Abstract
Introduction:
Health systems in high-income countries face a variety of challenges calling for a systemic approach to improve quality and efficiency. Putting people in the centre is the main idea of the WHO model of people-centred and integrated health services. Integrating health services is fuelled by an integration of health data with great potentials for decision support based on big data analytics. The research question of this paper is “How can big data analytics support people-centred and integrated health services?”
Methods:
A scoping review following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses – Scoping Review (PRISMA-ScR) statement was conducted to gather information on how big data analytics can support people-centred and integrated health services. The results were summarized in a role model of a people-centred and integrated health services platform illustrating which data sources might be integrated and which types of analytics might be applied to support the strategies of the people-centred and integrated health services framework to become more integrated across the continuum of care. Additional rapid literature reviews were conducted to generate frequency distributions of the most often used data types and analytical methods in the medical literature. Finally, the main challenges connected with big data analytics were worked out based on a content analysis of the results from the scoping literature review.
Results:
Based on the results from the rapid literature reviews the most often used data sources for big data analytics (BDA) in healthcare were biomarkers (39.3%) and medical images (30.9%). The most often used analytical models were support vector machines (27.3%) and neural networks (20.4%). The people-centred and integrated health services framework defines different strategic interventions for health services to become more integrated. To support all aspects of these interventions a comparably integrated platform of health-related data would be needed, so that a role model labelled as people-centred health platform was developed. Based on integrated data the results of the scoping review (n = 72) indicate, that big data analytics could for example support the strategic intervention of tailoring personalized health plans (43.1%), e.g. by predicting individual risk factors for different therapy options. Also BDA might enhance clinical decision support tools (31.9%), e.g. by calculating risk factors for disease uptake or progression. BDA might also assist in designing population-based services (26.4% by clustering comparable individuals in manageable risk groups e.g. mentored by specifically trained, non-medical professionals. The main challenges of big data analytics in healthcare were categorized in regulatory, (information-) technological, methodological, and cultural issues, whereas methodological challenges were mentioned most often (55.0%), followed by regulatory challenges (43.7%).
Discussion:
The BDA applications presented in this literature review are based on findings which have already been published. For some important components of the framework on people-centred care like enhancing the role of community care or establishing intersectoral partnerships between health and social care institutions only few examples of enabling big data analytical tools were found in the literature. Quite the opposite does this mean that these strategies have less potential value, but rather that the source systems in these fields need to be further developed to be suitable for big data analytics.
Conclusions:
Big data analytics can support people-centred and integrated health services e.g. by patient similarity stratifications or predictions of individual risk factors. But BDA fails to unfold its full potential until data source systems are still disconnected and actions towards a comprehensive and people-centred health-related data platform are politically insufficiently incentivized. This work highlighted the potential of big data analysis in the context of the model of people-centred and integrated health services, whereby the role model of the person-centered health platform can be used as a blueprint to support strategies to improve person-centered health care. Likely because health data is extremely sensitive and complex, there are only few practical examples of platforms to some extent already capable of merging and processing people-centred big data, but the integration of health data can be expected to further proceed so that analytical opportunities might also become reality in the near future.
Keywords: Big Data, people-centred and integrated health services, advanced analytics, Personal Health Record, health platform, machine Learning
Abstract
Einleitung:
Gesundheitssysteme in entwickelten Ländern stehen vor vielen Herausforderungen in Bezug auf die Verbesserung von Qualität und Effizienz, die zum Teil nur durch Veränderungen auf der Systemebene bewältigt werden können. Die Versorgung im Einklang mit den Bedürfnissen von Personen zu gestalten ist der zentrale Gedanke des WHO-Modells der personen-zentrierten und integrierten Versorgung (people-centred and integrated health services). Die Integration der Gesundheitsversorgung wird zusätzlich durch die Integration von Gesundheitsdaten mit enormen Potenzialen zur Entscheidungsunterstützung durch die Analyse von Big Data vorangetrieben. Die Forschungsfrage dieser Arbeit ist „Wie kann die Analyse von Big Data eine personenzentrierte und integrierte Gesundheitsversorgung unterstützen?“
Methoden:
Es wurde ein Scoping Review gemäß „Preferred Reporting Items for Systematic Reviews and Meta-Analyses – Scoping Review (PRISMA-ScR)“ durchgeführt, um Informationen zusammenzutragen, wie Big Data Analysen eine personen-zentrierte und integrierte Versorgung unterstützen können. Sämtliche Ergebnisse wurden in einem Modell einer personen-zentrierten und integrierten Gesundheitsplattform zusammengefasst, die veranschaulicht, welche Datenquellen integriert und welche analytischen Modelle darauf angewendet werden könnten, um Strategien zu unterstützen, die darauf ausgerichtet sind, eine personen-zentrierte Versorgung voranzutreiben. Übersichtsartige Rapid Reviews wurden genutzt, um Häufigkeitsverteilungen der zum Zeitpunkt der Durchführung dieses Reviews am häufigsten verwendeten Datentypen und analytischen Methoden zur Analyse von Big Data in der medizinischen Literatur zu erstellen. Außerdem wurden die am häufigsten erwähnten Herausforderungen im Zusammenhang mit der Analyse von Big Data im Gesundheitswesen auf Basis einer Inhaltsanalyse der Artikel aus dem Scoping Review herausgearbeitet.
Ergebnisse:
Basierend auf den Ergebnissen der Rapid Reviews sind die derzeit am häufigsten genutzten Datenquellen für Big Data Analysen (BDA) im Gesundheitswesen genetische Biomarker-Daten (39,3%) und Daten der medizinischen Bildgebung (30,9%). Die am häufigsten genutzten analytischen Methoden sind Support Vector Machines (27,3%) und neurale Netzwerke (20,4%). Das WHO-Modell der personenzentrierten und integrierten Versorgung definiert verschiedene strategische Interventionen. Um sämtliche Aspekte dieser Interventionen unterstützen zu können, sollte die Datenintegration ähnlich umfassend ausfallen. Das im Zuge dieser Publikation entwickelte Modell der Integration von Gesundheitsdaten wird als „personen-zentrierte Gesundheitsplattform“ bezeichnet. Die Ergebnisse des Scoping Reviews legen nahe, dass, sofern ein Zugriff auf integrierte Daten möglich ist, die darauf basierenden BDA genutzt werden könnten, um (bessere) individualisierte Gesundheitspläne zu erstellen (43,1%), z.B. durch Prädiktion persönlicher Risikofaktoren in Bezug auf verschiedene Therapieoptionen. Außerdem könnten BDA klinische Systeme der Entscheidungsunterstützung erweitern und verbessern (31,9%), z.B. durch die Berechnung von Risikofaktoren für Krankheitsentstehung oder -entwicklung. Zusätzlich könnten BDA hilfreich für die Ausgestaltung populations-basierter Modelle der Gesundheitsversorgung (26,4%) sein, etwa durch Kategorisierung von Personen in vergleich- und steuerbare Risikogruppen, die u.a. von speziell ausgebildeten, nicht-medizinischen Spezialisten betreut werden könnten. Die am häufigsten erwähnten Herausforderungen im Zusammenhang mit BDA im Gesundheitswesen wurden in regulatorisch-gesetzgeberische, (informations-)technologische, methodische und kulturelle Aspekte kategorisiert, wobei die methodischen Herausforderungen am häufigsten erwähnt wurden (55,0%), gefolgt von regulatorischen Herausforderungen (43,7%).
Diskussion:
Die Ergebnisse dieses Reviews basieren ausschließlich auf Erkenntnissen, die bereits veröffentlicht wurden. Für einige wichtige Komponenten des WHO-Modell einer personen-zentrierten Versorgung wie z.B. der verstärkten Bedeutung wohnortnaher Versorgungsangebote oder der Etablierung von Kooperationen zwischen Institutionen der Gesundheits- und Sozialversorgung wurden nur wenige Beispiele in der Literatur gefunden, wie diese Aspekte durch die Auswertung von Big Data verbessert werden könnten. Dies soll nicht den Anschein erwecken, dass diese Interventionen einen geringeren strategischen Wert haben, sondern es ist eher ein Indiz dafür, dass die Datenquellen in diesen Bereichen noch weiterentwickelt und integriert werden müssen, um Big Data Analytics zu ermöglichen.
Schlussfolgerung:
BDA können die Interventionen einer personen-zentrierten und integrierten Versorgung unterstützen, z.B. durch Risikostratifizierung oder durch Prädiktion individueller Erfolgs- und Risikofaktoren. Aber derzeit sind BDA noch nicht in der Lage ihr volles Potenzial zu entfalten, da die Quellsysteme von Gesundheitsdaten größtenteils nicht verknüpft bzw. integriert sind und politisch zu wenig Anreize für die Entwicklung personen-zentrierter Gesundheitsplattformen gesetzt werden. Diese Arbeit beleuchtete die Potenziale von Big Data Analysen im Zusammenhang mit dem Modell einer personenzentrierten Gesundheitsversorgung, wobei das Modell der personen-zentrierten Gesundheitsplattform als Blaupause zur Unterstützung von Strategien zur Verbesserung personenzentrierter Gesundheitsversorgung genutzt werden kann. Aufgrund der Komplexität und Sensibilität von Gesundheitsdaten gibt es nur wenige Beispiele von Gesundheitsplattformen, die bereits sämtliche Typen von personenzentrierten Gesundheitsdaten für Big Data Analysen integrieren, aber es darf erwartet werden, dass die Integration von Gesundheitsdaten weiter voranschreiten wird, so dass auch die analytischen Anwendungen in naher Zukunft eine echte Unterstützung der Versorgungsrealität werden können.
Introduction
Despite differing institutional arrangements, health systems in developed countries face a variety of similar challenges including financial constraints, a rising demand for health services due to demographic changes, increasing multi-morbidity and unhealthy behaviours as well as growing expectations of citizens [1]. These challenges arise from and are reinforced by misaligned financing and highly fragmented processes of health care delivery [2]. To meet these challenges, there is a need for a systemic approach to improve treatment processes focusing on improvements of quality and efficiency [3,4]. Transformation toward value-based healthcare is accompanied by a change in focus from provider-centred models, with a lack of coordination across sectors, to more patient-centred models of healthcare delivery [5] as described in the people-centred and integrated health services (PCIHS) framework [6]. Putting people rather than providers or diseases in the centre, PCIHS will foster people-centred models of data integration and vice versa will progresses in computational storage and processing power [7] as well as accelerating adoptions of electronic data sources facilitate health service integration [8,9,10,11] and support activities towards the triple aim [12,13]. The emerging data sets and advanced analytical capabilities are believed to be part of the most important innovations in healthcare [14,15].
The research question “How can big data analytics support people-centred and integrated health services?” was investigated by performing a scoping literature review. Big data analytical applications which might act as enablers to the five strategical domains proposed by the WHO for health services to become more integrated and people-centred were thereby worked out. To the best of the authors’ knowledge a combination of the concepts of PCIHS and big data analytics (BDA) was not presented in any previous publication. The estimation, that transforming the already existing big data assets into actionable knowledge could reduce costs only in the healthcare system of the USA by $300 to $450 billion per year [16] demonstrates the potential impact of BDA. The results presented in this work might be helpful for health policy in reinventing health systems as well as for providers and other healthcare decision makers struggling to work collaboratively within the context of their health systems.
Materials and methods
At first some key terms will be briefly defined before describing the methodology of the scoping literature review and the additional rapid literature reviews.
People-centred, integrated health services (PCIHS)
Designing health services in accordance with the determinants of health spanning biophysical, lifestyle-related, social, health system-related, and environmental factors challenges traditional disease-centred, fragmented models of health service delivery [17,18]. In response to the challenges in healthcare, different concepts of integrated care emerged, centred on the needs of patients, their families, and their communities [19]. The concepts vary in size and scope and are designed around the idea to put people in the centre of service delivery to improve value-creation [3,20]. Several of these approaches including the rainbow model of care were considered when researches designed the framework for people-centred and integrated health services (PCIHS) for the World Health Organization (WHO) [18]. In the WHO’s global vision, not only does it outline achieving a seamless patient experience but also focusing on health promotion and disease prevention for the people, which may not necessarily be patients yet [2]. Improving healthcare following this people-centred perspective must focus on all the potential interrelations of the determinants of health and uniting the diverse objectives of healthcare stakeholders [21,22,23,24,25,26] across the continuum of health promotion, disease prevention, disease detection and acute, chronic, and palliative care [24,25,27,28,29].
The PCIHS framework proposes five strategies for health services to become more integrated [30,31,32]:
Empowering and engaging people and communities(e.g. personalized health plans, shared decision making, access to health records)
Strengthening governance and accountability(e.g. acting upon user experience, decentralization, performance evaluation)
Reorienting the model of care(e.g. strengthening primary and community care, population health, prevention)
Coordinating services within and across sectors(e.g. care coordination, effective referral and discharge systems, coordinated systems)
Creating an enabling environment(e.g. large scale systems change, strong leadership, financial support, cultural change)
Big data
Although a consensus about the definition does not exist, it can be agreed upon that massive data storage alone does not define big data [27,33]. The definition referenced most often is rooting in the 3-V model focusing on the characteristics of volume, velocity, and variety [34], which was gradually enhanced to the 5-V model by adding veracity and value [14,35,36,37,38,39,40]. Accordingly big data is characterized by
| • | high volume | (big amount of data, often referred to as exceeding tera- or petabytes), |
| • | high velocity | (fast speed of data generation like streaming data close to real-time), |
| • | high variety | (many diverse data formats and structures from multiple sources), |
| • | high veracity | (conformity with facts and closely related to data quality), |
| • | high value | (the information derived provides benefits to decision makers which in healthcare is closely related to the triple aim). |
Big data types in healthcare
The fragmentation of patient care is also reflected in the decentralization of health data [41,42]. In general, any source contributing information to one of the factors influencing people’s health can be valuable [22], although not all data types abide by all criteria of the 5V-model. The most common types data in healthcare are billing data, clinical data, patient- or people-generated data, health-related research data and data collected externally to the health care environment including socio-economical, societal, community-based, demographical, environmental, and other health-related data (see Table 1) [27,43,44].
Table 1.
Data types for big data analytics in healthcare by data generation point.
|
| ||
|---|---|---|
| DATA GENERATION POINTS | DATA TYPES | EXAMPLES ON TYPICAL DATA CONTENT |
|
| ||
| Transactions/billing with different payer organizations | Administrative data | Patient demographics, plan types, type of provider, location, … |
|
| ||
| Medical claims | In-/outpatient visits, diagnosis/procedure coding, referrals, … | |
|
| ||
| Pharmaceutical claims | Drug codes, dosages, prescription dates, manufacturer, … | |
|
| ||
| Ancillary claims | Medical equipment, physiotherapy, home health assistance, … | |
|
| ||
| Clinical/diagnostic processes of different provider organizations (e.g., health, social, aged or disability care) | Institutional data | Educational background, work experience, working times, … |
|
| ||
| EMR/EHR data | Vital signs, medical history, disease conditions, lab results, … | |
|
| ||
| Medical imaging | X-ray, magnetic resonance, computed tomography, ultrasonography, … | |
|
| ||
| Biomarker | “-omics”: genomics, proteomics, metabolomics, lipidomics, … | |
|
| ||
| Registries | Structured collection of disease/population specific measures | |
|
| ||
| Patient- or people-generated | Smart sensor/device data | Biometric data, physical activity, gait/sleep patterns, location, … |
|
| ||
| Web usage data | Social media posts, internet search logs, health forum activity, … | |
|
| ||
| Health-related research | Clinical trial data | Study size, clinically defined parameters and outcomes, … |
|
| ||
| Drug surveillance data | Adverse drug effects, population size, regional uptake/variation, … | |
|
| ||
| (Health) Survey data | Patient-reported outcome measures (PROMs), health literacy, … | |
|
| ||
| Health-related systems | Socio-economic/community-based data | Income, deprivation, education, living situation, marital status, … |
|
| ||
| Environmental/spatial data | Air/noise pollution, temperature, neighbourhood characteristics, … | |
|
| ||
A good overview on sources, stakeholders and capabilities in the health data ecosystem is provided by Vayena et al. [45].
Big data analytics (BDA) in healthcare
For big data analytics there is also no consented definition. Compliant to other industries analytical types in healthcare [38,46,47] can be categorized in
descriptive analytics (What happened or is happening?),
predictive analytics (What is likely to happen next?),
explorative analytics (Why is it happening? What is unknown yet?),
prescriptive analytics (Which decision is best to reach a desired outcome?).
From a methodological perspective the terms “prediction” and “exploration” do not define different approaches, but different analytical purposes [48]. Taken together predictive and explorative analytics are also referred to as advanced analytics [49]. Performing advanced analytics on big data is one approach to define big data analytics (BDA) [14,15]. In a broader sense all kinds of predictive or explorative models applied to big data would meet this definition, also including statistical methods [50] and most often when the aspect of high velocity is inconclusively. In a narrower sense only inductive approaches like data mining or machine learning suited for high-dimensional data sets define big data analytics [10,27,46,51,52]. For this paper the broader focus was adapted. Big data analytics (BDA) can provide complementary information to those derived from hypothesis-based experiments which have a long tradition in healthcare [46,51,53,54,55]. As there is plenty of literature on statistical methods they are not further explained (see e.g., Hohmann et al. [56]). Machine learning has the potential to enhance statistical analytics by providing models that allow for more multivariate effects and complex relationships. While supervised learning is used to train algorithms in predictions, unsupervised learning is used for exploring unknown patterns within data sets [7,57], whereas the analytical methods are basically the same as for both tasks [48,58].
Machine learning models
Supervised machine learning encompasses hypothesis-free algorithms which do not need assumptions about the data distribution. Furthermore, an inclusion of high-dimensional and highly correlated input variables is often appropriate for model optimization [36,56]. In course of supervised learning the target variable has to be (human-)labelled and the prediction is deducted normally based on three stages in a causal chain: training, validation and testing [56,59]. To train the model it analyses a set of observations to identify discriminating features of the predictor variable and performs optimization algorithms to reproduce the outcome [38,60].
Unsupervised machine learning models
Unsupervised learning algorithms are not provided with human labelled target variables and leave the probability of the input variables undefined [48]. They search for the most frequent simultaneous occurrence of certain (patient) characteristics not having a potential structure or hypothesis in mind [61]. By using unspecified criteria cohorts are not necessarily disease-derived but feature-derived enabling dynamic risk groups [22]. The algorithms shall separate low dimensional, unlabelled samples to find a hidden structure represented by the deduction of as many reasonable distinctive classes as possible [7]. Humans are normally reintegrated during the process of data interpretation, which is supported by visualizing the results using graphical models [62,63].
Review method and content analysis
To first of all provide a comparative overview on the “data types” and “analytical methods” used most often in healthcare, rapid literature reviews were conducted in Medline/PubMed combining the search terms of the scoping review described in the following with terms specifying the data types and analytical models (see Table 4 and Table 5 in the appendix).
To answer the main research question “How can big data analytics support people-centred and integrated health services?” a scoping review following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses – Scoping Review (PRISMA-ScR) statement [64] was conducted. To better define the search term text mining algorithms were applied [65]. The search term “big data analytics” was used as a starting point and checked for similarities and thesaurus on Medline/PubMed using the search results clustering algorithm Lingo [66,67]. The clustering was based on the first 200 results from a search conducted on April 1st, 2019 and revealed overlap of BDA with the terms “predictive analytics”, “advanced analytics”, “machine learning” and “big data analysis methods”.
A combination of these overlapping terms and Boolean operators was used to build the final search term. The search was conducted in Medline/PubMed as well as in the computer science database dblp (see Table 6 in the appendix). To limit the search results some inclusion and exclusion criteria were applied followed by a qualitative classification of two researchers working independently (see Table 7 in the appendix). For instance articles before 2013 were excluded as the number of articles meeting the inclusion criteria before that date were rather low and Natural Language Processing as a subfield of BDA was excluded because it yielded too much technical articles with few links to integrated care interventions as most often textual information were extracted and analysed from one single source of medical documentation.
To further extract information about strategic interventions in context of the PCIHS framework and about challenges for big data analytics in healthcare content analyses were performed during which the articles chosen for the review were classified (see Tables 8 and 9 in the appendix).
Results
After elimination of eight duplicates, the search set included 313 articles which were independently categorized by two researchers in “relevant” or “irrelevant” based on titles and abstracts. Disagreements were discussed after the screening process and a consented categorization was agreed upon. This led to 57 articles which were retrieved for full text screening during which two articles were rated as “irrelevant”. The bibliographies of the chosen 55 articles were scanned for a thorough review. Thereby 17 additional publications were added, so that 72 articles were included in the final set (see Figure 4 in the appendix). From the articles in the final set 64% were written by authors in North America, 22% in Europe (incl. UK), 7% in Asia, 3% in the Middle East, 3% in Australia and 1% in Africa (see Table in the appendix). The study type can be broken down in review (33%), case report (24%), quantitative study (18%), technical report (17%), guideline (7%) and survey (1%) (see Table 11 in the appendix). The study settings were scientific research (45%), hospital care (20%), population health management (19%), health insurance (7%), pharmaceutical care (4%), public health (3%) and community care (1%) (see Table 12 in the appendix).
A first and central result of the scoping review was that PCIHS fuel but are also dependent on people-centred models of health data integration and vice versa. If an idealistic model of health service delivery is people-centred and integrated, an idealistic health data analytical platform supporting strategies towards this aim would have to be equally people-centred and integrated. So to answer the research question “How can big data analytics support people-centred and integrated health services?” it seemed helpful to previously develop a role model labelled as people-centred health platform which frames the subsequently presented results of the review. This role model combines the health-related data types across the continuum of care with BDA methods to support the strategies of enabling people-centred care. Which of the data types and analytical methods displayed in the role model are currently used most often in the literature will be presented in the following section. The main research question how BDA can support PCIHS is answered subsequent via the scoping review. Finally, challenges arising from big data analytics in healthcare will be worked out by the content analysis.
Development of a role model of a people-centred health platform (PCHP)
The role model of a people-centred health platform presented in Figure 1 is purposely meant as a roadmap for decision makers to realize data analytical capabilities in healthcare like the PCIHS framework also is an illustration of options healthcare decision makers might consider in optimizing health services dependent on and adapted to their context conditions.
Figure 1.
![Role model of a people-centred health platform for big data analytics (EHR = electronic health record; PROMs = patient-reported outcome measures, with elements of [37])](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/463c/9205381/0eab33720c47/ijic-22-2-5543-g1.jpg)
Role model of a people-centred health platform for big data analytics (EHR = electronic health record; PROMs = patient-reported outcome measures, with elements of [37]).
In compliance with the concept of PCIHS all data potentially contributing relevant information about people’s health (rainbow model) were taken into account. Integrating these data in a central health platform as timely as possible (high velocity) would create a data asset of tremendous extent (high volume) and distinctness (high variety). In the data analytics layer big data analytical methods might be applied to the data with the purpose to produce results of high veracity which, interpreted and used by well-informed health decision makers, providers or even patients shall lead to decisions of high value in terms of the five strategies towards people-centred and integrated health services. Comprehensive personal health records are developed and tested by some research institutions [10,53,68,69] as well as in some real-world initiatives such as the national health platforms of Finland [70], Estonia or Australia [71] or from the US Veterans Health Administration [72].
Types of big data and big data analytical methods in healthcare – Results of the rapid literature review
According to the search results of the rapid literature review biomarker (39.3%) and medical imaging data (30.9%) are currently used most often in publications (see Figure 2). Biomarker data include the whole spectrum of ‘-omics’ like genomic, proteomic, or metabolomic data [73,74,75]. Medical images are often part of electronic health records. The most common technologies are ultrasound, computed tomography, magnetic resonance, and x-ray imaging [38,52].
Figure 2.
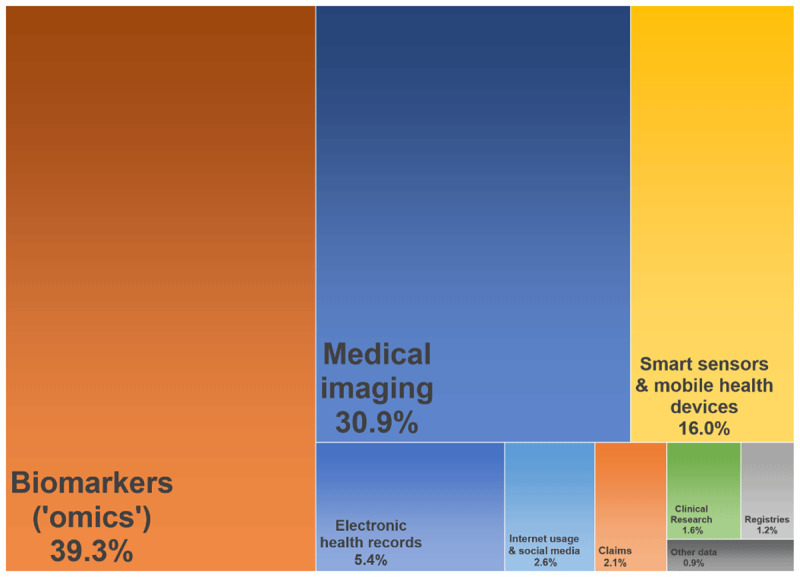
Data types most often applied for big data analyses in healthcare (April 2019), illustrated as tree map.
Considerably high rates were also found for smart sensor data (16,0%) and data from electronic health records (5.4%). A smart sensor can be used to constantly track individuals and is often embedded in a smart phone/watch or in telemonitoring devices, sometimes with several devices communicating with each other (Internet of Things). A smart sensor can continuously measure large volumes of data in terms of health, fitness, behaviour or lifestyle regardless of location, potentially in real-time and even supplemented by self-reported data (quantified-self) [22,27,38,43,74]. A side-specific electronic medical record (EMR) or a cross-institutional electronic health record (EHR) stores data stemming from different source systems which is why technically speaking EMR and EHR are rather data platforms than data types. The volume of data in EHR is massive on the health system level while it varies on the organizational level [37,76]. A typical EHR contains structured data (e.g. medical coding), semi-structured data (e.g. laboratory results) and unstructured data (e.g. narrative clinical notes, medical images) [43,77].
Data types used rather seldom were internet usage or social media data (2.6%), claims data (2.1%, most often health care data, rarely social care data), data from clinical trials (1.6%) and registry data (1.2%). Data generated by using internet technologies include access log data or click streams from websites, search engines, or forums or posts and network relationships from social media platforms or messaging services [22,38,78]. The most common claims data types are medical, pharmaceutical, and ancillary claims while payers hold additional administrative information [26,79]. Claims data are rather homogenous due to specific coding schemes, but at least the data provides a rather full picture of services utilization regardless of the point of care [80], whereas an all-payer database would be ideal for BDA supporting PCIHS so that analytics are not limited to the population covered by a single payer [81].
Other sources like patient surveys, drug surveillance, aged or community care data or other health-related systems together only accounted for less than 1% of current research articles on BDA in healthcare. For example, aged or community care data were presumably underrepresented because most of the provider organization are lacking the financial opportunities to build up and work with large, standardized databases although there would be additional value in using high level information technology and analytics in these contexts [82,83,84]. For PCIHS the integration of as many data sources as possible seems most beneficial.
Figure 3 displays the most often used BDA models in healthcare based on the rapid literature review. Support vector machines (27.3%), neural networks (20.4%) and random forests (19.5%) were used most often. Further models used occasionally were decision trees (6.7%), k-nearest neighbour models (6.1%), k-means clustering (1.9%) and Bayesian networks (1.4%). Traditional prediction models in healthcare are primarily parametric regression models based on assumptions regarding the data distribution and a predefined set of input variables [85]. Several studies retrieved in this review labelled their analytics as BDA by applying statistical models to data sources meeting more or less the definition of big data. Therefore considerably high rates were also found for statistical models like logistic regression (12.0%) and linear regression (3.7%) while other methods like multiple regression or proportional hazard models were used rather seldom (~1.0%). The results point to the fact that non-parametric models rather meet the general understanding of BDA in healthcare than traditional statistics.
Figure 3.
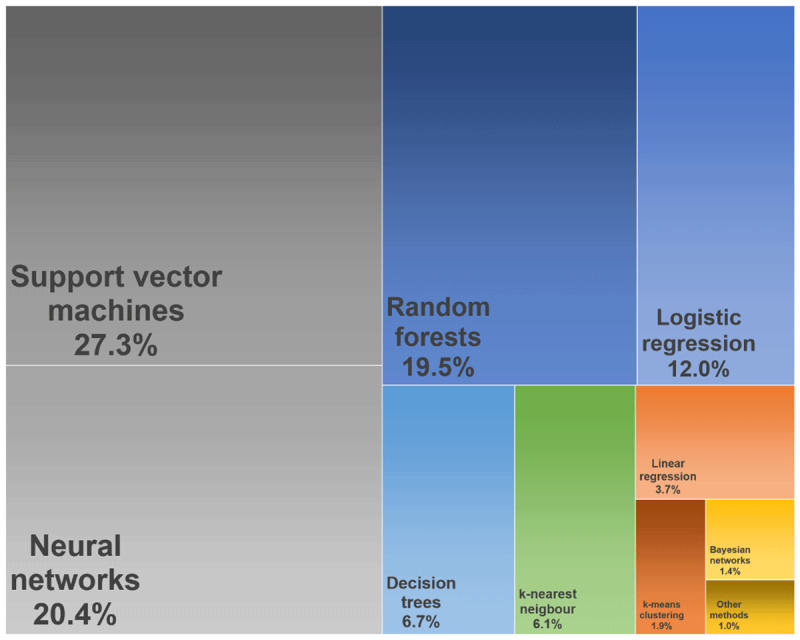
Distribution of the most often used big data analytical models in healthcare (April 2019), illustrated as tree map.
How can big data analytics support people-centred health services
A people-centred coordination of preventative, health, and social services (including aged and disability care) is likely impossible without an equally comprehensive integration of the underlying health information technology infrastructure [9,86]. In the scoping literature review articles were screened for analytical applications with the potential to support the five strategies for health services to become more integrated and people-centred. Based on a matrix table (see Table 8 in the appendix) all articles retrieved in the scoping review were categorized with respect to the five strategies of the PCIHS framework or rather to the respective policy options and strategical interventions. The results are summarized in Table 2.
Table 2.
The strategic interventions of the people-centred and integrated health services framework that might incorporate big data analytics (results of the in this scoping review and a content analysis, see also Table 8).
|
| |||||
|---|---|---|---|---|---|
| STRATEGIC DIRECTION | POLICY OPTIONS AND STRATEGICAL INTERVENTIONS POTENTIALLY SUPPORTED BY BDA | NUMBER OF PUBLICATIONS IN THE REVIEW (N = 72) | |||
|
| |||||
| Empowering and engaging people | 36 | (51%) | |||
|
| |||||
| Personalized care plans | 31 | 43% | |||
|
| |||||
| Self-management activities | 5 | 7% | |||
|
| |||||
| Shared decision making | 4 | 6% | |||
|
| |||||
| Health education | 3 | 4% | |||
|
| |||||
| Access to personal health records | 2 | 3% | |||
|
| |||||
| Peer support | 1 | 1% | |||
|
| |||||
| Patient satisfaction surveys | 1 | 1% | |||
|
| |||||
| Strengthening governance and accountability | 23 | 32% | |||
|
| |||||
| Performance evaluation | 15 | 21% | |||
|
| |||||
| Performance-based contracting | 8 | 11% | |||
|
| |||||
| Decentralization | 8 | 11% | |||
|
| |||||
| Patient-reported outcomes | 1 | 1% | |||
|
| |||||
| Reorienting the model of care | 56 | 79% | |||
|
| |||||
| Clinical decision support | 23 | 32% | |||
|
| |||||
| Tailoring population-based services | 19 | 27% | |||
|
| |||||
| Surveillance and control systems | 13 | 18% | |||
|
| |||||
| Mobile health technologies | 10 | 14% | |||
|
| |||||
| Health promotion and disease prevention | 9 | 13% | |||
|
| |||||
| Home and nursing care | 5 | 7% | |||
|
| |||||
| Coordinating services | 20 | 28% | |||
|
| |||||
| Care pathways | 8 | 11% | |||
|
| |||||
| Sharing of medical records | 6 | 8% | |||
|
| |||||
| Intersectoral partnerships | 5 | 7% | |||
|
| |||||
| District-based healthcare delivery | 1 | 1% | |||
|
| |||||
| Creating an enabling environment | 17 | 24% | |||
|
| |||||
| Resource allocation | 11 | 15% | |||
|
| |||||
| System research | 6 | 8% | |||
|
| |||||
| Quality assurance | 3 | 4% | |||
|
| |||||
| Workforce training | 2 | 3% | |||
|
| |||||
BDA as supporting tool to empower and engage people
At least one of the strategical interventions summarized under empowering and engaging people was named in 36 (51%) of the screened publications. Not only in this domain, but in general the ability of BDA to support the development of personalized care plans was mentioned most often (43%). This could for example be by accurately and timely predicting individual health risks (lifestyle, socio-economics, environment, genetic predisposition, etc.) [26,28,87], by predicting risk scores for disease conversion or progression [8,24], by deciding about the best intervention type based on patient similarity analyses or by predicting the probability for side effects or adverse events [59,88]. Examples found during the review are predictions for chronic diseases, heart failure, type 2 diabetes and severity stages for lung cancer or potential vaccination benefits and risks (see Table 8 for all references). Besides genome-wide association studies uncovering individual genetic predispositions for disease development [93], the full potential of BDA stems from the integration of data on all factors influencing health including also population-based, socio-economic, community-based or environmental factors. By providing information about the likelihood of an individual to benefit from different therapy options more targeted decision aids and medications could be developed and greater satisfaction on the patients’ side be achieved [9,62]. Also, self-diagnostics and self-management activities (7%) could be supported as people could regularly and timely be updated about their situation, their status and their current treatment options [22], e.g. based on sensor or patient-reported data (quantified self) [89]. By sending targeted information accessible via the personal health record or PCHP (3%) the support of peoples health education based on their individual risk factors might be improved (4%), as well as the process of shared decision making (6%) as patients can better define their individual care plans and therefore better adhere to their personal health goals. The PCHP could allow patients not only to access but also to administer and share their health-related data and to use the platform as a tool to communicate e.g. with providers.
BDA as supporting tool to strengthen governance and accountability
In 23 (32%) of the screened publications BDA was mentioned as a tool to strengthen governance and accountability. BDA could facilitate a deeper understanding of underlying factors for variation across providers, interventions, or regions (appropriate versus avoidable variation) to improve risk adjustment systems or performance evaluations (21%) supporting a transparent competition for outcome improvements [51,80,90], e.g. in performance-based contracts (11%). Also, results could be made publicly available, e.g. in league tables. Geocoded analyses could uncover community-based, regional, or environmental risk factors as well as supplier-induced problems and local disease hot spots [91,92] and be used to establish more decentralized systems (11%) with enhanced scope for local governments or community-care to implement regional health programs enhanced with patient-reported outcomes (1%). This would offer new opportunities for people in local communities to participate in the decision making process via the PCHP as a communication tool and become co-producers of population health.
BDA as supporting tool to reorient the model of care
The biggest share of articles in the scoping review described potential applications of BDA belonging to the strategy domain of reorienting the model of care (79%). Most often mentioned in this area was incorporating BDA in clinical decision support systems (32%), informing the provider about risks for disease uptake, progression, conversion, decompensation or the development of comorbidities [58,93]. A key factor of the PCIHS strategy of reorienting the model of care is strengthening primary and community care, whereas BDA could support more accurate diagnostics at these points of care [51,94,95,96]. Clinical judgements in these sectors might e.g. benefit from proactive alerts which inform about individual risks for preventable events like (re-)admissions to hospital, for intensified resource use, for (post-surgical) complications or disease progression [93,94,95,96], in the best case based on intersectoral health data from the PCHP also allowing for interdisciplinary communication. According to a survey in the USA, 15% of the healthcare providers already have access to some kind of predictive analytics and the conditions most often targeted were hospital readmissions (27%), the development of a sepsis (27%), patient deterioration (18%) and general health (10%) [97]. Using intersectoral data to stratify individuals into (chronic) care groups and identify comparable or manageable populations could support additional population health management activities (26%) in which the role of nurses and community health workers could be enhanced [22,24,26,35,98]. Also surveillance and control systems (18%) could benefit from BDA based on real world health data assets, e.g. the surveillance of adverse drug and vaccination effects or the monitoring of disease transmission patterns or outspread speed of epidemics or pandemics [91,92] enabling for example faster reaction and better targeted campaigns [88]. Using real-world data would additionally allow for rather small risk groups or (geographically) isolated communities already suffering from under-coordination to also be taken into consideration in healthcare decision making [44,99]. Furthermore, activities like health promotion and disease prevention (13%) might be better tailored to individuals if certain risk factors are specifically addressed. By using sensing devices as well as mobile technologies (14%) or devices within the patients’ ambient (6%) therapy results might be better tracked by patients as well as by providers.
BDA as supporting tool to coordinate services within and across sectors
In the scoping review 20 publications (28%) described BDA as a tool to support service coordination. Most articles mentioned the development and evaluation of intersectoral care pathways (11%) by exploring comparable patterns and then setting up multidisciplinary task forces of medical and non-medical providers for such multi-layered problems structured around an individual’s social experiences and comorbidities. Also, BDA respective the PCHP as enabler would simplify the exchange of medical records (8%), especially in the transition between hospital and home. Four publications (6%) described BDA as an enabling tool for intersectoral partnerships across the health sector (e.g. with social security, housing, education) to provide holistic care and one publication described a model in which BDA is used for district-based healthcare delivery [100].
BDA supporting the creation of an enabling environment
The strategy of creating an enabling environment is supporting the aforementioned strategies and is rather broad in scope. BDA itself is an enabler for people-centred health services, but 17 publications (24%) mentioned BDA as incorporated in other enabling factors as well. On the level of resource planning and allocation (15%) BDA might be capable of reducing financial waste by identifying common patterns of fraud and abuse or by uncovering disincentives of the renumeration system towards finding the right payment mix [79,80,101]. BDA could also support system research comparing the effects of different system architectures (9%). Assisting in quality assurance (4%), BDA could, e.g. by exploring care patterns, identify clinical waste and provide the opportunity to get rid of ineffective or unnecessary interventions or to reduce over- and undertreatment [37,44]. Two publications described BDA as tool to identify those professionals benefitting the most from additional training and education, e.g. on team-based culture or open feedback (3%).
Challenges of big data analytics in healthcare discussed in the literature
As BDA has the potential to improve PCIHS it seems valuable to find solutions for the challenges stemming from big data in healthcare [102]. Currently the situation for most stakeholders is characterized by confusion or uncertainty [54]. Of the 72 articles in this review, 45 (62.5%) discussed at least one BDA challenge. Most often discussed were methodological challenges (54.2%) followed by regulatory (43.1%) and technological challenges (41.7%). Cultural challenges were less often discussed (25.0%). The five issues mentioned most often in making better use of BDA were missing modelling standards and potential bias (36.1%), a questionable evidence-base of BDA results (33.3%), poor data quality (27.8%), the lack of an appropriate framework for privacy protection (26.4%) and the lack of interoperability requirements for data linkage (26.4%). In the successive descriptions only the most relevant publications will be referenced (see Table 9 in the appendix for more details).
Regulatory challenges
From a regulatory perspective it is challenging to set up a framework to coordinate, support and financially incentivize the efforts in building a big data platform for health data [15]. Besides ensuring for targeted investments this means describing the policies of appropriate data storage [27,36]. As the relevance of analytical results in clinical processes diminishes over time it is also a challenge to facilitate user friendly processes for data entry and timely exchange to finally enable (real-time) recommendations at the point of care [9,36,103]. To overcome legal or commercial barriers across domains intellectual property rights must be clearly defined, penalizing e.g. the unwillingness to share relevant (clinical) data for economic reasons or unintended uses [41]. To avoid underperforming models from mis-informing clinical decision making, a framework for transparent model development and evaluation would be needed [46,104,105]. Analytical modelling standards could, comparable to drug licensing, be transparently developed by quality controlled institutions which incorporate the technical and methodological expertise but also contribute domain knowledge to determine how to provide accurate, reliable and actionable information for patient care [44,106]. Likewise, this is touching ethical issues, e.g. if a BDA model at the beginning of the learning curve provides seriously harmful recommendations for some individuals [88,107,108]. The most often mentioned regulatory challenge was the design of an appropriate framework finding the sweet spot between transparency and protecting privacy enabling as effective decision supporting analytics as possible without enabling a potentially manipulative misuse of the data [54,77]. To enable as many beneficial analytics as possible, it might be an option to make deidentified data extracts from the PCHP accessible for chosen academic or even commercial purposes [9,54,77].
Technological challenges
Despite prices for data storage are steadily going down from a technological perspective the design of an infrastructure appropriate for storing and curating massive amounts of diverse health data is still a complex task [37,38,77,108]. Also, it is challenging to deal with high-velocity data depending on considerable computational processing resources and then to use appropriate software tools for data analytics [27,85]. Blending the extremely diverse and often unstructured health data from heterogenous sources leads to the challenge of establishing technological standards of interoperability [77,89]. Furthermore, inaccurately calibrated measurement systems as well as hard- and software failures (e.g., wrong auto-fill-in functions) inadequate data transfer protocols or not adequately developed software pose risks for data quality. Data quality problems can possibly arise at every step during data generation while the chance for bias might be lower for recorded medical signals than for manually documented features [36,39,54]. Finally, all layers of a big data platform (storage, transfer, analytics, presentation) have to be technically protected against unintended uses or breaches, e.g. by data encryption, certification or access authentication [72,77]. Big data technologies were out of the scope of this review, but at least it shall be referenced to articles discussing tools for big data storage & transformation like MongoDB or Apache HBase [9,38,43,74,108], for big data processing & analysing like Hadoop or MapReduce [38,43,74,85,109] as well as methods for (big) data security [77,110,111,112].
Methodological challenges
From a methodological perspective it is challenging to work on a high-dimensional database likely to contain more feature variables than observable subjects [44] and to develop real-time analytical models as most documentation processes in healthcare traditionally are rather slow [36,72]. Regarding human documentation also data entry errors like incomplete, incongruent, or missing data and a poor update status pose risks for data quality [39]. As a priori it is unclear which model is most appropriate for the targeted type of application and which model offers clinically more meaningful interpretations, the process of evaluating analytical models is quite challenging [113]. It affects the analytical results that no commonly accepted methodological standard for modelling exists offering nearly unlimited different options for the combination of variables whereas currently there is a lack of knowledge about which methods to use for which purposes and the black box design of some machine learning algorithms even exacerbates their comprehensibility. Additionally, external validity or generalizability is a challenge as it is difficult to compare the performance of different BDA models based on different data types from different regions [77,113,114]. It is also problematic that in some source systems data is recorded for specific reasons (e.g., medical billing) or with different coding standards potentially limiting interpretability beyond the original purpose. In a greater extent the same limitations as for observational studies also apply for BDA such that it is extremely difficult to exclude potential bias (e.g. selection bias, confounding bias, measurement bias), that due to missing randomization no causal relationships can be determined and that especially BDA has a high risk for modelling artefacts like random noise or overfitting [27,56,87]. Designing a methodology on how to evaluate the clinical usefulness and evidence-base of the analytical models or their effectiveness and safety in part also is a methodological issue [115]. To date, there is only minimal evidence that BDA in healthcare revealed anything surprisingly new and can effectively improve decision making or medical outcomes [93,94,116]. Furthermore, is has not been proven that machine learning models outperform traditional statistical models in predictive or exploratory tasks. Most often only sparse differences in the model performance are observed, maybe because they were often applied to rather small data sets limiting the ability of BDA models to optimize the inductive feature selection process [7,8,113]. To disseminate information about the most effective treatments to the intended providers at the point of care requires that information overload is prevented, and analytical results are timely and easily accessible, appropriately simplified, appealingly visualized and well-integrated in clinical workflows [93,117]. A comprehensive discussions of methodological issues of BDA in healthcare is e.g. provided by Hoffman/Podgurski [54] and Van Poucke et al. [46].
Cultural challenges
An adaption of BDA models in healthcare requires appropriate education as well as a shift towards team-based analytics enhancing medical domain knowledge with skills e.g. from data science and health economics [37,85]. Form an organizational perspective also resistances against expanding and speeding-up electronic data exchange and against redesigning clinical workflows with data-driven feedback need to be overcome by communicating potential benefits and by putting media-hyped expectations into perspective [25,72]. A data quality culture must be developed to reduce behaviours like unreflective copy-pasting and strategical manipulation of data. From the societal perspective, a data sharing culture would be helpful to counteract personal and organizational concerns. This might be accompanied by an open science culture which ensures that peoples’ data are used as intended [22,36,118]. Exploratory studies point to the fact that the majority of people is willing to share health data for population-based health research, but fewer individuals are comfortable to have their data used to improve medical decision making or to adapt insurance rates [119,120] with country-specific, cultural differences [121,122]. As the mere existence of BDA tools does not influence value improvement a learning culture with engaged providers needs to be achieved with (clinical) usability as a precondition.
In Table 3 all challenges mentioned above were systematized by combining the domains of technological, methodological, regulatory, and cultural challenges [37,74] with the 5-V model as each big data characteristic entails specific obstacles [36,54,77,85].
Table 3.
Challenges in designing a people-centred and integrated health platform to enable big data analytics in healthcare.
|
| ||||
|---|---|---|---|---|
| CHALLENGE DOMAIN BIG DATA CHARACTERISTIC | REGULATORY | TECHNOLOGICAL | METHODOLOGICAL | CULTURAL |
|
| ||||
| Volume | Investment & technology framework | Data infrastructure | High-dimensional analytics | Teamwork culture |
|
| ||||
| Velocity | Communication framework | Data processing | Real-time analytics | Delivery process redesign |
|
| ||||
| Variety | Intellectual property framework | Data linkage | Modelling standards & bias | Data sharing culture |
|
| ||||
| Veracity | Evaluation framework | Data quality | Evidence- base | Data governance |
|
| ||||
| Value | Privacy & ethics framework | Data access & data security | Interpretation & usability | Culture of learning & change |
|
| ||||
Potential success factors of big data analytics or strategies to overcome the challenges can be derived as countermovement to each challenge displayed in Table 3. For example the success factor of data quality assurance would be a strategical reaction to the described data quality challenges as well as the success factor of implementing a big data governance would be a reaction to the fact that healthcare organizations are often missing a data governance. The enabling factors of the PCIHS framework [32] as well as some articles from the scoping review provide further information [105,123].
Limitations
The results presented in this article depend on the literature found by using the defined search terms and also depend on the timing of the literature review. Although text mining algorithms were applied to refine the search terms it may be that a subclass of potentially relevant articles was not covered because domain-specific words were used or that relevant articles were unintentionally excluded by the exclusion criteria. If further literature databases as well as other languages than English or such literature being published between conduction and publication of this review were also included in the review, this would have enhanced the number of articles. As indicated by the frequency distribution of the authors’ country affiliation, experiences of middle- and low-income countries seem underrepresented. And also from high-income countries it may be that there is a certain number of data analytical applications nothing has been published about yet. The topic of Natural Language Processing (NLP) was intentionally excluded which does not mean that is does not also pose potential in supporting integrated care activities. Publication bias might have limited the results to scientifically relevant articles on rather novel topics, on articles with rather positive outcomes or on health-related issues where large databases already exist. Therefore, in the results part, data types and areas of applications are highlighted which were already described by researchers performing big data analytics, while areas of application, for which large datasets do not exist to the same extend (e.g., for social care, public health or preventative care, community care, education, or disability services) were underrepresented. Quite the opposite does this mean that additional data analytics might have less potential value, but rather that the source systems need to be further developed to be suitable for big data analytics. For some important components of the framework on people-centred care like enhancing the role of community care or establishing intersectoral partnerships between health and social care only few examples of enabling big data analytical tools were found in the literature.
Conclusion and outlook
This review aimed to make a contribution to the research question “How can big data analytics support people-centred and integrated health services”. The role model of the people-centred health platform may in combination with the PCIHS framework be used by health policy and healthcare decision makers as a design principle to guide (national) strategies, whereas no universally valid approach that can be applied in all contexts. Rather should the strategical options and potentials gathered be prioritized with respect to the specific circumstances and financial opportunities to enable developments in the desired direction. The BDA methods and practical applications have a tremendous potential to improve integrated care interventions with respect to better health quality and efficiency and at least the methods can already be incorporated by health professionals or health management organizations. But it has also to be stated that up to now big data analytics does not fulfil the oversized expectations and already constitutes better outcome with respect to the triple aim. Likely this is because health-related data is extremely sensitive and complex and there are few practical examples of data platforms to some extent already capable of merging and providing people-centred big data so that the models and applications described in this work cannot evolve their full potential. But anyhow the integration of health data can be expected to further proceed. Every foreseeable integration of health data – e.g., genetic data in electronic health records – is at least a small step to also improve people-centred care and in the near future these sources will be merged with additional health-related data types on individual level. It might be a long way until BDA enable a faster reaction on dynamic situations like pandemics, a more need-based distribution of resources across the continuum of care and a more detailed understanding of the complex factors that have an impact on individual and population-based health but although the challenges are big and efforts are high this movement will further proceed as the potential benefits cannot be neglected.
Additional File
The additional file for this article can be found as follows:
Figure 4 and Tables 4 to 12.
Acknowledgement
The APC is paid for under the ATLAS project “Innovation and digital transformation in healthcare” funded by the State of North Rhine-Westphalia, Germany [grant number: ITG-1-1].
Funding Statement
The APC is paid for under the ATLAS project “Innovation and digital transformation in healthcare” funded by the State of North Rhine-Westphalia, Germany [grant number: ITG-1-1].
Reviewers
David Peiris, The George Institute for Global Health, UNSW Sydney, Australia.
Dr. Alexander Pimperl, Director Data Insights & Business Intelligence, AstraZeneca GmbH, Germany.
Prof. Dr. Eva-Maria Wild, Assistant Professor at the Department of Health Care Management, Hamburg Center for Health Economics, University of Hamburg, Germany.
References
- 1.The Commonwealth Fund. Commonwealth Fund international health policy survey; 2013. https://www.commonwealthfund.org/publications/surveys/2013/nov/2013-commonwealth-fund-international-health-policy-survey. Accessed: May 2019.
- 2.Stein V, Barbazza ES, Tello J, Kluge H. Towards people-centred health services delivery: a framework for action for the World Health Organization (WHO) European region. Int J Integr Care; 2013. DOI: 10.5334/ijic.1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter ME. What Is Value in Health Care? New England Journal of Medicine. 2010; 363(26): 2477–2481. DOI: 10.1056/NEJMp1011024 [DOI] [PubMed] [Google Scholar]
- 4.Porter ME, Teisberg E. Redefining health care – Creating value-based competition on results. Boston: Harvard Business School Press; 2006. [Google Scholar]
- 5.Leijten FRM, Struckmann V, van Ginneken E, et al. The SELFIE framework for integrated care for multi-morbidity: Development and description. Health Policy. 2018; 122(1): 12–22. DOI: 10.1016/j.healthpol.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Framework on integrated, people-centred health services; 2016. http://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_39-en.pdf. Accessed: May 2019.
- 7.Hashimoto DA, Rosman G, Rus D, Meireles OR. Artificial intelligence in surgery: promises and perils. Annals of Surgery. 2018; 1. DOI: 10.1097/SLA.0000000000002693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad T, Lund L, Rao P, et al. Machine learning methods improve prognostication, identify clinically distinct phenotypes, and detect heterogeneity in response to therapy in a large cohort of heart failure patients. J Am Heart Assoc; 2018. DOI: 10.1161/JAHA.117.008081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger ML, Doban V. Big data, advanced analytics and the future of comparative effectiveness research. Journal of Comparative Effectiveness Research. 2014; 3(2): 167–176. DOI: 10.2217/cer.14.2 [DOI] [PubMed] [Google Scholar]
- 10.Martin-Sanchez FJ, Aguiar-Pulido V, Lopez-Campos GH, et al. Secondary use and analysis of big data collected for patient care: contribution from the IMIA working group on data mining and big data analytics. Yearbook of Medical Informatics. 2017; 26(01): 28–37. DOI: 10.15265/IY-2017-008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA. 2013; 309(13): 1351. DOI: 10.1001/jama.2013.393 [DOI] [PubMed] [Google Scholar]
- 12.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Affairs. 2008; 27(3): 759–769. DOI: 10.1377/hlthaff.27.3.759 [DOI] [PubMed] [Google Scholar]
- 13.Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. The Annals of Family Medicine. 2014; 12(6): 573–576. DOI: 10.1370/afm.1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghupathi W, Raghupathi V. Big data analytics in healthcare: promise and potential. Health Information Science and Systems. 2014; 2(1): 3. DOI: 10.1186/2047-2501-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roski J, Bo-Linn GW, Andrews TA. Creating value in health care through big data: opportunities and policy implications. Health Affairs. 2014; 33(7): 1115–1122. DOI: 10.1377/hlthaff.2014.0147 [DOI] [PubMed] [Google Scholar]
- 16.Groves P, Kayyali B, Knott D, et al. The “Big Data” revolution in healthcare. Accelerating value and innovation; 2013. https://www.mckinsey.com/~/media/mckinsey/industries/healthcaresystemsandservices/ourinsights/thebigdatarevolutioninushealthcare/the_big_data_revolution_in_healthcare.ashx. Accessed: May 2019.
- 17.Dahlgren G, Whitehead M. Policies and strategies to promote social equity in health: background document to WHO-strategy paper for Europe. Institute for Futures Studies; 1991. [Google Scholar]
- 18.World Health Organization. WHO global strategy on people-centred and integrated health services; 2015. http://www.who.int/servicedeliverysafety/areas/people-centred-care/global-strategy/en/. Accessed: May 2019.
- 19.Goodwin N. Towards People-Centred Integrated Care: From Passive Recognition to Active Co-production? Int J Integr Care; 2016. DOI: 10.5334/ijic.2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valentijn PP, Schepman S, Opheij W, Bruijnzeels MA. Understanding integrated care: a comprehensive conceptual framework based on the integrative functions of primary care. International Journal of Integrated Care. 13: 2013; e010. DOI: 10.5334/ijic.886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ham C, Walsh N. Making integrated care happen at scale and pace. Lessons from experience; 2013. [Google Scholar]
- 22.Schatz BR. National surveys of population health: big data analytics for mobile health monitors. Big Data. 2015; 3(4): 219–229. DOI: 10.1089/big.2015.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrence DM. How to forge a high-tech marriage between primary care and population health. Health Affairs. 2010; 29(5): 1004–1009. DOI: 10.1377/hlthaff.2010.0167 [DOI] [PubMed] [Google Scholar]
- 24.Bhardwaj N, Wodajo B, Spano A, et al. The impact of big data on chronic disease management. The Health Care Manager. 2017; 1. DOI: 10.1097/HCM.0000000000000194 [DOI] [PubMed] [Google Scholar]
- 25.Cottle M, Hoover W, Kanwal S, et al. Transforming health care through big data; 2013. http://c4fd63cb482ce6861463-bc6183f1c18e748a49b87a25911a0555.r93.cf2.rackcdn.com/iHT2_BigData_2013.pdf. Accessed: January 2019.
- 26.Bradley PS. Implications of big data analytics on population health management. Big Data. 2013; 1(3): 152–159. DOI: 10.1089/big.2013.0019 [DOI] [PubMed] [Google Scholar]
- 27.Mehta N, Pandit A. Concurrence of big data analytics and healthcare: a systematic review. International Journal of Medical Informatics. 2018; 114: 57–65. DOI: 10.1016/j.ijmedinf.2018.03.013 [DOI] [PubMed] [Google Scholar]
- 28.Dawson NV, Davis DA. Bringing big data to personalized healthcare: A patient-centered framework. Journal of General Internal Medicine. 2013; 28(S3): 660–665. DOI: 10.1007/s11606-013-2455-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain S, Wilk A, Thorpe K, Hammond S. A Model for Delivering Population Health Across the Care Continuum. Am. J. Accountable Care. 2018; 6. [Google Scholar]
- 30.World Health Organization SD and SD. The WHO Framework on integrated people-centred health services; 2016. [Google Scholar]
- 31.World Health Organization. People-centred and integrated health services: an overview of the evidence – Interim Report; 2015. [Google Scholar]
- 32.World Health Organization. WHO global strategy on people-centred and integrated health services – Interim Report; 2015. [Google Scholar]
- 33.Ward JS, Barker A, University of St Andrews, School of Computer Science. Undefined by data: a survey of big data definitions; 2013. https://arxiv.org/pdf/1309.5821v1.pdf. Accessed: May 2019.
- 34.Gartner Research. Big data; 2019. https://www.gartner.com/it-glossary/big-data. Accessed: May 2019.
- 35.Bates DW, Saria S, Ohno-Machado L, et al. Big data in health care: using analytics to identify and manage high-risk and high-cost patients. Health Affairs. 2014; 33(7): 1123–1131. DOI: 10.1377/hlthaff.2014.0041 [DOI] [PubMed] [Google Scholar]
- 36.Dinov ID. Volume and value of big healthcare data. Journal of Medical Statistics and Informatics. 2016; 4(1): 3. DOI: 10.7243/2053-7662-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kruse CS, Goswamy R, Raval Y, Marawi S. Challenges and opportunities of big data in health care: a systematic review. JMIR Medical Informatics. 2016; 4(4): e38. DOI: 10.2196/medinform.5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakr S, Elgammal A. Towards a comprehensive data analytics framework for smart healthcare services. Big Data Research. 2016; 4: 44–58. DOI: 10.1016/j.bdr.2016.05.002 [DOI] [Google Scholar]
- 39.Sukumar SR, Natarajan R, Ferrell RK. Quality of big data in health care. International Journal of Health Care Quality Assurance. 2015; 28(6): 621–634. DOI: 10.1108/IJHCQA-07-2014-0080 [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Hajli N. Exploring the path to big data analytics success in healthcare. Journal of Business Research. 2017; 70: 287–299. DOI: 10.1016/j.jbusres.2016.08.002 [DOI] [Google Scholar]
- 41.Amarasingham R, Audet AMJ, Bates DW, et al. Consensus statement on electronic health predictive analytics: a guiding framework to address challenges. eGEMs (Generating Evidence & Methods to improve patient outcomes). 2016; 4(1): 3. DOI: 10.13063/2327-9214.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson S, Varvel S, Sasinowski M, Burke JP. From value assessment to value cocreation: informing clinical decision-making with medical claims data. Big Data. 2016; 4(3): 141–147. DOI: 10.1089/big.2015.0030 [DOI] [PubMed] [Google Scholar]
- 43.Alonso SG, de la Torre Díez I, Rodrigues JJ, et al. A systematic review of techniques and sources of big data in the healthcare sector. J Med Syst.; 2017. DOI: 10.1007/s10916-017-0832-2 [DOI] [PubMed] [Google Scholar]
- 44.Szlezák N, Evers M, Wang J, Pérez L. The role of big data and advanced analytics in drug discovery, development, and commercialization. Clinical Pharmacology & Therapeutics. 2014; 95(5): 492–495. DOI: 10.1038/clpt.2014.29 [DOI] [PubMed] [Google Scholar]
- 45.Vayena E, Dzenowagis J, Brownstein JS, Sheikh A. Policy implications of big data in the health sector. Bulletin of the World Health Organization. 2018; 96(1): 66–68. DOI: 10.2471/BLT.17.197426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Poucke S, Thomeer M, Heath J, Vukicevic M. Are randomized controlled trials the (g)old standard? From clinical intelligence to prescriptive analytics. Journal of Medical Internet Research. 2016; 18(7): e185. DOI: 10.2196/jmir.5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohamed K. Health analytics types, functions and levels: a review of literature. Studies in Health Technology and Informatics. 2018; 137–140. DOI: 10.3233/978-1-61499-880-8-137 [DOI] [PubMed] [Google Scholar]
- 48.Alanazi HO, Abdullah AH, Qureshi KN. A critical review for developing accurate and dynamic predictive models using machine learning methods in medicine and health care. J Med Syst.; 2017. DOI: 10.1007/s10916-017-0715-6 [DOI] [PubMed] [Google Scholar]
- 49.Bayrak T. A review of business analytics: a business enabler or another passing fad. Procedia – Social and Behavioral Sciences. 2015; 195: 230–239. DOI: 10.1016/j.sbspro.2015.06.354 [DOI] [Google Scholar]
- 50.Callaghan CW. Developing the transdisciplinary aging research agenda: new developments in big data. Current Aging Science. 2018; 11(1): 33–44. DOI: 10.2174/1874609810666170719100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krumholz HM. Big data and new knowledge in medicine: the thinking, training, and tools needed for a learning health system. Health Affairs. 2014; 33(7): 1163–1170. DOI: 10.1377/hlthaff.2014.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holzinger A. Machine learning for health informatics. In: Holzinger A (ed.), Mach. Learn. Health Inform. Cham: Springer International Publishing. 2016; 1–24. DOI: 10.1007/978-3-319-50478-0_1 [DOI] [Google Scholar]
- 53.Elliott JH, Grimshaw J, Altman R, et al. Informatics: make sense of health data. Nature. 2015; 527(7576): 31–32. DOI: 10.1038/527031a [DOI] [PubMed] [Google Scholar]
- 54.Hoffman S, Podgurski A. The use and misuse of biomedical data: is bigger really better? American Journal of Law & Medicine. 2013; 39: 497–538. DOI: 10.1177/009885881303900401 [DOI] [PubMed] [Google Scholar]
- 55.Kitchin R. Big data, new epistemologies and paradigm shifts. Big Data & Society. 2014; 1(1). DOI: 10.1177/2053951714528481 [DOI] [Google Scholar]
- 56.Hohmann E, Arevalo MJ, D’Agostino RB. Research pearls: the significance of statistics and perils of pooling. Predictive modeling. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2017; 33(7): 1423–1432. DOI: 10.1016/j.arthro.2017.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kotsiantis SB, Zaharakis ID, Pintelas PE. Machine learning: a review of classification and combining techniques. Artificial Intelligence Review. 2006; 26(3): 159–190. DOI: 10.1007/s10462-007-9052-3 [DOI] [Google Scholar]
- 58.Cichosz SL, Johansen MD, Hejlesen O. Toward big data analytics: review of predictive models in management of diabetes and its complications. Journal of Diabetes Science and Technology. 2015; 10(1): 27–34. DOI: 10.1177/1932296815611680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernandez I, Zhang Y. Using predictive analytics and big data to optimize pharmaceutical outcomes. American Journal of Health-System Pharmacy. 2017; 74(18): 1494–1500. DOI: 10.2146/ajhp161011 [DOI] [PubMed] [Google Scholar]
- 60.Sanchez-Morillo D, Fernandez-Granero MA, Leon-Jimenez A. Use of predictive algorithms in home monitoring of chronic obstructive pulmonary disease and asthma: a systematic review. Chronic Respiratory Disease. 2016; 13(3): 264–283. DOI: 10.1177/1479972316642365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ozminkowski RJ, Wells TS, Hawkins K, et al. Big data, little data, and care coordination for Medicare beneficiaries with Medigap coverage. Big Data. 2015; 3(2): 114–125. DOI: 10.1089/big.2014.0034 [DOI] [PubMed] [Google Scholar]
- 62.Gotz D, Wang F, Perer A. A methodology for interactive mining and visual analysis of clinical event patterns using electronic health record data. Journal of Biomedical Informatics. 2014; 48: 148–159. DOI: 10.1016/j.jbi.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 63.Bettencourt-Silva JH, Mannu GS, de la Iglesia B. Visualisation of integrated patient-centric data as pathways: enhancing electronic medical records in clinical practice. Holzinger A (ed.), Mach. Learn. Health Inform. Cham: Springer International Publishing. 2016; 99–124. DOI: 10.1007/978-3-319-50478-0_5 [DOI] [Google Scholar]
- 64.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Annals of Internal Medicine. 2018; 169(7): 467. DOI: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 65.Ananiadou S, Rea B, Okazaki N, et al. Supporting systematic reviews using text mining. Social Science Computer Review. 2009; 27(4): 509–523. DOI: 10.1177/0894439309332293 [DOI] [Google Scholar]
- 66.Osinski S, Stefanowski J, Weiss D. Lingo: search results clustering algorithm based on singular value decomposition. Intelligent Information Processing and Web Mining Advances in Soft Computing. 2004; 25: 359–368. DOI: 10.1007/978-3-540-39985-8_37 [DOI] [Google Scholar]
- 67.Osinski S, Weiss D. Carrot2: Design of a flexible and efficient web information retrieval framework. Advances in Web Intelligence AWIC 2005 Lecture Notes in Computer Science. 2005; 3528: 439–444. DOI: 10.1007/11495772_68 [DOI] [Google Scholar]
- 68.Gottesman O, Kuivaniemi H, Tromp G, et al. The electronic medical records and genomics (eMERGE) network: past, present, and future. Genetics in Medicine. 2013; 15(10): 761–771. DOI: 10.1038/gim.2013.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kho AN, Pacheco JH, Peissig PL, et al. Electronic medical records for genetic research: results of the eMERGE consortium. Science Translational Medicine. 2011; 3(79): 79re1–79re1. DOI: 10.1126/scitranslmed.3001807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jormanainen V. Large-scale implementation and adoption of the Finnish national Kanta services in 2010–2017: a prospective, longitudinal, indicator-based study. Finn J EHealth EWelfare; 2018. DOI: 10.23996/fjhw.74511 [DOI] [Google Scholar]
- 71.Nøhr C, Parv L, Kink P, et al. Nationwide citizen access to their health data: analysing and comparing experiences in Denmark, Estonia and Australia. BMC Health Serv Res. 2017. DOI: 10.1186/s12913-017-2482-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Affairs. 2014; 33(7): 1203–1211. DOI: 10.1377/hlthaff.2014.0054 [DOI] [PubMed] [Google Scholar]
- 73.Stephens ZD, Lee SY, Faghri F, et al. Big data: astronomical or genomical? PLOS Biology. 2015; 13(7): e1002195. DOI: 10.1371/journal.pbio.1002195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang T, Lan L, Fang X, et al. Promises and challenges of big data computing in health sciences. Big Data Research. 2015; 2(1): 2–11. DOI: 10.1016/j.bdr.2015.02.002 [DOI] [Google Scholar]
- 75.Marx V. The big challenges of big data. Nature. 2013; 498(7453): 255–260. DOI: 10.1038/498255a [DOI] [PubMed] [Google Scholar]
- 76.Peters SG, Buntrock JD. Big data and the electronic health record. Journal of Ambulatory Care Management. 2014; 37(3): 206–210. DOI: 10.1097/JAC.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 77.Cyganek B, Graña M, Krawczyk B, et al. A survey of big data issues in electronic health record analysis. Applied Artificial Intelligence. 2016; 30(6): 497–520. DOI: 10.1080/08839514.2016.1193714 [DOI] [Google Scholar]
- 78.Allen C, Tsou MH, Aslam A, et al. Applying GIS and machine learning methods to Twitter data for multiscale surveillance of influenza. PLOS ONE. 2016; 11(7): e0157734. DOI: 10.1371/journal.pone.0157734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Srinivasan U, Arunasalam B. Leveraging big data analytics to reduce healthcare costs. IT Professional. 2013; 15(6): 21–28. DOI: 10.1109/MITP.2013.55 [DOI] [Google Scholar]
- 80.Handmaker K, Hart J. 9 steps to effective population health management. Healthcare Financial Management. 2015; 69 (4): 70–76. [PubMed] [Google Scholar]
- 81.Kreis K, Neubauer S, Klora M, et al. Status and perspectives of claims data analyses in Germany—A systematic review. Health Policy. 2016; 120(2): 213–226. DOI: 10.1016/j.healthpol.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 82.Douglas HE, Georgiou A, Tariq A, et al. Implementing Information and Technology to Support Community Aged Care Service Integration: Lessons from an Australian Aged Care Provider. Int J Integr Care. 2017; DOI: 10.5334/ijic.2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grayson S, Doerr M, Yu J-H. Developing pathways for community-led research with big data: a content analysis of stakeholder interviews. Health Res Policy Syst; 2020. DOI: 10.1186/s12961-020-00589-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson M. Data, Analytics and Community-Based Organizations: Transforming Data to Decisions for Community Development. I/S: A Journal of Law and Policy for the Information Society. 2015; 11(1): 49–96. [Google Scholar]
- 85.Alharthi H. Healthcare predictive analytics: an overview with a focus on Saudi Arabia. J Infect Public Health; 2018. DOI: 10.1016/j.jiph.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 86.Institute of Medicine. Best care at lower cost: the path to continuously learning health care in America; 2013. DOI: 10.17226/13444 [DOI] [PubMed] [Google Scholar]
- 87.Binder H, Blettner M. Big data in medical science – a biostatistical view. Dtsch Aerzteblatt Online; 2015.DOI: 10.3238/arztebl.2015.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liyanage H, de Lusignan S, Liaw S-T, et al. Big data usage patterns in the health care domain: a use case driven approach applied to the assessment of vaccination benefits and risks. IMIA Yearbook. 2014; 9(1): 27–35. DOI: 10.15265/IY-2014-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Swan M. The quantified self: fundamental disruption in big data science and biological discovery. Big Data. 2013; 1(2): 85–99. DOI: 10.1089/big.2012.0002 [DOI] [PubMed] [Google Scholar]
- 90.Choudhry SA, Li J, Davis D, et al. A public-private partnership develops and externally validates a 30-day hospital readmission risk prediction model. Online J Public Health Inform; 2013. DOI: 10.5210/ojphi.v5i2.4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flahault A, Bar-Hen A, Paragios N. Public health and epidemiology informatics. IMIA Yearbook. 2016; 1: 240–246. DOI: 10.15265/IY-2016-021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen M, Hao Y, Hwang K, et al. Disease prediction by machine learning over big data from healthcare communities. IEEE Access. 2017; 5: 8869–8879. DOI: 10.1109/ACCESS.2017.2694446 [DOI] [Google Scholar]
- 93.Rumsfeld JS, Joynt KE, Maddox TM. Big data analytics to improve cardiovascular care: promise and challenges. Nature Reviews Cardiology. 2016; 13(6): 350–359. DOI: 10.1038/nrcardio.2016.42 [DOI] [PubMed] [Google Scholar]
- 94.Sharafoddini A, Dubin JA, Lee J. Patient similarity in prediction models based on health data: a scoping review. JMIR Medical Informatics. 2017; 5(1): e7. DOI: 10.2196/medinform.6730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee J. Patient-specific predictive modeling using random forests: an observational study for the critically ill. JMIR Medical Informatics. 2017; 5(1): e3. DOI: 10.2196/medinform.6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ross EG, Shah N, Dalman RL, et al. Use of predictive analytics for the identification of latent vascular disease and future adverse cardiac events. Journal of Vascular Surgery. 2016; 63(6): 28S-29S. DOI: 10.1016/j.jvs.2016.03.209 [DOI] [Google Scholar]
- 97.Jvion. Jvion predictive analytics in healthcare survey; 2015. https://chimecentral.org/jvion-releases-findings-latest-predictive-analytics-healthcare-survey/. Accessed: May 2019.
- 98.Sheets L, Petroski G, Zhuang Y, et al. Combining contrast mining with logistic regression to predict healthcare utilization in a managed care population. Applied Clinical Informatics. 2017; 8(02): 430–446. DOI: 10.4338/ACI-2016-05-RA-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.White RW, Tatonetti NP, Shah NH, et al. Web-scale pharmacovigilance: listening to signals from the crowd. Journal of the American Medical Informatics Association. 2013; 20(3): 404–408. DOI: 10.1136/amiajnl-2012-001482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Batarseh FA, Latif EA. Assessing the quality of service using big data analytics. Big Data Research. 2016; 4: 13–24. DOI: 10.1016/j.bdr.2015.10.001 [DOI] [Google Scholar]
- 101.Kose I, Gokturk M, Kilic K. An Interactive machine-learning-based electronic fraud and abuse detection system in healthcare insurance. Applied Soft Computing. 2015; 36: 283–299. DOI: 10.1016/j.asoc.2015.07.018 [DOI] [Google Scholar]
- 102.Gottlieb L, Tobey R, Cantor J, et al. Integrating social and medical data to improve population health: opportunities and barriers. Health Affairs. 2016; 35(11): 2116–2123. DOI: 10.1377/hlthaff.2016.0723 [DOI] [PubMed] [Google Scholar]
- 103.Stadler JG, Donlon K, Siewert JD. et al. Improving the efficiency and ease of healthcare analysis through use of data visualization dashboards. Big Data. 2016; 4(2): 129–135. DOI: 10.1089/big.2015.0059 [DOI] [PubMed] [Google Scholar]
- 104.Huang BE, Mulyasasmita W, Rajagopal G. The path from big data to precision medicine. Expert Review of Precision Medicine and Drug Development. 2016; 1(2): 129–143. DOI: 10.1080/23808993.2016.1157686 [DOI] [Google Scholar]
- 105.Amarasingham R, Patzer RE, Huesch M, et al. Implementing electronic health care predictive analytics: considerations and challenges. Health Affairs. 2014; 33(7): 1148–1154. DOI: 10.1377/hlthaff.2014.0352 [DOI] [PubMed] [Google Scholar]
- 106.Buchanan V, Lu Y, McNeese N, et al. The role of teamwork in the analysis of big data: a study of visual analytics and box office prediction. Big Data. 2017; 5(1): 53–66. DOI: 10.1089/big.2016.0044 [DOI] [PubMed] [Google Scholar]
- 107.Davis K, Patterson D. Ethics of big data. Sebastopol, CA: O’Reilly. 2012. [Google Scholar]
- 108.Kuo M, Sahama T, Kushniruk A, et al. Health big data analytics: current perspectives, challenges and potential solutions. International Journal of Big Data Intelligence. 2014; 1(1/2): 114. DOI: 10.1504/IJBDI.2014.063835 [DOI] [Google Scholar]
- 109.Zhang H, Chen G, Ooi BC, et al. In-memory big data management and processing: a survey. IEEE Transactions on Knowledge and Data Engineering. 2015; 27(7): 1920–1948. DOI: 10.1109/TKDE.2015.2427795 [DOI] [Google Scholar]
- 110.Press G. Top 10 hot data security and privacy technologies; 2017. https://www.forbes.com/sites/gilpress/2017/10/17/top-10-hot-data-security-and-privacy-technologies/. Accessed.
- 111.Zhang X, Dou W, Pei J, et al. Proximity-aware local-recoding anonymization with MapReduce for scalable big data privacy preservation in cloud. IEEE Transactions on Computers. 2015; 64(8): 2293–2307. DOI: 10.1109/TC.2014.2360516 [DOI] [Google Scholar]
- 112.Xu L, Jiang C, Wang J, et al. Information security in big data: privacy and data mining. IEEE Access. 2014; 2: 1149–1176. DOI: 10.1109/ACCESS.2014.2362522 [DOI] [Google Scholar]
- 113.Ng K, Ghoting A, Steinhubl SR, et al. PARAMO: A PARAllel predictive MOdeling platform for healthcare analytic research using electronic health records. Journal of Biomedical Informatics. 2014; 48: 160–170. DOI: 10.1016/j.jbi.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Walsh C, Hripcsak G. The effects of data sources, cohort selection, and outcome definition on a predictive model of risk of thirty-day hospital readmissions. Journal of Biomedical Informatics. 2014; 52: 418–426. DOI: 10.1016/j.jbi.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Walsh CG, Sharman K, Hripcsak G. Beyond discrimination: a comparison of calibration methods and clinical usefulness of predictive models of readmission risk. Journal of Biomedical Informatics. 2017; 76: 9–18. DOI: 10.1016/j.jbi.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang R, Simon G, Yu F. Advancing Alzheimer’s research: a review of big data promises. International Journal of Medical Informatics. 2017; 106: 48–56. DOI: 10.1016/j.ijmedinf.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gigerenzer G, Gaissmaier W, Kurz-Milcke E, et al. Helping doctors and patients make sense of health statistics. Psychological Science in the Public Interest. 2007; 8(2): 53–96. DOI: 10.1111/j.1539-6053.2008.00033.x [DOI] [PubMed] [Google Scholar]
- 118.Kohane I. Secondary use of health information: are we asking the right question? JAMA Internal Medicine. 2013; 173(19): 1806. DOI: 10.1001/jamainternmed.2013.8276 [DOI] [PubMed] [Google Scholar]
- 119.Grande D, Mitra N, Shah A, et al. Public preferences about secondary uses of electronic health information. JAMA Internal Medicine. 2013; 173(19): 1798. DOI: 10.1001/jamainternmed.2013.9166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weitzman ER, Kaci L, Mandl KD. Sharing medical data for health research: The early personal health record experience. Journal of Medical Internet Research. 2010; 12(2): e14. DOI: 10.2196/jmir.1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vodafone Institute for Society and Communications. Big data: a European survey on the opportunities and risks of data analytics; 2016. https://www.vodafone-institut.de/wp-content/uploads/2016/01/VodafoneInstitute-Survey-BigData-en.pdf. Accessed: May 2019.
- 122.Skovgaard LL, Wadmann S, Hoeyer K. A review of attitudes towards the reuse of health data among people in the European Union: The primacy of purpose and the common good. Health Policy. 2019; 123(6): 564–571. DOI: 10.1016/j.healthpol.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mehta N, Pandit A. Concurrence of big data analytics and healthcare: a systematic review. International Journal of Medical Informatics. 2018; 114: 57–65. DOI: 10.1016/j.ijmedinf.2018.03.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 4 and Tables 4 to 12.


