Abstract
A method for the in situ production of formaldehyde from dimethylsulfoxide, bromine, and cesium carbonate is reported for reactions with difluoroenolates and difluorobenzyl carbanions. This process also generates formaldehyde-d2 for the production of 2,2-difluoro-1,1-deuteroethanols. Mechanistic and computational studies further characterize the production of hydroxymethylated and hydroxydeuteromethylated difluorinated organic molecules.
Hydroxymethylation is a well-established synthetic process to generate valuable organic compounds.1,2 Notable examples have appeared recently in the total synthesis of natural products,3,4 drug discovery,5 and biosynthesis.6 The typical methods for hydroxymethylation require the use of formaldehyde or a reagent that serves as a formaldehyde equivalent.3–5,7,8 Formaldehyde is present in formalin or produced from trioxane or paraformaldehyde; other typical sources are 1-benzotriazole-1-methanol, N-(hydroxymethyl)phthalimide, and 1,3-oxathiolane-3,3-dioxide (Figure 1). Alternatively, methods for the in situ generation of formaldehyde from dimethylsulfoxide (DMSO) or DMSO-like structures have been reported; however, they are not as convenient because excess heat is required and many side products are generated.9–12 Two examples are the production of a formaldehyde equivalent from DMSO and P2O59 and formaldehyde from DMSO and CuBr (10 mol %) at 100 °C.10
Figure 1.
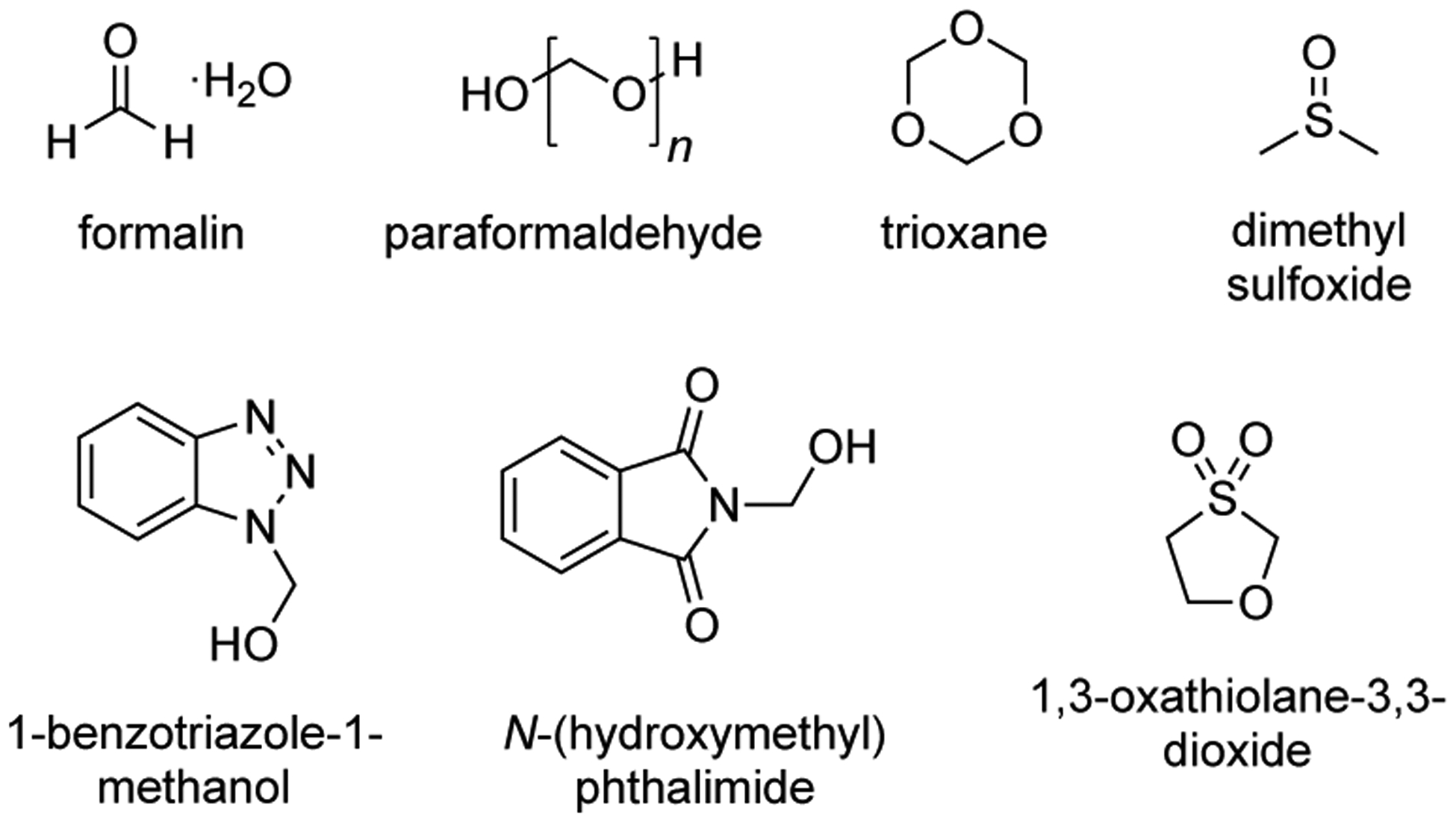
Typical sources of formaldehyde.
The incorporation of fluorine atoms on organic molecules is a common objective during drug development.13 Synthetic methods for the fluorination and trifluoromethylation of compounds are significantly more advanced than strategies that create a difluoromethyl group. In 2011, we designed a mild process for the generation of α,α-difluoroenolates from the fragmentation of pentafluoro-gem-diols (Figure 2).14 Although these difluorinated intermediates react with aldehydes14,15 and imines,16,17 the compatibility of the transformation with formaldehyde has not been established. Moreover, there is only one reaction of an α,α-difluoroenolate with formaldehyde in the existing literature.18 Indeed, a broader expansion of the reactivity of formaldehyde to difluorocarbanions would allow access to valuable 2,2-difluoroethanols and open new avenues for the synthesis of difluorinated targets. To address this need, we report a protocol for the in situ generation of formaldehyde for the hydroxymethylation of difluoroenolates to create 2,2-difluoroethanols. The method also allows the production of formaldehyde-d2 for hydroxydeuteromethylations, which constitutes a synthetic method for 2,2-difluoro-1,1-deuteroethanols. Also, this reaction is compatible with α,α-difluorobenzyl carbanions generated from the release of trifluoroacetate from electron-deficient aromatic and heteroaromatic rings.19
Figure 2.
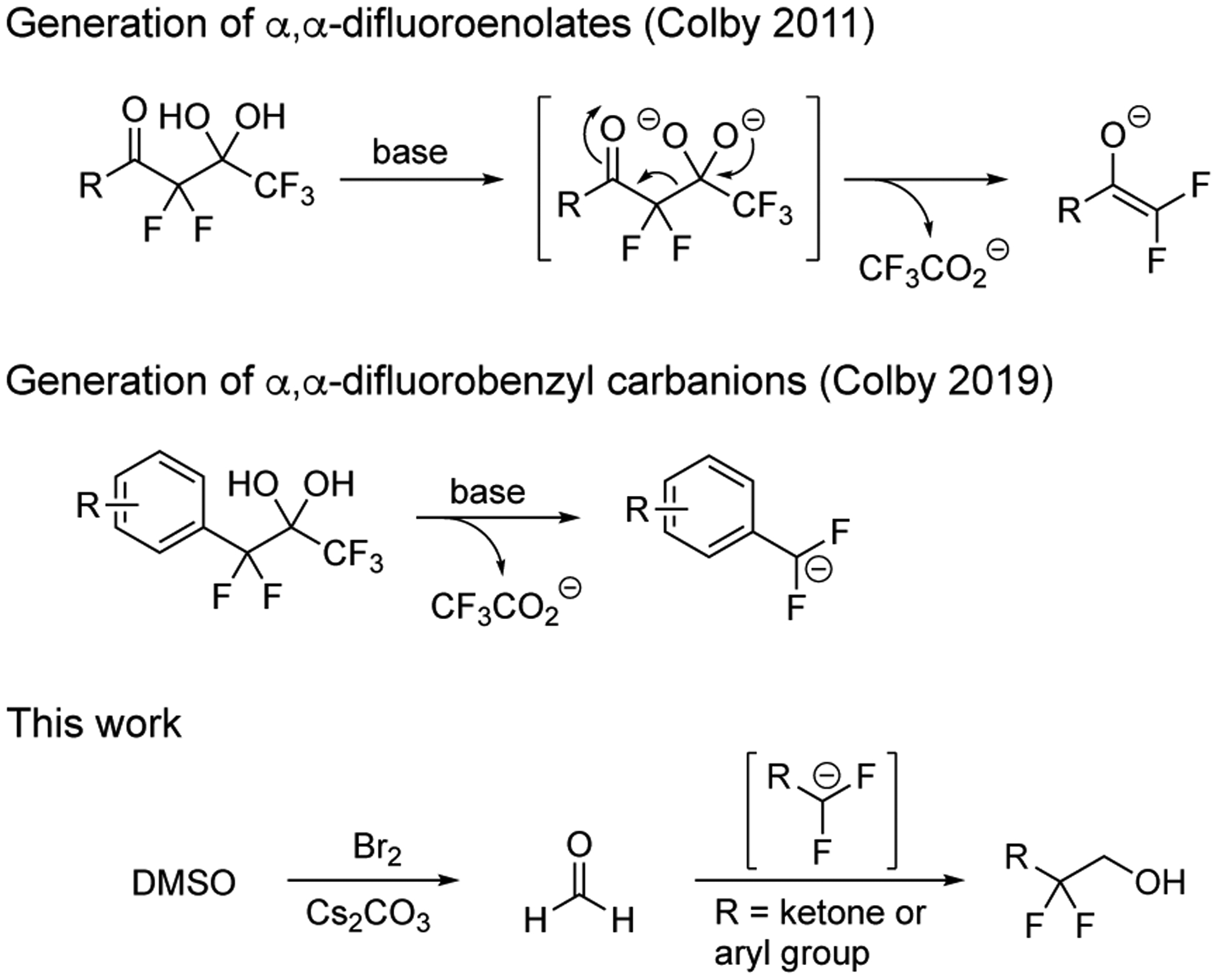
The generation of difluorocarbanions from the release of trifluoroacetate and the preparation of 2,2-difluoroethanols from the generation of formaldehyde in the presence of these reactive intermediates.
In 2019, we reported the production of difluorocarbanions from pentafluoro-gem-diols following the addition of K2CO3 in the solvent, DMSO.19 The use of DMSO is an ideal starting point for the development of a method for hydroxymethylation of difluorocarbanions, because this solvent is a known precursor of formaldehyde.9,10 Accordingly, we determined that the addition of bromine and 4Å molecular sieves along with K2CO3 in DMSO generates formaldehyde. We optimized these conditions for the simultaneous production of difluoroenolates from pentafluoro-gem-diols by adding LiBr14 and proton sponge (see Table S1 for optimization experiments). Lastly, K2CO3 was replaced with Cs2CO3 after screening other carbonate bases (e.g, Na2CO3 and Li2CO3). Using these conditions, the pentafluoro-gem-diols were hydroxymethylated and formed the products 1–6 in 32–68% isolated yields (Scheme 1). The higher conversions were observed with substrates bearing a naphthyl ring 1, a benzodioxoyl ring 2, an adamantyl group 3, or a benzothiophene 4, whereas the lower yields were obtained from the p-CF3 benzene 5 and the styrene derivative 6. The incorporation of deuterium into the structure of organic molecules is a growing field, especially for metabolic probes, leads in drug discovery,20,21 and internal standards in analytical techniques.22,23 Moreover, the presence of both deuterium and fluorine atoms on organic structures is an under-explored area, and few synthetic strategies are available to create these types of molecules.24–26 In order to address this shortcoming, we have adapted this method for hydroxydeuteromethylation by exchanging DMSO with DMSO-d6. The pentafluoro-gem-diols were subjected to these modified conditions (see Scheme 1). The difluorodideuteroethanols 7–9 were synthesized with high levels of incorporation of deuterium (88–94%) in similar conversions as the hydroxymethylations.
Scheme 1.
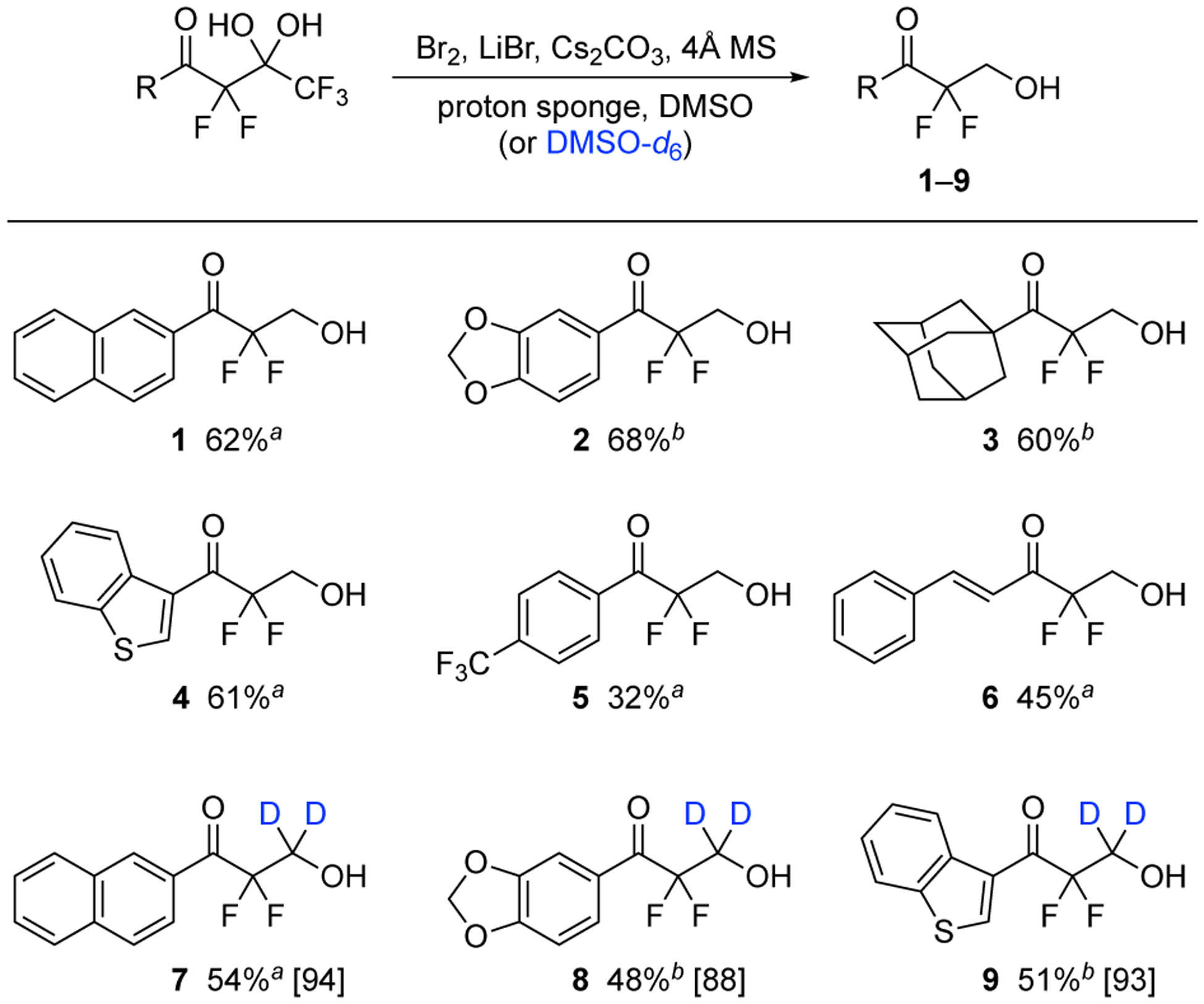
Synthesis of difluoroethanols 1–6 by hydroxymethylation from the in situ generation of formaldehyde and difluoroenolates, and synthesis of difluorodideuteroethanols 7–9 by hydroxydeuteromethylation (percent deuterium incorporation is listed in brackets). aStirred at rt. bStirred at 40 °C.
This process for hydroxymethylation could be simplified in the case of difluorobenzyl carbanions; however, higher temperatures were required. Specifically, only Cs2CO3, bromine, and 4Å molecular sieves were added in the presence of the pentafluoro-gem-diols shown in Scheme 2 and warmed to 60–65 °C. The transformation produces the aryl substituted difluoroethanols displaying 4-nitrobenzenes 10–11 or 5-nitro-2-pyridines 12–13 in isolated yields of 55–58%. The 5-trifluoromethyl-2-pyridine adduct 14 was produced in a lower 25% yield, but this observation was anticipated from our previous findings.19 The quinoxaline 15 represents another application of this reaction for the creation of heterocycles. Also, the difluorodideuteroethanols 16–18 were synthesized, by replacing DMSO with DMSO-d6, with high levels of incorporation of deuterium (93–100%) in similar yields as the respective hydroxymethylations.
Scheme 2.
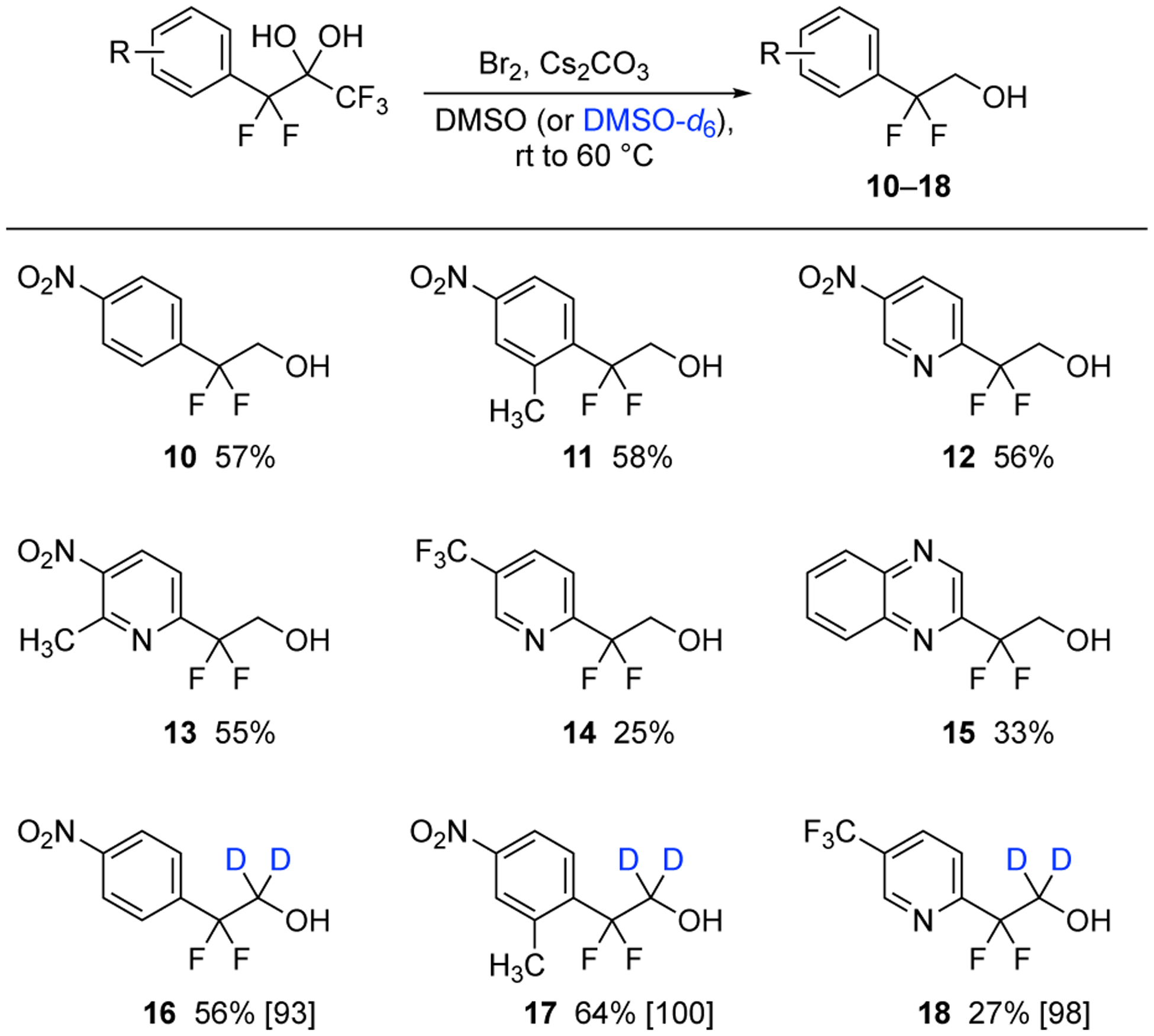
Synthesis of difluoroethanols 10–15 by hydroxymethylation from the in situ generation of formaldehyde and difluorobenzyl carbanions, and synthesis of difluorodideuteroethanols 16–18 by hydroxydeuteromethylation (percent deuterium incorporation is listed in brackets).
A Pummerer-like process is expected in this hydroxymethylation reaction.27 A plausible mechanism is proposed that initiates with the generation of methyl(methylene)sulfonium cation (Figure 3). Next, a carbonate base (e.g., K2CO3) serves as a nucleophile and adds to the electrophilic thionium cation. Then, the methylsulfide group is oxidized by bromine, and the resulting intermediate displaying the bromosulfonium ion fragments by the release of methanesulfenyl bromide and decarboxylation. The final result is the production of formaldehyde. Accordingly, after stirring K2CO3, Br2, and 4Å molecular sieves in DMSO at 60 °C for two hours, formaldehyde is observed in the 1H NMR spectrum with the characteristic peak at 9.68 ppm (in acetone-d6). Computational studies were performed using density functional theory28 in DMSO. The M06-2X29 level of theory and 6-311++G(3df,3pd) basis set were utilized. The free energies and enthalpy (i.e., ΔG = −36.12 and ΔH = −13.97 kcal/mol) are lower for the concerted fragmentation of the key bromosulfonium intermediate compared to both the reverse process, the regeneration of the (methylthio)methyl carbonate intermediate, and the stepwise fragmentation (see Figure S1 and Table S2). These results support a tandem fragmentation/decarboxylation process. The production of formaldehyde from this reaction allows the hydroxymethylation of the difluorobenzyl carbanion.
Figure 3.
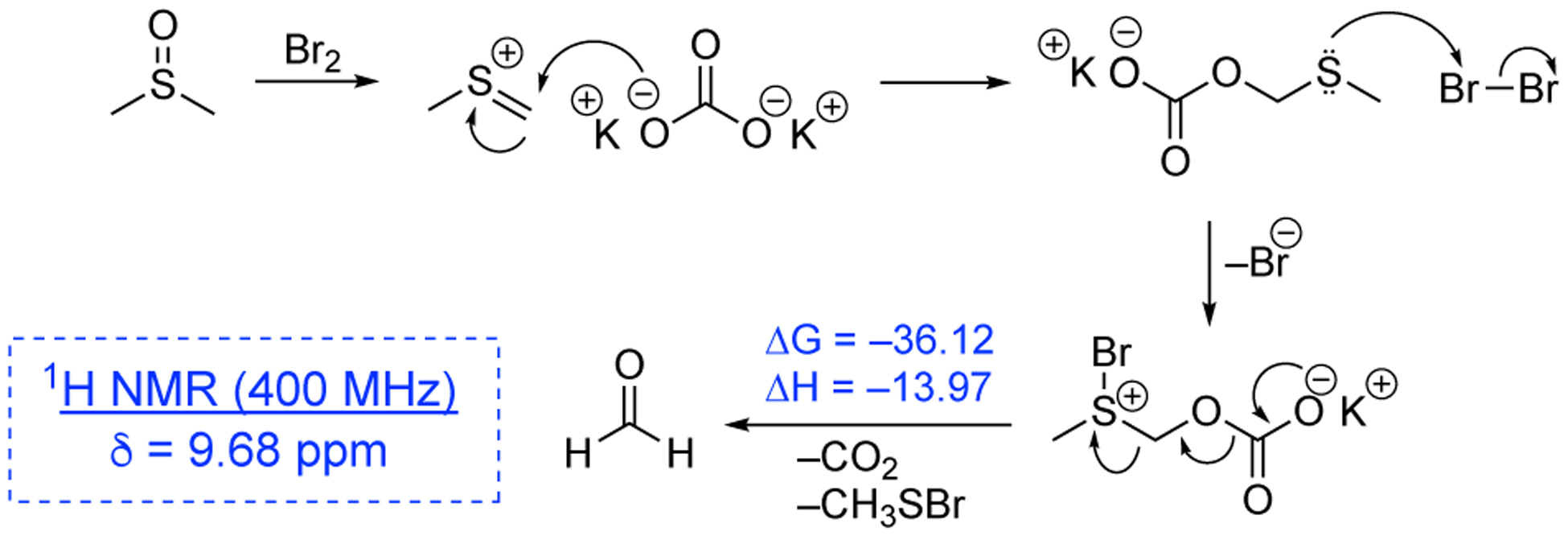
Proposed mechanism for the generation of formaldehyde from K2CO3, Br2, and DMSO starting from the methyl(methylene)sulfonium cation. The changes in free energy and enthalpy for the fragmentation step are shown (in kcal/mol at 298.15 K). 1H NMR data was obtained in acetone-d6 at 400 MHz at rt.
Additional mechanistic insight for this process was gathered from the reaction of 1,1,1,3,3-pentafluoro-3-(4-nitrophenyl)propane-2,2-diol with K2CO3 and H2O in DMSO. First, if this reaction is conducted at 65 °C without the presence of an electrophile, such as formaldehyde, the 1-(difluoromethyl)-4-nitrobenzene 19 is produced (Figure 4). Product 19 arises from the slow protonation of the difluorobenzyl carbanion. Second, if this same reaction is conducted with D2O instead of H2O, the 1-(deuterodifluoromethyl)-4-nitrobenzene 20 is isolated. Methods for the production of deuterodifluoromethylbenzenes are rare in the literature.30 Overall, the data obtained from the protonation and deuteration studies with the difluoromethylbenzenes compare favorably to our similar studies with difluoromethyl ketones.26
Figure 4.
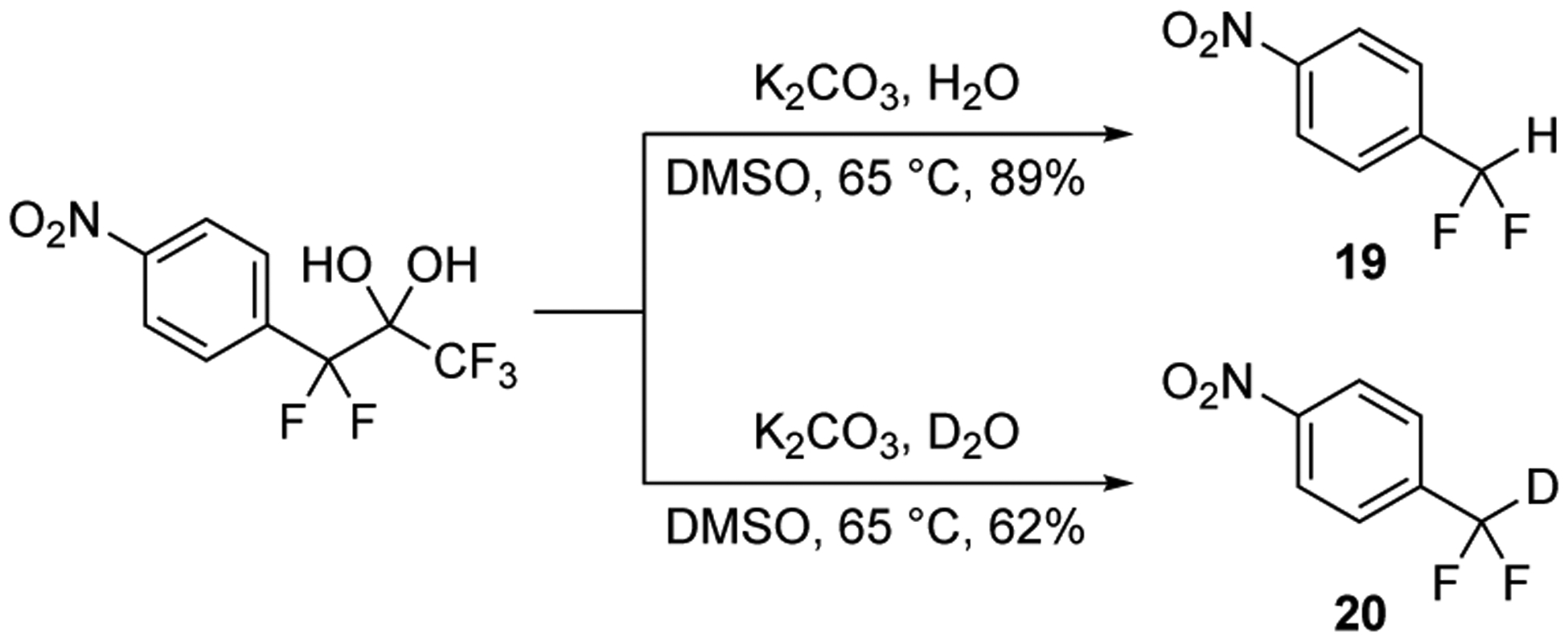
Conversion of 1,1,1,3,3-pentafluoro-3-(4-nitrophenyl)propane-2,2-diol into 1-(difluoromethyl)-4-nitrobenzene 19 and 1-(deuterodifluoromethyl)-4-nitrobenzene 20 with K2CO3 in DMSO at 65 °C.
In summary, we have reported an approach for the hydroxymethylation and hydroxydeuteromethylation of difluoroenolates and difluorobenzyl carbanions generated from pentafluoro gem-diols. This process produces formaldehyde or formaldehyde-d2 in the presence of a weak base, and in the latter case, high levels of deuterium incorporation are observed. The synthesis of a 2,2-difluoro-1,1-dideuteroethanol (i.e., RCF2CD2OH) has been previously reported via reduction of a difluorinated ester or amide using the deuterated reducing agents, NaBD4 or LiAlHD4;31–33 however, these reagents can concomitantly reduce other carbonyl or susceptible groups.33 No other preparations of 2,2-difluoro-1,1-dideuteroethanols exist in the literature, to our knowledge; therefore, our methodology provides a viable option for these targets. A plausible mechanism for hydroxymethylation is proposed and supported with experimental and computational studies; formaldehyde was observed by NMR. This strategy not only demonstrates new reactions for difluoroenolates and difluorobenzyl carbanions but also presents another method for the in situ formation of formaldehyde.
Supplementary Material
Footnotes
Conflicts of interest
D.A.C. serves as a scientific advisor for GnuPharma, and some of the studies in this manuscript were conducted with unrestricted support from GnuPharma. The authors declare no other competing financial interests.
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Alexanian EJ and Shenouda H, Org. Lett 2019, 21, 9268–9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tokala R, Bora D, Sana S, Nachtigall FM, Santos LS and Shankaraiah N, J. Org. Chem, 2019, 84, 5504–5513. [DOI] [PubMed] [Google Scholar]
- 3.Min L, Lin X and Li C-C, J. Am. Chem. Soc, 2019, 141, 15773–15778. [DOI] [PubMed] [Google Scholar]
- 4.Boeckman RK Jr., Niziol JM and Biegasiewicz KF, Org. Lett, 2018, 20, 5062–5065. [DOI] [PubMed] [Google Scholar]
- 5.Barcan GA, Conde JJ, Mokhallati MK, Nilson MG, Xie S, Alle CL, Andemichael YW, Calandra NA, Leitch DC, Li L and Morris MJ, Org. Process. Res. Dev, 2019, 23, 1396–1406. [Google Scholar]
- 6.Marin-Valls R, Hernandez K, Bolte M, Joglar J, Bujons J and Clapes P, ACS Catal, 2019, 9, 7568–7577. [Google Scholar]
- 7.Kutwal MS, Dev S and Appayee C, Org. Lett, 2019, 21, 2509–2513. [DOI] [PubMed] [Google Scholar]
- 8.Rauch M, Strater Z and Parkin G, J. Am. Chem. Soc, 2019, 141, 17754–17762. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Rochon FD, Ying Y, Hua L and Kayser MM, Tetrahedron: Asymmetry, 2007, 18, 1115–1123. [Google Scholar]
- 10.Ni Y, Zuo H, Yu H, Wu Y and Zhong F, Org. Lett, 2018, 20, 5899–5904. [DOI] [PubMed] [Google Scholar]
- 11.Jones-Mensah E, Karki M and Magolan J, Synthesis, 2016, 48, 1421–1436. [Google Scholar]
- 12.Forrester J, Jones RVH, Preston PN, Simpson ESC, J. Chem. Soc. Perkin Trans. 1, 1995, 18, 2289–2291. [Google Scholar]
- 13.Gillis EP, Eastman KJ, Hill MD, Donnelly DJ and Meanwell NA, J. Med. Chem, 2015, 58, 8315–8359. [DOI] [PubMed] [Google Scholar]
- 14.Han C, Kim EH and Colby DA, J. Am. Chem. Soc 2011, 130, 5802–5805. [DOI] [PubMed] [Google Scholar]
- 15.Zhang P and Wolf C, Angew. Chem. Int. Ed 2013, 52, 7869–7873. [DOI] [PubMed] [Google Scholar]
- 16.Xie C, Wu L, Mei H, Soloshonok VA, Han J and Pan Y, Org. Biomol. Chem, 2014, 12, 7836–7843. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen AL, Khatri HR, Woods JR, Baldwin CS, Fronczek FR and Colby DA, J. Org. Chem, 2018, 83, 3109–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balnaves AS, Gelbrich T, Hursthouse MB, Light ME, Palmer MJ and Percy JM, J. Chem. Soc., Perkins Trans 1, 1999, 2525–2535. [Google Scholar]
- 19.Khatri HR, Han C, Luong E, Pan X, Adam AT, Alshammari MD, Shao Y and Colby DA, J. Org. Chem, 2019, 84, 11665–11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng Y, Yu B, Huang H, Peng Y, Li E, Yao Y, Song C, Yu W, Zhu K, Wang K, Yi D and Du J, J. Med. Chem, 2021, 64, 925–937. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt C, Nat. Biotechnol, 2017, 35, 493–494. [DOI] [PubMed] [Google Scholar]
- 22.Atzrodt J, Derdau V, Kerr WJ and Reid M, Angew. Chem. Intl. Ed, 2018, 57, 1758–1784. [DOI] [PubMed] [Google Scholar]
- 23.Gant TG, J. Med. Chem, 2014, 57, 3595–3611. [DOI] [PubMed] [Google Scholar]
- 24.Huang L, Liu W, Zhao L-L and Yan X, J. Org. Chem, 2021, 86, 3981–3988. [DOI] [PubMed] [Google Scholar]
- 25.Fu WC and Jamison TF, Angew. Chem. Int. Ed 2020, 59, 13885–13890. [DOI] [PubMed] [Google Scholar]
- 26.Sowaileh MF, Han C, Hazlitt RA, Kim EH, John JP and Colby DA, Tetrahedron Lett., 2017, 58, 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bur SK and Padwa A, Chem. Rev, 2004, 104, 2401–2432. [DOI] [PubMed] [Google Scholar]
- 28.Becke AD, J. Chem. Phys, 2014, 140, 18A301. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y and Truhlar DG, Theor. Chem. Acc, 2008, 120, 215–241. [Google Scholar]
- 30.Miao W, Zhao Y, Ni C, Gao B, Zhang W and Hu J, J. Am. Chem. Soc, 2018, 140, 880–883. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Mikeš F, Koike Y and Okamoto Y, Macromolecules, 2004, 37, 7918–7923. [Google Scholar]
- 32.King JF and Gill MS, J. Org. Chem, 1998, 63, 808–811. [DOI] [PubMed] [Google Scholar]
- 33.Andersen TL, Frederiksen MW, Domino K and Skrydstrup T, Angew. Chem. Int. Ed, 2016, 55, 10396–10400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


