Abstract
Background and Aims:
Cancer cachexia is manifested by loss in muscle, adipose, weight, and appetite. PET 18F-FDG uptake identifies tumor metabolic and inflammatory changes, potentially associated with cachexia development. We examined if primary gastroesophageal tumor 18F-FDG uptake correlates with cachexia development and survival in cancer patients.
Methods:
One hundred twenty-six esophageal (n=87) and gastroesophageal junction (n=39) cancer patients, with a median age at diagnosis of 63 years (IQR 54–71), evaluated between 2006–2014 with pre-treatment PET imaging and cachexia determination at diagnosis were included in the study cohort (22.1% female; 6.7%, 24.4%, 50.4%, and 18.5% with tumor stage I, II, III, and IV respectively). Maximum primary tumor standardized uptake values (SUVMax) were obtained and dichotomized based off the calculated cut-point SUVMax of 8.5 (P=.0018). Associations between survival, cachexia development and primary tumor 18F-FDG uptake were evaluated using univariate and multivariate analyses.
Results:
Cancer-associated weight loss (cachexia) and primary tumor SUVMax at or above the statistically determined cut-point of 8.5 were present in 54% and 57% of patients, respectively. Primary tumor SUVMax above the cut-point was significantly associated with pre-treatment cancer-associated weight loss (P=.0033) and, in multivariate analysis, correlated with a 2.3-fold increased risk of death (95% CI 1.4, 3.7; P=.0010). When divided into cohorts defined by their combined cachexia and high versus low SUVMax tumor status, positive cachexia status or/and high SUVMax tumors were associated with similar significant decrements in survival.
Conclusion:
A positive association was present between cancer-associated weight loss and SUVMax of the primary tumor, suggesting greater glycolytic metabolism in gastroesophageal tumors that induce cachexia. This interpretation of routinely administered PET scans could lead to earlier categorization of patients with cachexia-inducing tumors. Both cachexia and high SUVMax status were independently associated with worsened survival outcomes, further supporting their prognostic relevance in patients with gastroesophageal cancer.
Keywords: Cachexia, Gastroesophageal cancer, Positron emission tomography, Cancer metabolism, Weight loss, Appetite
Introduction
Cachexia is a multifactorial syndrome observed across a wide degree of cancer types. It is characterized by continuous unintentional weight loss through the depletion of skeletal muscle mass, often with a depletion of fat mass [1, 2]. In patients with esophageal or gastroesophageal junction tumors, the presence of overt, clinically significant cancer-associated weight loss has been associated with nearly a threefold reduction in median survival when compared to patients without significant weight loss [3]. Cachexia management represents an unmet need in cancer treatment, as cachectic wasting is rarely identified, assessed, or actively factored into treatment. More specifically, the prognostic utility of early identification of cancer-associated weight loss can be particularly important in patients with esophageal or gastroesophageal junction tumors.
Positron Emission Tomography (PET) is an imaging modality often used in the staging and re-staging of solid tumors. Increased uptake of the radioactive glucose tracer 18F-FDG (fluorine-18-fluorodeoxyglucose) denotes an increased expression of glucose transporters such as GLUT-1 and hexokinase [4]. This leads to high glucose utilization, which implies tumor growth and a shift of tumor cells to a more malignant, metabolically demanding state. SUV (standardized uptake value) is a measurement that normalizes measurement of 18F-FDG uptake, taking patient size and amount of 18F-FDG administered into account. In gastroesophageal junction tumors, higher SUV values are correlated with higher grade malignancies [5]. Overall, PET-based imaging can identify altered metabolic and inflammatory states associated with malignancy, conditions also prevalent in cancer cachexia.
Therefore, the purpose of this study was to identify an association between primary tumor 18F-FDG uptake and the presence of cachexia-associated weight loss at diagnosis in esophageal and gastroesophageal junction cancer patients. We also aimed to identify the influence of 18F-FDG tumor uptake and cachexia on survival in this cohort. Establishing associations between cachexia and PET findings in these patients could offer mechanisms for early identification of individuals at high risk for cachexia development, thereby informing the timing of palliative care utilization.
Methods:
Population Cohort
Through an IRB-approved retrospective review of patient charts, a cohort of 224 patients from a tertiary care center diagnosed with esophageal or gastroesophageal junction tumors between January 1, 2006 to December 31, 2013 was established. If records were incomplete, or if synchronous or metachronous malignancy was present, these patients were excluded from the study. Patients were also excluded from this study if their records lacked weight data prior to the initiation of therapy, or if there was no information on PET imaging. After exclusion criteria were applied, 126 patients with pre-treatment staging PET available were evaluated. Patient characteristics including sex, race, age, date of last contact/death, and comorbidities were recorded. Tumor characteristics including primary site, grade, and stage were also reported. AJCC staging relevant to the time of diagnosis was utilized in our evaluations, primarily incorporating the 6th and 7th editions of the American Joint Committee on Cancer (AJCC) staging system.
Assessment of Cachexia Status
In order to identify patients with weight loss and cachexia-associated symptoms at diagnosis, we conducted a retrospective medical record review and obtained the following patient information from physician and nutrition notes and the clinical chart: body weight, tumor characteristics, survival outcomes, and treatments among other parameters.
To evaluate the presence of cancer-associated pre-treatment weight loss, the international consensus definition was used. Unintentional weight loss greater than 5% of total weight within 6 months before documented cancer diagnosis in patients with a BMI ≥ 20 kg/m2, or unintentional weight loss greater than 2% in patients with a BMI < 20 kg/m2 was classified as cancer-associated weight loss. Patients demonstrating weight gain, stable weight, or minimal weight loss not falling into the aforementioned parameters would not be categorized as having cancer-associated weight loss [6].
PET Review
Only patients with 18F-FDG PET obtained at the time of diagnosis and prior to any treatment intervention were included in this study. Oral contrast was used in all patients (unless otherwise indicated) and each patient underwent computed tomography (CT) after a standard uptake phase of 60–90 minutes. After this 18F-FDG infusion and time for uptake, 3D emission images were obtained through the scan range of 3mm. Maximum SUV (SUVMax) of the primary tumor, blood glucose level in the serum at the time of the scan, and the duration of the uptake phase before scan initiation were recorded when available.
Statistics
The cut-point for tumor SUVMax from PET/CT imaging was generated using Contal and O’Quigley’s method, which translates the continuous variable of tumor SUVMax into a categorical one. This approach utilizes log-rank test statistics to calculate the optimal dichotomization cut-point through outcome-oriented analysis on the basis of survival time from diagnosis [7]. Chi square testing was performed to measure the significance of the relationship between cancer-associated weight loss and tumor SUVMax. To estimate overall survival, the Kaplan-Meier approach was utilized. The log-rank test was carried out to compare survival outcomes between groups. All tests were performed at the 5% significance level and were conducted in a two-sided approach. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and SPSS Statistics version 24.0 (International Business Machines, Armonk, NY).
EGD and EUS Review
When available, esophagogastroduodenoscopy (EGD) and endoscopic ultrasound (EUS) results for patients at the time of diagnosis were obtained detailing the gastroenterologist’s interpretation of the degree of obstruction and his/her ability to extend the scope beyond the site of the tumor in the esophagus/stomach. Other measurements included were tumor size and the presence of lymph nodes greater than 1 cm in diameter as detected by EUS.
Results
Patient Cohort
The median age at cancer diagnosis for this cohort was 63 years (range 38–86). Median follow up time after diagnosis was 13.5 months (range: 1–93). Consistent with the Surveillance, Epidemiology, and End Results (SEER) registry data for gastroesophageal cancer, the majority of patients were male (77.9%) and non-Hispanic Caucasian (72.2%), with a positive alcohol (57.4%) or smoking history (73.6%) [8]. The majority of primary tumors were classified as having an adenocarcinoma (62.7%) or squamous cell carcinoma (29.4%) histology. Slightly over half of the patients had locally advanced stage III disease (50.4%), with similar numbers of patients having stage II (24.4%) or stage IV (18.5%) disease. The majority of patient primary tumors had grade 2 (40.5%) or 3 (53.2%; Table 1) features.
Table 1.
Patient Characteristics
| Characteristic | (n=126) |
|---|---|
| Age at diagnosis, median (IQR) | 63 (54, 71) |
| Female sex, n (%) | 30 (22.1%) |
| Race, n (%) | |
| Asian | 6 (4.8%) |
| Black | 17 (13.5%) |
| Caucasian | 91 (72.2%) |
| Hispanic | 12 (9.5%) |
| Tumor Site | |
| Esophagus | 87 (69.1%) |
| Cardia | 39 (31.0%) |
| Diabetes, n (%) | 25 (19.8%) |
| Tobacco history, n (%) | |
| None | 32 (25.6%) |
| Current or prior use | 92 (73.6%) |
| Unknown | 1 (.8%) |
| Alcohol history, n (%) | |
| None | 51 (41.8%) |
| Current or prior use | 70 (57.4%) |
| Unknown | 1 (.8%) |
| Cachexia, n (%) | 68 (54.0%) |
| Histology, n (%) | |
| Adenocarcinoma | 79 (62.7%) |
| Squamous cell carcinoma | 37 (29.4%) |
| Signet ring cell carcinoma | 6 (4.8%) |
| Other | 4 (3.2%) |
| Tumor grade, n (%) | |
| 1 | 7 (6.3%) |
| 2 | 45 (40.5%) |
| 3 | 59 (53.2%) |
| Tumor stage, n (%) | |
| I | 8 (6.7%) |
| II | 29 (24.4%) |
| III | 60 (50.4%) |
| IV | 22 (18.5%) |
Primary Tumor SUVMax and Patient Cachexia Characteristics
After calculating a dichotomized cut-point for tumor SUVMax of 8.5 (P=.0018), 72 (57.1%) of the 126 patients in this study were found to have a high tumor SUVMax value (SUVMax at or above the determined cut-point value of 8.5). There was a significantly lower number of patients with stage 1 disease (P=.0084), and a significantly higher number of patients with stage 4 disease (P=.0101) in a high tumor SUVMax versus low tumor SUVMax comparison. A significantly lower number of patients had grade 3 tumors in the high SUVMax group than in the low SUVMax group (P=.0393; Table 2) among evaluable patients.
Table 2.
Patient Characteristics at Diagnosis Separated by Cachexia Status or Tumor SUVMax Cut-point of 8.5. IQR, interquartile range
| Cachexia | SUVMax | |||
|---|---|---|---|---|
| No cachexia (%)a | Cachexia (%)a | < 8.5 (%)a | ≥ 8.5 (%)a | |
| Number of patients | 58 | 68 | 54 | 72 |
| Median age | 66, IQR: 59–74 | 59, IQR: 54–69 | 62.5, IQR: 55–69.5 | 63, IQR: 54.8–71 |
| Female | 12 (20.7%) | 14 (20.6%) | 13 (24.1%) | 13 (18.1%) |
| Race: | ||||
| Asian | 2 (3.5%) | 4 (5.9%) | 3 (5.6%) | 3 (4.2%) |
| Black | 4 (6.9%) | 13 (19.1%)b | 5 (9.3%) | 12 (16.7%) |
| Caucasian | 50 (86.2%)b | 41 (60.3%) | 40 (74.1%%) | 51 (70.8%) |
| Hispanic | 2 (3.5%) | 10 (14.7%)b | 6 (11.1%) | 6 (8.3%) |
| Tumor Site | ||||
| Esophagus | 39 (67.2%) | 48 (70.6%) | 30 (55.6%) | 57 (79.2%) |
| Cardia | 19 (32.8%) | 20 (29.4%) | 24 (44.4%) | 15 (20.8%) |
| Diabetes | 15 (25.9%) | 10 (14.7%) | 15 (27.8%) | 10 (13.9%) |
| Tobacco | 44 (75.9%) | 48 (71.6%) | 35 (66.0%) | 57 (79.2%) |
| Alcohol | 34 (61.8%) | 36 (53.7%) | 28 (56.0%) | 42 (58.3%) |
| Histology: | ||||
| Adenocarcinoma | 38 (65.5%) | 41 (60.3%) | 38 (70.4%) | 41 (56.9%) |
| Squamous carcinoma | 15 (25.9%) | 22 (32.4%) | 12 (22.2%) | 25 (34.7%) |
| Signet ring carcinoma | 2 (3.5%) | 4 (5.9%) | 3 (5.6%) | 3 (4.2%) |
| Other | 3 (5.2%) | 1 (1.5%) | 1 (1.9%) | 3 (4.2%) |
| Tumor Grade: | ||||
| 1 | 3 (6.0%) | 4 (6.6%) | 1 (2.1%) | 6 (9.5%) |
| 2 | 20 (40.0%) | 25 (41.0%) | 16 (33.3%) | 29 (46.0%) |
| 3 | 27 (54.0%) | 32 (52.5%) | 31 (64.6%)b | 28 (44.4%) |
| Tumor Stage: | ||||
| 1 | 5 (9.1%) | 1 (4.7%) | 7 (13.7%)b | 1 (1.5%) |
| 2 | 17 (30.9%) | 12 (18.8%) | 15 (29.4%) | 14 (20.6%) |
| 3 | 26 (47.3%) | 34 (53.1%) | 25 (49.0%) | 35 (51.5%) |
| 4 | 7 (12.7%) | 15 (23.4%) | 4 (7.8%) | 18 (26.5%)b |
% derived from division by characteristic column total available, ex: (female with no cachexia ÷ total no cachexia)×100 = 20.7% or (12÷58)×100 = 20.7%
Significantly higher incidence of row category (P<.05) within population characteristic group
Through our analysis of patient charts and use of the international consensus definition for cancer cachexia, we demonstrated that 68 (54.0%) of the 126 patients in the study had cancer associated weight loss at diagnosis. A significantly higher number of Black (P=.0454) and Hispanic (P=.0319) patients, as well as a significantly lower number of non-Hispanic Caucasian patients (P=.0012) were found to have cachexia at diagnosis. Otherwise, all other clinical metrics evaluated were balanced between high tumor SUVMax/low tumor SUVMax and no cachexia/cachexia cohorts (Table 2).
The Association Between Primary Tumor SUVMax and Cachexia
Of the 126 evaluable gastroesophageal cancer patients, 33 did not present with cachexia or a tumor SUVMax at or above the determined cut-point of 8.5, the latter representing our definition of patients with high SUVMax tumors. Twenty-one patients had cachexia and a tumor SUVMax below the 8.5 cut-point. Twenty-five patients had no cachexia at diagnosis but did have a tumor with a tumor SUVMax value at or above the 8.5 cut-point. Finally, 47 patients had both cachexia at diagnosis and a tumor SUVMax at or above the 8.5 cut-point. Using univariate analyses, the presence of pre-treatment cancer associated weight loss was significantly associated with primary tumor SUVMax at or above the determined cut-point of 8.5 (P=.0033; Supplemental Table 1), suggesting that patients with high SUVMax tumors at diagnosis are more apt to also having cachexia-associated weight loss at diagnosis.
Multivariate Analysis of Predictors of Survival
Through multivariate Cox regression analysis, the three variables found to significantly associate with overall survival were high tumor SUVMax (at or above the 8.5 cut-point), the administration of systemic therapy, and the incorporation of surgical resection into therapy. Patients with high tumor SUVMax values had a 2.3-fold risk of death than patients with low tumor SUVMax after controlling for the effect of surgical and systemic treatment (95% CI 1.4, 3.7; P=.0010; Supplemental Table 2).
Overall Survival
The median overall survival for the entire cohort was 19.5 months. Median overall survival was greater in patients with low tumor SUVMax, i.e. below versus at or above the 8.5 cut-point (26.7 vs 13.8 months; P=.0016; Figure 1). Patients without cachexia at diagnosis were also found to have greater median overall survival than those with cachexia (32.8 vs 14.7 months; P=.0119; Figure 2, Table 3).
Fig. 1.
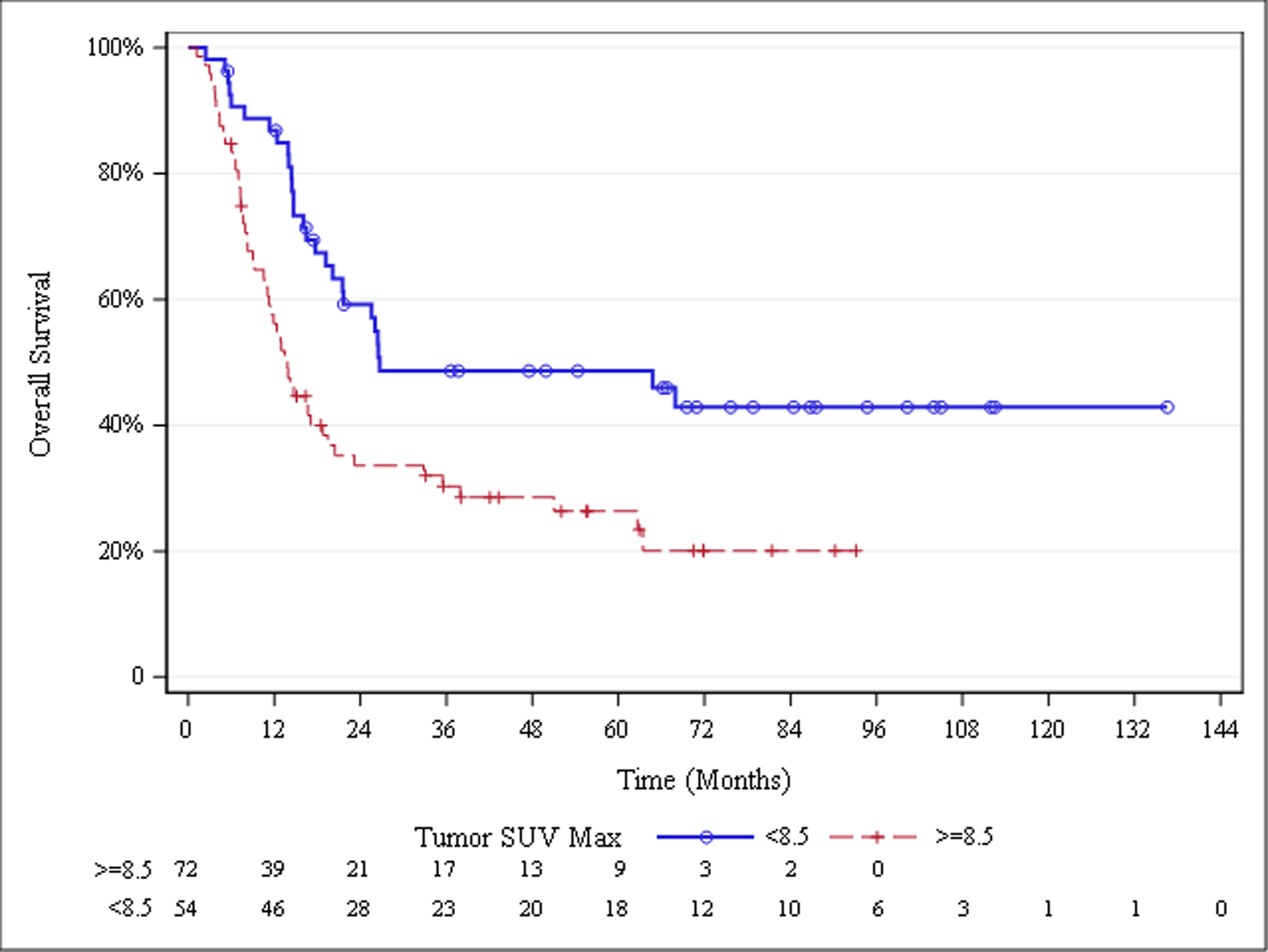
Overall Survival Stratified by Primary Tumor SUVMax
Fig. 2.
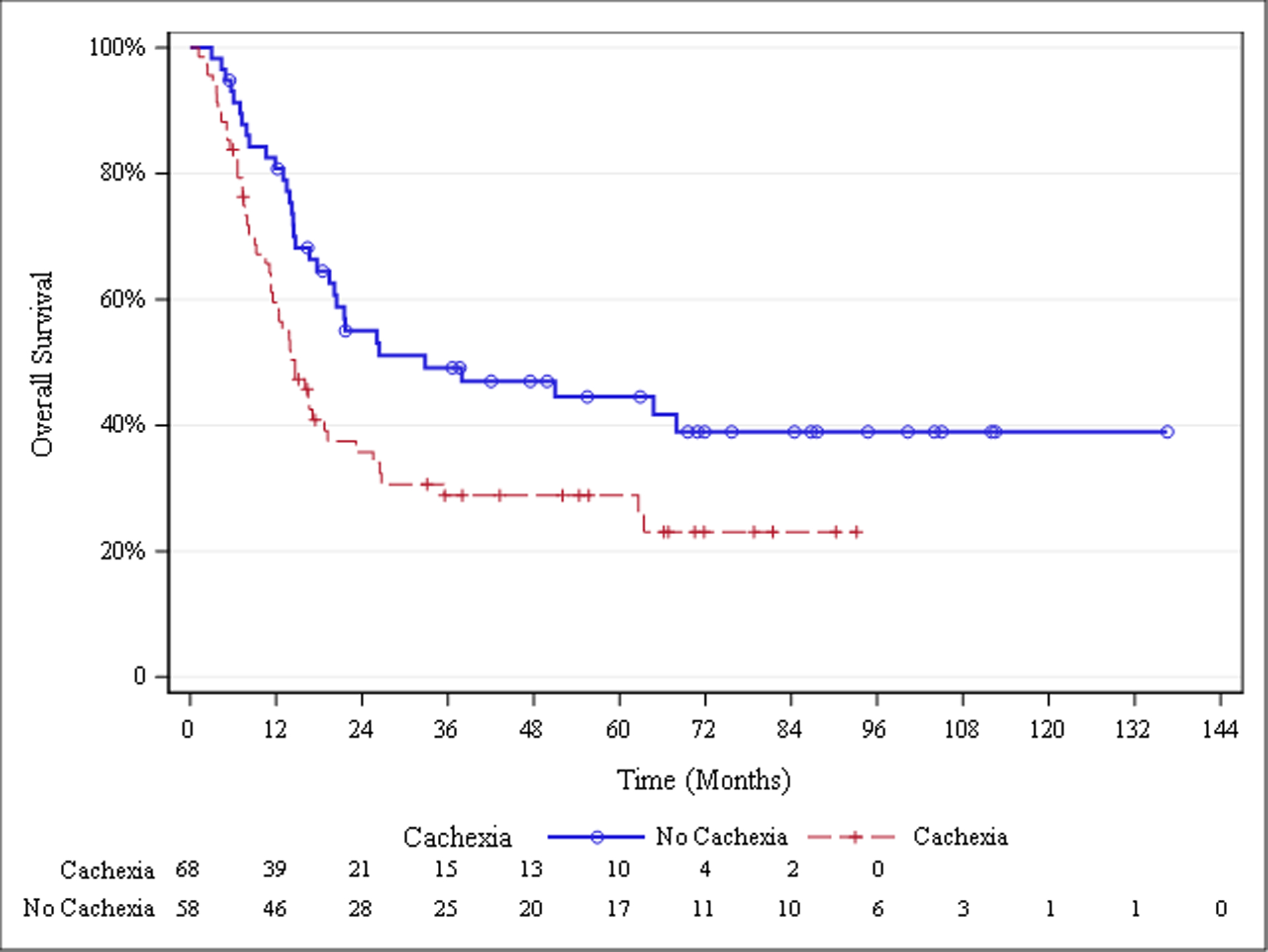
Overall Survival Stratified by Cachexia Status
Table 3.
Log-Rank Analysis for Overall Survival
| Group | Median survival time (95% CI) (Months) | P value | Survival comparison group | P value |
|---|---|---|---|---|
| All patients (n=126) | 19.5 (14.7, 26.5) | Cachexia, SUVMax ≥8.5 vs no cachexia, SUVMax ≥8.5 |
.7832 | |
| Cachexia* SUV Max | .0007 | |||
| Cachexia, SUVMax≥8.5 (n=47) | 12.4 (8.2, 23.2) | Cachexia, SUVMax ≥8.5 vs cachexia, SUVMax <8.5 |
.8313 | |
| No cachexia, SUVMax≥8.5 (n=25) | 14.2 (10.6, 20.4) | |||
| Cachexia, SUVMax <8.5 (n=21) | 16.0 (12.4, 26.5) | Cachexia, SUVMax ≥8.5 vs no cachexia, SUVMax <8.5 |
.0003 | |
| No cachexia, SUVMax <8.5 (n=33) | Did not reach median | |||
| Primary tumor SUV Max | .0016 | No cachexia, SUVMax ≥8.5 vs cachexia, SUVMax <8.5 |
.7345 | |
| <8.5 (n=54) | 26.7 (20.1, .) | |||
| ≥8.5 (n=72) | 13.8 (10.6, 18.8) | No Cachexia, SUVMax ≥8.5 vs no cachexia, SUVMax <8.5 |
<.0001 | |
| Cachexia at diagnosis | .0119 | |||
| No cachexia (n=58) | 32.8 (19.5, .) | Cachexia, SUVMax <8.5 vs no cachexia, SUVMax <8.5 |
.0007 | |
| Cachexia (n=68) | 14.7 (11.3, 19.2) |
When grouped into four cohorts based off of high vs low tumor SUVMax and the absence or presence of cachexia-associated weight loss, median overall survival was not significantly different between cohorts of patients with cachexia and low tumor SUVMax, no cachexia and high tumor SUVMax, and both cachexia and high tumor SUVMax (16.0, 14.2, and 12.4 months). There was a trend towards reduced median overall survival as patients demonstrated cachexia and high tumor SUVMax. Patients with no cachexia and low SUVMax did not reach a median overall survival time due to high rates of survival and the lack of events in the time period evaluated. Ultimately, significantly different survival times were found exclusively between patients negative for cachexia and high SUVMax, and every other patient group with either cachexia, high SUVMax, or both (Figure 3, Table 3).
Fig. 3.
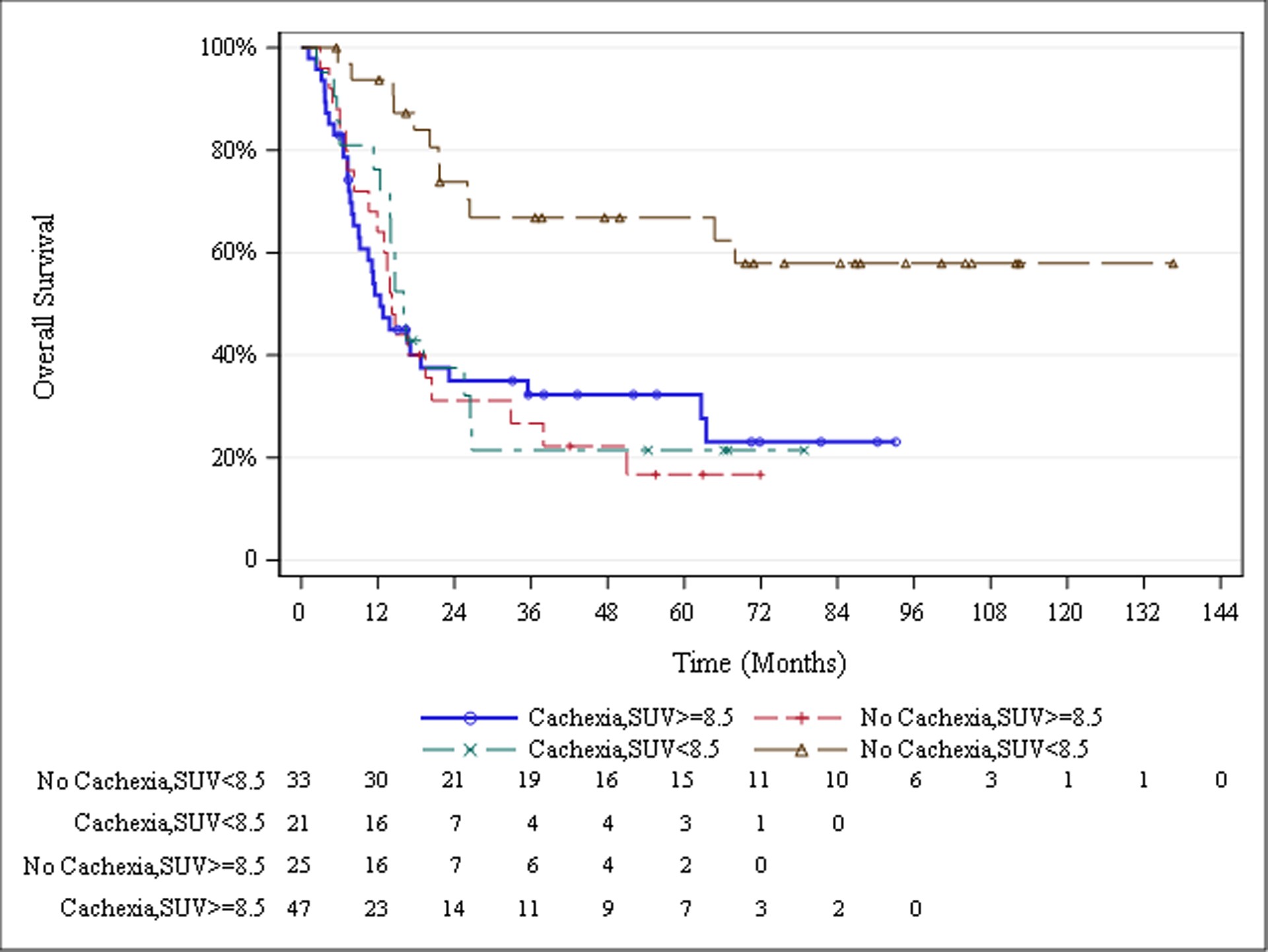
Overall Survival Stratified by Cachexia Status and Primary Tumor SUVMax
EGD/EUS Findings in the Esophageal or Gastroesophageal Junction Cancer Patients Cohort
As a means of assessing the cause of cancer-associated weight loss in the cohort of patients without and with cachexia, we evaluated findings from their EGD/EUS reports. A significantly higher number of cachectic patients had tumors resulting in any or complete obstruction compared to patients without cachexia (82.3% vs 55.6%, P=.0075 Supplemental Table 3). When divided into high tumor SUVMax and low tumor SUVMax cohorts, cachexia was significantly associated with an increase in any obstruction in only the high tumor SUVMax group (P=.0216, Supplemental Table 4). We also collected findings from the EGD/EUS evaluation that suggested that the scope was unable to traverse the esophagus as another surrogate for obstruction that could promote cancer-associated weight loss. Patients with cachexia had a significantly higher incidence of procedures with findings that the scope could not traverse the esophagus when compared to patients without cachexia (31.4% vs 8.5%, P=.0050, Supplemental Table 3). When the cachexia cohort was divided into high tumor SUVMax and low tumor SUVMax groups, there were no significant differences observed with scope traversal (Supplemental Table 4).
Discussion:
The aim of our study was to determine whether there were associations between primary tumor SUVmax for esophageal or gastroesophageal tumors and a risk for developing cancer-associated weight loss, a surrogate for cachexia. We also sought to identify a synergistic or additive effect on overall survival in patients with both higher tumor FDG uptake on PET imaging and cancer-associated weight loss. Our results demonstrate a significant association between higher tumor SUVMax values and the incidence of cancer-associated weight loss at diagnosis. A high tumor SUVMax was also associated with reduced patient survival in both univariate and multivariate analysis. At tumor SUVMax values either below or above a cut-point dichotomized to maximize survival differences, cancer-associated weight loss was found to be significantly associated with decreased survival.
The association we found between elevated cachexia incidence and higher SUVMax values is consistent with a preclinical study by Penet et al, in which the well-established cachexia- inducing murine adenocarcinoma MAC16 tumor model was shown to exhibit more than 2-fold higher tumor FDG uptake than its histologically similar non-cachexia inducing MAC13 counterpart [9]. It should be noted that in these mice with higher tumor FDG uptake, a significantly greater depletion of lipids was observed from the adipose, further implying the relationship between increased glycolysis and lipolysis in cancer cachexia.
High SUVMax values in primary tumor suggesting an altered level of glycolysis may account for the association of these high SUVMax tumors with cachexia-associated weight loss. The FDG measured increase of glucose uptake in tumor cells in PET scans might be a result of the Warburg effect, where anaerobic glycolysis becomes the predominant mechanism of glucose utilization. In this process, higher amounts of lactate are produced, which is recycled through gluconeogenesis in the liver in the well-known metabolic process of the Cori cycle [10]. The increased demands of hepatic gluconeogenesis are hypothesized to induce increased lipolysis and proteolysis, leading to the fat and muscle wasting observed in cachexia, which has further been demonstrated in a separate pancreatic cancer mouse model [11].
A study conducted by Leij-Halfwerk found evidence of increased liver gluconeogenesis in lung cancer patients with weight loss when compared to their weight stable counterparts, further suggesting the contribution of the Cori cycle (and therefore the Warburg effect) to cancer associated weight loss [12]. Gehman et al found that cachexia-inducing tumors in rats led to higher levels of hepatic gluconeogenesis than non-cachectic tumors through the evaluation of hepatic Pi/ATP ratios. These ratios were observed through 31P-Magnetic Resonance Spectroscopy, which provides relaive measures of phosphorous metabolites [13]. Further studies need to be conducted to further assess the involvement of the Warburg effect with increased FDG uptake and cancer cachexia. However, the effects of increased glycolytic demand of tumor cells measured by PET scans on hepatic gluconeogenesis and altered energy metabolism in cancer patients is apparent.
The combination of a high tumor SUVMax value and cachexia at diagnosis did not demonstrate synergy or even additive effects in reducing survival, suggesting that one process may be relevant and/or critical to the etiology of the other. For instance, the development of cachexia may promote increased glucose uptake by the tumors, resulting in higher tumor SUVMax values. Inversely, higher SUVMax values in tumors may suggest a biology supportive of secreting cachexia-inducing factors that act to reduce appetite, adipose mass, muscle mass, and weight. Cachexia and high tumor SUVMax values are also associated with advanced stage and progression of disease, which could imply that a less specific process drives the relationship of these factors and their contributions to survival prognoses. However, cachexia has been demonstrated to reduce survival in patients independent of stage across a variety of cancer types [3]. This study demonstrates the utility of detecting cachexia and high tumor FDG uptake in patients with esophageal or gastroesophageal junction tumors, as cachexia progression might interact with tumor glycolysis in a PET-detectable fashion, leading to worse survival outcomes.
There were limitations in this analysis that should be noted. Due to the retrospective nature of this review, cachexia was defined through review of routinely available clinical data, specifically body weight, which results in a narrow but accepted view of cachexia as a syndrome. The use of a cut-point for the interpretation of SUVMax facilitated the categorization of the variable and grouping for survival trend analysis. However, this can lead to a loss of statistical power, as there is inherent information loss when a continuous variable is converted into a dichotomous one. Moreover, this approach does not account for the possibility of multiple cut-point scenarios [7, 14]. The EDG/EUS results at the time of diagnosis were included in this study to identify and account for obstructive causes of cachexia. An understanding of these causes of cachexia are critical in this cohort as obstruction might be more readily alleviated than an inflammatory mechanism, reducing the burden of cachexia on patient survival. This might be especially relevant in cachectic patients with low tumor SUVMax values, as we found their survival to be decreased similarly to patients with high tumor SUVMax. If cachexia is a strong determinant of survival in these patients, early detection and intervention could be critical in improving their survival outcomes.
In prospective studies, cancer cachexia should be defined on intake and include longitudinal assessment of sarcopenia, adipose loss, and other diagnostic criterion (patient functionality inventories, reported appetite, and levels of inflammatory biomarkers). Sarcopenia specifically is heavily implicated in cachexia, as both part of cachexia’s definition, and as one of its most functionally impairing manifestations [6]. Including an assessment of sarcopenic wasting, evaluating its relationship with SUV and cachectic outcomes, and determining the effects of these variables on both survival and subjective functional outcomes would provide meaningful elucidation to our clinical understanding of tissue wasting in cancer syndromes. Current literature supports the evaluation of sarcopenia through methods including muscle tissue evaluation from diagnostic CT scans and grip strength testing, which are variables of interest for developing patient databases of this type. [15, 16].
Through a better understanding of the manifestations of cachexia in patients with gastroesophageal cancer, earlier and more specific interventions can be applied to minimize its effects on disease progression. Notably, the finding of statistically significant increases in incidence of cachexia at diagnosis in Black and Hispanic patients mirrors a separate study conducted by our group on a non-small cell lung cancer cachexia patient cohort [17]. Further insight into racial and socioeconomic disparities in cancer cachexia prevalence and outcomes are crucial to improving diagnosis and treatment.
Through retrospective review of a cohort of patients with esophageal or gastroesophageal junction tumors, this study provides novel insight into the poorly understood relationship between PET-based tumor FDG avidity and cancer-associated weight loss. Although a stronger mechanistic understanding is yet to be developed to further ascertain how tumor glucose utilization influences cachectic development or vice versa, we have observed a significant association between higher tumor SUVMax values and cachexia incidence at diagnosis. The correlation between cachexia and high tumor SUVMax was also associated with decreased patient survival, providing further insight into the utility of these measurements as prognostic indicators for patients with esophageal and gastroesophageal junction cancer. Ultimately, this knowledge may inform early palliative interventions that may increase survival outcomes independent of tumor-directed therapies.
Supplementary Material
Acknowledgments
This work was supported by the Burroughs Wellcome Fund Career Awards for Medical Scientists (1019692); American Gastroenterological Association Scholar Award (2019AGARSA3); Hartwell Foundation Fellowship grant, American Cancer Society grant (133889-RSG-19-195-01-TBE); Cancer Prevention and Research Institute of Texas (RP200170); V Foundation Scholar Award (V2019-014); and National Institutes of Health grants (P30CA142543).
RI laboratory has received funding from Pfizer unrelated to this work.
We thank the UTSW Department of Radiation Oncology Clinical Research Team for their support of our study
Abbreviations:
- EGD
esophagogastroduodenoscopy
- EUS
endoscopic ultrasound
- 18F-FDG
fluorine-18-fluorodeoxyglucose
- PET
positron emission tomography
- 31P MRS
31P-magnetic resonance spectroscopy
- SUV
standardized uptake value
- SUVMax
maximum standardized uptake value
Footnotes
SO, BSG, AG, and CA have nothing to disclose.
PI has worked with AstraZeneca in an advisory capacity unrelated to this work.
All relevant deidentified data, analytic methods, and study materials will be made available to other researchers as relevant.
References
- 1.Evans WJ, et al. , Cachexia: a new definition. Clin Nutr, 2008. 27(6): p. 793–9. [DOI] [PubMed] [Google Scholar]
- 2.Fonseca G, et al. , Cancer Cachexia and Related Metabolic Dysfunction. Int J Mol Sci, 2020. 21(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gannavarapu BS, et al. , Prevalence and Survival Impact of Pretreatment Cancer-Associated Weight Loss: A Tool for Guiding Early Palliative Care. J Oncol Pract, 2018. 14(4): p. e238–e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen B, et al. , Revisit 18F-fluorodeoxyglucose oncology positron emission tomography: “systems molecular imaging” of glucose metabolism. Oncotarget, 2017. 8(26): p. 43536–43542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stagg J, et al. , Significance of 18F-Fluorodeoxyglucose Uptake at the Gastroesophageal Junction: Comparison of PET to Esophagogastroduodenoscopy. Dig Dis Sci, 2015. 60(5): p. 1335–42. [DOI] [PubMed] [Google Scholar]
- 6.Fearon K, et al. , Definition and classification of cancer cachexia: an international consensus. Lancet Oncol, 2011. 12(5): p. 489–95. [DOI] [PubMed] [Google Scholar]
- 7.Contal C and O’Quigley J, An application of changepoint methods in studying the effect of age on survival in breast cancer. Computational Statistics & Data Analysis, 1999. 30(3): p. 253–270. [Google Scholar]
- 8.Mathieu LN, et al. , Age and sex differences in the incidence of esophageal adenocarcinoma: results from the Surveillance, Epidemiology, and End Results (SEER) Registry (1973–2008). Dis Esophagus, 2014. 27(8): p. 757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penet MF, et al. , Metabolic signatures imaged in cancer-induced cachexia. Cancer Res, 2011. 71(22): p. 6948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold J, Cancer cachexia and gluconeogenesis. Ann N Y Acad Sci, 1974. 230: p. 103–10. [DOI] [PubMed] [Google Scholar]
- 11.Wang F, et al. , The Warburg effect in human pancreatic cancer cells triggers cachexia in athymic mice carrying the cancer cells. BMC Cancer, 2018. 18(1): p. 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leij-Halfwerk S, et al. , Decreased energy and phosphorylation status in the liver of lung cancer patients with weight loss. J Hepatol, 2000. 32(6): p. 887–92. [DOI] [PubMed] [Google Scholar]
- 13.Gehman KE, et al. , Early detection of cancer cachexia in the rat using 31P magnetic resonance spectroscopy of the liver and a fructose stress test. NMR Biomed, 1996. 9(6): p. 271–5. [DOI] [PubMed] [Google Scholar]
- 14.Mandrekar J, Mandrekar S, and Cha S, Cutpoint Determination Methods in Survival Analysis using SAS. 2003. [Google Scholar]
- 15.Mori N, et al. , Prognostic role of low muscle mass and strength in palliative care patients with incurable cancer: a retrospective study. JCSM Clinical Reports, 2021. 6(3): p. 93–99. [Google Scholar]
- 16.Bhasin S, et al. , Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc, 2020. 68(7): p. 1410–1418. [DOI] [PubMed] [Google Scholar]
- 17.Lau SKM, et al. , Impact of Socioeconomic Status on Pretreatment Weight Loss and Survival in Non-Small-Cell Lung Cancer. J Oncol Pract, 2018. 14(4): p. e211–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


