Abstract
The central and peripheral nervous systems play critical roles in regulating pancreatic islet function and glucose metabolism. Over the last century, in vitro and in vivo studies along with examination of human pancreas samples have revealed the structure of islet innervation, investigated the contribution of sympathetic, parasympathetic and sensory neural pathways to glucose control, and begun to determine how the structure and function of pancreatic nerves are disrupted in metabolic disease. Now, state-of-the art techniques such as 3D imaging of pancreatic innervation and targeted in vivo neuromodulation provide further insights into the anatomy and physiological roles of islet innervation. Here, we provide a summary of the published work on the anatomy of pancreatic islet innervation, its roles, and evidence for disordered islet innervation in metabolic disease. Finally, we discuss the possibilities offered by new technologies to increase our knowledge of islet innervation and its contributions to metabolic regulation.
Keywords: 3D imaging, Innervation, Islet, Nerve, Neuromodulation, Pancreas, Parasympathetic, Review, Sensory, Sympathetic
Introduction
Metabolic regulation requires integration across multiple organs to maintain normal blood glucose levels. The pancreas, a highly heterogeneous organ with both exocrine and endocrine components, is critical to this metabolic control. The endocrine pancreas comprises the islets of Langerhans, cell clusters composed of the following diverse cell types: insulin-producing beta cells; glucagon-producing alpha cells; somatostatin-producing delta cells; and pancreatic polypeptide (PP)-producing cells. Insulin and glucagon, the major regulators of blood glucose, limit high (hyperglycaemia) and low (hypoglycaemia) blood glucose levels, respectively. To maintain normoglycaemia, pancreatic hormones are rapidly released in response to ingested nutrients or hypoglycaemia, or in anticipation of increased glucose requirements such as during stress or exercise. While pancreatic beta and alpha cells respond directly to blood glucose changes, significant evidence suggests that neural signals are critical for physiological pancreatic hormone release and metabolic regulation.
The pancreas is innervated by the autonomic nervous system, which regulates organ function in response to internal signals and external cues. The autonomic nervous system is composed of sympathetic and parasympathetic pathways. The sympathetic nervous system provides motor (efferent) inputs to the pancreas while the parasympathetic circuit has both a sensory (afferent) pathway relaying sensory information from the pancreas and an efferent pathway to regulate pancreatic function. Spinal afferent nerves also detect pancreatic sensory signals. Additionally, fibres project from the gastrointestinal tract to the pancreas to form enteropancreatic innervation (Fig. 1).
Fig. 1.
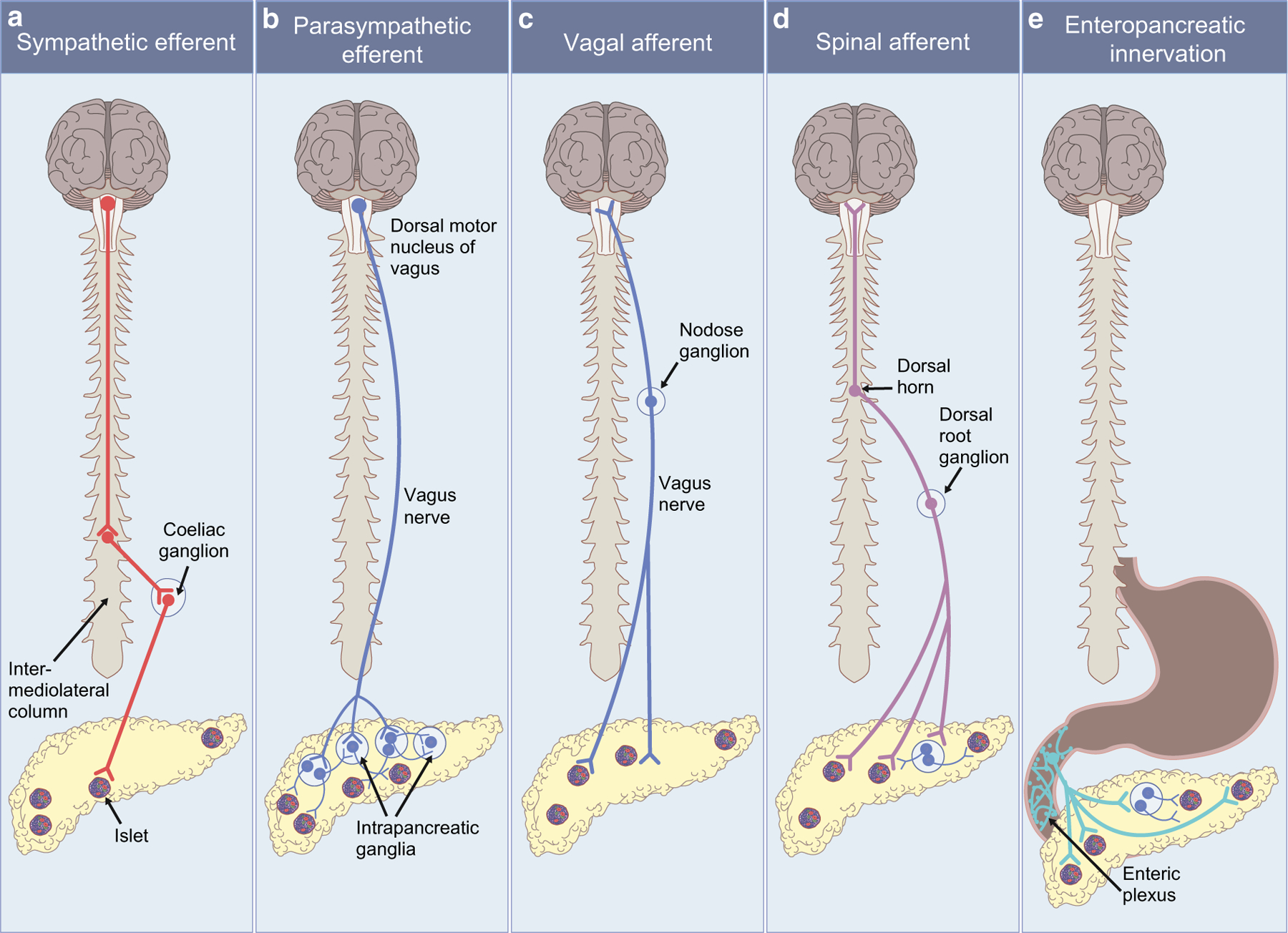
Summary of pancreatic innervation. (a) Pancreatic sympathetic preganglionic neurons arise from the intermediolateral column of the spinal cord and project to the coeliac ganglion. Postganglionic sympathetic neurons project from the coeliac ganglion to the pancreas. (b) Pancreatic parasympathetic efferent preganglionic neurons originate from the dorsal motor nucleus of the vagus and travel in the vagus nerve to intrapancreatic ganglia. Postganglionic parasympathetic neurons project from intrapancreatic ganglia to the islets and to other intrapancreatic ganglia to form a network within the pancreas. (c) Pancreatic parasympathetic afferent neurons with terminals in the pancreas and cell bodies in the nodose ganglia travel in the vagus nerve to provide sensory information from the pancreas to the nucleus of the solitary tract. (d) Pancreatic spinal sensory neurons with cell bodies in the dorsal root ganglia convey sensory information from the pancreas to the dorsal horn of the spinal cord and then to the CNS. (e) Enteropancreatic neurons with cell bodies in the myenteric ganglia of the stomach and duodenum project to the pancreas. This figure is available as part of a downloadable slideset
Here, we provide an overview of the existing literature on the anatomy of pancreatic islet innervation and its function and discuss the effects of metabolic disease on islet innervation. Finally, we explore the exciting opportunities provided by new technologies to extend our understanding of islet innervation in metabolic regulation.
Anatomy of pancreatic innervation
Sympathetic efferent innervation
The pancreas is innervated by sympathetic efferent nerves. The cell bodies of sympathetic preganglionic neurons, which express acetylcholine (ACh), are located in the intermediolateral columns of the spinal cord at levels T6–L2 [1–3]. Their axons travel in splanchnic nerves to the superior mesenteric and coeliac ganglia. Postganglionic neurons from these ganglia then project to the pancreas [4, 5] (Fig. 1a). Retrograde tracing from the pancreas demonstrates that numerous neurons in the coeliac ganglia innervate the pancreas [5].
Pancreatic postganglionic sympathetic fibres express tyrosine hydroxylase (TH) [6–8] and vesicular monoamine transporter 2 [9]. Immunohistochemistry for these markers demonstrates that sympathetic fibres innervate both islet endocrine cells and vasculature [7, 10] (Fig. 2). Species differences have been reported. Rodriguez-Diaz and Caicedo [11] described sympathetic fibre contacts on endocrine cells (alpha > delta > beta) in mouse islets but few contacts on endocrine cells in human islets (delta > alpha > beta). In contrast, recent 3D imaging studies suggested significant sympathetic innervation of human islets [6, 8, 12], with contacts on alpha and delta cells [6] that would allow direct sympathetic regulation of pancreatic endocrine function.
Fig. 2.
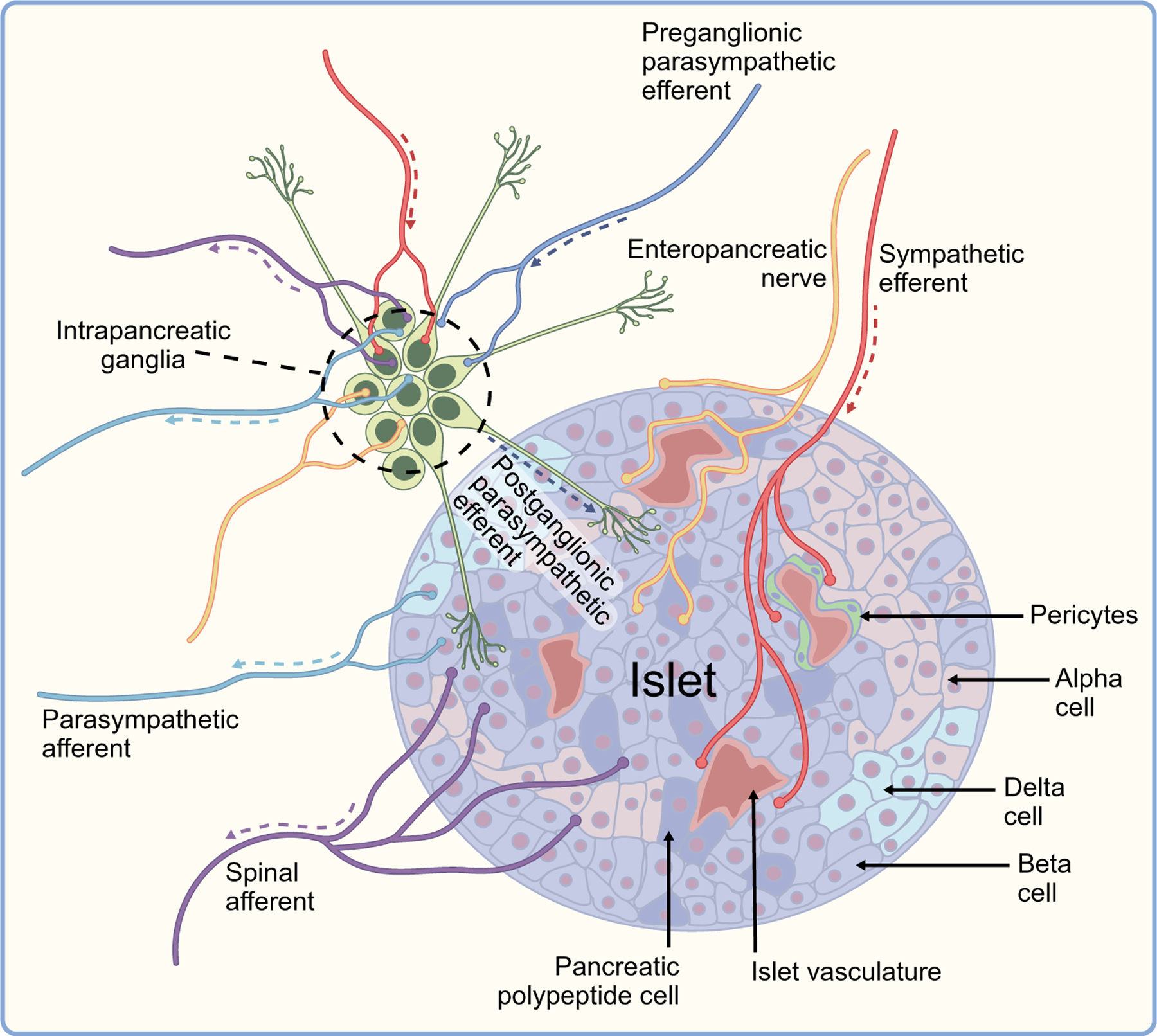
Summary of mouse islet innervation. Pancreatic islets are innervated by the autonomic nervous system. Preganglionic parasympathetic efferent fibres (dark blue) innervate intrapancreatic ganglia. Postganglionic parasympathetic efferent fibres project from intrapancreatic ganglia to the islets (green). Sympathetic efferent fibres (red) innervate intrapancreatic ganglia, islets and blood vessels. Islets also receive input from enteric neurons (yellow). Sensory information is transmitted through parasympathetic afferents (light blue) and spinal afferents (purple). Arrows show the direction of transmission. This figure is available as part of a downloadable slideset
The main sympathetic neurotransmitter is noradrenaline (norepinephrine), although pancreatic sympathetic nerves also express and release neuropeptides, including neuropeptide Y and galanin [13–15]. Their known effects on islet function are summarised in Table 1. All three neuropeptides have been reported to inhibit insulin and stimulate glucagon secretion (see below [Physiological roles of pancreatic innervation: sympathetic efferent innervation] for more details on the effects of noradrenaline). Adrenergic receptors are expressed in pancreatic islets: α2-adrenergic receptors on beta cells; β2-adrenergic receptors on alpha cells; and other receptor subtypes [15, 16]. All three isoforms of galanin receptors are expressed in islets [17] and neuropeptide Y receptors are present in beta cells [18].
Table 1.
Major neurotransmitters and neuropeptides of pancreatic innervation
| Neurotransmitter/neuropeptide | Receptor mediating effects of neurotransmission | Relevant cell type receiving neural signals | Effects of neural input to effector cell | References |
|---|---|---|---|---|
| Sympathetic nervous system | α2-adrenoceptors β2-adrenoceptors |
Beta cells | Inhibits insulin secretion | [6, 177,178] |
| Noradrenaline | β2-adrenoceptors | Alpha cells | Increases glucagon secretion | [6, 177, 178] |
| Neuropeptide Y | Neuropeptide Y1 receptor | Beta cells | Inhibits insulin and somatostatin secretion and stimulates glucagon secretion | [15] |
| Galanin | GalR1 | Islet cells | Inhibits insulin and somatostatin secretion and stimulates glucagon secretion | [15] |
| PACAP | PAC1 | Intrapancreatic ganglia, beta cells | Increases insulin secretion | [62] |
| Parasympathetic nervous system | ||||
| ACh | M3 | Beta cells | Increases insulin secretion | [15] |
| VIP | VPAC1 | Beta cells | Increases insulin secretion | [92] |
| VIP | VPAC2 | Intrapancreatic ganglia | Increases insulin secretion | [92] |
| VIP | Unknown | Alpha cells | Increases glucagon secretion during hypoglycaemic episodes | [62] |
| PACAP | PAC1 (PACAP-specific receptor) | Beta cells | Increases insulin secretion (generally increases pancreatic secretions) | [62, 92] |
| PACAP | VPAC2 | Intrapancreatic ganglia | Increases insulin secretion | [62, 92] |
| PACAP | Unknown | Alpha cells | Increases glucagon secretion during hypoglycaemic episodes | [92] |
| PP | NPY4R | Delta cells | Reduces somatostatin secretion, increases insulin secretion and reduces plasma glucose | [93] |
| GRP | GRPR | Beta cells, intrapancreatic ganglia | Stimulates insulin secretion | [33] |
| Sensory nervous system | ||||
| CGRP | RAMP1 | Beta cells | Inhibits insulin production | [115] |
| CGRP | CLR | Beta cells | Inhibits insulin production | [115] |
| PACAP | Unknown | Unknown | Unknown | [34, 179] |
| SP | NK-1R | Alpha cells | Unknown | [116] |
CLR, calcitonin-like receptor; NK-1R, neurokinin 1 receptor; RAMP1, receptor activity-modifying protein 1
The anatomy of pancreatic innervation has been examined across several species, and there are conflicting findings for human vs mouse innervation. While there are likely to be biological differences, the technical difficulties associated with examining innervation in human samples are considerable. Human samples present inherent variability as a consequence of several factors: premorbid conditions and medications; age; sex; site of biopsy; fixation methods; shipping conditions; and differences in antibodies and staining methods. Hence, any conclusion derived from examining small cohorts of human tissues should be taken with caution.
Gene expression in sympathetic neurons specifically innervating the pancreas remains unknown. This information might indicate whether a specific neural population defined by expression of a particular neuropeptide or combination of neuropeptides contributes to pancreatic innervation and so allow targeted modulation. The use of new viral tracing and genetic tools (e.g. single-cell RNA sequencing) that have been applied to peripheral ganglia such as the nodose [19] could provide significant new information about the nature and function of sympathetic neurons innervating the pancreas.
Parasympathetic efferent innervation
The pancreas is also innervated by efferent parasympathetic nerves (Fig. 1b). Pancreatic preganglionic parasympathetic efferent neurons originate in the dorsal motor nucleus of the vagus, particularly on the left [20]. These neurons are largely cholinergic and their axons contribute to the vagus nerve [20]. The vagus nerve divides into five branches in the abdomen: hepatic; anterior and posterior gastric; and anterior and posterior coeliac [21]. Pancreas-projecting parasympathetic neurons travel in the hepatic and anterior gastric branches of the vagus and synapse on intrapancreatic ganglia [22, 23].
Intrapancreatic ganglia are dispersed throughout the pancreas, often close to islets [8, 22, 24, 25]. These ganglia contain cholinergic postganglionic cell bodies [26, 27] and their axons project to other intrapancreatic ganglia and to islets, forming a neuro-insular network [24, 27]. As well as receiving inputs from preganglionic parasympathetic neurons, intrapancreatic ganglia also integrate inputs from several types of fibre: postganglionic sympathetic fibres expressing noradrenaline, neuropeptide Y and galanin; sensory fibres expressing substance P, calcitonin gene-related peptide (CGRP) and pituitary adenylate cyclase activating polypeptide (PACAP); and enteric fibres expressing ACh, serotonin, PACAP and nitric oxide [27].
Parasympathetic islet innervation has been verified in many species, including cats, rabbits, rodents and humans, by light and electron microscopy after immunolabelling for choline acetyltransferase or vesicular ACh transporter [26–29]. In mice, postganglionic parasympathetic fibres contact alpha, beta, delta and PP cells [24] (Fig. 2). However, results are conflicting about the contribution of parasympathetic innervation to human islets. Some 2D immunohistochemical studies report sparse vesicular ACh transporter-positive fibres [11] while recent 3D imaging studies suggest that parasympathetic fibres are present in human islets [25].
Pancreatic postganglionic parasympathetic neurons primarily release ACh (Table 1), and subpopulations of these neurons express vasoactive intestinal polypeptide (VIP), gastrin-releasing peptide (GRP), PACAP and nitric oxide [20, 30–32]. GRP acts directly on islets via GRP receptors [33]. PACAP and VIP are often colocalised in ganglionic neurons in the rat [34]. PACAP and VIP have common receptors (VIP1 and VIP2), while the PACAP receptor PAC1 is specific for PACAP. PACAP is also co-expressed with galanin, substance P and corticotropin-releasing factor in the intrapancreatic ganglia of the sheep pancreas [35]. Galanin and neuropeptide Y have also been reported in parasympathetic nerve terminals and intrapancreatic ganglia of pigs and rodents [13, 14, 36]. The actions of these neurotransmitters and neuropeptides are described in detail below (Physiological roles of pancreatic innervation: parasympathetic efferent innervation, and Table 1).
Our understanding of the anatomy and neurochemical composition of intrapancreatic ganglia and postganglionic parasympathetic efferent innervation has been limited by their small size and scattered distribution. It is difficult to accurately map these structures using traditional 2D immunolabelling, but 3D imaging techniques should enable continued characterisation of intrapancreatic ganglia and their projections.
Vagal afferent innervation
Pancreatic sensory innervation can be divided into spinal afferent and vagal afferent innervation (Fig. 1c,d). Retrograde tracing studies suggest that these pathways contribute equally to pancreatic sensory innervation [37]. Vagal sensory neurons have cell bodies in nodose ganglia and their fibres travel in the vagus nerve from the pancreas to the brainstem nucleus of the solitary tract and area postrema [38]. Pancreatic vagal sensory neurons express neuropeptides such as CGRP (mouse) [37], substance P (rat) [39] and cocaine-and-amphetamine-regulated transcript [40], as well as transient receptor potential cation channel subfamily V member 1 (TRPV1, mouse) [37], voltage-gated sodium channel, α subunit, type X (NaV1.8, mouse) [41], serotonin type 3 receptor (5HT3R, mouse) [42] and insulin receptors (mouse, rat) [43, 44] (Table 1). These are not separate neural populations. For example, insulin receptor expression overlaps with the expression of TRPV1, CGRP and substance P in vagal afferent neurons. In rats, intranodose injection of an anterograde tracer demonstrated vagal sensory innervation of the islet core, exocrine pancreas, vasculature and major ducts. However, labelled fibres were present in only 16–25% of islets [45]. In mice, pancreatic vagal afferent fibres are primarily activated by chemical signals such as serotonin [42], insulin [43], capsaicin [46], and cholecystokinin and its analogues [42]. Recent in vivo imaging studies provided direct evidence that vagal sensory fibres detect islet activity. Makhmutova et al [42] demonstrated that chemogenetic activation of pancreatic beta cells activates calcium signalling in nodose ganglia neurons. Their studies suggest that serotonin co-released with insulin from beta cells acts via 5HT3R to stimulate pancreatic vagal afferents. Mechanically activated vagal sensory neurons may also be involved, as lamellar corpuscles have been identified in the human [47] and cat pancreas [48].
Spinal afferent innervation
Pancreas-projecting spinal sensory neurons have their cell bodies in dorsal root ganglia from thoracic and upper lumbar segments (T3–L5 [cat] [49], T8–T11 [rat] [50], T6–T12 [mouse] [37]). Their fibres travel in splanchnic nerves to innervate the pancreas. Myelinated and unmyelinated fibres [51] transmit sensory signals to lamina I and IV in the dorsal horn of the spinal cord. Postsynaptic neurons then ascend in the dorsal column of the spinal cord to terminate in the gracile and cuneate nuclei [51]. Spinal sensory fibres express substance P, CGRP, TRPV1, insulin receptor [44], and NaV1.8 [41] (Table 1). While many markers are common to both vagal and spinal sensory fibres, CGRP immunoreactivity predominantly marks spinal sensory fibres. Almost 90% of pancreatic spinal sensory neurons express CGRP compared with 8–15% of vagal afferents (mice and rats) [37, 50]. In rats, CGRP-positive neurons co-express TRPV1 [46], tachykinin [52] and PACAP [34]. CGRP-positive fibres innervate islets, exocrine pancreas and intrapancreatic ganglia, with greater sensory innervation reported in male mice [46, 53, 54]. Substance P-positive fibres appear to be a separate, smaller neural population in rats [55]. These fibres innervate intrapancreatic ganglia and the peri-islet area in human pancreatic tissue and both the islet periphery and core in mice [56]. The presence of substance P-positive neurons in intrapancreatic ganglia raises the possibility of a local reflex pathway within the pancreas, similar to that in the enteric nervous system [57]. Pancreatic spinal afferents primarily respond to mechanical stimulation of the parenchyma and vasculature but subsets of spinal sensory neurons also respond to chemical stimuli such as histamine and bradykinin [58]. While there has been significant progress in understanding pancreatic afferent innervation, many details about the 3D structure, activity, and neurochemical identity of pancreatic vagal and spinal afferent innervation remain unknown.
Gut–pancreas innervation
In addition to autonomic and spinal innervation of the pancreas, there are also direct neural inputs from enteric neurons to the pancreas [59] (Fig. 1e). However, there is limited information about the anatomy or function of this pathway. Developmental studies have suggested that common neuronal precursors contribute to gut and pancreas innervation [60–62]. Furthermore, structural and neurochemical similarities between the enteric and intrapancreatic innervation support a common embryological origin [59]. Enteric fibres projecting to the pancreas are likely serotonergic and cholinergic. In rats, serotonergic (5-hydroxytryptamine) fibres from the enteric neurons in the duodenum and stomach project to intrapancreatic ganglia and islets [59]. 5-HT1A receptors are expressed in intrapancreatic ganglia neurons and alpha cells in rats [63]. Several studies have shown that serotonin modulates intrapancreatic ganglia activity in pancreatic explants [63–65]. PACAP may also be expressed in nerve bundles from the duodenum to the pancreas, projecting to intrapancreatic ganglia and islets [62], with PACAP (PAC1) expressed in intrapancreatic ganglia, enteric neurons and beta cells [62].
The structure and gene expression of enteropancreatic innervation are understudied compared with other pancreatic innervation circuits but application of newer tissue clearing methods and methods to interrogate gene expression in defined neural populations will likely provide important information about this pathway.
Physiological roles of pancreatic innervation
Sympathetic efferent innervation
Pancreatic sympathetic activation occurs when increased blood glucose is required (e.g. during hypoglycaemia, or the fight or flight response). Ex vivo and in vivo studies in many species suggest that sympathetic activation increases blood glucose through increased glucagon and reduced insulin secretion (Table 1). Direct application of adrenergic agonists inhibited insulin secretion from rat pancreas ex vivo [66]. In vivo, electrical stimulation of splanchnic nerves in anaesthetised cats reduced plasma insulin, increased glucagon secretion and blood glucose, without an effect on plasma somatostatin. Similar results have been reported in other species [67–71]. Clinical studies are consistent with these findings. Noradrenaline infusion decreased glucose-induced insulin secretion via α-adrenergic receptors in humans [66]. However, conflicting findings have been reported. Kaneto et al [72] described a slow increase in insulin levels after splanchnic stimulation in anaesthetised dogs. Additionally, splanchnic stimulation in pigs increased insulin secretion when basal glucose levels were above 5 mmol/l [73]. Pharmacological studies aimed at dissecting the contribution of sympathetic innervation to glucose control have also been contradictory. In one study, the selective β-receptor antagonist propranolol decreased glucagon release basally but not release during sympathetic stimulation [72], and in another, it decreased glucagon secretion from isolated canine pancreases during catecholamine/adrenergic stimulation [74]. Splanchnic nerve transection in anaesthetised dogs reduced blood glucose but did not alter plasma insulin [75]. More recently, ablation of sympathetic innervation using systemic 6-hydroxydopamine, a neurotransmitter analogue that reduces noradrenaline, decreased glucagon secretion [9]. Sympathetic fibres are in close contact with islet vasculature [76–78]. Almaça et al [77] found that pericytes, endothelial cells adjacent to capillaries, are contacted by sympathetic fibres and express adrenergic β2 and α2 receptors [79]. Sympathetic stimulation reduces capillary diameter and islet blood flow in murine and human pancreatic slices [78] (Table 2). These studies suggest that sympathetic activity regulates islet vasculature and glucagon and insulin release to control blood glucose. Additional studies to precisely identify and manipulate sympathetic innervation to the pancreas and, ideally, to the islets, are needed to better understand its contribution to pancreatic function.
Table 2.
Summary of the effects of pancreatic innervation on vasculature and blood glucose
| Peripheral nervous system | Insulin secretion | Glucagon secretion | PP secretion | Somatostatin secretion | Islet vasculature | Blood glucose |
|---|---|---|---|---|---|---|
| Sympathetic | Decrease [66] | Increase [67, 68] | Unknown | Decrease [15] | Vasoconstriction [77] | Increase |
| Parasympathetic | Increase [26, 27] | Modestly increase [27, 111] | Increase [81, 110] | Increase [81] | Increase [82] | Decrease |
| Parasympathetic afferent innervation | Decrease [109, 113] | Increase [111] | Unknown | Unknown | Unknown | Increase [112, 113] |
| Spinal afferent innervation | Conflicting evidence [112, 124] | Conflicting evidence [120, 124, 122] | Unknown | Unknown | Responds to mechanical stimulation of vasculature [180, 181] | Conflicting evidence [124,125, 162] |
Parasympathetic efferent innervation
Pancreatic parasympathetic efferent innervation is activated during the pre-absorptive cephalic phase of insulin secretion and postprandially. Extensive ex vivo and in vivo studies suggest that parasympathetic efferent neurotransmitters and neuropeptides act on islets, increasing insulin release and lowering blood glucose via muscarinic receptors (Table 2). Muscarinic agonist treatment and ACh stimulate beta cell insulin secretion in vitro and in vivo [80]. Electrical stimulation of the vagus nerve results in increased PP and somatostatin release, which is mediated by muscarinic mechanisms [81]. In vivo, activation of postganglionic cholinergic nerves by electrical stimulation and optogenetic tools results in insulin secretion [81, 82]. Furthermore, parasympathetic activation of transplanted mouse islets in the anterior chamber of the mouse eye improves glucose tolerance. This effect is blunted by atropine, a muscarinic antagonist [83]. Atropine also blunts postprandial insulin release in monkeys [84] and after nutrient infusion in rats [85]. In dogs, vagal blockade during intraduodenal nutrient infusion halves insulin release [86]. Similarly, vagal transection inhibits insulin secretion, increases plasma glucagon and impairs glucose tolerance [87–89]. The effects of parasympathetic efferent activity on insulin release are likely mediated by M3 receptors. M3 receptor antagonist treatment inhibits insulin secretion from isolated rat islets and in vivo in mice [90] while M1 and M2 receptor antagonists have no effect on insulin release from isolated mouse islets [91]. Mice lacking M3 muscarinic receptors have impaired glucose tolerance and reduced insulin secretion in response to glucose and carbachol.
The neuropeptides VIP and PACAP stimulate insulin release in a glucose-dependent manner from mouse islets in vitro as well as in vivo in many species including mice and humans [62, 92]. VIP and PACAP also increase glucagon secretion in perfused rat pancreas [92]. PP acts on neuropeptide Y4 receptors on somatostatin-producing delta cells to reduce somatostatin secretion and so increase insulin secretion and lower blood glucose in humans [93]. GRP regulates insulin secretion from beta cells in vivo in rats and mice [33]. GRP receptor antagonists reduced vagal-stimulated insulin release, suggesting GRP’s involvement in parasympathetic control of insulin secretion [33].
Parasympathetic efferent innervation also contributes to cephalic-phase insulin release. In this response, gustatory, olfactory and visual sensory information along with metabolic signals [94] are integrated in the hypothalamus, leading to activation of parasympathetic efferent innervation. This results in insulin and PP secretion before any elevation in blood glucose. Infusion of trimetophane, an autonomic ganglionic blocker, in healthy individuals reduced cephalic-phase insulin release by 75%, impairing glucose clearance [80]. Although cephalic-phase insulin secretion constitutes a fraction of postprandial insulin secretion, loss of this response (via vagotomy or atropine blockade [95–97]) significantly impairs glucose tolerance [13, 98–100].
Pancreatic parasympathetic efferent innervation may contribute to pulsatile insulin release. In islets, synchronised beta cell electrical impulses increase cytosolic Ca2+ concentrations, with peaks every 5–7 min, resulting in insulin secretion [101]. These peaks match plasma insulin oscillations in vivo [102–106]. Inputs from postganglionic neurons in intrapancreatic ganglia may synchronise insulin secretion across islets [106, 107]. Electrophysiological studies reveal that insulin oscillations during electrical stimulation of intrapancreatic ganglia are prevented by nicotinic blockers or tetrodotoxin, a chemical agent used to prevent action potentials [108]. Fendler et al showed insulin synchronisation in model islets with ACh-induced inositol trisphosphate pulses [109]. Additionally, plasma insulin oscillations are only restored 200 days after intrahepatic islet transplantation, possibly representing the time taken for reinnervation of transplanted islets [106].
Parasympathetic efferent activity may also contribute to the glucagon response to hypoglycaemia. Plasma PP, which is controlled by and serves as an indirect measurement of parasympathetic activity, is increased by mild hypoglycaemia [110]. Stimulation of the vagus nerve augments glucagon release, with hypoglycaemia in non-diabetic rodents and humans [110]. In rats, muscarinic antagonists, adrenergic antagonists, or a combination of the two, blunt the glucagon response to hypoglycaemia, suggesting that both parasympathetic and sympathoadrenal pathways regulate glucagon secretion with hypoglycaemia [111].
Vagal afferent innervation
Pancreatic vagal afferent nerves may contribute to glycaemic control, although their precise physiological roles remain unclear. Ex vivo studies demonstrate that pancreatic vagal afferents are activated by postprandial hormones such as cholecystokinin, serotonin and insulin. Selective electrical activation of vagal afferent fibres increased blood glucose and glucagon in rats [111, 112] and inhibited glucose-stimulated insulin release in mice, resulting in increased blood glucose and impaired glucose tolerance [113, 114] (Table 2). While these studies suggest vagal afferent signalling may regulate glucose metabolism, the approaches used do not specifically target pancreatic vagal afferent innervation. Newer approaches allowing more targeted modulation should define the specific contribution of pancreatic vagal afferent circuits to glucose regulation.
Spinal afferent innervation
Pancreatic spinal afferent nerves are activated by pathological conditions such as pancreatitis and pancreatic cancer. However, their activity under normal physiological circumstances is not well understood. Ex vivo, pancreatic spinal afferent nerves are activated by mechanical distension of pancreatic vasculature and chemical signals such as histamine and bradykinin [58]. Most investigations into the contribution made by pancreatic spinal innervation have used chemical ablation, which targets vagal and spinal sensory fibres across several organs, so dissecting the contribution of spinal sensory innervation is challenging. However, receptors for neurotransmitters/neuropeptides released from spinal sensory terminals are found in islets (Tables 1 and 2). CGRP receptor complexes (calcitonin receptor-like receptor/receptor activity-modifying protein 1) are expressed in murine beta cells [115] while neurokinin 1 receptors activated by substance P are expressed in murine alpha cells [116]. Ex vivo studies demonstrate that CGRP inhibits insulin production from rat islets [115]. In vivo, ablation of TRPV1+/CGRP+ fibres increases glucose-stimulated insulin release in male but not female mice [54, 117] and in obese Zucker rats [46], suggesting that sensory innervation may restrain insulin release. In keeping with this, TRPV1 null mice show enhanced glucose-stimulated insulin release and increased beta cell mass [46]. However, there are significant species differences: CGRP has no effect on insulin release in calves [118] and increases insulin in pigs [119]. TRPV1+/CGRP+ fibre ablation also blunts the glucagon response to glucopenia [120]. The effects of capsaicin ablation of TRPV1+/CGRP+ fibres on glycaemic control are likely via spinal rather than vagal afferent neurons since co-culture of dorsal root ganglia and islets reduces glucose-induced insulin secretion [121]. In addition, the effects of capsaicin are reproduced by removing dorsal root ganglia that innervate the pancreas [117].
Substance P-positive sensory innervation may also regulate islet hormone release. In vitro, substance P dose-dependently increases insulin and glucagon release from the canine pancreas [122] but can both stimulate [123] and inhibit insulin [120] from rat pancreas and inhibits glucagon release [124]. In vivo, substance P administration also regulates pancreatic hormone release, inhibiting insulin and stimulating glucagon release resulting in hyperglycaemia in rats [124, 125], and increasing both insulin and glucagon release in pigs [119]. Targeted neuromodulation of vagal and spinal pancreatic afferent innervation may provide more information about their physiological roles and the contribution made by specific neuropeptides to glucose regulation.
Gut–pancreas innervation
The functional role of enteropancreatic innervation has not been fully elucidated. Most published studies have used gut–pancreas explants to examine whether the enteric nervous system modulates activity of pancreatic nerves. Kirchgessner and Gershon [59] demonstrated that local duodenal application of veratridine, an activator of voltage-dependent Na+ channels that stimulates enteric neurons, activated pancreatic neurons and islets. This effect was diminished when the myenteric plexus was partially removed.
Bariatric surgery may disrupt gut–pancreas innervation, so studying its effects on pancreatic hormone release could provide insights into the possible roles of the enteropancreatic innervation. Bariatric surgery in obese individuals with type 2 diabetes rapidly improves insulin sensitivity and islet function [126, 127] even before significant weight loss. The mechanisms responsible for the beneficial metabolic effects are unclear, although improved secretion of gut and pancreatic hormones (e.g. ghrelin, glucagon-like-peptide-1, insulin, glucagon) could be partially responsible. Interestingly, changes in peripheral innervation after the procedure may be also involved. Fibres from the gut project to intrapancreatic ganglia and there is some evidence that bariatric surgery may modulate pancreatic parasympathetic function. For example, parasympathetic-mediated early insulin release is still present in individuals with type 2 diabetes after bariatric surgery but is blunted in individuals with type 2 diabetes without bariatric surgery. Plasma PP levels (an indirect measure of parasympathetic activity) are also modified by bariatric surgery but the results are conflicting, with reported increase [128] or decrease [129] in meal-induced PP levels after bariatric surgery. Indirect measures of parasympathetic activity (heart rate variability) suggest that bariatric surgery may increase parasympathetic tone [130].
Additional evidence may be gained about the role of gut–pancreas innervation from studying type 3c diabetes, which develops secondary to pancreatic diseases involving exocrine and digestive pancreatic functions. Diabetes has a high prevalence (40%) in pancreatic cancer and may develop before cancer symptoms appear [131]. Clinical studies suggest that type 3c diabetes is associated with a blunted or absent PP response to a mixed meal challenge, suggesting impaired parasympathetic activation [132, 133]. Interestingly, Kang et al and others [134, 135] report that pancreatoduodenectomy, a procedure used to treat pancreatic cancer, improves glycaemic control. Specifically, these studies show a decrease in insulin resistance and enhanced glucose-stimulated insulin secretion, independent of BMI. As pancreatoduodenectomy alters enteropancreatic innervation by disrupting both the pancreas and duodenum, it is plausible that loss or reorganisation in the enteropancreatic innervation may contribute to improved blood glucose control. However, PP responses are not improved by pancreatoduodenectomy [136].
The beneficial effects of both bariatric surgery and pancreatoduodenectomy on glycaemic control in pancreatic cancer patients suggest that enteropancreatic communication may have an important role. Detailed preclinical studies are needed to address the large gaps in our understanding of gut–pancreas innervation and its contribution to regulating islet function in the healthy state as well as after surgical interventions.
Pancreatic innervation in transplantation and metabolic disease
Islet transplantation
Islet transplantation is typically indicated for individuals with unstable type 1 diabetes complicated by severe hypoglycaemic episodes refractory to other treatments [137]. Revascularisation and reinnervation contribute to the success of islet transplants but the time course and extent of reinnervation varies with transplantation site. In a study in mice, reinnervation of islets transplanted into the anterior chamber of the eye began within days [83]. Sympathetic TH+ innervation contacted blood vessels at day 15 post-transplantation and increased in density up to day 30. Sympathetic fibres were present in the islet parenchyma after day 30 and plateaued by day 90. In the same mice, parasympathetic vesicular ACh transporter-positive reinnervation was slower but followed a similar pattern [83]. In another mouse study, islet cells transplanted into the liver were reinnervated by beta tubulin III-positive nerves after 3 weeks [138]. Sympathetic fibres and varicosities contacted alpha cells but no parasympathetic fibres were detected in the liver or engrafted islets. Using 3D graft histology of islets transplanted under the kidney capsule in mice, Juang et al [139] found that sympathetic TH+ nerves extend from renal parenchyma into the graft boundary at 3 weeks post-transplantation. TH+ nerves are associated with glucagon-positive alpha cells and vasculature [139]. Houwing et al [140] examined islets transplanted into the liver, spleen or kidney capsule of rats and demonstrated immunoreactivity to pan-neuronal (neuron-specific enolase), parasympathetic (choline acetyl-transferase) and sympathetic markers (TH and dopamine β-hydroxylase), with innervation patterns similar to pancreatic islets. However, other studies suggest increased sympathetic reinnervation with relatively sparse sensory reinnervation, as determined by CGRP and substance P immunoreactivity [141]. Reinnervation may play a role in islet graft survival. Islets cultured with neurotrophic factors had improved survival in vitro and in vivo after transplantation in streptozotocin-induced diabetic mice [142]. In support of this, addition of growth factors in streptozotocin-induced diabetic mice enhanced islet sympathetic innervation and enhanced endocrine and exocrine pancreatic function [143].
Revascularisation is particularly important to islet transplantation secretory function in response to blood glucose [144]. Many studies indicate that islet survival, insulin content and cell mass decline rapidly 1–3 days after transplantation, while the transplanted islets are avascular [145]. Islet transplantation in highly vascularised organs such as the liver can promote survival of the transplanted islets. Islets transplanted into the liver begin revascularisation within 3 days, connecting to the hepatic arterial tree, and revascularisation is complete by day 14 [146, 147]. Revascularisation of mouse islets transplanted into the anterior chamber of the eye begins within a few days and reaches a similar density as pancreatic islets at 4 weeks post-surgery [148, 149]. However, transplanted islets in rats and mice may be less vascularised than native islets, with lower oxygen tension even when revascularisation is completed, irrespective of transplantation site [144].
Several studies suggest that reinnervation is important in transplanted islet function. Atropine, a muscarinic antagonist, significantly impaired glucose tolerance in mice with intraocular islet transplants, suggesting cholinergic input to the transplanted islets improved glycaemic control [83]. In addition, exercise reduced insulin secretion from islets implanted into the rat liver, suggesting intact sympathetic inputs to these islets [150]. Islets implanted into the liver parenchyma demonstrated pulsatile insulin secretion, suggesting re-established synchronisation of transplanted islets, possibly via neural mechanisms [151]. Furthermore, direct electrical stimulation of hepatic nerves of rats with hepatic neonatal islets transplants inhibited insulin release [152]. Finally, islet blood flow did not differ between transplanted and native pancreatic tissue 4 weeks after pancreatic transplantation in rats, suggesting that sympathetic regulation of vascular tone was maintained in transplanted tissue [153]. However, several studies show impaired neural function in transplanted islets. Omer et al [154] demonstrated that exercise did not adequately suppress insulin or increase glucagon release from islets 4–6 weeks after transplantation into the liver, kidney or peritoneal cavity of diabetic rats, resulting in hypoglycaemia. These results suggest inadequate sympathetic function in transplanted islets [154]. In addition, Jansson et al [155] found that direct stimulation of nerves innervating islets transplanted under the kidney capsule in rats had minor effects on insulin release, suggesting that islet reinnervation had minimal effects on function. However, electrical stimulation does not distinguish between efferent vs afferent nerves or sympathetic vs parasympathetic fibres. Furthermore, diabetic mice with intraportal islet transplantation lack early insulin release during an OGTT, suggesting dysfunction of parasympathetic circuits in the transplanted islets [156].
Together these studies suggest that the site of transplantation may lead to differences in islet reinnervation, such as a lack of parasympathetic innervation in islets implanted into the liver. On balance, existing functional studies suggest that innervation may regulate transplanted islet function; however, longitudinal studies to examine islet function before, during and after full reinnervation would provide important insights, as would additional studies with targeted modulation of sympathetic or parasympathetic innervation to transplanted islets.
Metabolic disorders
A growing number of studies have examined whether defects in pancreatic innervation contribute to metabolic disease. Hypothalamic pathologies may lead to obesity but can also result in hyperinsulinaemia and impaired glucose tolerance relative to weight-matched individuals. Obesity may also result in hypothalamic inflammation and contribute to excess sympathetic excitation and reduced parasympathetic tone [157]. Similarly, individuals with a family history of type 2 diabetes may have reduced parasympathetic tone and increased sympathetic activity (as measured by cardiovascular variables) but the effects on pancreatic autonomic activity are not known [158]. Spinal cord injuries may also contribute to metabolic disease through increased sympathetic activity leading to hyperinsulinaemia and insulin resistance [159, 160].
Studies examining pancreatic sympathetic innervation have conflicting results in humans and animal models of type 1 diabetes. Early experiments described the loss of sympathetic innervation in the islets of Biobreeder rats but not in streptozotocin-induced diabetic rats [161]. Similarly, islet sympathetic innervation was decreased in pancreases from individuals with either recently diagnosed or long-established type 1 diabetes but not in samples from individuals with type 2 diabetes [162]. However, recent studies using tissue clearing techniques have not confirmed these findings. Tang and co-workers [12] reported an increase in intra-islet TH+ axons in streptozotocin-injected mice. In diabetic NOD mice, islet sympathetic innervation was unaltered but sympathetic fibre density was increased in regions of immune infiltration. Campbell-Thompson et al [6] identified reduced sympathetic islet innervation in cleared pancreatic tissue from non-diabetic, islet autoantibody-positive individuals but normal innervation in tissue from type 1 diabetes individuals. Several studies have suggested that there may be impaired pancreatic sympathetic transmission due to longstanding hyperglycaemia in type 1 diabetes and type 2 diabetes [9, 163]. While sympathetic overactivity may lead to basal hyperglucagonaemia (a hallmark of type 1 diabetes), blunted ability to increase sympathetic function further may impair the counter-regulatory response to hypoglycaemia in diabetes. Women with gestational diabetes show increased sympathetic activity, impaired glucose tolerance, insulin resistance, and chronic hyperglycaemia [164, 165] Sympathetic innervation may also modulate immune activity in diabetes. Activation of sympathetic innervation to pancreatic lymph nodes delayed diabetes onset in non-obese diabetic mice [166]. However, sympathetic denervation and adrenoceptor blockade limited inflammation and improved blood glucose levels in a mouse model of type 1 diabetes [167].
Compromised parasympathetic pancreatic innervation has been implicated in obesity and diabetes in some studies: parasympathetic innervation density was reduced in the exocrine pancreas of individuals with type 1 diabetes [168]; intrapancreatic ganglia were significantly enlarged in pancreatic tissue from donors with type 2 diabetes [25]; and impaired glucose tolerance and type 2 diabetes were characterised by disturbed insulin pulsatility [169]. Whether this is a consequence of neural or islet dysfunction remains unknown.
The effects of diabetes and obesity on the structure and function of pancreatic sensory innervation remain understudied. CGRP and substance P innervation were unchanged in streptozotocin-treated diabetic rats [123] but there is some evidence that pancreatic sensory innervation contributes to type 1 and type 2 diabetes. In type 1 diabetes, TRPV1+ and substance P innervation may exacerbate islet inflammation since TRPV1+ ablation reduced islet inflammation and delayed diabetes onset [170] while substance P acting via NK1 receptors blunted T cell proliferation [123] in non-obese diabetic mice. TRPV1+ innervation may also contribute to type 2 diabetes and insulin resistance. TRPV1+ fibre ablation prevented insulin resistance in ageing rats [170], improved glucose tolerance in diabetic streptozotocin-treated rats [171] and improved insulin release and glucose tolerance in obese Zucker rats [172]. However, these studies used systemic administration of TRPV1 agonists to ablate sensory innervation across many organs so the contribution of pancreatic sensory innervation to type 1 diabetes and type 2 diabetes remains unclear.
Unanswered questions and opportunities
A significant body of work supports islet innervation from sympathetic, parasympathetic and spinal sensory pathways as well as the existence of enteropancreatic communication. Despite the enormous progress that has been made, many questions remain unanswered about the anatomy, neurotransmitters and functional roles of pancreatic innervation. There are conflicting findings on the anatomy, density and targets of islet innervation between species. Recent advances in electron microscopy and light-sheet imaging may help clarify possible species differences, likely regional variation, contacts between innervation and endocrine cells, and the architecture of pancreas-projecting neurons in peripheral ganglia in health and metabolic disease.
The molecular profile of pancreas-projecting neurons in peripheral and intrapancreatic ganglia also remains incomplete. Approaches such as single-cell/single-nucleus RNAseq with viral targeting to tag pancreas-projecting neurons may help identify specific neuropeptides, neurotransmitters and receptors. Further studies examining the activity of pancreas-projecting neurons as well as specific targeting of pancreatic innervation are crucial for determining the latter’s physiological function. In particular, we know little about the specific roles of intrapancreatic ganglia or how signals from autonomic, enteric and sensory innervation are integrated by these ganglia. New viral approaches specifically targeting pancreatic innervation in intrapancreatic ganglia, coeliac ganglia and nodose ganglia are needed to elucidate the precise contribution of pancreatic innervation to metabolic regulation and its roles in metabolic disease. Genetically targeted neuromodulatory technologies such as chemogenetics [173], optogenetics [174] and magnetogenetics [175] could be suitable for this purpose. Combining neuromodulatory approaches with novel imaging techniques and methods such as long-term culture of pancreatic slices may allow a more detailed understanding of the contribution of pancreatic innervation to islet function and metabolic control.
Summary
Our understanding of islet innervation and its importance in metabolic regulation has grown enormously in the decades since Claude Bernard’s seminal work [176]. Multiple studies have revealed extensive islet innervation by autonomic, sensory and enteric pathways that contribute to modulating islet function via actions on endocrine cells and vasculature. This knowledge provides insights into key roles for pancreatic innervation and raises intriguing questions about the contribution of pancreatic innervation to metabolic disease. Novel tools and innovative approaches should allow us to uncover the precise contributions made by islet innervation to glucose control and diabetes with even greater precision in the coming years and possibly allow us to harness this understanding to provide novel avenues for treating metabolic disease.
Summary of the effects of pancreatic innervation on islet function.
Pancreatic sympathetic efferent activation during hypoglycaemia or the fight/flight response increases glucagon secretion and blood glucose
Pancreatic parasympathetic efferent nerve activation during the cephalic pre-absorptive phase of insulin secretion lowers blood glucose
Pancreatic sensory innervation can be divided into spinal afferent and vagal afferent innervation and may increase blood glucose
Enteropancreatic innervation is understudied but may modulate pancreatic hormone release
Acknowledgements
We would like to thank A. Alvarsson, R. Li and K. Devarakonda (Diabetes, Obesity and Metabolism Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA) for their assistance with preparation of the manuscript.
Funding
RFH is supported by NIH grant F31DK129016. MJG is supported by the Naomi Berries Diabetes Center Russell Berrie Foundation Award. Work in the authors’ laboratories is supported by the American Diabetes Association Pathway to Stop Diabetes Grant ADA no. 1-17-ACE-31 and by grants from the NIH (R01NS097184, OT2OD024912 and R01DK124461), NSF (1930163) and Department of Defense (W81XWH-20-1-0345, W81XWH-20-1-0156).
Abbreviations
- ACh
Acetylcholine
- CGRP
Calcitonin gene-related peptide
- GRP
Gastrin-releasing peptide
- 5HT3R
Serotonin type 3 receptor
- NaV1.8
Voltage gated sodium channel, α subunit, type X
- PACAP
Pituitary adenylate cyclase activating polypeptide
- PP
Pancreatic polypeptide
- TH
Tyrosine hydroxylase
- TRPV1
Transient receptor potential cation channel subfamily V member 1
- VIP
Vasoactive intestinal polypeptide
Footnotes
Supplementary Information The online version contains a slideset of the figures for download, which is available to authorised users at https://doi.org/10.1007/s00125-022-05691-9.
References
- 1.Luiten PGM, ter Horst GJ, Koopmans SJ, Rietberg M, Steffens AB (1984) Preganglionic innervation of the pancreas islet cells in the rat. J Auton Nerv Syst 10(1):27–42. 10.1016/0165-1838(84)90065-1 [DOI] [PubMed] [Google Scholar]
- 2.Jansen AS, Hoffman JL, Loewy AD (1997) CNS sites involved in sympathetic and parasympathetic control of the pancreas: a viral tracing study. Brain Res 766(1–2):29–38. 10.1016/s0006-8993(97)00532-5 [DOI] [PubMed] [Google Scholar]
- 3.Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K (2001) Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol 431(4):405–423. [DOI] [PubMed] [Google Scholar]
- 4.Chen XH, Itoh M, Sun W, Miki T, Takeuchi Y (1996) Localization of sympathetic and parasympathetic neurons innervating pancreas and spleen in the cat. J Auton Nerv Syst 59(1–2): 12–16. 10.1016/0165-1838(95)00136-0 [DOI] [PubMed] [Google Scholar]
- 5.Quinson N, Robbins HL, Clark MJ, Furness JB (2001) Locations and innervation of cell bodies of sympathetic neurons projecting to the gastrointestinal tract in the rat. Arch Histol Cytol 64(3):281–294. 10.1679/aohc.64.281 [DOI] [PubMed] [Google Scholar]
- 6.Campbell-Thompson M, Butterworth EA, Boatwright JL et al. (2021) Islet sympathetic innervation and islet neuropathology in patients with type 1 diabetes. Sci Rep 11(1):6562. 10.1038/s41598-021-85659-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang S-C, Shen C-N, Lin P-Y et al. (2018) Pancreatic neuro-insular network in young mice revealed by 3D panoramic histology. Diabetologia 61(1):158–167. 10.1007/s00125-017-4408-y [DOI] [PubMed] [Google Scholar]
- 8.Alvarsson A, Jimenez-Gonzalez M, Li R et al. (2020) A 3D atlas of the dynamic and regional variation of pancreatic innervation in diabetes. Sci Adv 6(41):eaaz9124. 10.1126/sciadv.aaz9124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mundinger TO, Mei Q, Figlewicz DP, Lernmark Å, Taborsky GJ (2003) Impaired glucagon response to sympathetic nerve stimulation in the BB diabetic rat: effect of early sympathetic islet neuropathy. Am J Physiol Metab 285(5):E1047–E1054. 10.1152/ajpendo.00136.2003 [DOI] [PubMed] [Google Scholar]
- 10.Lindsay TH, Halvorson KG, Peters CM et al. (2006) A quantitative analysis of the sensory and sympathetic innervation of the mouse pancreas. Neuroscience 137(4):1417–1426. 10.1016/j.neuroscience.2005.10.055 [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Diaz R, Caicedo A (2014) Neural control of the endocrine pancreas. Best Pract Res Clin Endocrinol Metab 28(5):745–756. 10.1016/j.beem.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 12.Chiu Y-C, Hua T-E, Fu Y-Y, Pasricha PJ, Tang S-C (2012) 3-D imaging and illustration of the perfusive mouse islet sympathetic innervation and its remodelling in injury. Diabetologia 55(12): 3252–3261. 10.1007/s00125-012-2699-6 [DOI] [PubMed] [Google Scholar]
- 13.Ahrén B (2000) Autonomic regulation of islet hormone secretion–implications for health and disease. Diabetologia 43(4):393–410. 10.1007/s001250051322 [DOI] [PubMed] [Google Scholar]
- 14.Sheikh SP, Holst JJ, Skak-Nielsen T et al. (1988) Release of NPY in pig pancreas: dual parasympathetic and sympathetic regulation. Am J Physiol Liver Physiol 255(1):G46–G54. 10.1152/ajpgi.1988.255.1.G46 [DOI] [PubMed] [Google Scholar]
- 15.Dunning BE, Taborsky GJ (1989) Galanin release during pancreatic nerve stimulation is sufficient to influence islet function. Am J Physiol Metab 256(1):E191–E198. 10.1152/ajpendo.1989.256.1.E191 [DOI] [PubMed] [Google Scholar]
- 16.Hu X, Friedman D, Hill S et al. Proteomic exploration of pancreatic islets in mice null for the α2A adrenergic receptor. J Mol Endocrinol 35(1):73–88. 10.1677/jme.1.01764 [DOI] [PubMed] [Google Scholar]
- 17.Barreto SG, Bazargan M, Zotti M et al. (2011) Galanin receptor 3 – a potential target for acute pancreatitis therapy. Neurogastroenterol Motil 23(3):e141–e151. 10.1111/j.1365-2982.2010.01662.x [DOI] [PubMed] [Google Scholar]
- 18.Franklin ZJ, Tsakmaki A, Fonseca Pedro P et al. (2018) Islet neuropeptide Y receptors are functionally conserved and novel targets for the preservation of beta-cell mass. Diabetes Obes Metab 20(3):599–609. 10.1111/dom.13119 [DOI] [PubMed] [Google Scholar]
- 19.Bai L, Mesgarzadeh S, Ramesh KS et al. (2019) Genetic identification of vagal sensory neurons that control feeding. Cell 179(5): 1129–1143.e23. 10.1016/j.cell.2019.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Love JA, Yi E, Smith TG (2007) Autonomic pathways regulating pancreatic exocrine secretion. Auton Neurosci 133(1):19–34. 10.1016/j.autneu.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 21.Fox EA, Powley TL (1985) Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res 341(2):269–282. 10.1016/0006-8993(85)91066-2 [DOI] [PubMed] [Google Scholar]
- 22.Lkhagvasuren B, Mee-Inta O, Zhao Z-W, Hiramoto T, Boldbaatar D, Kuo Y-M (2021) Pancreas-brain crosstalk. Front Neuroanat 15: 691777. 10.3389/fnana.2021.691777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berthoud HR, Powley TL (1990) Identification of vagal preganglionics that mediate cephalic phase insulin response. Am J Phys 258(2 Pt 2):R523–R530. 10.1152/ajpregu.1990.258.2.R523 [DOI] [PubMed] [Google Scholar]
- 24.Li W, Yu G, Liu Y, Sha L (2019) Intrapancreatic ganglia and neural regulation of pancreatic endocrine secretion. Front Neurosci 13:21. 10.3389/fnins.2019.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang SC, Baeyens L, Shen CN et al. (2018) Human pancreatic neuro-insular network in health and fatty infiltration. Diabetologia 61(1):168–181. 10.1007/s00125-017-4409-x [DOI] [PubMed] [Google Scholar]
- 26.Ahrén B, Taborsky GJJ, Porte DJ (1986) Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia 29(12):827–836. 10.1007/BF00870137 [DOI] [PubMed] [Google Scholar]
- 27.Brunicardi FC, Shavelle DM, Andersen DK (1995) Neural regulation of the endocrine pancreas. Int J Pancreatol Off J Int Assoc Pancreatol 18(3):177–195. 10.1007/BF02784941 [DOI] [PubMed] [Google Scholar]
- 28.Esterhuizen AC, Spriggs TL, Lever JD (1968) Nature of islet-cell innervation in the cat pancreas. Diabetes 17(1):33–36. 10.2337/diab.17.1.33 [DOI] [PubMed] [Google Scholar]
- 29.Coupland RE (1958) The innervation of pan creas of the rat, cat and rabbit as revealed by the cholinesterase technique. J Anat 92(1):143–149 [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Zheng H, Berthoud HR (1999) Functional vagal input to chemically identified neurons in pancreatic ganglia as revealed by Fos expression. Am J Phys 277(5):E958–E964. 10.1152/ajpendo.1999.277.5.E958 [DOI] [PubMed] [Google Scholar]
- 31.Gilon P, Henquin JC (2001) Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev 22(5):565–604. 10.1210/edrv.22.5.0440 [DOI] [PubMed] [Google Scholar]
- 32.Di Cairano ES, Moretti S, Marciani P et al. (2016) Neurotransmitters and neuropeptides: new players in the control of islet of Langerhans’ cell mass and function. J Cell Physiol 231(4):756–767. 10.1002/jcp.25176 [DOI] [PubMed] [Google Scholar]
- 33.Karlsson S, Sundler F, Ahrén B (1998) Insulin secretion by gastrin-releasing peptide in mice: ganglionic versus direct islet effect. Am J Phys 274(1):E124–E129. 10.1152/ajpendo.1998.274.1.E124 [DOI] [PubMed] [Google Scholar]
- 34.Hannibal J, Fahrenkrug J (2000) Pituitary adenylate cyclase-activating polypeptide in intrinsic and extrinsic nerves of the rat pancreas. Cell Tissue Res 299(1):59–70. 10.1007/s004419900124 [DOI] [PubMed] [Google Scholar]
- 35.Arciszewski MB, Mozel S, Sienkiewicz W (2015) Pituitary adenylate cyclase-activating peptide-27 (PACAP-27) is co-stored with galanin, substance P and corticotropin releasing factor (CRF) in intrapancreatic ganglia of the sheep. Pol J Vet Sci 18(2): 343–350. 10.1515/pjvs-2015-0044 [DOI] [PubMed] [Google Scholar]
- 36.Pettersson M, Ahrén B, Lundquist I, Böttcher G, Sundler F (1987) Neuropeptide Y: intrapancreatic neuronal localization and effects on insulin secretion in the mouse. Cell Tissue Res 248(1):43–48. 10.1007/BF01239960 [DOI] [PubMed] [Google Scholar]
- 37.Fasanella KE, Christianson JA, Chanthaphavong RS, Davis BM (2008) Distribution and neurochemical identification of pancreatic afferents in the mouse. J Comp Neurol. 10.1002/cne.21736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalia M, Sullivan JM (1982) Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol 211(3):248–265. 10.1002/cne.902110304 [DOI] [PubMed] [Google Scholar]
- 39.Shen Q, Wang Y, Zhang N, Gao D, Liu Y, Sha L (2016) Substance P expresses in intrapancreatic ganglia of the rats. Neuropeptides 59:33–38. 10.1016/j.npep.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 40.Wierup N, Sundler F (2006) CART is a novel islet regulatory peptide. Peptides 27(8):2031–2036. 10.1016/j.peptides.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 41.Gautron L, Sakata I, Udit S, Zigman JM, Wood JN, Elmquist JK (2011) Genetic tracing of Nav1.8-expressing vagal afferents in the mouse. J Comp Neurol 519(15):3085–3101. 10.1002/cne.22667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makhmutova M, Weitz J, Tamayo A et al. (2021) Pancreatic β-cells communicate with vagal sensory neurons. Gastroenterology 160(3):875–888.e11. 10.1053/j.gastro.2020.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwasaki Y, Shimomura K, Kohno D et al. (2013) Insulin activates vagal afferent neurons including those innervating pancreas via insulin Cascade and ca(2+) influx: its dysfunction in IRS2-KO mice with Hyperphagic obesity. PLoS One 8(6):e67198. 10.1371/journal.pone.0067198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lázár BA, Jancsó G, Oszlács O, Nagy I, Sántha P (2018) The insulin receptor is Colocalized with the TRPV1 nociceptive Ion Channel and neuropeptides in pancreatic spinal and vagal primary sensory neurons. Pancreas 47(1):110–115. 10.1097/MPA.0000000000000959 [DOI] [PubMed] [Google Scholar]
- 45.Neuhuber WL (1989) Vagal afferent fibers almost exclusively innervate islets in the rat pancreas as demonstrated by anterograde tracing. J Auton Nerv Syst 29(1):13–18. 10.1016/0165-1838(89)90015-5 [DOI] [PubMed] [Google Scholar]
- 46.Gram DX, Ahrén B, Nagy I et al. (2007) Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci 25(1):213–223. 10.1111/j.1460-9568.2006.05261.x [DOI] [PubMed] [Google Scholar]
- 47.Varga I, Nosál M, Babál P (2020) Ectopic lamellar Pacinian corpuscle within the thymus. Atypical or abnormal location? Rom J Morphol Embryol Rev Roum Morphol Embryol 61(1): 273–276. 10.47162/RJME.61.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharkey KA, Williams RG (1983) Extrinsic innervation of the rat pancreas: demonstration of vagal sensory neurones in the rat by retrograde tracing. Neurosci Lett 42(2):131–135. 10.1016/0304-3940(83)90395-6 [DOI] [PubMed] [Google Scholar]
- 49.Furuzawa Y, Ohmori Y, Watanabe T (1996) Anatomical localization of sympathetic postganglionic and sensory neurons innervating the pancreas of the cat. J Vet Med Sci 58(3):243–248. 10.1292/jvms.58.243 [DOI] [PubMed] [Google Scholar]
- 50.Su HC, Bishop AE, Power RF, Hamada Y, Polak JM (1987) Dual intrinsic and extrinsic origins of CGRP- and NPY-immunoreactive nerves of rat gut and pancreas. J Neurosci 7(9): 2674–2687. 10.1523/JNEUROSCI.07-09-02674.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lew WY, Longhurst JC (1986) Substance P, 5-hydroxytryptamine, and bradykinin stimulate abdominal visceral afferents. Am J Phys 250(3 Pt 2):R465–R473. 10.1152/ajpregu.1986.250.3.R465 [DOI] [PubMed] [Google Scholar]
- 52.Sternini C, Anderson K (1992) Calcitonin gene-related peptide-containing neurons supplying the rat digestive system: differential distribution and expression pattern. Somatosens Mot Res 9(1):45–59. 10.3109/08990229209144762 [DOI] [PubMed] [Google Scholar]
- 53.Sternini C, Card JP (1991) Ultrastructural characterization of calcitonin gene-related peptide-containing fibers and islet cells in the rat pancreas. Pancreas 6(4):375–384. 10.1097/00006676-199107000-00001 [DOI] [PubMed] [Google Scholar]
- 54.McEwan S, Kwon H, Tahiri A et al. (2021) Deconstructing the origins of sexual dimorphism in sensory modulation of pancreatic β cells. Mol Metab 53:101260. 10.1016/j.molmet.2021.101260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharkey KA, Williams RG, Dockray GJ (1984) Sensory substance P innervation of the stomach and pancreas. Demonstration of capsaicin-sensitive sensory neurons in the rat by combined immunohistochemistry and retrograde tracing. Gastroenterology 87(4): 914–921 [PubMed] [Google Scholar]
- 56.Chien H-J, Chiang T-C, Peng S-J et al. (2019) Human pancreatic afferent and efferent nerves: mapping and 3-D illustration of exocrine, endocrine, and adipose innervation. Am J Physiol Gastrointest Liver Physiol 317(5):G694–G706. 10.1152/ajpgi.00116.2019 [DOI] [PubMed] [Google Scholar]
- 57.Furness JB, Callaghan BP, Rivera LR, Cho H-J (2014) The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol 817:39–71. 10.1007/978-1-4939-0897-4_3 [DOI] [PubMed] [Google Scholar]
- 58.Schloithe AC, Sutherland K, Woods CM et al. (2008) A novel preparation to study rat pancreatic spinal and vagal mechanosensitive afferents in vitro. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc 20(9):1060–1069. 10.1111/j.1365-2982.2008.01141.x [DOI] [PubMed] [Google Scholar]
- 59.Kirchgessner AL, Gershon MD (1990) Innervation of the pancreas by neurons in the gut. J Neurosci 10(5):1626–1642. 10.1523/JNEUROSCI.10-05-01626.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirchgessner AL, Pintar JE (1991) Guinea pig pancreatic ganglia: projections, transmitter content, and the type-specific localization of monoamine oxidase. J Comp Neurol 305(4):613–631. 10.1002/cne.903050407 [DOI] [PubMed] [Google Scholar]
- 61.Tharakan T, Kirchgessner AL, Baxi LV, Gershon MD (1995) Appearance of neuropeptides and NADPH-diaphorase during development of the enteropancreatic innervation. Brain Res Dev Brain Res 84(1):26–38. 10.1016/0165-3806(94)00142-m [DOI] [PubMed] [Google Scholar]
- 62.Kirchgessner AL, Liu MT (2001) Pituitary adenylate cyclase activating peptide (PACAP) in the enteropancreatic innervation. Anat Rec 262(1):91–100. [DOI] [PubMed] [Google Scholar]
- 63.Kirchgessner AL, Liu MT, Raymond JR, Gershon MD (1996) Identification of cells that express 5-hydroxytryptamine1A receptors in the nervous systems of the bowel and pancreas. J Comp Neurol 364(3):439–455. [DOI] [PubMed] [Google Scholar]
- 64.Sha L, Ou LL, Miller SM, Ma R, Szurszewski JH (1996) Cat pancreatic neurons: morphology, electrophysiological properties, and responses to 5-HT. Pancreas 13(2):111–124 [PubMed] [Google Scholar]
- 65.Ma RC, Szurszewski JH (1996) 5-Hydroxytryptamine depolarizes neurons of cat pancreatic ganglia. J Auton Nerv Syst 57(1):78–86. 10.1016/0165-1838(95)00100-X [DOI] [PubMed] [Google Scholar]
- 66.Malaisse W, Malaisse-Lagae F, Wright PH, Ashmore J (1967) Effects of adrenergic and cholinergic agents upon insulin secretion in vitro. Endocrinology 80(5):975–978. 10.1210/endo-80-5-975 [DOI] [PubMed] [Google Scholar]
- 67.Bloom SR, Edwards AV, Vaughan NJ (1973) The role of the sympathetic innervation in the control of plasma glucagon concentration in the calf. J Physiol 233(2):457–466. 10.1113/jphysiol.1973.sp010317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bloom SR, Edwards AV (1975) The release of pancreatic glucagon and inhibition of insulin in response to stimulation of the sympathetic innervation. J Physiol 253(1):157–173. 10.1113/jphysiol.1975.sp011185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller RE (1975) Neural inhibition of insulin secretion from the isolated canine pancreas. Am J Phys 229(1):144–149. 10.1152/ajplegacy.1975.229.1.144 [DOI] [PubMed] [Google Scholar]
- 70.Porte DJ, Robertson RP (1973) Control of insulin secretion by catecholamines, stress, and the sympathetic nervous system. Fed Proc 32(7):1792–1796 [PubMed] [Google Scholar]
- 71.Girardier L, Seydoux J, Campfield LA (1976) Control of a and B cells in vivo by sympathetic nervous input and selective hyper or hypoglycemia in dog pancreas. J Physiol Paris 72(6):801–814 [PubMed] [Google Scholar]
- 72.Kaneto A, Kajinuma H, Kosaka K (1975) Effect of splanchnic nerve stimulation on glucagon and insulin output in the dog. Endocrinology 96(1):143–150. 10.1210/endo-96-1-143 [DOI] [PubMed] [Google Scholar]
- 73.Holst JJ, Grønholt R, Schaffalitzky de Muckadell OB, Fahrenkrug J (1981) Nervous control of pancreatic endocrine secretion in pigs. V. Influence of the sympathetic nervous system on the pancreatic secretion of insulin and glucagon, and on the insulin and glucagon response to vagal stimulation. Acta Physiol Scand 113(3):279–283. 10.1111/j.1748-1716.1981.tb06897.x [DOI] [PubMed] [Google Scholar]
- 74.Iversen J (1973) Adrenergic receptors and the secretion of glucagon and insulin from the isolated, perfused canine pancreas. J Clin Invest 52(9):2102–2116. 10.1172/JCI107395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hell NS, de Aguiar Pupo A (1979) Influence of the vagus and splanchnic nerves on insulin secretion and glycemia. J Auton Nerv Syst 1(1):93–101. 10.1016/0165-1838(79)90008-0 [DOI] [PubMed] [Google Scholar]
- 76.Jansson L, Eizirik DL, Sandler S (1989) Terbutaline decreases the blood flow of the pancreatic islets but does not reduce the diabetogenic action of streptozotocin in the rat. Eur J Pharmacol 161(1): 79–83. 10.1016/0014-2999(89)90182-9 [DOI] [PubMed] [Google Scholar]
- 77.Almaça J, Weitz J, Rodriguez-Diaz R, Pereira E, Caicedo A (2018) The Pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab 27(3):630–644.e4. 10.1016/j.cmet.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mateus Gonçalves L, Almaça J (2021) Functional characterization of the human islet microvasculature using living pancreas slices. Front Endocrinol (Lausanne) 11:1015. 10.3389/fendo.2020.602519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rucker HK, Wynder HJ, Thomas WE (2000) Cellular mechanisms of CNS pericytes. Brain Res Bull 51(5):363–369. 10.1016/s0361-9230(99)00260-9 [DOI] [PubMed] [Google Scholar]
- 80.Ito Y, Kaji M, Sakamoto E, Terauchi Y (2019) The beneficial effects of a muscarinic agonist on pancreatic β-cells. Sci Rep 9(1):16180. 10.1038/s41598-019-52691-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahrén B, Paquette TL, Taborsky GJJ (1986) Effect and mechanism of vagal nerve stimulation on somatostatin secretion in dogs. Am J Phys 250(2 Pt 1):E212–E217. 10.1152/ajpendo.1986.250.2.E212 [DOI] [PubMed] [Google Scholar]
- 82.Fontaine AK, Ramirez DG, Littich SF et al. (2021) Optogenetic stimulation of cholinergic fibers for the modulation of insulin and glycemia. Sci Rep 11(1):3670. 10.1038/s41598-021-83361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodriguez-Diaz R, Speier S, Molano RD et al. (2012) Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proc Natl Acad Sci. 10.1073/pnas.1211659110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ahrén B, Holst JJ (2001) The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes 50(5):1030–1038. 10.2337/diabetes.50.5.1030 [DOI] [PubMed] [Google Scholar]
- 85.Schusdziarra V, Bender H, Torres A, Pfeiffer EF (1983) Cholinergic mechanisms in intestinal phase insulin secretion in rats. Regul Pept 6(2):81–87. 10.1016/0167-0115(83)90001-0 [DOI] [PubMed] [Google Scholar]
- 86.Greenberg GR, Pokol-Daniel S (1994) Neural modulation of glucose-dependent insulinotropic peptide (GIP) and insulin secretion in conscious dogs. Pancreas 9(4):531–535. 10.1097/00006676-199407000-00018 [DOI] [PubMed] [Google Scholar]
- 87.Teff KL (2008) Visceral nerves: vagal and sympathetic innervation. JPEN J Parenter Enteral Nutr 32(5):569–571. 10.1177/0148607108321705 [DOI] [PubMed] [Google Scholar]
- 88.Fabris SE, Thorburn A, Litchfield A, Proietto J (1996) Effect of parasympathetic denervation of liver and pancreas on glucose kinetics in man. Metabolism 45(8):987–991. 10.1016/s0026-0495(96)90268-1 [DOI] [PubMed] [Google Scholar]
- 89.Balbo SL, Ribeiro RA, Mendes MC et al. (2016) Vagotomy diminishes obesity in cafeteria rats by decreasing cholinergic potentiation of insulin release. J Physiol Biochem 72(4):625–633. 10.1007/s13105-016-0501-9 [DOI] [PubMed] [Google Scholar]
- 90.Verspohl EJ, Tacke R, Mutschler E, Lambrecht G (1990) Muscarinic receptor subtypes in rat pancreatic islets: binding and functional studies. Eur J Pharmacol 178(3):303–311. 10.1016/0014-2999(90)90109-j [DOI] [PubMed] [Google Scholar]
- 91.Henquin JC, Nenquin M (1988) The muscarinic receptor subtype in mouse pancreatic B-cells. FEBS Lett 236(1):89–92. 10.1016/0014-5793(88)80290-4 [DOI] [PubMed] [Google Scholar]
- 92.Winzell MS, Ahrén B (2007) Role of VIP and PACAP in islet function. Peptides 28(9):1805–1813. 10.1016/j.peptides.2007.04.024 [DOI] [PubMed] [Google Scholar]
- 93.Kim W, Fiori JL, Shin Y-K et al. (2014) Pancreatic polypeptide inhibits somatostatin secretion. FEBS Lett 588(17):3233–3239. 10.1016/j.febslet.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katschinski M (2000) Nutritional implications of cephalic phase gastrointestinal responses. Appetite 34(2):189–196. 10.1006/appe.1999.0280 [DOI] [PubMed] [Google Scholar]
- 95.Louis-Sylvestre J (1978) Relationship between two stages of prandial insulin release in rats. Am J Phys 235(2):E103–E111. 10.1152/ajpendo.1978.235.2.E103 [DOI] [PubMed] [Google Scholar]
- 96.Teff KL, Townsend RR (1999) Early phase insulin infusion and muscarinic blockade in obese and lean subjects. Am J Phys 277(1):R198–R208. 10.1152/ajpregu.1999.277.1.R198 [DOI] [PubMed] [Google Scholar]
- 97.Strubbe JH, Steffens AB (1993) Neural control of insulin secretion. Horm Metab Res Horm und Stoffwechselforsch Horm Metab 25(10):507–512. 10.1055/s-2007-1002162 [DOI] [PubMed] [Google Scholar]
- 98.Steffens AB (1976) Influence of the oral cavity on insulin release in the rat. Am J Phys 230(5):1411–1415. 10.1152/ajplegacy.1976.230.5.1411 [DOI] [PubMed] [Google Scholar]
- 99.Berthoud HR, Bereiter DA, Trimble ER, Siegel EG, Jeanrenaud B (1981) Cephalic phase, reflex insulin secretion neuroanatomical and physiological characterization. Diabetologia 20(Suppl 1): 393–401. 10.1007/BF00254508 [DOI] [PubMed] [Google Scholar]
- 100.Teff KL, Engelman K (1996) Oral sensory stimulation improves glucose tolerance in humans: effects on insulin, C-peptide, and glucagon. Am J Phys 270(6 Pt 2):R1371–R1379. 10.1152/ajpregu.1996.270.6.R1371 [DOI] [PubMed] [Google Scholar]
- 101.MacDonald PE, Rorsman P (2007) The ins and outs of secretion from pancreatic beta-cells: control of single-vesicle exo- and endocytosis. Physiology (Bethesda) 22:113–121. 10.1152/physiol.00047.2006 [DOI] [PubMed] [Google Scholar]
- 102.Bergsten P, Grapengiesser E, Gylfe E, Tengholm A, Hellman B (1994) Synchronous oscillations of cytoplasmic Ca2+ and insulin release in glucose-stimulated pancreatic islets. J Biol Chem 269(12):8749–8753 [PubMed] [Google Scholar]
- 103.Zhang M, Goforth P, Bertram R, Sherman A, Satin L (2003) The Ca2+ dynamics of isolated mouse beta-cells and islets: implications for mathematical models. Biophys J 84(5):2852–2870. 10.1016/S0006-3495(03)70014-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cook DL (1983) Isolated islets of Langerhans have slow oscillations of electrical activity. Metabolism 32(7):681–685. 10.1016/0026-0495(83)90124-5 [DOI] [PubMed] [Google Scholar]
- 105.Bergsten P, Westerlund J, Liss P, Carlsson P-O (2002) Primary in vivo oscillations of metabolism in the pancreas. Diabetes 51(3): 699–703. 10.2337/diabetes.51.3.699 [DOI] [PubMed] [Google Scholar]
- 106.Pørksen N (2002) The in vivo regulation of pulsatile insulin secretion. Diabetologia 45(1):3–20. 10.1007/s125-002-8240-x [DOI] [PubMed] [Google Scholar]
- 107.Satin LS, Butler PC, Ha J, Sherman AS (2015) Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol Asp Med 42:61–77. 10.1016/j.mam.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sha L, Westerlund J, Szurszewski JH, Bergsten P (2001) Amplitude modulation of pulsatile insulin secretion by intrapancreatic ganglion neurons. Diabetes 50(1):51–55. 10.2337/diabetes.50.1.51 [DOI] [PubMed] [Google Scholar]
- 109.Fendler B, Zhang M, Satin L, Bertram R (2009) Synchronization of pancreatic islet oscillations by intrapancreatic ganglia: a modeling study. Biophys J 97(3):722–729. 10.1016/j.bpj.2009.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taborsky GJJ, Mundinger TO (2012) Minireview: the role of the autonomic nervous system in mediating the glucagon response to hypoglycemia. Endocrinology 153(3):1055–1062. 10.1210/en.2011-2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Havel PJ, Parry SJ, Stern JS et al. (1994) Redundant parasympathetic and sympathoadrenal mediation of increased glucagon secretion during insulin-induced hypoglycemia in conscious rats. Metabolism 43(7):860–866. 10.1016/0026-0495(94)90267-4 [DOI] [PubMed] [Google Scholar]
- 112.Payne SC, Ward G, MacIsaac RJ, Hyakumura T, Fallon JB, Villalobos J (2020) Differential effects of vagus nerve stimulation strategies on glycemia and pancreatic secretions. Physiol Rep 8(11):e14479. 10.14814/phy2.14479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meyers EE, Kronemberger A, Lira V, Rahmouni K, Stauss HM (2016) Contrasting effects of afferent and efferent vagal nerve stimulation on insulin secretion and blood glucose regulation. Physiol Rep 4(4):e12718. 10.14814/phy2.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stauss HM, Stangl H, Clark KC, Kwitek AE, Lira VA (2018) Cervical vagal nerve stimulation impairs glucose tolerance and suppresses insulin release in conscious rats. Physiol Rep 6(24): e13953. 10.14814/phy2.13953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martínez A, Kapas S, Miller MJ, Ward Y, Cuttitta F (2000) Coexpression of receptors for adrenomedullin, calcitonin gene-related peptide, and amylin in pancreatic beta-cells. Endocrinology 141(1):406–411. 10.1210/endo.141.1.7261 [DOI] [PubMed] [Google Scholar]
- 116.Persson-Sjögren S, Lejon K, Holmberg D, Forsgren S (2005) Expression of the NK-1 receptor on islet cells and invading immune cells in the non-obese diabetic mouse. J Autoimmun 24(4):269–279. 10.1016/j.jaut.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 117.Bou Karam J, Cai W, Mohamed R et al. (2018) TRPV1 neurons regulate β-cell function in a sex-dependent manner. Mol Metab 18:60–67. 10.1016/j.molmet.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Edwards AV, Bloom SR (1994) Pancreatic endocrine responses to substance P and calcitonin gene-related peptide in conscious calves. Am J Phys 267(6 Pt 1):E847–E852. 10.1152/ajpendo.1994.267.6.E847 [DOI] [PubMed] [Google Scholar]
- 119.Schmidt PT, Tornøe K, Poulsen SS, Rasmussen TN, Holst JJ (2000) Tachykinins in the porcine pancreas: potent exocrine and endocrine effects via NK-1 receptors. Pancreas. 20(3):241–247. 10.1097/00006676-200004000-00004 [DOI] [PubMed] [Google Scholar]
- 120.Karlsson S, Cand FS, Ahrdn B (1992) Neonatal capsaicin-treatment in mice: effects on pancreatic peptidergic nerves and 2-deoxy-D-glucose-induced insulin and glucagon secretion. J Auton Nerv Syst 39(1):51–59. 10.1016/0165-1838(92)90250-k [DOI] [PubMed] [Google Scholar]
- 121.Kozlova EN, Jansson L (2005) In vitro interactions between insulin-producing beta cells and embryonic dorsal root ganglia. Pancreas 31(4):380–384. 10.1097/01.mpa.0000181489.35022.4a [DOI] [PubMed] [Google Scholar]
- 122.Hermansen K (1980) Effects of substance P and other peptides on the release of somatostatin, insulin, and glucagon in vitro. Endocrinology 107(1):256–261. 10.1210/endo-107-1-256 [DOI] [PubMed] [Google Scholar]
- 123.Adeghate E (1999) Distribution of calcitonin-gene-related peptide, neuropeptide-Y, vasoactive intestinal polypeptide, cholecystokinin-8, substance P and islet peptides in the pancreas of normal and diabetic rats. Neuropeptides 33(3):227–235. 10.1054/npep.1999.0022 [DOI] [PubMed] [Google Scholar]
- 124.Chiba Y, Kawai K, Okuda Y, Munekata E, Yamashita K (1985) Effects of substance P and substance P-(6–11) on hormone release from isolated perfused pancreas: their opposite actions on rat and canine islets. Endocrinology 117(5):1996–2000. 10.1210/endo-117-5-1996 [DOI] [PubMed] [Google Scholar]
- 125.Brown M, Vale W (1976) Effects of neurotensin and substance P on plasma insulin, glucagon and glucose levels. Endocrinology 98(3):819–822. 10.1210/endo-98-3-819 [DOI] [PubMed] [Google Scholar]
- 126.Kashyap SR, Bhatt DL, Schauer PR (2010) Bariatric surgery vs. advanced practice medical management in the treatment of type 2 diabetes mellitus: rationale and design of the surgical therapy and medications potentially eradicate diabetes efficiently trial (STAMPEDE). Diabetes Obes Metab 12:452–454 [DOI] [PubMed] [Google Scholar]
- 127.Abegg K, Corteville C, Docherty NG et al. (2015) Effect of bariatric surgery combined with medical therapy versus intensive medical therapy or calorie restriction and weight loss on glycemic control in Zucker diabetic fatty rats. Am J Physiol Regul Integr Comp Physiol 308(4):R321–R329. 10.1152/ajpregu.00331.2014 [DOI] [PubMed] [Google Scholar]
- 128.Park JM, Chiu C-F, Chen S-C, Lee W-J, Chen C-Y (2020) Changes in post-oral glucose challenge pancreatic polypeptide hormone levels following metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Neuropeptides 81: 102032. 10.1016/j.npep.2020.102032 [DOI] [PubMed] [Google Scholar]
- 129.Schrumpf E, Linnestad P, Nygaard K, Giercksky KE, Fausa O (1981) Pancreatic polypeptide secretion before and after gastric bypass surgery for morbid obesity. Scand J Gastroenterol 16(8): 1009–1014. 10.3109/00365528109181020 [DOI] [PubMed] [Google Scholar]
- 130.Katsogiannos P, Kamble PG, Wiklund U et al. (2020) Rapid changes in neuroendocrine regulation may contribute to reversal of type 2 diabetes after gastric bypass surgery. Endocrine 67(2): 344–353. 10.1007/s12020-020-02203-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chari ST, Leibson CL, Rabe KG et al. (2008) Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology 134(1):95–101. 10.1053/j.gastro.2007.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hart PA, Baichoo E, Bi Y, Hinton A, Kudva YC, Chari ST et al. (2015) Pancreatic polypeptide response to a mixed meal is blunted in pancreatic head cancer associated with diabetes mellitus. Pancreatol Off J Int Assoc Pancreatol 15(2):162–166. 10.1016/j.pan.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 133.Rickels MR, Bellin M, Toledo FGS et al. (2013) Detection, evaluation and treatment of diabetes mellitus in chronic pancreatitis: recommendations from PancreasFest 2012. Pancreatol Off J Int Assoc Pancreatol 13(4):336–342. 10.1016/j.pan.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kang MJ, Jung HS, Jang J-Y et al. (2016) Metabolic effect of pancreatoduodenectomy: resolution of diabetes mellitus after surgery. Pancreatol Off J Int Assoc Pancreatol 16(2):272–277. 10.1016/j.pan.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 135.Wu J-M, Kuo T-C, Yang C-Y et al. (2013) Resolution of diabetes after pancreaticoduodenectomy in patients with and without pancreatic ductal cell adenocarcinoma. Ann Surg Oncol 20(1): 242–249. 10.1245/s10434-012-2577-y [DOI] [PubMed] [Google Scholar]
- 136.Lamers CB, Diemel JM (1981) Basal and postprandial serum concentrations of pancreatic polypeptide in pancreatitis and after pancreaticoduodenectomy. Postgrad Med J 57(672):617–621. 10.1136/pgmj.57.672.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shapiro AMJ, Pokrywczynska M, Ricordi C (2017) Clinical pancreatic islet transplantation. Nat Rev Endocrinol 13(5):268–277. 10.1038/nrendo.2016.178 [DOI] [PubMed] [Google Scholar]
- 138.Chen C-C, Peng S-J, Wu P-Y et al. (2021) Heterogeneity and neurovascular integration of intraportally transplanted islets revealed by 3-D mouse liver histology. Am J Physiol Endocrinol Metab 320(6):E1007–E1019. 10.1152/ajpendo.00605.2020 [DOI] [PubMed] [Google Scholar]
- 139.Juang J-H, Peng S-J, Kuo C-H, Tang S-C (2014) Three-dimensional islet graft histology: panoramic imaging of neural plasticity in sympathetic reinnervation of transplanted islets under the kidney capsule. Am J Physiol Endocrinol Metab 306(5): E559–E570. 10.1152/ajpendo.00515.2013 [DOI] [PubMed] [Google Scholar]
- 140.Houwing H, Van Asperen RM, Van der Zee EA et al. (1996) Noradrenergic and cholinergic reinnervation of islet grafts in diabetic rats. Cell Transplant 5(1):21–30. 10.1016/0963-6897(95)02019-5 [DOI] [PubMed] [Google Scholar]
- 141.Fike EA 4th, Simons E, Boswell C, Smith PG (1992) Sensory nerves impair sympathetic reinnervation and recovery of smooth muscle function. Exp Neurol 118(1):85–94. 10.1016/0014-4886(92)90025-l [DOI] [PubMed] [Google Scholar]
- 142.Miao G, Mace J, Kirby M et al. (2006) In vitro and in vivo improvement of islet survival following treatment with nerve growth factor. Transplantation 81(4):519–524. 10.1097/01.tp.0000200320.16723.b3 [DOI] [PubMed] [Google Scholar]
- 143.Stagner J, Mokshagundam S, Wyler K et al. (2004) Beta-cell sparing in transplanted islets by vascular endothelial growth factor. Transplant Proc 36(4):1178–1180. 10.1016/j.transproceed.2004.04.036 [DOI] [PubMed] [Google Scholar]
- 144.Carlsson PO, Palm F, Andersson A, Liss P (2001) Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes 50(3):489–495. 10.2337/diabetes.50.3.489 [DOI] [PubMed] [Google Scholar]
- 145.Pepper AR, Gala-Lopez B, Ziff O, Shapiro AMJ (2013) Revascularization of transplanted pancreatic islets and role of the transplantation site. Clin Dev Immunol 2013:352315. 10.1155/2013/352315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meloche RM (2007) Transplantation for the treatment of type 1 diabetes. World J Gastroenterol 13(47):6347–6355. 10.3748/wjg.v13.i47.6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nyqvist D, Speier S, Rodriguez-Diaz R et al. (2011) Donor islet endothelial cells in pancreatic islet revascularization. Diabetes 60(10):2571–2577. 10.2337/db10-1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Almaça J, Molina J, Arrojo E, Drigo R et al. (2014) Young capillary vessels rejuvenate aged pancreatic islets. Proc Natl Acad Sci U S A 111(49):17612–17617. 10.1073/pnas.1414053111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Speier S, Nyqvist D, Cabrera O et al. (2008) Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med 14(5):574–578. 10.1038/nm1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Houwing H, Fränkel KM, Strubbe JH, van Suylichem PT,Steffens AB (1995) Role of the sympathoadrenal system in exercise-induced inhibition of insulin secretion. Effects of islet transplantation. Diabetes 44(5):565–571. 10.2337/diab.44.5.565 [DOI] [PubMed] [Google Scholar]
- 151.Pørksen N, Munn S, Ferguson D, O’Brien T, Veldhuis J, Butler P (1994) Coordinate pulsatile insulin secretion by chronic intraportally transplanted islets in the isolated perfused rat liver. J Clin Invest 94(1):219–227. 10.1172/JCI117310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gardemann A, Jungermann K, Grosse V et al. (1994) Intraportal transplantation of pancreatic islets into livers of diabetic rats. Reinnervation of islets and regulation of insulin secretion by the hepatic sympathetic nerves. Diabetes 43(11):1345–1352. 10.2337/diab.43.11.1345 [DOI] [PubMed] [Google Scholar]
- 153.Jansson L, Carlsson PO, Bodin B, Andersson A, Källskog O (2001) Capillary blood pressure in the endocrine and exocrine parenchyma of rat pancreas transplants. Surgery 129(2):196–202. 10.1067/msy.2001.110022 [DOI] [PubMed] [Google Scholar]
- 154.Omer A, Duvivier-Kali VF, Aschenbach W, Tchipashvili V, Goodyear LJ, Weir GC (2004) Exercise induces hypoglycemia in rats with islet transplantation. Diabetes 53(2):360–365. 10.2337/diabetes.53.2.360 [DOI] [PubMed] [Google Scholar]
- 155.Jansson L, Kampf C, Källskog O (2013) Functional stimulation of graft nerves has minor effects on insulin release from transplanted rat pancreatic islets. Ups J Med Sci 118(4):209–216. 10.3109/03009734.2013.818601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Siegel EG, Trimble ER, Renold AE, Berthoud HR (1980) Importance of preabsorptive insulin release on oral glucose tolerance: studies in pancreatic islet transplanted rats. Gut 21(11): 1002–1009. 10.1136/gut.21.11.1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jais A, Brüning JC (2017) Hypothalamic inflammation in obesity and metabolic disease. J Clin Invest 127(1):24–32. 10.1172/JCI88878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.De Angelis C, Perelli P, Trezza R et al. (2001) Modified autonomic balance in offsprings of diabetics detected by spectral analysis of heart rate variability. Metabolism 50(11):1270–1274. 10.1053/meta.2001.27225 [DOI] [PubMed] [Google Scholar]
- 159.Groah SL, Nash MS, Ward EA et al. (2011) Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J Cardiopulm Rehabil Prev 31(2):73–80. 10.1097/HCR.0b013e3181f68aba [DOI] [PubMed] [Google Scholar]
- 160.Colachis SC 3rd (1992) Autonomic hyperreflexia with spinal cord injury. J Am Paraplegia Soc 15(3):171–186. 10.1080/01952307.1992.11735871 [DOI] [PubMed] [Google Scholar]
- 161.Mei Q, Mundinger TO, Lernmark A, Taborsky GJ (2002) Early, selective, and marked loss of sympathetic nerves from the islets of BioBreeder diabetic rats. Diabetes 51(10):2997–3002. 10.2337/diabetes.51.10.2997 [DOI] [PubMed] [Google Scholar]
- 162.Mundinger TO, Mei Q, Foulis AK, Fligner CL, Hull RL, Taborsky GJJ (2016) Human type 1 diabetes is characterized by an early, marked, sustained, and islet-selective loss of sympathetic nerves. Diabetes 65(8):2322–2330. 10.2337/db16-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Campanucci V, Krishnaswamy A, Cooper E (2010) Diabetes depresses synaptic transmission in sympathetic ganglia by inactivating nAChRs through a conserved intracellular cysteine residue. Neuron 66(6):827–834. 10.1016/j.neuron.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 164.Reyes LM, Khurana R, Usselman CW et al. (2020) Sympathetic nervous system activity and reactivity in women with gestational diabetes mellitus. Physiol Rep 8(13):e14504. 10.14814/phy2.14504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH (2018) The pathophysiology of gestational diabetes mellitus. Int J Mol Sci 19(11):3342. 10.3390/ijms19113342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guyot M, Simon T, Ceppo F et al. (2019) Pancreatic nerve electrostimulation inhibits recent-onset autoimmune diabetes. Nat Biotechnol 37(12):1446–1451. 10.1038/s41587-019-0295-8 [DOI] [PubMed] [Google Scholar]
- 167.Christoffersson G, Ratliff SS, von Herrath MG (2020) Interference with pancreatic sympathetic signaling halts the onset of diabetes in mice. Sci Adv 6(35):eabb2878. 10.1126/sciadv.abb2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lundberg M, Lindqvist A, Wierup N, Krogvold L, Dahl-Jørgensen K, Skog O (2017) The density of parasympathetic axons is reduced in the exocrine pancreas of individuals recently diagnosed with type 1 diabetes. PLoS One 12(6):e0179911. 10.1371/journal.pone.0179911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Polonsky KS, Sturis J, Van Cauter E (1998) Temporal profiles and clinical significance of pulsatile insulin secretion. Horm Res 49(3–4):178–184. 10.1159/000023168 [DOI] [PubMed] [Google Scholar]
- 170.Razavi R, Chan Y, Afifiyan FN et al. (2006) TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell 127(6):1123–1135. 10.1016/j.cell.2006.10.038 [DOI] [PubMed] [Google Scholar]
- 171.Melnyk A, Himms-Hagen J (1995) Resistance to aging-associated obesity in capsaicin-desensitized rats one year after treatment. Obes Res 3(4):337–344. 10.1002/j.1550-8528.1995.tb00159.x [DOI] [PubMed] [Google Scholar]
- 172.Moesgaard SG, Brand CL, Sturis J et al. (2005) Sensory nerve inactivation by resiniferatoxin improves insulin sensitivity in male obese Zucker rats. Am J Physiol Endocrinol Metab 288(6): E1137–E1145. 10.1152/ajpendo.00356.2004 [DOI] [PubMed] [Google Scholar]
- 173.Roth BL (2016) DREADDs for neuroscientists. Neuron 89(4): 683–694. 10.1016/j.neuron.2016.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Petreanu L, Huber D, Sobczyk A, Svoboda K (2007) Channelrhodopsin-2–assisted circuit mapping of long-range callosal projections. Nat Neurosci 10(5):663–668. 10.1038/nn1891 [DOI] [PubMed] [Google Scholar]
- 175.Stanley SA, Kelly L, Latcha KN et al. (2016) Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature 531(7596):647–650. 10.1038/nature17183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bernard C (1855) Leçons de physiologie expérimentale appliquée à la médecine. Ballière et Fils, Paris, France [Google Scholar]
- 177.Yang S-N, Shi Y, Yang G, Li Y, Yu J, Berggren P-O (2014) Ionic mechanisms in pancreatic β cell signaling. Cell Mol Life Sci 71(21):4149–4177. 10.1007/s00018-014-1680-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hamilton A, Zhang Q, Salehi A et al. (2018) Adrenaline stimulates glucagon secretion by Tpc2-dependent Ca2+ mobilization from acidic Stores in Pancreatic α-cells. Diabetes 67(6):1128–1139. 10.2337/db17-1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Winzell MS, Ahrén B (2007) G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacol Ther 116(3):437–448. 10.1016/j.pharmthera.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 180.Jin C, Naruse S, Kitagawa M et al. (2001) The effect of calcitonin gene-related peptide on pancreatic blood flow and secretion in conscious dogs. Regul Pept 99(1):9–15. 10.1016/s0167-0115(01)00214-2 [DOI] [PubMed] [Google Scholar]
- 181.Pawlik WW, Konturek SJ, Gustaw P et al. (1992) Role of tachykinins in the control of pancreatic secretion and circulation. J Physiol Pharmacol an Off J Polish Physiol Soc 43(1):43–57 [PubMed] [Google Scholar]


