Abstract
Purpose:
Decision aids have been found to improve patients’ knowledge of treatments and decrease decisional regrets. Despite these benefits, there is not widespread use of decision aids for newly diagnosed prostate cancer (PCa). This analysis investigates factors that impact men’s choice to use a decision aid for newly diagnosed prostate cancer.
Materials and Methods:
This is a retrospective analysis of a PCa registry from the Michigan Urological Surgery Improvement Collaborative (MUSIC). We included data from men with newly diagnosed, clinically localized PCa seen from 2018-21 at practices offering a PCa decision aid (Personal Patient Profile-Prostate; P3P). The primary outcome was men’s registration to use P3P. We fit a multilevel logistic regression model with patient-level factors and included urologist specific random intercepts. We estimated the intra-class correlation (ICC) and predicted the probability of P3P registration among urologists.
Results:
A total of 2629 men were seen at practices that participated in P3P and 1174 (45%) registered to use P3P. Forty-one percent of the total variance of P3P registration was attributed to clustering of men under a specific urologist’s care. In contrast, only 1.5% of the variance of P3P registration was explained by patient factors. Our model did not include data on socioeconomic, literacy or psychosocial factors, which limits the interpretation of the results.
Conclusions:
These results suggest that urologists’ effect far outweighs patient factors in a man’s decision to enroll in P3P. Strategies that encourage providers to increase decision aid adoption in their practices are warranted.
Keywords: Decision aid, Decision Making, Shared, Urologic Disease, Communication, Patient-Centered Care, Prostatic Neoplasms, Urologists, Decision Support Techniques, Health Services Research
INTRODUCTION
Shared decision making is the process of balancing the best available evidence with patients’ preferences and values to reach a medical decision.1 Decision aids are tools that facilitate shared decision making. High quality evidence suggests that decision aid use leads to increased patient knowledge, more accurate risk perceptions, and alignment between care options and patients’ values. 2 For these reasons, guidelines recommend that clinicians engage in shared decision making with patients facing localized prostate cancer (PCa) treatment decisions. 3,4
Considerable effort has been devoted to creating and disseminating PCa treatment decision aids over the past thirty years.5 Despite these efforts, the rates of regular use of shared decision making & decision aid use beyond research settings in PCa is uncertain.6 While recent self-reported data of urologists suggest high uptake and use, these data have yet to be corroborated with clinical practice patterns, a divergence that has been seen in other fields.7-9 Further, there is little clarity regarding which patient or clinician factors are associated with their use in routine practice settings. Measuring and understanding factors which influence the use of shared decision making and decision aids is an essential step to effective implementation.10
The Michigan Urological Surgery Improvement Collaborative (MUSIC) is a physician-led consortium of urology practices across the state of Michigan focused on improving the quality and cost efficiency of urologic care. In 2018, MUSIC implemented a novel pilot initiative through the Movember Foundation and True North (TrueNTH USA) to use the Personal Patient Profile-Prostate (P3P), a web-based, prostate cancer decision aid. 11-15 MUSIC maintains prospectively collected data regarding P3P use at the patient, urologist and practice level. The aim of this study was to assess the factors that were associated with patients’ registration to use P3P. We also explore variations in practice and clinician use of decision aids. Previous research has documented barriers and facilitators to decision aid use at the clinic, physician and patient level.16,17 We hypothesize that there will be variation in use of P3P within our cohort based on these factors.16,17 This study adds to the current literature by providing pragmatic data from a varied set of practices across a state-wide collaborative.
METHODS
Data Source:
This study used MUSIC data collected between May 2018 and February 2021. MUSIC includes about 90% of urologists within Michigan and captures data that represents urologic practices with varied characteristics which serve diverse patient communities. MUSIC’s data acquisition and quality control has been previously described.18,19 In brief, MUSIC collects patient demographics, health status, clinical characteristics, and data regarding treatments and their outcomes into a central registry. Each MUSIC urology practice obtained regulatory exemption or approval for participation from a local institutional review board. This study was deemed exempt by the institutional review board.
Study Population:
The study cohort consisted of patients with newly diagnosed, clinically localized (T1-T2N0M0), very low, low, or intermediate risk PCa who participated in MUSIC at the 13 practices that adopted the P3P initiative. Patients younger than 40, older than 90 years, and those missing Gleason scores or with Gleason scores less than 6 were excluded.
Practice data:
Data regarding the characteristics of individual urology practices that registered prostate cancer patients into MUSIC were analyzed. This included the number of MUSIC-participating urologists, number of patients registered into MUSIC annually, whether practices are academic (defined as having a residency training program), and location type (metropolitan or rural)
Decision aid data:
P3P is a widely tested, web-based decision aid designed to educate and prepare newly diagnosed PCa patients for conversations about their treatment options 11-14. P3P includes a questionnaire, tailored patient education and a summary report. The questionnaire comprises of a disease-specific health-related quality of life assessment for localized prostate cancer (Expanded Prostate Cancer Index Composite, EPIC-26) and questions about patients’ readiness to make a decision, decisional control preferences, and the degree to which the patients’ health status, concerns and relationships influence treatment decisions. Based on their responses, patients are guided through tailored video and text material about PCa care and survivorship. Patients are provided with a summary report and coaching tools to guide their treatment discussions with their physicians. A standalone version of P3P is available at P3P4me.org.
MUSIC implemented P3P in May 2018. Practices that elected to join the P3P initiative received individual site-visit training from MUSIC. Participating urologists determined which MUSIC patients were appropriate for P3P. A trained clinic employee offered these patients voluntary registration into P3P. Patients accessed the P3P tool via a personalized web link sent to their email address. For those unable to complete P3P in advance (i.e., those without access to email or internet), an in-office tablet was provided to access P3P on the day of their appointment.
Analysis:
The primary outcome was to assess factors associated with patient registration to use P3P. The secondary outcome was to describe differences between practices that participated in P3P or not. For the primary outcome, we limited our analysis to men from practices participating in the P3P initiative and patient data obtained after practices had implemented P3P. A multivariable mixed-effects logistic regression model was used to identify patient-level factors associated with P3P use. Patient-level factors included race, age, ethnicity, family history of PCa, pre-treatment prostate specific antigen, Gleason score and clinical stage. The model included random intercepts for each urologist to account for within-physician correlation (intraclass correlation, ICC), since the choice of P3P participation is likely to be influenced by physician preference. The intraclass correlation represents the degree of correlation in the outcome of interest (registration to use P3P) between patients nested under a urologist. The intraclass correlation was computed from the model to estimate the impact of a urologist on patients’ registration into P3P (urologist’s effect). Higher intraclass correlation values indicate that patients seen by the same urologist would have similar rates of registration into P3P. The adjusted probability of P3P registration was estimated among urologists who treated more than 25 patients, visualized graphically in a caterpillar plot.
For the secondary outcome, factors were evaluated that contributed to practice-level registration in the P3P initiative. Practices were dichotomized into those who have joined P3P versus not at 24 months after the P3P initiative began. This was treated as a binary choice as opposed to time-to-event since data collection initiatives, site visits, and site recruitment efforts occurred at differing times over the 24-month period between practices. The 24-month time point was selected to ensure that all MUSIC practices who had the intention to join the P3P initiative had sufficient time to go through the on-boarding process and join P3P by the time of data analysis. Due to the limited sample size, bivariate analyses were used to assess the impact of practice-level factors on P3P participation.
Fisher’s exact tests for categorical factors and Wilcoxon rank-sum tests for continuous measures were used to examine differences in practice size (number of urologists), patient volume, location and participation in prior MUSIC initiatives. All calculations were performed in STATA version 16. The level of significance was pre-specified at 5%.
RESULTS
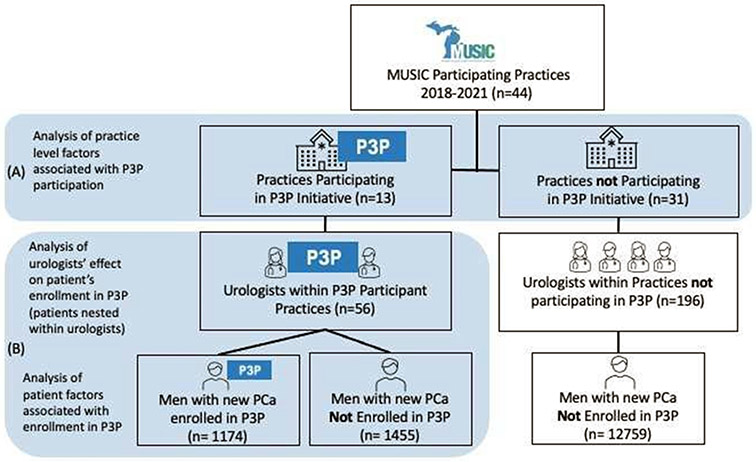
During the study period, 15,418 patients with localized PCa were registered into the MUSIC registry among 44 MUSIC practices comprising 252 urologists (Figure 1). After exclusions for age younger than 40 (n=8), older than 90 (n=6), missing Gleason scores (n=12) or Gleason scores less than 6 (n=4), 15,388 remained. Of these, 2629 (17%) patients were seen among the 13 practices (comprising 53 urologists) that participated in the P3P initiative. Of these patients, 1174 (45%) registered to use P3P. Table 1 describes patient demographics and PCa attributes among patients by registration status. A larger proportion of P3P-users identified as African American (10% vs 8%, p<0.001) and non-Hispanic (79% vs 24%, p=0.03) than those who did not register. More patients with a family history of PCa registered to use P3P (30% vs 22%, p=0.001). Registered patients tended to have lower severity of disease as demonstrated by lower PSA scores (5.7 vs 6.1, p<0.001), a higher proportion of NCCN low risk group (36% vs 29%, p < 0.001), and higher proportion of Gleason 6 (41% vs 33%, p<0.001) disease. (Table 1)
Figure 1: Depiction of Sample and Sampling frame.
(A) At the practice level, practices that participated in P3P are compared to practices who elected not to participate in P3P initiative. (B) Among patients seen within P3P participating practices, we assess patient factors associated with registration into P3P while controlling for nesting of patients under specific urologists.
Table 1:
Patient Demographic and clinical characteristics by P3P participation and registration status
| Men with PCa registered within MUSIC |
Men within P3P participatory Practices |
|||||
|---|---|---|---|---|---|---|
| Men seen at P3P practices |
Men seen at practices NOT participating in P3P |
Registered to use P3P |
Not Registered to use P3P |
|||
| n=2,629 | n=12,759 | p-value | n=1,174 | n=1,455 | p-value | |
| Age at PCa Diagnosis (mean, SD) | 65.0 (7.4) | 65.5 (7.7) | 0.002 | 64.7 (7.2) | 65.3 (7.5) | 0.03 |
| Race (n, %) | <0.001 | <0.001 | ||||
| African American | 225 (8.6) | 1,702 (13) | 116 (10) | 109 (7.5) | ||
| Caucasian | 1,862 (71) | 9,970 (78) | 853 (73) | 1,009 (69) | ||
| Other | 42 (1.6) | 302 (2.4) | 12 (1.0) | 30 (2.1) | ||
| Unknown/Declined | 500 (19.) | 785 (6.1) | 193 (16) | 307 (21) | ||
| Ethnicity (n, %) | <0.001 | 0.03 | ||||
| Non-Hispanic | 2,010 (77) | 11,221 (88) | 923 (79) | 1,087 (75) | ||
| Hispanic | 33 (1.3) | 136 (1.1) | 17 (1.4) | 16 (1.1) | ||
| Unknown/Declined | 586 (22) | 1,402 (11) | 234 (20) | 352 (24) | ||
| Family History of PCa (n, %) | 675 (26) | 4,221 (33) | <0.001 | 351 (30) | 324 (22) | <0.001 |
| PSA at PCa Diagnosis (median, IQR) | 6.0 (4.7-7.9) | 5.9 (4.6-8.1) | 0.5 | 5.7 (4.61-7.6) | 6.1 (4.8-8.2) | 0.001 |
| NCCN risk group (n, %) | ||||||
| Intermediate | 1,791 (68) | 8,850 (69) | 0.2 | 756 (64) | 1,035 (71) | <0.001 |
| Stage (TNM) (n, %) | 0.01 | 0.2 | ||||
| T1a-T1c, TX | 2,208 (84) | 10,533 (83) | 985 (84) | 1,223 (84) | ||
| T2, T2x-T2c | 378 (14) | 2,127 (17) | 181 (15) | 197 (14) | ||
| Grade Group (n, %)* | 0.02 | <0.001 | ||||
| 1 | 953 (36) | 4,456 (35) | 477 (33) | 476 (41) | ||
| 2 | 1,165 (44) | 5,519 (43) | 682 (47) | 483 (41) | ||
| 3 | 511 (19) | 2,784 (22) | 296 (20) | 215 (18) | ||
PCa: Prostate Cancer; PSA: Prostate Specific Antigen; P3P: Personal Patient Profile for Prostate; TNM - Tumor Nodes Metastasis stage, only patients with T1-T2N0M0 disease included; NCCN: National Comprehensive Cancer Network; SD: Standard Deviation
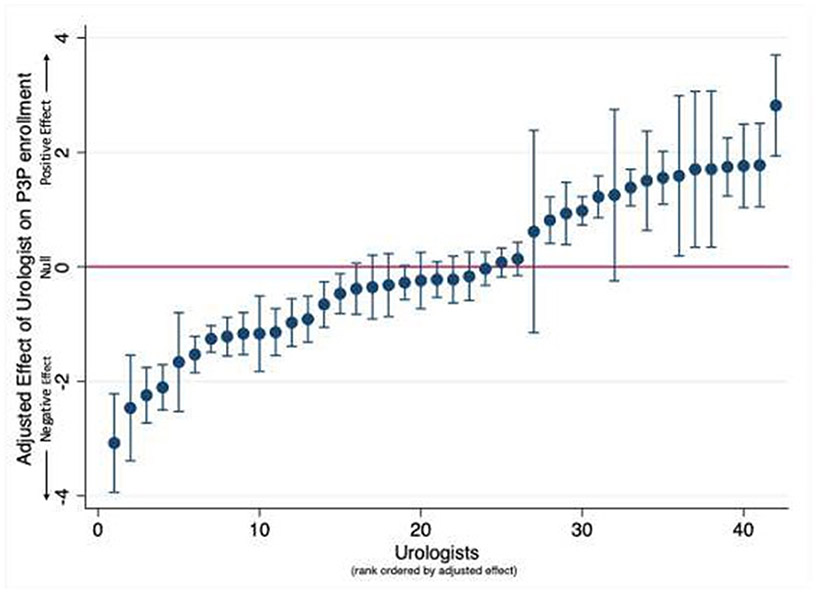
Within the multilevel logistic regression model 41% of the total variance of P3P registration was attributed to clustering of patients under a specific urologist (ICC = 0.41). In contrast, only 1.5% of the variance of P3P registration (R2 = 0.015) was explained by patient-level factors. There were no clinically significant associations between patient characteristics and registration to use P3P (Supplemental Figure 1). The multilevel logistic regression model was used to estimate the adjusted probability of P3P registration among patients of urologists who treated more than 25 patients. The resultant caterpillar plot showed significant variation in the probability of patient P3P registration between urologists (Figure 2).
Figure 2: Adjusted Probability of Registration into Prostate Cancer Decision Aid (Caterpillar Plot).
Each point represents a urologist. Data is rank ordered based on point estimate of the adjusted probability, error bars denote standard error. Urologists with less than 25 patients in the sample were excluded.
On bivariate analysis, practices participating had similar characteristics to those not participating, except for metropolitan setting (77% vs 48% participating vs not, respectively), a difference that did not achieve statistical significance (Supplemental Table 1).
DISCUSSION
This study of a prostate cancer treatment decision aid (P3P) used in a statewide urologic collaborative showed that patients seen by the same urologist tend to make similar decisions on whether to register to use the decision aid. No clinically meaningful patient factors contributed to whether patients enrolled in P3P. Taken together, this shows that urologists have a significant impact on decision aid implementation and use by patients.
While we did not investigate reasons for variation in clinician decision aid use, prior studies have shown that physicians have profound effects on patient decision making and decision aid use. 17,20,21 In a recent study of the implementation of P3P, verbal physician instruction followed by email and telephone invitations was associated with the highest rates of patient access to P3P, much higher than providing written materials alone. 17 These findings are consistent with prior studies showing that physician sentiments towards decision aids influence whether patients would use a decision aid. 20 Data from our study supports the notion that physician engagement is central to implementation of these aids.
The differences in clinicians’ perspectives on use of decision aids in urology may explain the variation in urologists’ effect on their use. 7,15,22 On multivariable analyses of data from the American Urological Association’s Annual Census, we found that urologists’ level of training, practice environment, and clinical volume influenced self-reported shared decision making and decision aid use.7 In a similar study of Dutch practitioners caring for patients with metastatic castrate resistant PCa, de Angst et al. found that less than half of urologists stated that a decision aid is appropriate for treatment decisions when patients have a choice between two or more treatment options.22 Qualitative surveys of physicians in practices using P3P within MUSIC found that over ⅓ of urologists felt that decision aids were not necessary, echoing the sentiment that provider skepticism of decision aid use is an ongoing barrier to implementation.15 This suggests that individual physicians' engagement is central to implementation of decision aids.
The concept of physician engagement has been recently defined as “regular participation of physicians in (1) deciding how their work is done, (2) making suggestions for improvement, (3) goal setting, (4) planning, and (5) monitoring of their own performance in activities targeted at the micro (patient), meso (organization), and/or macro (health system) levels”.23 Our study finds physician engagement at the patient and organization level to be critical to patients’ use of P3P. At the organizational level, we noted that there was low physician engagement, with only 13 of 46 MUSIC practices choosing to offer P3P to their patients, despite considerable support and encouragement from MUSIC. At the patient level, we find significant variation between urologists regarding their effect on patient registration to use P3P, implying differences in physician engagement. Data comparing organization versus health system implementation has found a higher level of PCa decision aid use with execution at the health systems (macro) level. 24
Several publications have evaluated how to increase physician engagement in quality improvement initiatives, including shared decision making. 10,25 A narrative review outlined two barriers to physician engagement in quality improvement: 1) quality may be defined in different ways by different clinicians and 2) clinicians may not feel that quality of care needs to be improved.26 These themes are echoed as barriers to shared decision making use in ours and prior studies. One reason for the divergence in physician engagement with decision aids may be that despite strong evidence to support that decision aids improve several patient centered metrics (decisional regret, knowledge), these metrics may not align with outcomes that clinicians traditionally value (health outcomes).7,15,25 Efforts to evaluate the impact of shared decision making and decision aid use on health outcomes beyond decisional impact may increase clinician engagement.
Our study has potential limitations that warrant comment. First, while the prospectively maintained database included demographic and health characteristics, it did not include information on several patient factors that could impact decision aid registration and have been found to influence decision aid use, such as psychosocial, socioeconomic and literacy factors. 9,27-29 Future efforts to regularly collect and incorporate additional patient and provider factors into research may aid in a comprehensive understanding of decision aid use. Another limitation is that the overall participation of practices in the decision aid initiative was low and therefore participating urologists may represent a unique subset that cannot be generalized to larger populations of clinicians. However, beyond metropolitan setting, practice level characteristics were similar between participating and non-participating practices. In addition, patient enrollment rates were within range of prior literature regarding PCa decision aid use. 24
Our findings provide pragmatic information for policymakers and health systems designing methods to implement decision aids into regular clinical practice; engaging individual physicians may be a critical part of decision aid implementation. This underscores the message that a urologist has significant influence on a patient’s decision making, even on the patient’s choice to use a decision aid. This concept is one that patients and caregivers may find beneficial to understand as they navigate prostate cancer treatment decisions.
CONCLUSION
Urologists are key facilitators in patient registration into a decision aid for prostate cancer treatment.
While decision aids are steadily gaining traction in medicine, there is a long way to go before such tools become a mainstay in clinical practice, especially in urology. Future research focused on increasing integration of decision aids in routine clinical practice should evaluate factors that motivate clinicians to adopt decision aids.
Supplementary Material
Supplemental Table 1: Practice characteristics stratified by participation in MUSIC prostate cancer treatment decision aid initiative.
Supplemental Figure 1: Patient Factors associated with registration in the prostate cancer decision aid, P3P. Results from multilevel logistic regression model adjusting for clustering within urologists.
Funding:
NIDDK Grant F32 DK126232, The Michigan Urological Surgery Improvement Collaborative (MUSIC) is funded by Blue Cross Blue Shield of Michigan. The Movember Foundation and True North (TrueNTH) USA provided funding for the P3P initiative.
Key of Abbreviations
- ICC
Interclass Correlation
- MUSIC
Michigan Urological Surgery Improvement Collaborative
- NCCN
National Comprehensive Cancer Network
- P3P
Personal Patient Profile-Prostate
- PCa
Prostate Cancer
Footnotes
This data has been accepted in abstract form to be presented at the 2021 American Urological Association Annual Meeting.
REFERENCES
- 1.United States. President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research: Making Health Care Decisions: The Ethical and Legal Implications of Informed Consent in the Patient-practitioner Relationship. President’s Commission for the Study of; 1982. Available at: https://play.google.com/store/books/details?id=dFdfcgReXlgC. [Google Scholar]
- 2.Stacey D, Légaré F, Lewis K, et al. : Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst. Rev 2017; 4: CD001431. Available at: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anon: Prostate cancer: Clinically localized guideline - American urological association. Available at: https://www.auanet.org/guidelines/prostate-cancer-clinically-localized-guideline, accessed March 2, 2021.
- 4.Mottet N, van den Bergh RCN, Briers E, et al. : EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol 2021; 79: 243–262. Available at: 10.1016/j.eururo.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 5.Violette PD, Agoritsas T, Alexander P, et al. : Decision aids for localized prostate cancer treatment choice: systematic review and meta-analysis. CA Cancer J. Clin 2015; 65: 239–251. Available at: https://onlinelibrary.wiley.com/doi/abs/10.3322/caac.21272. [DOI] [PubMed] [Google Scholar]
- 6.Makarov DV, Chrouser K, Gore JL, et al. : AUA White Paper on Implementation of Shared Decision Making into Urological Practice. Urology Practice 2016; 3: 355–363. Available at: http://www.sciencedirect.com/science/article/pii/S2352077915002733. [DOI] [PubMed] [Google Scholar]
- 7.Lane GI, Ellimoottil C, Wallner L, et al. : Shared Decision-making in Urologic Practice: Results From the 2019 AUA Census. Urology 2020; 145: 66–72. Available at: 10.1016/j.urology.2020.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin GA, Halley M, Rendle KAS, et al. : An effort to spread decision aids in five California primary care practices yielded low distribution, highlighting hurdles. Health Aff. 2013; 32: 311–320. Available at: 10.1377/hlthaff.2012.1070. [DOI] [PubMed] [Google Scholar]
- 9.Graham ID, Logan J, Bennett CL, et al. : Physicians’ intentions and use of three patient decision aids. BMC Med. Inform. Decis. Mak 2007; 7: 20. Available at: 10.1186/1472-6947-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Quality Forum: National Quality Partners Playbook TM: Shared Decision Making in Healthcare.; 2018. Available at: https://store.qualityforum.org/products/national-quality-partners-playbook%E2%84%A2-shared-decision-making.
- 11.Berry DL, Halpenny B, Hong F, et al. : The Personal Patient Profile-Prostate decision support for men with localized prostate cancer: a multi-center randomized trial. Urol. Oncol 2013; 31: 1012–1021. Available at: 10.1016/j.urolonc.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry DL, Hong F, Blonquist TM, et al. : Decision Support with the Personal Patient Profile-Prostate: A Multicenter Randomized Trial. J. Urol 2018; 199: 89–97. Available at: 10.1016/j.juro.2017.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry DL, Wang Q, Halpenny B, et al. : Decision preparation, satisfaction and regret in a multi- center sample of men with newly diagnosed localized prostate cancer. Patient Educ. Couns 2012; 88: 262–267. Available at: 10.1016/j.pec.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry DL, Halpenny B, Wolpin S, et al. : Development and evaluation of the personal patient profile-prostate (P3P), a Web-based decision support system for men newly diagnosed with localized prostate cancer. J. Med. Internet Res 2010; 12: e67. Available at: https://www.jmir.org/2010/4/e67/?utm_source=twitterfeed&utm_medium=twitter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paudel R, Ferrante S, Woodford J, et al. : Implementation of prostate cancer treatment decision aid in Michigan: a qualitative study. Implement Sci Commun 2021; 2: 27. Available at: 10.1186/s43058-021-00125-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gravel K, Légaré F and Graham ID: Barriers and facilitators to implementing shared decision- making in clinical practice: a systematic review of health professionals’ perceptions. Implement. Sci 2006; 1: 16. Available at: 10.1186/1748-5908-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry DL, Hong F, Halpenny B, et al. : Evaluating clinical implementation approaches for prostate cancer decision support. Urology Practice 2019; 6: 93–99. Available at: https://www.auajournals.org/doi/abs/10.1016/j.urpr.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Womble PR, Linsell SM, Gao Y, et al. : A Statewide Intervention to Reduce Hospitalizations after Prostate Biopsy. J. Urol 2015; 194: 403–409. Available at: 10.1016/j.juro.2015.03.126. [DOI] [PubMed] [Google Scholar]
- 19.Womble PR, Montie JE, Ye Z, et al. : Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur. Urol 2015; 67: 44–50. Available at: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Tong WT, Ng CJ, Lee YK, et al. : What will make patients use a patient decision aid? A qualitative study on patients’ perspectives on implementation barriers and facilitators. J. Eval. Clin. Pract 2020; 26: 755–764. Available at: 10.1111/jep.13161. [DOI] [PubMed] [Google Scholar]
- 21.Leishman NJ, Wiener RS, Fagerlin A, et al. : Variation in Eligible Patients’ Agreeing to and Receiving Lung Cancer Screening: A Cohort Study. Am. J. Prev. Med 2021; 60: 520–528. Available at: 10.1016/j.amepre.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Angst IB, Kil PJM, Bangma CH, et al. : Should we involve patients more actively? Perspectives of the multidisciplinary team on shared decision-making for older patients with metastatic castration-resistant prostate cancer. J. Geriatr. Oncol 2019; 10: 653–658. Available at: 10.1016/j.jgo.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Perreira TA, Perrier L, Prokopy M, et al. : Physician engagement: a concept analysis. J Healthc Leadersh 2019; 11: 101–113. Available at: 10.2147/JHL.S214765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stacey D, Taljaard M, Breau RH, et al. : A Patient Decision Aid for Men With Localized Prostate Cancer: A Comparative Case Study of Natural Implementation Approaches. Cancer Nurs. 2020; 43: E10–E21. Available at: 10.1097/NCC.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 25.Légaré F, Adekpedjou R, Stacey D, et al. : Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst. Rev 2018; 7: CD006732. Available at: 10.1002/14651858.CD006732.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies H, Powell A and Rushmer R: Healthcare professionals’ views on clinician engagement in quality improvement. A literature review 2007; 4. Available at: https://www.health.org.uk/sites/default/files/HealthcareProfessionalsViewsClinicianEngagementQualityImprovement.pdf. [Google Scholar]
- 27.Delanoë A, Lépine J, Turcotte S, et al. : Role of Psychosocial Factors and Health Literacy in Pregnant Women’s Intention to Use a Decision Aid for Down Syndrome Screening: A Theory-Based Web Survey. J. Med. Internet Res 2016; 18: e283. Available at: 10.2196/jmir.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomko C, Davis KM, Luta G, et al. : A comparison of web-based versus print-based decision AIDS for prostate cancer screening: participants’ evaluation and utilization. J. Gen. Intern. Med 2015; 30: 33–42. Available at: 10.1007/s11606-014-2994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Partin MR, Nelson D, Flood AB, et al. : Who uses decision aids? Subgroup analyses from a randomized controlled effectiveness trial of two prostate cancer screening decision support interventions. Health Expect. 2006; 9: 285–295 Available at: 10.1111/j.1369-7625.2006.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Practice characteristics stratified by participation in MUSIC prostate cancer treatment decision aid initiative.
Supplemental Figure 1: Patient Factors associated with registration in the prostate cancer decision aid, P3P. Results from multilevel logistic regression model adjusting for clustering within urologists.