Abstract
Hypoglycaemia is a complication associated with the management of both type 1 and type 2 diabetes. Despite newer technologies to help minimize the risk of hypoglycaemia, it remains a barrier for some patients to achieve optimal glycaemic control. In this review, the definitions and risk factors for hypoglycaemia will be briefly discussed and an in-depth review of the management for a conscious or unconscious patient in the outpatient and inpatient settings is provided. Rapid-acting glucose is the preferred treatment for a conscious patient regardless of the setting. For an unconscious patient, glucagon is preferred if the patient does not have intravenous (IV) access and dextrose can be used for patients with IV access. Until recently, there was only one formulation of glucagon, which had limitations due to the multiple steps required for reconstitution prior to administration in an emergency setting. This review also discusses the different glucagon formulations currently available for the management of hypoglycaemia.
Keywords: diabetes mellitus, glucagon, glucose, hypoglycaemia, management, treatment
Introduction
Hypoglycaemia is a scary and potentially life-threatening complication associated with diabetes management. It is a major limiting factor for the management of both type 1 diabetes (T1D) and type 2 diabetes (T2D), which could potentially interfere with the patient’s ability to achieve control of their diabetes.1 The PANORAMA study found that, for patients with T2D, experiencing any severe or non-severe hypoglycaemia episode strongly predicted further fear and worry of hypoglycaemia.2 A systemic review evaluating patients with T1D found a significant positive association between hypoglycaemia and negative psychological outcomes, which ranged from a greater fear of hypoglycaemia for both severe and self-treated hypoglycaemia to diabetes distress and reduced general emotional well-being 6–24 months following episodes of severe hypoglycaemia.3 Balancing glycaemic control to reduce the risk of microvascular disease and minimizing the risk for hypoglycaemia is an important element for the safe and effective management of diabetes. Intensive insulin therapy is often associated with hypoglycaemic events; however, there are also high-risk oral medications used in T2D management associated with hypoglycaemia (sulfonylureas, meglitinides). It is estimated that patients with T1D experience severe hypoglycaemia approximately once a year with an average of two to three episodes of symptomatic hypoglycaemia per week.4 Reported episodes of severe hypoglycaemia in T1D range from 115 to 320 per 100 patient years and range from 35 to 70 per 100 patient-years for T2D.5 Despite significant advances in diabetes management to help reduce the incidence of hypoglycaemia, such as continuous glucose monitoring (CGM), individualized patient glycaemic goals and new agents for T2D with minimal risk of hypoglycaemia, the risk of hypoglycaemia is still present. Although individual patient risk factors and different medication regimens are associated with varying risks of hypoglycaemia, occurrence and risk should be assessed at each follow-up appointment. If hypoglycaemia occurs, action should be taken to mitigate future risk.
Hypoglycaemia is reversed with either rapid-acting glucose or glucagon. The American Diabetes Association (ADA) Standards of Care recommends oral glucose as the preferred treatment for all conscious patients with hypoglycaemia, defined as blood glucose <70 mg/dL (3.9 mmol/L). It also states that individuals who are at risk for significant hypoglycaemia, defined as blood glucose <54 mg/dL (3.0 mmol/L), should be prescribed glucagon to have readily available if needed. In addition to educating the patient regarding use and administration of glucagon, an emphasis should be placed on the importance of education to caregivers, school personnel, or family members in case the patient develops neuroglycopenic symptoms and requires assistance.1
Prior to 2019, there was only one formulation for glucagon available. Since 2019, there have been easier to use, more stable formulations of glucagon that are now available in varying dosage forms and administration routes.6–10 The purpose of this article is to review the risk factors, symptoms, management, and prevention of hypoglycaemia. Specifically, provide an updated review for the management of hypoglycaemia in a conscious and unconscious patient, describe the recommendations in the community setting compared to an inpatient setting, and describe the different glucagon products available.
Methods
A PubMed search was performed with Clinical Queries through September 21, 2021, using the key term “hypoglycemia”. The search strategy included meta-analyses, randomized controlled trials, clinical trials, observational studies and reviews. The search was restricted to English literature.
Review
Definition of hypoglycaemia
The classification of hypoglycaemia varies slightly across diabetes organizations as does the definition of hypoglycaemia used in clinical trials. In some instances, hypoglycaemia is defined strictly on the plasma blood glucose value whilst, in others, it is driven by the severity of symptoms the patient experiences or the level of care needed to address the hypoglycaemic episode (e.g. requiring emergency medical service or hospitalization). The variation has posed challenges when comparing the safety and tolerability of various medication regimens across clinical trials. In response, the International Hypoglycaemia Study Group was formed to standardize and validate the definition of hypoglycaemia and to raise awareness on the importance of hypoglycaemia and its effects on clinicians’ ability to effectively manage diabetes. Their proposed criteria (Table 1) have been adopted by the ADA, the European Association for the Study of Diabetes and the European Medicines Agency. These definitions have since been validated in a series of clinical trials and will be referenced for this review.11
Table 1.
Classification of hypoglycaemia.
| Glycaemic criteria or scenario | |
|---|---|
| Level 1 | Blood glucose <70 mg/dL (3.9 mmol/L) and ≥54 mg/dL (3.0 mmol/L) |
| Level 2 | Blood glucose <54 mg/dL (3.0 mmol/L) |
| Level 3 | A hypoglycaemic event accompanied by altered mental status and/or that requires physical assistance for treatment of hypoglycaemia |
Adapted from the American Diabetes Association.1
Even in the absence of symptoms, a blood glucose concentration of <70 mg/dL (3.9 mmol/L) should be considered of clinical significance as this is the recognized threshold that elicits a neuroendocrine response. At level 2 hypoglycaemia (blood glucose concentration <54 mg/dL; 3.0 mmol/L), neuroglycopenic symptoms (e.g. confusion, sensation of warmth, weakness or fatigue, impaired cognition) begin to occur and require prompt treatment. Finally, a level 3 hypoglycaemic event is defined not by any blood glucose concentration value but by physical or mental symptoms severe enough to require assistance from another person.1,11
It should be noted that there is a range of symptoms associated with hypoglycaemia and the degree at which patients may notice symptoms is specific for each person. Common symptoms include hunger, fatigue, shakiness, dizziness, confusion, irritability and diaphoresis. Severe consequences include loss of consciousness, seizure, coma or even death. Hypoglycaemia can be particularly problematic in patients who experience an onset of neuroglycopenia before the onset of autonomic warning symptoms, which is known as hypoglycaemia unawareness. Hypoglycaemia unawareness occurs in up to 40% of patients with T1D and is more common in patients with long-standing diabetes, advanced age, history of frequent hypoglycaemic episodes and intense medication regimens.12 Conversely, some patients may instead experience symptoms of hypoglycaemia despite blood glucose concentrations well above 70 mg/dL (3.9 mmol/L). These episodes are most often noted in patients with sustained high baseline blood glucose concentration and hypoglycaemia symptoms present as blood glucose normalizes. These episodes are not associated with neuroglycopenia and do not pose risks of patient harm.
Risk factors for hypoglycaemia
Risk factors for non-severe hypoglycaemia are difficult to quantify due to inadequate reporting from patients as non-severe hypoglycaemia is often self-managed. Nevertheless, a fairly consistent predictor of hypoglycaemic risk in both T1D and T2D is a history of a prior severe or non-severe hypoglycaemic episode.13–15 In two US claims-based studies, a history of hypoglycaemia was strongly associated with an additional episode of hypoglycaemia, requiring an escalation of care upon recurrence.16,17
With increasing age, noticeable symptoms of hypoglycaemia may become less evident or manifest differently.15,18 Older patients may be at a higher risk due to alterations in the counterregulatory response to hypoglycaemic events, where glucagon and epinephrine responses are diminished during mild hypoglycaemia.19 Lifestyle factors also play an important role in hypoglycaemia amongst T1D and T2D and across paediatric, adolescent and adult populations. Delayed eating, fewer carbohydrate-containing meals and snacks, skipped meals, and an increase in exercise patterns or higher intensity exercise can result in a decrease of blood glucose concentration that can also potentiate hypoglycaemia.15,20–22
Medication use for the treatment of both T1D and T2D can increase the risk of hypoglycaemia. Insulin and insulin secretagogues, such as sulfonylureas and meglitinides, pose the highest risk. Of the sulfonylureas, those with a longer half-life and active metabolites are associated with the highest risk (glyburide, 21.3%). In the presence of additional factors, such as renal impairment and advanced age, even the sulfonylureas thought to pose less risk can still result in serious, even potentially fatal, hypoglycaemic consequences (glipizide, 3.4%; glimepiride, 4–19.7%).23,24 All insulin types have the potential to cause hypoglycaemia but some forms may pose a greater risk. As compared to the insulin analogues glargine and detemir, NPH (isophane) insulin and pre-mixed insulins have been associated with higher rates of hypoglycaemia (67% hypoglycaemia; 5.1% severe hypoglycaemia).23,25–29 Similar rates of hypoglycaemia are described with regular, short-acting insulins, which are more dangerous than the rapid-acting insulin analogues, such as lispro, aspart and glulisine, as it relates to hypoglycaemia.23,30,31 Medication-related hypoglycaemia risk is a modifiable risk factor to a certain extent, but does pose additional challenges in patients with T1D, where insulin regimens are required. Side effects or cost of other medications that do not pose hypoglycaemic risk may also prove to be a barrier when considering alternate medication regimens.
Additional risk factors that have been noted include hypoglycaemia unawareness, longer duration of diabetes and a higher comorbidity burden, particularly chronic kidney disease.13,32–34
Management for an alert patient
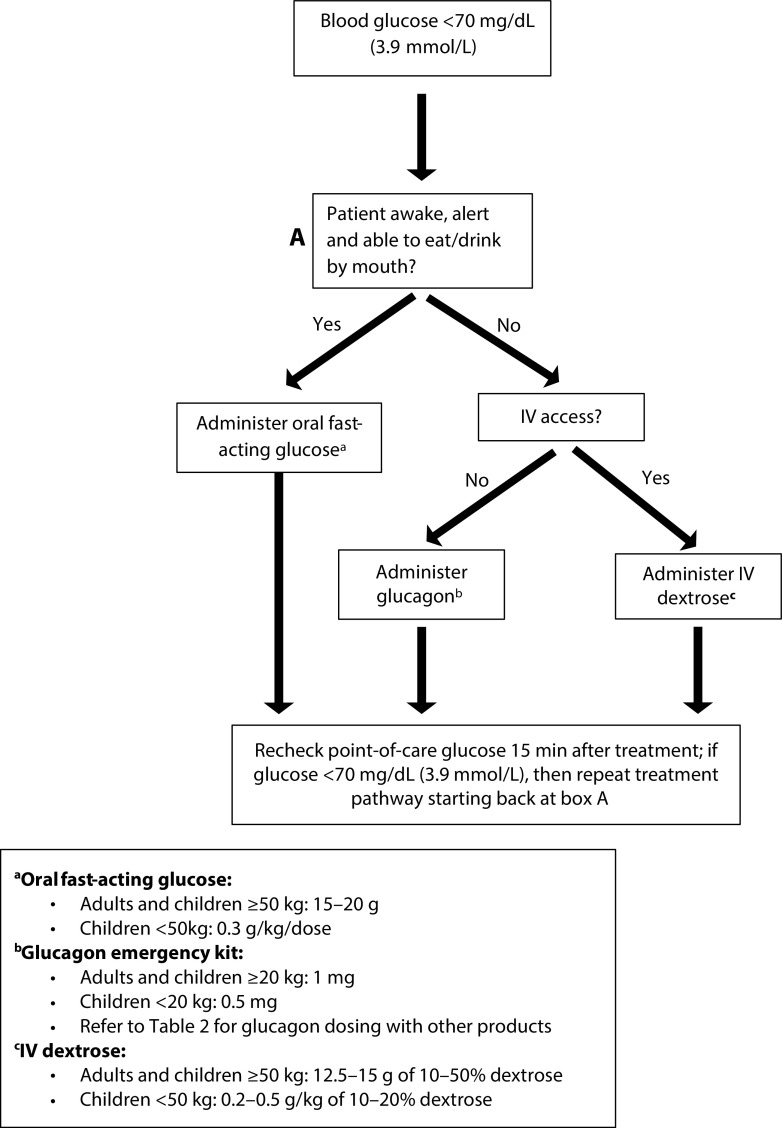
The comprehensive management of hypoglycaemia (Figure 1) is first dependent on the mental status of the patient. In the case of an alert patient, fast-acting carbohydrates are the mainstay for treatment of a hypoglycaemic event and should be initiated at any blood glucose concentration ≤70 mg/dL (3.9 mmol/L). There are hundreds of forms of fast-acting carbohydrates and sugars that can be used to effectively manage hypoglycaemia. In one systematic review and meta-analysis, the blood glucose response to various types of dietary sugars, including sucrose, fructose, orange juice, jelly beans, Mentos, corn-starch hydrolysate, skittles and milk, was compared to that induced by glucose tablets. There was no difference in the comparison of individual outcomes. However, in the pooled analysis of all dietary sugars compared to glucose tablets, a higher proportion of patients experienced relief of a hypoglycaemic episode with glucose tablets (RR 0.89, 95% CI 0.83–0.96).35 These results suggest that glucose tablets infer greater symptom resolution and should be the preferred treatment option, when available.
Figure 1. Hypoglycaemia management.
Few studies have evaluated the appropriate dose of carbohydrate to treat a hypoglycaemic episode. For adults, 15 g of fast-acting carbohydrates (e.g. 3–4 glucose tablets, 4 ounces of juice or non-diet soda, 1 tablespoon of sugar, 6–8 hard candies) is commonly recommended by practitioners to treat a hypoglycaemic episode in a conscious patient. After 15 minutes, the blood glucose concentration should be re-tested and the 15 g of carbohydrate repeated, if necessary, until the blood glucose concentration is >70 mg/dL (3.9 mmol/L). There are data to suggest that higher doses of carbohydrates (e.g. 20 g) and potentially a weight-based approach of 0.3 g/kg may be more effective at resolving hypoglycaemia.36,37
For children, data extrapolated from adult trials suggests that a dose of 0.3 g/kg should result in a similar increase in blood glucose.22 A randomized trial of children (aged 8–12 years) with T1D evaluated the effect on blood glucose when hypoglycaemic children were administered 0.3 g/kg of glucose tablets, orange juice, jelly beans or sugar mints. The study found that this dose effectively resolved hypoglycaemia in most children though those treated with jelly beans had the slowest glycaemic response.38 Similar to adults, the blood glucose concentration should be retested after 15 minutes, and the dose of carbohydrates repeated until blood glucose is >70 mg/dL (3.9 mmol/L).22 Patients and/or their parents or caregivers should be counselled on the importance of recognizing early hypoglycaemia signs and symptoms and how to appropriately treat in a timely manner. Whilst pure glucose is the preferred treatment option, any glucose-containing carbohydrate is a reasonable treatment option. Patients should be counselled to avoid treatment with foods high in fat or protein content until blood glucose returns to >70 mg/dL (3.9 mmol/L) as these can impair the blood glucose response. Once the blood glucose returns to normal a meal or snack is recommended to prevent blood glucose from decreasing again.1
Management for an unconscious ambulatory patient
Glucagon is the first-line and only approved treatment for severe hypoglycaemia in a person out of the hospital with impaired consciousness who is unable to administer fast-acting carbohydrates orally. Glucagon is a 29-amino acid chain peptide secreted by the α-cells of the pancreas. It is an important counterregulatory hormone to the insulin that stimulates hepatic glycogenolysis and gluconeogenesis, which raises blood glucose concentrations.6–10,39 Recently, additional glucagon options have been approved, including a new glucagon analogue, a stable glucagon solution and a formulation available for intranasal administration (Table 2).
Table 2.
| Generic name (proprietary name) | Reconstituted glucagon | Glucagon solution (Gvoke™) | Dasiglucagon (Zegalogue®) | Intranasal glucagon (Baqsimi™) | |
|---|---|---|---|---|---|
| Glucagen® | Glucagon emergency kit | ||||
| Approved age | All ages | ≥2 years | ≥6 years | ≥4 years | |
| Route | IM, SubQ, IVa | SubQ | SubQ | Intranasal | |
| Age or weight-based dose adjustments | Yes | Yes | No | No | |
| Dosage | 0.5 mg, 1 mg | 0.5 mg, 1 mg | 0.6 mg | 3 mg | |
| Administration | <25 kg or <6 years of unknown weight:
|
<20 kg:
|
<45 kg or <12 years:
|
0.6 mg injection; repeat after 15 minutes if no response using new device | One actuation of the intranasal device into one nostril; if no response, repeat in 15 minutes using a new device |
| Storage |
|
|
|
Up to 30°C in the shrink-wrapped tube provided | |
| How supplied | Kit containing 1-mg single-dose vial of glucagon and one prefilled syringe containing sterile water | Kit containing 1-mg glucagon for injection in a single-dose vial and one prefilled syringe containing diluent | Single-dose auto-injector or prefilled syringe containing either 0.5 mg/0.1 mL or 1 mg/0.2 mL solution | Single-dose auto-injector or prefilled syringe containing 0.6 mg/0.6 mL solution | Baqsimi one pack (one intranasal device) Baqsimi two pack (two intranasal devices) |
Only under medical supervision.
IM, intramuscular; IV, intravenous; SubQ, subcutaneous.
In general, glucagon has a rapid onset of action and has been shown to correct hypoglycaemia in about 10–20 minutes and is similar between formulations and routes of administration. It is well tolerated though nausea and vomiting are the most commonly reported adverse events with all the formulations. All glucagon formulations are contraindicated in patients with known hypersensitivity, pheochromocytoma or insulinoma.6–10
After glucagon is administered, emergency medical services should be contacted as soon as possible, and the unconscious patient should be turned on their side to reduce the risk of choking or aspiration if vomiting occurs. If the patient does not respond after 15 minutes and emergency medical services have not arrived, another dose of glucagon should be administered. Once the patient is awake and able to swallow, they should take fast-acting glucose and then, similar to the conscious patient, eat a meal or snack to prevent recurrent hypoglycaemia.6–10
Injectable glucagon
Reconstituted glucagon
Reconstituted glucagons for injection (GlucaGen® HypoKit® and Glucagon Emergency Kit) are similar formulations of glucagon that are unstable in an aqueous solution. These are supplied as a lyophilized powder and a diluent in a prefilled syringe, which requires reconstitution prior to administration.7,8,40 Both can be stored at room temperature up to 2 years from the manufacture date if not reconstituted, though once reconstituted it needs to be used immediately.7,8 This was the only available glucagon product for many years, though use was limited due to the multiple steps required during an emergency, including dosing (based on weight and age), preparation (reconstituting and drawing up the solution accurately) and administration. Several studies have shown a low rate of full and accurate intramuscular (IM) dosing by caregivers and family members despite training. One simulation study had only 13% of caregivers and 0% of acquaintances administer the full dose of glucagon.41 Another evaluated 136 parents and found that, despite over 85% of them receiving previous training by a diabetes educator or specialist, 69% had difficulties with the kit, which ranged from opening the container to drawing up the correct dose, and 4% did not administer any glucagon due to only water or air being injected.42 Despite difficulty with use, when administered correctly, treatment response was quick, with increases in plasma glucose observed within 10 minutes after administration, and this is the standard of care to which newer agents are compared.7,8
Stable glucagon (reconstitution not required)
Glucagon solution
Glucagon solution by auto-injection (Gvoke HypoPen™) and prefilled syringe (Gvoke™ PFS) is a liquid stable, non-aqueous formulation of native glucagon. It is stabilized in a dimethyl sulfoxide-based solvent that allows it to remain at room temperature for up to 2 years after the date of manufacture.9,43 The auto-injector glucagon solution can be administered in a few simple steps. This device allows for decreased drug preparation times compared to reconstituted glucagon (0.79 minutes for glucagon solution versus 1.76 minutes for reconstituted glucagon (p<0.001)); however, once injected, time to resolution of hypoglycaemia is similar between products (15.69 minutes for glucagon solution versus 15.32 for reconstituted glucagon).9,44 Results from a comparative usability study showed that 88% (14/16) of participants were able to successfully administer the glucagon autoinjector solution compared with only 31% (5/16) of participants administering the reconstituted glucagon (p<0.05).45 In a phase III non-inferiority study, 99.2% of participants in the glucagon autoinjector group versus 100% of participants in the reconstituted glucagon group met the primary endpoint of plasma glucose >70 mg/dL (3.9 mmol/L) or an increase of ≥20 mg/dL (1.1 mmol/L) within 30 minutes after study drug administration, proving non-inferiority for the glucagon autoinjector device.44
Dasiglucagon
Dasiglucagon (Zegalogue®) is available as both an autoinjector and prefilled syringe. It is a stable soluble peptide analogue of human glucagon consisting of 29 amino acids with 7 amino acid substitutions relative to native glucagon, which improves its chemical and physical stability. It is meant to be stored in the refrigerator but can be safely stored at room temperature for up to 12 months.10,46 In a randomized, double blind, placebo-controlled trial comparing dasiglucagon 0.6 mg with glucagon 1 mg and placebo, the median time to plasma glucose recovery (defined as an increase in serum glucose by ≥20 mg/dL (1.1 mmol/L) from baseline) was similar between medications and significantly better compared to placebo (10 minutes with dasiglucagon versus 12 minutes with glucagon versus 40 minutes with placebo).47 Similar results were found when dasiglucagon was studied in paediatric and adolescent patients aged between 6 and 17 years with T1D.48
Non-injectable glucagon
Intranasal glucagon
Intranasal glucagon (Baqsimi™) is the first needle-free glucagon option for the treatment of severe hypoglycaemia in patients aged 4 years and older. Intranasal glucagon is absorbed through the nasal mucosa and the dose does not need to be inhaled.6 Nasal congestion or the use of a nasal decongestant did not significantly impact the pharmacokinetic or pharmacodynamic parameters.6,49 This can be stored at room temperature for up to 2 years from the manufacture date and must remain in the shrink-wrap tube.6 After administering the intranasal glucagon 3 mg dose, blood glucose increases within 5 minutes and peak plasma concentration is reached within 15–20 minutes for adults and children above 4 years of age.6,50,51 Intranasal glucagon was found to be non-inferior to IM glucagon in the comparison studies for both paediatric and adult patients, with successful treatment in over 98% of hypoglycaemic events. In the paediatric study, 58 of 59 (98.3%) patients receiving intranasal glucagon achieved treatment success, defined by a ≥25 mg/dL (1.4 mmol/L) increase in plasma glucose within 20 minutes after administration of glucagon.50 In an adult comparison trial, 74 of 75 (98.7%) patients receiving intranasal glucagon achieved treatment success, which was defined as an increase of ≥20 mg/dL (1.1 mmol/L) from glucose nadir or an increase in plasma glucose ≥70 mg/dL (3.9 mmol/L) within 30 minutes of receiving glucagon.51
Inpatient management
In hospitalized adult patients, hypoglycaemia is associated with an increase in both short-term and long-term mortality.52 Hypoglycaemia can be seen in hospitalized patients with or without diabetes and is a consequence of dysregulated metabolism and/or diabetes treatment. Due to the potentially severe and life-threatening nature of hypoglycaemia, it is imperative to initiate treatment as soon as it is identified. Hypoglycaemia management protocols should be implemented within each hospital or hospital system and should detail prevention and treatment plans.53 These protocols should be nurse-driven and standardized to ensure prompt evidence-based care is provided to all patients at risk of hypoglycaemia. Additionally, protocol development should be interdisciplinary and include, at minimum, physicians, nurses, pharmacists and diabetes educators.
Hypoglycaemia management for hospitalized children and adolescents follows a similar treatment algorithm to adults with the primary difference being weight-based dosing for children. The initial management of hypoglycaemia in a hospitalized patient depends on the severity of hypoglycaemia, whether the patient has intravenous (IV) access, and if they can take food and/or drink by mouth. If the patient is alert/oriented/responsive and taking food/drink by mouth, they should be administered oral fast-acting carbohydrates as described in the previous section (15–20 g for adults ≥50 kg; 0.3 g/kg for children <50 kg).53 If a patient is unresponsive, has neuroglycopenic symptoms, or is unable/unwilling to take food or drink by mouth, they must be treated with either IV dextrose or glucagon. Dextrose is preferred for those with IV access as glucagon is more costly, can cause nausea and vomiting, which would be dangerous in an unconscious or altered patient, and will not be as effective in patients with depleted glycogen stores.54–56 A few studies from the late 80s and early 90s compared IV dextrose with IM glucagon in adults and found that blood glucose response was quicker with IV dextrose once the treatment had been administered, though the time to obtain an IV line is not accounted for.57–59
For adults, any IV dextrose concentration from 10% to 50% can be used for hypoglycaemia treatment. Higher concentrations of dextrose (25% or 50% solutions) are more irritating to the veins when administered peripherally and extravasation can cause tissue necrosis. Lower concentrations of dextrose (10–15% solutions) are less irritating to the veins; however, it would require a larger volume and may put certain patients (i.e. those with congestive heart failure, renal failure) at risk for volume overload.54,60 A randomized controlled trial comparing IV dextrose 10% (D10W) with 50% (D50W) in patients with hypoglycaemia treated by paramedics out of hospital, there was no difference in median time to recovery (8 minutes) or in median post-treatment Glasgow Coma Scores between groups. Although the patients in the D10W group received significantly less IV dextrose overall compared with the D50W group and post-treatment blood glucose levels were also significantly lower with D10W. This study suggests that D10 may have a lower risk of overshooting hyperglycaemia.61 Dextrose concentrations <10% should be avoided for acute hypoglycaemia treatment as the volume required for an adequate dose is too large and may lead to delays in treatment. Typically, a starting bolus dose of 12.5–15 g of IV dextrose (equivalent to 25–30 mL of D50W or 125–150 mL of D10W) is recommended and can be administered by slow IV push over 3–5 minutes. Should the treatment need to be repeated due to continued hypoglycaemia, higher doses of 25 g can be used. In rare cases, continuous dextrose infusions may be needed, especially if a patient has severe hypoglycaemia secondary to excess basal insulin or use of a sulfonylurea. Overtreating hypoglycaemia risks rebound hyperglycaemia, which can cause endogenous insulin secretion and recurrent hypoglycaemia.62
If a patient requires non-oral treatment for hypoglycaemia when IV access is not already established, then glucagon must be used until IV access can be obtained. A single dose of glucagon 1 mg should be administered, either IM or subcutaneously (SQ), and the patient should be turned on their side to prevent aspiration in case the medication induces vomiting.63 Both routes of administration are comparable and will result in a similar increase in blood sugar concentration. Maximum plasma concentrations of glucagon after the IM injection occurs on average about 7 minutes sooner compared with SQ injections.7–9
For children, treatment of hypoglycaemia using dextrose concentrations >20% are typically avoided due to the risk for extravasation and tissue necrosis (when administered peripherally). The recommend initial bolus dose of dextrose 10–20% is 0.2–0.5 g/kg (max dose: 25 g).22 Similar to adults, IM or SQ glucagon should be used in children when IV access has not yet been established. The dose of glucagon depends on the device being used though, for the reconstituted formulations, children <20–25 kg should be administered 0.5 mg and those weighing above that should be given 1 mg.7,8 See Table 2 for paediatric dosing of various glucagon formulations.
Regardless of the treatment administered, blood glucose should be rechecked within 15 minutes and, if it is still <70 mg/dL (3.9 mmol/L), then the treatment should be repeated until blood sugar is >70 mg/dL (3.9 mmol/L) and stable. Once the patient is no longer hypoglycaemic, the cause of the event should be determined, and appropriate changes must be made. If the event was caused secondary to insulin, then the basal and/or bolus insulin doses should be decreased based on the time of day when the event occurred. If a patient was administered oral diabetes medications with a high risk for causing hypoglycaemia such as sulfonylureas or meglitinides, then they should be held for the remainder of their admission or until their risk of hypoglycaemia has diminished.63,64
Prevention/patient education
Minimizing risk and preventing episodes of hypoglycaemia is an important part of diabetes management and ensuring quality of life for the patient. Assessing a patient’s overall risk for hypoglycaemia and mitigating any modifiable risk factors should be a part of the decision process when determining a treatment plan for a patient. When medications associated with hypoglycaemia risk are prescribed, patients and their caregivers should be counselled on the signs and symptoms, treatment strategies to raise blood glucose quickly, and the balance between the medication with diet and exercise. Patients should also be aware of situations that can increase the risk of hypoglycaemia such as fasting or delay in meals, alcohol and intense exercise.1 At each appointment, episodes of hypoglycaemia, the glucose level at the time of the event, symptoms associated with hypoglycaemia and how it was treated should be assessed. High-risk medications should be adjusted or discontinued, if possible, based on glucose patterns to balance minimizing overall risk with optimal glycaemic control.65 Where there is a compelling need to minimize hypoglycaemia, the ADA preferentially recommends the use of dipeptidyl peptidase 4 (DPP4) inhibitors, glucagon-like peptide 1 (GLP1) receptor agonists, sodium–glucose cotransporter 2 (SGLT2) inhibitors, and/or thiazolidinediones over insulin agents or sulfonylureas.
For high-risk patients, either due to non-modifiable risk factors or the need for medications associated with hypoglycaemia, adjusting blood glucose and A1c goals should be considered to ensure patient safety. For patients at high risk, frequent self-monitoring of blood glucose or CGM is essential to help identify patterns and quickly adjust regimens. The ADA Standards of Care recommend that CGM devices be considered for any patient with diabetes that requires insulin management. There are two general categories of CGM devices, those that display glucose levels continuously (real-time CGM) and those that only display glucose readings when scanned (intermittently scanned CGM).66 Significant reductions in hypoglycaemia are seen in adults with T1D using either multiple daily insulin injections or insulin pump devices. These studies have shown that real-time CGM devices are particularly useful in T1D patients with hypoglycaemia unawareness or frequent hypoglycaemic episodes.67–69 The data for hypoglycaemia reduction in patients with T2D are less robust, though one study using an intermittently scanned CGM device showed that patients with T2D on a variety of insulin regimens spent 43% less time in the hypoglycaemic range.70 Any patient prescribed a CGM device requires extensive patient education to ensure it is being used safely and effectively.
Conclusion
Hypoglycaemia is a potentially life-threatening medical emergency that can occur when managing T1D and T2D. Despite ways to mitigate the overall risk of hypoglycaemia, such as adjusting glycaemic goals, choosing medications with lower risk of hypoglycaemia when possible, and utilizing CGM technology, unfortunately for some patients, that is not enough. At each follow-up appointment, a patient’s overall hypoglycaemic risk should be assessed and appropriate counselling should be provided. All patients at high risk for hypoglycaemia should have glucagon available. Prior to prescribing a glucagon product, a discussion should take place to determine the preferred glucagon formulation based on device and administration to ensure timely treatment of a hypoglycaemic event. Family members, friends and caregivers should know where the glucagon is stored and how to administer this in the event of an emergency in the outpatient, community setting. For inpatient management, all hospitals should have a detailed protocol, developed by an interdisciplinary team, to allow for prompt management of a hypoglycaemic emergency.
Key practice points
Hypoglycaemia is considered a medical emergency and needs immediate treatment
Level 1 hypoglycaemia is defined as a blood glucose <70 mg/dL (3.9 mmol/L); level 2 hypoglycaemia is defined as <54 mg/dL (3.0 mmol/L); and level 3 hypoglycaemia is defined as any event accompanied by altered mental status or the need for assistance in hypoglycaemia treatment
Risk factors for hypoglycaemia may include older age, longer duration of diabetes, irregular eating patterns, and use of certain medications such as insulin products and sulfonylureas
Alert patient management: 15 g of fast-acting carbohydrates (e.g. 3–4 glucose tablets, 4 ounces of juice or non-diet soda, 1 tablespoon of sugar, 6–8 hard candies)
Unconscious patient management: glucagon (no IV access) or dextrose (for inpatient with IV access)
All patients: after 15 minutes, if the patient is not responsive or blood glucose is not >70 mg/dL (3.9 mmol/L), repeat with the initial treatment
Once the patient is conscious and blood glucose is >70 mg/dL (3.9 mmol/L), patient should eat a meal or snack to help maintain blood glucose
All patients at high risk for hypoglycaemia should have glucagon available; product device and administration should be discussed to determine preferred glucagon product
Medication options with lower risk of hypoglycaemia should be utilized, where appropriate, in patients with recurrent hypoglycaemia
Continuous glucose monitoring devices should be considered for any patient with diabetes that requires insulin management to help achieve A1C treatment goals and reduce episodes of hypoglycaemia
Acknowledgements
None.
Footnotes
Contributions: All authors contributed equally to the preparation of this manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/02/dic.2021-9-11-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2022 Lowe RN, Williams B, Claus LW. https://doi.org/10.7573/dic.2021-9-11. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/diabetes-how-to-manage-patients-experiencing-hypoglycaemia
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes – 2021. Diabetes Care. 2021;44(Suppl 1):S73–S84. doi: 10.2337/dc21-S006. [DOI] [PubMed] [Google Scholar]
- 2.Bradley C, Eschwège E, de Pablos-Velasco P, et al. Predictors of quality of life and other patient-reported outcomes in the PANORAMA multinational study of people with type 2 diabetes. Diabetes Care. 2018;41(2):267–276. doi: 10.2337/dc16-2655. [DOI] [PubMed] [Google Scholar]
- 3.Chatwin H, Broadley M, Speight J, et al. The impact of hypoglycaemia on quality of life outcomes among adults with type 1 diabetes: a systematic review. Diabetes Res Clin Pract. 2021;174:108752. doi: 10.1016/j.diabres.2021.108752. [DOI] [PubMed] [Google Scholar]
- 4.Cryer PE, David SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 5.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.BaqsimiTM (glucagon nasal powder) [package insert] Indianapolis, IN: Eli Lilly and Company; Oct, 2020. [Accessed March 21, 2022]. https://uspl.lilly.com/baqsimi/baqsimi.html#pi . [Google Scholar]
- 7.GlucaGen® (glucagon for injection) [package insert] Plainsboro, NJ: Novo Nordisk Inc; Mar, 2021. [Accessed March 21, 2022]. https://www.novo-pi.com/glucagenhypokit.pdf . [Google Scholar]
- 8.Glucagon Emergency Kit (glucagon) [package insert] Indianapolis, IN: Eli Lilly and Company; Jan, 2021. [Accessed March 21, 2022]. https://uspl.lilly.com/glucagon/glucagon.html#pi . [Google Scholar]
- 9.GvokeTM (glucagon for injection) [package insert] Chicago, IL: Xeris Pharmaceuticals Inc; Jul, 2021. [Accessed March 21, 2022]. https://www.gvokeglucagon.com/pdf/gvoke-prescribing-information.pdf . [Google Scholar]
- 10.Zegalogue® (dasiglucagon) [package insert] Søborg, Denmark: Zealand Pharma A/S; Mar, 2021. [Accessed March 21, 2022]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214231s000lbl.pdf . [Google Scholar]
- 11.Heller SR, Buse JB, Ratner R, et al. Redefining hypoglycemia in clinical trials: validation of definitions recently adopted by the American Diabetes Association/European Association for the study of diabetes. Diabetes Care. 2020;43(2):398–404. doi: 10.2337/dc18-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martín-Timón I, Del Cañizo-Gómez FJ. Mechanisms of hypoglycemia unawareness and implications in diabetic patients. World J Diabetes. 2015;6(7):912–926. doi: 10.4239/wjd.v6.i7.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller CD, Phillips LS, Ziemer DC, et al. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med. 2001;161(13):1653–1659. doi: 10.1001/archinte.161.13.1653. [DOI] [PubMed] [Google Scholar]
- 14.Festa A, Heller SR, Seaquist E, et al. Association between mild and severe hypoglycemia in people with type 2 diabetes initiating insulin. J Diabetes Complications. 2017;31(6):1047–1052. doi: 10.1016/j.jdiacomp.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Giorda CB, Ozzello A, Gentile S, et al. Incidence and risk factors for severe and symptomatic hypoglycemia in type 1 diabetes. Results of the HYPOS-1 study. Acta Diabetol. 2015;52(5):845–853. doi: 10.1007/s00592-015-0713-4. [DOI] [PubMed] [Google Scholar]
- 16.Quilliam BJ, Simeone JC, Ozbay AB. Risk factors for hypoglycemia-related hospitalization in patients with type 2 diabetes: a nested case-control study. Clin Ther. 2011;33(11):1781–1791. doi: 10.1016/j.clinthera.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Misra-Hebert AD, Pantalone KM, Ji X, et al. Patient characteristics associated with severe hypoglycemia in a type 2 diabetes cohort in a large, integrated health care system from 2006 to 2015. Diabetes Care. 2018;41(6):1164–1171. doi: 10.2337/dc17-1834. [DOI] [PubMed] [Google Scholar]
- 18.Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care. 2005;28(12):2948–2961. doi: 10.2337/diacare.28.12.2948. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz-Alonso FJ, Galecki A, Herman WH, Smith MJ, Jacquez JA, Halter JB. Hypoglycemia counterregulation in elderly humans: relationship to glucose levels. Am J Physiol. 1994;267:E497–E506. doi: 10.1152/ajpendo.1994.267.4.E497. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell BD, Vietri J, Zagar A, et al. Hypoglycaemic events in patients with type 2 diabetes in the United Kingdom: associations with patient-reported outcomes and self-reported HbA1c. BMC Endocr Disord. 2013;13:59. doi: 10.1186/1472-6823-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallad A, Hinshaw L, Schiavon M, et al. Exercise effects on postprandial glucose metabolism in type 1 diabetes: a triple-tracer approach. Am J Physiol Endocrinol Metab. 2015;308:E1106–E1115. doi: 10.1152/ajpendo.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abraham MB, Jones TW, Naranjo D, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):178–192. doi: 10.1111/pedi.12698. [DOI] [PubMed] [Google Scholar]
- 23.Micromedex® (electronic version) Greenwood Village, CO: IBM Watson Health; [Accessed January 2, 2022]. https://www.micromedexsolutions.com . [Google Scholar]
- 24.Asplund K, Wilholm B-E, Lundman B. Severe hypoglycaemia during treatment with glipizide. Diabet Med. 1991;8:726–731. doi: 10.1111/j.1464-5491.1991.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 25.The HOE 901/204 Study Investigators Group. Safety and efficacy of insulin glargine (HOE 901) versus NPH insulin in combination with oral treatment in type 2 diabetic patients. Diabet Med. 2003;20:545–551. doi: 10.1046/j.1464-5491.2003.00999.x. [DOI] [PubMed] [Google Scholar]
- 26.Riddle MC, Rosenstock J, Gerich J the Insulin Glargine 4002 Study Investigators. Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 27.Hermansen K, Derezinski T, Kim H, Gall M-A. Treatment with insulin detemir in combination with oral agents is associated with less risk of hypoglycaemia and less weight gain than NPH insulin at comparable levels of glycaemic improvement in people with type 2 diabetes (Abstract) Diabetologia. 2004;47(Suppl 1):A273. [Google Scholar]
- 28.Janka HU, Plewe G, Riddle MC, KliebFrisch C, Schweitzer MA, Yki-Järvinen H. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial therapy for type 2 diabetes. Diabetes Care. 2005;28:254–259. doi: 10.2337/diacare.28.2.254. [DOI] [PubMed] [Google Scholar]
- 29.Raskin P, Allen E, Hollander P, et al. Initiating insulin theapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;26:260–265. doi: 10.2337/diacare.28.2.260. [DOI] [PubMed] [Google Scholar]
- 30.Bastyr EJ, Huang Y, Brunelle RL, Vignati L, Cox DJ, Kotsamos JG. Factors associated with nocturnal hypoglycemia among patients with type 2 diabetes new to insulin therapy. Diabetes Obes Metab. 2000;2:39–46. doi: 10.1046/j.1463-1326.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 31.Dailey G, Rosenstock J, Moses RG, Ways K. Insulin glulisine provides improved glycemic control in patients with type 2 diabetes. Diabetes Care. 2004;27:2363–2368. doi: 10.2337/diacare.27.10.2363. [DOI] [PubMed] [Google Scholar]
- 32.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 33.Davis TM, Brown SG, Jacobs IG, et al. Determinants of severe hypoglycemia complicating type 2 diabetes: the Fremantle diabetes study. J Clin Endocrinol Metab. 2010;95(5):2240–2247. doi: 10.1210/jc.2009-2828. [DOI] [PubMed] [Google Scholar]
- 34.Schopman JE, Geddes J, Frier BM. Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract. 2010;87(1):64–68. doi: 10.1016/j.diabres.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Carlson JN, Schunder-Tatzber S, Neilson CJ, Hood N. Dietary sugars versus glucose tablets for first-aid treatment of symptomatic hypoglycaemia in awake patients with diabetes: a systematic review and meta-analysis. Emerg Med J. 2017;34(2):100–106. doi: 10.1136/emermed-2015-205637. [DOI] [PubMed] [Google Scholar]
- 36.Vindedzis S, Marsh B, Sherriff J, et al. Dietary treatment of hypoglycaemia: should the Australian recommendation be increased? Intern Med J. 2012;42:830–833. doi: 10.1111/j.1445-5994.2012.02831.x. [DOI] [PubMed] [Google Scholar]
- 37.McTavish L, Krebs JD, Weatherall M, et al. Weight-based hypoglycaemia treatment protocol for adults with type 1 diabetes: a randomized crossover clinical trial. Diabet Med. 2015;32:1143–1148. doi: 10.1111/dme.12730. [DOI] [PubMed] [Google Scholar]
- 38.McTavish L, Wiltshire E. Effective treatment of hypoglycemia in children with type 1 diabetes: a randomized controlled clinical trial. Pediatr Diabetes. 2011;12(4 Pt 2):381–387. doi: 10.1111/j.1399-5448.2010.00725.x. [DOI] [PubMed] [Google Scholar]
- 39.Beato-Víbora PI, Arroyo-Díez FJ. New uses and formulations of glucagon for hypoglycaemia. Drugs Context. 2019;8:212599. doi: 10.7573/dic.212599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawkes CP, De Leon DD, Rickels MR. Novel preparations of glucagon for the prevention and treatment of hypoglycemia. Curr Diab Rep. 2019;19(10):97. doi: 10.1007/s11892-019-1216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yale JF, Dulude H, Egeth M, et al. Faster use and fewer failures with needle-free nasal glucagon versus injectable glucagon in severe hypoglycemia rescue: a simulation study. Diabetes Technol Ther. 2017;19(7):423–432. doi: 10.1089/dia.2016.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrism G, Diment A, Sulway M, Wilkinson M. Glucagon administration – underevaluated and undertaught. Pract Diab Int. 2001;18:22–25. doi: 10.1002/pdi.138. [DOI] [Google Scholar]
- 43.Isaacs D, Clements J, Turco N, Hartman R. Glucagon: its evolving role in the management of hypoglycemia. Pharmacotherapy. 2021;41(7):623–633. doi: 10.1002/phar.2534. [DOI] [PubMed] [Google Scholar]
- 44.Pieber T, Cummins M, Junaidi K, Close N, Nguyen A. A phase 3 comparison of drug preparation time and the symptomatic relief of severe hypoglycemia in a ready-to-use liquid stable glucagon versus powder glucagon. Diabetes Technol Ther. 2020;22:A-188. doi: 10.1089/dia.2020.2525.abstracts. [DOI] [Google Scholar]
- 45.Valentine V, Newswanger B, Prestrelski S, Andre A, Garibaldi M. Human factors usability and validation studies of a glucagon autoinjector in a simulated severe hypoglycemia rescue situation. Diabetes Technol Ther. 2019;21(9):522–530. doi: 10.1089/dia.2019.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hövelmann U, Bysted B, Mouritzen U, et al. Pharmacokinetic and pharmacodynamic characteristics of dasiglucagon, a novel soluble and stable glucagon analog. Diabetes Care. 2018;41(3):531–537. doi: 10.2337/dc17-1402. [DOI] [PubMed] [Google Scholar]
- 47.Pieber T, Aronson R, Hövelmann U, et al. Dasiglucagon: a next-generation glucagon analog for rapid and effective treatment of severe hypoglycemia results of phase 3 randomized double-blind clinical trial. Diabetes Care. 2021;44(6):1361–1367. doi: 10.2337/DC20-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Battelino T, Tehranchi R, Bailey T, et al. Dasiglucagon, a next-generation ready-to-use glucagon analog, for treatment of severe hypoglycemia in children and adolescents with type 1 diabetes: results of a phase 3, randomized controlled trial. Pediatr Diabetes. 2021;22(5):734–741. doi: 10.1111/pedi.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guzman CB, Dulude H, Piché C, et al. Effects of common cold and concomitant administration of nasal decongestant on the pharmacokinetics and pharmacodynamics of nasal glucagon in otherwise healthy participants: a randomized clinical trial. Diabetes Obes Metab. 2018;20(3):646–653. doi: 10.1111/dom.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherr JL, Ruedy KJ, Foster NC, et al. Glucagon nasal powder: a promising alternative to intramuscular glucagon in youth with type 1 diabetes. Diabetes Care. 2016;39(4):555–562. doi: 10.2337/dc15-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rickels MR, Ruedy KJ, Foster NC, et al. Intranasal glucagon for treatment of insulin-induced hypoglycemia in adults with type 1 diabetes: a randomized crossover noninferiority study. Diabetes Care. 2016;39(2):264–270. doi: 10.2337/dc15-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akirov A, Grossman A, Shochat T, Shimon I. Mortality among hospitalized patients with hypoglycemia: insulin related and noninsulin related. J Clin Endocrinol Metab. 2017;102(2):416–424. doi: 10.1210/jc.2016-2653. [DOI] [PubMed] [Google Scholar]
- 53.American Diabetes Association. 15. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S211–S220. doi: 10.2337/dc21-S015. [DOI] [PubMed] [Google Scholar]
- 54.Dextrose 20%, 30%, 40%, 50%, and 70% injection [package insert] Lake Forest, IL: Hospira Inc; May, 2018. [Accessed March 21, 2022]. https://dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=155f0dfd-657a-4fc4-11a7-5b84cc216c4c&type=pdf . [Google Scholar]
- 55.Bosse GM. Antidiabetics and hypoglycemics/antiglycemics. In: Nelson LS, Howland M, Lewin NA, Smith SW, Goldfrank LR, Hoffman RS, editors. Goldfrank’s Toxicologic Emergencies. 11e. McGraw Hill; 2019. [Accessed February 18, 2022]. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2569§ionid=210272153 . [Google Scholar]
- 56.O’Brien K, Chock A, Shea J. An overview of hypoglycemia. US Pharm. 2012;37(6):50–56. https://www.uspharmacist.com/article/an-overview-of-hypoglycemia . [Google Scholar]
- 57.Collier A, Steedman DJ, Patrick AW, et al. Comparison of intravenous glucagon and dextrose in treatment of severe hypoglycemia in an accident and emergency department. Diabetes Care. 1987;10(6):712–715. doi: 10.2337/diacare.10.6.712. [DOI] [PubMed] [Google Scholar]
- 58.Patrick AW, Collier A, Hepburn DA, Steedman DJ, Clarke BF, Robertson C. Comparison of intramuscular glucagon and intravenous dextrose in the treatment of hypoglycemic coma in an accident and emergency department. Arch Emerg Med. 1990;7(2):73–77. doi: 10.1136/emj.7.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howell MA, Guly HR. A comparison of glucagon and glucose in prehospital hypoglycaemia. Emerg Med J. 1997;14(1):30–32. doi: 10.1136/emj.14.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thome J, Byon D. Addressing hypoglycemic emergencies. [Accessed February 18, 2022];US Pharm. 2018 43(10):HS2–HS6. https://www.uspharmacist.com/article/addressing-hypoglycemic-emergencies . [Google Scholar]
- 61.Moore C, Woollard M. Dextrose 10% or 50% in the treatment of hypoglycaemia out of hospital: a randomised controlled trial. Emerg Med J. 2005;22(7):512. doi: 10.1136/emj.2004.020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yale J-F, Paty B., Senior Mbbs PA Diabetes Canada Clinical Practice Guidelines Expert Committee. Hypoglycemia. Can J Diabetes. 2018;42(Suppl 1):S104–S108. doi: 10.1016/j.jcjd.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 63.Tomky D. Detection, prevention, and treatment of hypoglycemia in the hospital. Diabetes Spectr. 2005;18(1):39–44. doi: 10.2337/diaspect.18.1.39. [DOI] [Google Scholar]
- 64.Cruz P. Inpatient hypoglycemia: the challenge remains. J Diabetes Sci Technol. 2020;14(3):560–566. doi: 10.1177/1932296820918540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S90–S102. doi: 10.2337/dc19-S009. [DOI] [PubMed] [Google Scholar]
- 66.American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S85–S99. doi: 10.2337/dc21-S007. [DOI] [PubMed] [Google Scholar]
- 67.Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254–2263. doi: 10.1016/S0140-6736(16)31535-5. [DOI] [PubMed] [Google Scholar]
- 68.van Beers Cornelis A, Wit Maartje D, Kleijer Susanne J, et al. Continuous glucose monitoring in patients with type 1 diabetes and impaired awareness of hypoglycemia: also effective in patients with psychological distress? Diabetes Technol Ther. 2017;19(10):595–599. doi: 10.1089/dia.2017.0141. [DOI] [PubMed] [Google Scholar]
- 69.Deiss D, Bolinder J, Riveline J, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 70.Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline J-P, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55–73. doi: 10.1007/s13300-016-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]