Abstract
Background:
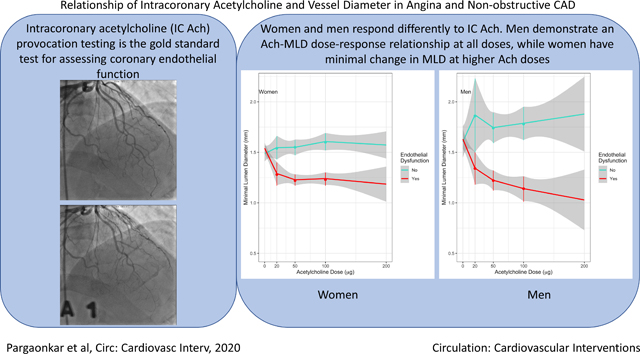
Intracoronary (IC) acetylcholine (Ach) provocation testing is the gold standard for assessing coronary endothelial function. However, dosing regimens of Ach are quite varied in the literature and there are limited data evaluating the optimal dose. We evaluated the dose-response relationship between Ach and minimal lumen diameter (MLD) by sex and studied whether incremental IC Ach doses given during endothelial function testing improve its diagnostic utility.
Methods:
We evaluated 65 men and 212 women with angina and no obstructive coronary artery disease (ANOCA) who underwent endothelial function testing using the highest tolerable dose of IC Ach, up to 200μg. Epicardial endothelial dysfunction was defined as a decrease in MLD > 20% after IC Ach by quantitative coronary angiography (QCA). We used a linear mixed effects model to evaluate the dose-response relationship. Deming regression analysis was done to compare the %MLD constriction after incremental doses of IC Ach.
Results:
The mean age was 53.5 years. Endothelial dysfunction was present in 186 (68.1%). Among men with endothelial dysfunction, there was a significant decrease in MLD/10µg of Ach at doses above 50μg and 100µg, while this decrease in MLD was not observed in women (p<0.001). The %MLD constriction at 20μg vs. 50μg and 50μg vs. 100μg were not equivalent while the %MLD constriction at 100μg vs. 200μg were equivalent.
Conclusion:
Women and men appear to have different responses to Ach during endothelial function testing. In addition to having a greater response to IC Ach at all doses, men also demonstrate an Ach-MLD dose-response relationship with doses up to 200μg, while women have minimal change in MLD with doses above 50µg. An incremental dosing regimen during endothelial function testing appears to improve the diagnostic utility of the test, and should be adjusted based on the sex of the patient.
Keywords: endothelial dysfunction, acetylcholine, angina, vasospasm, sex-specific
Graphical Abstract
Introduction
More than 20% of patients undergoing coronary angiography have no obstructive coronary artery disease (CAD).1 One potential cause of symptoms in patients having angina with no obstructive CAD (ANOCA) is epicardial endothelial dysfunction, also known as coronary vasomotor dysfunction.2,3 Impaired endothelial function is suggested to be an early step in the development of atherosclerosis and studies have reported its association with poor cardiovascular outcomes, including rehospitalization, stroke, myocardial infarction, and death in both women and men with ANOCA.4–7 These data suggest that abnormal endothelial function is central to the pathogenesis of CAD, and highlight the importance of better diagnostic tools for early detection for favorable prognosis.
Intracoronary (IC) acetylcholine (Ach) provocation testing is considered the gold standard for assessing coronary endothelial function.8–10 Ach induces endothelial-dependent dilation and smooth muscle mediated constriction in the artery. In patients with normal endothelial function, endothelial-dependent dilation dominates, resulting in vasodilatation after IC Ach injection. On the other hand, in patients with endothelial dysfunction, smooth muscle mediated constriction dominates, resulting in vasoconstriction after IC Ach.11 Previous studies using IC Ach provocation testing in ANOCA patients have reported the prevalence of epicardial endothelial dysfunction ranging from 27%−62% depending on the cutoff value of %vasoconstriction used.2,4,5 However, the dosing regimens of IC Ach used for provocation testing are quite varied in the literature, with some using incremental doses starting at 2µg to up to 200µg, while others use only a single dose.5,12 This limits the ability to establish dosing recommendations for invasive vascular function testing.9 In addition, no data are available regarding the dose-response relationship between IC Ach and coronary minimal lumen diameter (MLD), and whether it differs by sex.
Accordingly, we studied the dose-response relationship between IC Ach and MLD, and studied whether incremental IC Ach doses given during endothelial function testing improve its diagnostic utility in women and men with ANOCA.
Methods
Study population
We prospectively enrolled adult patients between August 2007 and August 2017 who were electively referred for coronary angiography. These patients were referred because of a clinical suspicion of ischemia based on the presence of persistent (>3 months) typical or atypical angina,13 with or without an abnormal stress test. Before invasive testing, patient’s symptoms were quantified using the Seattle Angina Questionnaire (SAQ).14 Exclusion criteria included the presence of an acute coronary syndrome, prior heart transplantation, coronary artery bypass grafting, serum creatinine>1.5mg/dL, ejection fraction<55%, or presence of another likely explanation of angina such as pulmonary hypertension, hypertrophic cardiomyopathy, or valvular heart disease. The study was approved by Stanford’s institutional review board and informed, written consent was obtained from all patients.
The data, methods used in the analysis, and materials used to conduct the research will be made available to researchers for purposes of reproducing the results or replicating the procedure. The data that support the findings of this study are available from the corresponding author on reasonable request.
Invasive Coronary Angiography
All coronary vasodilating drugs, including nitrates and calcium channel blockers, were discontinued 48 hours prior to testing except for sublingual nitroglycerin as needed. A baseline coronary angiogram was performed using a 6-French guiding catheter from the femoral approach to rule out significant obstructive CAD (epicardial stenosis>50%) in the right and left coronary arteries. Once obstructive CAD was ruled out, further invasive evaluation was conducted.
Coronary Endothelial Function Testing
Intravenous heparin (50–70units/kg) was administered and a 6 French guiding catheter without side-holes was used to engage the left main coronary artery. We tested coronary endothelial function by injecting IC Ach directly into the left coronary artery over ∼1 minute, starting at a dose of 20µg, followed by 50µg, then 100µg, and up to a maximum dose of 200µg unless there was significant bradycardia, atrioventricular block, paroxysmal atrial fibrillation, or severe vasoconstriction. Details of preparing Ach are shown in Supplemental material 1. After each injection, coronary angiography was performed, and the angiograms were later compared to baseline by quantitative coronary angiography (QCA) to determine if endothelial dysfunction was present. Endothelial dysfunction was defined as a decrease in the epicardial coronary artery MLD by >20% compared with baseline following the maximally tolerated dose of Ach in at least one segment (proximal, mid, distal) of the left anterior descending artery (LAD).4 Lastly, a 200μg bolus of IC nitroglycerin was administered to evaluate endothelium-independent vasodilation of the epicardial artery. We also recorded patient’s symptoms, ischemic electrocardiographic (ECG) changes, and complications/side effects if any, during the IC Ach infusion.
Quantitative Coronary Angiography
The Stanford Cardiovascular Core Analysis Laboratory, blinded to the clinical, physiologic, and IVUS data, performed QCA on the LAD using the computer‐assisted method QAngio XA7.3 (Medis) to determine the lumen diameter at baseline, after each IC Ach injection, and after IC nitroglycerin administration.
Coronary physiology measurements
After endothelial function testing, coronary flow reserve (CFR), index of microcirculatory resistance (IMR), and fractional flow reserve (FFR) were measured by methods described previously.2 IMR was calculated as the distal pressure at maximal hyperemia divided by the inverse of the hyperemic mean transit time (Tmn).15 CFR was calculated as resting Tmn divided by hyperemic Tmn, and FFR was calculated by the ratio of mean distal pressure/mean aortic pressure at maximal hyperemia. Microvascular dysfunction (MVD) was defined as an IMR ≥25.16,17 An abnormal FFR was defined as ≤0.80.
Intravascular Ultrasound
IVUS was performed with a 40‐MHz mechanical transducer ultrasound catheter (Atlantis SR Pro2, Boston Scientific, Natick, MA) in the LAD. An automated and manual pullback at 0.5 mm/s was performed, and the IVUS images were stored onto DVD for offline analysis. Standard 2- and 3-dimensional measurements were performed as previously described.18 All measurements were performed by the Stanford Cardiovascular Core Analysis Laboratory, blinded to clinical, physiologic, and angiographic information.
The presence of a myocardial bridge (MB) was defined by IVUS, by either the presence of an echo-lucent halo and/or ≥10% systolic compression during the cardiac cycle.19
Statistical analysis
Variables are presented as means or medians (continuous) or percentages (categorical), with differences tested using the t-test, χ2 test, or for cell frequencies less than 5, Fisher’s exact test. Piecewise linear mixed effects models (random intercept and random slope) were fit using all participants (diagnosed with endothelial dysfunction or not). Likelihood ratio tests were done to compare the simple linear model, to a random-intercept model, to a random-intercept-slope model. The simplest model with the best fit was selected. Models were adjusted for sex and vessel segment, and included piecewise linear splines at 20µg, 50µg, and 100µg Ach doses. P-values from the likelihood ratio test are to be interpreted descriptively, and not as a formal hypothesis test. Body surface area (BSA) was calculated using the formula of Du Bois and Du Bois.20
Equivalence between incremental doses of Ach were assessed using Deming regression models (R package “mcr”)21 to compare percent constriction between each pair of doses with a 20% equivalence margin selected a-priori. The estimated regression line and 95% confidence intervals between paired measurements of percent constriction were provided and any 95% confidence intervals that were fully contained within the 20% equivalence margin were considered statistically significant. We considered the constriction by two doses to be clinically equivalent if the 95% CI of the slope of regression line fell between 0.80 and 1.20 (20% difference from the slope of 1 on each side). If the 95% CI was either below 0.80 or higher than 1.20, then the constriction by two doses were considered clinically not equivalent.
All data management and analyses were conducted using Stata 15 (StataCorp College Station, TX) and R 3.5.1 (R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinical characteristics
Supplemental Table 1 shows the clinical characteristics of the patients by sex. Of the 288 patients recruited in the study, 215 (74.7%) were women with a mean age of 54.1±12.2 years and 73 (25.3%) were men with a mean age of 51.8±14.0 years. Typical and atypical angina was present in 193 (67%) and 95 (33%), respectively. Baseline SAQ scores confirmed significant anginal symptoms in patients, with women having a significantly lower anginal frequency score, representing worse symptoms, than men (57.7±23.2 vs. 65.1±22.2, p=0.03).
Of the total 288 patients, 3 had an EF<55% and QCA analysis was not done in 8 patients for technical reasons, and hence were excluded. Finally, we included 277 patients (212 women and 65 men) in the analysis.
Endothelial function testing
Of the total, 20µg Ach dose was given in 81 patients (63 women, 18 men), 50µg in 266 (206 women, 60 men), 100µg in 206 (164 women, 42 men), and 200µg in 45 patients (38 women, 7 men). Table 1 represents QCA and coronary physiology findings by sex. Epicardial endothelial dysfunction was present in 186 (68.1%). In patients with endothelial dysfunction, men had a significantly higher maximum %constriction of MLD after IC Ach than women. Forty-eight (17.5%) patients reproduced their symptom of chest pain and 27 (9.8%) had ischemic ECG changes. With IC Ach injection, 19 (6.9%) developed atrioventricular block (2 at 20 µg, 8 at 50µg, and 9 at 100µg), 9 (3.3%) developed significant bradycardia (8 at 50µg and 1 at 100µg), and 3 (1.1%) developed paroxysmal atrial fibrillation (1 at 20µg, 100µg, and 200µg). We did not give higher doses of IC Ach if the above conditions developed.
Table 1.
QCA, coronary physiology and IVUS findings by Sex
| Variable | Total (n=277) | Women (n=212) | Men (n=65) |
|---|---|---|---|
|
| |||
| QCA findings | |||
| Minimal lumen diameter, mm | |||
| Baseline | 1.54±0.31 | 1.52±0.31 | 1.61±0.30 |
| After Ach 20µ dose | 1.41±0.35 | 1.42±0.33 | 1.39±0.42 |
| After Ach 50µ dose | 1.35±0.39 | 1.34±0.37 | 1.37±0.48 |
| After Ach 100µ dose | 1.37±0.43 | 1.37±0.40 | 1.37±0.54 |
| After Ach 200µ dose | 1.40±0.42 | 1.43±0.38 | 1.27±0.64 |
| After nitroglycerin 200µ dose | 1.69±0.30 | 1.66±0.29 | 1.76±0.32 |
| Endothelial dysfunction | 186 (68.1) | 140 (66.4) | 46 (74.2) |
| Max %MLD constriction* | 45±17 | 43±16 | 51±18 |
| Chest pain during Ach testing | 48 (17.5) | 38 (18.0) | 10 (15.6) |
| Ischemic ECG changes during Ach testing | 27 (9.8) | 23 (10.9) | 4 (6.2) |
| Complications during Ach testing | |||
| Atrioventricular block | 19 (6.9) | 14 (6.6) | 5 (7.8) |
| Bradycardia | 9 (3.3) | 6 (2.8) | 3 (4.7) |
| Atrial fibrillation | 3 (1.1) | 2 (0.9) | 1 (1.6) |
| Physiology findings | |||
| IMR | 19.65±8.77 | 19.96±9.19 | 18.62±7.19 |
| Microvascular dysfunction† | 61 (22.0) | 53 (25.0) | 8 (12.3) |
| CFR | 4.32±3.53 | 4.17±3.91 | 4.81±1.79 |
| CFR<2.0 | 20 (7.2) | 19 (9.0) | 1 (1.5) |
| CFR<2.5 | 43 (15.5) | 38 (17.9) | 5 (7.7) |
| FFR | 0.86±0.05 | 0.86±0.06 | 0.84±0.05 |
| FFR<0.80 | 30 (10.8) | 23 (10.8) | 7 (10.8) |
| IVUS findings | |||
| MPB, % | 39.9 [29.5, 52.2] | 38.9 [28.8, 50.8] | 44.9 [32.6, 57.4] |
| MLA, mm2 | 4.54±1.85 | 4.51±1.89 | 4.65±1.71 |
| Myocardial bridge | 164 (59.2) | 123 (58.0) | 41 (63.1) |
Data presented for patients with endothelial dysfunction;
defined as IMR≥25; CFR=coronary flow reserve; FFR=fractional flow reserve; IMR=index of microcirculatory resistance; QCA=Quantitative Coronary angiography;
Dose-response relationship between IC Ach and MLD
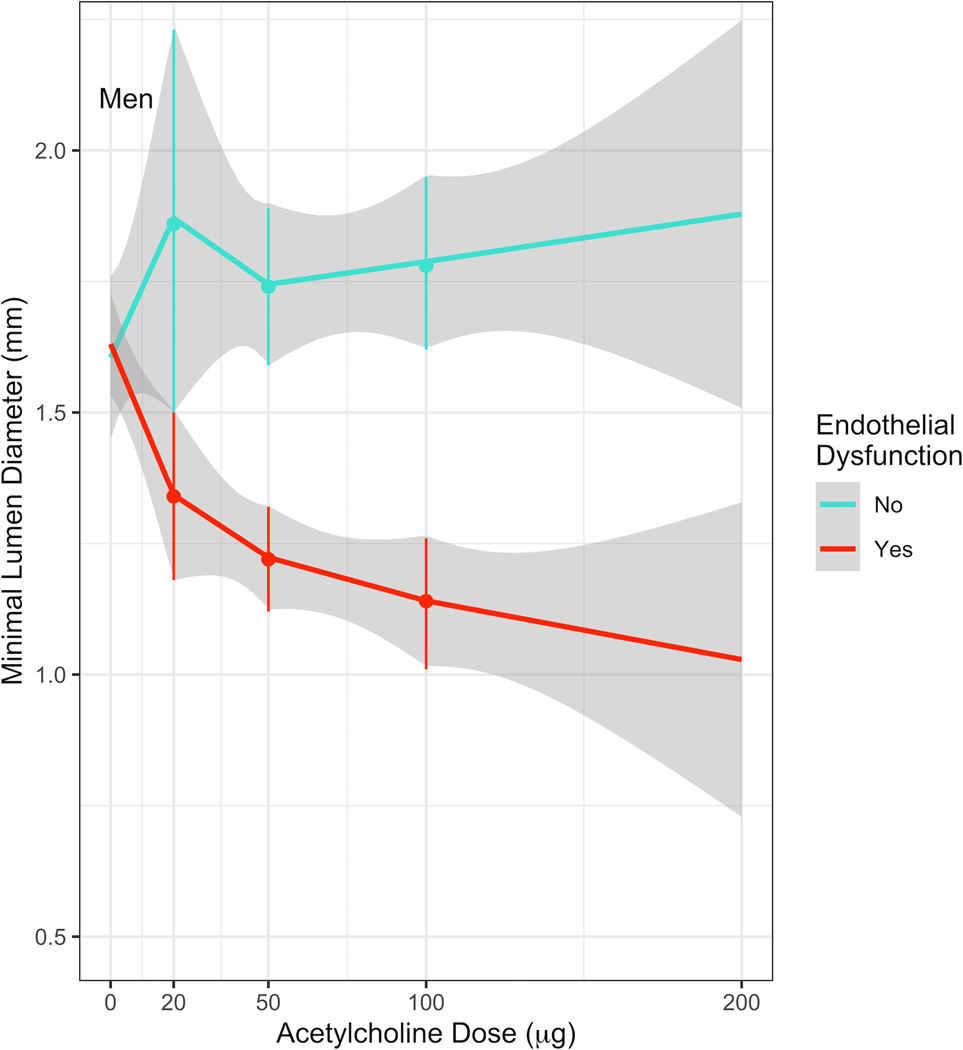
Figures 1A and 1B show the dose-response relationship between IC Ach and MLD in women and men, respectively. Among men with endothelial dysfunction, the MLD decreased significantly at doses above 50µg (−14.0µm/10µg (95% CI: −27.3 – −0.76)), which remained consistent at doses above 100μg (−18.7µm/10µg (95% CI −32.3 – −5.1)) (Table 2). This decrease in MLD was not observed in women with endothelial dysfunction at doses above 50µg (1.8µm/10µg (95% CI: −9.3 – 12.8)) or above 100 μg (−2.9µm/10µg (95% CI −14.9 – 9.1)). The decrease in MLD/10 μg at Ach doses above 50μg and 100μg was significantly greater in men than in women (p<0.001).
Figure 1. Dose-response relationship between IC Ach and MLD.
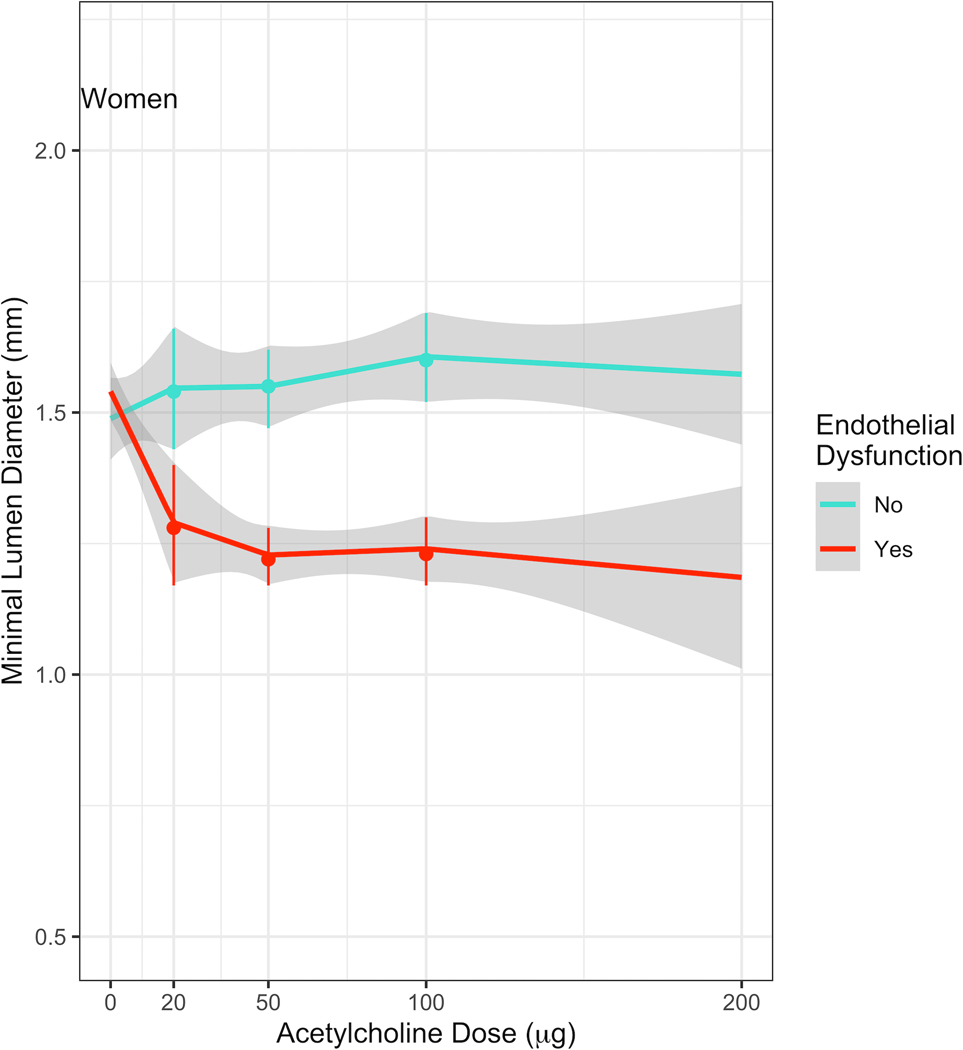
(A) Estimates of MLD at each dose of Acetylcholine in women. Among women with endothelial dysfunction, there was a decrease in the MLD up to a dose of 20μg Ach, which was not observed at higher doses.
(B) Estimates of MLD at each dose of Acetylcholine in men. Among men with endothelial dysfunction, there was a dose-response relationship up to a dose of 200μg.
Table 2.
Estimated change in MLD in μmeter for every 10μg change in Ach dosage
| Slopes | Women (estimates of MLD change/10µg Ach) | Men (estimates of MLD change/10µg Ach) | ||
|---|---|---|---|---|
|
| ||||
| Ach | No endothelial dysfunction | Endothelial dysfunction | No endothelial dysfunction | Endothelial dysfunction |
|
| ||||
| doses >20µg | −2.7 (−31.8 – 26.4) | −21.6 (−47.4 – 4.2) | 3.9 (−27.3 – 35.1) | −37.4 (−63.8 – −11.0) |
| doses >50µg | 9.5 (−4.4 – 23.3) | 1.8 (−9.3 – 12.8) | 16.1 (−0.67 – 32.8) | −14.0 (−27.3 – −0.76) |
| doses >100µg | −2.4 (−12.2 – 7.3) | −2.9 (−14.9 – 9.1) | 4.2 (−10.8 – 19.1) | −18.7 (−32.3 – −5.1) |
Slope estimates presented in µmeter/10µg; Ach = acetylcholine
To study if these sex differences in the dose response between women and men were due to differences in their body surface area, we adjusted the models for BSA. We found no significant difference in the results between the adjusted and unadjusted models, suggesting that the differences in BSA did not affect the dose response relationship (Supplemental Table 2).
We also assessed the dose response relationship between IC Ach and MLD when endothelial dysfunction was defined as %MLD constriction ≥1% and ≥50%. We found no significant difference in the change in MLD/10µg with different thresholds for the definition of endothelial dysfunction (Supplemental Table 3).
Comparison of %MLD constriction at incremental IC Ach doses
We performed Deming regression analysis to compare the %MLD constriction induced by the incremental doses of IC Ach to study the diagnostic benefit of giving a higher dose. In comparing 50µg and 20µg Ach doses, we found that the 95% CI of the slope of regression line (95% CI 0.60 – 0.76) did not fall within the predefined equivalence limit of 0.80 and 1.20, suggesting that the %MLD constrictions at these doses were not clinically equivalent (Figure 2A). Similarly, we found that the %MLD constriction at 100µg Ach was not equivalent to that of 50µg (95% CI 0.71 – 0.86) (Figure 2B), suggesting that the %MLD constrictions at these doses were not clinically equivalent either.
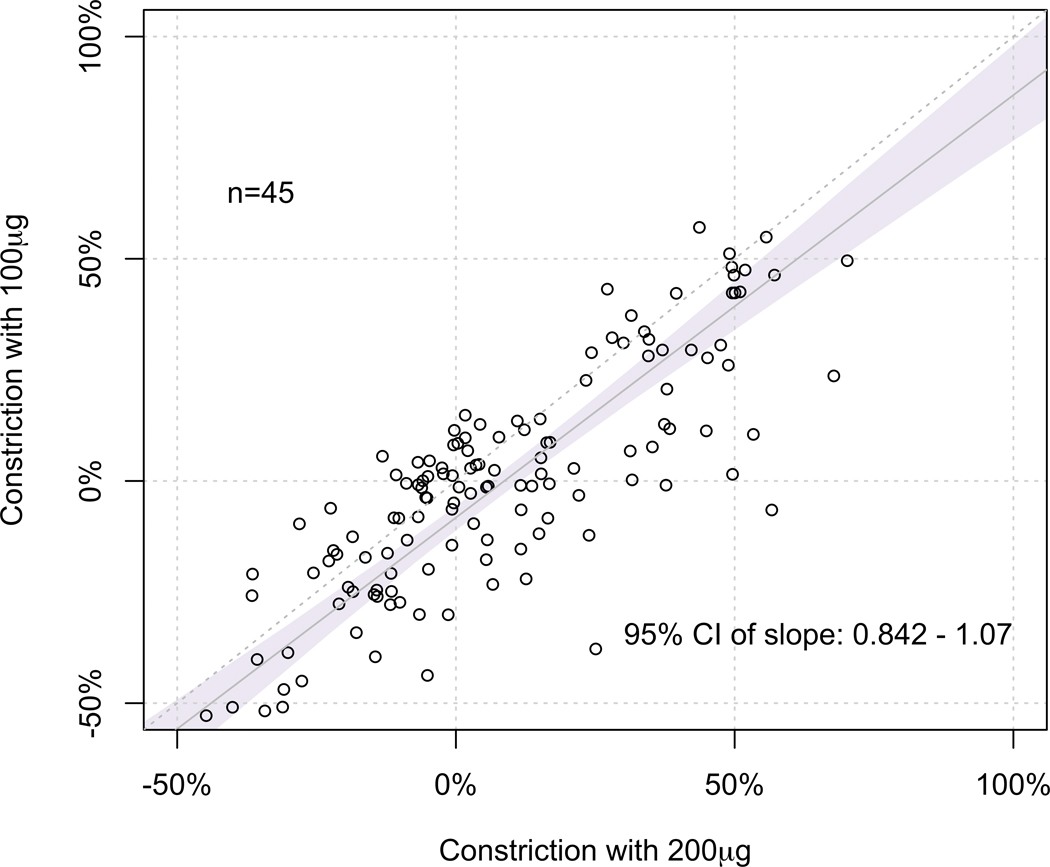
Figure 2. Comparison between %MLD constriction induced at each dose.
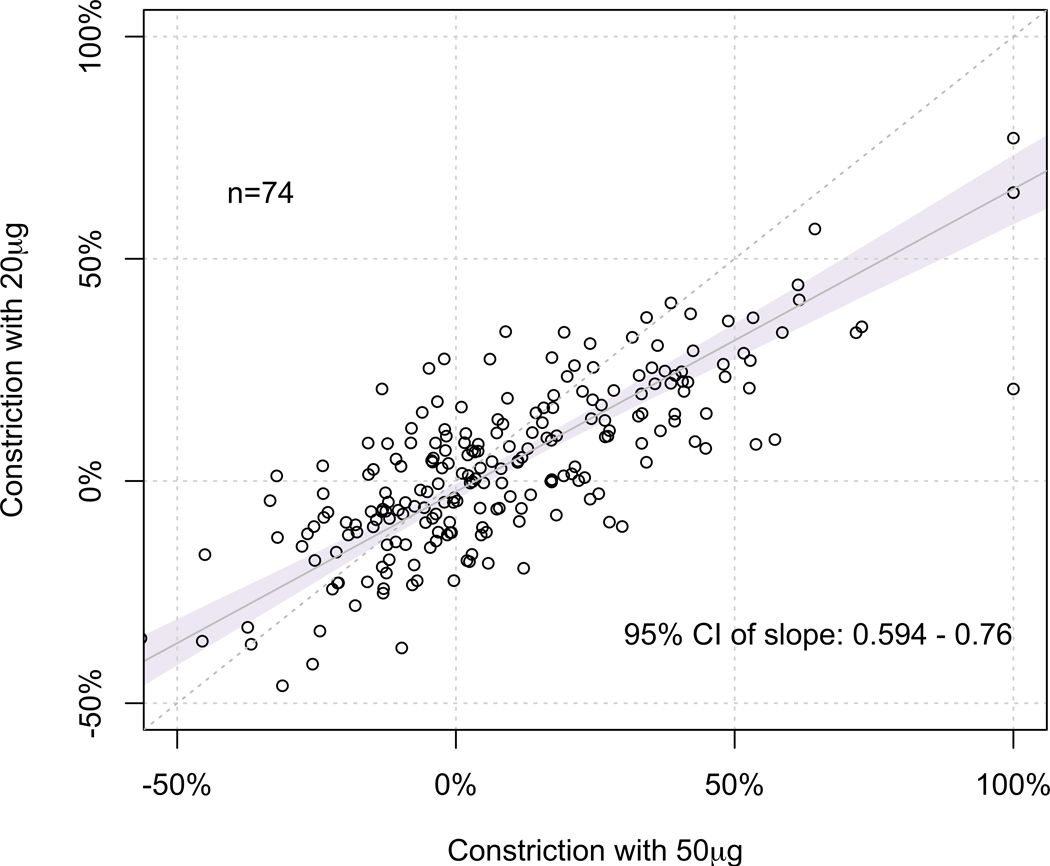
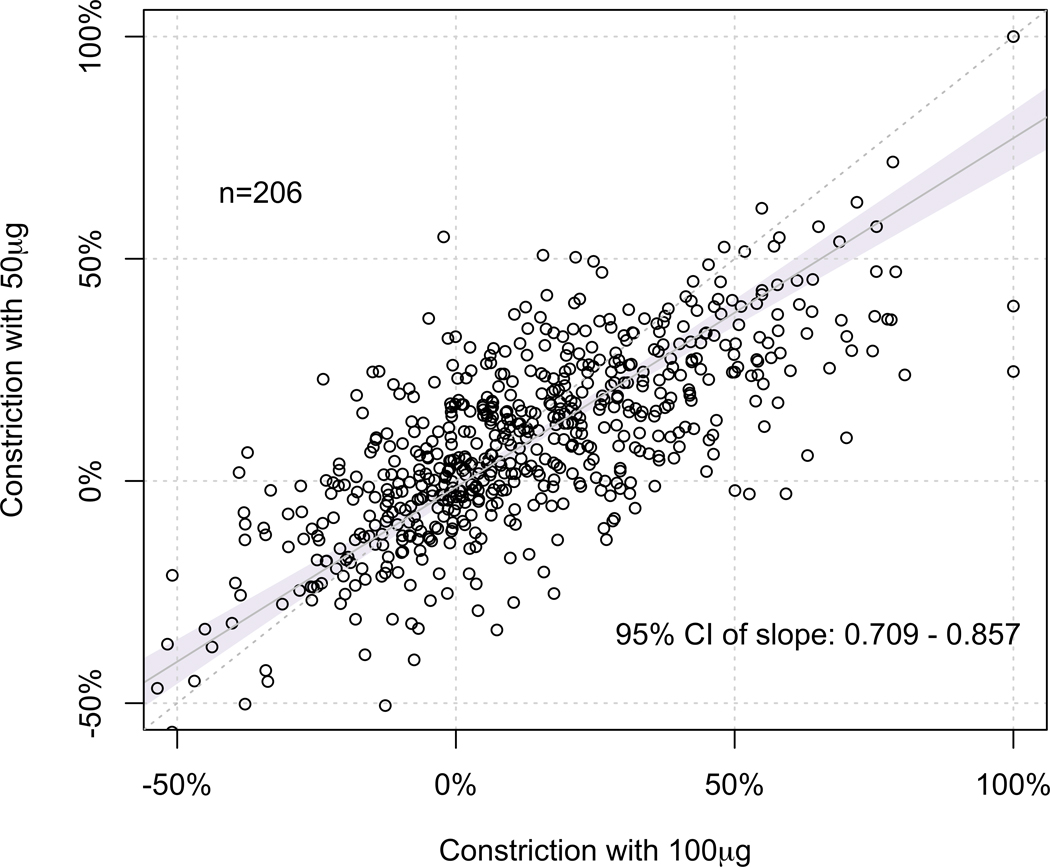
(A) At 50μg vs. 20μg, the 95% CI of the slope of regression line (95% CI 0.60 – 0.76) did not fall within the predefined equivalence limit of 0.80 and 1.20, suggesting that the %MLD constrictions at these doses were not clinically equivalent.
(B) At 100μg vs. 50μg, the 95% CI of the slope of regression line (95% CI 0.71 – 0.86) did not fall within the predefined equivalence limit of 0.80 and 1.20, suggesting that the %MLD constrictions at these doses were not clinically equivalent.
(C) At 200μg vs. 1000μg, the 95% CI of the slope of regression line (95% CI 0.84 – 1.06) fell within the predefined equivalence limit of 0.80 and 1.20, suggesting that the %MLD constrictions at these doses were clinically equivalent.
In comparing 200µg and 100µg Ach doses, we found that the 95% CI of the slope of regression line (95% CI 0.84 – 1.06) fell within the predefined equivalence limit of 0.80 and 1.20, suggesting that the %MLD constrictions at these doses were clinically equivalent (Figure 2C).
Coronary physiology testing
The mean IMR was 19.6±8.77. MVD was present in 61 (22%) patients. Mean CFR was 4.32±3.53. Mean FFR was 0.86±0.05. While no patients had obstructive CAD (>50% stenosis), FFR was ≤0.80 in 30 (10.8%), with mild-moderate diffuse atherosclerosis seen on IVUS in most cases.
Intravascular Ultrasound
An MB was present in 164 (59.2%) patients, when defined as the presence of a halo and/or systolic compression ≥10% on IVUS. We found significant overlap in the prevalence of an MB and epicardial endothelial dysfunction. Of the 164 patients with an MB, endothelial dysfunction was present in 125 (76.2%) patients.
Discussion
The salient findings of our study are: 1) Women and men have different responses to Ach during endothelial function testing, with men having a greater response to IC Ach at all doses when compared with women. 2) Men also demonstrate an Ach-MLD dose-response relationship with doses up to 200μg, while women have minimal change in MLD with doses above 50µg. 3) Incremental dosing of Ach, starting at 20µg and increasing to 100µg, appears to improve the diagnostic utility of identifying a clinically significant MLD constriction during endothelial function testing. This benefit was not observed at Ach doses above 100µg.
ANOCA is a common, but poorly understood condition that can be challenging for patients and providers, and puts an economic strain on the healthcare system.22,23 Occult coronary abnormalities, including endothelial and microvascular dysfunction, are a common findings in these patients.2,24 Non-invasive cardiac stress tests have limited diagnostic accuracy in identifying these occult abnormalities,25 and invasive tests such as IC Ach provocation testing and coronary physiology testing and are the preferred methods of diagnosis.8,10,26
The current knowledge of efficacy of Ach dosing regimens during provocation testing has primarily focused on coronary epicardial and microvascular vasospasm.27–29 Ong et al studied 921 patients and reported that IC Ach provocation testing with incremental doses of Ach is a safe technique to assess coronary vasomotor function in identifying epicardial and microvascular spasm.30 In a separate analysis, they also reported that women were more sensitive to Ach, leading to abnormal test results at lower doses.31 On the other hand, studies assessing epicardial endothelial function have primarily used a single dose of Ach that is lower than 20 μg to diagnose endothelial dysfunction.5,32,33 Our study shows that women and men respond differently to IC Ach, and a single dose of IC Ach may not be sufficient to identify maximum constriction, especially in men with endothelial dysfunction. In addition, we also show that there is a diagnostic benefit in giving incremental doses of Ach during endothelial function testing.
We found that women and men respond differently to IC Ach, with men not only having an overall greater response to Ach doses than women, but also showing a dose-response relationship to higher doses. This relationship was not seen in women, with only minimal change in the MLD at higher doses of Ach. One of the reasons for this could be that the coronary arteries in women are smaller in diameter and have thinner walls with more tortuosity than men, which may attenuate the response to Ach provocation.34,35 In addition, sex differences in the effects of hormones and body mass on vessel remodeling may also play an important role in the coronary reactivity to Ach. A previous study by Aziz et. al. also reported that women were more sensitive to a lower dose of Ach to achieve a positive test than men during IC Ach provocation testing.31 Also, one of the underlying mechanisms for the difference in response to Ach in women and men could be due to the sex difference in muscarinic receptors demonstrated at the higher doses of IC Ach, which warrants further research.
In our study, we did not observe any fatal or serious non-fatal complications with Ach testing. While 6.9% patients developed atrioventricular block and 3.3% developed significant bradycardia, these conditions readily reversed once the administration of Ach was stopped. Paroxysmal atrial fibrillation is a rare complication with Ach infusion (1.1% in our population) and may be more common in patients with existing paroxysmal atrial fibrillation or a propensity toward atrial fibrillation. Previous studies have reported similar incidences,30,36 and its prognostic significance is currently unknown. When atrial fibrillation occurs during Ach testing, it generally self-converts, but pharmacologic or electrical cardioversion may be required. Finally, for patients with severe vasoconstriction resulting in reduced coronary flow and ECG changes, IC nitroglycerin is the treatment of choice, which we routinely give at the conclusion of Ach provocative testing.
Finally, it has been demonstrated that a comprehensive invasive assessment during coronary angiography, including testing for endothelial and microvascular dysfunction, and IVUS for detection of an MB and subclinical atherosclerosis, is valuable for the full evaluation of patients with ANOCA and may help to guide therapy, and improve patient’s symptoms and quality of life.37 However, developing unified standards for testing remains a work in progress.9,26 This study gives guidance as to the optimal dosing of Ach for women and men undergoing vascular function testing and can contribute to evidence-based dosing recommendations.
Limitations
This is a single center study with a relatively smaller sample size, but ours is the first study to report the dose-response relationship between IC Ach and MLD by sex in ANOCA patients. All investigations were performed in the LAD only, thus potentially limiting the applicability of the findings to other coronary branches. Not all patients in this study received all four Ach doses. We began administering the maximum dose of 200μg Ach in April 2015, after a safety evaluation report was published by Ong et. al. in 2014.30 At that same time, we added the lower dose of 20µg to more closely mirror early Women’s Ischemia Syndrome Evaluation (WISE) studies,7 and ultimately, to conduct this study. For equivalence testing between Ach doses, we choose 20% constriction as an equivalence margin, but this value is not clinically validated, and further studies are needed to validate our findings. Finally, endothelial dysfunction and/or spasm isolated to the microvasculature was not specifically assessed.
Conclusion
Women and men with ANOCA appear to have different responses to Ach during epicardial endothelial function testing. In addition to having a greater response to IC Ach at all doses, men also demonstrate an Ach-MLD dose-response relationship with doses up to 200μg, while women have minimal change in MLD with doses above 50µg. An incremental dosing regimen of IC Ach during endothelial function testing, adjusted based on sex, appears to improve the diagnostic utility of endothelial function testing, and may help balance the diagnostic yield vs. safety.
Supplementary Material
What is Known.
Epicardial coronary endothelial dysfunction is a major cause of angina in patients with no obstructive coronary artery disease (ANOCA)
Intracoronary acetylcholine (IC Ach) provocation testing is the gold standard test for assessing coronary endothelial function
What the Study Adds
Women and men respond differently to IC Ach, with men having a greater response to IC Ach at all doses
Men also demonstrate an Ach-minimal lumen diameter (MLD) dose-response relationship with doses up to 200μg, while women have minimal change in MLD with doses above 50µg
An incremental dosing regimen of IC Ach appears to improve the diagnostic utility of endothelial function testing, which may help balance the diagnostic yield vs. safety
Acknowledgment:
This work was supported in part by a generous gift from The Ron and Sanne Higgins Women’s Heart Health Fund.
Funding Support: NIH K23 HL092233–05 (PI: JAT)
Financial Disclosure: VSP: Research fellowship support, Gilead Sciences. JAT: Honoraria, Boston Scientific and Terumo; Stocks/equity, Recor. Others nothing to disclose.
Non-standard Abbreviations and Acronyms
- Ach
acetylcholine
- ANOCA
angina with no obstructive coronary artery disease
- CFR
coronary flow reserve
- FFR
fractional flow reserve
- IC
intracoronary
- IMR
index of microcirculatory resistance
- IVUS
intravascular ultrasound
- LAD
left anterior descending artery
- MB
myocardial bridge
- MLD
minimal lumen diameter
- MVD
microvascular dysfunction
- QCA
quantitative coronary angiography
- SAQ
Seattle Angina Questionnaire
- Tmn
hyperemic mean transit time
References
- 1.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA. Invasive Evaluation of Patients with Angina in the Absence of Obstructive Coronary Artery Disease. Circulation. 2015;131:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halcox J, Schenke W, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KA, Quyyumi AA. Prognostic Value of Coronary Vascular Endothelial Dysfunction. Circulation. 2002;106:653–658. [DOI] [PubMed] [Google Scholar]
- 4.Suwaidi J Al, Hamasaki S, Higano ST, Nishimura RA, David R, Lerman A. Long-Term Follow-Up of Patients with Mild Coronary Artery Disease and Endothelial Dysfunction. Circulation. 2000;101:948–954. [DOI] [PubMed] [Google Scholar]
- 5.AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G et al. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. J Am Coll Cardiol. 2019;73:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. [DOI] [PubMed] [Google Scholar]
- 7.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:722–725. [DOI] [PubMed] [Google Scholar]
- 8.Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779–2801. [DOI] [PubMed] [Google Scholar]
- 9.Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Bairey Merz CN. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J [Internet]. 2015;38:2565–2568. [DOI] [PubMed] [Google Scholar]
- 10.Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 11.Jean D, Peter G. Role of Endothelial Dysfunction in Atherosclerosis. Circulation. 2004;109:III-27-III–32. [DOI] [PubMed] [Google Scholar]
- 12.Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High Prevalence of a Pathological Response to Acetylcholine Testing in Patients With Stable Angina Pectoris and Unobstructed Coronary Arteries: The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries. J Am Coll Cardiol. 2012;59:655–662. [DOI] [PubMed] [Google Scholar]
- 13.Diamond GA. A clinically relevant classification of chest discomfort. J. Am. Coll. Cardiol. 1983;1:574–575. [DOI] [PubMed] [Google Scholar]
- 14.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 15.Fearon WF, Balsam LB, Farouque HMO, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–3132. [DOI] [PubMed] [Google Scholar]
- 16.Ng MKC, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: Superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113:2054–2061. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi Y, Fearon WF, Honda Y, Tanaka S, Pargaonkar V, Fitzgerald PJ, Lee DP, Stefanick M, Yeung AC, Tremmel JA. Effect of Sex Differences on Invasive Measures of Coronary Microvascular Dysfunction in Patients With Angina in the Absence of Obstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fearon WF, Nakamura M, Lee DP, Rezaee M, Vagelos RH, Hunt SA, Fitzgerald PJ, Yock PG, Yeung AC. Simultaneous assessment of fractional and coronary flow reserves in cardiac transplant recipients: Physiologic Investigation for Transplant Arteriopathy (PITA Study). Circulation. 2003;108:1605–1610. [DOI] [PubMed] [Google Scholar]
- 19.Ge J, Jeremias A, Rupp A, Abels M, Baumgart D, Liu F, Haude M, Görge G, Von Birgelen C, Sack S, Erbel R. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and Doppler. Eur Heart J. 1999;20:1707–1716. [DOI] [PubMed] [Google Scholar]
- 20.Du Bois D, Du Bois EF. Clinical calorimetry: Tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;XVII:863–871. [Google Scholar]
- 21.Manuilova E, Schuetzenmeister A, Model F. mcr: Method Comparison Regression. R package version 1.2.1. 2014. [Google Scholar]
- 22.Jespersen L, Abildstrom SZ, Hvelplund A, Madsen JK, Galatius S, Pedersen F, Hojberg S, Prescott E. Burden of Hospital Admission and Repeat Angiography in Angina Pectoris Patients with and without Coronary Artery Disease: A Registry-Based Cohort Study. PLoS One. 2014;9:e93170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw L, Merz C, Pepine C, Reis S, Bittner V. The Economic Burden of Angina in Women With Suspected Ischemic Heart Disease. Circulation. 2006;114:894–904. [DOI] [PubMed] [Google Scholar]
- 24.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–741. [DOI] [PubMed] [Google Scholar]
- 25.Pargaonkar VS, Kobayashi Y, Kimura T, Schnittger I, Chow EKH, Froelicher VF, Rogers IS, Lee DP, Fearon WF, Yeung AC et al. Accuracy of non-invasive stress testing in women and men with angina in the absence of obstructive coronary artery disease. Int J Cardiol. 2019;282:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 27.Saito Y, Kitahara H, Shoji T, Tokimasa S, Nakayama T, Sugimoto K, Fujimoto Y, Kobayashi Y. Feasibility of omitting provocation test with 50 mug of acetylcholine in left coronary artery. Heart Vessels. 2017;32:685–689. [DOI] [PubMed] [Google Scholar]
- 28.Saito Y, Kitahara H, Shoji T, Tokimasa S, Nakayama T, Sugimoto K, Fujimoto Y, Kobayashi Y. Intracoronary Acetylcholine Provocation Testing- Omission of the 20-microg Dose Is Feasible in Patients Without Coronary Artery Spasm in the Other Coronary Artery. Circ J. 2016;80:1820–1823. [DOI] [PubMed] [Google Scholar]
- 29.Sueda S, Kohno H, Miyoshi T, Sakaue T, Sasaki Y, Habara H. Maximal acetylcholine dose of 200 mug into the left coronary artery as a spasm provocation test: comparison with 100 mug of acetylcholine. Heart Vessels. 2015;30:771–778. [DOI] [PubMed] [Google Scholar]
- 30.Ong P, Athanasiadis A, Borgulya G, Vokshi I, Bastiaenen R, Kubik S, Hill S, Schäufele T, Mahrholdt H, Kaski JC, Sechtem U. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129:1723–1730. [DOI] [PubMed] [Google Scholar]
- 31.Aziz A, Hansen HS, Sechtem U, Prescott E, Ong P. Sex-Related Differences in Vasomotor Function in Patients With Angina and Unobstructed Coronary Arteries. J Am Coll Cardiol. 2017;70:2349–2358. [DOI] [PubMed] [Google Scholar]
- 32.Kitahara H, Kobayashi Y, Iwata Y, Fujimoto Y, Komuro I. Effect of pioglitazone on endothelial dysfunction after sirolimus-eluting stent implantation. Am J Cardiol. 2011;108:214–219. [DOI] [PubMed] [Google Scholar]
- 33.Aoki Y, Ishikawa K, Miura K, Sugimoto K, Nakayama T, Fujimoto Y, Kobayashi Y. Protective effect of angiotensin II receptor blocker and calcium channel blocker on endothelial vasomotor function after everolimus-eluting stent implantation. J Cardiol. 2016;67:236–240. [DOI] [PubMed] [Google Scholar]
- 34.Paulsen S, Vetner M, Hagerup LM. Relationship between heart weight and the cross ual area of the coronary ostia. Acta Pathol Microbiol Scand A. 1975;83:429–432. [DOI] [PubMed] [Google Scholar]
- 35.KITZMAN DW, SCHOLZ DG , HAGEN PT, ILSTRUP DM , EDWARDS WD. Age-Related Changes in Normal Human Hearts During the First 10 Decades of Life. Part II (Maturity): A Quantitative Anatomic Study of 765 Specimens From Subjects 20 to 99 Years Old. Mayo Clin Proc. 1988;63:137–146. [DOI] [PubMed] [Google Scholar]
- 36.Sueda S, Fukuda H, Watanabe K, Ochi N, Kawada H, Hayashi Y, Uraoka T. Clinical characteristics and possible mechanism of paroxysmal atrial fibrillation induced by intracoronary injection of acetylcholine. Am J Cardiol. 2001;88:570–573. [DOI] [PubMed] [Google Scholar]
- 37.Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K et al. Stratified Medical Therapy Using Invasive Coronary Function Testing In Angina: CorMicA Trial. J Am Coll Cardiol. 2018;72:2814–2855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


