Abstract
Policy Points
Only a small minority of new drugs in “nonprotected” classes are widely covered by Part D plans nationwide in the year after US Food and Drug Administration (FDA) approval.
Part D plans frequently apply utilization management restrictions such as prior authorizations to newly approved drugs in both protected and nonprotected classes.
Drug price influences both formulary inclusion (in nonprotected classes) and coverage restrictions (in both protected and nonprotected classes), while other drug characteristics such as therapeutic benefits are not consistently associated with formulary design. Plans do not seem to favor the minority of drugs that are determined to offer added therapeutic benefit over existing alternatives.
Context
Medicare Part D is an outpatient prescription drug benefit for older Americans covering more than 46 million beneficiaries. Except for mandatory coverage for essentially all drugs in six protected classes, plans have substantial flexibility in how they design their formularies: which drugs are covered, which drugs are subject to restrictions, and what factors determine formulary placement. Our objective in this paper was to document the extent to which Part D plans limit coverage of newly approved drugs.
Methods
We examined the formulary design of 4,582 Part D plans from 2014 through 2018 and measured (1) the decision to cover newly approved drugs in nonprotected classes, (2) use of utilization management tools in protected and nonprotected classes, and (3) the association between plan design and drug‐level characteristics such as 30‐day cost, therapeutic benefit, and the US Food and Drug Administration (FDA) expedited regulatory pathway.
Findings
The FDA approved 109 new drugs predominantly used in outpatient settings between 2013 and 2017. Of these, 75 fell outside of the six protected drug classes. One‐fifth of drugs in nonprotected classes (15 out of 75) were covered by more than half of plans during the first year after approval. Coverage was often conditional on utilization management strategies in both protected and nonprotected classes: only seven drugs (6%) were covered without prior authorization requirements in more than half of plans. Higher 30‐day drug costs were associated with more widespread coverage in nonprotected classes: drugs that cost less than $150 for a 30‐day course were covered by fewer than 20% of plans while those that cost more than $30,000 per 30 days were covered by more than 50% of plans. Plans were also more likely to implement utilization management tools on high‐cost drugs in both protected and nonprotected classes. A higher proportion of plans implemented utilization management strategies on covered drugs with first‐in‐class status than drugs that were not first in class. Other drug characteristics, including availability of added therapeutic benefit and inclusion in FDA expedited regulatory approval, were not consistently associated with plan coverage or formulary restrictions.
Conclusions
Newly approved drugs are frequently subject to formulary exclusions and restrictions in Medicare Part D. Ensuring that formulary design in Part D is linked closely to the therapeutic value of newly approved drugs would improve patients’ welfare.
Keywords: Medicare Part D, US Food and Drug Administration, prescription drugs
Medicare part d is a voluntary outpatient prescription drug benefit for older or disabled Americans that covers more than 46 million beneficiaries. 1 Since its implementation in January 2006, Part D has improved prescription drug use among Medicare beneficiaries and lowered cost‐related medication nonadherence. 2 The program was also associated with reduced hospitalizations and non‐drug‐related medical spending among older Americans who had poor drug coverage before 2006. 3
Not all drugs are covered in Medicare Part D. 4 Private plans administering Part D benefits use formularies to define the list of covered drugs. The program gives plans some flexibility in how to design formularies, although the statute authorizing Part D requires plans to include “all or substantially all” drugs approved by the US Food and Drug Administration (FDA) within six “protected classes” (immunosuppressants for prophylaxis of organ transplant rejection, antidepressants, antipsychotics, anticonvulsants, antiretrovirals, and antineoplastic agents). 5 Plans must also cover at least two drugs within other therapeutic classes.
It is important to understand how contemporary Part D plans design their formularies: which drugs are covered, which drugs are subject to restrictions, and what factors determine formulary placement. 4 Mandatory coverage of new drugs in protected classes has been linked to limited payer bargaining power and high drug prices as a result. 6 , 7 Nonprotected drugs excluded from formularies can be approved for coverage only via appeals. Short of exclusion from formulary, plans may impose restriction strategies—known as utilization management tools—that aim to ensure appropriate use and control costs. For example, plans may adopt prior authorization policies that require physicians to obtain insurance coverage approval from the plan sponsor for certain prescribed drugs. Plans may also require patients to try a different drug before using the prescribed option (known as step therapy) or set quantity limits that restrict the amount covered over a certain period of time.
If plans design formularies based on evidence of clinical or cost‐effectiveness, then their formularies should be broadly similar, more frequently including drugs that have documented evidence of added therapeutic benefits over alternatives. In such cases, patients can choose a plan based on premiums. However, if plans use other criteria to design formularies, then patients may have to choose plans that best fit their medical needs, and there is considerable evidence that patients are unable to pick plans or respond to cost‐sharing without harming their health. 8 , 9 For example, net drug prices (taking into account manufacturer discounts and rebates to insurers) may affect formulary exclusions and restrictions. From 2007 to 2018, net drug prices increased by an estimated 60%. 10 Plan administrators frequently voice concerns about rising drug prices. Therefore, plans may be reluctant to cover expensive drugs in nonprotected classes or may opt to implement formulary restrictions to limit access. By contrast, plans may be compelled to cover expensive drugs if these drugs are perceived to offer greater benefit. Plans may also use other information available at the time of regulatory approval as a signal of a new drug's therapeutic usefulness or value. For example, drugs that address life‐threatening conditions or unmet need may be eligible for inclusion in one of the FDA's expedited development or review pathways. Drugs approved through the FDA expedited programs may have different rates of plan coverage and utilization management requirements.
We therefore sought to examine the extent to which Part D plans limit coverage of newly approved drugs, whether by excluding them from formularies (in the case of nonprotected classes) or by imposing utilization management tools (for both protected and nonprotected classes). We also examined the association between coverage and utilization management tools and different relevant characteristics of recently approved therapies, including price, therapeutic benefit, and FDA regulatory pathway.
Methods
Sample Drugs
We used the publicly available Drugs@FDA database to identify novel therapeutic agents that received FDA approval between January 2013 and December 2017. Existing drugs that received supplemental approvals for new indications were excluded. To determine eligibility for Medicare Part D coverage, we reviewed drug labeling at the time of initial FDA approval to identify the indication, dosage, and route of administration. The following drug categories were excluded because they are typically not covered by Part D plans: agents used for anorexia, weight loss, or weight gain; fertility drugs; cosmetic drugs; and drugs for symptomatic relief of coughs and colds. We also excluded drugs that required physician administration, as these could be covered under Medicare Part B.
We categorized drugs according to Medicare Part D protected and nonprotected class status, using the World Health Organization Anatomical Therapeutic Chemical (ATC) codes L04 (immunosuppressants for prophylaxis of organ transplant rejection), N06A (antidepressants), N05A (antipsychotics), N03A (anticonvulsants), J05A (antiretrovirals), and L01 (antineoplastic agents) to identify the drugs in the six protected classes.
Part D Plan Data
Data on Medicare Part D plans were obtained from the Centers for Medicare and Medicaid Services (CMS) Prescription Drug Plan Formulary files from 2014 through 2018. 11 We included Medicare Advantage and stand‐alone prescription drug plans offered in 50 states and Washington, DC. Special‐needs plans were excluded. We also excluded employer‐only group health plans that were not open for general enrollment (i.e., were only available for retirees of a former employer or union). The annual number of available Part D plans ranged from 2,373 to 2,760. The selected plans had a total enrollment of 32.9 million in 2018, representing more than three‐quarters (77%) of the Medicare Part D beneficiary population.
CMS data included information on formulary design (list of covered drugs, prior authorization requirements, step therapy, and quantity limits) and beneficiary enrollment. We matched drugs in our sample to Part D plans using National Drug Code (NDC) identifiers and RxNorm concept unique identifiers (RXCUI), available from the National Library of Medicine. Within each calendar year, we used monthly data from June, allowing a minimum of six months for NDC and RXCUI numbers for new drugs to be issued and incorporated into CMS data files.
Drug Characteristics
We examined several characteristics of drugs hypothesized to influence coverage in Part D formularies. First, by reviewing publicly available reports that provided a summary of annual drug approvals, we determined which drugs received designations intended to expedite their development or regulatory approval: accelerated approval, breakthrough therapy, fast‐track, and priority review. We cross‐checked this information with data collected from the Drugs@FDA entry for each approval (using either the new drug application or biologic license application numbers, as relevant) and the FDA's monthly drug approval reports database. We also reviewed the separate lists compiled by the FDA's Center for Drug Evaluation and Research on drugs with accelerated approvals, breakthrough therapy designations, and priority reviews.
Second, we determined whether drugs received an Orphan Drug Act designation for their approved indications using the list compiled by the FDA's Center for Drug Evaluation and Research.
Third, we examined medical review reports from the Drugs@FDA database to identify the characteristics of pivotal studies that established the drug's efficacy. We classified pivotal study designs as randomized controlled trials or nonrandomized studies.
Fourth, we noted if drugs were the first agents in their pharmacological class, as classified by the FDA in annual reports.
Therapeutic Benefit Assessment
We obtained assessments of the therapeutic value of a medicine from the health technology assessment authorities in Canada, France, Germany, and Italy to determine which drugs offered added therapeutic benefit in their approved indications. 12 Health authorities in these four countries—Canadian Human Drug Advisory Panel, Haute Autorité de Santé in France, Federal Joint Committee in Germany, and Italian Medicines Agency in Italy—use evidence‐based criteria to determine the added therapeutic benefit of new drugs on the basis of comparative clinical effectiveness data and make their judgments publicly available. 13
For example, sofosbuvir (Sovaldi), widely considered to be a transformative therapy for treating chronic hepatitis C virus infection, was consistently judged to offer added therapeutic benefit by health authorities in these countries. By contrast, albiglutide (Tanzeum), one of several antidiabetic drugs on the market with no superior efficacy than existing alternatives in lowering baseline hemoglobin A1c levels, was not.
Drug benefit assessments made in Canada, France, Germany, and Italy rely on clinical trial data available at the time of drug approval and additional clinical, safety, and health‐related quality‐of‐life data that may be requested from the manufacturers. In the absence of direct head‐to‐head comparisons between new drugs and existing alternatives or usual care, health authorities may use indirect comparisons to judge the comparative therapeutic benefits of drugs. These assessments of therapeutic benefit are made independent of drug cost. 14 Information available from health authorities in other countries such as Australia and England were excluded from the study, as their assessments often rely on cost‐effectiveness in addition to clinical effectiveness. 15 , 16
To be conservative about our categorizations, drugs were considered to offer added therapeutic benefit compared to existing alternatives in their approved indications if any one of the four health authorities concluded that such benefit existed. 12
Drug Cost
To explore the potential impact of drug price on formulary inclusion and coverage restrictions, we used the publicly available Medicare Provider Utilization and Payment data and calculated the 30‐day costs based on net (postrebate) prices for drugs approved between 2013 and 2017. For each drug, we first divided the total amount spent by CMS during the year after FDA approval by the number of standardized 30‐day fills. We obtained data on net prices (accounting for confidential manufacturer rebates and other concessions for payers other than Medicaid) from the investment firm SSR Health. 17 Net prices provided by SSR Health are constructed by combining publicly reported manufacturer revenue and the volume of filled prescriptions for each branded drug. Information on net prices was unavailable for 21 (19%) drugs in our sample. We imputed missing values with the median rebate amount (for payers other than Medicaid) within each drug class (as defined by ATC codes).
Analysis
Our analyses had two primary outcomes. We first counted the proportion of Part D plans that included each drug in their formularies during the first year after FDA approval. We then counted the proportion of plans in which coverage was conditional on any prior authorization, step therapy, or quantity limits.
To investigate the association between drug characteristics and coverage outcomes, we used logistic regression models. Our primary model weighted each plan by its enrollment in order to measure the experience of the typical beneficiary. We also ran a sensitivity analysis without enrollment weighting. Most drug characteristics (inclusion in the FDA's expedited development and review programs, Orphan Drug Act designation, availability of evidence on added therapeutic benefit, first‐in‐class status, and pivotal study designs) were modeled as binary variables. We modeled 30‐day cost as a continuous variable on the logarithmic scale. All multivariable models controlled for potentially confounding variables available for analysis—namely, approval year and plan type (Medicare Advantage versus a stand‐alone prescription drug plan). We clustered standard errors at the drug level.
In addition to our primary plan‐level analysis, we conducted two sensitivity analyses to check the robustness of our findings. First, we ran a multivariable logistic regression model at the formulary level to evaluate the association between drug characteristics and formulary inclusion and restriction outcomes. This model accounted for the observed overlap in coverage and utilization management decisions across plans that shared the same formulary. The sample for this analysis consisted of distinct plan formularies available during the five‐year time period of our study. Similar to our primary analysis, we clustered standard errors at the drug level. Finally, we ran a drug‐level multivariable fractional logistic regression model. This model computed robust standard errors.
A two‐tailed p‐value of ≤0.05 was considered significant. Analyses were performed using Stata version 14.2 (StataCorp, College Station, TX).
Results
From 2013 through 2017, the FDA approved 181 novel drugs. After excluding 72 drugs not eligible for Medicare Part D coverage (Table A1 in the online appendix), our sample included 109 drugs. Characteristics of our study sample are summarized in Table 1. Additional details of included novel therapeutic agents are shown in Table A2.
Table 1.
Characteristics of Outpatient Prescription Drugs That Received FDA Approval Between 2013 and 2017
| Characteristic | n (%) |
|---|---|
| Total sample | 109 |
| Year of approval | |
| 2013 | 21 (19%) |
| 2014 | 23 (21%) |
| 2015 | 28 (26%) |
| 2016 | 11 (10%) |
| 2017 | 26 (24%) |
| CMS protected class | |
| Yes | 34 (31%) |
| No | 75 (69%) |
| Inclusion in FDA's expedited programs | |
| Yes | 56 (51%) |
| No | 53 (49%) |
| Added therapeutic benefit | |
| Yes | 27 (25%) |
| No | 64 (59%) |
| Data not available | 18 (16%) |
| Orphan Drug Act designation | |
| Yes | 38 (35%) |
| No | 71 (65%) |
| First‐in‐class | |
| Yes | 35 (32%) |
| No | 74 (68%) |
| FDA approval with randomized trial | |
| Yes | 95 (87%) |
| No | 14 (13%) |
| 30‐day cost based on net (postrebate) price | |
| Lowest (<$500) | 35 (32%) |
| Medium ($500‐$10,000) | 51 (47%) |
| Highest (>$10,000) | 23 (21%) |
Abbreviations: CMS, Centers for Medicare and Medicaid Services; FDA, US Food and Drug Administration.
Thirty‐four drugs (31%) were included in one of CMS's six protected drug classes. Most drugs in protected classes were for cancer (n = 26). Novel drugs for diabetes accounted for the largest single category of drugs in nonprotected classes (n = 10), followed by non‐HIV antiviral agents (n = 9) and immunosuppressant drugs for psoriasis, rheumatoid arthritis, and multiple sclerosis (n = 8).
More than half (n = 56, 51%) qualified for at least one of the FDA's expedited development and approval programs. Eleven benefited from one program; twenty from two; twenty‐two from three; and three were associated with all four. Information on comparative therapeutic benefit was available for 91 drugs (83%), and 27 (25%) were judged to have added therapeutic benefit according to assessments conducted by at least one of the health authorities in Canada, France, Germany, and Italy. Thirty‐day costs based on net prices exceeded $10,000 for 23 drugs (21%).
Coverage in Part D Plans
Although all new drugs in CMS's six protected classes were included in all plan formularies, coverage was limited for new drugs in nonprotected classes. Only 15 drugs (20%) in nonprotected classes were covered by more than half of Part D plans, and only 5 drugs (7%) were covered by at least 80% of plans during the first year after FDA approval. Eight drugs (11%) in nonprotected classes were not immediately covered by any plans. Table 2 shows the annual proportion of Part D plans that included newly approved nonprotected drugs in their formularies during our study period.
Table 2.
Coverage of Recently Approved Nonprotected Drugs in Medicare Part D Formularies
| Drug Name | Coverage With Prior Authorizations | Coverage Without Prior Authorizations |
|---|---|---|
| A. Part D Plan Coverage in 2014 | ||
| riociguat | 62% | 8% |
| fluticasone furoate and vilanterol inhalation powder | 0% | 65% |
| dimethyl fumarate | 56% | 8% |
| macitentan | 38% | 6% |
| mipomersen | 35% | 8% |
| sofosbuvir | 40% | 3% |
| simeprevir | 39% | 3% |
| canagliflozin | 3% | 24% |
| alogliptin | 0% | 25% |
| umeclidinium and vilanterol inhalation powder | 0% | 11% |
| conjugated estrogens/bazedoxifene | 5% | 6% |
| luliconazole | 0% | 5% |
| ospemifene | 0% | 0% |
| B. Part D Plan Coverage in 2015 | ||
| ledipasvir and sofosbuvir | 97% | 0% |
| metreleptin | 41% | 5% |
| apremilast | 34% | 3% |
| vorapaxar | 19% | 17% |
| ombitasvir, paritaprevir, ritonavir and dasabuvir | 31% | 0% |
| empagliflozin | 2% | 30% |
| eliglustat | 20% | 11% |
| droxidopa | 25% | 5% |
| pirfenidone | 25% | 2% |
| tasimelteon | 22% | 3% |
| nintedanib | 23% | 2% |
| peginterferon beta‐1a | 10% | 11% |
| dulaglutide | 3% | 17% |
| albiglutide | 1% | 18% |
| dapagliflozin | 1% | 15% |
| olodaterol | 0% | 14% |
| suvorexant | 2% | 4% |
| tavaborole | 2% | 3% |
| efinaconazole | 2% | 4% |
| naloxegol | 0% | 0% |
| C. Part D Plan Coverage in 2016 | ||
| parathyroid hormone | 96% | 4% |
| lumacaftor and ivacaftor | 85% | 2% |
| alirocumab | 86% | 1% |
| sacubitril and valsartan | 73% | 5% |
| daclatasvir | 72% | 0% |
| evolocumab | 54% | 1% |
| insulin degludec | 0% | 53% |
| ivabradine | 26% | 12% |
| secukinumab | 33% | 2% |
| selexipag | 30% | 2% |
| edoxaban | 0% | 25% |
| patiromer | 2% | 13% |
| eluxadoline | 6% | 8% |
| rolapitant | 10% | 1% |
| lesinurad | 0% | 0% |
| uridine triacetate | 0% | 0% |
| D. Part D Plan Coverage in 2017 | ||
| sofosbuvir and velpatasvir | 84% | 0% |
| elbasvir and grazoprevir | 72% | 0% |
| obeticholic acid | 35% | 3% |
| daclizumab | 20% | 2% |
| lifitegrast | 7% | 8% |
| ixekizumab | 11% | 2% |
| crisaborole | 2% | 4% |
| lixisenatide | 0% | 4% |
| E. Part D Plan Coverage in 2018 | ||
| sofosbuvir, velpatasvir, and voxilaprevir | 55% | 1% |
| glecaprevir and pibrentasvir | 51% | 1% |
| abaloparatide | 38% | 4% |
| valbenazine | 31% | 2% |
| deutetrabenazine | 23% | 5% |
| telotristat | 17% | 3% |
| dupilumab | 15% | 2% |
| sarilumab | 10% | 2% |
| brodalumab | 9% | 2% |
| guselkumab | 9% | 2% |
| naldemedine | 3% | 4% |
| plecanatide | 0% | 7% |
| ertugliflozin | 0% | 3% |
| latanoprostene | 0% | 3% |
| netarsudil | 0% | 0% |
| safinamide | 0% | 0% |
| secnidazole | 0% | 0% |
| semaglutide | 0% | 0% |
In enrollment‐weighted multivariable analyses among 4,582 Part D plans over the five‐year period, regulatory and therapeutic characteristics of drugs were not associated with more favorable formulary coverage of nonprotected drugs in Part D plans (Figure 1). A similar proportion of plans covered drugs with and without added therapeutic benefit, FDA expedited review, Orphan Drug Act designation, first‐in‐class status, and approval on the basis of at least one randomized controlled trial.
However, 30‐day drug cost based on net (postrebate) prices was positively associated with formulary coverage in nonprotected classes (Figure 2), controlling for other drug characteristics. For example, nonprotected drugs that cost less than $150 for a 30‐day course were covered by fewer than 20% of plans. By contrast, nonprotected drugs that cost more than $30,000 per 30 days were covered by more than 50% of plans. Similar results were obtained in multivariable logistic regression analyses without enrollment weighting. Full results of these analyses are shown in Table A3 in the online appendix. The association between 30‐day drug cost and coverage in Part D plan formularies for nonprotected drugs was not affected when we used different prices to calculate 30‐day costs (Figure A1 in the online appendix).
Figure 2.
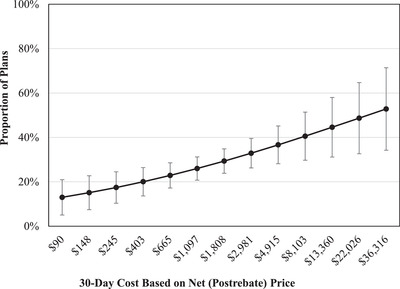
Association Between 30‐Day Drug Cost and Coverage in Part D Plan Formularies During the Year After FDA Approval for Drugs in Nonprotected Drug Classes
Results obtained from enrollment‐weighted multivariable logistic regression analysis, also controlling for plan type, approval year, FDA expedited approval, Orphan Drug Act designation, first‐in‐class status, pivotal trial design, and availability of added therapeutic benefit.
Coverage Restrictions in Part D Plans
Coverage was often conditional on utilization management strategies. Only seven drugs (6%) were covered without prior authorization requirements in more than half of plans. Most new drugs in protected classes (31 out of 34) were covered with restrictions in more than 80% of plans. The corresponding share of nonprotected drugs subject to widespread restrictions (in more than 80% of plans) was 42 out of 67 that had any formulary inclusion.
When controlling for therapeutic and regulatory drug characteristics in enrollment‐weighted multivariable analyses, there was no difference between the proportions of plans imposing coverage restrictions on drugs in protected and nonprotected classes (92% vs. 87%, p = 0.11) (Figure 3). Part D plan coverage with utilization management tools was higher for drugs with first‐in‐class status than for other drugs (93% vs. 88%, p = 0.01). There was no association between coverage restrictions and other drug features.
Figure 3.
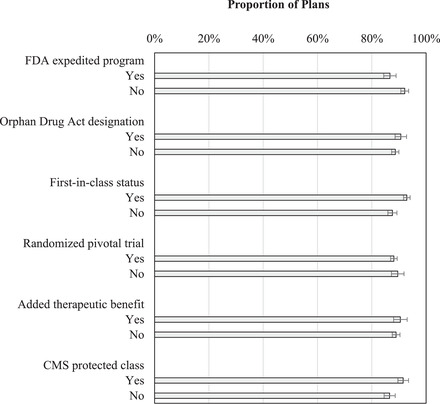
Coverage With Utilization Restrictions for Protected and Nonprotected Drugs According to Drug‐Related Characteristics
Abbreviations: CMS, Centers for Medicare and Medicaid Services; FDA, US Food and Drug Administration
Results obtained from enrollment‐weighted multivariable logistic regression analysis, also controlling for approval year and plan type. Bars represent the subset of plans that cover new drugs. For example, over 90% of plans that covered drugs in CMS protected classes had utilization management tools. None of the differences was statistically significant.
Figure 4 shows the association between 30‐day drug cost and plan coverage with utilization management tools during the year after FDA approval for drugs in protected and nonprotected drug classes, controlling for all other characteristics of drugs. Plans were more likely to implement coverage restrictions on drugs with higher 30‐day costs compared to those with lower 30‐day costs. More than 90% of plans implemented utilization management for drugs that cost more than $10,000 for a 30‐day course. We obtained similar results in multivariable logistic regression analyses without enrollment weighting. Full results of these analyses are shown in Table A4.
Figure 4.
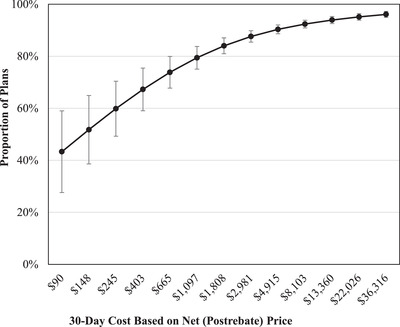
Association Between 30‐Day Drug Cost and Plan Coverage With Utilization Management Tools During the Year After FDA Approval for Drugs in Protected and Nonprotected Drug Classes [Colour figure can be viewed at wileyonlinelibrary.com]
Results obtained from enrollment‐weighted multivariable logistic regression analysis, also controlling for plan type, approval year, Food and Drug Administration expedited approval, Orphan Drug Act designation, first‐in‐class status, pivotal trial design, availability of added therapeutic benefit, and Centers for Medicare and Medicaid Services protected drug class.
Sensitivity Analyses
In sensitivity analyses, we observed no consistent association between regulatory and therapeutic characteristics of drugs and Part D coverage. Thirty‐day drug cost was positively associated with coverage in nonprotected classes, and with utilization management tools in protected and nonprotected classes, in both formulary and drug‐level analyses, controlling for other drug characteristics. Although availability of a pivotal randomized controlled trial was not associated with plan coverage in our primary analysis, the formulary‐level analysis showed a statistically significant increase in the odds of plan coverage for drugs that had at least one randomized controlled trial supporting FDA approval vs. those that did not (odds ratio [OR]: 1.90, 95% confidence interval [CI]: 1.09‐3.31, p = 0.023) (Table A5). First‐in‐class status was positively associated with the use of utilization management tools in protected and nonprotected drug classes; by contrast, inclusion in FDA expedited programs was negatively associated with the use of utilization management tools (Tables A6‐A8 in the online appendix). Figure 5 shows the consistency of findings obtained from primary and sensitivity analyses.
Figure 5.
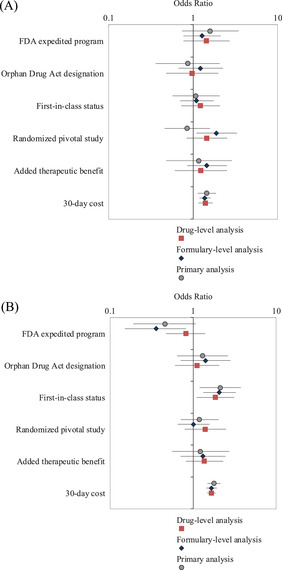
Sensitivity Analysis Results
Panel A. Coverage in Part D Plans. Association between drug characteristics and coverage in Part D plan formularies during the year after FDA approval for drugs in nonprotected drug classes.
Panel B. Coverage With Utilization Management Tools in Part D Plans. Association between drug characteristics and plan coverage with utilization management tools during the year after FDA approval for drugs in protected and nonprotected drug classes.
Discussion
Our evaluation of new drugs approved from 2013 to 2017 found that more than four‐fifths of drugs in nonprotected classes were covered by fewer than half of plans immediately following FDA approval. When drugs were included in Part D plan formularies, their coverage was typically subject to utilization management. A higher proportion of plans implemented utilization management strategies on covered drugs with first‐in‐class status than covered drugs without these characteristics, likely because of the high prices of such drugs. In fact, drug price influenced both formulary inclusion (in nonprotected classes) and coverage restrictions (in both protected and nonprotected classes), as coverage was more likely to be conditional on restrictions for high‐cost drugs than for low‐cost drugs.
Over our study period, only a small minority of new drugs in nonprotected classes were widely covered by Part D plans nationwide in the year after approval. That formulary exclusions were common in nonprotected classes challenges the widely held belief that new drugs are routinely available in Medicare. If the protected classes restrictions were relaxed, plans might similarly not cover expensive and marginally useful new medicines in those classes or use that leverage to obtain price concessions.
Lack of a consistent statistical association between plan coverage and drugs’ therapeutic and regulatory characteristics may be a result of plans making decisions in ad hoc ways. For example, drugs approved through the FDA's expedited programs had similar rates of plan coverage as nonexpedited drugs. These programs are aimed at expediting the development and approval of drugs that address life‐threatening conditions or unmet need. Previous studies suggested that the FDA does not limit expedited programs to such drugs, but instead applies them to many drugs that do not offer much clinical advantage over existing alternatives. 18 Our findings suggest that Part D plans may recognize that expedited drugs are associated with considerable uncertainty in their evidence base.
Similarly, plans did not favor the minority of drugs that were determined to offer added therapeutic benefit, as assessed by health authorities in Canada, France, Germany, and Italy. These assessments rely on the clinical trial data available at the time of drug approval and additional information that may be requested from manufacturers. 12 Other research has also found little consistency in evidence cited by commercial plans for specialty drug coverage, which may partly explain the substantial variation observed across plan formularies. 19
We also found that Part D plans were more likely to cover expensive drugs, although with more restrictions. One potential explanation is that the highest‐priced drugs tend to be for rare diseases, and hence reflect less overall spending by the plan. 20 Although some high‐priced drugs have high rebates, we found no association between drugs’ gross‐to‐net ratios and plan coverage in a sensitivity analysis (results not shown). Of course, expensive drugs may still be cost‐effective and may therefore warrant coverage when stacked against other high‐cost alternatives. 21
Our study makes numerous contributions to the literature. Several previous analyses documented Part D coverage of individual products or classes of drugs. 22 , 23 , 24 By considering drug‐related factors influencing formulary exclusions and coverage restrictions for a recent cohort of drug approvals, we extend and update earlier work, which characterized Part D plan coverage for an older cohort of drug approvals, from 2006 through 2012. 25 Our multivariable analyses also took into account drug price and therapeutic benefit, which were not considered in earlier studies. Comparing our results to those from previous studies suggests that formulary exclusions in Medicare Part D have increased over time. During our study period from 2014 to 2018, only 20% of newly approved drugs in nonprotected classes were covered by more than half of plans nationwide in their first year after approval. By contrast, earlier work found that 50% of drugs that received FDA approval between 2006 and 2012 were covered by more than half of Part D plans during the year following approval. 25
Limitations
This study is not without limitations. First, we calculated net drug prices using the proprietary SSR Health database, which is widely used by industry and academic researchers, because actual drug‐level discounts in Medicare Part D are confidential. 26 Second, information on postrebate, net prices was unavailable for 21 drugs (19%) in our sample. SSR Health data do not contain prices for drugs manufactured by private companies such as Boehringer Ingelheim (n = 3). However, our results were not sensitive to different assumptions for imputing missing price data (Appendix Figure 1). Third, findings of comparative therapeutic benefit assessments conducted by health authorities in Canada, France, Germany, and Italy were available for a subset of 91 drugs in our sample (83%). 12 The remaining 18 FDA‐approved drugs were either unavailable on the European or Canadian markets at the time of data collection or health authorities in these countries had not yet assessed these agents. Fourth, the comparative benefit assessments conducted by health authorities in other settings provide a proxy of therapeutic value because of the possibility that not all dimensions of value are considered by these approaches. 27 Fifth, CMS's formulary information files did not include details of the step therapy or prior authorization requirements. We therefore could not distinguish between utilization management requirements that posed major administrative burdens to prescribers and those that were not burdensome. Sixth, our multivariable regression analysis did not account for disease prevalence.
Figure 1.
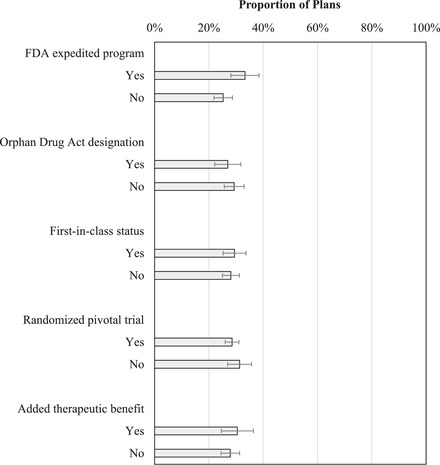
Probability of Medicare Part D Plan Coverage of Nonprotected Drugs According to Drug‐Related Characteristics
Results obtained from enrollment‐weighted multivariable logistic regression analysis, also controlling for approval year and plan type. None of the differences were statistically significant.
We also did not control for existing alternatives in our analyses, as it was not possible to accurately determine the number of therapeutic competitors for each newly approved drug. There is no established approach for grouping drugs in a clinically and pharmacologically meaningful way. Drugs within a specific pharmacologic class may have different clinical uses. For example, several targeted cancer drugs classified as kinase inhibitors have different molecular pathways and are indicated for different therapeutic uses. In addition, drugs with different pharmacologic mechanisms of action may have similar therapeutic indications. For example, several drug classes are indicated for individuals with type 2 diabetes. Although formulary inclusion may be lower for new “me‐too” drugs in already‐crowded therapeutic areas, there was no consistent association between the number of therapeutic alternatives and Part D coverage in our sample. For example, several “addition‐to‐class” antiviral drugs indicated for the treatment of hepatitis C had higher rates of plan coverage than earlier alternatives.
Relevance for Policy
Our findings reveal the complex dynamic between the market entry of new drugs and their subsequent inclusion in Medicare Part D. Currently, plans must cover all new drugs in CMS's six protected classes, which may have several unintended consequences. Mandatory coverage of new drugs, which often enter the market on the basis of limited evidence, may further erode already‐limited incentives for postapproval evaluation of clinical benefit. 28 Moreover, Part D plans seldom secure meaningful rebates for drugs in protected classes. 29 Increased flexibility in Part D formulary design may be able to strike the balance between beneficiary access to new drugs and cost containment for patients and payers.
Formulary restrictions in Part D plans may have important implications for Medicare beneficiaries. Previous studies have shown that prior authorization requirements can dampen use of targeted drugs. 30 , 31 Notably, such changes may have a positive effect on patient outcomes if certain drugs are FDA‐approved despite not demonstrating firm clinical benefits or are being improperly overprescribed prior to the implementation of the utilization management tools. 32 Plans may also implement tiered formularies and place certain drugs on higher tiers with greater patient cost‐sharing requirements. Most recently, maximum cost‐sharing requirements on the top specialty tiers in Part D plans amounted to 33% coinsurance. 33 During our study period, more than 90% of new drugs that were covered by Part D plans during the year after FDA approval were assigned to specialty tiers in more than half of plans (results not shown). Specialty tier placement with high out‐of‐pocket costs can lead to prescription abandonment, delayed initiation, poor adherence, and medication discontinuation, with adverse health consequences. 8 , 34 , 35
Older Americans who enroll in Medicare's Part D program need to compare and choose from multiple available private plans in their region. Evidence to date suggests that beneficiaries may not select the most financially optimal plan for their medication needs. 36 Also, beneficiaries are often reluctant to switch plans after their first enrollment due to transaction costs. 37 FDA approval of new drugs may further complicate plan selection decisions, as beneficiaries may not become immediately aware of new drugs for their existing conditions and cannot foresee future medication needs. Ensuring that formulary inclusion and utilization management decisions are linked closely to the comparative clinical effectiveness of new and existing drugs would improve patients’ welfare.
Funding/Support: This work was supported by a Harkness Fellowship in Health Care Policy and Practice from the Commonwealth Fund.
Conflict of Interest Disclosures: All authors have completed the ICMJE Form for Disclosure of Potential Conflicts of Interest. No conflicts were reported.
Supporting information
Table A1. List of excluded novel therapeutic agents approved by the FDA between 2013 and 2017
Table A2. List of included novel therapeutic agents approved by the FDA between 2013 and 2017
Table A3. Full results of the multivariable logistic regression analyses evaluating the association between drug characteristics and inclusion in Part D plans for nonprotected drugs
Table A4. Full results of the multivariable logistic regression analyses evaluating the association between drug characteristics and coverage with utilization management for protected and nonprotected drugs
Table A5. Full results of the formulary‐level multivariable logistic regression analysis evaluating the association between drug characteristics and inclusion in Part D formularies for nonprotected drugs
Table A6. Full results of the formulary‐level multivariable logistic regression analysis evaluating the association between drug characteristics and formulary coverage with utilization management for protected and nonprotected drugs
Table A7. Full results of the drug‐level multivariable fractional logistic regression analysis evaluating the association between drug characteristics and coverage decisions for nonprotected drugs
Table A8. Full results of the drug‐level multivariable fractional logistic regression analysis evaluating the association between drug characteristics and utilization management for protected and nonprotected drugs
Figure A1. Sensitivity of multivariable regression analysis evaluating the association between 30‐day drug cost and coverage in Part D plan formularies for nonprotected drugs when using different prices to calculate 30‐day costs
Figure A2. Sensitivity of multivariable regression analysis evaluating the association between 30‐day drug cost and coverage in Part D plan formularies for nonprotected drugs when using different approaches to impute missing net prices
References
- 1. Kaiser Family Foundation. An overview of the Medicare Part D prescription drug benefit. https://www.kff.org/medicare/fact‐sheet/an‐overview‐of‐the‐medicare‐part‐d‐prescription‐drug‐benefit/. Published October 13, 2020. Accessed August 2, 2021.
- 2. Madden JM, Graves AJ, Zhang F, et al. Cost‐related medication nonadherence and spending on basic needs following implementation of Medicare Part D. JAMA. 2008;299(16):1922‐1928. 10.1001/jama.299.16.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Y, Donohue JM, Lave JR, O'Donnell G, Newhouse JP. The effect of Medicare Part D on drug and medical spending. N Engl J Med. 2009;361(1):52‐61. 10.1056/NEJMsa0807998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tseng CW, Mangione CM, Brook RH, Keeler E, Dudley RA. Identifying widely covered drugs and drug coverage variation among Medicare Part D formularies. JAMA. 2007;297(23):2596‐2602. 10.1001/jama.297.23.2596. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Medicare and Medicaid Services . Prescription drug benefit manual. https://www.cms.gov/medicare/prescription‐drug‐coverage/prescriptiondrugcovcontra/partdmanuals.html. Published September 26, 2018. Accessed February 15, 2022.
- 6. Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA. 2016;316(8):858‐871. 10.1001/jama.2016.11237. [DOI] [PubMed] [Google Scholar]
- 7. Hwang TJ, Dusetzina SB, Feng J, Maini L, Kesselheim AS. Price increases of protected‐class drugs in Medicare Part D, relative to inflation, 2012‐2017. JAMA. 2019;322(3):267‐269. 10.1001/jama.2019.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chandra A, Flack E, Obermeyer Z. The health costs of cost‐sharing. National Bureau of Economic Research working paper 28439. February 2021. Accessed February 15, 2022. https://www.nber.org/papers/w28439.
- 9. Brot‐Goldberg ZC, Chandra A, Handel BR, Kolstad JT. What does a deductible do? The impact of cost‐sharing on health care prices, quantities, and spending dynamics. Q J Econ. 2017;132(3):1261‐1318. 10.1093/qje/qjx013. [DOI] [Google Scholar]
- 10. Hernandez I, San‐Juan‐Rodriguez A, Good CB, Gellad WF. Changes in list prices, net prices, and discounts for branded drugs in the US, 2007‐2018. JAMA. 2020;323(9):854‐862. 10.1001/jama.2020.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prescription drug plan formulary, pharmacy network, and pricing information files. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Files‐for‐Order/NonIdentifiableDataFiles/PrescriptionDrugPlanFormularyPharmacyNetworkandPricingInformationFiles.html. Accessed January 22, 2019.
- 12. Hwang TJ, Ross JS, Vokinger KN, Kesselheim AS. Association between FDA and EMA expedited approval programs and therapeutic value of new medicines: retrospective cohort study. BMJ. 2020;371. 10.1136/bmj.m3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kleijnen S, George E, Goulden S, et al. Relative effectiveness assessment of pharmaceuticals: similarities and differences in 29 jurisdictions. Value Health. 2012;15(6):954‐960. 10.1016/j.jval.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 14. Emanuel EJ, Zhang C, Glickman A, Gudbranson E, DiMagno SSP, Urwin JW. Drug reimbursement regulation in six peer countries. JAMA Intern Med. 2020;180(11):1510‐1517. 10.1001/jamainternmed.2020.4793. [DOI] [PubMed] [Google Scholar]
- 15. Chalkidou K, Tunis S, Lopert R, et al. Comparative effectiveness research and evidence‐based health policy: experience from four countries. Milbank Q. 2009;87(2):339‐367. 10.1111/j.1468-0009.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clement FM, Harris A, Li JJ, Yong K, Lee KM, Manns BJ. Using effectiveness and cost‐effectiveness to make drug coverage decisions: a comparison of Britain, Australia, and Canada. JAMA. 2009;302(13):1437‐1443. 10.1001/jama.2009.1409. [DOI] [PubMed] [Google Scholar]
- 17. US prescription brand net pricing data and analysis. SSR Health website. https://www.ssrhealth.com/. Accessed February 9, 2021. [Google Scholar]
- 18. Hwang TJ, Franklin JM, Chen CT, et al. Efficacy, safety, and regulatory approval of Food and Drug Administration–designated breakthrough and nonbreakthrough cancer medicines. J Clin Oncol. 2018;36(18):1805‐1812. 10.1200/JCO.2017.77.1592. [DOI] [PubMed] [Google Scholar]
- 19. Chambers JD, Panzer AD, Pope EF, Graff JS, Neumann PJ. Little consistency in evidence cited by commercial plans for specialty drug coverage. Health Aff (Millwood). 2019;38(11):1882‐1886. 10.1377/hlthaff.2019.00201. [DOI] [PubMed] [Google Scholar]
- 20. Dusetzina SB, Conti RM, Yu NL, Bach PB. Association of prescription drug price rebates in Medicare Part D with patient out‐of‐pocket and federal spending. JAMA Intern Med. 2017;177(8):1185‐1188. 10.1001/jamainternmed.2017.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bach PB. New math on drug cost‐effectiveness. N Engl J Med. 2015;373(19):1797‐1799. 10.1056/NEJMp1512750. [DOI] [PubMed] [Google Scholar]
- 22. Hartung DM, Johnston KA, Irwin A, Markwardt S, Bourdette DN. Trends in coverage for disease‐modifying therapies for multiple sclerosis in Medicare Part D. Health Aff (Millwood). 2019;38(2):303‐312. 10.1377/hlthaff.2018.05357. [DOI] [PubMed] [Google Scholar]
- 23. Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474‐1480. 10.1002/art.39079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tseng C, Yazdany J, Dudley R, et al. Medicare Part D plans’ coverage and cost‐sharing for acute rescue and preventive inhalers for chronic obstructive pulmonary disease. JAMA Intern Med. 2017;177(4):585‐588. 10.1001/jamainternmed.2016.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shaw DL, Dhruva SS, Ross JS. Coverage of novel therapeutic agents by Medicare prescription drug plans following FDA approval. J Manag Care Spec Pharm. 2018;24(12):1230‐1238. 10.18553/jmcp.2018.24.12.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feldman WB, Rome BN, Raimond VC, Gagne JJ, Kesselheim AS. Estimating rebates and other discounts received by Medicare Part D. JAMA Health Forum. 2021;2(6):e210626. 10.1001/jamahealthforum.2021.0626. [DOI] [PubMed] [Google Scholar]
- 27. Angelis A, Lange A, Kanavos P. Using health technology assessment to assess the value of new medicines: results of a systematic review and expert consultation across eight European countries. Eur J Health Econ. 2018;19(1):123‐152. 10.1007/s10198-017-0871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cipriani A, Ioannidis JPA, Rothwell PM, et al. Generating comparative evidence on new drugs and devices after approval. Lancet. 2020;395(10228):998‐1010. 10.1016/S0140-6736(19)33177-0. [DOI] [PubMed] [Google Scholar]
- 29. US Government Accountability Office . Medicare Part D: use of pharmacy benefit managers and efforts to manage drug expenditures and utilization. https://www.gao.gov/assets/710/700259.pdf. Published July 2019. Accessed February 10, 2021. [Google Scholar]
- 30. Fischer MA, Choudhry NK, Winkelmayer WC. Impact of Medicaid prior authorization on angiotensin‐receptor blockers: can policy promote rational prescribing? Health Aff (Millwood). 2007;26(3):800‐807. 10.1377/hlthaff.26.3.800. [DOI] [PubMed] [Google Scholar]
- 31. Dillender M. What happens when the insurer can say no? Assessing prior authorization as a tool to prevent high‐risk prescriptions and to lower costs. J Public Econ. 2018;165:170‐200. 10.1016/j.jpubeco.2018.07.006. [DOI] [Google Scholar]
- 32. Fischer MA, Schneeweiss S, Avorn J, Solomon DH. Medicaid prior‐authorization programs and the use of cyclooxygenase‐2 inhibitors. N Engl J Med. 2004;351(21):2187‐2194. 10.1056/NEJMsa042770. [DOI] [PubMed] [Google Scholar]
- 33. Cubanski Juliette, Koma Wyatt, Neuman Tricia. The out‐of‐pocket cost burden for specialty drugs in Medicare Part D in 2019. Kaiser Family Foundation website. https://www.kff.org/medicare/issue‐brief/the‐out‐of‐pocket‐cost‐burden‐for‐specialty‐drugs‐in‐medicare‐part‐d‐in‐2019/. Published February 1, 2019. Accessed February 7, 2019.
- 34. Trish E, Xu J, Joyce G. Medicare beneficiaries face growing out‐of‐pocket burden for specialty drugs while in catastrophic coverage phase. Health Aff (Millwood). 2016;35(9):1564‐1571. 10.1377/hlthaff.2016.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298(1):61‐69. 10.1001/jama.298.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou C, Zhang Y. The vast majority of Medicare Part D beneficiaries still don't choose the cheapest plans that meet their medication needs. Health Aff (Millwood). 2012;31(10):2259‐2265. 10.1377/hlthaff.2012.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ho K, Hogan J, Scott Morton F. The impact of consumer inattention on insurer pricing in the Medicare Part D program. RAND J Econ. 2017;48(4):877‐905. 10.1111/1756-2171.12207. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A1. List of excluded novel therapeutic agents approved by the FDA between 2013 and 2017
Table A2. List of included novel therapeutic agents approved by the FDA between 2013 and 2017
Table A3. Full results of the multivariable logistic regression analyses evaluating the association between drug characteristics and inclusion in Part D plans for nonprotected drugs
Table A4. Full results of the multivariable logistic regression analyses evaluating the association between drug characteristics and coverage with utilization management for protected and nonprotected drugs
Table A5. Full results of the formulary‐level multivariable logistic regression analysis evaluating the association between drug characteristics and inclusion in Part D formularies for nonprotected drugs
Table A6. Full results of the formulary‐level multivariable logistic regression analysis evaluating the association between drug characteristics and formulary coverage with utilization management for protected and nonprotected drugs
Table A7. Full results of the drug‐level multivariable fractional logistic regression analysis evaluating the association between drug characteristics and coverage decisions for nonprotected drugs
Table A8. Full results of the drug‐level multivariable fractional logistic regression analysis evaluating the association between drug characteristics and utilization management for protected and nonprotected drugs
Figure A1. Sensitivity of multivariable regression analysis evaluating the association between 30‐day drug cost and coverage in Part D plan formularies for nonprotected drugs when using different prices to calculate 30‐day costs
Figure A2. Sensitivity of multivariable regression analysis evaluating the association between 30‐day drug cost and coverage in Part D plan formularies for nonprotected drugs when using different approaches to impute missing net prices


