Abstract
Diabetes mellitus is a chronic condition affecting 1 out of every 11 people worldwide. Monitoring of blood glucose allows for therapeutic lifestyle and pharmacotherapy changes to reduce the occurrence of hyperglycaemia and hypoglycaemia. Advancements in technology over the past two decades have increased patient and clinician access to glucose data and trends with continuous glucose monitoring (CGM) systems. This narrative review seeks to investigate the efficacy and safety of CGM for the management of diabetes. In type 1 diabetes (T1DM) and type 2 diabetes, efficacy studies of real-time CGM (rtCGM) or intermittently scanned CGM (isCGM) have shown a decrease in HbA1C (0.3–0.6%) over traditional self-monitoring blood glucose. Percent time in the target glucose range also improved (6.8–17.6%). Rates of hypoglycaemia, including severe hypoglycaemia, decreased in studies of rtCGM and isCGM with most available data in T1DM. In pregnant women with T1DM, rtCGM has shown modest improvements in HbA1C and time in target glucose range and decreased risk of neonatal complications. Multiple studies have shown that the use of rtCGM or isCGM increased diabetes treatment satisfaction amongst patients. Head-to-head studies of rtCGM and isCGM are limited but one study indicates that a CGM system with alarms may be preferred in T1DM to reduce the risk of hypoglycaemia. Selection of a CGM device should depend on patient-specific factors and insurance coverage. The results of one study show that the benefits of CGM device use were not sustained after discontinuing use. Increasing widespread and long-term access to CGM devices is necessary to improve the management of diabetes amongst the greater population.
Keywords: blood glucose self-monitoring, diabetes mellitus, gestational diabetes, hyperglycaemia, hypoglycaemia, interstitial glucose, technology
Introduction
Diabetes mellitus (DM) is a chronic condition affecting 1 out of every 11 people worldwide, approximately 422 million total.1 When inadequately managed, it can lead to serious complications and death.1 Globally, diabetes was the ninth leading cause of death in 2019.2 Successfully managing diabetes often necessitates the use of a multifaceted approach and blood glucose monitoring is a mainstay of management.3 Monitoring of blood glucose allows for therapeutic lifestyle and pharmacotherapy changes to reduce the occurrence of hyperglycaemia and hypoglycaemia. In 1999, the FDA approved the first continuous glucose monitoring (CGM) device, a professional system worn by patients, which gathered glucose data to be downloaded and reviewed by clinicians.4 Since then, CGM technology has evolved allowing clinicians and patients improved access to glucose data and trends.
This narrative review seeks to investigate the efficacy and safety of CGM devices for the management of diabetes. A MEDLINE search of English-language articles from June 2011 through June 2021 was conducted using terms “diabetes”, “continuous glucose monitor” or “flash glucose monitor”, separately or in multiple combinations. We included human studies of type 1 DM (T1DM) and type 2 DM (T2DM) and manually searched for and included additional pertinent articles.
How the technology works
The CGM device typically comes with an adhesive sensor and a display device to collect glucose data. In contrast to traditional finger stick testing, where blood glucose is measured, CGM utilizes the interstitial fluid in the subcutaneous layer.5 Interstitial fluid surrounds adipocytes in the subcutaneous layer and provides cells with glucose.5 Glucose is delivered to the subcutaneous layer via capillaries, then moves by passive diffusion through the interstitial fluid into the adipocytes.5 The electrochemical sensor is inserted by the patient or clinician (depending on the type of device) and measures the interstitial glucose concentration. Data are then wirelessly transmitted to a receiver or smartphone.5 As glucose must diffuse from the capillaries into the interstitial fluid for a reading to be made, there is an approximate lag time of 8–10 minutes between plasma and interstitial concentrations under steady-state conditions.5 This lag time is increased when glucose levels are rapidly rising or falling.5 The delay has been accounted for by software programmes and is only clinically significant when glucose levels change suddenly, such as in hypoglycaemic episodes.5 Therefore, patients should perform fingerstick self-monitoring blood glucose (SMBG) in these situations, or when symptoms do not match CGM device readings. Additionally, certain CGM devices allow patients to track meals, physical activity and administered medications, which can then be reviewed with the provider to help inform treatment decisions.
Types of systems
Intermittently scanned CGM (isCGM) and real-time CGM (rtCGM) systems are the two types of CGM devices currently available. Intermittently scanned CGM systems require the patient to actively scan the sensor throughout the day with the device reader or through the use of a smartphone application to measure glucose levels and receive glucose data.6 rtCGM devices continuously collect glucose data and transmit it every 5 minutes to a receiver or smartphone application.6
Systems are also designated as personal or professional CGMs devices. Table 1 compares currently available devices.7–15 Personal-use CGM devices are the patients’ personal devices. In the United States, these are typically obtained through insurance or out of pocket. Patients can share their glucose data from these devices with caregivers and clinicians via smartphone or smartwatch applications or via manual upload at home or in the clinic. Professional use or practice-based CGM devices are used in the healthcare clinic setting. In the United States, these are covered by insurance and allow for provider billing for CGM application, removal and interpretation of CGM data. Patients utilizing a professional CGM device wear the sensor placed by the practice for 6–14 days. After the wear period, the patient returns to the clinic with the sensor and equipment, the sensor is removed, glucose data are downloaded and analysed, and treatment decisions can be made. Professional CGM devices may be blinded or unblinded depending on the device. In blinded mode, glucose levels are recorded without influencing patients’ behaviour, and the clinician retrospectively reviews the data. Conversely, in unblinded mode, both patients and clinicians can monitor glucose data in real time. Studies have demonstrated that professional CGM devices, especially unblinded, can assist clinicians and patients by allowing them to see the effects of food choices and exercise on glycaemic control and make individualized therapy adjustments.16–18
Table 1.
| Type | Manufacturer, name | FDA-approved population (age, years) | FDA-approved application site | Sensor wear (days) | Warm-up period (hours) | Technological capabilitiesa | Alarms, alerts | Calibrations (per day) | Insulin pump integration | Interacting substances and effect on glucose readings |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| isCGM | ||||||||||
|
| ||||||||||
| Personal | Abbott Freestyle Flash Libre 14 day and Libre 2b | Libre: ≥18 Libre 2: ≥4 |
Upper arm | 14 | 1 | Use Reader or App for smartphone Libre 14 day: FreeStyle LibreLink app for iPhone and Android Libre 2: FreeStyle Libre 2 app for iPhone and Android |
Libre 14 day: none Libre 2: customizable alarms Alerts: high glucose low glucose loss of signal urgent low glucose (<55 mg/dL)c |
None | No | Ascorbic acid (falsely elevated); salicylic acid (falsely decreased) |
| Professional blinded | Abbott Freestyle Flash Libre Prob | ≥18 | No | |||||||
|
| ||||||||||
| rtCGM | ||||||||||
|
| ||||||||||
| Personal | Dexcom G6d | ≥2 | Age 2–17 years: abdomen, upper buttocks | 10 | 2 | Use Reader or App for smartphone available for iPhone and Android | Customizable alerts for high/low glucose, including urgent low Soon (predicts glucose <55 mg/dL in 20 min), rise/fall readings, loss of signal alarm: urgent low (<55 mg/dL) | None if used with sensor code; 2 if used without sensor code | Yes | High doses of acetaminophen exceeding 1 g every 6 hours (falsely increased) Hydroxyurea (falsely increased) |
| Professional blinded or unblinded | Dexcom G6 Prod | ≥2 | Age ≥18 years: abdomen | Yes | ||||||
|
| ||||||||||
| Personal | Eversense Senseonicse | ≥18 | Implantable device in upper arm placed by healthcare provider | 90 | 24 | App for smartphone available for iPhone and Android, or smartwatch | Vibration alert for hypoglycaemia; predictive high and lows alerts | 2, and when indicated | No | Tetracyclines (falsely decreased); contains dexamethasone (avoid if contraindication) |
|
| ||||||||||
| Personal | Medtronic Guardian Connectf,g | ≥14–75 | Abdomen, upper arm | 7 | 2 | App for smartphone available iPhone or Android Sugar.IQ.app available for iOS | Customizable predictive alerts can be set for 10–60 min before a high or low glucose | ≥2 | Yes | Acetaminophen (falsely elevated) |
| Professional blinded | Medtronic iPro2f | ≥14–75 | 6 | ≥3 | No | |||||
Check the manufacturer’s website for a full list of smartphone compatibility.
Freestyle Flash Libre, Libre 2 and Libre Pro are registered trademarks of Abbott Laboratories, Abbott Park, IL, USA.
FreeStyle Libre 2 smartphone app features a mandatory Urgent Low Glucose alarm to signal glucose reading <55 mg/dL. The user must start the sensor with the phone app to receive this alert.
Dexcom G6 and G6 Pro are registered trademarks of Dexcom, Inc., San Diego, CA, USA.
Eversense Senseonics is a registered trademark of Senseonics, Inc., Germantown, MD, USA.
Medtronic Guardian Connect and Medtronic iPro2 is a registered trademark of Medtronic, Dublin, Ireland.
Medtronic Guardian Connect system and iPro2 are designed to be used in conjunction with or as a complement to fingerstick glucose monitoring.
CGM, continuous glucose monitor; isCGM, intermittently scanned continuous glucose monitoring; rtCGM, real-time continuous glucose monitoring.
Interpreting the data
At the patient appointment, the provider can connect the patient’s CGM device to a computer and download the data onto the corresponding CGM platform to access the ambulatory glucose profile (AGP) report to make therapeutic adjustments. Providers can also access data remotely from the patient’s CGM device through the use of device-specific secure websites, allowing data to be shared between clinic visits. The AGP report is standardized amongst devices and consists of key metrics: data sufficiency, average glucose, a glucose management index, time in or outside the target range, glucose variability, and a graphical depiction of glucose data.19
The data sufficiency section of the report shows the percentage of time or time in days that the CGM device was used in the last 14 days.19 Within a 14-day period, the CGM device should be utilized at least 70% of the time or for approximately 10 days.19 Ensuring that sufficient data have been collected adds confidence to the report and decision-making. This data point can also be used to educate the patient and encourage full-time use of the CGM device. The report provides the average glucose during the 14-day period and should not be used solely for pharmacotherapy changes as it does not identify glucose patterns throughout the day.19 An estimated haemoglobin A1C (HbA1C) based on the data from the 14-day period is reported as the glucose management index. Although glucose management index should not be used to replace lab monitoring of HbA1C, it allows patients and providers to continually evaluate patient progress towards their HbA1C goal between lab draws. Glucose variability signifies to what extent the patient’s glucose level differs from the average.19 The coefficient of variation is a common metric of glucose variability included on CGM reports.19 Randomized controlled trials and prospective studies have shown that increased glucose variability correlates to higher rates of hypoglycaemia.20 A coefficient of variation of ≤36% is considered stable glycaemic control.19
The AGP report also provides a percentage breakdown of how much time the patient’s glucose falls into different time ranges.19 Typically, the time ranges that are reported are as follows: very high (glucose >250 mg/dL), high (glucose 181–249 mg/dL), target (glucose 70–180 mg/dL), low (glucose 54–69 mg/dL) and very low (glucose <54 mg/dL).21 The relationship between time in target range (TTR) and haemoglobin A1c has estimated that TTRs of 70% and 50% correlate with estimated A1c values of 7% and 8%, respectively, and that a 10% increase in TTR corresponds with a 0.5% reduction in A1c.21 Time in the range provides the patient and provider another way to compare progress between visits, the goal being that the TTR increases and time above or below the target range decreases.
The AGP is critical to making informed lifestyle and pharmacotherapy changes as it depicts average glucose trends throughout the day.19 The AGP utilizes data from the entire CGM device wear period and consolidates the glucose data into a standard 24-hour day.19 Glucose data are displayed on a graph with the concentration of glucose on the vertical axis and the time in hours over a 24-hour wear period on the horizontal axis.19 There is a dark coloured median line and dark blue shading above and below which signifies the interquartile range (25th and 75th percentile).19 The dashed lines on the AGP identify the interdecile range (10th and 90th percentile).19 The interquartile range illustrates issues with patients’ average glucose profile and treatment regimen in contrast to the interdecile range, which correlates to irregular variations in patients’ daily lifestyle.22 Analysis of the interquartile range and interdecile range aids in the identification of whether pharmacotherapy adjustments or lifestyle interventions are needed.22
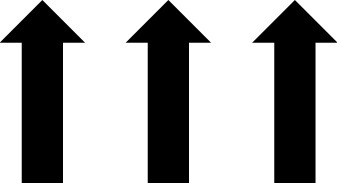
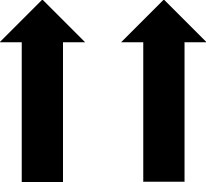
In addition to the AGP report, CGM devices can provide the patient with real-time glucose readings. The CGM device uses trend arrows to alert the patient on how their glucose is changing. Trend arrows may vary depending on the device, so it is important to educate the patient on the meaning of these (Table 2).23
Table 2.
Description of trend arrows across devices
| Trend arrows | CGM devices and effect on blood glucose | |||
|---|---|---|---|---|
| Freestyle Libre, Libre 2a | Dexcom G6b | Senseonics Eversensec | Medtronic MiniMed, Guardian 3, Guardian Connectd | |
|
— | — | — | Increasing ≥3 mg/dL/min |
|
— | Increasing >3 mg/dL/min | — | Increasing 2–3 mg/dL/min |
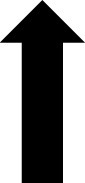
|
Increasing >2 mg/dL/min | Increasing 2–3 mg/dL/min | Increasing >2 mg/dL/min | Increasing 1–2 mg/dL/min |

|
Increasing 1–2 mg/dL/min | Increasing 1–2 mg/dL/min | Increasing 1–2 mg/dL/min | — |

|
Increasing or decreasing <1 mg/dL/min | Stable glucose, changing <1 mg/dL/min | Increasing or decreasing <1 mg/dL/min | — |

|
Decreasing 1–2 mg/dL/min | Decreasing 1–2 mg/dL/min | Decreasing 1–2 mg/dL/min | — |
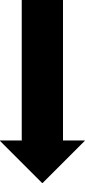
|
Decreasing >2 mg/dL/min | Decreasing 2–3 mg/dL/min | Decreasing >2 mg/dL/min | Decreasing 1–2 mg/dL/min |
|
— | Decreasing >2 mg/dL/min | — | Decreasing 2–3 mg/dL/min |
|
— | — | — | Decreasing >3 mg/dL/min |
Freestyle Flash Libre, Libre 2 and Libre Pro are registered trademarks of Abbott Laboratories, Abbott Park, IL, USA.
Dexcom G6 and G6 Pro are registered trademarks of Dexcom, Inc., San Diego, CA, USA.
Eversense Senseonics is a registered trademark of Senseonics, Inc., Germantown, MD, USA.
Medtronic MiniMed, Guardian 3 and Guardian Connect are registered trademarks of Medtronic, Dublin, Ireland.
CGM, continuous glucose monitor.
Adapted from ref.23
Review of clinical trials
Several studies have demonstrated the benefits of CGM technology in reducing HbA1C and improving safety (Table 3).
Table 3.
| Study | N | Patients | Methods | Primary outcome (HbA1C change unless otherwise noted) | Select secondary outcomes | Safety/other |
|---|---|---|---|---|---|---|
| EFFICACY: Adults with T1DM – HbA1C reduction | ||||||
| Beck et al.24 | 158 | Age ≥25 years (mean 48); HbA1C 7.5–9.9% (mean 8.6%); MDI | rtCGM (Dexcom G4) versus SMBG; 24 weeks | −0.6% (p<0.001) | Mean TTR: 736 versus 650 min/day; adjusted difference 77 (p=0.005) | Severe hypoglycaemia: 4.2 versus 12.2 events/100 person-years); 93% used rtCGM 6 days/week |
| Lind et al.25 | 161 | Age ≥18 years (mean 44 years); HbA1C ≥7.5% (mean 8.6%) | rtCGM (Dexcom G4) versus SMBG; crossover 26 weeks, 17-week washout | −0.43% (p<0.001) | Increased treatment satisfaction (DTSQ) with rtCGM, mean score increase 3.43 (p<0.001) | Hypoglycaemia: 1 (0.6%) versus 5 (3.3%) |
| Sequeira et al.26 | 25 | Age ≥18 years (mean 40 years); mean HbA1C 8.5%; 76% Latinx, uninsured; MDI | rtCGM (Dexcom G7) versus SMBG; crossover; 28 weeks, no washout | Not SS | TTR: no SS difference; 80% of participants wanted to continue using CGM, felt insulin adjustment was easier, felt CGM prevented hypoglycaemia | Hypoglycaemia: no SS difference |
| Tumminia et al.27 | 20 | Age 18–60 years (mean 34 years); A1C ≥8% (mean 8.7%); MDI or CSII; analysed n=14 used sensor ≥40% of time | rtCGM (Medtronic Guardian REAL-Time) versus SMBG; crossover; 24 weeks, 2-week washout | Mean HbA1C decreased from 8.5% to 7.8% (p<0.05) with rtCGM | Risk of hyperglycaemia reduced (p<0.05) with rtCGM; MDI subgroup had further decreased risk of hyperglycaemia and hypoglycaemia (p<0.05) | No severe hypoglycaemia; risk of hypoglycaemia reduced (p<0.05) |
| Visser et al.28 | 254 | Age ≥18 years (mean 43 years); T1DM; HbA1C ≤10% (mean 7.4%); MDI or CSII; isCGM use for at least 6 months (median use >2 years); median scans per day: 11 | rtCGM (Dexcom G6) versus isCGM (FreeStyle Libre); 6 months | Mean TTR higher with rtCGM (59.6% versus 51.9%; mean difference 6.85%; p<0.0001) | HbA1C further decreased with rtCGM (7.1% versus 7.4%; p<0.0001); fear of hypoglycaemia (survey) decreased with rtCGM (mean difference −2.62 points; p=0.0071) | Mean time spent in severe hypoglycaemia: 0.47% versus 0.84% (p=0.007); bleeding with sensor insertion in rtCGM group only (9%); skin reaction more common in isCGM group (7% versus 13%) |
| EFFICACY: Young people with T1DM – HbA1C reduction or TTR | ||||||
| Boucher et al.29 | 64 | Age 13–20 years (mean 16 years); HbA1C ≥9% (mean 10.9%) | isCGM (Freestyle Libre) versus SMBG; 24 weeks | −0.2%, (p=0.576) not SS | isCGM group checked glucose 3.2 times more than SMBG group (p<0.001); improved diabetes treatment satisfaction (DTSQ) difference between groups 0.47 (p=0.048) | No severe hypoglycaemia events; no SS difference in DKA (18% versus 16%) |
| Laffel et al.30 | 153 | Age 14–24 years (mean 17 years); HbA1C 7.5–10.9% (mean 8.9%); 38% non-white; 41% public insurance | rtCGM (Dexcom G5) versus SMBG; 26 weeks | −0.37% (p<0.01) | TTR: 43% versus 35%, (p<0.001); mean time in hypoglycaemia was lower with rtCGM (−0.7%; p=0.002) | Severe hypoglycaemia: 8.3 versus 7.8 events/100 person-years); DKA: 8.3 versus 2.6 events/100 person-years) |
| Thabit, et al.31 | 31 | Age 16–24 years (mean 21 years); HbA1C ≥7.5% (mean 9.3%) | rtCGM (Dexcom G6) versus SMBG; crossover; 8 weeks, 3–4-week washout | Higher TTR with rtCGM mean difference 11.1% (p<0.001) | HbA1C reduction: −0.76% (p<0.001) | No SS difference in hypoglycaemia; 84% sensor usage |
| EFFICACY: Adults with T2DM – HbA1C reduction | ||||||
| Beck et al.32 | 158 | Age ≥25 years (mean 60 years); HbA1C 7.7–9.9% (mean 8.5%); EGFR ≥45 mL/min/1.73 m2; 46% non-white (CGM group), 27% (SMBG group) | rtCGM (Dexcom G4) versus SMBG; 24 weeks | −0.3 (p=0.022) | TTR (median) increased further with rtCGM (802–882 min/day versus 794 to 836 min/day); no SS difference in glucose variability | No severe hypoglycaemia; no SS difference in time in hypoglycaemic range; mean rtCGM use: 6.7 days/week |
| Ehrhardt et al.33 | 100 | Age ≥18 years (mean 57.7 years); HbA1C 7–11.9% (mean 8.3%); not on prandial insulin; rtCGM group slightly younger, more men | rtCGM (Dexcom SEVEN) versus SMBG; 12 weeks; 4 cycles of 2 weeks on/1 week off | −0.6 (p=0.002) | New basal insulin starts: 6% versus 16%; net medication/dose changes similar between groups | Hypoglycaemia: not available |
| Haak et al.34 | 224 | Age ≥18 years (mean 59 years); HbA1C 7.5–12% (mean 8.7%); MDI or prandial only (95%), CSII (5%) | isCGM (Freestyle Libre) versus SMBG; randomized 2:1; 6 months | −0.03, not SS (p=0.822) Age <65 years: −0.53% (p=0.03) |
TTR: no difference; higher mean DTSQ satisfaction score with isCGM (13.1 versus 9; p<0.0001) | Severe hypoglycaemia: 2% versus 1%; isCGM decrease time in hypoglycaemia by 43% (p=0.0006) |
| Martens et al.35 | 175 | Age ≥30 years (mean 57 years); HbA1C 7.8–11.5% (mean 9.1%); primary care; on basal insulin, other agents, no prandial insulin; 53% ethnic minorities | rtCGM (Dexcom G6) versus SMBG; randomized 2:1; 8 months | −0.4% (p=0.02) | TTR: 59% versus 43%, adjusted difference 15% (p<0.001); mean time glucose >250 mg/dL: 11% versus 27%, adjusted difference 16% (p<0.001) | Severe hypoglycaemia: 1% versus 2%; median rtCGM use: 6.1 days/week |
| Grace et al.37 | 38 | Adults (mean age 55 years); HbA1C >7.5% (mean 10.1%); on various therapies – excluding prandial insulin; basal insulin (42%), non-insulin injectable (24%), oral diabetes medication, i.e. metformin (55%), sulfonylurea (39%) and/or diet/exercise regimen | rtCGM (Dexcom G6) – single arm/no comparator group; open-label, interventional, cohort study; 6 months | −3.0% (p<0.01) | TTR: increased from 57% to 72.2% (mean change 15.2; p<0.001); body weight: mean change of −3.1 kg (p=0.002) Subgroup with 0–1 medications (n=13) showed further improvement in TTR (mean change 17.6; p=0.002) |
No severe hypoglycaemia; therapy intensified: 53% of patients; medication changes or reduction: 39% |
| EFFICACY: Special populations – type 1 or 2 diabetes | ||||||
| Ruedy et al.39 | 116 | Older adults, age ≥60 years (mean 67 years); T1DM (29%) or T2DM (71%); HbA1C 7.5–10% (mean 8.5%) | rtCGM (Dexcom G4) versus SMBG; 24 weeks | −0.4% (p<0.001) | TTR: CGM group: increased from 796 to 889 min/day; control group: decreased from 753 to 732 min/day (p<0.001) | No severe hypoglycaemia; 97% used rtCGM 6 days/week |
| Joubert et al.40 | 15 | Haemodialysis 3x/week; −age 18–80 years (mean 61 years); mean HbA1C 6.9%; T1DM (n=2), T2DM (n=9), or secondary DM; 20% managed with diet only | rtCGM (Medtronic iPro2) versus SMBG; 6 weeks with SMBG, 6 weeks with blinded CGM (5 day recording every 2 weeks) | HbA1C decreased from 6.9% to 6.5% (p<0.05); mean glucose decreased from 150 to 139 mg/dL (p<0.05) | Treatment changes occurred more often with rtCGM (2.1 versus 1.4; p<0.05); mean glucose lower on dialysis days (137 versus 141 mg/dL; p<0.05) | No SS difference in hypoglycaemia |
| EFFICACY and SAFETY – Pregnant – T1DM, T2DM or GDM – HbA1C reduction | ||||||
| Feig et al.41 | 325 | Age 18–40 years; T1DM, MDI or CSII; pregnant (66%): HbA1C 6.5–10% (mean 6.8%), mean age 31 years; planning pregnancy (34%): HbA1C 7–10% (mean 7.5%), mean age 33 years | rtCGM (Guardian RT or MiniMed Minilink) versus SMBG; pregnant: 34 weeks; planning pregnancy: 24 weeks | −0.19% (p=0.0207) if pregnant; no SS difference in planning pregnancy subgroup | TTR: pregnant: 68% versus 61% (p=0.0034) | Severe hypoglycaemia: 3% versus 4% (p=0.10) not SS; LGA: OR 0.51 (95% CI: 0.28–0.90; p=0.0210); neonatal ICU stay >24 hours: OR 0.48 (95% CI: 0.26–0.86; p=0.0157); neonatal hypoglycaemia: OR 0.45 (95% CI: 0.22–0.89; p=0.0250) |
| Secher et al.42 | 154 | T1DM (80%), T2DM (20%); adult women (mean age 31); median HbA1C 6.7%; MDI or CSII | rtCGM (Medtronic Guardian RT) versus SMBG; rtCGM use × 6 days at weeks 8, 12, 21, 27 and 33 | No difference in HbA1C | TTR: no difference | Severe hypoglycaemia: no difference (16% in both); no difference in LGA, preterm delivery, or severe neonatal hypoglycaemia |
| Wei et al.43 | 106 | Adult women with GDM at 24–28 weeks; mean HbA1C 5.8% | rtCGM (Medtronic Gold) versus SMBG; subgroups: early CGM (worn second trimester), late CGM (worn third trimester) | No SS difference in HbA1C | Excessive gestational weight gain decreased: 33.3% versus 56.4% (p=0.039); early rtCGM subgroup (second trimester) gained less weight: 12.7 versus 14.3 kg (p=0.017) | No differences in neonatal outcomes (LGA, neonatal hypoglycaemia, macrosomia) |
| SAFETY – T1DM | ||||||
| Bolinder et al.44 | 328 | Age ≥18 years (mean 44 years); T1DM; HbA1C ≤7.5% (mean 6.7%); MDI or CSII | isCGM (Freestyle Libre) versus SMBG; 6 months | Time in hypoglycaemia decreased: −1.24 h/day (p<0.0001), 38% reduction | Difference between groups with DTSQ score: 6.1 (p<0.0001) | Severe hypoglycaemia: similar between groups |
| Heinemann et al.45 | 149 | Age ≥18 years (mean 47 years); T1DM; HbA1C ≥9% (mean 7.5%); MDI; severe hypoglycaemia in the past 12 months (requiring assistance) or impaired hypoglycaemia awareness | rtCGM (Dexcom G5) versus SMBG; 26 weeks | Mean number of hypoglycaemia events/28 days, IRR 0.28 (p<0.0001); incidence of hypoglycaemia decreased 72% | Lower glucose variability: CV decreased from 39.3% to 34.1% (p<0.0001) | Severe hypoglycaemia: 0.64 versus 1.18 events/patient-year; IRR 0.36 (p=0.0247): 64% decrease in severe hypoglycaemia |
| Olafsdottir et al.46 | 161 | Age ≥18 years (mean 45 years); T1DM; HbA1C ≥7.5% (mean 8.7%); MDI | rtCGM (Dexcom G4) versus SMBG; crossover; 26 weeks, 17-week washout | Decreased time spent in nocturnal hypoglycaemia: 2.63% versus 4.74%; mean difference −2.11 (p<0.001) | Decreased time spent in daytime hypoglycaemia: 2.81% versus 4.77%; mean difference −1.93 (p<0.001); lower glucose variability: CV 0.37 versus 0.40): difference −0.03 (p<0.001) | Severe hypoglycaemia occurred less often (1 versus 5) |
| Pratley et al.47 | 203 | Age ≥60 years (median 68 years); T1DM; HbA1C <10% (mean 7.5%); MDI or CSII | rtCGM (Dexcom G5) versus SMBG; 6 months | Time in hypoglycaemia decreased; −1.9% (p<0.001) | Lower glucose variability: CV decreased from 41% to 37% (−4.7%; p<0.001) | Severe hypoglycaemia: 1.9 versus 22.4 events/100 person-years |
CGM, continuous glucose monitor; CSII, continuous subcutaneous insulin infusion; CV, coefficient of variation; DKA, diabetic ketoacidosis; DM, diabetes mellitus; DTSQ, Diabetes Treatment Satisfaction Questionnaire; GDM, gestational diabetes mellitus; IRR, incidence rate ratio; IS, intermittently scanned; LGA, large for gestational age; MDI, multiple daily injections; OR, odds ratio; RT, real time; SMBG, self-monitoring blood glucose; SS, statistically significant; TTR, time in target range blood glucose 70–180 mg/dL; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Skin Tac is a registered trademark of Torbot Group, Inc., Cranston, RI, United States.
T1DM – adults
A trial recruiting adults from endocrinology clinics with T1DM on multiple daily injections (MDI) of insulin randomized 158 patients to rtCGM or SMBG.24 The primary endpoint was the difference in HbA1C from baseline to 24 weeks. With rtCGM, mean HbA1C reduction was 1% compared to 0.4% with SMBG (p<0.001).24 The mean adjusted difference between groups was −0.6% (p<0.001).24 Severe hypoglycaemia occurred in two patients in each group, resulting in an overall lower event rate in the rtCGM group (4.2 versus 12.2 events per 100 person-years).24
A study conducted in Sweden enrolled patients with T1DM on MDI in an open-label crossover trial. It included 161 patients randomized to rtCGM or SMBG for 26 weeks with a 17-week washout period between treatment arms.25 The primary outcome was the difference in HbA1C between the two treatments. Mean HbA1C during rtCGM use was 7.9% compared to 8.4% with SMBG, resulting in a mean difference of −0.43 (p<0.001).25 The study also found a statistically significant increase in diabetes treatment satisfaction with rtCGM on the Diabetes Treatment Satisfaction Questionnaire (DTSQ).25
A smaller crossover trial studied a diverse population of uninsured patients with T1DM on MDI.26 Of the 25 included individuals, 76% identified as Latinx. Participants were randomized to rtCGM or SMBG for 28 weeks before switching treatment arms without a washout period.26 The results showed no difference with change in HbA1C, TTR or rates of hypoglycaemia. However, patient satisfaction increased with the use of rtCGM; 80% of participants wanted to continue using the technology after the study, noting they felt it made adjusting insulin easier and helped prevent and manage hypoglycaemia.26
A third crossover study included patients on MDI or continuous subcutaneous insulin infusion (CSII) and studied rtCGM versus SMBG for 24 weeks, separated by a 2-week washout period.27 The final analysis included 14 participants who used rtCGM for at least 40% of the time. They found a statistically significant decrease in mean HbA1C during rtCGM use (8.5–7.8%; p<0.05).27 During SMBG use, HbA1C decreased from 8.6% to 8.4% but the difference was not statistically significant.27 Risk of hyperglycaemia decreased with rtCGM usage. Specifically, the subgroup of patients treated with MDI had a further decreased risk of hyperglycaemia and hypoglycaemia during the rtCGM period.27
Lastly, a study of patients with T1DM on MDI or CSII included those who had been using isCGM for at least 6 months.28 Less than 20% of patients experienced hypoglycaemia unawareness at baseline. They randomized 254 participants to rtCGM or continued use of isCGM. The primary outcome was the mean difference in TTR between groups at 6 months and found that rtCGM resulted in higher TTR (59.6% versus 51.9%; p<0.0001).28 Mean HbA1C at baseline between the two groups was 7.4% and decreased in the rtCGM group (7.1% versus 7.4%; p<0.0001).28 Time spent in severe hypoglycaemia was lower with rtCGM (0.47% versus 0.84%; p=0.007).28 It is unclear if these results are due to the real-time connection or alert functionality of rtCGM. Notably, bleeding with sensor insertion only occurred with rtCGM (9%) and skin reactions occurred more commonly with isCGM (13%) compared to rtCGM (7%).28 Additional studies are needed to compare rtCGM to alert-enabled isCGM.
T1DM – young people
A multicentre study randomized 64 individuals aged 13–20 years with T1DM to isCGM or SMBG.29 The mean difference in HbA1C between groups after 24 weeks was −0.2% but was not statistically significant.29 The study did find that the number of glucose checks per day increased with isCGM and decreased with SMBG. At 3 months, the isCGM group checked glucose 3.2 times more often than the SMBG group (p<0.001).29 Additionally, diabetes treatment satisfaction was increased with isCGM.29
A second study included 153 participants aged 14–24 years with T1DM randomized to rtCGM or SMBG.30 The primary outcome was HbA1C change at 26 weeks. A greater change in mean HbA1C was observed with rtCGM use and the adjusted difference between groups was −0.37% (p<0.01).30 The rtCGM group also had higher TTR (43% versus 35%; p<0.001). With rtCGM, authors also discovered a statistically significant improvement in average time in hypoglycaemia (–0.7%; p=0.002) but a non-statistically significantly higher rate of severe hypoglycaemia and diabetic ketoacidosis.30
In a randomized crossover trial of individuals aged 16–24 years with T1DM, 31 participants used rtCGM or SMBG for 8 weeks followed by a 3–4-week washout period.31 The primary outcome was TTR and the results showed TTR increased with rtCGM with a mean difference between groups of 11.1% (p<0.001).31 HbA1C was also reduced by 0.76% with rtCGM (p<0.001) and participants adhered to sensor use 84% of the time.31
T2DM – adults
Evidence for use of CGM devices to reduce HbA1C is also emerging for patients with T2DM. A study randomized 158 adults with T2DM and normal renal function to rtCGM or SMBG.32 Approximately 46% of patients in the rtCGM group identified as non-white, compared to 27% in the SMBG group. The primary outcome was the change in HbA1C after 24 weeks and an adjusted mean difference of −0.3% was found between groups in favour of rtCGM. Median TTR was further increased with rtCGM compared to SMBG.32 New medication initiation occurred at a similar frequency between the two groups.32 There was no statistically significant difference in glucose variability or time in hypoglycaemia and no episodes of severe hypoglycaemia occurred in either group.32
A second study included 100 adults with T2DM who were not on prandial insulin.33 They were randomized to rtCGM or SMBG use with four cycles following the pattern: 2 weeks on, 1 week off. The primary endpoint was a change in HbA1C at 12 weeks. The rtCGM group had a mean HbA1C decrease of 1% compared to 0.5% with SMBG, which was statistically significant (p=0.006).33 The mean adjusted difference in HbA1C between groups was −0.6% favouring rtCGM (p=0.002).33 There was a similar frequency of medication or dose changes between groups. However, the rtCGM users were less likely to be started on basal insulin than the SMBG group (6% versus 16%).33 This study concluded that short-term rtCGM use can be beneficial as a non-pharmacological intervention that may improve HbA1C for some patients without adjustment of medications, which can precipitate hypoglycaemia.33
Another study of adults with T2DM treated with MDI or CSII randomized 224 individuals in a 2:1 fashion to isCGM or SMBG.34 No statistically significant difference was found in the primary endpoint of HbA1C reduction. In a subgroup analysis, participants under 65 years had a greater reduction in HbA1C with isCGM (mean difference −0.53%; p=0.03).34 Alternatively, HbA1C reduction in the subgroup of older individuals favoured SMBG with a mean HbA1C change of −0.49% compared to −0.05% with isCGM (p=0.008).34 Time spent in hypoglycaemia was reduced by 43% with isCGM (p=0.0006).34 Use of isCGM also correlated with a higher mean satisfaction score (DTSQ) compared to usual care (13.1 versus 9%; p<0.0001).34
A study of adults with T2DM recruited patients from primary care clinics with a stable antidiabetic regimen including basal (but not prandial) insulin for at least 3 months.35 About 53% of participants represented ethnic minorities. They randomized 175 individuals to rtCGM or SMBG in a 2:1 fashion. The primary outcome was a change in HbA1C after 8 months, and the mean adjusted difference in HbA1C was −0.4% in favour of rtCGM (p=0.02).35 Users of rtCGM had a higher TTR than SMBG users (59% versus 43%, adjusted difference 15%; p<0.001).35 Average time spent in hyperglycaemia was reduced with rtCGM (11%) versus SMBG (17%) (adjusted difference 15%; p<0.001).35 The occurrence of severe hypoglycaemia was similar between groups. After study completion, a 6-month follow-up phase was conducted in which 106 of the original rtCGM users were randomized to continue rtCGM use or discontinue rtCGM and resume SMBG.36 The group that continued rtCGM showed little change in TTR during these additional 6 months (56% versus 57%; p=0.89).36 However, in those that discontinued rtCGM, TTR worsened with a mean change of −12% (62% versus 50%; p=0.01).36 Mean HbA1C during this period did not show much change in the continuation group (−0.03; p=0.89) but increased in the discontinuation group (0.43%; p=0.06).36 This indicates that the benefits of rtCGM use on glucose lowering in this population were not sustained after discontinuing rtCGM and returning to SMBG.
A smaller, single-arm study of rtCGM was conducted in 38 adults with T2DM, including patients using various pharmacological diabetes therapies (except prandial insulin) and/or non-pharmacological methods of diabetes control.37 The primary outcome was a change in HbA1C from baseline to 6 months, where a mean HbA1C decrease of 3.0% was found (p<0.001).37 TTR increased (mean change 15.2; p<0.001) and body weight decreased during the study period (mean change −3.1 kg; p=0.002).37 About 53% of patients had their therapy intensified, and 39% had their medications changed or reduced during the study period.37
Whilst these studies do demonstrate the effectiveness of CGM devices in improving glycaemic control in T1DM and T2DM, it is important to note that the extent to which glucose is improved may depend on multiple factors. Patients with higher HbA1C at baseline may have a greater decrease in HbA1C as evidenced by subgroup analyses of the DIAMOND study.38 Those using rtCGM with HbA1C ≥9% had the greatest change in HbA1C, compared to those in lower HbA1C subgroups.38
T1DM or T2DM
Older adults
A multicentre study randomized 116 older adults (aged 60 years and older) with T1DM or T2DM to rtCGM or SMBG for 24 weeks.39 The primary endpoint was the change in HbA1C at 24 weeks and found an adjusted mean difference in HbA1C of −0.4% favouring rtCGM use (p<0.001).39 In this trial, adherence to rtCGM use was high with 97% of participants using the technology at least 6 days per week.39
Haemodialysis
A small study enrolled 15 adults with T1DM or T2DM on haemodialysis for 6 weeks of SMBG then 6 weeks of blinded professional CGM devices.40 Twenty per cent of patients were managed with diet control only. The primary outcome was the change in mean glucose level, which decreased from 150 to 139 mg/dL (p<0.05) over the 12-week period.40 Mean HbA1C also decreased from 6.9% to 6.5% (p<0.005).40 The study found medication changes were more likely to occur during the CGM period (2.1 versus 1.4; p<0.05).40
Pregnancy
CGM use in pregnancy has a growing level of evidence. A study randomized 325 participants with T1DM who were pregnant or planning pregnancy to rtCGM or SMBG.41 The primary outcome was a change in HbA1C after 34 weeks in pregnant women or 24 weeks in those planning pregnancy. There was a slight reduction in HbA1C in the pregnant group only (mean difference −0.19%; p=0.0207).41 Pregnant users also had higher TTR (68% versus 61%; p=0.0034).41 With rtCGM use, there were superior neonatal health outcomes, decreasing the risk of large for gestational age births by 49%, neonatal ICU stay longer than 24 hours by 52% and neonatal hypoglycaemia by 55%.41
Another study of 143 pregnant adult women with T1DM or T2DM on MDI or CSII compared rtCGM to SMBG.42 Participants used rtCGM 6 days per week during weeks 8, 12, 21, 27 and 33. This periodic approach found no statistically significant difference in HbA1C change, occurrence of severe hypoglycaemia or neonatal health outcomes.42
A third study included 106 pregnant women with gestational diabetes mellitus who were randomized to rtCGM or SMBG.43 There was no statistically significant difference in HbA1C or neonatal health outcomes between groups. However, the proportion of women who gained excessive weight was lower with rtCGM versus SMBG (33.3% versus 56.4%; p=0.039).43 A subgroup analysis also found that those who wore a rtCGM device during the second trimester gained less weight than those who used it in the third trimester (12.7 versus 14.3 kg; p=0.017).43
Safety – T1DM
More CGM safety data currently exist for T1DM. A study of adults with well-controlled T1DM compared isCGM and SMBG over 6 months.44 The primary outcome was a change in time spent in hypoglycaemia. They randomized 328 individuals treated with MDI or CSII and found time in hypoglycaemia with isCGM decreased from 3.28 to 2.03 h/day, resulting in a 38% reduction in time in hypoglycaemia.44
Another study randomized 149 adults treated with MDI with severe hypoglycaemia or hypoglycaemia unawareness to rtCGM or SMBG for 26 weeks.45 The primary outcome was the mean number of hypoglycaemic events per 28 days. The rtCGM group had a 72% decreased incidence of hypoglycaemic events (p<0.0001) compared to SMBG.45
A third study included adults treated with MDI.46 They randomized 161 individuals to rtCGM or SMBG for 26 weeks before a 17-week washout period, then crossover to the opposite treatment group. The primary endpoint was time spent in nocturnal hypoglycaemia. The rtCGM group spent less time in nocturnal hypoglycaemia, regardless of the time frame or glucose cut-off utilized.46 Corresponding time in daytime hypoglycaemia also was lower during rtCGM use.46
One trial has investigated hypoglycaemia risk in older adults (aged 60 years and older) treated with MDI or CSII.37 They randomized 203 participants to rtCGM or SMBG for 6 months. Time spent in hypoglycaemia decreased further with rtCGM (median between group difference −1.9%; p<0.001).47 Glucose variability also decreased by 4.7% with rtCGM.47 Severe hypoglycaemia occurred less frequently with rtCGM than with SMBG (1.9 versus 22.4 events/100 person-years).47
Considerations for use
An increase in technology and glucose data can be met with challenges for the end users. A study of children with T1DM described some common barriers to CGM device use, including pain with sensor insertion, disruption from alarms and alerts, gaps in data, adhesion issues, and skin sensitivity.48 Other concerns from caregivers were receiving overwhelming amounts of data requiring attention and difficulty interpreting data.48 Some patients may also struggle with the device set up and linking remote data sharing. Studies have found that providing adequate initial device training and ongoing support contributes to increased successful use of isCGM.49,50 Certain CGM devices can utilize smartphone technology and internet access to interpret and share glucose data. In patients with financial insecurity, access to CGM devices and supporting technology may be limited. Additionally, if literacy and health literacy concerns are present, this could result in less interpretation of CGM data and reduced translation into real-time health behaviour changes.
Although CGM devices are generally convenient to use, issues may arise. Dermatological concerns are common with long-term CGM use, including skin irritation, lipodystrophy and scarring.51 To prevent skin irritation, the patient should be instructed to thoroughly clean the area before inserting the sensor into the skin.51 If skin becomes red and irritated, the sensor should be removed and a new sensor inserted elsewhere.52 To help prevent lipodystrophy and scarring, the insertion site should be rotated through 6–10 different spots with each use.51 Another frequent issue is difficulty keeping the sensor in place for the full wear time of the product. It is important to instruct the patient to place the sensor in an area that will not rub against another part of the body, clothing or sports equipment.51 Patients can use a medical bandage, patches or adhesive products made for CGM sensors such as Skin Tac1, to help keep the sensor in place.51 Each CGM also has unique malfunction codes that can be identified and resolved by consulting the manufacturer.
CGM devices have specific interactions and precautions to consider when selecting a product for use. These are also summarized in Table 1. Freestyle Libre’s manufacturer identifies an interaction with ascorbic acid, which may falsely increase glucose readings, as well as salicylate, which may falsely decrease readings.11 Hydroxyurea may falsely increase glucose when using Dexcom G6 and, therefore, another CGM device should be selected if the patient uses this medication.12 The Senseonics Eversense sensor contains small amounts of dexamethasone acetate in a silicone ring (eluting approximately 3 mcg per day) that helps prevent an inflammatory response and should therefore be avoided if dexamethasone use is contraindicated.13,14 Tetracycline can also falsely decrease Eversense readings.14 Medtronic Guardian 3 is affected by acetaminophen and may falsely increase readings.9 Dexcom G6 is not affected by typical oral acetaminophen doses (1000 mg taken every 6 hours), but higher doses may falsely increase readings.12,15
Each CGM system also has a unique temperature range where the system can function. The Freestyle Libre has a temperature range of 10–45°C and with temperature excursions, data collection will be halted presenting an error message on the display device.53 Additionally, all CGM systems must be removed prior to the use of MRI, CT scans or diathermy treatment.9,10,12,14,19,54
Place in therapy
The 2022 American Diabetes Association (ADA) guidelines recommend the use of rtCGM in adult patients on MDI and CSII (Grade A), or basal insulin (Grade A), or the use of isCGM in adult patients on these regimens (Grades B, C).55 Additionally, rtCGM should be offered to youths with T1DM on MDI or CSII (Grade B) or isCGM (Grade E). A new recommendation is to offer rtCGM or isCGM for youths with T2DM on MDI or CSII (Grade E).55 All of these recommendations should be offered to patients who are capable of using these devices safely either alone or with a caregiver, and the decision on which device to use should be driven by patient needs and circumstances.55 The ADA recommends specifics on the frequency of use, especially in patients on MDI or CSII. For example, rtCGM ‘should be used as close to daily as possible for maximal benefit’ and isCGM should be scanned frequently, at a minimum of every 8 hours (Grades A).55 CGM monitoring can also help achieve HbA1C targets in pregnant patients with diabetes when used as an adjunct to preprandial and postprandial SMBG (Grade B).55
The ADA also notes that the use of professional CGM, isCGM or rtCGM can help with the recognition and correction of hyperglycaemia and hypoglycaemia, improving HbA1C levels in patients with diabetes on insulin and non-insulin therapy (Grade C).55 CGM monitoring can help achieve HbA1C targets in pregnant patients with diabetes when used as an adjunct to preprandial and postprandial SMBG (Grade B).55 Lastly, the ADA recommends that patients should have access to CGM at the onset of diabetes diagnosis requiring insulin therapy and, if attained, patients should have consistent access across third-party payors (Grade E).55 Importantly, they recognize the importance of robust diabetes education, and recommend ongoing education, training and support for the optimal implementation and use of CGM.55
The American Association of Clinical Endocrinology published their 2021 guidelines on diabetes technology and strongly recommend CGM devices for all patients with diabetes treated with three or more insulin injections per day or insulin pump therapy (Grade A).56 CGM is also strongly recommended for the following individuals: those with problematic hypoglycaemia, hypoglycaemia unawareness, nocturnal hypoglycaemia, and frequent or severe hypoglycaemia, adolescents, children/adolescents with T1DM, pregnant women with T1DM and T2DM on intensive insulin therapy, and women with gestational diabetes mellitus on insulin therapy (Grade A) and not on insulin therapy (Grade B), as well as T2DM treated with less intensive insulin therapy (Grade B).56
The decision to use professional or personal use CGM devices and type of device should be driven by patient preference and patient-specific factors, including but not limited to hypoglycaemia unawareness and need for alerts/alarms, insurance coverage and cost, dexterity issues, neuropathy or circulatory problems, ease of insertion and technology, need for insulin pump therapy integration, calibrations, and adhesive sensitivity.16 Real-time CGM may be the preferred option for patients with hypoglycaemia unawareness, frequent nocturnal hypoglycaemia, frequent severe hypoglycaemia, significantly variability with the goal of trying to improve time in range, and those that would benefit from data sharing, on insulin pump therapy.6 Clinicians may consider isCGM for patients newly diagnosed with T2DM who may be unwilling or unable to perform SMBG, patients with prediabetes, T2DM on oral antidiabetic therapy or only on basal insulin needing titration, or patients with limited risk for hypoglycaemia and zero to no degree of hypoglycaemia unawareness.16,56 Data suggest that patients with T1DM may especially benefit from a system with alerts, regardless of the presence of hypoglycaemia unawareness.28 An increasing number of insurance companies in the United States are covering CGM devices either through the durable medical equipment benefit or through the pharmacy benefit but coverage may vary in other countries. If a personal use CGM device is not an option, clinicians may consider a professional CGM device. In general, isCGM devices are less costly compared to rtCGM devices. It is important to note that, at this time, all personal CGM devices mentioned in this article are approved by the FDA to facilitate insulin dose changes, except for Medtronic Guardian, which requires adjunctive use of fingerstick glucose checks to guide treatment decisions.10
Conclusion
CGM technology is now approved for use in T1DM and T2DM. Across studies of multiple age groups, it has been shown to effectively reduce HbA1C, improve glucose TTR, increase diabetes treatment satisfaction, and importantly reduce the risk of hypoglycaemia and severe hypoglycaemia. CGM device use in pregnant women with diabetes has been shown to decrease the risk of neonatal complications versus SMBG use. Recent advancements in CGM technology have increased access to glucose levels and trends by patients and clinicians alike. Successfully expanding the widespread use of CGM devices will require improved affordability and coverage by more third-party payors. It will also involve increasing patient and clinician familiarity with a new system and the data interpretation that accompanies it.
Key practice points
Continuous glucose monitoring (CGM) devices have shown improvement in efficacy (haemoglobin A1C, time in target glucose range) and safety (time in hypoglycaemia, rates of severe hypoglycaemia) metrics key to managing type 1 and 2 diabetes.
CGM device use improves diabetes treatment satisfaction in patients across multiple age groups.
Barriers to CGM device use may include sensor insertion or adhesion issues, skin reactions, disruption from alerts, data inundation, technology issues and cost. Addressing these barriers through adequate training, ongoing support and improved coverage is key to successful use.
Acknowledgements
None.
Footnotes
Contributions: JK conducted the literature search, reviewed the literature, wrote portions of the manuscript and edited and approved the full manuscript. AS and BW reviewed the literature, wrote portions of the manuscript and edited and approved the full manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/02/dic.2021-9-13-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2022 Kluemper JR, Smith A, Wobeter B. https://doi.org/10.7573/dic.2021-9-13. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.World Health Organization. Diabetes. [Accessed August 4, 2021]. https://www.who.int/news-room/fact-sheets/detail/diabetes .
- 2.World Health Organization. The top 10 causes of death. [Accessed August 4, 2021]. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death .
- 3.American Diabetes Association Professional Practice Committee; American Diabetes Association Professional Practice Committee. Draznin B, et al. 5. Facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S60–S82. doi: 10.2337/dc22-S005. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch IB. Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington, VA: American Diabetes Association; Aug 1, 2018. Introduction: history of glucose monitoring. [DOI] [PubMed] [Google Scholar]
- 5.Cengiz E, Tamborlane WV. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol Ther. 2009;11(Suppl 1):S11–S16. doi: 10.1089/dia.2009.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edelman S, Argento N, Pettus J, et al. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care. 2018;41(11):2265–2274. doi: 10.2337/dc18-1150. [DOI] [PubMed] [Google Scholar]
- 7.Resources for healthcare professionals: FreeStyle Libre CGM systems. Resources for your practice, Personal CGM, FreeStyle libre providers. [Accessed August 5, 2021]. https://provider.myfreestyle.com/freestyle-libre-resources.html .
- 8.Dexcom. Continuous glucose monitoring. Dexcom; [Accessed September 29, 2021]. https://www.dexcom.com/en-IE/ie-dexcom-g6-cgm-system . [Google Scholar]
- 9.Ascensia Diabetes. [Accessed September 29, 2021];Eversense continuous glucose monitoring system. https://www.ascensiadiabetes.com/eversense/eversense-cgm-system . [Google Scholar]
- 10.Medtronic Diabetes. [Accessed September 13, 2021];Guardian Sensor 3 user guide. https://www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Sensor-3-user-guide.pdf . [Google Scholar]
- 11.Freestyle Libre. Full indications and important safety information. [Accessed August 14, 2021]. https://www.freestyle.abbott/us-en/safety-information.html#:~:text=Taking%20ascorbic%20acid%20(vitamin%20C)%20supplements%20while%20wearing%20the%20Sensor,a%20severe%20low%20glucose%20event .
- 12.Dexcom. [Accessed September 28, 2021];Safety information. https://www.dexcom.com/safety-information . [Google Scholar]
- 13.Deiss D, Szadkowska A, Gordon D, et al. Clinical practice recommendations on the routine use of Eversense, the first long-term implantable continuous glucose monitoring system. Diabetes Technol Ther. 2019;21(5):254–264. doi: 10.1089/dia.2018.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eversense. [Accessed September 29, 2021];CGM sensor insertion and removal instructions. https://www.fda.gov/media/112159/download . [Google Scholar]
- 15.Calhoun P, Johnson TK, Hughes J, Price D, Balo AK. Resistance to acetaminophen interference in a novel continuous glucose monitoring system. J Diabetes Sci Technol. 2018;12(2):393–396. doi: 10.1177/1932296818755797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longo R, Sperling S. Personal vs professional continuous glucose monitoring: when to use which on whom. Diabetes Spectrum. 2019;32(3):183–193. doi: 10.2337/ds18-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn D, Pettus J, Edelman S. Unblinded CGM should replace blinded CGM in the clinical management of diabetes. J Diabetes Sci Technol. 2016;110:793–798. doi: 10.1177/1932296816632241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy M, Jugnee N, Anantharaja S, Oliver N. Switching from flash glucose monitoring to continuous glucose monitoring on hypoglycemia in adults with type 1 diabetes at high hypoglycemia risk: the extension phase of the I HART CGM study. Diabetes Tech & Therap. 2018;20(11):751–757. doi: 10.1089/dia.2018.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergenstal RM. Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington, VA: American Diabetes Association; Aug 20–23, 2018. Understanding continuous glucose monitoring data. [DOI] [PubMed] [Google Scholar]
- 20.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kröger J, Reichel A, Siegmund T, Ziegler R. Clinical recommendations for the use of the ambulatory glucose profile in diabetes care. J Diabetes Sci Technol. 2020;14(3):586–594. doi: 10.1177/1932296819883032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aleppo G, Webb K. Continuous glucose monitoring integration in clinical practice: a stepped guide to data review and interpretation. J Diabetes Sci Technol. 2019;13(4):664–673. doi: 10.1177/1932296818813581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371–378. doi: 10.1001/jama.2016.19975. [DOI] [PubMed] [Google Scholar]
- 25.Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379–387. doi: 10.1001/jama.2016.19976. [DOI] [PubMed] [Google Scholar]
- 26.Sequeira PA, Montoya L, Ruelas V, et al. Continuous glucose monitoring pilot in low-income type 1 diabetes patients. Diabetes Technol Ther. 2013;15(10):855–858. doi: 10.1089/dia.2013.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tumminia A, Crimi S, Sciacca L, et al. Efficacy of real-time continuous glucose monitoring on glycaemic control and glucose variability in type 1 diabetic patients treated with either insulin pumps or multiple insulin injection therapy: a randomized controlled crossover trial. Diabetes Metab Res Rev. 2015;31(1):61–68. doi: 10.1002/dmrr.2557. [DOI] [PubMed] [Google Scholar]
- 28.Visser MM, Charleer S, Fieuws S, et al. Comparing real-time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): a 6-month, prospective, multicentre, randomised controlled trial. Lancet. 2021;397:2275–2283. doi: 10.1016/S0140-6736(21)00789-3. [DOI] [PubMed] [Google Scholar]
- 29.Boucher SE, Gray AR, Wiltshire EJ, et al. Effect of 6 months of flash glucose monitoring in youth with type 1 diabetes and high-risk glycemic control: a randomized controlled trial. Diabetes Care. 2020;43(10):2388–2395. doi: 10.2337/dc20-0613. [DOI] [PubMed] [Google Scholar]
- 30.Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2388–2396. doi: 10.1001/jama.2020.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thabit H, Prabhu JN, Mubita W, et al. Use of factory-calibrated real-time continuous glucose monitoring improves time in target and HbA1c in a multiethnic cohort of adolescents and young adults with type 1 diabetes: the MILLENNIALS study. Diabetes Care. 2020;43(10):2537–2543. doi: 10.2337/dc20-0736. [DOI] [PubMed] [Google Scholar]
- 32.Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365–374. doi: 10.7326/M16-2855. [DOI] [PubMed] [Google Scholar]
- 33.Ehrhardt NM, Chellappa M, Walker MS, Fonda SJ, Vigersky RA. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5(3):668–675. doi: 10.1177/193229681100500320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55–73. doi: 10.1007/s13300-016-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262–2272. doi: 10.1001/jama.2021.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aleppo G, Beck RW, Bailey R, et al. The effect of discontinuing continuous glucose monitoring in adults with type 2 diabetes treated with basal insulin. Diabetes Care. 2021;44:2729–2737. doi: 10.2337/dc21-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grace T, Salyer J. Use of real-time continuous glucose monitoring improves glycemic control and other clinical outcomes in type 2 diabetes patients treated with less intensive therapy. Diabetes Technol Ther. 2022;24:26–31. doi: 10.1089/dia.2021.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Billings LK, Parkin CG, Price D. Baseline glycated hemoglobin values predict the magnitude of glycemic improvement in patients with type 1 and type 2 diabetes: subgroup analyses from the DIAMOND Study Program. Diabetes Technol Ther. 2018;20:561–565. doi: 10.1089/dia.2018.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C. Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol. 2017;11(6):1138–1146. doi: 10.1177/1932296817704445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joubert M, Fourmy C, Henri P, Ficheux M, Lobbedez T, Reznik Y. Effectiveness of continuous glucose monitoring in dialysis patients with diabetes: the DIALYDIAB pilot study. Diabetes Res Clin Pract. 2015 Mar;107(3):348–354. doi: 10.1016/j.diabres.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 41.Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390(10110):2347–2359. doi: 10.1016/S0140-6736(17)32400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care. 2013;36(7):1877–1883. doi: 10.2337/dc12-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei Q, Sun Z, Yang Y, Yu H, Ding H, Wang S. Effect of a CGMS and SMBG on maternal and neonatal outcomes in gestational diabetes mellitus: a randomized controlled trial. Sci Rep. 2016;27(6):19920. doi: 10.1038/srep19920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked randomised controlled trial. Lancet. 2016;388(10057):2254–2263. doi: 10.1016/S0140-6736(16)31535-5. [DOI] [PubMed] [Google Scholar]
- 45.Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391(10128):1367–1377. doi: 10.1016/S0140-6736(18)30297-6. [DOI] [PubMed] [Google Scholar]
- 46.Ólafsdóttir AF, Polonsky W, Bolinder J, et al. A randomized clinical trial of the effect of continuous glucose monitoring on nocturnal hypoglycemia, daytime hypoglycemia, glycemic variability, and hypoglycemia confidence in persons with type 1 diabetes treated with multiple daily insulin injections (GOLD-3) Diabetes Technol Ther. 2018;20(4):274–284. doi: 10.1089/dia.2017.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pratley RE, Kanapka LG, Rickels MR, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2397–2406. doi: 10.1001/jama.2020.6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493–498. doi: 10.1089/dia.2019.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hermanns N, Ehrmann D, Schipfer M, Kröger J, Haak T, Kulzer B. The impact of a structured education and treatment programme (FLASH) for people with diabetes using a flash sensor-based glucose monitoring system: results of a randomized controlled trial. Diabetes Res Clin Pract. 2019;150:111–121. doi: 10.1016/j.diabres.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Pintus D, Ng SM. Freestyle libre flash glucose monitoring improves patient quality of life measures in children with Type 1 diabetes mellitus (T1DM) with appropriate provision of education and support by healthcare professionals. Diabetes Metab Syndr. 2019;13(5):2923–2926. doi: 10.1016/j.dsx.2019.07.054. [DOI] [PubMed] [Google Scholar]
- 51.Messer LH, Berget C, Beatson C, Polsky S, Forlenza GP. Preserving skin integrity with chronic device use in diabetes. Diabetes Technol Ther. 2018;20(S2):S254–S264. doi: 10.1089/dia.2018.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Medtronic Diabetes. [Accessed August 14, 2021];Sensor & transmitter troubleshooting tips. https://www.medtronicdiabetes.com/customer-support/sensors-and-transmitters-support/troubleshoot-sensor . [Google Scholar]
- 53.Freestyle Libre. [Accessed September 29, 2021];FAQ. https://www.freestyle.abbott/us-en/support/faq.html?page=device%2Ffreestyle-libre-14-day-system%2Ffaq%2Ftopic%2Fsystem . [Google Scholar]
- 54.Medtronic Diabetes. [Accessed September 29, 2021];Important safety information. https://www.medtronicdiabetes.com/important-safety-information . [Google Scholar]
- 55.American Diabetes Association Professional Practice Committee. 7. Diabetes technology: standards of medical care in diabetes – 2022. Diabetes Care. 2022;45(Suppl 1):S97–S112. doi: 10.2337/dc22-S007. [DOI] [PubMed] [Google Scholar]
- 56.Grunberger G, Sherr J, Allende M, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: the use of advanced technology in the management of persons with diabetes Mellitus. Endocrine Practice. 2021;27:505–537. doi: 10.1016/j.eprac.2021.04.008. [DOI] [PubMed] [Google Scholar]

