Abstract
Pyriproxyfen (PPF) mimics a natural hormone in insects and disrupts their growth. It is a well-known synthetic insecticide and aromatic juvenile hormone analog frequently used in agriculture and vegetable crops to control various insect species. At present, scanty information is available about the possible potential threats of PPF in aquatic organisms. Therefore, in this study, different toxico-pathologic endpoints of PPF like DNA damage, biomarkers of oxidative stress, and status of antioxidant enzymes were determined in Labeo rohita (freshwater fish). In our study, 60 active, free from any external obvious ailments, same size, age, and body mass were randomly allocated to four glass aquaria (T0-T3) separately containing 100 L water. The fish present in groups T1, T2, and T3 were administered PPF dissolved in water 300, 600, and 900 μg/L for 30 days. Different tissues including the blood and visceral organs were obtained from each fish on days 10, 20, and 30 of the experiment. Results on various morphological and nuclear changes in red blood cells of PPF-exposed Labeo rohita fish including pear-shaped erythrocytes, spherocytes, red blood cells with a blebbed nucleus, micronucleus, and nuclear remnants were significantly increased. Our results on genotoxicity (comet assay) recorded significantly (P ≤ 0.05) increased DNA damage in various tissues of insecticide-exposed fish. The results on oxidative stress profile (reactive oxygen species and thiobarbituric acid reactive substances) and antioxidant enzymes (reduced glutathione superoxide dismutase, peroxidase, and catalase) in multiple tissues of Labeo rohita fish concluded significantly (P ≤ 0.05) higher quantity of biomarkers of oxidative stress and lower concentrations of different antioxidant enzymes in treated fish. Hence, the findings of our experimental research determine that PPF could induce adverse toxic impacts on multiple tissues of Labeo rohita fish.
1. Introduction
Monitoring of potentially hazardous impacts of different environmental pollutants such as insecticides herbicides, pesticides, and industrial effluents has gotten a lot of attention in the recent several decades all over the world [1–3]. Numerous chemicals have been widely used in public health, agriculture, protection of environmental, aquatic ecosystems, and in different industries for the production of various materials causing great threat to encountered species, biodiversity, and food products [4–6].
The majority of pesticides and insecticides are not biodegradable, and they tend to remain in the soil and water bodies for years [7]. Various chemicals from multiple sources directly and quickly enter into the body of several animals through contaminated water and food products, ultimately inducing different disorders in normal physiological status [1, 8, 9]. Pyriproxyfen (PPF) stimulates a natural hormone in insects that disrupts their growth. It is a well-known aromatic juvenile hormone analog for controlling insect species among other insecticides [10]. Studies have revealed that PPF being a registered insecticide is commonly used in agriculture and on citrus fruit to control a variety of insects like jassids, whitefly, aphids, bollworm, and cutworms throughout the world [11]. Studies indicate that PPF can induce death during mosquito control in different nontarget animals including fish living in the aquatic environments [12]. Previously, different concentrations (89.66 ng/L) of PPF in water samples collected from the river have been detected [13]. Furthermore, the different lethal concentrations of PPF (LC 50) have also been investigated in different species of fish like rainbow trout [14], Labeo rohita [15], and embryos of zebrafish [16]. Reports highlighted those different insecticides, herbicides, and pesticides commonly used in various fields like agriculture, industries, and public health adversely affect the early developmental stages of different aquatic animals [17–20]. The direct and indirect exposure to various environmental pollutants causes overproduction and release of reactive oxygen species in animals [21] leading to induction of oxidative stress [1] and depletion of antioxidant enzymes, injury to different organelles of cells including lipids, proteins, and damage to DNA biomolecules [21, 22]. Studies have reported that morphological and nuclear changes in red blood cells, genotoxicity, oxidative stress, and biomarkers of antioxidant enzymes assays are reliable and useful tools for the exact and early screening of toxicity of various synthetic chemicals in birds [23, 24] and other aquatic organisms including fish [21, 25].
DNA damage assessment using comet assay is of great importance and is frequently used in aquatic animals [21, 26, 27]. However, to date, no literature is found in previous data regarding the various toxicological events like nuclear and morphological disorders in red blood cells, oxidative stress, genotoxicity, and status of antioxidant biomarkers due to PPF insecticide in Labeo rohita. Therefore, the current study was executed to measure the deleterious effects of PPF on different multiple endpoints including nuclear changes in red blood cells, genotoxicity, oxidative stress, and antioxidant enzymes of Labeo rohita fish.
2. Materials and Methods
2.1. Fish Management
The current study was carried out at the labs of the Islamia University of Bahawalpur's departments of zoology (life sciences) and pathology (veterinary sciences). The total quantity of freshwater fish Labeo rohita with body mass (130-140 g), size, and age was collected from a commercial fish farm in the Punjab region of Pakistan (District Bahawalnagar). Following the capture of the fish, all samples were packed in oxygen-rich plastic bags and sent to the laboratory. Fish were housed in a glass tank (10″ L 14″ W 12″ H) for ten days as a means of accommodation. 2-3% food was chosen as body weight and supplied to all of the fishes twice a day, early in the morning, and late in the evening. The aquarium medium was cleaned every day since cleanliness was a big component.
2.2. Chemicals
Pyriproxyfen was acquired for research purposes from M/S Ali Akbar Enterprises in Pakistan's main market area of Lodhran. Many more compounds were bought from Merck (Germany) and Sigma Aldrich throughout this investigation (USA). Company (Pvt.) Pakistan provided many commercial kits for the assessment of serum biochemical parameters.
2.3. Experimental Strategy and Handling
Following adaption, the fish were chosen at random, separated, and assigned into four groups (T0, T1, T2, and T3). Each had a total of 20 species. Each tank held 100 liters of water. The control group (T0) did not receive PPF dose at any stage and served as negative control. The experimental groups T1, T2, and T3 served as positive groups and received PPF 300, 600, and 900 g/L in distilled water for one month, respectively. Daily, all aquariums were cleansed of residual debris and fecal material for the sake of cleanliness. According to the requirements, all findings and observations were data-recorded each day.
2.4. Genotoxicity Assessment and Blood Sampling
On days 10, 20, and 30 of the experiment, each fish was subjected to draw blood from caudal vein utilizing a 26-gauge sterile hypodermic needle. Thin smear from each fish was prepared from fresh blood without the use of any anticoagulant medications to evaluate morphological and nuclear alterations in erythrocytes. The blood films were immediately dried, fixed with 100% alcohol, and stained with Giemsa. A computer-assisted examination of 1500 red blood cells from each fish was carried out using a light microscope with an oil immersion lens [28]. Single-cell gel electrophoresis or the comet test technique were used to evaluate DNA damage in diverse organs such as the liver, gills, and kidneys under alkaline circumstances [29]. After dissection, the liver, kidneys, and gills of each fish were removed and immersed separately in a chilled normal saline solution. The tissues (0.2 g) were combined and homogenized in a centrifuge. Every tissue's single cells was separated and put through a comet test [1]. The slides were rinsed in a cold buffer solution after they were produced. After being lysed, the slides were placed in a horizontal electrophoresis tank with a refrigerated electrophoresis solution. At a voltage of 25 volts, electrophoresis was performed for 25-30 minutes [1]. The slides were after electrophoresis (pH 7.5) and then stained with ethidium bromide solution and viewed at a magnification of 400× using a fluorescence microscope. The range of DNA damage (percent DNA) in each sample was estimated after seeing 500 cells on a fish slide.
2.5. Tissue Preparation and Biochemical Analyses
Fish were dissected at days 10, 20, and 30 of the experiment for biochemical analysis. The liver, kidneys, brain, and gills were taken from each fish. All of the tissues were soaked in an ice-cold saline solution. Oxidative stress-causing agents such as reactive oxygen species, lipid peroxidation, thiobarbituric acid reactive species, reduced glutathione, total protein contents, and variant antioxidant enzymes such as superoxide dismutase, catalase, and peroxidase were all examined in samples. Homogenate from various visceral organs was separately prepared, and various antioxidant biomarkers include peroxidase, catalase, superoxide dismutase [1, 30], reduced glutathione [30, 31], and reactive oxygen species [32], and thiobarbituric acid reactive substance [1, 33].
2.6. Statistical Analysis
Data thus collected were subjected to statistical analysis by applying ANOVA using SPSS statistics (version 20). The group means were compared by post hoc Tukey's test. Data are presented as mean ± SE. The level of significance was considered at P ≤ 0.05.
3. Results
3.1. Gross Pathology
At necropsy, all the visceral organs such the brain, liver, gills, and kidneys of Labeo rohita fish were normal in appearance and consistency throughout the trial. The heart of PPF (900 μg/L) treated Labeo rohita exhibited hyperemia, edema, and dark black color after day 20 of the experiment. No obvious gross signs of toxicity of PPF on the liver, brain, and kidneys of Labeo rohita are treated with 900 μg/L. The gills of PPF-treated Labeo rohita (900 μg/L) were moderately hyperemic. Mild gross signs of toxicity of PPF (600 μg/L) on brain and gills of Labeo rohita were observed after day 20 of our study.
3.2. Morphological and Nuclear Abnormalities in Red Blood Cells
Results on different cellular abnormalities in erythrocyte of PPF-treated Labeo rohita fish at various doses including nuclear abnormalities (erythrocytes with the lobed nucleus, erythrocytes with the blabbed nucleus, notched nucleus, erythrocyte with two nuclei, erythrocyte with micronucleus, and erythrocyte with a condensed nucleus) and morphological abnormalities (pear shape erythrocyte, spindle shape erythrocyte, and spherocyte) are recorded in Table 1 and Figures 1–2. The results exhibited a substantial increase in frequencies of erythrocytes with the lobed nucleus, blabbed nucleus, vacuolated nucleus, and notched nucleus in fish exposed to various amounts of PPF at day 20 in group T2 and at day 30 in group T3 of our research. Notably, the frequencies of formation of micronuclei were substantially high in erythrocytes obtained from fish exposed to PPF in groups T2 and T3 throughout the trial (Figure 1). Remarkably, increased frequencies of erythrocytes with bi-nucleus/dividing nucleus in fish kept in group T3 at day 20 while at day 30 in groups T2 and T3 were recorded (Figure 2). Significantly increased abnormalities in morphology of erythrocyte including pear shape, spindle shape, and spherocyte were detected in blood smear prepared from Labeo rohita of fish exposed to higher doses of PPF in comparison to untreated control fish.
Table 1.
Various morphological and nuclear alterations in erythrocytes of Labeo rohita fish exposed to different pyriproxyfen concentrations.
| Parameters/days | Groups/treatment | |||
|---|---|---|---|---|
| T0 (0.0) | T1 (300 μg/L) | T2 (600 μg/L) | T3 (900 μg/L) | |
| Erythrocytes with lobed nucleus (%) | ||||
| 10 | 1.39 ± 0.08 | 1.42 ± 0.06 | 1.57 ± 0.09 | 3.43 ± 0.18∗ |
| 20 | 1.40 ± 0.02 | 1.43 ± 0.04 | 1.59 ± 0.08 | 3.45 ± 0.23∗ |
| 30 | 1.41 ± 0.03 | 1.47 ± 0.04 | 3.61 ± 0.09∗ | 4.46 ± 0.18∗ |
| Erythrocytes with blabbed nucleus (%) | ||||
| 10 | 1.22 ± 0.17 | 1.39 ± 0.22 | 1.54 ± 0.13 | 2.99 ± 0.12∗ |
| 20 | 1.24 ± 0.15 | 1.48 ± 0.17 | 1.57 ± 0.11 | 3.18 ± 0.09∗ |
| 30 | 1.29 ± 0.14 | 1.49 ± 0.16 | 2.64 ± 0.15∗ | 3.19 ± 0.07∗ |
| Erythrocytes with vacuolated nucleus (%) | ||||
| 10 | 2.36 ± 0.04 | 2.43 ± 0.02 | 2.63 ± 0.12 | 2.99 ± 0.06∗ |
| 20 | 2.38 ± 0.02 | 2.44 ± 0.04 | 2.65 ± 0.05 | 3.03 ± 0.09∗ |
| 30 | 2.40 ± 0.03 | 2.47 ± 0.04 | 3.69 ± 0.06∗ | 3.90 ± 0.16∗ |
| Notched nucleus (%) | ||||
| 10 | 1.79 ± 0.01 | 1.86 ± 0.03 | 2.92 ± 0.08 | 2.66 ± 0.10∗ |
| 20 | 1.80 ± 0.02 | 1.89 ± 0.02 | 1.95 ± 0.09 | 2.70 ± 0.07∗ |
| 30 | 1.81 ± 0.01 | 1.90 ± 0.03 | 2.67 ± 0.12∗ | 2.81 ± 0.09∗ |
| Binucleate nucleus (%) | ||||
| 10 | 1.44 ± 0.18 | 1.50 ± 0.13 | 1.64 ± 0.09 | 1.77 ± 0.05 |
| 20 | 1.48 ± 0.18 | 1.55 ± 0.05 | 1.71 ± 0.09 | 2.89 ± 0.09∗ |
| 30 | 1.49 ± 0.13 | 1.63 ± 0.07 | 3.88 ± 0.05∗ | 3.91 ± 0.08∗ |
| Pear shaped (%) | ||||
| 10 | 3.87 ± 0.34 | 4.04 ± 0.18 | 4.10 ± 0.14 | 7.07 ± 0.04∗ |
| 20 | 3.89 ± 0.31 | 4.19 ± 0.16 | 6.68 ± 0.32∗ | 7.16 ± 0.19∗ |
| 30 | 3.66 ± 0.21 | 4.20 ± 0.43 | 6.75 ± 0.28∗ | 7.19 ± 0.08∗ |
| Micronucleus (%) | ||||
| 10 | 1.73 ± 0.18 | 1.83 ± 0.19 | 2.24 ± 0.08∗ | 3.92 ± 0.08∗ |
| 20 | 1.74 ± 0.24 | 1.96 ± 0.09 | 2.58 ± 0.13∗ | 4.04 ± 0.05∗ |
| 30 | 1.75 ± 0.21 | 2.10 ± 0.13 | 3.13 ± 0.14∗ | 4.26 ± 0.06∗ |
| Condensed nucleus (%) | ||||
| 10 | 2.11 ± 0.57 | 2.24 ± 0.21 | 2.53 ± 0.66 | 3.41 ± 0.12∗ |
| 20 | 2.30 ± 0.33 | 2.35 ± 0.51 | 3.82 ± 0.16∗ | 4.30 ± 0.05∗ |
| 30 | 2.45 ± 0.48 | 2.47 ± 0.13 | 4.95 ± 0.69∗ | 6.25 ± 0.26∗ |
| Spindle shaped erythrocyte (%) | ||||
| 10 | 1.83 ± 0.21 | 2.09 ± 0.15 | 3.22 ± 0.19∗ | 4.40 ± 0.26∗ |
| 20 | 1.95 ± 0.19 | 2.16 ± 0.19 | 3.35 ± 0.14∗ | 4.88 ± 0.21∗ |
| 30 | 1.84 ± 0.34 | 2.23 ± 0.33 | 3.49 ± 0.17∗ | 5.12 ± 0.09∗ |
| Spherocytes (%) | ||||
| 10 | 1.94 ± 0.03 | 2.18 ± 0.17 | 3.46 ± 0.08∗ | 4.20 ± 0.23∗ |
| 20 | 1.98 ± 0.08 | 2.21 ± 0.15 | 3.56 ± 0.09∗ | 4.45 ± 0.16∗ |
| 30 | 2.01 ± 0.03 | 2.25 ± 0.09 | 3.78 ± 0.16∗ | 4.69 ± 0.31∗ |
In each row, values (Mean ± SE) bearing asterisks differ significantly (P ≤ 0.05) from that of values in untreated (T0 negative control) fish. T1, T2, and T3 are positive control and dose based.
Figure 1.
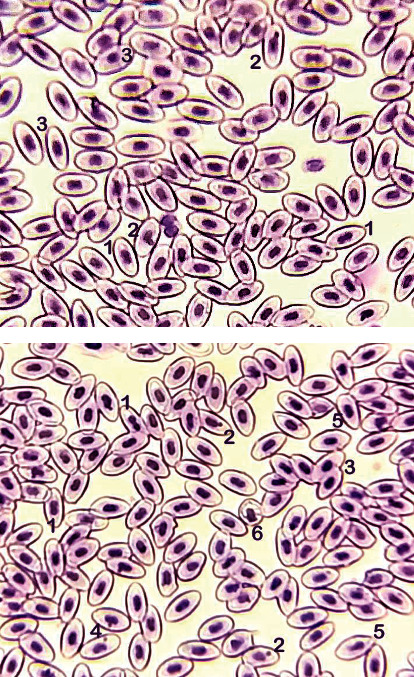
Blood smear of Labeo rohita treated with pyriproxyfen (T3: 900 μg/L at 30 days experiment). Upper and lower figures showing (1) bi-nucleus/dividing nucleus, (2) micronucleus, (3) condensed nuclei, (4) notched nuclei, (5) pear-shaped erythrocyte, and (6) macrocyte (immature erythrocytes). Stain: Giemsa. 1000×.
Figure 2.
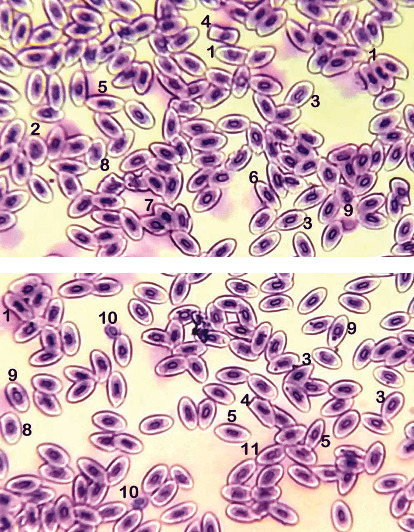
Blood smear of Labeo rohita treated with pyriproxyfen (T3: 900 μg/L at 30-day experiment). Upper and lower figures showing (1) notched nucleus, (2) macrocyte (immature erythrocytes), (3) pear-shaped erythrocyte, (4) abnormal erythrocytes, (5) spindle-shaped erythrocyte, (6) elliptical erythrocyte, (7) condensed nuclei, (8) macrocyte, (9) micronucleus, (10) microcyte, and (11) bi-nucleus/dividing nucleus. Stain: Giemsa. 1000×.
3.3. Oxidative Stress and Antioxidant Responses
3.3.1. Oxidative Stress and Antioxidant Responses in Liver
The result measured on ROS and TBARS from the liver of PPF-treated fish at day 10 in group T3 and in groups T2 and T3 at days 20 and 30 shows a significantly increased quantity of these biomarkers than the liver obtained from normal Labeo rohita fish (Table 2). The result measured on the level of SOD from the liver of PPF-treated fish at day 20 in group T3 and in groups T2 and T3 at day 30 shows a significantly (P ≤ 0.05) lower quantity than the liver obtained from untreated organisms. The results measured on the concentration CAT from the liver of PPF-treated fish at day 10 in group T3 and in groups T2 and T3 at days 20 and 30 showed a significantly (P ≤ 0.05) reduced quantity than the liver of normal fish. The result computed on the concentration POD from the liver of PPF-treated fish showed significantly (P ≤ 0.05) reduced quantity throughout the study in comparison to the normal liver of Labeo rohita (Table 2).
Table 2.
Oxidative stress indices and antioxidant enzyme levels in liver tissues of Labeo rohita exposed to pyriproxyfen.
| Parameters/days | Groups/treatment | |||
|---|---|---|---|---|
| T0 (0.0) | T1 (300 μg/L) | T2 (600 μg/L) | T3 (900 μg/L) | |
| ROS (optical density) | ||||
| 10 | 0.36 ± 0.03 | 0.37 ± 0.02 | 0.40 ± 0.03 | 0.84 ± 0.07∗ |
| 20 | 0.37 ± 0.01 | 0.39 ± 0.01 | 0.67 ± 0.02∗ | 0.86 ± 0.05∗ |
| 30 | 0.39 ± 0.04 | 0.42 ± 0.03 | 0.75 ± 0.02∗ | 0.92 ± 0.05∗ |
| TBARS (nmol/TBARS formed/mg protein/min) | ||||
| 10 | 37.42 ± 2.93 | 39.23 ± 1.95 | 41.07 ± 1.15 | 54.93 ± 2.74∗ |
| 20 | 38.89 ± 1.94 | 40.68 ± 2.95 | 50.44 ± 1.97∗ | 56.25 ± 3.92∗ |
| 30 | 40.02 ± 3.94 | 41.95 ± 2.93 | 51.88 ± 2.34∗ | 58.83 ± 4.53∗ |
| Reduced GSH (μmol/g tissue) | ||||
| 10 | 8.66 ± 1.17 | 7.65 ± 0.06 | 6.64 ± 0.13 | 5.64 ± 0.15∗ |
| 20 | 8.38 ± 1.12 | 7.44 ± 1.10 | 6.05 ± 0.02 | 5.56 ± 0.12∗ |
| 30 | 8.35 ± 1.14 | 7.38 ± 1.05 | 5.93 ± 1.15∗ | 5.48 ± 0.14∗ |
| Antioxidant enzymes | ||||
| SOD (units/mg protein) | ||||
| 10 | 11.65 ± 0.12 | 10.97 ± 0.12 | 10.29 ± 0.13 | 10.27 ± 0.11 |
| 20 | 11.64 ± 0.15 | 10.32 ± 0.15 | 9.46 ± 0.15 | 7.58 ± 0.14∗ |
| 30 | 10.75 ± 0.18 | 9.68 ± 0.18 | 7.10 ± 0.18∗ | 7.04 ± 0.17∗ |
| CAT (units/min) | ||||
| 10 | 8.70 ± 0.19 | 7.52 ± 0.19 | 7.14 ± 0.19 | 5.16 ± 0.19∗ |
| 20 | 7.99 ± 0.17 | 7.35 ± 0.16 | 5.63 ± 0.16∗ | 5.03 ± 0.16∗ |
| 30 | 7.95 ± 0.16 | 7.02 ± 0.16 | 5.46 ± 0.16∗ | 4.95 ± 0.16∗ |
| POD (units/μg) | ||||
| 10 | 4.08 ± 0.09 | 3.56 ± 0.09 | 2.78 ± 0.09∗ | 2.47 ± 0.08∗ |
| 20 | 4.03 ± 0.08 | 3.47 ± 0.08 | 2.63 ± 0.08∗ | 2.41 ± 0.08∗ |
| 30 | 3.96 ± 0.08 | 3.39 ± 0.08 | 2.55 ± 0.08∗ | 2.38 ± 0.08∗ |
In each row, values (Mean ± SE) bearing asterisks differ significantly (P ≤ 0.05) from that of values in untreated (T0 negative control) fish. T1, T2, and T3 are positive control and dose based.
3.3.2. Oxidative Stress and Antioxidant Responses in Kidneys
Reduced GSH is a name. Therefore, sentence will beincrease, while in reduced GSH decresaed significantly than the kidneys obtained from normal Labeo rohita fish (Table 3). The results of SOD, CAT, and POD from the kidneys of PPF-treated fish at day 20 and 30 in group T2 and T3 showed significantly (P ≤ 0.05) lower values than the values obtained from the kidneys of untreated (T0) Labeo rohita (Table 3).
Table 3.
Oxidative stress parameters and antioxidant enzyme levels in Labeo rohita kidneys tissues subjected to pyriproxyfen dosages.
| Parameters/days | Groups/treatment | |||
|---|---|---|---|---|
| T0 (0.0) | T1 (300 μg/L) | T2 (600 μg/L) | T3 (900 μg/L) | |
| ROS (optical density) | ||||
| 10 | 0.56 ± 0.03 | 0.57 ± 0.01 | 0.63 ± 0.01 | 0.71 ± 0.02∗ |
| 20 | 0.53 ± 0.01 | 0.59 ± 0.02 | 0.65 ± 0.07∗ | 0.74 ± 0.03∗ |
| 30 | 0.57 ± 0.01 | 0.62 ± 0.02 | 0.70 ± 0.05∗ | 0.78 ± 0.07∗ |
| TBARS (nmol/TBARS formed/mg protein/min) | ||||
| 10 | 28.51 ± 1.51 | 30.21 ± 1.11 | 35.92 ± 0.5∗ | 39.63 ± 1.39∗ |
| 20 | 29.08 ± 1.63 | 31.08 ± 1.16 | 37.07 ± 0.6∗ | 41.06 ± 1.83∗ |
| 30 | 29.72 ± 1.71 | 33.79 ± 1.17 | 37.79 ± 0.7∗ | 41.83 ± 1.77∗ |
| Reduced GSH (μmol/g tissue) | ||||
| 10 | 7.70 ± 0.3 | 6.45 ± 0.3 | 5.21 ± 0.2∗ | 3.95 ± 0.2∗ |
| 20 | 7.66 ± 0.3 | 6.35 ± 0.2 | 5.18 ± 0.2∗ | 3.82 ± 0.2∗ |
| 30 | 7.54 ± 0.3 | 6.31 ± 0.2 | 5.10 ± 0.2∗ | 3.79 ± 0.2∗ |
| Antioxidant enzymes | ||||
| SOD (units/mg protein) | ||||
| 10 | 15.51 ± 0.36 | 13.53 ± 0.35 | 11.56 ± 0.33∗ | 9.55 ± 0.32∗ |
| 20 | 15.40 ± 0.36 | 13.14 ± 0.35 | 10.99 ± 0.33∗ | 8.77 ± 0.32∗ |
| 30 | 15.26 ± 0.36 | 13.10 ± 0.35 | 10.86 ± 0.33∗ | 8.68 ± 0.32∗ |
| CAT (units/min) | ||||
| 10 | 5.12 ± 0.1 | 4.58 ± 0.09 | 3.84 ± 0.09∗ | 3.46 ± 0.07∗ |
| 20 | 5.08 ± 0.1 | 4.56 ± 0.09 | 3.78 ± 0.08∗ | 3.43 ± 0.07∗ |
| 30 | 5.04 ± 0.1 | 4.45 ± 0.09 | 3.62 ± 0.08∗ | 3.30 ± 0.06∗ |
| POD (units/μg) | ||||
| 10 | 5.98 ± 0.13 | 5.33 ± 0.13 | 4.17 ± 0.11∗ | 4.04 ± 0.10∗ |
| 20 | 5.92 ± 0.13 | 5.30 ± 0.12 | 4.13 ± 0.11∗ | 3.95 ± 0.10∗ |
| 30 | 5.89 ± 0.13 | 5.27 ± 0.12 | 4.03 ± 0.10∗ | 3.89 ± 0.10∗ |
In each row, values (Mean ± SE) bearing asterisks differ significantly (P ≤ 0.05) from that of values in untreated (T0 negative control) fish. T1, T2, and T3 are positive control and dose based.
3.3.3. Oxidative Stress and Antioxidant Responses in Gills
Our results on oxidative stress biomarkers reveal a significantly (P ≤ 0.05) higher quantity of ROS and TBARS from gills of PPF-treated fish at day 30 in groups T2 and T3 than the gills of untreated fish (Table 4). The result recorded on the level of GSH from gills of PPF-treated fish at day 30 in group T3 shows significantly lower values than the gills of unexposed fish at all sampling days. The result obtained at day 10 in fish of group T3 while in groups T2 and T3 at day 20 and on the concentration different antioxidant responses including SOD, CAT, and POD from gills of PPF-treated fish shows significantly (P ≤ 0.05) reduced quantity than the gills of Labeo rohita (Table 4).
Table 4.
Oxidative stress parameters and antioxidant enzyme levels in Labeo rohita gills tissues subjected to pyriproxyfen dosages.
| Parameters/days | Groups/treatment | |||
|---|---|---|---|---|
| T0 (0.0) | T1 (300 μg/L) | T2 (600 μg/L) | T3 (900 μg/L) | |
| ROS (optical density) | ||||
| 10 | 0.34 ± 0.03 | 0.37 ± 0.01 | 0.49 ± 0.07∗ | 0.55 ± 0.09∗ |
| 20 | 0.35 ± 0.05 | 0.39 ± 0.04 | 0.53 ± 0.06∗ | 0.57 ± 0.07∗ |
| 30 | 0.37 ± 0.04 | 0.41 ± 0.01 | 0.56 ± 0.08∗ | 0.59 ± 0.09∗ |
| TBARS (nmol/TBARS formed/mg protein/min) | ||||
| 10 | 40.67 ± 1.63 | 44.47 ± 2.12 | 58.27 ± 2.22∗ | 61.07 ± 2.33∗ |
| 20 | 41.13 ± 2.61 | 44.88 ± 2.31 | 58.64 ± 3.41∗ | 62.39 ± 2.29∗ |
| 30 | 41.20 ± 1.61 | 45.04 ± 1.36 | 61.82 ± 2.39∗ | 63.70 ± 2.27∗ |
| Reduced GSH (μmol/g tissue) | ||||
| 10 | 2.58 ± 0.05 | 2.24 ± 0.10 | 1.90 ± 0.04∗ | 1.56 ± 0.16∗ |
| 20 | 2.44 ± 0.11 | 2.23 ± 0.14 | 1.83 ± 0.03∗ | 1.52 ± 0.17∗ |
| 30 | 2.34 ± 0.15 | 2.05 ± 0.17 | 1.76 ± 0.08∗ | 1.47 ± 0.25∗ |
| Antioxidant enzymes | ||||
| SOD (units/mg protein) | ||||
| 10 | 10.79 ± 1.2 | 9.73 ± 1.12 | 8.98 ± 0.04 | 7.24 ± 0.12∗ |
| 20 | 10.68 ± 1.1 | 9.53 ± 1.17 | 8.13 ± 0.21∗ | 7.22 ± 0.18∗ |
| 30 | 10.57 ± 1.4 | 9.32 ± 1.11 | 8.07 ± 0.13∗ | 6.83 ± 0.31∗ |
| CAT (units/min) | ||||
| 10 | 3.03 ± 0.14 | 2.89 ± 0.12 | 2.48 ± 0.08 | 2.19 ± 0.13∗ |
| 20 | 2.98 ± 0.11 | 2.79 ± 0.17 | 2.33 ± 0.08∗ | 2.15 ± 0.19∗ |
| 30 | 2.95 ± 0.09 | 2.73 ± 0.15 | 2.19 ± 0.14∗ | 2.08 ± 0.21∗ |
| POD (units/μg) | ||||
| 10 | 0.41 ± 0.05 | 0.37 ± 0.01 | 0.36 ± 0.01 | 0.26 ± 0.03∗ |
| 20 | 0.40 ± 0.04 | 0.36 ± 0.02 | 0.30 ± 0.05∗ | 0.24 ± 0.04∗ |
| 30 | 0.39 ± 0.05 | 0.34 ± 0.04 | 0.29 ± 0.06∗ | 0.22 ± 0.02∗ |
In each row, values (Mean ± SE) bearing asterisks differ significantly (P ≤ 0.05) from that of values in untreated (T0 negative control) fish. T1, T2, and T3 are positive control and dose based.
3.3.4. Oxidative Stress and Antioxidant Responses in Brain
We observed a significantly (P ≤ 0.05) increased quantity of ROS and TBARS from the brain of PPF-treated fish at days 20 and 30 in groups T2 and T3 and showed significantly increased quantity than the brain of untreated fish (Table 5). The result recorded on the level of GSH from the brain of PPF-treated fish at day 20 in group T3 and at day 30 in groups T2 and T3 indicates significantly lower values than the brain of unexposed fish at all sampling days. The result obtained at day 20 in fish of group T3 while in groups T2 and T3 at day 30 on the concentration different antioxidant responses including SOD, CAT, and POD from the brain of PPF treated fish shows significantly (P ≤ 0.05) reduced quantity than the brain of normal fish (Table 5).
Table 5.
Oxidative stress parameters and antioxidant enzyme levels in Labeo rohita brain tissue subjected to pyriproxyfen dosages.
| Parameters/days | Groups/treatments | |||
|---|---|---|---|---|
| T0 (0.0) | T1 (300 μg/L) | T2 (600 μg/L) | T3 (900 μg/L) | |
| ROS (optical density) | ||||
| 10 | 0.48 ± 0.02 | 0.50 ± 0.02 | 0.51 ± 0.03 | 0.53 ± 0.03 |
| 20 | 0.46 ± 0.02 | 0.52 ± 0.02 | 0.59 ± 0.03∗ | 0.72 ± 0.04∗ |
| 30 | 0.51 ± 0.02 | 0.53 ± 0.03 | 0.66 ± 0.03∗ | 0.76 ± 0.04∗ |
| TBARS (nmol/TBARS formed/mg protein/min) | ||||
| 10 | 18.60 ± 2.11 | 19.29 ± 1.03 | 20.97 ± 1.12 | 21.66 ± 1.06 |
| 20 | 19.08 ± 2.01 | 20.77 ± 1.21 | 26.46 ± 1.13∗ | 30.14 ± 1.13∗ |
| 30 | 19.18 ± 2.04 | 22.92 ± 1.5 | 26.66 ± 1.17∗ | 30.40 ± 2.13∗ |
| Reduced GSH (μmol/g tissue) | ||||
| 10 | 3.01 ± 0.06 | 2.96 ± 0.06 | 2.79 ± 0.05 | 2.89 ± 0.04 |
| 20 | 2.96 ± 0.06 | 2.77 ± 0.05 | 2.66 ± 0.04 | 1.77 ± 0.03∗ |
| 30 | 2.88 ± 0.06 | 2.75 ± 0.05 | 2.10 ± 0.04∗ | 1.73 ± 0.03∗ |
| Antioxidant enzymes | ||||
| SOD (units/mg protein) | ||||
| 10 | 13.65 ± 0.3 | 12.09 ± 0.3 | 12.02 ± 0.2 | 11.96 ± 0.2 |
| 20 | 13.56 ± 0.3 | 12.20 ± 0.2 | 11.73 ± 0.2 | 8.87 ± 0.1∗ |
| 30 | 13.45 ± 0.3 | 11.88 ± 0.2 | 10.32 ± 0.2∗ | 8.75 ± 0.1∗ |
| CAT (units/min) | ||||
| 10 | 4.16 ± 0.08 | 3.93 ± 0.08 | 3.85 ± 0.07 | 3.76 ± 0.06 |
| 20 | 4.12 ± 0.08 | 3.68 ± 0.08 | 3.77 ± 0.07 | 2.46 ± 0.06∗ |
| 30 | 4.07 ± 0.08 | 3.59 ± 0.07 | 2.98 ± 0.07∗ | 2.23 ± 0.06∗ |
| POD (units/μg) | ||||
| 10 | 3.04 ± 0.06 | 2.79 ± 0.06 | 2.64 ± 0.05 | 2.59 ± 0.04 |
| 20 | 3.01 ± 0.06 | 2.73 ± 0.05 | 2.35 ± 0.05 | 1.98 ± 0.04∗ |
| 30 | 3.03 ± 0.06 | 2.69 ± 0.05 | 2.03 ± 0.05∗ | 1.94 ± 0.04∗ |
In each row, values (Mean ± SE) bearing asterisks differ significantly (P ≤ 0.05) from that of values in untreated (T0 negative control) fish. T1, T2, and T3 are positive control and dose based.
3.4. DNA Damage Assessment by Comet Assay
The results on DNA damage by comet assay (Figure 3) in different visceral organs of Labeo rohita fish treated with various concentrations of PPF showed a significantly (P ≤ 0.05) increased percentile rate of DNA damage in isolated cells of the liver, kidneys, and gills at day 10 in group T3 while at day 20 and 30 in groups T2 and T3 compared to untreated fish (Table 6).
Figure 3.
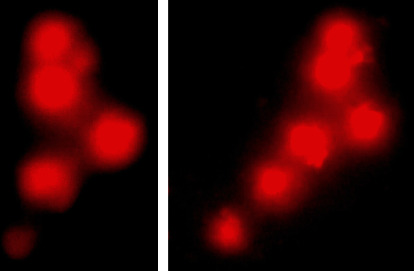
Comet assay showing DNA damage in isolated cells of the liver of fish treated with pyriproxyfen. (a) 600 μg/L (T2) and (b) 900 μg/L (T3) at 30-day experiment. Note the frequency and intensity is increasing with the dose of pyriproxyfen increasing.
Table 6.
DNA damage in different visceral organs of Labeo rohita (as observed by Comet assay) treated with various doses of pyriproxyfen.
| Parameters/days | Groups/treatment | |||
|---|---|---|---|---|
| T0 (0.0) | T1 (300 μg/L) | T2 (600 μg/L) | T3 (900 μg/L) | |
| DNA damage by comet assay in liver | ||||
| 10 | 2.35 ± 0.04 | 2.37 ± 0.05 | 2.39 ± 0.03 | 3.42 ± 0.14∗ |
| 20 | 2.37 ± 0.09 | 2.42 ± 0.06 | 3.47 ± 0.02∗ | 4.52 ± 0.19∗ |
| 30 | 2.43 ± 0.02 | 2.56 ± 0.02 | 4.70 ± 0.01∗ | 4.84 ± 0.22∗ |
| DNA damage by comet assay in kidneys | ||||
| 10 | 2.55 ± 0.19 | 2.67 ± 0.17 | 2.69 ± 0.27 | 4.47 ± 0.45∗ |
| 20 | 2.57 ± 0.21 | 2.72 ± 0.16 | 4.87 ± 0.51∗ | 5.53 ± 0.49∗ |
| 30 | 2.63 ± 0.25 | 2.76 ± 0.21 | 5.70 ± 0.61∗ | 5.81 ± 0.28∗ |
| DNA damage by comet assay in gills | ||||
| 10 | 2.23 ± 0.23 | 2.41 ± 0.11 | 2.51 ± 0.21 | 3.94 ± 0.44∗ |
| 20 | 2.31 ± 0.31 | 2.44 ± 0.14 | 3.87 ± 0.02∗ | 4.55 ± 0.39∗ |
| 30 | 2.23 ± 0.26 | 2.46 ± 0.12 | 4.77 ± 0.01∗ | 5.89 ± 0.42∗ |
In each row, values (Mean ± SE) bearing asterisks differ significantly (P ≤ 0.05) from that of values in untreated (T0 negative control) fish. T1, T2, and T3 are positive control and dose based.
4. Discussion
The pesticide used unwisely can cause many serious environmental dangers and also pollute the groundwater. These chemical residues do not dissolve in soil for a long period of time and remain in underground water [34]. This contamination is dangerous in agricultural land areas and can be a serious hazard for crops particularly in water resources and should be evaluated in agricultural countries like India and Pakistan [3]. One of these pesticides, PPF is a pesticide that works against a wide range of insects [10]. It was first launched in the USA in 1996 as a whitefly repellent for cotton crops. Other crops have also benefited from it. It is also used to keep domestic pets flea-free, as well as to eliminate ants and roaches both indoors and out [11, 35, 36]. Pyriproxyfen is an insect growth regulator and a juvenile hormone analog [37] that affects their growth. It inhibits larvae from maturing into adults, preventing them from reproducing. Pyriproxyfen damages the liver in mice, rats, and dogs at high dosages surpassing 5000 mg/kg body weight [38].
Therefore, prolonged monitoring and evaluation of the potential toxicity of PPF due to low concentrations of long-term exposure are incredibly important in an attempt to lessen its public health risks. In this research when Labeo rohita was treated with PPF, different morphological changes were observed including the pear-shaped nucleus, in different cells, and in some cases, bilobed nucleus like in erythrocyte micronucleus was observed like in red blood cells and in white blood cell types. While in the earlier report, the greater strength of nuclear anomalies including nuclear aberrations of erythrocyte, micronucleus, terminal nucleus, extended and swollen nucleus, and karyopyknotic was observed in silver barb (Barbonymus gonionotus) [39] treated with various concentrations of the toxicant. Previously, swollen erythrocytes identified as “spherocytes” change in size and shape of cells like elongated cells, cells with tapered ends, numerous spherocytes, erythrocytes showing contraction from one side and with small projections, the disrupted lipid membrane, and increased lipid peroxidation altered shapes of red blood cells in Ctenopharyngodon idellus [1, 40] exposed to the toxicant. Moreover, Channa punctatus abnormality occurred in red blood cells (acanthocytes with cytoplasmic blebbing and badly disrupted cell membrane) due to depressed adenosine triphosphate under hypoxic conditions [41] at higher concentrations of the toxicant was found. It was noticed that PPF alone had side effects on fish health; however, the combination of PPF with vitamin E and naringenin used had a better effect on fish health towards recovery.
Under normal physiological conditions, cells are capable of counterbalancing the noxious effects of ROS with the antioxidant defense system which consists of free radical scavengers such as SOD, GSH, glutathione peroxidase (GPx), and CAT. When production of free radicals exceeds the body's antioxidant defense system, it results in oxidative stress [42]. It is imposed on cells due to increase in oxidant generation, a decrease in antioxidant protection, and failure in the repair of oxidative damage [43–46] in the form of severe damage to cellular macromolecules such as proteins, lipids, and DNA, resulting in detrimental effects on cells [47–50]. Pesticides are known to induce ROS and cause oxidative stress in fish [11, 51, 52]. The antioxidant enzymes (SOD, CAT, GPx, and GST) prevent oxidative stress, and the actions of these enzymes are routinely used to monitor the risk of pesticides [53]. Glutathione reductase is a suitable biomarker to evaluate the impact of pesticides in aquatic organisms [54].
The activities of hepatic enzymatic antioxidants SOD, CAT, glutathione peroxidase, glutathione S-transferase, and glutathione reductase were previously examined in similar work. Catalase is a key enzyme that plays an essential role in cell defense against oxidative stress [55]. Several investigators noted changes in liver CAT activity in fish exposed to pesticides and thus considered this enzyme a useful marker of chemical-mediated tissue oxidation [56]. Catalase activity is higher in organs with high oxidative potential such as the liver, kidney, and erythrocytes [57]. Several studies have shown changes in liver catalase of fish exposed to pesticides, and catalase has been considered a useful marker of liver changes due to damage by toxic substances [56, 58].
With a high dose of PPF, the values of the ROS increased considerably in liver tissues. The POD enzymes remarkably reduced in liver of fish received high doses of PPF. In the present study, with little bit different results of parameters like ROS, POD, TBARS were observed in the kidney, gills, and brain. Scanty of work available previously as similar work on different chemicals and species like rats [59] was studied previously. The number of liver cells with damaged DNA increased in this research when the dosage of PPF was increased. The concentrations of ROS and TBARS were assessed in the gills, liver, and kidneys of PPF-treated Labeo rohita fish in this study. Previously, no data on PPF-induced oxidative stress (ROS and TBARS) in the Labeo rohita's brain, gills, liver, or kidneys could be located in the literature. However, due to the detoxifying systems of exposed animals, exposure to diverse toxicants produces quick and increased formation of ROS. The formation of ROS starts the process of lipid peroxidation, which leads to cellular membrane irregularities and the development of TBARS [1, 33, 60]. As a result, elevated levels of oxidative stress indices in fish exposed to PPF in the current investigation might be related to antioxidant enzyme depletion and misbalancing. Earlier investigations in rare minnow [61] and bigheaded carp [1] found increased levels of oxidative stress parameters owing to toxicants such as lipid peroxidation product, nitric oxide, and ROS.
Furthermore, several investigations have discovered that DNA damage in various tissues of organisms is mostly caused by the formation of free radicals and oxidative stress [29, 41]. Increased levels of ROS and H2O2 owing to toxicants have also been observed in rats, which is similar to our findings [62]. ROS production is primarily influenced by toxicant concentrations, cellular backgrounds, duration, and exposure time [1]. Pyriproxyfen also causes oxidative stress by lowering antioxidant enzymes (CAT, SOD, glutathione peroxidase, and glutathione reductase) and increasing lipid peroxidation in both target and non-target animals [11, 63, 64].
Pyriproxyfen, a pesticide and its metabolites, also showed oxidative stress damage by inhibiting the activity of CAT and SOD and increasing MDA [65]. In the present study, fish exposed to PPF caused a decrease in SOD activity in the gill tissue which indicates the adaptive response of fish to pesticides. Catalase (CAT), an important antioxidant enzyme, protects the aquatic organisms from oxidative stress. It catalyzes hydrogen peroxide into water and oxygen consequently completing the detoxification process imitated by SOD [66]. The observed decrease in CAT activity in gill tissue of Catla catla treated with ACE and TMX indicates overproduction of ROS due to pesticide stress. Furthermore, inhibition of protein synthesis due to pesticide stress may be another possible reason for the inhibition of CAT activity which has also been reported [67].
The amounts of GSH and total proteins in the gills, livers, and kidneys of fish were shown to be lower in this experimental investigation. To present, there is no information on the effects of PPF on the contents of GSH and total proteins in various Labeo rohita tissues. The lower values of GSH and total proteins in various tissues of fish in the current study might be due to dysfunctions of tissues and increased utilization of energy (body proteins) to overcome oxidative stress [64, 68–71]. Previously, it is well-established that different toxicants are responsible for the reduction of proteins in different tissues of fish (Oreochromis spilurus, Mystusvittatus, Channa punctatus, and Labeo rohita) including gills, kidneys, and livers [41, 72]. However, other than fish species, DNA damage was also observed in other organisms like birds and mammals [72], rats [6], chickens [73], liver cancer cell line [74, 75], and HepG2 cell line [76]. In contrast to the results on comet assay, no significant increase in DNA damage due to toxicant has been observed in fish [77–79]. Moreover, it can be speculated that DNA damage in different tissues of Labeo rohita might also be related to genetic abnormalities induced by PPF leading to the activation of abnormal and physiologically nonfunctional proteins responsible for mitochondrion dysfunctioning and breakage of nuclear proteins [80, 81]. Furthermore, DNA damage in multiple organs of the Labeo rohita might be linked to genetic abnormalities generated by PPF, which could lead to the activation of aberrant and physiologically nonfunctional proteins that cause mitochondrion dysfunction and nuclear protein breakdown [1, 82, 83].
5. Conclusions
From the findings of the current trial, our results indicated that pyriproxyfen induced deleterious effects on red blood cells and different vital organs of Labeo rohita. Exposure of specimen to PPF at 600 μg/L and 900 μg/L causes DNA damage in isolated blood lymphocytes, the brain, gills, liver, and kidneys cells. Moreover, PPF with vitamin C causes low oxidative stress and also causes less reduction in antioxidant enzymes in the brain, livers, kidneys, and gills of Labeo rohita in a concentration and time-dependent manner.
Abbreviations
- ANOVA:
Analysis of variance
- ATP:
Adenosine triphosphate
- CAT:
Catalase
- GSH:
Glutathione
- POD:
Peroxidase
- PPF:
Pyriproxyfen
- ROS:
Reactive oxygen species
- SOD:
Superoxide dismutase
- TBARS:
Thiobarbituric acid reactive species.
Contributor Information
Kun Li, Email: lk3005@njau.edu.cn.
Ahrar Khan, Email: ahrar1122@yahoo.com.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
Abdul Ghaffar and Riaz Hussain planned and designed the research work. Riaz Hussain and Sumaira Naseem executed the study and obtained the data. Riaz Hussain and Ahrar Khan analyzed the collected data. Xuwen Li, Kun Li, Riaz Hussain, and Ahrar Khan interpreted the data. Xuwen Li and Kun Li prepared the manuscript paper. All authors read and approved the final version of the manuscript. Xuwen Li and Abdul Ghaffar are the first authors as they equally contributed to this study.
References
- 1.Akram R., Ghaffar A., Hussain R., et al. Hematological, serum biochemistry, histopathological and mutagenic impacts of triclosan on fish (bighead carp) Agrobiological Records . 2022;7:18–28. [Google Scholar]
- 2.Namratha M. L., Lakshman M., Jeevanalatha M., Kumar B. A. Hematological alterations induced by glyphosate and ameliorative effect of ascorbic acid in Wistar rats. Continental Veterinary Journal . 2020;1:32–36. [Google Scholar]
- 3.Hussain R., Ghaffar A., Ali H. M., et al. Analysis of different toxic impacts of fipronil on growth, hemato-biochemistry, protoplasm and reproduction in adult cockerels. Toxin Reviews . 2018;37(4):294–303. doi: 10.1080/15569543.2017.1366921. [DOI] [Google Scholar]
- 4.Amaroli A., Gallus L., Ferrando S. Permethrin drastically affects the developmental cycle of the non-target slime mould Dictyostelium discoideum. Chemosphere . 2018;193:1–7. doi: 10.1016/j.chemosphere.2017.10.127. [DOI] [PubMed] [Google Scholar]
- 5.Tahir R., Ghaffar A., Abbas G., et al. Pesticide induced hematological, biochemical and genotoxic changes in fish: a review. Agrobiological Records . 2021;3:41–57. doi: 10.47278/journal.abr/2021.005. [DOI] [Google Scholar]
- 6.Ahmed H. H., El-Toukhey N. E. S., Abd El-Rahman S. S., Hendawy A. K. Efficacy of melatonin against oxidative stress, DNA damage and histopathological changes induced by nicotine in liver and kidneys of male rats. International Journal of Veterinary Science . 2021;10(1):31–36. doi: 10.47278/journal.ijvs/2020.022. [DOI] [Google Scholar]
- 7.EL-Saeid M. H., BaQais A., Alshabanat M. Study of the photocatalytic degradation of highly abundant pesticides in agricultural soils. Molecules . 2022;27(3):p. 634. doi: 10.3390/molecules27030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabeen G., Manzoor F., Arshad M., Barbol B. Effect of cadmium exposure on hematological, nuclear and morphological alterations in erythrocyte of fresh water fish (Labeo rohita) Continental Veterinary Journal . 2021;1(1):20–24. [Google Scholar]
- 9.Haque M. A., Quan H., Zuo Z., Khan A., Siddique N., He C. Pathogenicity of feed-borne Bacillus cereus and its implication on food safety. Agrobiological Records . 2021;3:1–16. doi: 10.47278/journal.abr/2020.015. [DOI] [Google Scholar]
- 10.Ginjupalli G. K., Baldwin W. S. The time- and age-dependent effects of the juvenile hormone analog pesticide, pyriproxyfen on Daphnia magna reproduction. Chemosphere . 2013;92(9):1260–1266. doi: 10.1016/j.chemosphere.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H., Li P., Wang P., Liu D., Zhou H. Toxicity risk assessment of pyriproxyfen and metabolites in the rat liver: A vitro study. Journal of Hazardous Materials . 2020;389, article id 121835 doi: 10.1016/j.jhazmat.2019.121835. [DOI] [PubMed] [Google Scholar]
- 12.Caixeta E. S., Silva C. F., Santos V. S. V., Olegário de Campos Júnior E., Pereira B. B. Ecotoxicological assessment of pyriproxyfen under environmentally realistic exposure conditions of integrated vector management for Aedes aegypti control in Brazil. Journal of Toxicology Environmental Health A . 2016;79(18):799–803. doi: 10.1080/15287394.2016.1191400. [DOI] [PubMed] [Google Scholar]
- 13.Belenguer V., Martinez-Capel F., Masiá A., Picó Y. Patterns of presence and concentration of pesticides in fish and waters of the Júcar River (Eastern Spain) Journal of Hazardous Materials . 2014;265:271–279. doi: 10.1016/j.jhazmat.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Little E. E., Archeski R. D., Flerov B. A., Kozlovskaya V. I. Behavioral indicators of sublethal toxicity in rainbow trout. Archives of Environmental Contamination and Toxicology . 1990;19(3):380–385. doi: 10.1007/BF01054982. [DOI] [PubMed] [Google Scholar]
- 15.Naseem S., Ghaffar A., Hussain R., Khan A. Inquisition of toxic effects of pyriproxyfen on physical, hemato-biochemical and histopathological parameters in Labeo rohita fish. Pakistan Veterinary Journal . 2022 doi: 10.29261/pakvetj/2022.014. [DOI] [Google Scholar]
- 16.Kannan M., Sellamani M., Bojan N., Ramesh M., Kadirvelu K. Toxicity assessment of pyriproxyfen in vertebrate model zebrafish embryos (Danio rerio): a multi biomarker study. Aquatic Toxicology . 2018;196:132–145. doi: 10.1016/j.aquatox.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Fulton M. H., Key P. B., DeLorenzo M. E. Insecticide toxicity in fish. Fish Physiology . 2013;33:309–368. doi: 10.1016/B978-0-12-398254-4.00006-6. [DOI] [Google Scholar]
- 18.Azevedo-Linhares M., Souza A. T. C., Lenz C. A., et al. Microcystin and pyriproxyfen are toxic to early stages of development in Rhamdia quelen: an experimental and modelling study. Ecotoxicology and Environmental Safety . 2018;166:311–319. doi: 10.1016/j.ecoenv.2018.09.064. [DOI] [PubMed] [Google Scholar]
- 19.Farag M. R., Alagawany M., Bilal R. M., et al. An overview on the potential hazards of pyrethroid insecticides in fish, with special emphasis on cypermethrin toxicity. Animals . 2021;11, article 1880 doi: 10.3390/ani11071880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang C., Lim W., Song G. Immunotoxicological effects of insecticides in exposed fishes. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology . 2021;247, article 109064 doi: 10.1016/j.cbpc.2021.109064. [DOI] [PubMed] [Google Scholar]
- 21.Hussain R., Ghaffar A., Abbas G., et al. Thiamethoxam at sublethal concentrations induces histopathological, serum biochemical alterations and DNA damage in fish (Labeo rohita) Toxin Review . 2020;41(1):154–164. doi: 10.1080/15569543.2020.1855655. [DOI] [Google Scholar]
- 22.Hussein N. M., Saeed R. M. A., Shaheen A. A., Hamed H. S. Ameliorative role of chitosan nanoparticles against bisphenol-A induced behavioral, biochemical changes and nephrotoxicity in the African catfish, Clarias gariepinus. Egyptian Journal of Aquatic Biology and Fisheries . 2021;25(1):493–510. doi: 10.21608/ejabf.2021.145446. [DOI] [Google Scholar]
- 23.Merdana I. M., Watiniasih N. L., Sudira I. W., et al. The effect of ethanolic extract of Myrmecodia pendans on gentamicin induced nephrotoxicity in Wistar rats. International Journal of Veterinary Science . 2021;10(2):96–101. doi: 10.47278/journal.ijvs/2020.025. [DOI] [Google Scholar]
- 24.Taha M. G., El-Hamamsy S. M. A., Ahmed N. S., Ali M. M. Amelioration effect of Carica papaya fruit extracts on doxorubicin – induced cardiotoxicity in rats. International Journal of Veterinary Science . 2020;9:349–354. [Google Scholar]
- 25.Ghaffar A., Hussain R., Abbas G., et al. Assessment of genotoxic and pathologic potentials of fipronil insecticide in Labeo rohita (Hamilton, 1822) Toxin Reviews . 2021;40(4):1289–1300. doi: 10.1080/15569543.2019.1684321. [DOI] [Google Scholar]
- 26.Ghaffar A., Hussain R., Ahmad N., et al. Evaluation of hemato-biochemical, antioxidant enzymes as biochemical biomarkers and genotoxic potential of glyphosate in freshwater fish (Labeo rohita) Chemistry and Ecology . 2021;37(7):646–667. doi: 10.1080/02757540.2021.1937141. [DOI] [Google Scholar]
- 27.Yamin A., Naz S., Hussain R., et al. Exposure to low concentrations of heavy metals alone and in combination induces histopathological and genotoxic effects in fish (Labeo rohita) Advancement in Life Sciences . 2020;7:240–246. [Google Scholar]
- 28.Singh N. N., Srivastava A. K. Haematological parameters as bioindicators of insecticide exposure in teleosts. Ecotoxicology . 2010;19(5):838–854. doi: 10.1007/s10646-010-0465-4. [DOI] [PubMed] [Google Scholar]
- 29.Hussain R., Ali F., Rafique A., et al. Exposure to sub-acute concentrations of glyphosate induces clinico-hematological, serum biochemical and genotoxic damage in adult cockerels. Pakistan Veterinary Journal . 2019;39(2):181–186. doi: 10.29261/pakvetj/2019.064. [DOI] [Google Scholar]
- 30.Kakkar P., Das B., Viswanathan P. A modified spectrophotometric assay of superoxide dismutase. Indian Journal of Biochemistry and Biophysics . 1984;21(2):130–132. [PubMed] [Google Scholar]
- 31.Jollow D. J., Mitchell J. R., Zampaglione N., Gillette J. R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology . 1974;11(3):151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi I., Morishita Y., Imai K., Nakamura M., Nakachi K., Hayashi T. High through put spectrophotometric assay of reactive oxygen species in serum. Mutation Research . 2007;631(1):55–61. doi: 10.1016/j.mrgentox.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Iqbal M., Sharma S., Rezazadeh H., Hasan N., Abdulla M., Athar M. Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate mediated hepatic injury. Redox Reports . 1996;2(6):385–391. doi: 10.1080/13510002.1996.11747079. [DOI] [PubMed] [Google Scholar]
- 34.Kida M., Myangan O., Oyuntsetseg B., Khakhinov V., Kawahigashi M., Fujitake N. Dissolved organic matter distribution and its association with colloidal aluminum and iron in the Selenga River Basin from Ulaanbaatar to Lake Baikal. Environmental Science, Pollution Research . 2018;25(12):11948–11957. doi: 10.1007/s11356-018-1462-z. [DOI] [PubMed] [Google Scholar]
- 35.Mujahid Q., Khan A., Qadir M. F., et al. Allethrin induced toxicopathological alterations in adult male albino rats. Agrobiological Records . 2021;5:8–14. doi: 10.47278/journal.abr/2020.019. [DOI] [Google Scholar]
- 36.Ahmad L., Gul S. T., Saleemi M. K., et al. The effect of different repeated doses of cypermethrin on the behavioral and histological alterations in the brain of rabbits (Oryctolagus cuniculi) International Journal of Veterinary Science . 2021;10(4):347–354. doi: 10.47278/journal.ijvs/2021.092. [DOI] [Google Scholar]
- 37.Fiaz M., Martínez L. C., Plata-Rueda A., et al. Pyriproxyfen, a juvenile hormone analog, damages midgut cells and interferes with behaviors of Aedes aegypti larvae. Peer J . 2019;7, article e7489 doi: 10.7717/peerj.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gwaltney-Brant S. M. Chapter 70. In: Small Animal Toxicology (Third Edition) Elsevier B.V.; 2013. Atypical topical spot-on products. [Google Scholar]
- 39.Islam S. M., Khan M. M., Moniruzzaman M. Recuperation patterns in fish with reference to recovery of erythrocytes in Barbonymus gonionotus disordered by an organophosphate. International Journal of Environment, Science and Technology . 2019;16:7535–7544. doi: 10.1007/s13762-019-02425-0. [DOI] [Google Scholar]
- 40.Abdel-Tawwab M., Hamed H. S. Effect of bisphenol A toxicity on growth performance, biochemical variables and oxidative stress biomarkers of Nile tilapia, Reochromis niloticus (L.) Journal of Applied Ichthyology . 2018;34(5):1117–1125. doi: 10.1111/jai.13763. [DOI] [PubMed] [Google Scholar]
- 41.Ghaffar A., Hussain R., Noreen S., et al. Dose and time-related pathological and genotoxic studies on thiamethoxam in fresh water fish (Labeo rohita) in Pakistan. Pakistan Veterinary Journal . 2020;40(2):151–156. doi: 10.29261/pakvetj/2020.002. [DOI] [Google Scholar]
- 42.Ali M. M., Sahar T., Firyal S., et al. Assessment of cytotoxic, genotoxic, and oxidative stress of dibutyl phthalate on cultured bovine peripheral lymphocytes. Oxidative Medicine and Cellular Longevity . 2022;2022:6. doi: 10.1155/2022/9961513.9961513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dianzani M. U. Lipid peroxidation and cancer. Critical Reviews in Oncology and Hematology . 1993;15(2):125–147. doi: 10.1016/1040-8428(93)90052-6. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J., Jiang S., Zhang M., Wu X. Monitoring the acute and subacute toxicity effects of herbicide acetochlor by bacterivorous nematode. International Conference on New Technology of Agricultural; 2011; Zibo, China. pp. 669–672. [DOI] [Google Scholar]
- 45.Liman R., Ali M. M., Ciğerci İ. H., İstifli E. S., Sarıkurkcu C. Cytotoxic and genotoxic evaluation of copper oxychloride through allium test and molecular docking studies. Environmental Science and Pollution Research International . 2021;28(33):44998–45008. doi: 10.1007/s11356-021-13897-4. [DOI] [PubMed] [Google Scholar]
- 46.Liman R., Ali M. M., Istifi E. S., Ciğerci İ. H., Bonciu E. Genotoxic and cytotoxic effects of pethoxamid herbicide on Allium cepa cells and its molecular docking studies to unravel genotoxicity mechanism. Environmental science and Pollution Research International . 2022 doi: 10.1007/s11356-022-20166-5. [DOI] [PubMed] [Google Scholar]
- 47.Mahmood Y., Ghaffar A., Hussain R. New insights into hemato-biochemical and histopathological effects of acetochlor in bighead carp (Aristichthys nobilis) Pakistan Veterinary Journal . 2021;41:538–544. [Google Scholar]
- 48.Khanna P., Ong C., Bay B. H., Baeg G. H. Nanotoxicity: an interplay of oxidative stress, inflammation and cell death. Nanomaterials . 2015;5(3):1163–1180. doi: 10.3390/nano5031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liman R., Acikbas Y., Ciğerci İ. H., Ali M. M., Kars M. D. Cytotoxic and genotoxic assessment of silicon dioxide nanoparticles by allium and comet tests. Bulletin of Environmental Contamination and Toxicology . 2020;104(2):215–221. doi: 10.1007/s00128-020-02783-3. [DOI] [PubMed] [Google Scholar]
- 50.Liman R., Başbuğ B., Ali M. M., Acikbas Y., Ciğerci İ. H. Cytotoxic and genotoxic assessment of tungsten oxide nanoparticles in Allium cepa cells by Allium ana-telophase and comet assays. Journal of Applied Genetics . 2021;62(1):85–92. doi: 10.1007/s13353-020-00608-x. [DOI] [PubMed] [Google Scholar]
- 51.Adeyemi J. A., da Cunha Martins-Junior A., Barbosa F., Jr. Teratogenicity, genotoxicity and oxidative stress in zebrafish embryos (Danio rerio) co-exposed to arsenic and atrazine. Compartive Biochemistry and Physiology . 2015;172-173:7–12. doi: 10.1016/j.cbpc.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Mahmood Y., Hussain R., Ghaffar A., et al. Acetochlor affects bighead carp (Aristichthys nobilis) by producing oxidative stress, lowering tissue proteins, and inducing genotoxicity. BioMed Research International . 2022;2022:12. doi: 10.1155/2022/9140060.9140060 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Jin Y., Zhang X., Shu L., et al. Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio) Chemosphere . 2010;78(7):846–852. doi: 10.1016/j.chemosphere.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 54.Khare A., Chhawani N., Kumari K. Glutathione reductase and catalase as potential biomarkers for synergistic intoxication of pesticides in fish. Biomarkers . 2019;24(7):666–676. doi: 10.1080/1354750X.2019.1651902. [DOI] [PubMed] [Google Scholar]
- 55.Santana M. S., Domingues de Melo G., Sandrini-Neto L., Di Domenico M., Prodocimo M. M. A meta-analytic review of fish antioxidant defense and biotransformation systems following pesticide exposure. Chemosphere . 2022;291(Part 1, article id 132730) doi: 10.1016/j.chemosphere.2021.132730. [DOI] [PubMed] [Google Scholar]
- 56.Clasen B., Loro V. L., Murussi C. R., Tiecher T. L., Moraes B., Zanella R. Bioaccumulation and oxidative stress caused by pesticides in Cyprinus carpio reared in a rice-fish system. Science of the Total Environment . 2018;626:737–743. doi: 10.1016/j.scitotenv.2018.01.154. [DOI] [PubMed] [Google Scholar]
- 57.Glorieux C., Zamocky M., Sandoval J. M., Verrax J., Calderon P. B. Regulation of catalase expression in healthy and cancerous cells. Free Radical Biology and Medicine . 2015;87:84–97. doi: 10.1016/j.freeradbiomed.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 58.Moraes B. S., Loro V. L., Glusczak L., et al. Effects of four rice herbicides on some metabolic and toxicology parameters of teleost fish (Leporinus obtusidens) Chemosphere . 2007;68(8):1597–1601. doi: 10.1016/j.chemosphere.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Renugadevi N., Sangeetha M., Kavitha B. Methylene blue removal using a low–cost activated carbon adsorbent from mimusops elengi. Journal of Industrial Pollution Control . 2010;26:19–22. [Google Scholar]
- 60.Akram R., Iqbal R., Hussain R., Jabeen F., Ali M. Evaluation of oxidative stress, antioxidant enzymes and genotoxic potential of bisphenol A in fresh water bighead carp (Aristichthys nobils) fish at low concentrations. Environmental Pollution . 2021;268(Part A, article 115896) doi: 10.1016/j.envpol.2020.115896. [DOI] [PubMed] [Google Scholar]
- 61.Tao S., Wang L., Zhu Z., et al. Adverse effects of bisphenol A on Sertoli cell blood-testis barrier in rare minnow Gobiocypris rarus. Ecotoxicology and Environmental Safety . 2019;171:475–483. doi: 10.1016/j.ecoenv.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Tiwari D., Vanage G. Bisphenol A induces oxidative stress in bone marrow cells, lymphocytes, and reproductive organs of Holtzman rats. International Journal of Toxicology . 2017;36(2):142–152. doi: 10.1177/1091581817691224. [DOI] [PubMed] [Google Scholar]
- 63.Robea M. A., Jijie R., Nicoara M., et al. Vitamin C attenuates oxidative stress and behavioral abnormalities triggered by fipronil and pyriproxyfen insecticide chronic exposure on zebrafish juvenile. Antioxidants . 2020;9(10):p. 944. doi: 10.3390/antiox9100944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noaman N. H., Abdel-Halim K. Y., ElDin S. M. M., El-Abasy M. M. Cytotoxicity, oxidative stress and biochemical alterations induced by traditional and nanoform of pendimethalin in freshwater alga Chlorella vulgaris. Nano World Journal . 2020;6(1):13–25. doi: 10.17756/nwj.2020-076. [DOI] [Google Scholar]
- 65.Wei Y., Cui J., Zhai W., et al. Toxicity and fate of chiral insecticide pyriproxyfen and its metabolites in zebrafish (Danio rerio) Environmental Pollution . 2021;280, article 116894 doi: 10.1016/j.envpol.2021.116894. [DOI] [PubMed] [Google Scholar]
- 66.Chelikani P., Fita I., Loewen P. C. Diversity of structures and properties among catalases. Cellular and Molecular Life Sciences . 2004;61(2):192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li B., Xia X., Wang J., Zhu L., Wang J., Wang G. Evaluation of acetamiprid-induced genotoxic and oxidative responses in Eisenia fetida. Ecotoxicology and Environmental Safety . 2018;161:610–615. doi: 10.1016/j.ecoenv.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 68.Ameur W. B., de Lapuente J., El Megdiche Y., et al. Oxidative stress, genotoxicity and histopathology biomarker responses in mullet (Mugil cephalus) and sea bass (Dicentrarchus labrax) liver from Bizerte Lagoon (Tunisia) Marine Pollution Bulletin . 2012;64(2):241–251. doi: 10.1016/j.marpolbul.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 69.Gupta P., Verma S. K. Evaluation of genotoxicity induced by herbicide pendimethalin in fresh water fish Clarias batrachus (linn.) and possible role of oxidative stress in induced DNA damage. Drug and Chemical Toxicology . 2022;45(2):750–759. doi: 10.1080/01480545.2020.1774603. [DOI] [PubMed] [Google Scholar]
- 70.Ahmad I., Ahmad M. Fresh water fish, Channa punctatus, as a model for pendimethalin genotoxicity testing: a new approach toward aquatic environmental contaminants. Environmental Toxicology . 2016;31(11):1520–1529. doi: 10.1002/tox.22156. [DOI] [PubMed] [Google Scholar]
- 71.Latif M., Faheem M. Study of oxidative stress and histo-biochemical biomarkers of diethyl phthalate induced toxicity in a cultureable fish, Labeo rohita. Pakistan Veterinary Journal . 2020;40(2):202–208. doi: 10.29261/pakvetj/2019.108. [DOI] [Google Scholar]
- 72.Whittemore K., Martínez-Nevado E., Blasco M. A. Slower rates of accumulation of DNA damage in leukocytes correlate with longer lifespans across several species of birds and mammals. Aging (Albany NY) . 2019;11:9829–9845. doi: 10.18632/aging.102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luckmann M. R., de Melo M. S., Spricigo M. C., da Silva N. M., Nazari E. M. Pyriproxyfen exposure induces DNA damage, cell proliferation impairments and apoptosis in the brain vesicles layers of chicken embryos. Toxicology . 2021;464, article 152998 doi: 10.1016/j.tox.2021.152998. [DOI] [PubMed] [Google Scholar]
- 74.Ali M. M., Ciğerci İ. H. Anti-cancerous efficacy of alcoholic and aqueous extracts from an endemic plant Thermopsis turcica on liver carcinoma. British Journal of Pharmaceutical Research . 2017;16(3):1–5. doi: 10.9734/BJPR/2017/32413. [DOI] [Google Scholar]
- 75.Ali M. M., Ciğerci İ. H. Genotoxic evaluation of an endemic plant Thermopsis turcica extracts on liver cancer cell line. Pakistan Journal of Zoology . 2018;51:355–357. [Google Scholar]
- 76.Ali M. M., Ciğerci İ. H. Evaluation of anti-cancerous and genotoxic mechanisms via gene expression analysis of various extracts from Thermopsis turcica in HepG2 cell line. Gut . 2019;68(Suppl 1):p. A123. [Google Scholar]
- 77.Cervantes-Camacho I., Guerrero-Estévez S. M., López M. F., Alarcón-Hernández E., López-López E. Effects of Bisphenol A on Foxl2 gene expression and DNA damage in adult viviparous fish Goodea atripinnis. Journal of Toxicology and Environmental Health, Part A . 2020;83(3):95–112. doi: 10.1080/15287394.2020.1730282. [DOI] [PubMed] [Google Scholar]
- 78.Wang J. Q., Hussain R., Ghaffar A., et al. Clinico-hematological, mutagenic, and oxidative stress induced by pendimethalin in freshwater fish bighead carp (Hypophthalmichthys nobilis) Oxidative Medicine and Cellular Longevity . 2022;2022:15. doi: 10.1155/2022/2093822.2093822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Faheem M., Lone K. P. Oxidative stress and histopathologic biomarkers of exposure to bisphenol-A in the freshwater fish, Ctenopharyngodon idella. Brazilian Journal of Pharmaceutical Sciences . 2017;53(3, article e17003) doi: 10.1590/s2175-97902017000317003. [DOI] [Google Scholar]
- 80.Silva F. F. D., Silva J. M. D., Silva T. D. J. D., et al. Evaluation of Nile tilapia (Oreochromis niloticus) fingerlings exposed to the pesticide pyriproxyfen. Latin American Journal of Aquatic Research . 2020;48(5):826–835. doi: 10.3856/vol48-issue5-fulltext-2556. [DOI] [Google Scholar]
- 81.Koenig N., Almunia C., Bonnal-Conduzorgues A., et al. Co-expression network analysis identifies novel molecular pathways associated with cadmium and pyriproxyfen testicular toxicity in Gammarus fossarum. Aquatic Toxicology (Amsterdam, Netherlands) . 2021;235, article 105816 doi: 10.1016/j.aquatox.2021.105816. [DOI] [PubMed] [Google Scholar]
- 82.Trapp J., Gouveia D., Almunia C., et al. Digging deeper into the pyriproxyfen-response of the amphipod Gammarus fossarum with a next-generation ultra-high-field orbitrap analyser: new perspectives for environmental toxicoproteomics. Frontiers in Environmental Science . 2018;6, article 54 [Google Scholar]
- 83.Mahim S. S., Anjali V. R., Remya V. S., Reshmi S., Aruna Devi C. Oxidative stress responses of a freshwater fish, Labeo rohita, to a xenobiotic, bisphenol S. Journal of Biochemical and Molecular Toxicology . 2021;35(8, article e22820) doi: 10.1002/jbt.22820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


