Abstract
The kidneys, heart and lungs are vital organ systems evaluated as part of acute or chronic toxicity assessments. New methodologies are being developed to predict these adverse effects based on in vitro and in silico approaches. This paper reviews the current state of the art in predicting these organ toxicities. It outlines the biological basis, processes and endpoints for kidney toxicity, pulmonary toxicity, respiratory irritation and sensitization as well as functional and structural cardiac toxicities. The review also covers current experimental approaches, including off-target panels from secondary pharmacology batteries. Current in silico approaches for prediction of these effects and mechanisms are described as well as obstacles to the use of in silico methods. Ultimately, a commonly accepted protocol for performing such assessment would be a valuable resource to expand the use of such approaches across different regulatory and industrial applications. However, a number of factors impede their widespread deployment including a lack of a comprehensive mechanistic understanding, limited in vitro testing approaches and limited in vivo databases suitable for modeling, a limited understanding of how to incorporate absorption, distribution, metabolism, and excretion (ADME) considerations into the overall process, a lack of in silico models designed to predict a safe dose and an accepted framework for organizing the key characteristics of these organ toxicants.
Keywords: In Silico, Computational toxicology, Organ toxicity, In Silico toxicology protocols, Kidney, Heart, Lung, Hazard identification, Risk assessment, QSAR, Expert alerts, Read-across
Introduction
Chemical safety assessment of substances encompasses the assessment of acute and chronic toxicities, which in turn often includes examination of the adverse effects induced on different organs (e.g., kidney, heart, lung). In repeated-dose toxicity studies, organs and tissues are investigated to monitor changes (e.g., physiological, functional and morphological), leading to an adverse effect and to identify organs that are most affected (i.e., target organs) by a particular chemical [1,2]. Adverse effects on target organs are also relevant in the context of acute systemic toxicity [3]. Whereas, dysregulations and alterations of complex biological pathways result in organ toxicity that can occur as a primary effect on a given organ, they can also be a result of secondary effects in organs and tissues that have a physiological dependence on the primary target [4].
Use of in silico toxicology (IST) methods to predict organ toxicity can be sustained and advanced by development of IST protocols that are formulated to offer a standardized way to exploit in silico methods [5]. Such a standardization process promotes acceptability of both the methods and the corresponding predictions by end users, colleagues, collaborators, and regulators as well as provides a means to support a more transparent analysis of the results. Protocols that describe the integration of in silico methods with existing experimental data to identify potential genotoxicants [6] and skin sensitizers [7] have been developed based on the experience of a cross-industry consortium comprising many organizations.
An IST protocol is a description of the in silico prediction workflow within a consistent and well-documented structure and includes [5]:
identification of adverse effects or mechanisms to predict alongside the corresponding experimental data and/or in silico methodologies and approaches to use;
recommendation on generation of the predictions and on assessment of relevant experimental data;
indications on the performance of the in silico analysis to generate results including expert review;
recommendation on the reporting formats to share the results and the corresponding uncertainties.
An IST protocol then consists of the definition of experimental data and in silico methodologies associated with each effect or mechanism, the definition of rules underlying the combination of information, the definition of expert review guidelines, and the definition of a documentation guideline (see Fig. 1). Hence the development of an IST protocol first requires the definition of an assessment framework that outlines how to integrate data originating from different sources, e.g., in vivo and alternative methods including in silico predictions. A basic assessment framework has been drafted and proposed for liver toxicity and this is shown in Fig. 2 [8]. The current work is a preparatory step for the development of IST protocols for other organ toxicities, and more specifically for the development of a framework that integrates in silico methods predicting potential adverse effects from the molecular structure of chemicals. The focus is on toxicity to specific organ systems, namely kidney toxicity (i.e., nephrotoxicity or renal toxicity), heart toxicity (i.e., cardiotoxicity or cardiac toxicity), and lung toxicity (i.e., pulmonary toxicity). It was recently noted that the term kidney should be preferred over the use of either “renal” or the prefix “nephro-” to generally describe kidney disease and function especially in non-technical contexts [9].
Fig. 1.
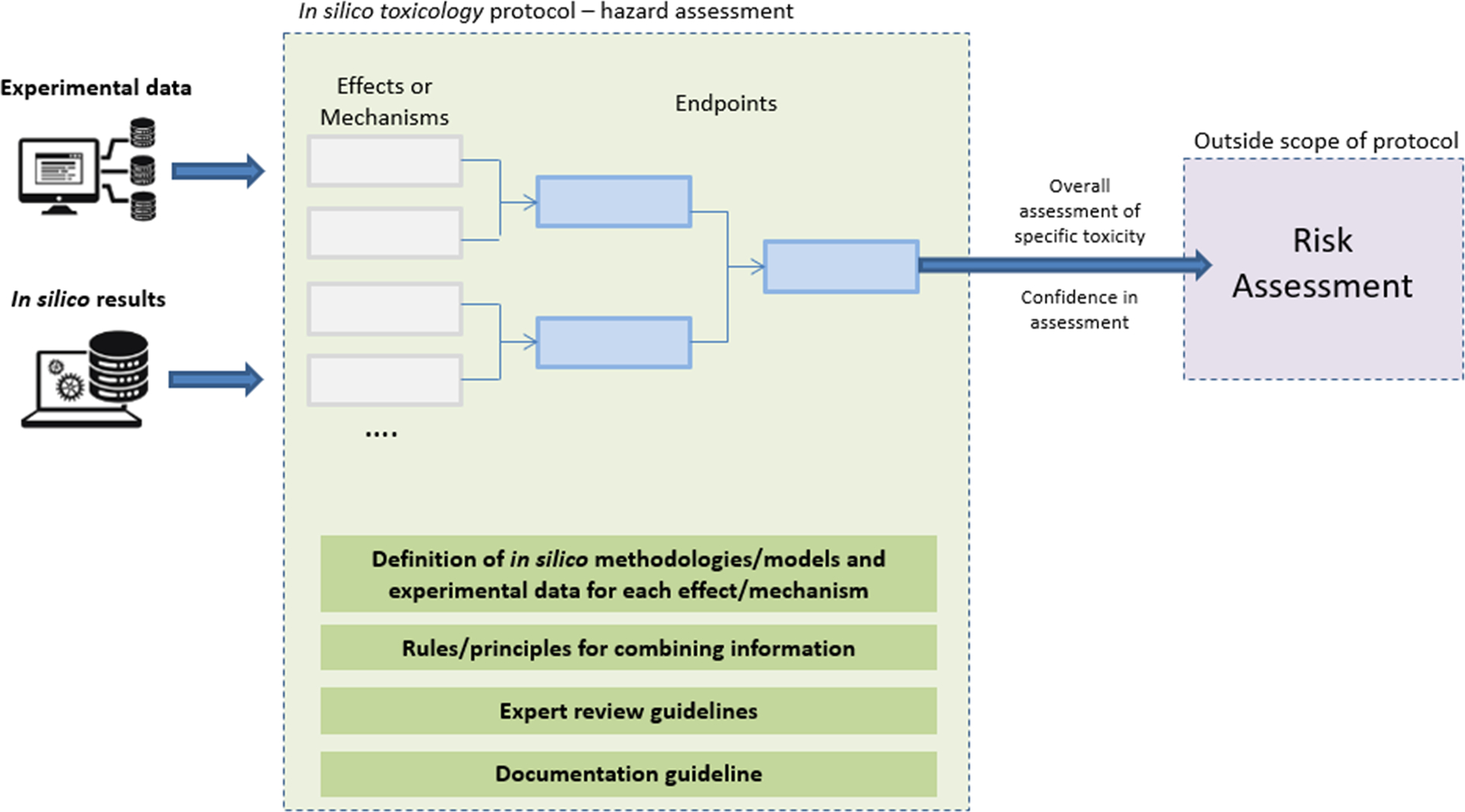
Schematic workflow encoded in the in silico toxicology protocols [5].
Fig. 2.
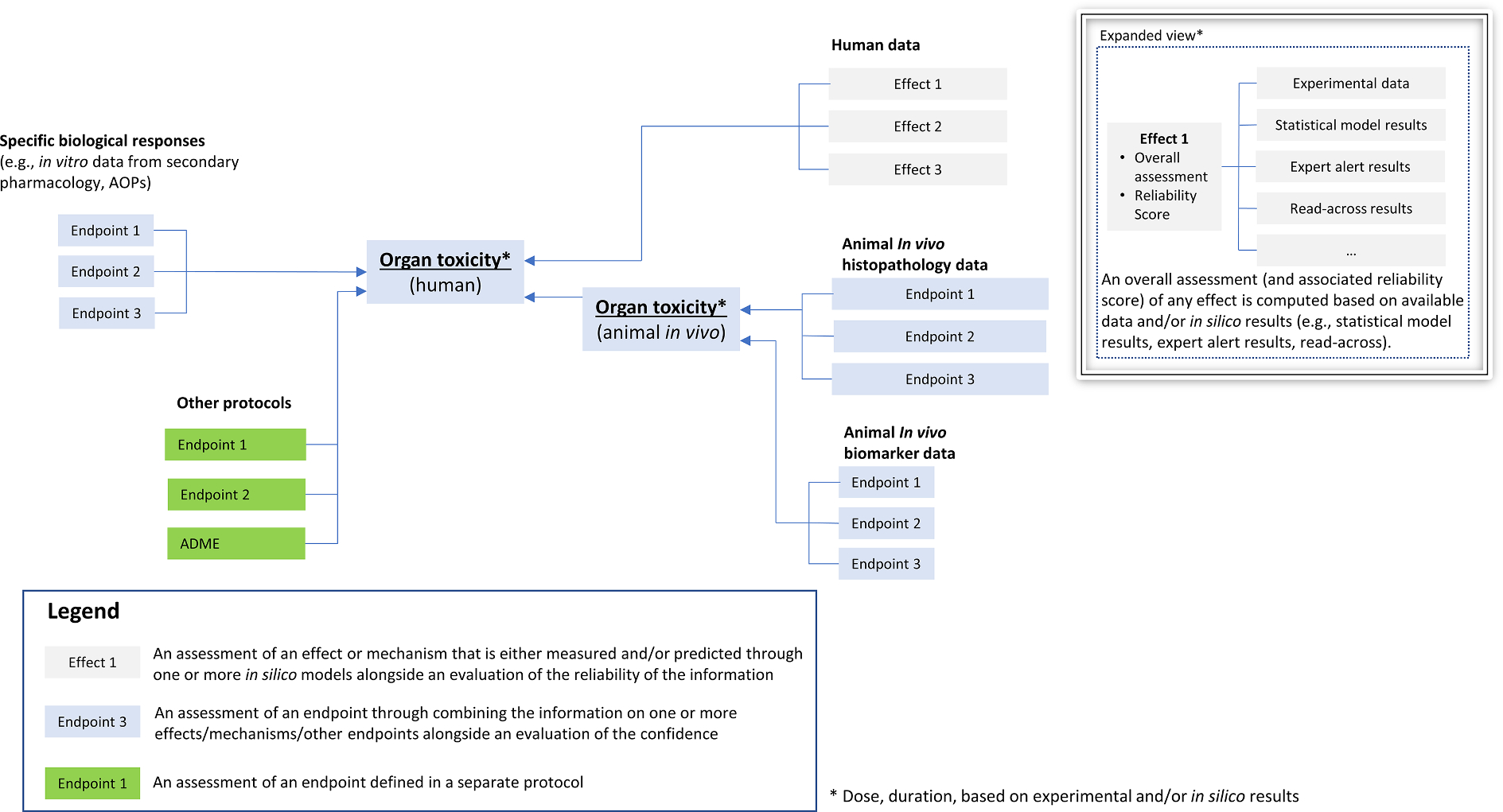
Draft outline of potential hazard assessment framework for organ toxicity (adapted from [8]). The draft framework combines information from in vitro approaches (e.g., biological responses from receptor-based assays), in vivo experiments, and human data. Other protocols (e.g., ADME or other organs) may feed a protocol for a given organ. Exposure scenarios (e.g., environmental, drug, consumer, accidental) may also be used to supplement the protocol. Effects (predicted by in silico methods or measured experimentally) are combined for the assessment of a given endpoint.
The review material collected here provides the basis for identification of endpoints and definition of their relationships in a mechanistically-informed framework that constitutes the basis for the development of the IST protocol. These organ systems are reviewed, and this includes a description of organ toxicity along with processes and endpoints. These are outlined to provide context to what needs to be predicted. Current in vivo and in vitro methods are generally discussed, as this information is essential to incorporate within the weight of evidence (WoE) in any hazard assessment in addition to supporting the development of in silico methods. An outline of the current state of the art in predicting organ toxicity is provided together with a discussion on what progress is needed to improve such predictions. Finally, the discussion summarizes key issues to address across all organ systems highlighted.
Kidney toxicity
Kidney toxicity (nephrotoxicity) is defined as disease or dysfunction of the kidney caused by chemical insult following acute or chronic exposure to drugs or xenobiotics [10]. It relates to toxicity to the nephron, the functional unit of the kidney. The primary functions of kidneys are clearance of waste products from the blood, maintenance of electrolyte and acid-base balance, regulation of extracellular fluid volume, and endocrine activity [11–13]. Vulnerability of this organ to chemical injury is related to its specialized role in the filtration, metabolism, and excretion of exogenous compounds [14,15] resulting in high local concentration of potentially toxic substances and/or formation of reactive metabolites.
A number of physiological and biochemical factors contribute to renal liabilities. First, the small mass of the kidney as compared to the resting cardiac output that it receives exposes this organ to high levels of circulating xenobiotics and of corresponding metabolites mainly produced in the liver [12,16]. Second, the renal processes of glomerular filtration, tubular reabsorption, and secretion contribute to concentrate toxicants in the kidneys; glomerular filtration is the first step of production of urine and results in an ultrafiltrate of the plasma; during tubular reabsorption and secretion, glomerular filtrate passes through the different segments of renal tubules where filtered solutes and water are reabsorbed, allowing the elimination of waste products [17]. Importantly, transport proteins play a critical role in concentrating potential toxicants [16]. Third, kidneys have high energy requirements to maintain their reabsorptive and secretory functions and this makes them susceptible to oxidative stress, resulting in an imbalance between free radical production and antioxidant defense [10,11]. This effect is particularly exacerbated in patients with common systemic diseases such as hypertension, diabetes mellitus and hypercholesterolemia [18,19]. Fourth, the renal system includes enzymes such as CYP450 and flavin-containing mono-oxygenases that mediate the metabolic degradation of xenobiotics possibly leading to the formation of reactive metabolites that are nephrotoxic [12].
Given the central role of this organ in the filtration and active elimination of foreign compounds, kidney toxicity may arise from exposure to a wide variety of substances including pharmaceuticals, agrochemicals, and industrial and environmental chemicals; growing concern is also posed by substances such as herbal remedies, natural products, and nutritional supplements [12,20,21]. After hepatotoxicity, toxicity to kidney significantly accounts for drug candidate failure in drug discovery and development; it is also a rather common problem in standard clinical care [22,23] and it contributes to acute or chronic functional changes of kidneys [24]. Prolonged cumulative lifetime exposure to chemicals in conjunction with age factors may accelerate the deterioration of kidney function and lead to chronic kidney disease (CKD) [25]. Exposure to pesticides has been clearly linked to kidney adverse effects [26,27]. As such, kidney toxicity is a specific concern in the context of occupational health too.
Kidney toxicity – Processes and endpoints
The spectrum of kidney toxicity manifestations is wide, and it reflects the diverse damage that can occur along the different segments of the nephron. Each nephron consists of glomerulus, proximal tubule, loop of Henle, distal tubule, and collecting duct; the different segments of the nephron comprise cells designed to perform specific functions and express various transporters and receptors. Notably, drug-induced kidney injuries frequently affect the proximal tubules, and it results in acute or chronic functional changes as a consequence of their key function in glomerular filtrate concentration and drug transport [24,28].
How toxicants cause injury to the nephron has been extensively studied in the context of drug-induced kidney injury, highlighting that different mechanisms of toxicity exist with drugs selectively targeting specific cell types, or non-selectively injuring multiple cell types [29]. Chemically induced kidney injury specifically depends on the intrinsic nephrotoxic potential of the chemical and the corresponding exposure (dose, route of administration, duration). A simplistic way to picture progression of kidney toxicity involves a first step where the foreign substances can undergo metabolic degradation that potentially forms reactive metabolites; toxic compounds can interact with organelles in the cells, interfere with signaling pathways, and ultimately lead to cell death and inflammation [30]. Kidney injury may progress to specific diseases including glomerulonephritis (injury to the glomeruli), acute kidney injury (AKI), CKD, and kidney failure. While AKI entails an abrupt change in kidney function, CKD is characterized by lasting structural and functional abnormalities. Kidney failure is defined as the final stage of chronic kidney disease (i.e., the disease stage where kidneys cannot function on their own).
Notably, oxidative stress is known to play an important role in the development of kidney injury or diseases, where an imbalance between the generation and elimination of reactive oxygen species can elicit damaging processes including inflammation, cell death (necrosis or apoptosis), fibrosis, tissue damage, and finally abnormal kidney function [11,30–32].
Extensive studies on kidney toxicity for pharmaceuticals have linked the adverse effects of kidney toxicants to general pathogenic mechanisms (see Table 1) that may be further related to specific molecular and biological events within the Adverse Outcome Pathway (AOP) construct (see Table 2) [33].
Table 1.
Pathogenic mechanisms of kidney toxicity [12,33,37–40]. It should be noted that rhabdomyolysis and thrombotic microangiopathy are two forms of kidney toxicity that have a systemic origin [33].
| Pathogenic mechanisms | Details |
|---|---|
|
| |
| Altered intraglomerular hemodynamics | Regulation of intraglomerular pressure is mediated by circulation of prostaglandins (vasodilation) and the action of angiotensin-II (vasoconstriction). Alteration of glomerular pressure and a decrease of the glomerular filtration rate can be promoted by substances with antiprostaglandin activity (e.g., nonsteroidal anti-inflammatory drugs) or with antiangiotensin-II activity (e.g., inhibitors of ACE receptor or blockers of ARB receptor). |
| Tubular injury (proximal and distal) | Tubules, especially the proximal segments, are vulnerable to toxicants that can elicit cytotoxicity by affecting mitochondrial function, impairing tubular transport, increasing oxidative stress, or favoring free radical formation. |
| Nephritis (tubular, interstitial, and glomerular) | Nephritis is inflammation of the kidneys that occurs in glomerulus, renal tubular cells, and/or the surrounding interstitium to promote regeneration and repair of the kidney injury; unresolved inflammation can progressively lead to renal fibrosis and impairment of the kidney function. Nephritis involves both cells of the immune system and activation of intrinsic renal cells. |
| Tubular obstruction | Insoluble crystals are formed in the nephron tubules, primarily in the distal segments, obstructing urine flow and driving disorder in kidney function. |
| Rhabdomyolysis | Rhabdomyolysis is a syndrome caused by skeletal muscle injury leading to death of muscle fibers and release of intracellular contents (myoglobin and creatine kinase) into the plasma that in turn induce adverse effects in the kidneys. |
| Thrombotic microangiopathy | Thrombotic microangiopathy is a vascular issue, where platelet thrombi in the microcirculation induce kidney damage. |
Table 2.
Molecular initiating events identified for the pathogenic mechanisms of kidney toxicity [33]. The table shows mechanisms involving enzymes such as cyclooxygenase (COX) and ornithine aminotransferase (OAT).
| Pathogenic mechanism | Molecular Initiating Event in the AOP |
|---|---|
|
| |
| Hemodynamic alteration | COX-1 and/or COX-2 inhibition leading to reduced prostaglandin synthesis and uncontrolled renal vasoconstriction [41,42] |
| Proximal and distal tubular cell toxicity | Mitochondrial toxicity pathways: a) Mitochondrial DNA incorporation [43] b) Mitochondrial DNA polymerase gamma inhibition [43] c) Depletion of SH-groups leading to reactive oxygen species (ROS) induction [44] Metabolization by oxidase in hepatocyte to benzoquinoneimine, followed by formation of GSH (glutathione) S-conjugates [45] |
| Tubular, interstitial, tubulointerstitial and glomerular nephritis | Interaction with hOAT1 and 3, accumulation within proximal tubule cells, followed by uncoupling/inhibition of mitochondrial oxidative phosphorylation and tubular/papillary necrosis [41] |
| Tubular obstruction | OAT interaction causing secretion via proximal tubule cells, accumulation and crystal formation in urine leading to concentration in renal tissue/tubule and obstructive nephropathy [43] |
The AOPs associated with kidney toxicity as included in the AOP-Wiki are instead listed in Table S1 of the supplemental material [34,35], which shows that all of the mechanisms need to be finalized. The AOP-Wiki is a platform overseen by the Organisation for Economic Co-operation and Development (OECD).
Histopathology-related findings included in preclinical toxicity study reports for regulatory submissions can be organized in two-level clusters of terms (see Table 3) related to similar findings (and, possibly, similar mechanisms) [36]. As demonstrated in our sister publication on liver [8], such organization is important for the development of an assessment framework for kidney toxicity (as outlined in Fig. 2), where the consistent use of defined terminology and ontologies is crucial to map actual data.
Table 3.
The hierarchical organization used to group histopathology terms of similar findings (and mechanism) for kidney toxicity; findings were extracted from preclinical toxicity study reports for regulatory submissions [36].
| KIDNEY TOXICITY | |
|---|---|
|
| |
| General clusters | Specific clusters |
|
| |
| Tissue damage | Necrosis Degeneration Nephropathy |
| Inflammatory changes | Inflammation Infiltration |
| Structural alterations | Dilation Adaption cell size/number |
| Accumulative lesions | Accumulation Vacuolation Mineralization |
Kidney toxicity – in vivo and in vitro methods
Identification of kidney toxicity traditionally relies on in vivo testing. For pharmaceuticals, kidneys do not fall within the safety pharmacology core battery and supplemental studies on the renal system are required when there is cause of concern not addressed by the core battery [46] or repeated-dose toxicity studies [47].Together with histopathological observations, changes in the kidney function are detected by assessing clinical markers such as glomerular filtration rate (GFR), blood urea nitrogen (BUN) and serum creatinine (sCr) [11]. Much effort is underway to identify novel biomarkers that could ideally allow for an early detection of chemically induced kidney toxicity, differentiate it from other causes, and predict long-term kidney outcome and mortality; some promising biomarkers include Kidney Injury Molecule-1, Beta-2 Microglobulin, and albuminuria [16,48].
Animal models have been challenged by the insufficient level of prediction of kidney failure in humans and their inadequacy has been linked to the significant differences in expressions of transport proteins and metabolizing enzymes between species [11,29]. Kinetics needs to be evaluated in a human-relevant system (including a human-based mathematical model) to adequately assess internal exposure and dose–response relationships over time.
In vitro screens are also being used to evaluate chemically induced kidney injury, but a standardized approach is not currently available and existing models are found to be poorly predictive of human kidney toxicity [29,49,50]. Advanced 3D in vitro models such as organoids and kidney-on-a-chip platforms are emerging to overcome the limitations of the 2D in vitro assays including and improve kidney safety assessment [51].
Kidney toxicity – Molecular targets
In vitro safety pharmacology profiling panels are used by pharmaceutical companies to investigate organ toxicity [52]. In the safety panel by Bowes and co-workers [53], cyclooxygenase 1 (Cox1) and vasopressin V1A receptor (Table S2 of the supplemental material) are associated with kidney adverse effects. Additional molecular targets have been associated with [54], and Tables S3 and S4 of the supplemental material provide lists of targets derived from a genetic and pharmacological phenotype analysis [55] or other data curation processes [56], respectively.
Kidney toxicity – In silico methods
An IST protocol for the identification of potential kidney toxicants needs to account for a draft assessment framework that includes several types of data as depicted in Fig. 3. In terms of hazard identification, available IST approaches for kidney toxicity are based both on statistical-based (or QSAR) methods [36,57–61] and expert rule-based (or expert/structural alerts) methods [36,62,63]. Such methods are usually built on either in vivo data (e.g., rat and mouse) or human data, the latter originating in the pharmaceutical sector from clinical trials or post-marketing surveillance reports. The resulting in silico models must be expected to be generalistic in their predictive capabilities as the underlying broad database will be based on many mechanisms of action and potentially many different effects. As such, they may identify compounds with the potential for kidney toxicity, but the type of adverse effects and quantitative identification of the Point of Departure (PoD) will be difficult to determine unless detailed analysis is undertaken. In addition, in terms of risk assessment, since animal models have been challenged as to their ability to adequately predict kidney adverse effects in humans, particularly if these are driven by kinetics, integrating human data in predictive models is vital.
Fig. 3.
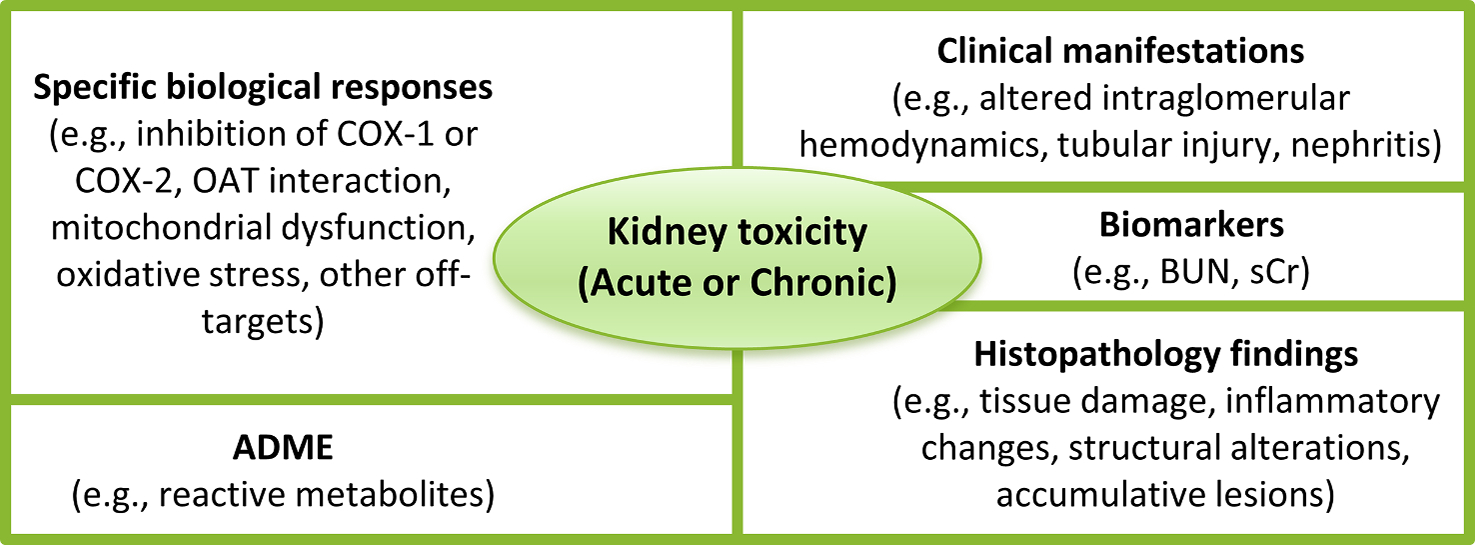
Types of data in a draft assessment framework that needs to be considered for the development of an IST protocol for the identification of potential kidney toxicants.
The prediction of the general endpoint (i.e., “kidney toxicity”) can be combined with the prediction of other toxicity subcategories to gain a better understanding of specific adverse effects. An illustration of this was reported by Matthews and coworkers, who constructed QSAR models based on the adverse events retrieved from FDA post-market reports. Their models predict six composite endpoints of the urinary tract: acute kidney disorders, nephropathies, bladder disorders, kidney function tests, blood in urine, and urolithiases [60]. Even within these groupings, whilst there will be greater homogeneity of mechanisms of action, there will be variability. It is likely that these QSARs for “groups” of effects will be more localized models, with less applicability. An “ontology” of some form, which organizes mechanisms linked to effects in a hierarchical manner, may be required to gain a more comprehensive overview of kidney toxicity and associated mechanisms. For instance, a good example of this approach is provided by an appropriate hierarchical clustering of histopathology data (see Table 3) [36]. The advantage of setting out adverse effects related to the kidney (or any organ level toxicity) is that Amberg and coworkers developed a number of models (i.e., structural alerts, fragment-based, molecular descriptor-based machine learning approaches) to predict specific kidney toxicity findings. This modeling approach, also applied in the context of other target organ toxicities (i.e., liver and heart), indicates that a proper clustering process, and hence grouping endpoints/effects in a meaningful way, is crucial for a good predictivity.
A number of structure–activity relationships (SARs) are available for kidney toxicity, as well as focusing on specific biological pathways [33] such as protein binding [64] and mitochondrial toxicity [65,66]. At the current time, a comprehensive, publicly available, in silico profiler for kidney toxicity is lacking. However, lists of alerts for kidney toxicity, e.g. from data mining approaches, are available [63]. These alerts are very useful starting places, although to allow for greater applicability, especially for regulatory approaches, they require adequate definition and linkage to mechanisms of action.
SAR based alerts can be used in a variety of ways but are generally useful either as direct predictions of toxicity, i.e., a qualitative estimation, or as a means of grouping to allow for read-across. There are a small number of published reports of attempting read-across for kidney toxicity and repeated dose toxicity driven by effects to the kidney. For instance, Fowles and co-workers identified adverse effects to the kidney as a significant factor in the toxicological assessment of ethylene glycols [67]. Use of metabolomics was demonstrated to support read-across for organ level toxicity including that to the kidney [68].
In conclusion, there are a variety of in silico approaches that predict kidney toxicity. At the current time there is no unified approach to toxicity prediction, for instance that may apply generalistic broad QSAR type models supplemented by more mechanistic models or confirmation through the use of structural alerts. In addition, little has been performed in terms of ensuring the toxicokinetic component of kidney toxicity is included [69]. Whilst the current models are satisfactory for prioritisation and possibly hazard identification, an integration of approaches (including ADME predictions) will be needed for risk assessment.
Kidney toxicity – In silico approaches: Data gaps and issues
In silico models for kidney toxicity bring with them a number of problems and issues to overcome, some of which are general for all organs, others are specific to kidney. There is no easy way to approach the topic of modeling kidney toxicity for two fundamental reasons: (1) the complexity of the endpoint and (2) the quality and relevance of the data to model. Starting with the complexity of the endpoint, clear guidance, or definition within a model, is required as to what constitutes kidney toxicity, e.g., general toxicity to the kidney, specific effects within the nephrons or kidney structure, or related adverse effects such as to the urinary tract or bladder. As noted above, there are a variety of means to obtain information relating to kidney toxicity from both in vitro and in vivo methods. It is crucial to decide for the modeling approach, what endpoint is to be predicted. Thus, a general in silico model for the presence of kidney toxicity from in vivo test results, for instance from a repeat dose experiment, may include a variety of mechanisms of action and apical effects. Such models should not be discounted, but they may be most appropriate for screening and prioritisation purposes, i.e., to identify those compounds with a strong probability of causing kidney toxicity. The use of in vivo data is also made more complex in that it will be difficult to prove a negative test, i.e., there is no adverse effect on the kidney. This may be because the test was not performed at a sufficiently high dose, or that other toxicities were observed at lower doses and no account was taken of adverse effects to kidneys. Thus, the use of such data must be considered for generalistic models. The biomarker and histopathology data are likely to be important to gain a more detailed approach of potential kidney toxicity. In other words, it is probable that there will be models based on localised areas of chemistry which may be suitable for risk assessment provided the quality of the original data is acceptable. The problem of predicting accurately Points of Departure (PoD) is particularly relevant for kidney toxicity. As noted above, kidney toxicity is largely driven by toxicokinetics and the ability to accumulate within the kidney. In terms of modeling, to obtain a PoD predictions will be required not only for relative hazard but also for bioavailability in the relevant compartment of the kidney, for which data are currently scarce. The use of techniques such as physiologically-based mechanistic modeling, an extension of PBK, is likely to become increasingly important to perform adequate risk assessment. There is also an opportunity for physiologically-based mechanistic modeling to assist in the proper incorporation of inter-species differences, e.g., for the extrapolation of rodent data to humans.
Lung toxicity
The lung is a primary target organ for potential chemically induced damage caused by inhaled material, such as gases and particles [70–72]; it acts as portal of entry for airborne chemicals into the human body facilitating gas exchange between blood and air. While pulmonary toxicity refers to toxicity to the lung as target organ, inhalation toxicity refers to the route of exposure through the respiratory system that includes the upper respiratory tract (mouth, nose, and pharyngeal region) and the lower respiratory tract (tracheobronchial region and the pulmonary parenchyma or alveolar region) [70,73]. Since the lung is highly perfused and receives the total cardiac output to be replenished with oxygen [74], this organ may also be injured through the vascular system, namely by xenobiotics entering the systemic circulation irrespective of the route of absorption [75].
Toxicity to the lungs may be caused by a great variety of chemical agents from intentional or non-intentional exposure including natural products, industrial chemicals, pesticides, environmental pollutants, combustible cigarettes, and drugs. Notably, evaluation of the adverse effects to the lungs is of paramount importance in the acute inhalation studies for hazard identification and characterization of chemicals, including classification and labelling [76,77]. Lungs are also a prominent target organ for occupational diseases caused by accidental or prolonged inhalation of xenobiotics. In the context of pharmaceuticals, drug-induced lung diseases are reported to be a significant subset of adverse drug reactions [78,79] with the most common form being the so called drug-induced interstitial lung disease (DILD), which is mainly caused by oral and parenteral administration [80]. Additionally, in the drug discovery and development of inhaled therapies, toxicity to the lungs represents a challenging hurdle to overcome [81].
Lung toxicity – Processes and endpoints
Lung toxicity following inhalation of airborne chemical agents concerns gases and vapors, as well as aerosols and particulate matter. Central to inhalation toxicity is the concept of dosimetry (rather than exposure concentration), that seeks to define the amount, rate, and form of a substance delivered to the target tissue [76,82]. Dosimetry involves evaluation of the deposition, clearance, and translocation patterns within the respiratory tract, and two key elements have been singled out to influence these patterns: a) respiratory anatomy and physiology that differs among species; and b) the physico-chemical characteristics of the inhaled chemical agents [76]. Deposition, clearance, and translocation patterns of particles are affected by properties such as size, shape, density, hygroscopicity, and surface characteristics [83]. For gases and vapors, solubility is critical in determining the depth of penetration of the substance; generally, low-water soluble substances penetrate lower in the respiratory tract [70].
Toxicity to the pulmonary tissue following inhalation exposure or systemic circulation of xenobiotics frequently depends on the metabolizing capability of this organ; phase I and II enzymes are involved in the lung disposition processes and they can catalyze biotransformation reactions resulting in the formation of toxic metabolites [71,84]. Potential bioactivation of parent compounds in highly reactive intermediates together with other factors (e.g., preferential exposure or accumulation of the xenobiotics or metabolites in given sites, specific cellular defense mechanisms) affect the types of lung cells that are injured by chemicals [71].
Irritation
Chemically induced transient effects to the lung are referred to as irritation. Irritation is a nonimmunological state of the respiratory tract that follows inhalation of substances at doses that cause inflammation [85]. Within the EU classification and labelling (C&L) perspective, the European Chemicals Agency (ECHA) states that respiratory tract irritation is “a transient target organ effect, i.e. an effect which adversely alters human function for a short duration after exposure and from which humans may recover in a reasonable period without leaving significant alteration of structure or function” [86]. For the U.S. Occupational Safety and Health Administration (OSHA), irritant chemicals cause a reversible inflammation in contrast to corrosive damage that is permanent and irreparable [87]. Under the Specific Target Organ Toxicity (Single Exposure) (STOT-SE) of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS), respiratory tract irritation falls in category 3, namely in the transient target organ effects category, where respiratory irritant effects (characterized by localized redness, edema, pruritis, and/or pain) impair function with symptoms such as cough, pain, choking, and breathing difficulties [88]. The OSHA implementation of the GHS emphasizes that such adverse effects are of short duration after exposure, and do not result in significant alterations of structure or function following recovery [89].
Adverse effects related to respiratory tract irritation are grouped into two different forms [85,86,90]: local cellular damage and effects caused by airborne chemicals that stimulate the peripheral nerve fibers innervating the respiratory tract from the nose to the alveoli (sensory irritation) [91]. Inhaled substances interacting with the nerve endings of the respiratory tract have been classified by Alarie [92,93] according to the “first level” of the respiratory tract at which they act as the exposure concentration increases from zero [90]. Sensory irritants, when inhaled via the nose, stimulate the trigeminal nerve endings, evoke a burning sensation of the nasal passages, and inhibit respiration. Bronchoconstrictors act on the conducting airways of the lung and induce an increase in resistance to air flow within the airways. Pulmonary irritants stimulate the nerve endings within the lung, increase the respiratory rate, and decrease tidal volume (rapid shallow breathing). According to the “by-first-level-of-action” classification by Alarie, respiratory irritants interacting with peripheral nerve fibers can act as a sensory irritant, a bronchoconstrictor, and a pulmonary irritant and they are capable of all three actions; there is little difference between the concentrations at which they induce an effect at the three levels: nose, conducting airways, and deep lung. Physico-chemical properties of the inhaled substances play a role in the Alarie classification: highly water soluble and/or reactive chemicals (e.g., formaldehyde) affect the upper airways while less water-soluble compounds deeply penetrate the lung and affect the lower respiratory tract [90].
Sensitization
In contrast to respiratory irritants, respiratory sensitizers lead to hypersensitivity of the airways following inhalation [88], an immune-mediated response to an otherwise innocuous antigen [94]. The immune-mediated hypersensitivity reactions are referred to as chemical respiratory allergy [95], and include two steps. The first phase is sensitization (induction) involving the development of specialized memory cells in the immune system of an individual following initial exposure to the respiratory sensitizer. The second phase is elicitation when, on repeated exposure, the heightened immunological responsiveness can provoke allergic reactions resulting in clinical manifestations such as asthma and rhinitis [95]. The number of chemicals confirmed with the potential to cause allergic sensitization of the respiratory tract are relatively low (less than a hundred) in contrast to the hundreds to thousands of confirmed dermal sensitizers [96]. Many dermal sensitizers have not been regarded as respiratory sensitizers and there are examples of respiratory allergens that have no potential to cause skin sensitization [97]. Commonly it was assumed that inhalation exposure was necessary for respiratory sensitization, but there is evidence that respiratory sensitization might also result from dermal exposure [98,99]. While there are similarities in the biological pathways that lead to the acquisition of dermal and respiratory sensitization, the differences are important to note. Respiratory sensitizers preferentially bond lysine and result in a cytokine profile that favors the generation of a T helper 2 (Th-2) response as opposed to a T helper 1 (Th-1) response with skin sensitization [99]. Furthermore, the Th-2 response promote IgE antibody production but the role of the IgE antibody in respiratory sensitization remains uncertain [100].
AOP
In the context of inhalation toxicity, a field tightly bound to lung toxicity, important global efforts are being undertaken to advance the use of alternatives methods and promote their global regulatory acceptance [76,77] and mechanistically-informed Integrated Approaches to Testing and Assessment (IATA) are being developed using the Aggregate Exposure Pathway (AEP) and AOP frameworks. Table S5 of the supplemental material lists some AOPs specifically targeting the lung as listed in the AOP-Wiki [34,35]. All of these AOPs needs further development.
Lung toxicity – In vitro and in vivo methods
Toxicity to lung can be induced by inhaled substances and several OECD Test Guidelines (TGs) provide the framework to measure the adverse effects in the upper part and lower part of the respiratory tract following inhalation exposure. More specifically, inhalation studies are conducted in animals and include tests for acute inhalation toxicity with death as endpoint (TG 403 [101], TG 436 [102], tests based on clear signs of toxicity as endpoint (TG 433 [103]), and repeated-dose inhalation testing (TG 412 [104], and TG 413 [105]) [76,77,106]. For pharmaceuticals, adverse effects to the respiratory tract are identified at a relatively late phase during the comprehensive pre-clinical assessment undertaken during in vivo toxicity studies [81]. Inhalation in vivo studies using rodents must account for significant species differences (e.g., different nasal/pharyngeal anatomy and obligate nose breathing) and translation of results to humans needs to be critically evaluated [107].
Inhalation toxicology studies are increasingly taking advantage of the 3D in vitro models (e.g., organ-on-chip, organoids) that better reflect cell interactions in their natural environment as compared to traditional 2D in vitro assays [108–111]. However, it is important when using these more sophisticated 3D tissues to mimic the in vivo exposure route with a more relevant exposure system to dose at the air/liquid interface [112,113].
Irritation
Whilst inhalation studies provide information related to respiratory irritation, in vitro methods that address lung irritation are limited compared to other organs (skin and eye). The use of cytotoxicity as a surrogate to investigate irritation is widely accepted in the development of in vitro models to predict irritation potential of chemicals [114,115]. Neilson and co-workers took this approach to develop an in vitro 3D airway tissue model to assess the potential irritancy of e-cigarette aerosols compared to cigarette smoke [116]. The Alarie test assesses the sensory irritation potential by measuring the inhaled concentration of a substance necessary to cause a 50% reduction in the respiratory rate in mice allowing for the quantification of irritating concentrations and ranking of chemicals for their sensory irritancy potential [117]. Sensory irritation is frequently identified as a critical endpoint for setting occupational exposure limits [118,119]. Notably, there is no generally accepted in vitro model for assessing respiratory irritation [119].
Sensitization
To date, no in vitro/in vivo test methods have been validated for the assessment of respiratory sensitization and test methods used for skin sensitization hazard assessment are employed as a surrogate for respiratory sensitization [86,98,120]. Of these, the Direct Peptide Reactivity Assay (DPRA) and Amino Acid Derivative Reactivity Assay (ADRA) both assess activation of the molecular initiating event (MIE), covalent modification of proteins. While respiratory sensitizers preferentially bind to lysine (a comparatively hard nucleophile), this selectivity is not absolute, and reactivity with cysteine also occurs with some respiratory sensitizers [121]. However, in more recent studies [122], the preference for lysine binding was not as apparent, but the use of the DPRA assay was still deemed useful within the testing strategy. The Local lymph Node Assay (LLNA) and Guinea Pig Maximization Tests (GPMT) also supports the weight of evidence assessment of respiratory sensitization [98,123]. Dermal exposure to a respiratory sensitizer triggers an immunological effect that could be detected in methods that assess skin sensitization; it is not possible to distinguish between the respiratory and dermal effects using standard methods. As such, a negative LLNA result is part of the evidence in support of a negative assessment for respiratory sensitization [100,123], although the possibility of false negatives needs to be considered carefully [124]. Modifications to the LLNA allow for cytokine profiling which can distinguish between the Th2 versus Th1 response types following either dermal or inhalation exposures [86]. Total IgE measurements have also been used to support an assessment of respiratory sensitization. None of these approaches, however, are validated or standardized. Additional experimental approaches that may support a weight of evidence assessment of respiratory sensitization could be found in the ECHA guidance [86].
Lung toxicity - molecular targets
The molecular targets associated with lung toxicity as derived from the in vitro safety pharmacology profiling panel of 44 targets discussed by Bowes and coworkers [53] are listed in Table S6 of the Supplemental material. Additional molecular targets associated with liabilities to the respiratory system have been discussed in the literature [54], and Tables S7 and S8 of the supplemental material report some collections as derived from the analysis of human genetics and pharmacology data [55] and other data curation processes [56].
Lung toxicity – In silico methods
An IST protocol to predict lung toxicity will be based on a draft assessment framework that accounts for different types of endpoints such as irritation and sensitization and that integrates information from several sources, e.g., human data, animal in vivo data, specific biologic responses (Fig. 4) [5,8] and ADME information (Fig. 2).
Fig. 4.

Toxicity to lung includes different endpoints such as irritation (transient effects) and sensitization (immune-mediated response). Experimental data on lung toxicity originates from different sources and they are combined in a decision framework for hazard assessment; for example, in vitro data may originate from assays investigating molecular targets associated with lung toxicity, such as TRPA1, an ion channel whose activation is proposed to induce sensory pulmonary irritation (see supplementary material). In silico methods build on available experimental data and they can thus be integrated in the overall hazard assessment framework.
In silico methods to predict lung toxicity can be sorted according to the type of adverse effects they predict and thus according to the type of data they are built on, including sensitization, irritation (i.e., cellular damage, sensory effects), other acute lung injury, and chronic effects (i. e., asthma, fibrosis, chronic obstructive pulmonary disease). Examples of in silico models are given in Table 4. In silico methods for the prediction of GHS classes based on acute inhalation toxicity studies address systemic toxicity rather than specifically pulmonary toxicity.
Table 4.
Some models for lung toxicity. Models for the prediction of inhalation toxicity are not included.
| Endpoint | Endpoint details | References |
|---|---|---|
|
| ||
| Irritation (sensory) | Model based on set of 145 diverse volatile organic compounds as sensory irritants | [137] |
| Irritation (pulmonary) | Data (either sensory irritation or tissue damage) on 1997 organic compounds | [135] |
| Respiratory sensitization | Training and validation sets have been built from chemicals that are negative for human sensitization potential (Graham et al 1997), tested negative in the LLNA, non-sensitizers based on occupational exposure limits (OELs) and no cases of occupational asthma (OA); in addition to, chemicals that are identified as respiratory sensitizers through case-reports, and asthmagens that cause OA | [126–130,132] |
| Inflammation | IL-8 gene expression: in vitro data on gene expression in A549 cells of IL-8, a well-known inflammatory cytokine | [138] |
| Drug-induced respiratory toxicity | Dataset with a series of toxicological end points of mouse intraperitoneal respiratory toxicity including: focal fibrosis (pneumoconiosis), acute pulmonary edema, bronchiolar constriction, bronchiolar dilation, changes in pulmonary vascular resistance, chronic pulmonary edema, cyanosis, dyspnea, pleural thickening, respiratory depression, respiratory obstruction, respiratory stimulation, structural or functional change in trachea or bronchi, and other changes | [59] |
Several in silico systems have been developed to predict respiratory sensitization including both expert systems and QSAR models [125–131] with some respiratory sensitization models specifically built on a dataset of asthmagenic chemicals [132]. Enoch et al. defined structural alerts which describe covalent protein binding in the lung; each structural alert is associated with a mechanistic domain, which could be used to support a read-across assessment [127]. Similarly, Mekenyan et al. reported a mechanistic approach for the assessment of respiratory sensitization potential or for grouping chemicals for subsequent read-across application [133]. Other efforts have resulted in similar profilers [134].
Within the project “Respiratox”, models for pulmonary irritation have been developed to predict the potential to induce tissue damage and/or sensory irritation effects [135]. Some other models have been developed using lung injuries data [59]. Jeong et al. reported the development of an adverse outcome pathway (AOP) to better define the linkage of PPARγ antagonism to the adverse outcome of pulmonary fibrosis using the ToxCast Database and a Deep Learning Artificial Neural Network Model-Based Approach [136].
Lung toxicity – In silico approaches: Data gaps and issues
As noted for other organ toxicities, the complexity of the pathways leading to adverse effects on the lung poses an obstacle for the development of in silico models, as does the heterogenous nature of compound properties (and their interplay) which can lead to lung toxicity. Such biological pathways that may lead to different types of adverse effects (e. g., sensitization, sensory irritation, tissue damage) need to be accounted for in the development of in silico models.
In relation to respiratory sensitization, it can be noted that the limited acceptance of in vitro/in vivo approaches for respiratory sensitization presents a challenge to the standardization of a robust training set. A tiered in silico approach using two SAR models could not conclude with a reliable classification on 65% of the chemicals tested in an external evaluation set [126]. While read-across could be used to fill these gaps, the overall conclusion points to the need for standardized testing methods for respiratory sensitization.
Future assessment of chemical respiratory sensitizer potential should take advantage of multiple lines of evidence to draw conclusions. While not the sole method to assess hazards, the weight of evidence approach remains the only available option within the absence of validated methods for hazard assessment.
It should then be noted the close relationship between toxicity to the lung and inhalation toxicity with the latter mostly referring to systemic toxicity rather than lung toxicity. An ontology that unifies existing knowledge on lung adverse effects may facilitate the advancement of in silico methods and their corresponding applications.
Heart toxicity
Toxicity affecting the heart, namely cardiac toxicity or cardiotoxicity, is particularly important in the context of pharmaceuticals, where it significantly contributes to the attrition of drug candidates in the preclinical phase of drug discovery and development. It is one of the major causes of human adverse drug reactions occurring both in the clinical phase and post-market approval phase [139,140]. Not only do cardiac safety liabilities remain a major obstacle for the pharmaceutical industry, they also pose an increasing concern in the context of environmental risks [141] as they have been associated with exposure to environmental chemicals [142] such as pesticides [143,144], flame retardants [145,146], and polycyclic aromatic hydrocarbons (PAHs) [147,148]. Cardiac safety is also becoming recognized as an issue with dietary supplements and herbal products [149].
Heart toxicity - processes and endpoints
Chemical insults initiate a series of events in cardiac cells (see Fig. 5) that may manifest as functional and/or structural perturbations of the heart [150–152]. Functional effects correspond to alteration of the mechanical (contractility) or electrical (ECG) function whereas structural effects correspond to morphological damage or loss of cellular/subcellular components. Structural change may precede dysfunction, or occur as a result of it [153–155]. In contrast, chemically-induced changes on myocardial contractility can arise from both electrophysiological and structural elements [150,153,156].
Fig. 5.
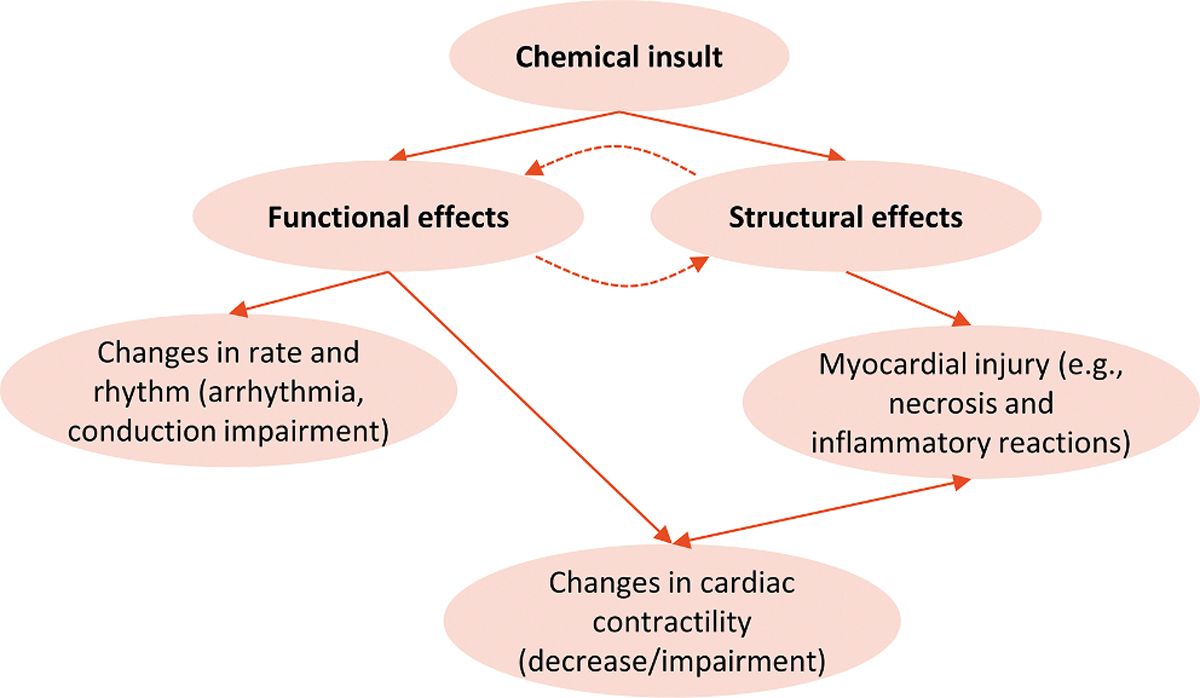
Heart’s possible response to toxic injury induced by xenobiotics [150,153]. Functional and structural adverse effects are interrelated: primary functional effects may occur with possible secondary structural effects; similarly, primary adverse effects on cardiac structure may occur with secondary functional changes. Myocardial contractility may be altered by functional effects (effects on contractile proteins, Ca2+ or mitochondria) or structural perturbations (loss of cardiomyocytes following apoptosis or necrosis and possible replacement with less contractile fibrotic tissue).
Pharmaceutical companies have summarized cardiac key liabilities faced throughout drug discovery, drug development and clinical practice (see Table 5). These include proarrhythmic potential, myocardial ischemia, myocardial necrosis, heart failure, coronary artery disorders, cardiac valve disorders and endocardial disorders [139].
Table 5.
Key cardiac liabilities reported by pharmaceutical industry (adapted from Laverty et al. [139]).
| Toxicity | Common standard assessment strategies§ |
|---|---|
|
| |
| Proarrhythmic potential | Drug discovery: hERG screening, other cardiac ion channel screening, in vitro profiling in cardiac tissue, in silico hERG and cardiac action potential assessment Drug development: QT interval determination in telemetry and or toxicology studies and mechanistic investigations (e.g., hERG trafficking, metabolites effects) Clinical practice: QT interval determination including concentration QTc modeling and assessment of other ECG parameters |
| Myocardial ischemia | Drug discovery: assessment of ECG morphology changes, histological examinations and functional assessments (e.g. LVEF) Drug development: observation of clinical signs, assessment of ECG morphology changes, histological examinations and functional assessments (e.g. LVEF) |
| Myocardial necrosis | Drug discovery: few predictive in vitro methods, histological examination from early repeated-dose toxicity studies Drug development: some reflective biomarkers available (e.g., troponin), histological examinations, imaging (e.g., echocardiography) |
| Heart failure | Drug discovery: assessment of some functional endpoints in vitro and in vivo (e.g., contractility), histological examinations and cardiac biomarkers (e.g. pro NT-BNP) Drug development: observation of clinical signs, imaging and cardiac biomarkers |
| Coronary artery disorders | Drug development: observation of clinical signs and imaging |
| Cardiac valve disorders | Drug discovery: evaluation of alerts from receptor (e.g., 5-HT2B) binding data Drug development: imaging, histological examinations |
| Endocardial disorders | Drug development: histological examinations |
hERG (human ether-à-go-go related gene); ECG (electrocardiogram); LVEF (left ventricular ejection fraction); pro-N terminal B-type natriuretic peptide; QT (duration of ventricular depolarization and repolarization); QTc (corrected QT interval); 5-HT2B (5-Hydroxytryptamine receptor 2B).
Drug-induced QT prolongation (delayed ventricular repolarization of the cardiac action potential) is one of the most investigated cardiac safety concerns. QT prolongation is a surrogate marker of proarrhythmia, for example Torsades de Pointes (TdP), a rare form of arrhythmia that is potentially lethal and has caused the removal from the market of several drugs [139,157–159]. QT prolongation has thus been extensively studied and the understanding of the underlying biological mechanism led to the development of a successful cardiac safety assessment paradigm for use in drug discovery and development. This paradigm was formalized in 2005 by the International Council on Harmonization (ICH) through the release of the S7B- and E14 regulatory guidelines [160,161]. From a mechanistic point of view, QT prolongation is associated with prolonged ventricular cardiac action potential, with the potassium channel encoded by the human ether-à-go-go related gene (hERG) being responsible for cardiac repolarization [162–165]. QT prolongation and associated arrhythmia arising from the inhibition of the hERG potassium channel is an example of functional cardiotoxicity.
The safety paradigm for cardiotoxicity defined by the ICH S7B and E14 guidelines focuses on the assessment of QT interval as marker of proarrhythmia [157]. ICH S7B addresses the nonclinical evaluation of the QT interval prolongation, recommending a testing strategy that includes both an in vitro assay to assess whether a compound or its metabolites block the repolarizing ionic current through inhibition of hERG and an in vivo animal assay to assess ventricular repolarization (it should be noted that hERG assessment is not appropriate for all pharmaceuticals). ICH E14 establishes the quality of the clinical evaluation required to understand drug-induced QT prolongation.
In contrast to pharmaceuticals, the hERG channel activity of dietary supplements and herbal products is not routinely assessed nor have regulatory guidelines been developed that specifically address this issue, despite their widespread use and evidence that some are potent hERG blockers [166].
Regarding structural cardiotoxicity, this may be described by a continuum of progression of cardiac cell injury spanning through degeneration, necrosis, responding inflammatory changes (inflammatory cell infiltrate) and eventually fibrosis, with the latter being a repair process which does not generate functional contractile tissues [152]. The number and distribution of the injured cells determines the ultimate effects on the myocardial contractile function [152].
Histopathological observations included in preclinical toxicity study reports for regulatory submissions have been organized in groups of similar findings (and mechanism) [36]; as in the case of other organ toxicities (i.e., toxicity to liver and kidney) [8], heart-related histopathology data can be structured in two-level clusters (i.e., tissue damage, inflammatory changes, structural alterations), that can be further separated into more specific groups of terms as shown in Table 6. As in case of other organs, the consistent use of terminology is key for later reuse of the data generated.
Table 6.
The hierarchical organization used to group histopathology terms of similar findings (and mechanism) for heart toxicity; findings were extracted from preclinical toxicity study reports for regulatory submissions [36].
| HEART TOXICITY | |
|---|---|
|
| |
| General clusters | Specific clusters |
|
| |
| Tissue damage | Necrosis Degeneration Myopathy |
| Inflammatory changes | Inflammation Infiltration |
| Structural alterations | Dilation Adaption cell size/number |
In contrast to ion-channel mediated mechanisms, other biological pathways leading to heart toxicity are in general poorly understood, particularly those underlying cardiac contractility and structural cardiotoxicity. Efforts are underway to elucidate such mechanisms possibly within an AOP framework [155]; this offers a means to organize the existing knowledge of adverse outcomes and to advance the mechanistic understanding of heart toxicity [140]. However, data coverage in both the chemical and biological domain is a limiting factor in the field.
Information on biological pathways that are associated with cardiac liabilities is being collated in the AOP-Wiki, and Table S9 of the supplemental material lists several AOPs as included in this repository [34,35]. The AOPs cited in the AOP-Wiki focus on ion channel activity.
Heart toxicity – In vivo and in vitro methods
In drug discovery and development, functional and structural cardiotoxicity is assessed using a variety of in vitro (e.g., over expressing cell lines, primary cardiomyocytes, stem cell derived cardiomyocytes), ex vivo (e.g., isolated heart, ventricular wedge) and in vivo (e.g., single and repeat-dose rodent and non-rodent species) models. In vitro approaches can be divided into molecular- and phenotypic-based assays. Phenotypic-based assays are primarily used to identify a potential cardiac safety risks (hazard detection) that can be further characterised in a more complex model system. These approaches allow the investigation of multiple cardiac effects, for example the assessment of cardiac contractility via measurement of calcium transients or impedance and cardiac structure via high content biology imaging. The phenotypic endpoints typically use integrated in vitro models, such as human induced pluripotent stem cell-derived cardiomyocytes that contain a milieu of kinases, ion channels, enzymes and receptors present within the heart facilitating the detection of potential adverse cardiac effects where the molecular understanding is limited [156].
Molecular in vitro approaches mainly focus on prediction of electrocardiogram abnormalities and QT-interval prolongation by ion channel screening and measurement of cardiac action potentials [152]. The assessment of QT interval prolongation and hERG inhibition has proven to be very sensitive and thus successful in eliminating drug candidates at risk of causing TdP. On the other hand, assessment of hERG block and QT prolongation is an imperfect biomarker for predicting proarrhythmia risk since it is known that multiple drugs inhibit hERG and/or prolong QT, albeit, not leading to TdP [159,167].
The current proarrhythmia testing paradigm relies on the predictive link between drug-induced hERG block and in vivo/clinical QT interval prolongation and TdP [157,159]. It provides a valuable example of a screening approach for hazard identification and elimination of compound with predicted toxicities on humans based on the AOP concept. Given the observation that blockade of multiple cardiac ion channels might be predictive of torsadogenic potential [167,168], the scientific community is moving towards an updated proarrhythmia paradigm promoted by the CIPA (Comprehensive in vitro Proarrhythmia Assay) initiative. This initiative is based on the integration of data from in vitro testing of multiple cardiac ion channels with mechanistic in silico electrophysiology modeling to predict proarrhythmic risk [159,169–173]. The ongoing improvements of the assessment strategy [157,159] through the CIPA initiative is expected to lead to further refinements via an ICH S7B-E14 Questions and Answers process enabling a more efficient, comprehensive and mechanism driven process with greater emphasis of non-clinical data [174–176].
Improved in vitro models are required to further enhance the ability to detect and risk assess heart toxicity in vitro,. 3D in vitro models are attracting interest and attention in drug discovery as promising approaches to investigate both structural and functional toxicity affecting the heart [177–179]. For example, human 3D cardiac microtissue is proposed as a model to capture drug-induced structural cardiotoxicity and gain mechanistic insights [180]; it is noted that this type of model overcomes some of the limitations of current in vitro preclinical testing that predominantly focuses on the prediction of functional changes.
Heart toxicity – Molecular targets
Heart toxicity is investigated by pharmaceutical companies using panels of safety molecular targets that have been associated with different adverse effects [52]. The molecular targets associated with cardiac liabilities as derived from the safety panel by Bowes and co-workers [53] are listed in Table S10 of the supplemental material. This target list is complemented with other off-target panels (see for example Tables S11 and S12 of the supplemental information) derived from different studies such as the analysis of human genetic and pharmacology data [55] or other data curation processes [54,56,181]. Associations between molecular targets and structural cardiotoxicity have also been investigated by mining data from FDA Adverse Event Reporting System and assay outcomes from ToxCast leading to the formulation of mechanistic hypotheses of toxicity [155].
Heart toxicity – In silico methods
The schema for the development of an IST protocol for the prediction of potential cardiotoxicants is shown in Fig. 6, which combines different types of information and where in silico methods can be integrated.
Fig. 6.
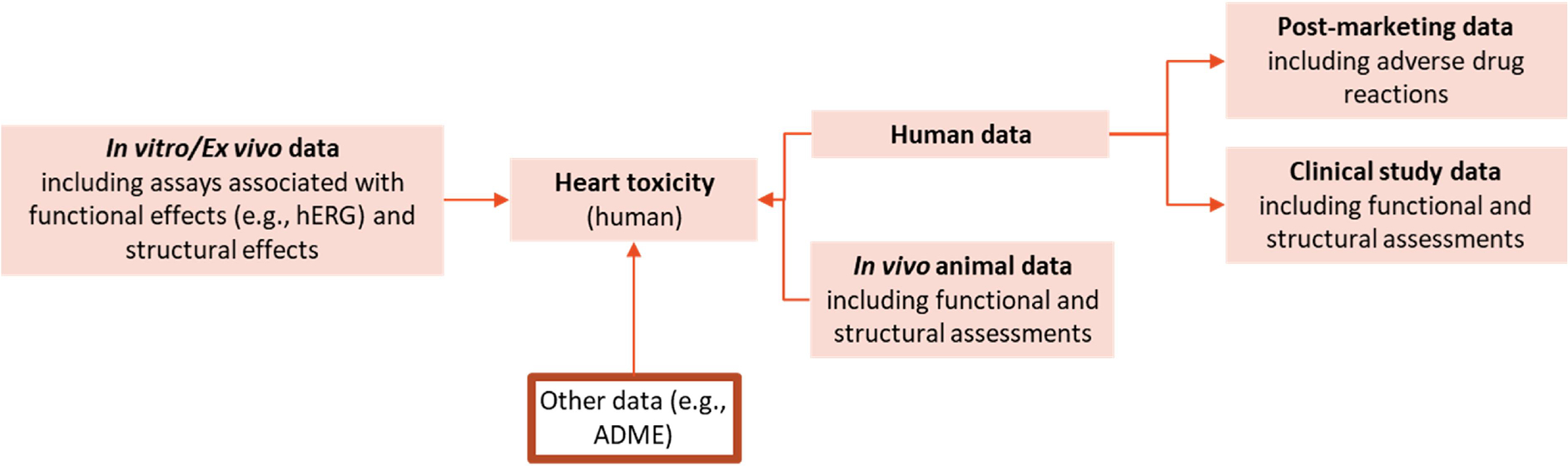
Schema for the assessment framework of heart toxicity. Human data (measured or predicted) include endpoints such as arrhythmia and heart failure. In vitro data may be collected from different types of assays such as binding assays, functional flux assays, patch clamp, Langendorff perfused heart assay, Microelectrode Arrays, impedance assays, high content imaging assays, cytotoxicity assays. Other types of data standardized in different protocols can be integrated such as in vitro ADME profiling and toxicokinetics data.
Current in silico models for the prediction of cardiac toxicity mainly address hERG inhibition, a surrogate marker for proarrhythmia, and they build on the in vitro hERG-related data from early screening in drug discovery and development. The most popular approaches for predicting pharmacological hERG blockade are ligand-based methods that correlate the biological activity to the structural information of chemicals [182,183]. Such methods use approaches such as QSAR (based on different techniques including machine learning), pharmacophore, and 3D QSAR methodologies. Most of these models are classification-based QSARs, but regression-based QSARs have also been proposed to predict activity [184]. In silico models using multiple ion channel data (hERG, Cav1.2 and Nav1.5) have been shown to more accurately predict TdP than models based on hERG effects alone [167,185]. Improving chemical space coverage and quantitative activity prediction remain areas of current research.
Structure-based approaches (e.g., docking) that make use of structural knowledge of the biological target (i.e., hERG) have also been applied to identify hERG blockers [164,186,187].
In silico predictions of inhibition of ion channels were integrated in the CIPA approach [188], the paradigm for the assessment of ventricular proarrhythmic liabilities based on in vitro methods and mathematical models simulating cellular cardiac electrophysiologic activity [169,172]. QSAR models based on human data [189–191] have been developed for the prediction of several cardiac adverse effects such as: arrhythmia, hypertension, bradycardia, conduction disorder, electrocardiogram, palpitations, QT prolongation, rate rhythm abnormality, tachycardia, Torsades de pointes, coronary artery disorders, heart failure, myocardial disorders, and myocardial infarction. One of the strengths of using post-market data is that idiosyncratic toxicities can be identified and incorporated into a QSAR model. Unfortunately, such databases suffer from various reporting biases and confounding factors and have been said to be more suitable for signal detection rather than validation (other types of data would be needed to draw reliable conclusions on the observed effects) [192]. Nonetheless, development of QSAR models using these data have been shown to provide useful predictions [190].
Using the hierarchical organization of similar findings (and mechanisms) collated from preclinical toxicity study reports for regulatory submissions (see Table 6), in silico models built on different methodologies (e.g., statistical fragment/fingerprint-based models, molecular descriptor-based machine learning models, expert-rule based models) were developed by Amberg and co-workers [36]. It was noted that the initial clustering of the effects affected the resulting predictivity of these models.
Heart toxicity – In silico approaches: Data gaps and issues
Currently, alternative approaches for heart toxicity prediction focus on cardiac electrophysiological effects, that pharmaceutical research investigates through an integration of in silico and in vitro methods; this is then supported by short-duration in vivo studies [152]. Regarding structural cardiac toxicity, integrated tiered approaches that exploit predictivity of in vitro and in silico models are instead generally limited [151].
Development of alternative methods (e.g., in silico and in vitro) that accurately predict the entire spectrum of cardiac toxicity must rely on robust understanding of the cellular and molecular mechanisms leading to cardiac liabilities. The AOP framework sustains the advance of such understanding (see Table S9 of the Supporting material addressing AOPs related to cardiotoxicity).
Available in vitro, in vivo, and human data on which in silico model can be constructed are sparse. As observed by Laverty and coworkers [139], the majority of the adverse effects reported in the FDA’s Adverse Event Reporting System are often not described in detail, and the causal relationship between an adverse effect and a drug is generally not established in the reports provided.
Discussion
Different computational methods (e.g., statistical-based methods, rule-based methods) can exist to identify chemicals that potentially induce organ toxicity. These methodologies can be used in a complementary manner, e.g., a statical-based method together with structural alerts. They can also be linked to the AOP framework. For example, structural alerts can be applied to categorize chemicals potentially linking a given class of compounds to a specific mechanism or even MIEs [193].
Application of in silico approaches should account for the specific use case, context and thus purpose (e.g., screening, prioritization, classification and labelling, risk assessment, and product development) [193,194]. For example, for consumer safety, a missed hazard may be crucial and lead to subsequent risks; in product development, in silico predictions may be used for flagging organ toxicity and prompting scientists to monitor the corresponding liability as the compound advances through discovery.
The present mechanistically-driven analysis of in silico methods to predict organ toxicity highlights a number of areas for further research that would enhance such predictions.
It is noted how organ toxicity involves a multitude of biological pathways associated with a plethora of endpoints, and how the underlying molecular mechanisms are often poorly understood. This complicates the development of predictive in silico models that are mechanistically-informed. Advances in the understanding of biology at a molecular level would fuel strategies for organ toxicity prediction, based on the integration of different alternative approaches and combination of information in a quantitative manner, such as through defined approaches or on the transcriptomics, proteomics, and metabolomics levels, that are currently lacking to a large extent.
As most in silico modeling approaches require a database of historically performed experimental in vivo or in vitro test results to build such models, the lack of appropriate experimental tests in certain areas provides some challenges. For example, a number of MIEs or key events (KEs) within existing organ toxicity AOPs do not have a corresponding experimental assay or the available assays have limitations such as the lack of metabolic competency. In some situations, as in the case of pulmonary toxicity, the in vivo models have strong limitations themselves, which are being addressed with the development of next generation in vitro models. Subjective grading (and terminology) of histopathology endpoints represents one of the problems with existing data; however, current digital pathology developments may help come up with more objective and consistent assessments in the future.
Databases containing appropriately annotated information are essential to support any in silico model building as well as to support an expert review of the results. There is currently a lack of large in vivo databases covering organ toxicity that (1) are linked to chemical structures, (2) are annotated with the necessary experimental design information, and (3) document both positive and negative (i.e., no treatment related findings) results on findings at tested timepoints and concentrations. These findings should also be linked to the endpoints within the assessment frameworks. Ontologies, standardized terminology, and other technology to support integration and linking of information from different sources are critical. The use of SEND and documents produced through the INHAND working groups will be important to support these databases [195,196]. Toxicogenomic databases are emerging tools that can be used to develop predictive approaches for the classification of chemicals in terms of their toxicogenomic signatures which are, in turn, related to the mechanisms underlying their toxicity. Toxicity is directly linked to gene expression data in databases [197] such as DrugMatrix [198], Open TG-GATEs (Toxicogenomics Project-Genomics Assisted Toxicity Evaluation System) [199] and the Comparative Toxicology Database (CTD) [200].
The number of in silico models being developed, as discussed in this paper, is rapidly expanding; however, a limited number of models fit in specific areas outlined in the proposed assessment framework, and this limitation concerns models to predict MIE’s or in vivo models for certain major toxicological endpoints such as kidney toxicity. The training sets used to build any models may also limit the chemical space that such models may predict (i.e., applicability domain consideration).
It is observed that models that predict dose/timepoints are limited in part due to technical limitations and the lack of properly annotated data. Current models for organ toxicity are mainly performing classification, delivering limited information on threshold levels, that, on the other hand, may be evaluated through read-across approaches provided that data on analogues are available (and properly annotated) and that a thorough analysis of the chemicals establishes a sound similarity between the source chemical and its analogues. Ordinal models (based on ranges of toxic concentration) that are technically more tractable may provide a way forward to support necessary risk assessment decisions. Such quantitative models can support the safety evaluation of compounds in different contexts including those frameworks where in vivo experiments are limited by regulations (e.g., cosmetics).
The importance of internal exposure and in general of the ADME processes has been highlighted, identifying factors (e.g., formation of reactive metabolites) that need to be accounted for when developing the IST organ toxicity protocol. Metabolism is an important element to evaluate for specific organ toxicity (e.g., lung, kidney). Xenobiotic enzyme activity in different organs should be considered as it affects the rate and extent of formation of reactive metabolites. For example, in silico technology to predict metabolites, identify the points of metabolism, or predict binding to CYP enzymes is available and should play a role in the integration of the information as well as incorporated into any expert review. Currently, the prediction of metabolites may result in a high number of many predicted metabolites originating from a multitude of potential pathways, that may need to be critically evaluated. Likewise, it remains difficult to predict absolute likelihoods (as opposed to relative likelihoods) of metabolism at particular sites. ADME considerations are also important in support of the extrapolation of any in vitro experiment data (or models derived from such data) to in vivo outcomes, as well as for inter-species extrapolation. Species differences is another important element that need to be critically evaluated (e.g., different nasal/pharyngeal anatomy in the context of lung toxicity) to translate results to humans.
The development of frameworks capturing the key characteristics of toxicants to a specific target organ, similar to the ten key characteristics of carcinogens [201–203], would provide valuable organizational principles for the IST framework. Key characteristics do not necessarily represent mechanisms nor are adverse outcome pathways, but they provide a broad and holistic structure to organize relevant mechanistic data for human health assessments of possible toxicants. This construct was first introduced for carcinogens and it is now under consideration in other contexts such as for hepatotoxicants, neurotoxicants and developmental neurotoxicants and cardiotoxicants [204,205].
Conclusion
This work is a mechanistically-driven analysis of the current state of the art with respect to the in silico prediction of organ toxicity (with focus on heart, lung and kidney) and it includes an overview of key characteristics/mechanisms and how they contribute to organ toxicity. A summary of the major topics discussed throughout the work is summarized in Table 7.
Table 7.
Main topics discussed in the present work.
| Main topics |
|---|
|
|
| • Overview of key characteristics/mechanisms is presented with reference to the AOP construct. |
| • Importance of mitochondrial dysfunction across different organ toxicities is highlighted. |
| • Relevant endpoints for each target organ are discussed. |
| • Binding to molecular targets that are associated with adverse effects to specific organs (i.e., off-target panels from secondary pharmacology batteries) is discussed. |
| • In vitro and/or in vivo models for investigating target organ toxicity and detecting corresponding toxic xenobiotics are discussed alongside emerging experimental approaches such as 3D in vitro models and toxicogenomics. |
| • An overview is given of computational methods (statistical models, expert alerts, read-across) that can be used to identify chemicals that potentially induce organ toxicity with reference to specific key characteristics/mechanisms, if any. |
| • Data gaps and challenges ahead for the development of computational methods predictive of target organ toxicity are discussed. |
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number R44ES026909. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CRediT authorship contribution statement
Arianna Bassan: Conceptualization, Writing – original draft, Writing - review & editing. Vinicius M. Alves: Writing - review & editing. Alexander Amberg: Writing - review & editing. Lennart T. Anger: Writing - review & editing. Lisa Beilke: Writing - review & editing. Andreas Bender: Writing - review & editing. Autumn Bernal: Writing - review & editing. Mark T.D. Cronin: Writing - review & editing. Jui-Hua Hsieh: Writing - review & editing. Candice Johnson: Writing - review & editing. Raymond Kemper: Writing - review & editing. Moiz Mumtaz: Writing - review & editing. Louise Neilson: Writing - review & editing. Manuela Pavan: Writing - review & editing. Amy Pointon: Writing - review & editing. Julia Pletz: Writing - review & editing. Patricia Ruiz: Writing - review & editing. Daniel P. Russo: Writing - review & editing. Yogesh Sabnis: Writing - review & editing. Reena Sandhu: Writing - review & editing. Markus Schaefer: Writing - review & editing. Lidiya Stavitskaya: Writing - review & editing. David T. Szabo: Writing - review & editing. Jean-Pierre Valentin: Writing - review & editing. David Woolley: Writing - review & editing. Craig Zwickl: Writing - review & editing. Glenn J. Myatt: Conceptualization, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Glenn J Myatt reports financial support was provided by National Institutes of Health.
Disclaimer
CDC Disclaimer
The findings and conclusions in this article are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
FDA disclaimer
This article reflects the views of the authors and should not be construed to represent FDA’s views or policies. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.comtox.2021.100188.
References
- [1].EMA, Repeated dose toxicity, Guidel. Repeated Dose Toxic. (2010). https://www.ema.europa.eu/en/repeated-dose-toxicity.
- [2].Klaassen C, Casarett LJ, Doull J, Casarett & Doull’s Toxicology, McGraw-Hill Publishing, Blacklick, 2013. https://public.ebookcentral.proquest.com/choice/publicfullrecord.aspx?p=4959412 (accessed March 26, 2021). [Google Scholar]
- [3].Prieto P, Graepel R, Gerloff K, Lamon L, Sachana M, Pistollato F, Gribaldo L, Bal-Price A, Worth A, Investigating cell type specific mechanisms contributing to acute oral toxicity, Altex 36 (2019) 39–64. 10.14573/altex.1805181. [DOI] [PubMed] [Google Scholar]
- [4].Kaufmann W, Jacobsen MC, Examination of organ toxicity, in: Reichl F-X, Schwenk M(Eds.), Regul. Toxicol, Springer-Verlag, Berlin, Heidelberg, 2014: pp. 89–98. 10.1007/978-3-642-35374-1_32. [DOI] [Google Scholar]
- [5].Myatt GJ, Ahlberg E, Akahori Y, Allen D, Amberg A, Anger LT, Aptula A, Auerbach S, Beilke L, Bellion P, Benigni R, Bercu J, Booth ED, Bower D, Brigo A, Burden N, Cammerer Z, Cronin MTD, Cross KP, Custer L, Dettwiler M, Dobo K, Ford KA, Fortin MC, Gad-McDonald SE, Gellatly N, Gervais V, Glover KP, Glowienke S, Van Gompel J, Gutsell S, Hardy B, Harvey JS, Hillegass J, Honma M, Hsieh J-H, Hsu C-W, Hughes K, Johnson C, Jolly R, Jones D, Kemper R, Kenyon MO, Kim MT, Kruhlak NL, Kulkarni SA, Kümmerer K, Leavitt P, Majer B, Masten S, Miller S, Moser J, Mumtaz M, Muster W, Neilson L, Oprea TI, Patlewicz G, Paulino A, Lo Piparo E, Powley M, Quigley DP, Reddy MV, Richarz A-N, Ruiz P, Schilter B, Serafimova R, Simpson W, Stavitskaya L, Stidl R, Suarez-Rodriguez D, Szabo DT, Teasdale A, Trejo-Martin A, Valentin J-P, Vuorinen A, Wall BA, Watts P, White AT, Wichard J, Witt KL, Woolley A, Woolley D, Zwickl C, Hasselgren C, In silico toxicology protocols, Regul. Toxicol. Pharmacol 96 (2018) 1–17, 10.1016/j.yrtph.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hasselgren C, Ahlberg E, Akahori Y, Amberg A, Anger LT, Atienzar F, Auerbach S, Beilke L, Bellion P, Benigni R, Bercu J, Booth ED, Bower D, Brigo A, Cammerer Z, Cronin MTD, Crooks I, Cross KP, Custer L, Dobo K, Doktorova T, Faulkner D, Ford KA, Fortin MC, Frericks M, Gad-McDonald SE, Gellatly N, Gerets H, Gervais Véronique, Glowienke S, Van Gompel J, Harvey JS, Hillegass J, Honma M, Hsieh J-H, Hsu C-W, Barton-Maclaren TS, Johnson C, Jolly R, Jones D, Kemper R, Kenyon MO, Kruhlak NL, Kulkarni SA, Kümmerer K, Leavitt P, Masten S, Miller S, Moudgal C, Muster W, Paulino A, Lo Piparo E, Powley M, Quigley DP, Reddy MV, Richarz A-N, Schilter B, Snyder RD, Stavitskaya L, Stidl R, Szabo DT, Teasdale A, Tice RR, Trejo-Martin A, Vuorinen A, Wall BA, Watts P, White AT, Wichard J, Witt KL, Woolley A, Woolley D, Zwickl C, Myatt GJ, Genetic toxicology in silico protocol, Regul. Toxicol. Pharmacol 107 (2019) 104403, 10.1016/j.yrtph.2019.104403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Johnson C, Ahlberg E, Anger LT, Beilke L, Benigni R, Bercu J, Bobst S, Bower D, Brigo A, Campbell S, Cronin MTD, Crooks I, Cross KP, Doktorova T, Exner T, Faulkner D, Fearon IM, Fehr M, Gad SC, Gervais Véronique, Giddings A, Glowienke S, Hardy B, Hasselgren C, Hillegass J, Jolly R, Krupp E, Lomnitski L, Magby J, Mestres J, Milchak L, Miller S, Muster W, Neilson L, Parakhia R, Parenty A, Parris P, Paulino A, Paulino AT, Roberts DW, Schlecker H, Stidl R, Suarez-Rodrigez D, Szabo DT, Tice RR, Urbisch D, Vuorinen A, Wall B, Weiler T, White AT, Whritenour J, Wichard J, Woolley D, Zwickl C, Myatt GJ, Skin sensitization in silico protocol, Regul. Toxicol. Pharmacol. 116 (2020) 104688, 10.1016/j.yrtph.2020.104688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bassan A, Alves VM, Amberg A, Anger LT, Auerbach S, Beilke L, Bender A, Cronin MTD, Cross KP, Hsieh J-H, Greene N, Kemper R, Kim MT, Mumtaz M, Noeske T, Pavan M, Pletz J, Russo DP, Sabnis Y, Schaefer M, Szabo DT, Valentin J-P, Wichard J, Williams D, Woolley D, Zwickl C, Myatt GJ, In silico approaches in organ toxicity hazard assessment: current status and future needs in predicting liver toxicity, (2021) Unpublished results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Levey AS, Eckardt K-U, Dorman NM, Christiansen SL, Hoorn EJ, Ingelfinger JR, Inker LA, Levin A, Mehrotra R, Palevsky PM, Perazella MA, Tong A, Allison SJ, Bockenhauer D, Briggs JP, Bromberg JS, Davenport A, Feldman HI, Fouque D, Gansevoort RT, Gill JS, Greene EL, Hemmelgarn BR, Kretzler M, Lambie M, Lane PH, Laycock J, Leventhal SE, Mittelman M, Morrissey P, Ostermann M, Rees L, Ronco P, Schaefer F, St. Clair Russell J, Vinck C, Walsh SB, Weiner DE, Cheung M, Jadoul M, Winkelmayer WC, Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference, Kid. Int 97 (6) (2020) 1117–1129, 10.1016/j.kint.2020.02.010. [DOI] [PubMed] [Google Scholar]
- [10].WHO, Principles and methods for the assessment of nephrotoxicity associated with exposure to chemicals, World Health Organization, Geneva, Switzerland, 1991. http://www.inchem.org/documents/ehc/ehc/ehc119.htm#PartNumber:2. [Google Scholar]
- [11].Barnett LMA, Cummings BS, Nephrotoxicity and renal pathophysiology: a contemporary perspective, Toxicol. Sci. 164 (2018) 379–390, 10.1093/toxsci/kfy159. [DOI] [PubMed] [Google Scholar]
- [12].Perazella MA, Renal vulnerability to drug toxicity, Clin. J. Am. Soc. Nephrol. CJASN 4 (7) (2009) 1275–1283, 10.2215/CJN.02050309. [DOI] [PubMed] [Google Scholar]
- [13].Tarloff JB, Wallace AD, Nephrotoxicity, in: Hodgson E (Ed.), Textb. Mod. Toxicol, 4th ed, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2010: pp. 291–302. [Google Scholar]
- [14].Cesta MF, Malarkey DE, Herbert RA, Brix A, Hamlin MH, Singletary E, Sills RC, Bucher JR, Birnbaum LS, The National Toxicology Program Web-based Nonneoplastic Lesion Atlas: a global toxicology and pathology resource, Toxicol. Pathol 42 (2) (2014) 458–460, 10.1177/0192623313517304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].NTP, Nonneoplastic Lesion Atlas - National Toxicology Program, (2014). https://ntp.niehs.nih.gov/nnl/ (accessed February 15, 2020).
- [16].Griffin BR, Faubel S, Edelstein CL, Biomarkers of drug-induced kidney toxicity, Ther. Drug Monit. 41 (2019) 213–226, 10.1097/FTD.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].National Research Council, Biologic Markers in Urinary Toxicology, National Academies Press, Washington, DC, 1995. 10.17226/4847. [DOI] [PubMed] [Google Scholar]
- [18].Fu Q, Colgan SP, Shelley CS, Hypoxia: the force that drives chronic kidney disease, Clin. Med. Res 14 (1) (2016) 15–39, 10.3121/cmr.2015.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ozbek E, Induction of oxidative stress in kidney, Int. J. Nephrol. 2012 (2012) 1–9, 10.1155/2012/465897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brown AC, Kidney toxicity related to herbs and dietary supplements: online table of case reports. Part 3 of 5 series, Food Chem. Toxicol. 107 (2017) 502–519, 10.1016/j.fct.2016.07.024. [DOI] [PubMed] [Google Scholar]
- [21].Koraishy FM, Moeckel GW, Geller DS, A case of severe nephrotoxicity associated with long-term dietary supplement use, Clin. Nephrol 5 (2017) 42–47, 10.5414/CNCS109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Institute of Medicine, Forum on Drug Discovery, Development, and Translation, Accelerating the Development of Biomarkers for Drug Safety: Workshop Summary, The National Academies Press, Washington, D.C., 2009. 10.17226/12587. [DOI] [Google Scholar]
- [23].Roth A, Singer T, The application of 3D cell models to support drug safety assessment: opportunities & challenges, Adv. Drug Deliv. Rev. 69–70 (2014) 179–189, 10.1016/j.addr.2013.12.005. [DOI] [PubMed] [Google Scholar]
- [24].Awdishu L, Mehta RL, The 6R’s of drug induced nephrotoxicity, BMC Nephrol 18 (2017) 124, 10.1186/s12882-017-0536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kataria A, Trasande L, Trachtman H, The effects of environmental chemicals on renal function, Nat. Rev. Nephrol 11 (10) (2015) 610–625, 10.1038/nrneph.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lebov JF, Engel LS, Richardson D, Hogan SL, Hoppin JA, Sandler DP, Pesticide use and risk of end-stage renal disease among licensed pesticide applicators in the Agricultural Health Study, Occup. Environ. Med 73 (1) (2016) 3–12, 10.1136/oemed-2014-102615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Valcke M, Levasseur M-E, Soares da Silva A, Wesseling C, Pesticide exposures and chronic kidney disease of unknown etiology: an epidemiologic review, Environ. Health 16 (2017) 49, 10.1186/s12940-017-0254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kandasamy K, Chuah JKC, Su R, Huang P, Eng KG, Xiong S, Li Y, Chia CS, Loo L-H, Zink D, Prediction of drug-induced nephrotoxicity and injury mechanisms with human induced pluripotent stem cell-derived cells and machine learning methods, Sci. Rep. 5 (2015) 12337, 10.1038/srep12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Soo J-C, Jansen J, Masereeuw R, Little MH, Advances in predictive in vitro models of drug-induced nephrotoxicity, Nat. Rev. Nephrol 14 (6) (2018) 378–393, 10.1038/s41581-018-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Barnett LMA, Cummings BS, Cellular and molecular mechanisms of kidney toxicity, Semin. Nephrol 39 (2) (2019) 141–151, 10.1016/j.semnephrol.2018.12.004. [DOI] [PubMed] [Google Scholar]
- [31].Hosohata K, Role of oxidative stress in drug-induced kidney injury, Int. J. Mol. Sci. 17 (2016) 1826, 10.3390/ijms17111826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS, Oxidant mechanisms in renal injury and disease, Antioxid. Redox Signal. 25 (3) (2016) 119–146, 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pletz J, Enoch SJ, Jais DM, Mellor CL, Pawar G, Firman JW, Madden JC, Webb SD, Tagliati CA, Cronin MTD, A critical review of adverse effects to the kidney: mechanisms, data sources, and in silico tools to assist prediction, Expert Opin. Drug Metab. Toxicol 14 (12) (2018) 1225–1253, 10.1080/17425255.2018.1539076. [DOI] [PubMed] [Google Scholar]
- [34].AOP Knowledgebase, AOPwiki, (2021). https://aopwiki.org/ (accessed March 5, 2021).
- [35].Pittman ME, Edwards SW, Ives C, Mortensen HM, AOP-DB: a database resource for the exploration of Adverse Outcome Pathways through integrated association networks, Toxicol. Appl. Pharmacol. 343 (2018) 71–83, 10.1016/j.taap.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Amberg A, Kopanska K, Anger LT, Schaefer M, Spirkl H-P, Stolte M, Durchfeld-Meyer B, Myatt G, Czich A, In silico prediction of organ toxicity – Development of in silico models from in vivo drug histopathology data from regulatory toxicity study reports, Toxicol. Suppl. Toxicol. Sci 174 (2020) Abstract #2050. https://www.toxicology.org/pubs/docs/Tox/2020Tox.pdf. [Google Scholar]
- [37].Kim S-Y, Moon A-R, Drug-induced nephrotoxicity and its biomarkers, Biomol. Ther 20 (3) (2012) 268–272, 10.4062/biomolther.2012.20.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Meng X-M, Nikolic-Paterson DJ, Lan HY, Inflammatory processes in renal fibrosis, Nat. Rev. Nephrol 10 (9) (2014) 493–503, 10.1038/nrneph.2014.114. [DOI] [PubMed] [Google Scholar]
- [39].Naughton CA, Drug-induced nephrotoxicity, Am. Fam. Phys. 78 (2008) 743–750. [PubMed] [Google Scholar]
- [40].Weber EJ, Himmelfarb J, Kelly EJ, Concise review: current and emerging biomarkers of nephrotoxicity, Curr. Opin. Toxicol. 4 (2017) 16–21, 10.1016/j.cotox.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Drew WC, Surfraz B, Adverse outcome pathways for the nephrotoxicity of nonsteroidal anti-inflammatory, Toxicol. Suppl. Toxicol. Sci. 144 (2015) Abstract #1326. https://www.toxicology.org/pubs/docs/Tox/2015Tox.pdf. [Google Scholar]
- [42].Naesens M, Kuypers DRJ, Sarwal M, Calcineurin inhibitor nephrotoxicity, Clin. J. Am. Soc. Nephrol. CJASN 4 (2) (2009) 481–508, 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- [43].Drew WC, Cayley A, Benz RD, Kruhlak NL, Surfraz B, Identification of adverse outcome pathways for the nephrotoxicity of nucleoside and nucleotide antiviral drugs, Toxicol. Suppl. Toxicol. Sci. 138 (2014) Abstract #2256. https://www.toxicology.org/pubs/docs/Tox/2014Tox.pdf. [Google Scholar]
- [44].Walker RJ, Endre ZH, Chapter 85 - Cellular mechanisms of drug nephrotoxicity, in: Alpern RJ, Moe OW, Caplan M(Eds.), Seldin Giebischs Kidney Fifth Ed, Fifth Edition, Academic Press, 2013: pp. 2889–2932. 10.1016/B978-0-12-381462-3.00085-9. [DOI] [Google Scholar]
- [45].Health and Safety Publications, Paris 2011 8&doclanguage=en http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono (2011).
- [46].ICH, Ich, S7A Safety pharmacology studies for human pharmaceuticals European Medicines Agency 2000. https://database.ich.org/sites/default/files/S7A_Guideline.pdf.
- [47].ICH, Ich, M3 (R2) Non-clinical safety studies for the conduct of human clinical trials pharmaceuticals European Medicines Agency 2009. https://database.ich.org/sites/default/files/M3_R2__Guideline.pdf.
- [48].Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM, Park J, Nair V, Schlafly A, Saulnier P-J, Satake E, Simeone CA, Shah H, Qiu C, Looker HC, Fiorina P, Ware CF, Sun JK, Doria A, Kretzler M, Susztak K, Duffin KL, Nelson RG, Krolewski AS, A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes, Nat. Med. 25 (5) (2019) 805–813, 10.1038/s41591-019-0415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li Z, Su W, Zhu Y, Tao T, Li D, Peng X, Qin J, Drug absorption related nephrotoxicity assessment on an intestine-kidney chip, Biomicrofluidics 11 (3) (2017) 034114, 10.1063/1.4984768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ramm S, Adler M, Vaidya VS, A high-throughput screening assay to identify kidney toxic compounds, Curr. Protoc. Toxicol. 69 (2016) 9.10.1–9.10.26. 10.1002/cptx.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Faria J, Ahmed S, Gerritsen KGF, Mihaila SM, Masereeuw R, Kidney-based in vitro models for drug-induced toxicity testing, Arch. Toxicol. 93 (12) (2019) 3397–3418, 10.1007/s00204-019-02598-0. [DOI] [PubMed] [Google Scholar]
- [52].Jenkinson S, Schmidt F, Rosenbrier Ribeiro L, Delaunois A, Valentin J-P, A practical guide to secondary pharmacology in drug discovery, J. Pharmacol. Toxicol. Methods 105 (2020) 106869, 10.1016/j.vascn.2020.106869. [DOI] [PubMed] [Google Scholar]
- [53].Bowes J, Brown AJ, Hamon J, Jarolimek W, Sridhar A, Waldron G, Whitebread S, Reducing safety-related drug attrition: the use of in vitro pharmacological profiling, Nat. Rev. Drug Discov. 11 (12) (2012) 909–922, 10.1038/nrd3845. [DOI] [PubMed] [Google Scholar]
- [54].Smit IA, Afzal AM, Allen CHG, Svensson F, Hanser T, Bender A, Systematic analysis of protein targets associated with adverse events of drugs from clinical trials and postmarketing reports, Chem. Res. Toxicol. 34 (2) (2021) 365–384, 10.1021/acs.chemrestox.0c00294. [DOI] [PubMed] [Google Scholar]
- [55].Deaton AM, Fan F, Zhang W, Nguyen PA, Ward LD, Nioi P, Rationalizing secondary pharmacology screening using human genetic and pharmacological evidence, Toxicol. Sci. 167 (2019) 593–603, 10.1093/toxsci/kfy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lynch JJ, Van Vleet TR, Mittelstadt SW, Blomme EAG, Potential functional and pathological side effects related to off-target pharmacological activity, J. Pharmacol. Toxicol. Methods 87 (2017) 108–126, 10.1016/j.vascn.2017.02.020. [DOI] [PubMed] [Google Scholar]
- [57].Hammann F, Gutmann H, Vogt N, Helma C, Drewe J, Prediction of adverse drug reactions using decision tree modeling, Clin. Pharmacol. Ther. 88 (1) (2010) 52–59, 10.1038/clpt.2009.248. [DOI] [PubMed] [Google Scholar]
- [58].Lee S, Kang Y-M, Park H, Dong M-S, Shin J-M, No KT, Human nephrotoxicity prediction models for three types of kidney injury based on data sets of pharmacological compounds and their metabolites, Chem. Res. Toxicol. 26 (11) (2013) 1652–1659, 10.1021/tx400249t. [DOI] [PubMed] [Google Scholar]
- [59].Lei T, Sun H, Kang Y, Zhu F, Liu H, Zhou W, Wang Z, Li D, Li Y, Hou T, ADMET evaluation in drug discovery. 18. Reliable prediction of chemical-induced urinary tract toxicity by boosting machine learning approaches, Mol. Pharm. 14 (11) (2017) 3935–3953, 10.1021/acs.molpharmaceut.7b00631. [DOI] [PubMed] [Google Scholar]
- [60].Matthews EJ, Ursem CJ, Kruhlak NL, Benz RD, Sabaté D.Aragonés, Yang C, Klopman G, Contrera JF, Identification of structure-activity relationships for adverse effects of pharmaceuticals in humans: Part B. Use of (Q)SAR systems for early detection of drug-induced hepatobiliary and urinary tract toxicities, Regul. Toxicol. Pharmacol. RTP 54 (1) (2009) 23–42, 10.1016/j.yrtph.2009.01.009. [DOI] [PubMed] [Google Scholar]
- [61].Zhang H, Ren J-X, Ma J-X, Ding L, Development of an in silico prediction model for chemical-induced urinary tract toxicity by using naïve Bayes classifier, Mol. Divers. 23 (2) (2019) 381–392, 10.1007/s11030-018-9882-8. [DOI] [PubMed] [Google Scholar]
- [62].Myshkin E, Brennan R, Khasanova T, Sitnik T, Serebriyskaya T, Litvinova E, Guryanov A, Nikolsky Y, Nikolskaya T, Bureeva S, Prediction of organ toxicity endpoints by QSAR modeling based on precise chemical-histopathology annotations, Chem. Biol. Drug Des. 80 (2012) 406–416, 10.1111/j.1747-0285.2012.01411.x. [DOI] [PubMed] [Google Scholar]
- [63].Pizzo F, Gadaleta D, Lombardo A, Nicolotti O, Benfenati E, Identification of structural alerts for liver and kidney toxicity using repeated dose toxicity data, Chem. Cent. J 9 (2015) 62, 10.1186/s13065-015-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Enoch SJ, Ellison CM, Schultz TW, Cronin MTD, A review of the electrophilic reaction chemistry involved in covalent protein binding relevant to toxicity, Crit. Rev. Toxicol. 41 (9) (2011) 783–802, 10.3109/10408444.2011.598141. [DOI] [PubMed] [Google Scholar]
- [65].Enoch S, Mellor C, Nelms M, Structure-activity modeling of mitochondrial dysfunction, in: Will Y, Dykens JA (Eds.), Mitochondrial Dysfunct. Caused Drugs Environ. Toxic, John Wiley & Sons Inc, Hoboken, NJ, USA, 2018, pp. 25–34, 10.1002/9781119329725.ch3. [DOI] [Google Scholar]
- [66].Nelms MD, Mellor CL, Cronin MTD, Madden JC, Enoch SJ, Development of an in silico profiler for mitochondrial toxicity, Chem. Res. Toxicol. 28 (10) (2015) 1891–1902, 10.1021/acs.chemrestox.5b00275. [DOI] [PubMed] [Google Scholar]
- [67].Fowles J, Banton M, Klapacz J, Shen H, A toxicological review of the ethylene glycol series: commonalities and differences in toxicity and modes of action, Toxicol. Lett. 278 (2017) 66–83, 10.1016/j.toxlet.2017.06.009. [DOI] [PubMed] [Google Scholar]
- [68].van Ravenzwaay B, Herold M, Kamp H, Kapp MD, Fabian E, Looser R, Krennrich G, Mellert W, Prokoudine A, Strauss V, Walk T, Wiemer J, Metabolomics: A tool for early detection of toxicological effects and an opportunity for biology based grouping of chemicals - From QSAR to QBAR, Mutat. Res. Genet. Toxicol. Environ. Mutagen. 746 (2) (2012) 144–150, 10.1016/j.mrgentox.2012.01.006. [DOI] [PubMed] [Google Scholar]
- [69].Pletz J, Allen TJ, Madden JC, Cronin MTD, Webb SD, A mechanistic model to study the kinetics and toxicity of salicylic acid in the kidney of four virtual individuals, Comput. Toxicol. 19 (2021) 100172, 10.1016/j.comtox.2021.100172. [DOI] [Google Scholar]
- [70].Bonner JC, Respiratory toxicology, in: Hodgson E (Ed.), Textb. Mod. Toxicol, 4th ed, John Wiley & Sons, Inc., Hoboken, NJ, 2010: pp. 363–386. [Google Scholar]
- [71].Castell JV, Teresa Donato M, Gómez-Lechón MJ, Metabolism and bioactivation of toxicants in the lung. The in vitro cellular approach, Exp. Toxicol. Pathol 57 (2005) 189–204, 10.1016/j.etp.2005.05.008. [DOI] [PubMed] [Google Scholar]
- [72].Weitnauer M, Mijošek V, Dalpke AH, Control of local immunity by airway epithelial cells, Mucosal Immunol 9 (2) (2016) 287–298, 10.1038/mi.2015.126. [DOI] [PubMed] [Google Scholar]
- [73].Pickrell JA, CHAPTER 12 - Respiratory toxicity, in: Gupta RC (Ed.), Vet. Toxicol, Academic Press, 2007: pp. 177–192. 10.1016/B978-012370467-2/50109-7. [DOI] [Google Scholar]
- [74].Suresh K, Shimoda LA, Lung circulation, in: Terjung R(Ed.), Compr. Physiol, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2016: pp. 897–943. 10.1002/cphy.c140049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].van der Merwe D, Respiratory toxicity, in: Gupta RC (Ed.), Vet, Third edition, Academic Press, Amsterdam, Toxicol. Basic Clin. Princ, 2018, p. 1238. [Google Scholar]
- [76].Clippinger AJ, Allen D, Jarabek AM, Corvaro M, Gaça M, Gehen S, Hotchkiss JA, Patlewicz G, Melbourne J, Hinderliter P, Yoon M, Huh D, Lowit A, Buckley B, Bartels M, BéruBé K, Wilson DM, Indans I, Vinken M, Alternative approaches for acute inhalation toxicity testing to address global regulatory and non-regulatory data requirements: an international workshop report, Toxicol. In Vitro 48 (2018) 53–70, 10.1016/j.tiv.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Clippinger AJ, Allen D, Behrsing H, BéruBé KA, Bolger MB, Casey W, DeLorme M, Gaça M, Gehen SC, Glover K, Hayden P, Hinderliter P, Hotchkiss JA, Iskandar A, Keyser B, Luettich K, Ma-Hock L, Maione AG, Makena P, Melbourne J, Milchak L, Ng SP, Paini A, Page K, Patlewicz G, Prieto P, Raabe H, Reinke EN, Roper C, Rose J, Sharma M, Spoo W, Thorne PS, Wilson DM, Jarabek AM, Pathway-based predictive approaches for non-animal assessment of acute inhalation toxicity, Toxicol. In Vitro 52 (2018) 131–145, 10.1016/j.tiv.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bhatia P, O’Reilly JF, Li-Kam-Wa E, Adverse drug reactions and the respiratory system, Prim. Care Respir. J. J. Gen. Pract. Airw. Group 10 (2) (2001) 39–43, 10.1038/pcrj.2001.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Camus P, Rosenow EC, Iatrogenic lung disease, Clin. Chest Med 25 (1) (2004) xiii–xix, 10.1016/S0272-5231(03)00146-1. [DOI] [PubMed] [Google Scholar]
- [80].Schwaiblmair M, Behr W, Haeckel T, Märkl B, Foerg W, Berghaus T, Drug induced interstitial lung disease, Open Respir, Med. J 6 (2012) 63–74, 10.2174/1874306401206010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Balogh Sivars K, Sivars U, Hornberg E, Zhang H, Brändén L, Bonfante R, Huang S, Constant S, Robinson I, Betts CJ, Åberg PM, A 3D human airway model enables prediction of respiratory toxicity of inhaled drugs in vitro, Toxicol. Sci. Off. J. Soc. Toxicol. 162 (2018) 301–308, 10.1093/toxsci/kfx255. [DOI] [PubMed] [Google Scholar]
- [82].Kuempel ED, Sweeney LM, Morris JB, Jarabek AM, Advances in inhalation dosimetry models and methods for occupational risk assessment and exposure limit derivation, J. Occup. Environ. Hyg 12 (sup1) (2015) S18–S40, 10.1080/15459624.2015.1060328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Braakhuis HM, Park MVDZ, Gosens I, De Jong WH, Cassee FR, Physicochemical characteristics of nanomaterials that affect pulmonary inflammation, Part. Fibre Toxicol 11 (1) (2014) 18, 10.1186/1743-8977-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhang JY, Wang Y, Prakash C, Xenobiotic-metabolizing enzymes in human lung, Curr. Drug Metab. 7 (2006) 939–948, 10.2174/138920006779010575. [DOI] [PubMed] [Google Scholar]
- [85].Borak J, Fields C, Andrews LS, Pemberton MA, Methyl methacrylate and respiratory sensitization: a critical review, Crit. Rev. Toxicol. 41 (3) (2011) 230–268, 10.3109/10408444.2010.532768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].ECHA, Guidance on information requirements and chemical safety assessment Chapter R.7a: endpoint specific guidance. Version 6.0, Publications Office of the EU, 2017. 10.2823/337352. [DOI] [Google Scholar]
- [87].OSHA, Hazard, Classification Guidance for Manufacturers, Importers, and Employers 2016. https://www.osha.gov/Publications/OSHA3844.pdf.
- [88].GHS, Globally Harmonised System of Classification and Labelling of Chemicals (GHS) - Fifth revised edition., United Nations, New York and Geneva, 2013. 10.18356/dbde9a22-en. [DOI] [Google Scholar]
- [89].SCHC-OSHA, Hazard communication information sheet reflecting the US OSHA implementation of the Globally Harmonized System of classification and labelling of chemicals (GHS) - Specific target organ toxicity - Single exposure 2017. https://www.schc.org/assets/docs/ghs_info_sheets/specific_target_organ_toxicity-single_exposure.pdf.
- [90].Arts JHE, de Heer C, Woutersen RA, Local effects in the respiratory tract: relevance of subjectively measured irritation for setting occupational exposure limits, Int. Arch. Occup. Environ. Health 79 (4) (2006) 283–298, 10.1007/s00420-005-0044-9. [DOI] [PubMed] [Google Scholar]
- [91].Alarie Y, Dose-response analysis in animal studies: prediction of human responses, Environ. Health Perspect. 42 (1981) 9–13, 10.1289/ehp.81429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Alarie Y, Lecture notes - Inhalation toxicology and toxic responses of the lung, (2014). http://www.pitt.edu/~rd50/Yves%20AlarieHandoutforMidAmericaLecture2014.pdf.
- [93].Alarie Y, Sensory irritation of the upper airways by airborne chemicals, Toxicol. Appl. Pharmacol. 24 (2) (1973) 279–297, 10.1016/0041-008X(73)90148-8. [DOI] [PubMed] [Google Scholar]
- [94].Holsapple MP, Jones D, Kawabata TT, Kimber I, Sarlo K, Selgrade MK, Shah J, Woolhiser MR, Assessing the potential to induce respiratory hypersensitivity, Toxicol. Sci. 91 (2006) 4–13, 10.1093/toxsci/kfj074. [DOI] [PubMed] [Google Scholar]
- [95].Cochrane SA, Arts JHE, Ehnes C, Hindle S, Hollnagel HM, Poole A, Suto H, Kimber I, Thresholds in chemical respiratory sensitisation, Toxicology 333 (2015) 179–194, 10.1016/j.tox.2015.04.010. [DOI] [PubMed] [Google Scholar]
- [96].Kimber I, Dearman RJ, Basketter DA, Boverhof DR, Chemical respiratory allergy: reverse engineering an adverse outcome pathway, Toxicology 318 (2014) 32–39, 10.1016/j.tox.2014.02.001. [DOI] [PubMed] [Google Scholar]
- [97].Basketter DA, Kimber I, Phthalic anhydride: illustrating a conundrum in chemical allergy, J. Immunotoxicol 13 (6) (2016) 767–769, 10.1080/1547691X.2016.1177149. [DOI] [PubMed] [Google Scholar]
- [98].Arts J, How to assess respiratory sensitization of low molecular weight chemicals? Int. J. Hyg. Environ. Health 225 (2020) 113469, 10.1016/j.ijheh.2020.113469. [DOI] [PubMed] [Google Scholar]
- [99].Sullivan KM, Enoch SJ, Ezendam J, Sewald K, Roggen EL, Cochrane S, An adverse outcome pathway for sensitization of the respiratory tract by low-molecular-weight chemicals: building evidence to support the utility of in vitro and in silico methods in a regulatory context, Appl. Vitro Toxicol 3 (3) (2017) 213–226, 10.1089/aivt.2017.0010. [DOI] [Google Scholar]
- [100].Basketter D, Poole A, Kimber I, Behaviour of chemical respiratory allergens in novel predictive methods for skin sensitisation, Regul. Toxicol. Pharmacol 86 (2017) 101–106, 10.1016/j.yrtph.2017.03.002. [DOI] [PubMed] [Google Scholar]
- [101].OECD, Test No. 403: Acute Inhalation Toxicity, OECD Publishing, Paris, 2009. 10.1787/9789264070608-en. [DOI] [Google Scholar]
- [102].OECD, Test No. 436: Acute Inhalation Toxicity – Acute Toxic Class Method, OECD Publishing, Paris, 2009. 10.1787/9789264076037-en. [DOI] [Google Scholar]
- [103].OECD, Test No. 433: Acute Inhalation Toxicity: Fixed Concentration Procedure, OECD Publishing, Paris, 2018. 10.1787/9789264284166-en. [DOI] [Google Scholar]
- [104].OECD, Test No. 412: Subacute Inhalation Toxicity: 28-Day Study, OECD Publishing, Paris, 2018. 10.1787/9789264070783-en. [DOI] [Google Scholar]
- [105].OECD, Test No. 413: Subchronic Inhalation Toxicity: 90-day Study, OECD Publishing, Paris, 2018. 10.1787/9789264070806-en. [DOI] [Google Scholar]
- [106].Sewell F, Ragan I, Marczylo T, Anderson B, Braun A, Casey W, Dennison N, Griffiths D, Guest R, Holmes T, van Huygevoort T, Indans I, Kenny T, Kojima H, Lee K, Prieto P, Smith P, Smedley J, Stokes WS, Wnorowski G, Horgan G, A global initiative to refine acute inhalation studies through the use of ‘evident toxicity’ as an endpoint: towards adoption of the fixed concentration procedure, Regul. Toxicol. Pharmacol 73 (3) (2015) 770–779, 10.1016/j.yrtph.2015.10.018. [DOI] [PubMed] [Google Scholar]
- [107].Brain J, Kreyling W, Godleski J, Inhalation toxicology, in: Hayes A, Kruger C(Eds.), Hayes Princ. Methods Toxicol, CRC Press, London, 2014: pp. 1385–1444. 10.1201/b17359-32. [DOI] [Google Scholar]
- [108].Benam KH, Mazur M, Choe Y, Ferrante TC, Novak R, Ingber DE, Human lung small airway-on-a-chip protocol, in: Koledova Z (Ed.), 3D Cell Cult, Humana Press, New York, NY, 2017: pp. 345–365. 10.1007/978-1-4939-7021-6_25. [DOI] [PubMed] [Google Scholar]
- [109].Gkatzis K, Taghizadeh S, Huh D, Stainier DYR, Bellusci S, Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease, Eur. Respir. J. 52 (5) (2018) 1800876, 10.1183/13993003.00876-2018. [DOI] [PubMed] [Google Scholar]
- [110].Hiemstra PS, Grootaers G, van der Does AM, Krul CAM, Kooter IM, Human lung epithelial cell cultures for analysis of inhaled toxicants: lessons learned and future directions, Toxicol. In Vitro 47 (2018) 137–146, 10.1016/j.tiv.2017.11.005. [DOI] [PubMed] [Google Scholar]
- [111].Mittal R, Woo FW, Castro CS, Cohen MA, Karanxha J, Mittal J, Chhibber T, Jhaveri VM, Organ-on-chip models: implications in drug discovery and clinical applications, J. Cell. Physiol. 234 (6) (2019) 8352–8380, 10.1002/jcp.v234.610.1002/jcp.27729. [DOI] [PubMed] [Google Scholar]
- [112].Barosova H, Maione AG, Septiadi D, Sharma M, Haeni L, Balog S, O’Connell O, Jackson GR, Brown D, Clippinger AJ, Hayden P, Petri-Fink A, Stone V, Rothen-Rutishauser B, Use of EpiAlveolar lung model to predict fibrotic potential of multiwalled carbon nanotubes, ACS Nano 14 (4) (2020) 3941–3956, 10.1021/acsnano.9b0686010.1021/acsnano.9b06860.s001. [DOI] [PubMed] [Google Scholar]
- [113].Czekala L, Simms L, Stevenson M, Tschierske N, Maione AG, Walele T, Toxicological comparison of cigarette smoke and e-cigarette aerosol using a 3D in vitro human respiratory model, Regul. Toxicol. Pharmacol. 103 (2019) 314–324, 10.1016/j.yrtph.2019.01.036. [DOI] [PubMed] [Google Scholar]
- [114].OECD, Test No. 439 Vitro Skin Irritation: Reconstructed Human Epidermis Test Method 2020. OECD Publishing; Paris: 10.1787/9789264242845-en. [DOI] [Google Scholar]
- [115].OECD, Test No. 492: Reconstructed human Cornea-like Epithelium (RhCE) test method for identifying chemicals not requiring classification and labelling for eye irritation or serious eye damage, OECD Publishing, Paris, 2019. 10.1787/9789264242548-en. [DOI] [Google Scholar]
- [116].Neilson L, Mankus C, Thorne D, Jackson G, DeBay J, Meredith C, Development of an in vitro cytotoxicity model for aerosol exposure using 3D reconstructed human airway tissue; application for assessment of e-cigarette aerosol, Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 29 (7) (2015) 1952–1962, 10.1016/j.tiv.2015.05.018. [DOI] [PubMed] [Google Scholar]
- [117].OECD, Guidance Document On Inhalation Toxicity Studies, second, OECD Environment, Health and Safety Publications, Paris, 2018. https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2009)28/rev1&doclanguage=en. [Google Scholar]
- [118].Nielsen GD, Sensory irritation of vapours of formic, acetic, propionic and butyric acid, Regul. Toxicol. Pharmacol. 99 (2018) 89–97, 10.1016/j.yrtph.2018.09.012. [DOI] [PubMed] [Google Scholar]
- [119].Nielsen GD, Wolkoff P, Evaluation of airborne sensory irritants for setting exposure limits or guidelines: a systematic approach, Regul. Toxicol. Pharmacol. 90 (2017) 308–317, 10.1016/j.yrtph.2017.09.015. [DOI] [PubMed] [Google Scholar]
- [120].North CM, Ezendam J, Hotchkiss JA, Maier C, Aoyama K, Enoch S, Goetz A, Graham C, Kimber I, Karjalainen A, Pauluhn J, Roggen EL, Selgrade M, Tarlo SM, Chen CL, Developing a framework for assessing chemical respiratory sensitization: a workshop report, Regul. Toxicol. Pharmacol. 80 (2016) 295–309, 10.1016/j.yrtph.2016.06.006. [DOI] [PubMed] [Google Scholar]
- [121].Lalko JF, Kimber I, Gerberick GF, Foertsch LM, Api AM, Dearman RJ, The direct peptide reactivity assay: selectivity of chemical respiratory allergens, Toxicol. Sci. 129 (2012) 421–431, 10.1093/toxsci/kfs205. [DOI] [PubMed] [Google Scholar]
- [122].Dik S, Rorije E, Schwillens P, van Loveren H, Ezendam J, Can the direct peptide reactivity assay be used for the identification of respiratory sensitization potential of chemicals? Toxicol. Sci. Off. J. Soc. Toxicol. 153 (2) (2016) 361–371, 10.1093/toxsci/kfw130. [DOI] [PubMed] [Google Scholar]
- [123].Dearman RJ, Basketter DA, Kimber I, Inter-relationships between different classes of chemical allergens: Interrelationships among classes of allergen, J. Appl. Toxicol. 33 (7) (2013) 558–565, 10.1002/jat.v33.710.1002/jat.1758. [DOI] [PubMed] [Google Scholar]
- [124].Roberts DW, Schultz TW, Api AM, Chemical applicability domain of the Local Lymph Node Assay (LLNA) for skin sensitisation potency. Part 3. Apparent discrepancies between LLNA and GPMT sensitisation potential: False positives or differences in sensitivity? Regul. Toxicol. Pharmacol. 80 (2016) 260–267, 10.1016/j.yrtph.2016.07.018. [DOI] [PubMed] [Google Scholar]
- [125].Cunningham AR, Cunningham SL, Consoer DM, Moss ST, Karol MH, Development of an information-intensive structure–activity relationship model and its application to human respiratory chemical sensitizers, SAR QSAR Environ. Res 16 (3) (2005) 273–285, 10.1080/10659360500036976. [DOI] [PubMed] [Google Scholar]
- [126].Dik S, Ezendam J, Cunningham AR, Carrasquer CA, van Loveren H, Rorije E, Evaluation of in silico models for the identification of respiratory sensitizers, Toxicol. Sci. 142 (2) (2014) 385–394, 10.1093/toxsci/kfu188. [DOI] [PubMed] [Google Scholar]
- [127].Enoch SJ, Roberts DW, Madden JC, Cronin MTD, Development of an in silico profiler for respiratory sensitisation, ATLA Altern. Lab. Anim 42 (6) (2014) 367–375, 10.1177/026119291404200606. [DOI] [PubMed] [Google Scholar]
- [128].Enoch SJ, Seed MJ, Roberts DW, Cronin MTD, Stocks SJ, Agius RM, Development of mechanism-based structural alerts for respiratory sensitization hazard identification, Chem. Res. Toxicol. 25 (11) (2012) 2490–2498, 10.1021/tx3003092. [DOI] [PubMed] [Google Scholar]
- [129].Graham C, Rosenkranz HS, Karol MH, Structure-activity model of chemicals that cause human respiratory sensitization, Regul. Toxicol. Pharmacol 26 (3) (1997) 296–306, 10.1006/rtph.1997.1170. [DOI] [PubMed] [Google Scholar]
- [130].Seed MJ, Agius RM, Progress with Structure-Activity Relationship modelling of occupational chemical respiratory sensitizers, Curr. Opin. Allergy Clin. Immunol. 17 (2017) 64–71, 10.1097/ACI.0000000000000355. [DOI] [PubMed] [Google Scholar]
- [131].Warne MA, Nicholson JK, Lindon JC, Guiney PD, Gartland KPR, A QSAR investigation of dermal and respiratory chemical sensitizers based on computational chemistry properties, SAR QSAR Environ. Res. 20 (5–6) (2009) 429–451, 10.1080/10629360903278768. [DOI] [PubMed] [Google Scholar]
- [132].Jarvis J, Seed MJ, Stocks SJ, Agius RM, A refined QSAR model for prediction of chemical asthma hazard, Occup. Med 65 (8) (2015) 659–666, 10.1093/occmed/kqv105. [DOI] [PubMed] [Google Scholar]
- [133].Mekenyan O, Patlewicz G, Kuseva C, Popova I, Mehmed A, Kotov S, Zhechev T, Pavlov T, Temelkov S, Roberts DW, A mechanistic approach to modeling respiratory sensitization, Chem. Res. Toxicol. 27 (2) (2014) 219–239, 10.1021/tx400345b. [DOI] [PubMed] [Google Scholar]
- [134].Wijeyesakere SJ, Wilson DM, Settivari R, Auernhammer TR, Parks AK, Marty MS, Development of a profiler for facile chemical reactivity using the open-source Konstanz Information Miner, Appl. Vitro Toxicol 4 (2) (2018) 202–213, 10.1089/aivt.2017.0040. [DOI] [Google Scholar]
- [135].Fraunhofer ITEM, Respiratox, (2018). https://www.item.fraunhofer.de/en/press-and-media/news/respiratox.html.
- [136].Jeong J, Garcia-Reyero N, Burgoon L, Perkins E, Park T, Kim C, Roh J-Y, Choi J, Development of adverse outcome pathway for PPARγ antagonism leading to pulmonary fibrosis and chemical selection for its validation: ToxCast database and a deep learning artificial neural network model-based approach, Chem. Res. Toxicol. 32 (6) (2019) 1212–1222, 10.1021/acs.chemrestox.9b00040. [DOI] [PubMed] [Google Scholar]
- [137].Gupta S, Basant N, Singh KP, Estimating sensory irritation potency of volatile organic chemicals using QSARs based on decision tree methods for regulatory purpose, Ecotoxicology 24 (4) (2015) 873–886, 10.1007/s10646-015-1431-y. [DOI] [PubMed] [Google Scholar]
- [138].Hosoya J, Tamura K, Muraki N, Okumura H, Ito T, Maeno M, A novel approach for a toxicity prediction model of environmental pollutants by using a quantitative structure-activity relationship method based on toxicogenomics, ISRN Toxicol. 2011 (2011) 1–9, 10.5402/2011/515724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Laverty H, Benson C, Cartwright E, Cross M, Garland C, Hammond T, Holloway C, McMahon N, Milligan J, Park B, Pirmohamed M, Pollard C, Radford J, Roome N, Sager P, Singh S, Suter T, Suter W, Trafford A, Volders P, Wallis R, Weaver R, York M, Valentin J, How can we improve our understanding of cardiovascular safety liabilities to develop safer medicines? Br. J. Pharmacol. 163 (2011) 675–693, 10.1111/j.1476-5381.2011.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Weaver RJ, Valentin J-P, Today’s challenges to de-risk and predict drug safety in human “Mind-the-Gap”, Toxicol. Sci. 167 (2019) 307–321, 10.1093/toxsci/kfy270. [DOI] [PubMed] [Google Scholar]
- [141].Sirenko O, Grimm FA, Ryan KR, Iwata Y, Chiu WA, Parham F, Wignall JA, Anson B, Cromwell EF, Behl M, Rusyn I, Tice RR, In vitro cardiotoxicity assessment of environmental chemicals using an organotypic human induced pluripotent stem cell-derived model, Toxicol. Appl. Pharmacol. 322 (2017) 60–74, 10.1016/j.taap.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Prüss-Üstün A, Corvalán C, Preventing disease through healthy environments: towards an estimate of the environmental burden of disease, WHO Press, Geneva, Switzerland, 2006. https://www.who.int/quantifying_ehimpacts/publications/preventing-disease/en/. [Google Scholar]
- [143].Anakwue R, Cardiotoxicity of pesticides: are Africans at risk? Cardiovasc. Toxicol 19 (2) (2019) 95–104, 10.1007/s12012-018-9486-7. [DOI] [PubMed] [Google Scholar]
- [144].Georgiadis N, Tsarouhas K, Tsitsimpikou C, Vardavas A, Rezaee R, Germanakis I, Tsatsakis A, Stagos D, Kouretas D, Pesticides and cardiotoxicity. Where do we stand? Toxicol. Appl. Pharmacol. 353 (2018) 1–14, 10.1016/j.taap.2018.06.004. [DOI] [PubMed] [Google Scholar]
- [145].Jing L, Sun Y, Wang Y, Liang B, Chen T, Zheng D, Zhao X, Zhou X, Sun Z, Shi Z, Cardiovascular toxicity of decabrominated diphenyl ethers (BDE-209) and decabromodiphenyl ethane (DBDPE) in rats, Chemosphere 223 (2019) 675–685, 10.1016/j.chemosphere.2019.02.115. [DOI] [PubMed] [Google Scholar]
- [146].Lema SC, Schultz IR, Scholz NL, Incardona JP, Swanson P, Neural defects and cardiac arrhythmia in fish larvae following embryonic exposure to 2,2’,4,4’-tetrabromodiphenyl ether (PBDE 47), Aquat. Toxicol. Amst. Neth. 82 (4) (2007) 296–307, 10.1016/j.aquatox.2007.03.002. [DOI] [PubMed] [Google Scholar]
- [147].Alhamdow A, Lindh C, Albin M, Gustavsson P, Tinnerberg H, Broberg K, Early markers of cardiovascular disease are associated with occupational exposure to polycyclic aromatic hydrocarbons, Sci. Rep. 7 (2017) 9426, 10.1038/s41598-017-09956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Burstyn I, Kromhout H, Partanen T, Svane O, Lang??rd S, Ahrens W, Kauppinen T, St??cker I, Shaham J, Heederik D, Ferro G, Heikkil?? P, Hooiveld M, Johansen C, Randem BG, Boffetta P, Polycyclic aromatic hydrocarbons and fatal ischemic heart disease, Epidemiol. Camb. Mass 16 (6) (2005) 744–750, 10.1097/01.ede.0000181310.65043.2f. [DOI] [PubMed] [Google Scholar]
- [149].Brown AC, Heart toxicity related to herbs and dietary supplements: online table of case reports. Part 4 of 5, J. Diet. Suppl 15 (4) (2018) 516–555, 10.1080/19390211.2017.1356418. [DOI] [PubMed] [Google Scholar]
- [150].Berridge BR, Van Vleet JF, Herman E, Chapter 9 - Cardiovascular system, in: Wallig MA, Haschek WM, Rousseaux CG, Bolon B (Eds.), Fundam. Toxicol. Pathol, Academic Press, 2018: pp. 153–194. 10.1016/B978-0-12-809841-7.00009-5. [DOI] [Google Scholar]
- [151].Collins TA, Rolf MG, Pointon A, Current and future approaches to nonclinical cardiovascular safety assessment, Drug Discov. Today 25 (7) (2020) 1129–1134, 10.1016/j.drudis.2020.03.011. [DOI] [PubMed] [Google Scholar]
- [152].Cross MJ, Berridge BR, Clements PJM, Cove-Smith L, Force TL, Hoffmann P, Holbrook M, Lyon AR, Mellor HR, Norris AA, Pirmohamed M, Tugwood JD, Sidaway JE, Park BK, Physiological, pharmacological and toxicological considerations of drug-induced structural cardiac injury, Br. J. Pharmacol. 172 (4) (2015) 957–974, 10.1111/bph.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Hanton G, Preclinical cardiac safety assessment of drugs, Drugs RD 8 (4) (2007) 213–228, 10.2165/00126839-200708040-00002. [DOI] [PubMed] [Google Scholar]
- [154].Pointon A, Edmunds N, Soluble biomarkers for drug-induced cardiotoxicity, in: Carini C, Fidock M, van Gool A (Eds.), Handb. Biomark. Precis. Med, 1st ed., Chapman and Hall/CRC, New York, 2019: p. 657. 10.1201/9780429202872. [DOI] [Google Scholar]
- [155].Svensson F, Zoufir A, Mahmoud S, Afzal AM, Smit I, Giblin KA, Clements PJ, Mettetal JT, Pointon A, Harvey JS, Greene N, Williams RV, Bender A, Information-derived mechanistic hypotheses for structural cardiotoxicity, Chem. Res. Toxicol. 31 (11) (2018) 1119–1127, 10.1021/acs.chemrestox.8b00159. [DOI] [PubMed] [Google Scholar]
- [156].Yang X, Papoian T, Moving beyond the comprehensive in vitro proarrhythmia assay: use of human-induced pluripotent stem cell-derived cardiomyocytes to assess contractile effects associated with drug-induced structural cardiotoxicity: iPSC-CMs to assess contractile and structural cardiotoxicity, J. Appl. Toxicol. 38 (9) (2018) 1166–1176, 10.1002/jat.v38.910.1002/jat.3611. [DOI] [PubMed] [Google Scholar]
- [157].Gintant G, Sager PT, Stockbridge N, Evolution of strategies to improve preclinical cardiac safety testing, Nat. Rev. Drug Discov. 15 (7) (2016) 457–471, 10.1038/nrd.2015.34. [DOI] [PubMed] [Google Scholar]
- [158].Li M, Ramos LG, Drug-induced QT prolongation and torsades de pointes, Pharm. Ther 42 (2017) 473–477. [PMC free article] [PubMed] [Google Scholar]
- [159].Vicente J, Zusterzeel R, Johannesen L, Mason J, Sager P, Patel V, Matta MK, Li Z, Liu J, Garnett C, Stockbridge N, Zineh I, Strauss DG, Mechanistic model-informed proarrhythmic risk assessment of drugs: review of the “CiPA” initiative and design of a prospective clinical validation study, Clin. Pharmacol. Ther. 103 (1) (2018) 54–66, 10.1002/cpt.v103.110.1002/cpt.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].ICH, ICH E14 Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs, 2005. https://database.ich.org/sites/default/files/E14_Guideline.pdf. [PubMed]
- [161].ICH, ICH S7B Non-clinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals, 2005. https://database.ich.org/sites/default/files/S7B_Guideline.pdf. [PubMed]
- [162].Fermini B, Fossa AA, The impact of drug-induced QT interval prolongation on drug discovery and development, Nat. Rev. Drug Discov. 2 (6) (2003) 439–447, 10.1038/nrd1108. [DOI] [PubMed] [Google Scholar]
- [163].Sanguinetti MC, Jiang C, Curran ME, Keating MT, A mechanistic link between an inherited and an acquired cardiac arrhythmia: hERG encodes the IKr potassium channel, Cell 81 (1995) 299–307, 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- [164].Saxena P, Zangerl-Plessl E-M, Linder T, Windisch A, Hohaus A, Timin E, Hering S, Stary-Weinzinger A, New potential binding determinant for hERG channel inhibitors, Sci. Rep. 6 (2016) 24182, 10.1038/srep24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Trudeau M, Warmke J, Ganetzky B, Robertson G, HERG, a human inward rectifier in the voltage-gated potassium channel family, Science 269 (5220) (1995) 92–95, 10.1126/science:7604285. [DOI] [PubMed] [Google Scholar]
- [166].Kratz JM, Grienke U, Scheel O, Mann SA, Rollinger JM, Natural products modulating the hERG channel: heartaches and hope, Nat. Prod. Rep. 34 (8) (2017) 957–980, 10.1039/C7NP00014F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Kramer J, Obejero-Paz CA, Myatt G, Kuryshev YA, Bruening-Wright A, Verducci JS, Brown AM, MICE models: superior to the hERG model in predicting torsade de pointes, Sci. Rep. 3 (2013) 2100, 10.1038/srep02100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Mirams GR, Cui Y, Sher A, Fink M, Cooper J, Heath BM, McMahon NC, Gavaghan DJ, Noble D, Simulation of multiple ion channel block provides improved early prediction of compounds’ clinical torsadogenic risk, Cardiovasc. Res. 91 (2011) 53–61, 10.1093/cvr/cvr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Cavero I, Holzgrefe H, Comprehensive in vitro proarrhythmia assay, a novel in vitro/in silico paradigm to detect ventricular proarrhythmic liability: a visionary 21st century initiative, Expert Opin. Drug Saf. 13 (2014) 745–758, 10.1517/14740338.2014.915311. [DOI] [PubMed] [Google Scholar]
- [170].Colatsky T, Fermini B, Gintant G, Pierson JB, Sager P, Sekino Y, Strauss DG, Stockbridge N, The Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative – Update on progress, J. Pharmacol. Toxicol. Methods 81 (2016) 15–20, 10.1016/j.vascn.2016.06.002. [DOI] [PubMed] [Google Scholar]
- [171].Li Z, Ridder BJ, Han X, Wu WW, Sheng J, Tran PN, Wu M, Randolph A, Johnstone RH, Mirams GR, Kuryshev Y, Kramer J, Wu C, Crumb WJ, Strauss DG, Assessment of an in silico mechanistic model for proarrhythmia risk prediction under the CiPA initiative, Clin. Pharmacol. Ther. 105 (2019) 466–475, 10.1002/cpt.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Sager PT, Gintant G, Turner JR, Pettit S, Stockbridge N, Rechanneling the cardiac proarrhythmia safety paradigm: a meeting report from the cardiac safety research consortium, Am. Heart J. 167 (3) (2014) 292–300, 10.1016/j.ahj.2013.11.004. [DOI] [PubMed] [Google Scholar]
- [173].Vicente J, Zusterzeel R, Johannesen L, Ochoa-Jimenez R, Mason JW, Sanabria C, Kemp S, Sager PT, Patel V, Matta MK, Liu J, Florian J, Garnett C, Stockbridge N, Strauss DG, Assessment of multi-ion channel block in a phase I randomized study design: results of the CiPA phase I ECG biomarker validation study, Clin. Pharmacol. Ther. 105 (2019) 943–953, 10.1002/cpt.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].ICH, ICH E14/S7B IWG Work Plan, 2020. https://database.ich.org/sites/default/files/Revised_E14%28S7B%29_IWG_Work%20Plan_2020_0430.pdf.
- [175].ICH, Final Concept Paper ICH S7B and E14 Q&A, 2018. https://database.ich.org/sites/default/files/E14S7B_IWG_Concept_Paper.pdf.
- [176].Vargas HM, Rolf MG, Wisialowski TA, Achanzar W, Bahinski A, Bass A, Benson CT, Chaudhary KW, Couvreur N, Dota C, Engwall MJ, Michael Foley C, Gallacher D, Greiter-Wilke A, Guillon J, Guth B, Himmel HM, Hegele-Hartung C, Ito M, Jenkinson S, Chiba K, Lagrutta A, Levesque P, Martel E, Okai Y, Peri R, Pointon A, Qu Y, Teisman A, Traebert M, Yoshinaga T, Gintant GA, Leishman DJ, Valentin J, Time for a fully integrated nonclinical-clinical risk assessment to streamline QT prolongation liability determinations: a pharma Industry perspective, Clin. Pharmacol. Ther. 109 (2) (2021) 310–318, 10.1002/cpt.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Ronaldson-Bouchard K, Vunjak-Novakovic G, Organs-on-a-chip: a fast track for engineered human tissues in drug development, Cell Stem Cell. 22 (3) (2018) 310–324, 10.1016/j.stem.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Zhang B, Korolj A, Lai BFL, Radisic M, Advances in organ-on-a-chip engineering, Nat. Rev. Mater 3 (8) (2018) 257–278, 10.1038/s41578-018-0034-7. [DOI] [Google Scholar]
- [179].Zuppinger C, 3D cardiac cell culture: a critical review of current technologies and applications, Front. Cardiovasc. Med. 6 (2019) 87, 10.3389/fcvm.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Archer CR, Sargeant R, Basak J, Pilling J, Barnes JR, Pointon A, Characterization and validation of a human 3D cardiac microtissue for the assessment of changes in cardiac pathology, Sci. Rep. 8 (2018) 10160, 10.1038/s41598-018-28393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Krishna S, Berridge B, Kleinstreuer N, High-throughput screening to identify chemical cardiotoxic potential, Chem. Res. Toxicol. 34 (2) (2021) 566–583, 10.1021/acs.chemrestox.0c00382. [DOI] [PubMed] [Google Scholar]
- [182].Burton J, Worth AP, Tsakovska I, Diukendjieva A, In silico models for acute systemic toxicity, in: Benfenati E (Ed.), Silico Methods Predict. Drug Toxic, Springer, New York, NY, 2016: pp. 177–200. 10.1007/978-1-4939-3609-0_11. [DOI] [PubMed] [Google Scholar]
- [183].Munawar S, Windley MJ, Tse EG, Todd MH, Hill AP, Vandenberg JI, Jabeen I, Experimentally validated pharmacoinformatics approach to predict hERG inhibition potential of new chemical entities, Front. Pharmacol. 9 (2018), 10.3389/fphar.2018.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Wacker S, Noskov SY, Performance of machine learning algorithms for qualitative and quantitative prediction drug blockade of hERG1 channel, Comput. Toxicol. 6 (2018) 55–63, 10.1016/j.comtox.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Zhou X, Qu Y, Passini E, Bueno-Orovio A, Liu Y, Vargas HM, Rodriguez B, Blinded in silico drug trial reveals the minimum set of ion channels for Torsades de Pointes risk assessment, Front. Pharmacol. 10 (2020) 1643, 10.3389/fphar.2019.01643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Braga RC, Alves VM, Silva MFB, Muratov E, Fourches D, Lião LM, Tropsha A, Andrade CH, Pred-hERG: a novel web-accessible computational tool for predicting cardiac toxicity, Mol. Inform 34 (10) (2015) 698–701, 10.1002/minf.201500040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Du-Cuny L, Chen L, Zhang S, A critical assessment of combined ligand- and structure-based approaches to hERG channel blocker modeling, J. Chem. Inf. Model 51 (11) (2011) 2948–2960, 10.1021/ci200271d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Schaefer M, Schmidt F, Czich A, Guillon J-M, Ballet V, Rampe D, Kang J, Bohme A, Matter H, Amberg A, Application of the CiPA in silico model in early drug research: validation results for different drug development phases, Toxicol. Suppl. Toxicol. Sci. 174 (1) (2020) Abstract #2965. https://www.toxicology.org/pubs/docs/Tox/2020Tox.pdf. [Google Scholar]
- [189].Cai C, Fang J, Guo P, Wang Q, Hong H, Moslehi J, Cheng F, In silico pharmacoepidemiologic evaluation of drug-induced cardiovascular complications using combined classifiers, J. Chem. Inf. Model 58 (5) (2018) 943–956, 10.1021/acs.jcim.7b00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Frid AA, Matthews EJ, Prediction of drug-related cardiac adverse effects in humans-B: use of QSAR programs for early detection of drug-induced cardiac toxicities, Regul. Toxicol. Pharmacol. 56 (3) (2010) 276–289, 10.1016/j.yrtph.2009.11.005. [DOI] [PubMed] [Google Scholar]
- [191].Sharifi M, Buzatu D, Harris S, Wilkes J, Development of models for predicting Torsade de Pointes cardiac arrhythmias using perceptron neural networks, BMC Bioinform. 18 (2017) 497, 10.1186/s12859-017-1895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Duan R, Zhang X, Du J, Huang J, Tao C, Chen Y, On the evidence consistency of pharmacovigilance outcomes between Food and Drug Administration Adverse Event Reporting System and electronic medical record data for acute mania patients, Health Inform. J 26 (2) (2020) 753–764, 10.1177/1460458219833093. [DOI] [PubMed] [Google Scholar]
- [193].Hemmerich J, Ecker GF, In silico toxicology: from structure–activity relationships towards deep learning and adverse outcome pathways, WIREs Comput. Mol. Sci 10 (4) (2020), 10.1002/wcms.v10.410.1002/wcms.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Cronin MTD, Madden JC, Yang C, Worth AP, Unlocking the potential of in silico chemical safety assessment – A report on a cross-sector symposium on current opportunities and future challenges, Comput. Toxicol. Amst. Neth. 10 (2019) 38–43, 10.1016/j.comtox.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Elmore SA, Cardiff R, Cesta MF, Gkoutos GV, Hoehndorf R, Keenan CM, McKerlie C, Schofield PN, Sundberg JP, Ward JM, A review of current standards and the evolution of histopathology nomenclature for laboratory animals, ILAR J. 59 (2018) 29–39, 10.1093/ilar/ily005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Keenan CM, Goodman DG, Regulatory Forum commentary: through the looking glass–SENDing the pathology data we have INHAND, Toxicol. Pathol 42 (5) (2014) 807–810, 10.1177/0192623313485451. [DOI] [PubMed] [Google Scholar]
- [197].Alexander-Dann B, Pruteanu LL, Oerton E, Sharma N, Berindan-Neagoe I, Módos D, Bender A, Developments in toxicogenomics: understanding and predicting compound-induced toxicity from gene expression data, Mol. Omics 14 (4) (2018) 218–236, 10.1039/C8MO00042E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Ganter B, Snyder RD, Halbert DN, Lee MD, Toxicogenomics in drug discovery and development: mechanistic analysis of compound/class-dependent effects using the DrugMatrix ® database, Pharmacogenomics 7 (7) (2006) 1025–1044, 10.2217/14622416.7.7.1025. [DOI] [PubMed] [Google Scholar]
- [199].Igarashi Y, Nakatsu N, Yamashita T, Ono A, Ohno Y, Urushidani T, Yamada H, Open TG-GATEs: a large-scale toxicogenomics database, Nucleic Acids Res. 43 (2015) D921–D927, 10.1093/nar/gku955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Davis AP, Grondin CJ, Johnson RJ, Sciaky D, King BL, McMorran R, Wiegers J, Wiegers TC, Mattingly CJ, The comparative toxicogenomics database: update 2017, Nucleic Acids Res. 45 (D1) (2017) D972–D978, 10.1093/nar/gkw838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].IARC, Tumour site concordance and mechanisms of carcinogenesis, WHO Press, Switzerland, 2019. https://publications.iarc.fr/_publications/media/download/5592/a0d74b9a500b62a4fb6d2ee6c2926b54b82cb9dc.pdf. [PubMed] [Google Scholar]
- [202].Smith MT, Guyton KZ, Kleinstreuer N, Borrel A, Cardenas A, Chiu WA, Felsher DW, Gibbons CF, Goodson WH, Houck KA, Kane AB, La Merrill MA, Lebrec H, Lowe L, McHale CM, Minocherhomji S, Rieswijk L, Sandy MS, Sone H, Wang A, Zhang L, Zeise L, Fielden M, The key characteristics of carcinogens: relationship to the hallmarks of cancer, relevant biomarkers, and assays to measure them, Cancer Epidemiol, Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 29 (10) (2020) 1887–1903, 10.1158/1055-9965.EPI-19-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, DeMarini DM, Caldwell JC, Kavlock RJ, Lambert PF, Hecht SS, Bucher JR, Stewart BW, Baan RA, Cogliano VJ, Straif K, Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis, Environ. Health Perspect. 124 (6) (2016) 713–721, 10.1289/ehp.1509912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Ahearn A, Key characteristics: a new approach to identifying potential toxicants, with Martyn Smith, Podcasts Res. Perspect. 2019 (1) (2019), 10.1289/EHP5776. [DOI] [Google Scholar]
- [205].Rusyn I, Arzuaga X, Cattley RC, Corton JC, Ferguson SS, Godoy P, Guyton KZ, Kaplowitz N, Khetani SR, Roberts R, Roth RA, Smith MT, Key characteristics of human hepatotoxicants as a basis for identification and characterization of the causes of liver toxicity, Hepatology (2021) hep.31999, 10.1002/hep.31999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


