Fehrentz JA, Martinez J, Torsello A, Biagini G, 2017. Involvement of PPARgamma in the Anticonvulsant Activity of EP-80317, a Ghrelin Receptor Antagonist. Frontiers in pharmacology 8, 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Cui XL., Wang Y., Li XW., Yang F., Wei D., Jiang W., 2012. Aspirin attenuates spontaneous recurrent seizures and inhibits hippocampal neuronal loss, mossy fiber sprouting and aberrant neurogenesis following pilocarpine-induced status epilepticus in rats. Brain Res 1469, 103–113. [DOI] [PubMed] [Google Scholar]
- Marchi N, Granata T, Freri E, Ciusani E, Ragona F, Puvenna V, Teng Q, Alexopolous A, Janigro D, 2011. Efficacy of anti-inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PloS one 6, e18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Granata T, Ghosh C, Janigro D, 2012. Blood-brain barrier dysfunction and epilepsy: pathophysiologic role and therapeutic approaches. Epilepsia 53, 1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Iori V, Wright CI, French J, Vezzani A, 2011. Interleukin-1beta biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics 8, 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A, 2010. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nature medicine 16, 413–419. [DOI] [PubMed] [Google Scholar]
- Marsh EB, Freeman JM, Kossoff EH, Vining EPG, Rubenstein JE, Pyzik PL, Hemingway C, 2006. The Outcome of Children with Intractable Seizures: A 3- to 6-Year Follow-up of 67 Children Who Remained on the Ketogenic Diet Less Than One Year. Epilepsia 47, 425–430. [DOI] [PubMed] [Google Scholar]
- Matin N, Tabatabaie O, Falsaperla R, Lubrano R, Pavone P, Mahmood F, Gullotta M, Serra A, Di Mauro P, Cocuzza S, Vitaliti G, 2015. Epilepsy and innate immune system: A possible immunogenic predisposition and related therapeutic implications. Hum Vaccin Immunother 11, 2021–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder AG., Patial V., Singh D., 2019. Mycophenolate mofetil contributes to downregulation of the hippocampal interleukin type 2 and 1beta mediated PI3K/AKT/mTOR pathway hyperactivation and attenuates neurobehavioral comorbidities in a rat model of temporal lobe epilepsy. Brain Behav Immun 75, 84–93. [DOI] [PubMed] [Google Scholar]
- McDaniel SS, Wong M, 2011. Therapeutic role of mammalian target of rapamycin (mTOR) inhibition in preventing epileptogenesis. Neurosci Lett 497, 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michinaga S, Koyama Y, 2019. Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage. Int J Mol Sci 20, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milikovsky DZ, Ofer J, Senatorov VV, Friedman AR, Prager O, Sheintuch L, Elazari N, Veksler R, Zelig D, Weissberg I, Bar-Klein G, Swissa E, Hanael E, Ben-Arie G, Schefenbauer O, Kamintsky L, Saar-Ashkenazy R, Shelef I, Shamir MH, Goldberg I, Glik A, Benninger F, Kaufer D, Friedman A, 2019. Paroxysmal slow cortical activity in Alzheimer’s disease and epilepsy is associated with blood-brain barrier dysfunction. Science Translational Medicine 11, eaaw8954. [DOI] [PubMed] [Google Scholar]
- Mogensen TH, 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22, 240–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Schwarze AB, Tandon P, Liu Z, Yang Y, Holmes GL, Stafstrom CE, 1999. Ketogenic diet reduces spontaneous seizures and mossy fiber sprouting in the kainic acid model. Neuroreport 10, 1517–1522. [DOI] [PubMed] [Google Scholar]
- Musto AE, Rosencrans RF, Walker CP, Bhattacharjee S, Raulji CM, Belayev L, Fang Z, Gordon WC, Bazan NG, 2016. Dysfunctional epileptic neuronal circuits and dysmorphic dendritic spines are mitigated by platelet-activating factor receptor antagonism. Sci Rep 6, 30298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Brewster AL, Clark ME, Regnier-Golanov A, Sunnen CN, Patil VV, D’Arcangelo G, Anderson AE, 2015. mTOR inhibition suppresses established epilepsy in a mouse model of cortical dysplasia. Epilepsia 56, 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Mahadeo T, Bordey A, 2019. mTOR Hyperactivity Levels Influence the Severity of Epilepsy and Associated Neuropathology in an Experimental Model of Tuberous Sclerosis Complex and Focal Cortical Dysplasia. J Neurosci 39, 2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niere F, Raab-Graham KF, 2017. mTORC1 Is a Local, Postsynaptic Voltage Sensor Regulated by Positive and Negative Feedback Pathways. Front Neurol 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova KA, Parker WE, Heuer GG, Tsai V, Yoon J, Baybis M, Fenning RS, Strauss K, Crino PB, 2010. STRADalpha deficiency results in aberrant mTORC1 signaling during corticogenesis in humans and mice. J Clin Invest 120, 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostendorf AP, Wong M, 2015. mTOR inhibition in epilepsy: rationale and clinical perspectives. CNS Drugs 29, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Stone KP, Hsuchou H, Manda VK, Zhang Y, Kastin AJ, 2011. Cytokine signaling modulates blood-brain barrier function. Curr Pharm Des 17, 3729–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT, 2011. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- Patel A, Pyzik PL, Turner Z, Rubenstein JE, Kossoff EH, 2010. Long-term outcomes of children treated with the ketogenic diet in the past. Epilepsia 51, 1277–1282. [DOI] [PubMed] [Google Scholar]
- Peltola J, Palmio J, Korhonen L, Suhonen J, Miettinen A, Hurme M, Lindholm D, Keranen T, 2000. Interleukin-6 and interleukin-1 receptor antagonist in cerebrospinal fluid from patients with recent tonic-clonic seizures. Epilepsy Res 41, 205–211. [DOI] [PubMed] [Google Scholar]
- Perluigi M, Di Domenico F, Butterfield DA, 2015. mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiology of disease 84, 39–49. [DOI] [PubMed] [Google Scholar]
- Polascheck N, Bankstahl M, Loscher W, 2010. The COX-2 inhibitor parecoxib is neuroprotective but not antiepileptogenic in the pilocarpine model of temporal lobe epilepsy. Exp Neurol 224, 219–233. [DOI] [PubMed] [Google Scholar]
- Pópulo H, Lopes JM, Soares P, 2012. The mTOR signalling pathway in human cancer. Int J Mol Sci 13, 1886–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Graham KF, Haddick PC, Jan YN, Jan LY, 2006. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science 314, 144–148. [DOI] [PubMed] [Google Scholar]
- Raffo E., Coppola A., Ono T., Briggs SW., Galanopoulou AS., 2011. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiology of disease 43, 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani AH, Alsahli MA, Aly SM, Khan MA, Aldebasi YH, 2018. Role of Curcumin in Disease Prevention and Treatment. Adv Biomed Res 7, 38–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A, Musto AE, 2018. The role of inflammation in the development of epilepsy. J Neuroinflammation 15, 144–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza T, Boer K, Redeker S, Spliet WG, van Rijen PC, Troost D, Vezzani A, Aronica E, 2006. The IL-1beta system in epilepsy-associated malformations of cortical development. Neurobiology of disease 24, 128–143. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A, 2008. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiology of disease 29, 142–160. [DOI] [PubMed] [Google Scholar]
- Reed CM, Mosher CP, Chandravadia N, Chung JM, Mamelak AN, Rutishauser U, 2019. Extent of single-neuron activity modulation by hippocampal interictal discharges predicts declarative memory disruption in humans. Journal of Neuroscience. [DOI] [PMC free article] [PubMed]
- Ren Z, Wang L, Cui J, Huoc Z, Xue J, Cui H, Mao Q, Yang R, 2013. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie 68, 689–694. [PubMed] [Google Scholar]
- Rodgers KM, Hutchinson MR, Northcutt A, Maier SF, Watkins LR, Barth DS, 2009. The cortical innate immune response increases local neuronal excitability leading to seizures. Brain 132, 2478–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A, Chen D, Ganesh T, Varvel NH, Dingledine R, 2019. The COX-2/prostanoid signaling cascades in seizure disorders. Expert Opin Ther Targets 23, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseti C, van Vliet EA, Cifelli P, Ruffolo G, Baayen JC, Di Castro MA, Bertollini C, Limatola C., Aronica E., Vezzani A., Palma E., 2015. GABAA currents are decreased by IL1beta in epileptogenic tissue of patients with temporal lobe epilepsy: implications for ictogenesis. Neurobiology of disease 82, 311–320. [DOI] [PubMed] [Google Scholar]
- Rosina E, Battan B, Siracusano M, Di Criscio L, Hollis F, Pacini L, Curatolo P, Bagni C, 2019. Disruption of mTOR and MAPK pathways correlates with severity in idiopathic autism. Transl Psychiatry 9, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma H, Tanuma N, Kuki I, Takahashi Y, Shiomi M, Hayashi M, 2015. Intrathecal overproduction of proinflammatory cytokines and chemokines in febrile infection-related refractory status epilepticus. J Neurol Neurosurg Psychiatry 86, 820–822. [DOI] [PubMed] [Google Scholar]
- Santoro C, Bernardo P, Coppola A, Pugliese U, Cirillo M, Giugliano T, Piluso G, Cinalli G, Striano S, Bravaccio C, Perrotta S, 2018. Seizures in children with neurofibromatosis type 1: is neurofibromatosis type 1 enough? Ital J Pediatr 44, 41–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM, 2006. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22, 159–168. [DOI] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM, 2017. mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B, 2012. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz F, Heit A, Dreher S, Eisenacher K, Mages J, Haas T, Krug A, Janssen KP, Kirschning CJ, Wagner H, 2008. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur J Immunol 38, 2981–2992. [DOI] [PubMed] [Google Scholar]
- Schneider M, de Vries PJ, Schonig K, Rossner V, Waltereit R, 2017. mTOR inhibitor reverses autistic-like social deficit behaviours in adult rats with both Tsc2 haploinsufficiency and developmental status epilepticus. Eur Arch Psychiatry Clin Neurosci 267, 455–463. [DOI] [PubMed] [Google Scholar]
- Seifert G, Carmignoto G, Steinhauser C, 2010. Astrocyte dysfunction in epilepsy. Brain Res Rev 63, 212–221. [DOI] [PubMed] [Google Scholar]
- Semple BD, O’Brien TJ, Gimlin K, Wright DK, Kim SE, Casillas-Espinosa PM, Webster KM, Petrou S, Noble-Haeusslein LJ, 2017. Interleukin-1 Receptor in Seizure Susceptibility after Traumatic Injury to the Pediatric Brain. J Neurosci 37, 7864–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha L-Z, Xing X-L, Zhang D, Yao Y, Dou W-C, Jin L-R, Wu L-W, Xu Q, 2012. Mapping the Spatio-Temporal Pattern of the Mammalian Target of Rapamycin (mTOR) Activation in Temporal Lobe Epilepsy. PloS one 7, e39152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, 2011. Promise of resveratrol for easing status epilepticus and epilepsy. Pharmacol Ther 131, 269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LM, Chen RJ, Zhang H, Jiang CM, Gong J, 2017. Cerebrospinal fluid neuron specific enolase, interleukin-1beta and erythropoietin concentrations in children after seizures. Childs Nerv Syst 33, 805–811. [DOI] [PubMed] [Google Scholar]
- Simeone TA, Matthews SA, Samson KK, Simeone KA, 2017. Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp Neurol 287, 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas HV, Shah U, 2017. Comorbidities of epilepsy. Neurol India 65, S18–s24. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Carmant L, 2015. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med 5, a022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D., Beattie EC., Seo JY., Malenka RC., 2005. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci 25, 3219–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SW, Cilio MR, Sogawa Y, Silveira DC, Holmes GL, Stafstrom CE, 2000. Timing of ketogenic diet initiation in an experimental epilepsy model. Brain Res Dev Brain Res 125, 131–138. [DOI] [PubMed] [Google Scholar]
- Sunnen CN, Brewster AL, Lugo JN, Vanegas F, Turcios E, Mukhi S, Parghi D, D’Arcangelo G, Anderson AE, 2011. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia 52, 2065–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A, 2006. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 281, 21362–21368. [DOI] [PubMed] [Google Scholar]
- Talos DM., Sun H., Zhou X., Fitzgerald EC., Jackson MC., Klein PM., Lan VJ., Joseph A., Jensen FE., 2012. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PloS one 7, e35885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Narazaki M, Kishimoto T, 2014. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6, a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM, 2002. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A 99, 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AW, Turnquist HR, Raimondi G, 2009. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 9, 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzot M, Soulillou JP, Dantal J, 2012. Mechanistic target of rapamycin inhibitors in solid organ transplantation: from benchside to clinical use. Curr Opin Organ Transplant 17, 626–633. [DOI] [PubMed] [Google Scholar]
- Troutman TD, Bazan JF, Pasare C, 2012. Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle 11, 3559–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang CK, Qi H, Liu LF, Zheng XF, 2007. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today 12, 112–124. [DOI] [PubMed] [Google Scholar]
- Tyan L, Sopjani M, Dermaku-Sopjani M, Schmid E, Yang W, Xuan NT, Shumilina E, Lang F, 2010. Inhibition of voltage-gated K+ channels in dendritic cells by rapamycin. Am J Physiol Cell Physiol 299, C1379–1385. [DOI] [PubMed] [Google Scholar]
- Uludag IF, Duksal T, Tiftikcioglu BI, Zorlu Y, Ozkaya F, Kirkali G, 2015. IL-1beta, IL6 and IL1Ra levels in temporal lobe epilepsy. Seizure 26, 22–25. [DOI] [PubMed] [Google Scholar]
- van Stuijvenberg M, Derksen-Lubsen G, Steyerberg EW, Habbema JD, Moll HA, 1998. Randomized, controlled trial of ibuprofen syrup administered during febrile illnesses to prevent febrile seizure recurrences. Pediatrics 102, E51. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Forte G, Holtman L, den Burger JCG, Sinjewel A, de Vries HE, Aronica E, Gorter JA, 2012. Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood–brain barrier leakage but not microglia activation. Epilepsia 53, 1254–1263. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Otte WM, Wadman WJ, Aronica E, Kooij G, de Vries HE, Dijkhuizen RM, Gorter JA, 2016. Blood-brain barrier leakage after status epilepticus in rapamycintreated rats II: Potential mechanisms. Epilepsia 57, 70–78. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Ravizza T, 2019. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol 15, 459–472. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Richichi C, Aliprandi M, Burrows SJ, Ravizza T, Perego C, De Simoni MG, 2002. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia 43 Suppl 5, 30–35. [DOI] [PubMed] [Google Scholar]
- Vignoli A, La Briola F, Peron A, Turner K, Vannicola C, Saccani M, Magnaghi E, Scornavacca GF, Canevini MP, 2015. Autism spectrum disorder in tuberous sclerosis complex: searching for risk markers. Orphanet J Rare Dis 10, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgili M, Contestabile A, 2000. Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine antioxidant agent, trans-resveratrol in rats. Neurosci Lett 281, 123–126. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M, 2003. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci 23, 8692–8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Bo QY, Zhao XH, Yang X, Chi ZF, Liu XW, 2013. Resveratrol pre-treatment reduces early inflammatory responses induced by status epilepticus via mTOR signaling. Brain Res 1492, 122–129. [DOI] [PubMed] [Google Scholar]
- Wang X, Sha L, Sun N, Shen Y, Xu Q, 2017. Deletion of mTOR in reactive astrocytes suppresses chronic seizures in a mouse model of temporal lobe epilepsy. Mol Neurobiol 54, 175–187. [DOI] [PubMed] [Google Scholar]
- Wa SW., Rozas NS., Wu HC., McKenna J 3rd, Reith RM., Hashmi SS., Dash PK., Gambello MJ., 2012. The differential effects of prenatal and/or postnatal rapamycin on neurodevelopmental defects and cognition in a neuroglial mouse model of tuberous sclerosis complex. Hum Mol Genet 21, 3226–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichhart T, Hengstschlager M, Linke M, 2015. Regulation of innate immune cell function by mTOR. Nat Rev Immunol 15, 599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman EC, Yancy WS Jr., Mavropoulos JC, Marquart M, McDuffie JR, 2008. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond) 5, 36–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, 2013a. A critical review of mTOR inhibitors and epilepsy: from basic science to clinical trials. Expert Rev Neurother 13, 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, 2013b. Mammalian target of rapamycin (mTOR) pathways in neurological diseases. Biomed J 36, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Xu Q, Zhang L, Kong D, Ma R, Wang L, 2009. Protective effect of resveratrol against kainate-induced temporal lobe epilepsy in rats. Neurochem Res 34, 1393–1400. [DOI] [PubMed] [Google Scholar]
- Yoon M-S, 2017. The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling. Nutrients 9, 1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K, Kita Y, Kishimoto K, Shimizu T, 2006. Profiling of eicosanoid production in the rat hippocampus during kainic acid-induced seizure: dual phase regulation and differential involvement of COX-1 and COX-2. J Biol Chem 281, 14663–14669. [DOI] [PubMed] [Google Scholar]
- Yuskaitis CJ, Rossitto LA, Gurnani S, Bainbridge E, Poduri A, Sahin M, 2019. Chronic mTORC1 inhibition rescues behavioral and biochemical deficits resulting from neuronal Depdc5 loss in mice. Hum Mol Genet 28, 2952–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M, 2009. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci 29, 6964–6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M, 2008. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol 63, 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF, 2009. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci 29, 1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Yang C., Iyaswamy A., Krishnamoorthi S., Sreenivasmurthy SG., Liu J., Wang Z., Tong BC., Song J., Lu J., Cheung KH., Li M., 2019. Balancing mTOR Signaling and Autophagy in the Treatment of Parkinson’s Disease. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]