Abstract
90% of blood clots originate from the left atrial appendage (LAA) in non-valvular atrial fibrillation (AF) patients and are a major cause of embolic stroke. Long-term anticoagulation therapy has been used to prevent thrombus formation, but its use is limited in patients at high risk for bleeding complications. Thus, left atrial appendage closure (LAAC) devices for LAA occlusion are well established as an alternative to the anticoagulation therapy. However, anticoagulation therapy is still required for at least 45 days post-implantation to bridge the time until complete LAA occlusion by neo-endocardium coverage of the device. In this study, we applied the endothelium-mimicking nanomatrix to the LAAC device membrane for delivery of nitric oxide (NO) to enhance endothelialization, with the goal of possibly being able to reduce the duration of anticoagulation therapy. The nanomatrix was uniformly coated on the LAAC device membranes and provided sustained release of NO for up to one month in vitro. In addition, the nanomatrix coating promoted endothelial cell proliferation and reduced platelet adhesion compared to the uncoated device membranes in vitro. The nanomatrix coated and uncoated LAAC devices were then deployed in a canine LAA model for 22 days as a pilot study. All LAAC devices were not completely covered by neo-endocardium 22 days post implantation. However, histology image analysis showed that the nanomatrix coated LAAC device may have thicker neo-endocardium coverage compared to the uncoated device. Therefore, our in vitro and in vivo results indicate that the nanomatrix coating has the potential to enhance endothelialization on the LAAC device membrane, which could improve patient outcomes by shortening the need for extended anticoagulation treatment.
Keywords: Nitric oxide, nanomatrix, left atrial appendage closure device, endothelialization, neo-endocardium coverage
Graphical Abstract

INTRODUCTION.
Atrial fibrillation (AF) is the most common heart rhythm disorder that occurs with increasing frequency as people age. It is estimated that more than 2.3 million people in the United States currently have AF, and this is expected to increase to more than 5.6 million by 20501–2. Cardioembolic strokes are the most serious complication of AF and carry a high mortality rate. About 15–20% of all strokes have been reported to be caused by AF, which are estimated to cost $5 billion in the United States3–4. The disorganized electrical activity of AF causes abnormal contractions of the atrium resulting in blood stasis and thrombus formation. The left atrial appendage (LAA) is a small pouch, shaped like a windsock, which is located in the muscle wall of the left atrium and is especially prone to thrombus formation5. In AF patients with intracardiac thrombi, 90% of the thrombi originate from the LAA6. The thrombus travels to and blocks major vessels in the body. Blockage of blood vessels in the brain leads to strokes and transient ischemic attacks7. The conventional therapy for stroke prevention in patients with AF is treatment with anticoagulants, but bleeding complications hinder its use in many eligible patients. Warfarin is an effective means for stroke reduction but can present challenges such as increased bleeding risk, poor adherence rates due to daily regimen requirements, the need to monitor international normalized ratio (INR), as well as potential interactions with foods and certain drugs. Compared to warfarin, novel oral anticoagulants, such as dabigatran, rivaroxaban, apixaban, and edoxaban demonstrate an effective reduction of stroke in patients with AF. However, the risk of bleeding, lack of readily available antidotes, and drug discontinuation rates are still unacceptable8–11.
Thus, in order to minimize the risk of embolic stroke in patients with non-valvular AF who are at an unacceptably high risk for long-term oral anticoagulant therapy, percutaneous LAA occlusion strategies have been developed in order to prevent blood clots in the LAA from entering the blood stream. In order to address this issue, several left atrial appendage closure (LAAC) devices have been developed. These permanently implanted devices function by occluding the LAA thereby reducing the risk of thromboembolism, as well as decreasing the risk of bleeding by lessening the duration of oral anticoagulation treatments required. The major challenges of LAAC device implantation are slow neo-endocardial coverage of the device-LA interface and thrombus formation on the LAAC devices12–13. In the commercially available LAAC device trials, warfarin treatment was still required for at least 45 days post-implantation to bridge the time until complete neo-endocardium coverage14. Additionally, if there was persistent leak due to peridevice flow, warfarin was continued longer14. Even after warfarin discontinuation, dual antiplatelet therapy (aspirin and clopidogrel) was continued at least 6 months. Additionally, there were rates of 4.1% device-related thrombi (34 of 835 successfully implanted patients) and 32.1% peridevice flow leakage (143 of 445 patients) observed 12 months post-implantation14–16. In a separate LAAC device trial, 17.6% device-related thrombi (6 of 34 patients) at 12 months post-implantation were observed despite dual antiplatelet therapy17. These adverse events are likely caused by incomplete neo-endocardial coverage of the device-LA interface. Thus, stimulation of the healing process at the device-LA interface by promoting faster and durable endothelialization is expected to reduce adverse events from LAAC devices, while also decreasing the duration of oral anticoagulation therapy. In fact, further reduction in duration of oral anticoagulation therapy is key, as patients receiving LAAC device implantation are by definition considered at high bleeding risk. The Left Atrial Appendage Occlusion (LAAO) Registry, using National Cardiovascular Data Registry (NCDR) data for 38,158 patients, who received LAAC devices, demonstrated a mean HAS-BLED score of 3.0 +/− 1.1, indicating a high bleeding risk population18. An additional reduction in the duration of anticoagulant therapy could significantly improve safety in patients who have a very high risk for bleeding.
In this study, we developed a new approach to coat the endothelium-mimicking nanomatrix on LAAC devices. This nanomatrix delivers an important biological “cue” to improve the healing process for LAA occlusion without significant modification of the LAAC device itself. This “cue” is sustained release of nitric oxide (NO) from the endothelium-mimicking nanomatrix coating applied to LAAC devices, widely known as a key mediator of endothelialization. NO is produced from the endothelium of blood vessels and plays an important role in cardiovascular physiology, as it promotes vascular endothelial cell proliferation and migration, prevents platelet activation and aggregation, and inhibits vascular smooth muscle cell proliferation19–22. We synthesized the endothelium-mimicking nanomatrix using self-assembled peptide amphiphiles (PAs) including various bio-active peptide sequences21, 23–24. The nanomatrix contains a matrix metallopeptidase 2 (MMP2) enzyme mediated degradation sequence (GTAGLIGQ) connected to either an endothelial cell adhesive ligand (YIGSR) or a NO-producing donor sequence (KKKKK). The endothelial cell adhesive ligand promotes retention of endothelial cells and differentiation of endothelial progenitor cells25–26. NO can be released by the dissociation of NO from the surface of the nanomatrix coating, and then gradual biodegradation of the nanomatrix, which exposes layers of the nanomatrix, and provides a further sustained release of NO21. In addition, the self-assembled nanomatrix is free of toxic solvents for coating application to LAAC devices and minimizes the risk of inflammation. Thus, we hypothesized that the endothelium-mimicking nanomatrix coating on LAAC devices will promote more rapid healing and durable neo-endocardium coverage by stimulating endothelialization and reducing the risks of inflammatory responses (Scheme 1). To support the hypothesis, we coated the nanomatrix on the LAAC device membranes and evaluated the coated devices in vitro and in vivo. First, the ultrasonic spray coating system was used for nanomatrix coating on the LAAC device membranes. Then, in vitro coating characterization and cell studies were performed. Based on in vitro results, we applied the nanomatrix coated LAAC device into a dog LAA model for 22 days and analyzed the implants by gross and histology analysis as a pilot study. Our study provides a potential approach for enhancing endothelialization on the LAAC devices, thereby improving LAAC device function as well as LAA occlusion.
Scheme 1.

The endothelium-mimicking nanomatrix coated LAAC device to enhance endothelialization and promote LAA occlusion.
MATERIALS AND METHODS
Nanomatrix Synthesis
First, peptides were synthesized using Fmoc-chemistry on an Apex 396 peptide synthesizer as previously described21, 27–28. The peptides contained MMP-2 sensitive sequences with YIGSR (cell-adhesive ligand) or KKKKK (NO donating residue). Then, peptide amphiphiles (PAs: PA-YIGSR and PA-KKKKK) were created by alkylating the created peptides with palmitic acid. The endothelium-mimicking nanomatrix was prepared by mixing of PA-YIGSR (1 wt.%) and PA-KKKKK (1 wt.%) in a 9:1 ratio, respectively, and then reacted with NO gas in a 100 mL round bottom flask under Argon gas and left overnight to form PA-YK-NO21, 26.
Nanomatrix Coating Conditions
The nanomatrix coating was applied to the polyester membranes of LAAC devices (Watchman, Boston Scientific) using an ultrasonic spray coating machine (Exactacoat, Sonotek Corporation, NY). Using an Impact nozzle, the LAAC device membrane was completely coated employing an overlapping back and forth pattern (flow rate: 0.2 mL/min; speed: 50 mm/secs; shaping air: 1.5 PSI). Each layer was allowed to completely dry before coating an additional layer (dwell time: 15 secs). For in vitro coating characterization, LAAC devices were coated with varying thicknesses of 20, 30, and 48-layers. The coated LAAC devices were inserted into the delivery device and flushed with medical grade saline to mimic the clinical preparation for in vivo implantation.
Coating Characterization
Coating was characterized using scanning electron microscopy (SEM, Quanta 650 FEG, FEI) on the LAAC device membrane29. In addition, energy dispersive X-ray analysis (EDX) and nitrogen mapping were performed to analyze the surface elements of the nanomatrix coating on LAAC devices30.
NO release kinetics
Each coated LAAC device membrane was placed in 1 mL of phosphate buffered saline (PBS) in a 12 well plate. PBS samples were collected, and replaced with fresh PBS at day 1 and every 3 or 4 days up to one month. Samples were stored at −80°C until analysis. NO release from the nanomatrix coating was quantified using the Total NO kit (ThermoFisher, NC) and absorbance was measured at 540 nm in a microplate reader. The kit contains nitrate reductase which converts nitrate to nitrite. The converted nitrite is combined with Griess reagents (sulfanilamide and N-(1-naphthyl) ethylenediamine dihydrochloride)) to create an azo compound for colorimetric analysis21.
HAEC Proliferation
To assess the coating’s effects on endothelialization, human aortic endothelial cells (HAEC) were seeded (density: 30,000 cells/cm2) and cultured for 3 days on coated and uncoated LAAC device membranes. After 3-day incubation at 37°C, the membranes were stained with Calcein-AM (0.75 μL/mL PBS) (ThermoFisher, NC) and incubated at 37°C for 20 mins. After incubation the stained membranes were evaluated by live fluorescent image analysis for comparison31.
Platelet Adhesion
To assess the coating’s effects on platelet adhesion, Human Whole Blood platelets (Innovative Research, MI) were seeded (density: 6×106 platelets/mL) with uncoated and coated membranes and incubated at 37°C for 30 mins in a 24 well plate. Then, each sample was stained with Calcein-AM (0.75 μL/mL PBS) and evaluated by live fluorescent image analysis31.
In vivo device implantation
All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) compliance with Animal Care regulations. Animals (mongrel adult dogs; 26.6 ± 1.3 kg, 8 to 15 months of age, acquired by Charles River Laboratory) were administered a combination of warfarin (6 mg/day) and aspirin (81 mg/day) 1 day prior to the procedure and every day afterwards until termination. Monitoring of the international normalized ratio (INR) was done throughout the study. Medications were adjusted to maintain the INR between 2.0 and 3.032. The nanomatrix coated (30 layers) and uncoated LAAC devices were implanted in the left atrial appendage (LAA) of two dogs through the femoral vein for 22 days as previously described32–33. TEE and fluoroscopy were used to evaluate LAA size as well as device position and stability before releasing the device into the LAA. Heparin was administered (200 U/kg) to maintain an active clotting time of 250 to 300 secs. The electrocardiogram, invasive blood pressure, end tidal CO2, functional O2 saturation, and body temperature were continuously monitored during the device implantation. After deployment, any leakage around the LAAC device, pericardial effusion, mitral valve (MV) function, and left circumflex coronary artery (LCX) flow were evaluated32. At 22 days, the animals were sacrificed and underwent necropsy and tissue harvest.
Gross examination
Hearts were perfused with lactated Ringer’s solution, neutral-buffered formalin (NBF), followed by immersion in NBF for storage until analysis. Gross examination was conducted on the side and front aspect of the LAA containing the implanted device. Digital photographs of the LAA with the device were taken.
SEM analysis
For SEM analysis, tissue on the LAAC device surfaces were first dehydrated in ethanol in a graded series of 50% to 100%. The prepared devices were then placed in a SEM (JEOL JSM-IT500) and examined under low vacuum. Device images were obtained at 50x, 100x, 250x, 500x magnifications. After imaging, rehydration of all samples was done using a graded series of ethanol, 100% to 50%, and finally returned to fixative solution for histopathology.
Histopathology
After SEM imaging, tissue from the LAAC device membranes were collected for histopathology analysis. The tissue and mesh were separated from the device frame in three areas (left side, center, right side) and embedded in paraffin. Hematoxylin and eosin staining (H&E) was conducted on paraffin embedded tissue samples and observed under a light microscope.
Statistical Analysis
The in vitro data represented in the present study were tested for statistical significance using ANOVA with Tukey post hoc analysis and *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001 were defined as significant (at least n=3 per each group).
RESULTS AND DISCUSSIONS.
Nanomatrix coating characterization
The self-assembled nanomatrix consisting of PA-YK-NO (1 wt.%) was coated on LAAC device membranes using an ultrasonic spray coating system (Exactacoat, Sonotek Corporation, NY). Using this coating system, we controlled coating thickness, pattern, and time by modifying various parameters including flow rate, nozzle transverse speed, shaping air, distance (z-height), and dwell time34. After coating, the nanomatrix coated LAAC devices were compressed and inserted into the delivery device and flushed with medical grade saline to mimic the clinical preparation for in vivo implantation. A uniform nanomatrix coating was desired on LAAC device membranes to promote endothelialization across the device surface. Thus, coating surface and uniformity before and after saline flushing process were analyzed by scanning electron microscope (SEM). SEM imaging analysis of varied numbers of nanomatrix coating layers (uncoated, 20-, 30-, and 48-layers; one layer coating per each spray) indicated a uniform coating on the LAAC device membranes and visibly there was no significant difference in all nanomatrix coating layer groups before and after saline flush process (Figure 1A–D and Figure S1A–D). However, the 48-layer coating group began to show some webbing generation between the fiber mesh of the device membrane. In addition, the presence of some salt deposition was observed among all groups of membranes after the saline flushing process. Energy dispersive X-ray analysis (EDX) was also conducted to analyze surface elements of the saline flushed nanomatrix coated device membranes. All coated membranes displayed the presence of carbon (C), oxygen (O), and nitrogen (N), demonstrating that the nanomatrix was present on the membrane surface. However, uncoated membranes did not show the presence of N, indicating that the nanomatrix was not present (Figure 1E–H and Table S1). The elemental mapping for nitrogen (N-mapping) on the all flushed nanomatrix coated membranes showed well distributed nitrogen through the whole membrane surface, indicating that the nanomatrix was evenly coated on the membrane surface (Figure 1I–L).
Figure 1.
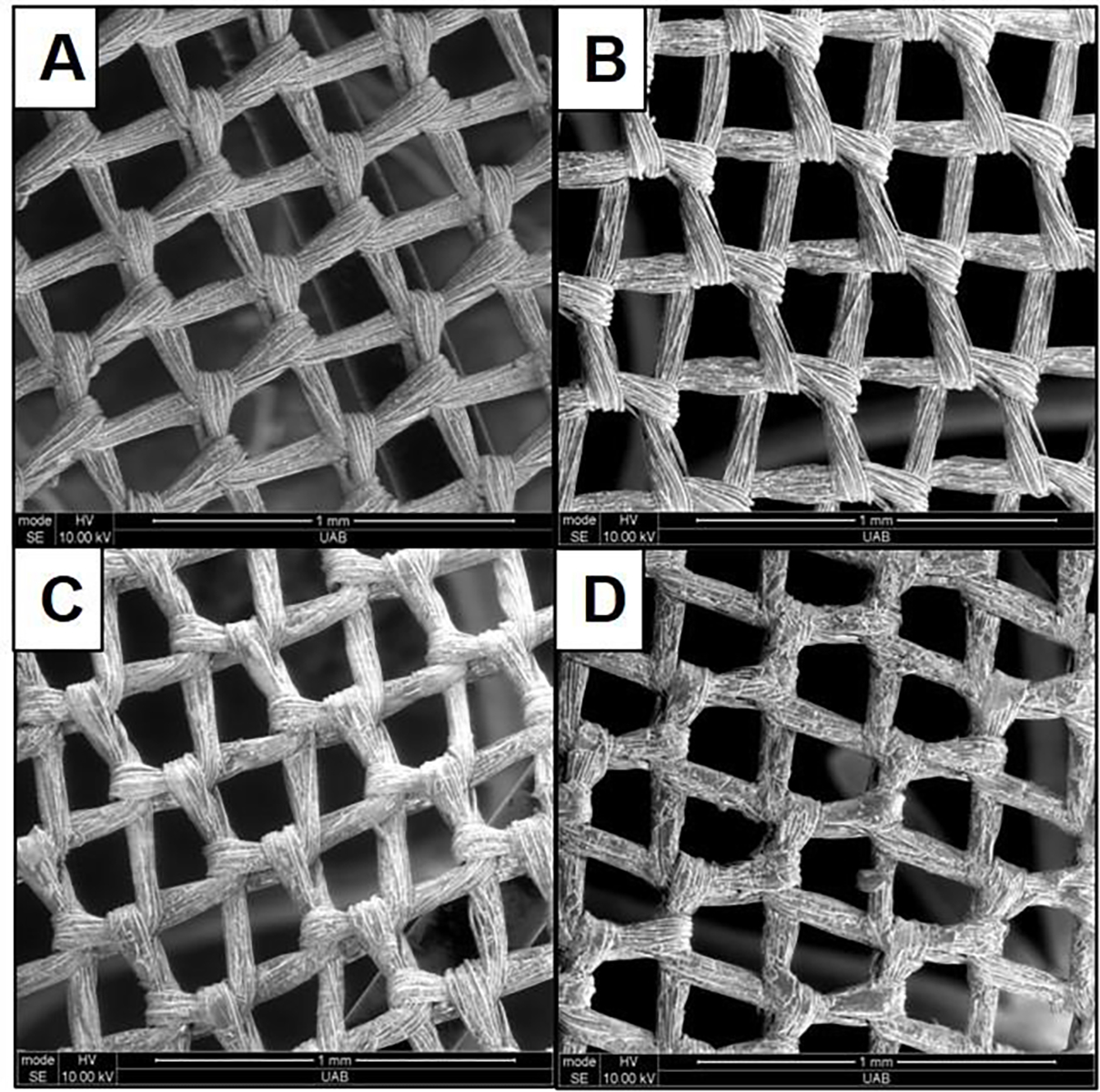


SEM images, EDX analyses, and N mapping of (a, e, and i) uncoated, (b, f, and j) 20-layers, (c, g, and k) 30-layers, and (d, h, and l) 48-layers nanomatrix coated LAAC device membranes after saline flushing, respectively. White scale bar (N-mapping): 200 μm.
In addition, the NO release from the different layers of nanomatrix coated membranes was evaluated using the Total NO assay. Successful NO release from all the different layers of nanomatrix coatings was found to be burst release in the first 24 hours, followed by slower, sustained release over a month-long period (Figure 2). Due to the saline flushing, all nanomatrix coatings showed similar NO release kinetics with a trend toward slightly more NO release from the 48-layer coating over time.
Figure 2.

Accumulated NO release kinetics from 20-, 30-, and 48-layers of nanomatrix coated LAAC devices after saline flushing for one month.
Effects of nanomatrix coating on endothelial cell proliferation and platelet adhesion on the LAAC device membranes in vitro
After coating characterization, an in vitro cell study using HAECs was performed to evaluate the effects of the nanomatrix coating on endothelial cell proliferation. Live fluorescent image analysis was conducted to assess HAEC proliferation on the nanomatrix coated membranes with different coating layers (20-, 30-, and 48-layers). HAECs showed greater cell adhesion and proliferation on all nanomatrix coated membranes compared to the uncoated membranes (Figure 3). There was no significant difference in HAEC proliferation among different degrees of layers of coating after 3 days. This may be attributed to the similar NO release from different coating layers after saline flushing could result in a lack of significant difference in endothelial cell proliferation on the different layers of nanomatrix coated membranes in vitro. Our results suggest that the nanomatrix coating could enhance endothelialization on the membranes in vivo. This was also confirmed by our previous studies that demonstrated similar results in which the nanomatrix coating promoted endothelial cell proliferation and migration23, 26. In addition, a platelet adhesion test on the nanomatrix coated membranes with different coating layers (20-, 30-, and 48-layers) was conducted by live fluorescent image analysis. Platelets were significantly less attached to the 30- and 48-layer nanomatrix coated membranes compared to the uncoated and 20-layer nanomatrix coated membranes 30 mins after treatment (Figure 4). This result may indicate that the nanomatrix coating could contribute to a reduction of inflammatory response on the device membranes since platelet adhesion and activation play an important role initiating inflammatory responses and promote thrombosis35–36. There was less platelet adhesion as the number of nanomatrix coating layers increased, so we may need to further investigate the effect of NO and the nanomatrix layer thickness on platelet adhesion.
Figure 3.

Live fluorescence image analysis of HAECs on (a) uncoated, (b) 20-layers, (c) 30-layers, and (d) 48-layers nanomatrix coated LAAC device membranes after saline flushing. (e) quantification fluorescent cell number for each group after 3 days. White scale bar: 500 μm. *p<0.05 vs. uncoated membrane.
Figure 4.

Live fluorescence image analysis of platelet adhesion on (a) uncoated, (b) 20-layers, (c) 30-layers, and (d) 48-layers nanomatrix coated LAAC device membranes after saline flushing. (e) quantification platelet number for each group after 30 minutes. White scale bar: 100 μm. **p<0.01 and ***p<0.001 vs. uncoated membrane.
The in vitro results demonstrate that the 30-layer nanomatrix coated membranes successfully promoted HAEC proliferation as well as reduced platelet adhesion. Importantly, the 30-layer nanomatrix coating displayed uniform nanomatrix coating without webbing. Our in vitro studies provide critical guidance for the coating conditions to be used for the in vivo application of the nanomatrix coated LAAC device, in order to enhance endothelialization of the device and occlusion of the LAA.
In vivo pilot study of nanomatrix coated LAAC device implantation in a LAA canine model
After in vitro characterization, we set up in vivo studies for the application of nanomatrix coated LAAC devices to the LAA of adult mongrel dogs. This pilot study provided proof of concept before setting up a large-scale animal study. Based on the results from in vitro studies, the 30-layer nanomatrix coated LAAC device was selected for in vivo implantation. The nanomatrix coated and uncoated devices were implanted in the LAA of two dogs for 22 days. After animal sacrifice, the implanted devices and surrounding tissues were harvested for analysis. Macroscopic gross examination of the atrial device surface was conducted in both nanomatrix coated and uncoated devices (Figure 5A and B). Both devices had good circumferential radial contact with the appendicular (auricular) wall resulting in complete occlusion of the LAA. In addition, both devices were not completely covered by neo-endocardium (light-tan-to-white tissue) 22 days post implantation. The nanomatrix coated device suggested visually higher neo-endocardium coverage compared to the uncoated device. SEM analysis also showed that the nanomatrix coated device surface had more tissue coverage than the uncoated device surface (Figure 5C and D). Microscopic histopathology evaluation was conducted using cross-sectional H&E staining of near the center of the device and surrounding tissues. The nanomatrix coated device showed a thicker layer of mature neo-endocardial/neo-intimal tissues with a continuous layer of neo-endothelium (black arrow) covering the mesh of the device membrane (blue arrow) (Figure 5F). However, the uncoated device group had a thinner layer of mature neo-endocardial/neo-intimal tissues compared to the nanomatrix coated device group (Figure 5E). There was no significant indication of foreign body responses on either device membrane nor infiltration of mononuclear cells (lymphocytes/macrophages) overlying the neo-endothelium. As a proof of concept, our results demonstrate the potential that nanomatrix coating could enhance neo-endothelium coverage. Therefore, our nanomatrix coating may provide a beneficial approach for LAAC device application as this biocompatible nanomatrix coating is easily applied without organic solvents and does not alter the function or characteristics of the device. We plan to perform a large scale in vivo study to confirm the findings from this pilot study.
Figure 5.

Gross and SEM images of atrial device surface for (a and c) uncoated and (b and d) nanomatrix coated devices at 22 days post implantation, respectively. Cross-sectional H&E staining images of (e) uncoated and (f) nanomatrix coated devices (center area) and surrounding tissues at 22 days. Black arrow: a layer of neo-endocardial/neo-intimal tissue with a continuous layer of neo-endothelium. Blue arrow: a device membrane. Black scale bar: 100 μm.
CONCLUSIONS
In summary, we successfully coated the NO releasing endothelium-mimicking nanomatrix on the LAAC device membranes and applied the nanomatrix coated LAAC devices in the canine LAA model. First, we characterized nanomatrix coating with different layers (20-, 30-, and 48-layers) before and after saline flushing to mimic the conditions of clinical application. The coatings were uniformly and evenly distributed on the device membranes. The nanomatrix coatings showed a sustained release of NO for at least 30 days, even after saline flushing. In addition, the nanomatrix coatings promoted endothelial cell proliferation and reduced platelet adhesion on the LAAC device membranes in vitro. Based on coating characterization and cell studies, the 30-layer nanomatrix coating was selected for the in vivo pilot study. The nanomatrix coated and uncoated LAAC devices were implanted in the canine LAA for 22 days. Both devices showed complete occlusion with good circumferential radial contact. Analysis of gross imaging, SEM imaging, and histology indicated that the nanomatrix coating may promote a thicker neo-endocardium coverage on the LAAC device membrane. Therefore, based on the results from this pilot study, the endothelium-mimicking nanomatrix coating may provide an approach to enhance endothelialization on the LAAC device membrane without significant modification to the device itself.
Supplementary Material
ACKNOWLEDGEMENT.
SEM and EDX analyses were performed at UAB Institutional Research Core. Animal studies were conducted at Charles River Laboratory.
Funding Sources
This work was supported by funding through NIH 1R43HL137515-01 (Co-PI: Patrick Hwang & Dongming Hou), NIH 1R43 NS110114-01 (PI: Patrick Hwang), and R01HL125391 (PI: Ho-Wook Jun).
REFERENCES.
- 1.Go AS; Hylek EM; Phillips KA; Chang Y; Henault LE; Selby JV; Singer DE, Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285 (18), 2370–5. [DOI] [PubMed] [Google Scholar]
- 2.Akinapelli A; Bansal O; P. Chen J; Pflugfelder A; Gordon N; Stein K; Huibregtse B; Hou D, Left Atrial Appendage Closure-The WATCHMAN Device. Current Cardiology Reviews 2015, 11 (4), 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petty GW; Brown RD Jr.; Whisnant JP; Sicks JD; O’Fallon WM; Wiebers DO, Ischemic stroke subtypes : a population-based study of functional outcome, survival, and recurrence. Stroke 2000, 31 (5), 1062–8. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL; Go AS; Lloyd-Jones DM; Benjamin EJ; Berry JD; Borden WB; Bravata DM; Dai S; Ford ES; Fox CS; Fullerton HJ; Gillespie C; Hailpern SM; Heit JA; Howard VJ; Kissela BM; Kittner SJ; Lackland DT; Lichtman JH; Lisabeth LD; Makuc DM; Marcus GM; Marelli A; Matchar DB; Moy CS; Mozaffarian D; Mussolino ME; Nichol G; Paynter NP; Soliman EZ; Sorlie PD; Sotoodehnia N; Turan TN; Virani SS; Wong ND; Woo D; Turner MB; American Heart Association Statistics, C.; Stroke Statistics, S., Heart disease and stroke statistics−-2012 update: a report from the American Heart Association. Circulation 2012, 125 (1), e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigel R; Wunderlich NC; Ho SY; Arsanjani R; Siegel RJ, The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging 2014, 7 (12), 1251–65. [DOI] [PubMed] [Google Scholar]
- 6.Blackshear JL; Odell JA, Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996, 61 (2), 755–9. [DOI] [PubMed] [Google Scholar]
- 7.Shehata M; Yeow WL; Kar S, Cardiology patient page: device interventions for stroke prevention in atrial fibrillation. Circulation 2014, 129 (9), e360–2. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ; Ezekowitz MD; Yusuf S; Eikelboom J; Oldgren J; Parekh A; Pogue J; Reilly PA; Themeles E; Varrone J; Wang S; Alings M; Xavier D; Zhu J; Diaz R; Lewis BS; Darius H; Diener HC; Joyner CD; Wallentin L; Committee R-LS; Investigators, Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009, 361 (12), 1139–51. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR; Mahaffey KW; Garg J; Pan G; Singer DE; Hacke W; Breithardt G; Halperin JL; Hankey GJ; Piccini JP; Becker RC; Nessel CC; Paolini JF; Berkowitz SD; Fox KA; Califf RM; Investigators RA, Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011, 365 (10), 883–91. [DOI] [PubMed] [Google Scholar]
- 10.Giugliano RP; Ruff CT; Braunwald E; Murphy SA; Wiviott SD; Halperin JL; Waldo AL; Ezekowitz MD; Weitz JI; Spinar J; Ruzyllo W; Ruda M; Koretsune Y; Betcher J; Shi M; Grip LT; Patel SP; Patel I; Hanyok JJ; Mercuri M; Antman EM; Investigators EA-T, Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013, 369 (22), 2093–104. [DOI] [PubMed] [Google Scholar]
- 11.Granger CB; Alexander JH; McMurray JJ; Lopes RD; Hylek EM; Hanna M; Al-Khalidi HR; Ansell J; Atar D; Avezum A; Bahit MC; Diaz R; Easton JD; Ezekowitz JA; Flaker G; Garcia D; Geraldes M; Gersh BJ; Golitsyn S; Goto S; Hermosillo AG; Hohnloser SH; Horowitz J; Mohan P; Jansky P; Lewis BS; Lopez-Sendon JL; Pais P; Parkhomenko A; Verheugt FW; Zhu J; Wallentin L; Committees A; Investigators, Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011, 365 (11), 981–92. [DOI] [PubMed] [Google Scholar]
- 12.Sick PB; Schuler G; Hauptmann KE; Grube E; Yakubov S; Turi ZG; Mishkel G; Almany S; Holmes DR, Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2007, 49 (13), 1490–5. [DOI] [PubMed] [Google Scholar]
- 13.Holmes DR Jr.; Lakkireddy DR; Whitlock RP; Waksman R; Mack MJ, Left atrial appendage occlusion: opportunities and challenges. J Am Coll Cardiol 2014, 63 (4), 291–8. [DOI] [PubMed] [Google Scholar]
- 14.Viles-Gonzalez JF; Kar S; Douglas P; Dukkipati S; Feldman T; Horton R; Holmes D; Reddy VY, The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol 2012, 59 (10), 923–9. [DOI] [PubMed] [Google Scholar]
- 15.Sedaghat A; Nickenig G; Schrickel JW; Ince H; Schmidt B; Protopopov AV; Betts TR; Gori T; Sievert H; Mazzone P; Grygier M; Wald C; Vireca E; Allocco D; Boersma LVA; group E. s., Incidence, predictors and outcomes of device-related thrombus after left atrial appendage closure with the WATCHMAN device-Insights from the EWOLUTION real world registry. Catheter Cardiovasc Interv 2021. [DOI] [PubMed] [Google Scholar]
- 16.Yu J; Bai Y; Jiang LS, Device related thrombus after left atrial appendage closure: State of the art. Pacing Clin Electrophysiol 2020. [DOI] [PubMed] [Google Scholar]
- 17.Plicht B; Konorza TF; Kahlert P; Al-Rashid F; Kaelsch H; Janosi RA; Buck T; Bachmann HS; Siffert W; Heusch G; Erbel R, Risk factors for thrombus formation on the Amplatzer Cardiac Plug after left atrial appendage occlusion. JACC Cardiovasc Interv 2013, 6 (6), 606–13. [DOI] [PubMed] [Google Scholar]
- 18.Freeman JV; Varosy P; Price MJ; Slotwiner D; Kusumoto FM; Rammohan C; Kavinsky CJ; Turi ZG; Akar J; Koutras C; Curtis JP; Masoudi FA, The NCDR Left Atrial Appendage Occlusion Registry. J Am Coll Cardiol 2020, 75 (13), 1503–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z; Yang Y; Xiong K; Li X; Qi P; Tu Q; Jing F; Weng Y; Wang J; Huang N, Nitric oxide producing coating mimicking endothelium function for multifunctional vascular stents. Biomaterials 2015, 63, 80–92. [DOI] [PubMed] [Google Scholar]
- 20.Cyr AR; Huckaby LV; Shiva SS; Zuckerbraun BS, Nitric Oxide and Endothelial Dysfunction. Crit Care Clin 2020, 36 (2), 307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kushwaha M; Anderson J; Minor W; Andukuri A; Bosworth C; Lancaster J; Brott B; Anderson P; Jun HW, Native Endothelium Mimicking Self-assembled Nanomatrix for Cardiovascular Devices. Biomaterials 2010, 31, 1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tousoulis D; Kampoli AM; Tentolouris C; Papageorgiou N; Stefanadis C, The role of nitric oxide on endothelial function. Curr Vasc Pharmacol 2012, 10 (1), 4–18. [DOI] [PubMed] [Google Scholar]
- 23.Alexander GC; Hwang PTJ; Chen J; Kim J; Brott BC; Yoon YS; Jun HW, Nanomatrix Coated Stent Enhances Endothelialization but Reduces Platelet, Smooth Muscle Cell, and Monocyte Adhesion under Physiologic Conditions. ACS Biomater Sci Eng 2018, 4 (1), 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander GC; Vines JB; Hwang P; Kim T; Kim JA; Brott BC; Yoon YS; Jun HW, Novel Multifunctional Nanomatrix Reduces Inflammation in Dynamic Conditions in Vitro and Dilates Arteries ex Vivo. ACS Appl Mater Interfaces 2016, 8 (8), 5178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andukuri A; Sohn Y; Anakwenze C; Lim D; Brott B; Yoon Y; Jun H, Enhanced human endothelial progenitor cell adhesion and differentiation by a bioinspired multifunctional nanomatrix. Tissue Engineering Part C Method 2013, 19, 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andukuri A; Kushwaha M; Tambralli A; Anderson JM; Dean DR; Berry JL; Sohn YD; Yoon YS; Brott BC; Jun HW, A hybrid biomimetic nanomatrix composed of electrospun polycaprolactone and bioactive peptide amphiphiles for cardiovascular implants. Acta Biomater 2011, 7 (1), 225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Ferzli GT; Andukuri A; Alexander G; Scopel M; Ambalavanan N; Patel RP; Jun HW, A Nitric Oxide-Releasing Self-Assembled Peptide Amphiphile Nanomatrix for Improving the Biocompatibility of Microporous Hollow Fibers. ASAIO J 2015, 61 (5), 589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andukuri A; Min I; Hwang P; Alexander G; Marshall LE; Berry JL; Wick TM; Joung YK; Yoon YS; Brott BC; Han DK; Jun HW, Evaluation of the effect of expansion and shear stress on a self-assembled endothelium mimicking nanomatrix coating for drug eluting stents in vitro and in vivo. Biofabrication 2014, 6 (3), 035019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang PT; Lim DJ; Fee T; Alexander GC; Tambralli A; Andukuri A; Tian L; Cui W; Berry J; Gilbert SR; Jun HW, A bio-inspired hybrid nanosack for graft vascularization at the omentum. Acta Biomater 2016, 41, 224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rokosz K; Hryniewicz T; Matysek D; Raaen S; Valicek J; Dudek L; Harnicarova M, SEM, EDS and XPS Analysis of the Coatings Obtained on Titanium after Plasma Electrolytic Oxidation in Electrolytes Containing Copper Nitrate. Materials (Basel) 2016, 9 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijayan VM; Tucker BS; Hwang PTJ; Bobba PS; Jun HW; Catledge SA; Vohra YK; Thomas V, Non-equilibrium organosilane plasma polymerization for modulating the surface of PTFE towards potential blood contact applications. J Mater Chem B 2020, 8 (14), 2814–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kar S; Hou D; Jones R; Werner D; Swanson L; Tischler B; Stein K; Huibregtse B; Ladich E; Kutys R; Virmani R, Impact of Watchman and Amplatzer devices on left atrial appendage adjacent structures and healing response in a canine model. JACC Cardiovasc Interv 2014, 7 (7), 801–9. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz RS; Holmes DR; Van Tassel RA; Hauser R; Henry TD; Mooney M; Matthews R; Doshi S; Jones RM; Virmani R, Left atrial appendage obliteration: mechanisms of healing and intracardiac integration. JACC Cardiovasc Interv 2010, 3 (8), 870–7. [DOI] [PubMed] [Google Scholar]
- 34.Bose S; Keller SS; Alstrom TS; Boisen A; Almdal K, Process optimization of ultrasonic spray coating of polymer films. Langmuir 2013, 29 (23), 6911–9. [DOI] [PubMed] [Google Scholar]
- 35.Wagner DD; Burger PC, Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol 2003, 23 (12), 2131–7. [DOI] [PubMed] [Google Scholar]
- 36.Koupenova M; Clancy L; Corkrey HA; Freedman JE, Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ Res 2018, 122 (2), 337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


