Abstract
Aim
To investigate whether the American Joint Committee on Cancer (AJCC) clinical category cT2b needs to be subclassified by the type and distribution of retinoblastoma (RB) seeding.
Methods
Multicentre, international registry-based data were collected from RB centres enrolled between January 2001 and December 2013. 1054 RB eyes with vitreous or subretinal seeds from 18 ophthalmic oncology centres, in 13 countries within six continents were analysed. Local treatment failure was defined as the use of secondary enucleation or external beam radiation therapy (EBRT) and was estimated with the Kaplan-Meier method.
Results
Clinical category cT2b included 1054 eyes. Median age at presentation was 16.0 months. Of these, 428 (40.6%) eyes were salvaged, and 430 (40.8%) were treated with primary and 196 (18.6%) with secondary enucleation. Of the 592 eyes that had complete data for globe salvage analysis, the distribution of seeds was focal in 143 (24.2%) and diffuse in 449 (75.8%). The 5-year Kaplan-Meier cumulative globe-salvage (without EBRT) was 78% and 49% for eyes with focal and diffuse RB seeding, respectively. Cox proportional hazards regression analysis confirmed a higher local treatment failure risk with diffuse seeds as compared with focal seeds (hazard rate: 2.8; p<0.001). There was insufficient evidence to prove or disprove an association between vitreous seed type and local treatment failure risk(p=0.06).
Conclusion
This international, multicentre, registry-based analysis of RB eyes affirmed that eyes with diffuse intraocular distribution of RB seeds at diagnosis had a higher risk of local treatment failure when compared with focal seeds. Subclassification of AJCC RB category cT2b into focal vs diffuse seeds will improve prognostication for eye salvage.
Keywords: pathology, neoplasia, retina, vitreous
Introduction
Retinoblastoma (RB) is typically discohesive, resulting in intraocular dissemination of tumour seeds in the subretinal or vitreous space on tumour growth.1 They are morphologically described as dust, sphere and cloud type, and especially the emergence of vitreous seeds is thought to worsen local tumour control.1–6 For example, the Reese-Ellsworth classification assigned the prognosis of eyes with vitreous seeds to the most unfavourable group, Vb group.7 Similarly, the International Intraocular Retinoblastoma Classification and the International Classification for Retinoblastoma also account for the presence and extent of vitreous and subretinal seeds by dividing eyes according to focal (group C) or diffuse (group D) seeding.8 9 In contrast, the eighth edition American Joint Committee on Cancer (AJCC) staging system does not account for characteristics or distribution of RB seeds.10
Despite advances in the conservative management of advanced RB, a major cause of local treatment failure remains the persistence or recurrence of resistant vitreous and subretinal RB seeding.2 3 5 Vitreous and subretinal seeds can remain viable in the relatively avascular subretinal and vitreous environments and even grow to spheres. This environment has been thought to help RB seeds elude systemic and intra-arterial chemotherapy.1 Their small size and often diffuse distribution also prevent control with sequential aggressive local therapy.1 This has resulted in the need for external beam radiotherapy, periocular, and intravitreal chemotherapy to avoid enucleation.3 11 12
In 2018, the AJCC eighth edition provided a comprehensive, evidence-based RB staging system which was then adopted by the Union for International Cancer Control (UICC).10 Its advantages include that AJCC-UICC was the first and only RB staging system to incorporate intraocular and extraocular RB as well as heredity to predict both local treatment failure and metastasis-related mortality.10 13 14
The AJCC Ophthalmic Oncology Task Force (OOTF) periodically updates RB staging based on the availability of significant medical evidence. In 2017, the AJCC-OOTF analysed the literature for the eighth edition AJCC RB staging system and found no statistically significant convincing evidence that focal and diffuse seeding should be separated.10 However, since that time, new evidence has suggested that not all types of seeds nor their distributions are equal. Differences resulted in variation in the number of required intravitreal injections, the speed of seed-regression, and the rates of local treatment failure1 6 12
Herein, we examine if clinical characteristics and intraocular distribution of RB seeds at presentation can be used to predict local treatment failure and improve RB staging.
Methods
This study was conducted in adherence to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act of 1996. A total of 18 RB centres from 13 countries in 6 continents participated. Data from RB patients diagnosed between 5 January 2001 and 31 December 2013 were collected and entered into a secure online database and used to analyse RB seeding.
The Registry
Select RB subspecialty AJCC-OOTF committee members developed the data fields employed in the internet-based registry to obtain statistically significant medical evidence and thus answer our questions about RB.10 13–15 The scope of this study was limited to assessing the risk of local treatment failure based on the clinical type and intraocular distribution of RB seeds. Our internet database and security methods have been described in our prior registry publications.13–15
Definitions
The eighth edition of the AJCC Cancer Staging Manual on RB was used to determine the primary clinical tumour (cT) extent (table 1). This study assessed clinically visible seeds assigned to category cT2b. The diagnostic and therapeutic protocols were determined by each centre. Data collected included: demographic and clinical information comprising size and location of the intraocular tumour, presence of glaucoma, iris neovascularisation, subretinal fluid (SRF, <5 mm of tumour or >5 mm) and the distribution of seeds (diffuse/local) and type of vitreous seeds (dust/sphere/cloud).
Table 1.
The American Joint Committee on Cancer (AJCC) eighth edition TNMH classification for retinoblastoma, Definitions for primary tumour staging (CT)
| cTX | Unknown evidence of intraocular tumour |
| cT0 | No evidence of intraocular tumour. |
| cT1 | Intraocular tumour(s) with subretinal fluid ≤5 mm from the base of any tumour. |
| cT1a | Tumours ≤3 mm and further than 1.5 mm from the disc and fovea. |
| cT1b | Tumours >3 mm or closer than 1.5 mm to the disc and fovea. |
| cT2 | Intraocular tumour(s) with retinal detachment, vitreous seeding or subretinal seeding. |
| cT2a | Subretinal fluid >5 mm from the base of any tumour. |
| cT2b | Tumours with vitreous seeding and/or subretinal seeding. |
| cT3 | Advanced intraocular tumour(s). |
| cT3a | Phthisis or prephthisis bulbi. |
| cT3b | Tumour invasion of the pars plana, ciliary body, lens, zonules, iris or anterior chamber. |
| cT3c | Raised intraocular pressure with neovascularisation and/or buphthalmos. |
| cT3d | Hyphema and/or massive vitreous haemorrhage. |
| cT3e | Aseptic orbital cellulitis. |
| cT4 | Extraocular tumour(s) involving the orbit, including the optic nerve. |
| cT4a | Radiological evidence of retrobulbar optic nerve involvement or thickening of the optic nerve or involvement of the orbital tissues. |
| cT4a | Extraocular tumour clinically evident with proptosis and orbital mass. |
cT, clinical tumour.
Seed distribution
Focal (within 3 mm of main tumour).
Diffuse (beyond 3 mm of main tumour).
Seed type
Dust: small granules of vitreous opacity.
Sphere: spherical shaped vitreous opacity.
Cloud: dense collection of punctate vitreous opacities.
In this study, clinical vitreous seed type characteristics were divided into dust, sphere, and cloud. While these seed type characteristics were formalised after the closure of our registry, they were commonly used widely by participating centres.1 Therefore, seed-type classification was analysed for all eyes where this information was entered. In cases where more than one seed type characteristic was recorded, the predominant type with the highest degree was recorded.
Treatment definitions
The removal of treatment-naive RB eyes was defined as primary enucleation.
The removal of an eye after an attempt at eye salvage was defined as secondary enucleation, irrespective of the reason for enucleation (eg, recurrent tumour, significant residual disease, recurrent seeding and treatment complications).
Local treatment failure after conservative treatment was defined as need for external beam radiation therapy (EBRT) or secondary enucleation.
The follow-up time for each eye was defined as the time from diagnosis to last visit for salvaged eyes; or enucleation or EBRT, if the eye had to be enucleated or received EBRT, respectively.
Exclusion criteria included: if key variables, such as clinical variables essential for RB classification (tumour location, size, extent), treatment data (date and type of treatment) and outcome (globe salvage or primary/secondary enucleation) were missing or inconsistent.
A total of 2190 patients were enrolled from 18 ocular oncology centres across the world. Records with data sufficient for this study were available for 2085 patients (95.2%). Of these, all 1054 eyes staged as cT2b category were analysed.
Statistical analysis
The median, range and IQR were used to describe continuous variables, and frequencies and proportions were employed for categorical variables. Kaplan-Meier plots, log-rank test for trend and Logistic and Cox proportional hazards regression models were used to test if distribution and type of RB seeding were independently related to local treatment failure. The statistical analysis was performed using SPSS (V.26.0, IBM). Statistical significance was set at p<0.05.
Results
Demographic and clinical features
Median age at diagnosis was 16.0 months (mean, 20.5; SD, 20.6; IQR, 8–27; range, 1.0 month-13.6 years) and the median follow-up duration was 44.0 months (mean, 54.7; SD, 42.7; IQR, 20–81; range, 1 month to 14.6 years). Of the 1054 eyes, 535 (50.8%) had right eye involved and 579 (54.9%) had unilateral RB.
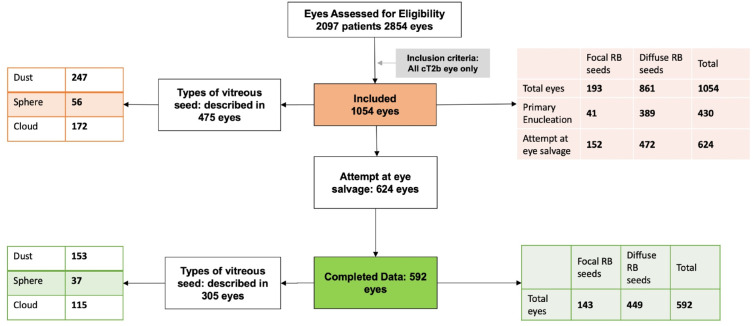
The distribution of predominant seeds was focal in 193 (18.3%) and diffuse in 861 (81.7%) eyes. The SRF was within 5 mm of the main tumour in 388 (36.8%) and beyond 5 mm in 666 (63.2%) eyes. The different types of vitreous seed were described in 475 eyes: dust 247 (52.0%), sphere 56 (11.8%) and cloud 172 (36.2%) eyes (figure 1).
Figure 1.
CONSORT flow diagram of all retinoblastoma eyes with vitreous seeding. CONSORT, Consolidated Standards of Reporting Trials; RB, retinoblastoma
Treatment outcomes
Of the 1054 eyes, 430 (40.8%) underwent primary enucleation and 624 (59.2%) had an attempt at eye salvage. Of those, 428 (40.6%) eyes were salvaged and 196 (18.6%) required secondary enucleation (table 2). There was a significant association between the distribution of RB seeds and treatment choice (χ(1, N=1054)=37.400, p<0.001). RB eyes with diffuse seeds were more commonly treated with primary enucleation. The various treatment strategies employed for eye salvage included: systemic chemotherapy: 980 (93%), intra-arterial chemotherapy: 69 (6.5%) and primary EBRT: 5 (0.5%) eyes. Intravitreal chemotherapy was employed in 53 (5.0%) and periocular chemotherapy in 64 (6.0%) eyes.
Table 2.
Treatment outcomes for eyes with retinoblastoma seeds by distribution
| Focal RB seeds, N (%) | Diffuse RB seeds, N (%) | Total, N (%) | |
| Total eyes | 193 (18.3) | 861 (81.7) | 1054 |
| Primary enucleation | 41 (21.2) | 389 (45.2) | 430 (40.8) |
| Attempt at eye salvage | 152 (78.8) | 472 (54.8) | 624 (59.2) |
| Eye salvage | 124 (64.2) | 304 (35.3) | 428 (40.6) |
| Secondary enucleation | 28 (14.5) | 168 (19.5) | 196 (18.6) |
Association between the distribution of seeds and treatment choice: χ(1)=37.400, p<0.001.
RB, retinoblastoma.
Cumulative proportion of avoiding local treatment failure
Focal versus diffuse seed distribution
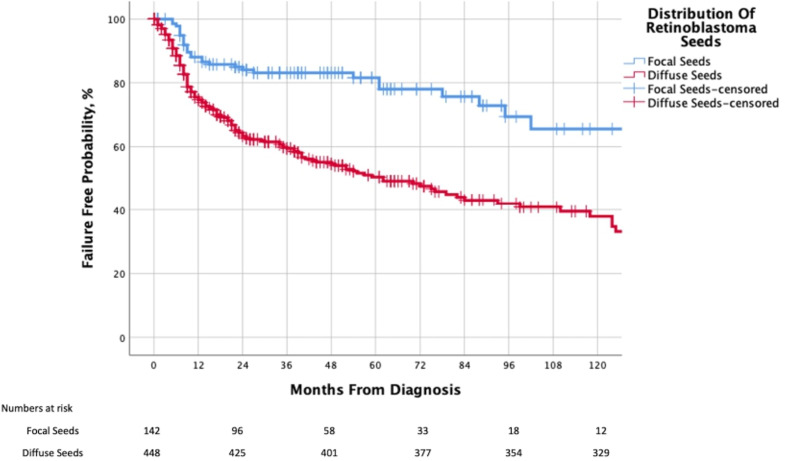
Of the 624 eyes, where eye salvage was attempted, 592 had complete data for globe salvage analysis. The distribution of seeds was focal in 143 (24.2%) and diffuse in 449 (75.8%) eyes. Associated SRF was within 5 mm from the main tumour in 237 (40.0%), and beyond 5 mm in 355 (60.0%) eyes. Though 375 (63.3%) eyes were salvaged, 217 (36.7%) were considered as local treatment failure (required secondary enucleation in 196 and EBRT in 21 eyes). The median follow-up duration was 22.0 months (mean, 36.7; SD, 39.4; IQR, 8–51; range, 1 month to 14.2 years). The 2-year and 5-year Kaplan-Meier cumulative proportions of avoiding local treatment failure by distribution of RB seeds were 83% (95% CI 80% to 86%) and 78% (95% CI 74% to 82%) for focal seeds and 60% (95% CI 57% to 63%) and 49% (95% CI 44% to 52%) for diffuse seeds, respectively. Diffuse seeds were associated with an increased risk of local treatment failure (p<0.001, log-rank test for trend) as compared with focal seeds (table 3A, figure 2).
Table 3.
(A) Kaplan-Meier cumulative proportion of avoiding local treatment failure according to distribution of retinoblastoma seeding, (B) Kaplan-Meier cumulative proportion of avoiding local treatment failure according to type of retinoblastoma vitreous seeding
| Classification | Kaplan-Meier point estimates (95% CI), % | |||
| 1 year | 2 years | 5 years | 10 years | |
| (A) | ||||
| All eyes (n=592) | 70 (68 to 72) | 66 (64 to 68) | 57 (54 to 60) | 25 (20 to 30) |
| Focal (n=143) | 85 (82 to 88) | 83 (80 to 86) | 78 (74 to 82) | 66 (59 to 73) |
| Diffuse (n=449) | 65 (63 to 67) | 60 (57 to 63) | 49 (44 to 52) | 14 (9 to 19) |
| (B) | ||||
| Dust (n=153) | 74 (70 to 78) | 66 (61 to 71) | 47 (39 to 55) | 38 (28 to 48) |
| Sphere (n=37) | 51 (42 to 60) | 47 (38 to 56) | 47 (38 to 56) | 8 (4 to 12) |
| Cloud (n=115) | 69 (64 to 74) | 67 (62 to 72) | 50 (42 to 58) | Not available |
(A) Log-rank test for trend: p<0.001.
(B) Log-rank test for trend, overall comparison: p=0.06, Wilcoxon (Gehan) Statistic: p=0.033, Tarone-Ware: p=0.04
N, Number.
Figure 2.
Kaplan-Meier curve showing the cumulative proportion of salvaged eyes (without need for EBRT), by distribution of retinoblastoma seeding in clinical category cT2b eyes. EBRT, external beam radiation therapy.
Cox proportional hazard regression univariable analysis confirmed that eyes with diffuse seeds (HR 2.8; 95% CI 1.9 to 4.1; p<0.001) had a greater risk relative to those with focal seeds, associated SRF >5 mm (HR 2.2; 95% CI 1.6 to 3.0; p<0.001) had a greater risk relative to those with SRF <5 mm and eyes of patients with unilateral disease (HR 1.9; 95% CI 1.4 to 2.4; p<0.001) had a greater risk of local treatment failure (need for EBRT or secondary enucleation) compared with those with bilateral disease (table 4A).
Table 4.
Cox proportional hazards regression model in retinoblastoma eyes for association of retinoblastoma clinical features with local treatment failure
| Variable | Eyes in category, no (total=592) | Reference | HR (95% CI) | P value |
| (A) Univariate analysis | ||||
| Diffuse seeding | n=449 | Focal seeding | 2.80 (1.89 to 4.14) | <0.001 |
| Unilateral | n=213 | Bilateral | 1.85 (1.40 to 2.42) | <0.001 |
| SRF >5 mm | n=355 | SRF <5 mm | 2.20 (1.64 to 2.95) | <0.001 |
| Sphere | n=37 | Dust | 1.77 (1.05 to 2.97) | 0.031 |
| Cloud | n=115 | Dust | 1.00 (0.64 to 1.55) | >0.99 |
| (B) Multivariate analysis | ||||
| Diffuse | n=449 | Focal | 1.64 (1.20 to 2.26) | 0.002 |
| Unilateral | n=213 | Bilateral | 1.76 (1.34 to 2.31) | <0.001 |
| SRF >5 mm | n=355 | SRF <5 mm | 2.20 (1.45 to 3.35) | <0.001 |
SRF, subretinal fluid.
Vitreous seed type
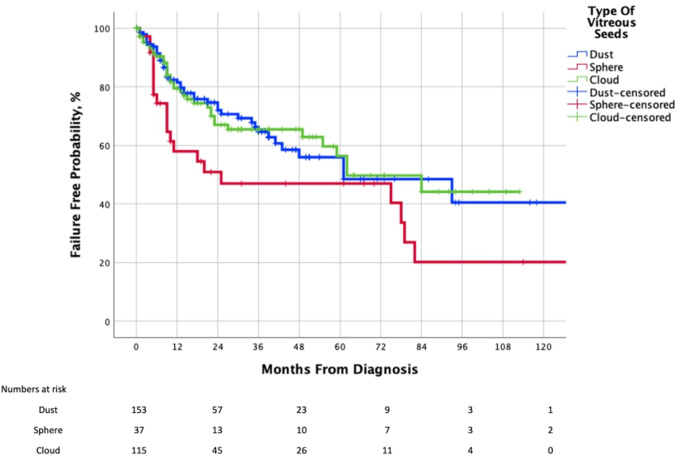
The predominant vitreous seed type was noted in 305 eyes: dust in 153 (50.1%), spheres 37 (12.1%), and cloud in 115 (37.8%). The 2-year and 5-year Kaplan-Meier cumulative proportions of avoiding local treatment failure by type of RB vitreous seeds were 66% (95% CI 61% to 71%) and 47% (95% CI 39% to 55%) for dust, and 47% (95% CI 38% to 56%, for both 2-year and 5-year) for sphere, and 67% (95% CI 62% to 72%) and 50% (95% CI 42% to 58%) for cloud, respectively. The overall p value for this analysis was log rank: p=0.06, Wilcoxon (Gehan) Statistic: p=0.033, Breslow: p=0.03 and Tarone-Ware: p=0.04 (table 3B, figure 3). We did not have enough evidence either to prove or disprove the possibility that there was no statistically significant difference in the risk of local treatment failure between the three groups.
Figure 3.
Kaplan-Meier curve showing the cumulative proportion of salvaged eyes (without need for EBRT), by type of retinoblastoma vitreous seeding in clinical category cT2b eyes. EBRT, external beam radiation therapy.
Cox proportional hazard regression univariable analysis revealed that eyes with spherical vitreous seeds are associated with a higher risk of local treatment failure (HR 1.8; 95% CI 1.1 to 3.0; p=0.031) as compared with dust. However, there was no increased risk of local treatment failure with cloud (p=0.99) compared with dust.
Multivariate analysis
Multivariable Cox regression showed a greater risk of local treatment failure in eyes in diffuse seeds (HR 1.6; 95% CI 1.2 to 2.3; p=0.002) compared with eyes with focal seeds, as were the patients with unilateral disease (HR 1.8; 95% CI 1.3 to 2.3; p<0.001) compared with those with bilateral disease and associated SRF >5 mm (HR 2.2; 95% CI 1.5 to 3.4; p<0.001) compared with those with SRF <5 mm (table 4B).
Discussion
Herein, we present an analysis of a multicentre, international, internet-based registry used to answer the question: Does the distribution and clinical characteristics of RB seeds affect local treatment failure and thus suggest that AJCC cT2b category should be further subdivided? We found that at presentation, diffuse RB seeding was significantly associated with increased risk of local treatment failure (defined as need for EBRT or secondary enucleation). When compared with focal RB-seeding, diffuse seeding was associated with a 2.8-fold risk of eventual local treatment failure. The clinical characteristics of vitreous seed type did not significantly increase the risk of secondary enucleation or need for EBRT. Though unrelated to seed type or distribution, the presence of SRF >5 mm has been a component of other staging systems (eg, WEH, CHLA) related to globe salvage.8 9 Separate analysis found that the presence of SRF >5 mm at presentation also affected local control, having a 2.2-fold risk of local treatment failure compared with SRF <5 mm.
The eighth edition AJCC staging system for RB was derived by the AJCC-OOTF through international consensus after evaluating evidence-based data.10 The AJCC manages a dynamic, ever-evolving staging system where multicentre international committees of subspecialists regularly convene to modify the staging. Our study provides statistically significant evidence that eyes with focal and diffuse seeding carry significantly different risks of local treatment failure. Therefore, a subcategory description should be considered in subsequent editions of the AJCC RB staging.
The eighth edition AJCC staging system for RB for risk of metastatic mortality demonstrated no difference between clinical categories cT2a and cT2b, reaffirming the fact that presence of RB seeding does not impact the overall risk of mortality.13 The main impediment in RB eyes with seeds has been local tumour control, with need for multiple treatments to tackle recurrent and resistant seeds. In addition, our recent national income-based evaluation of RB presentation across the world revealed that cT2b category was one of the most common RB stages at presentation.15 The large size and resultant heterogeneity of this category supports the need for further analysis to determine useful subclassification.
The worse outcomes associated with diffuse seeding have been attributable to their inaccessibility to focal therapy and poor reach of systemic treatments into avascular spaces. These seeds may also be radioresistant due to hypoxia.1 The presence of extensive SRF can complicate the use of intravitreal chemotherapy for vitreous seeds and laser treatment for subretinal seeds. Further, various studies have reviewed the impact of morphology of RB seeds on the efficacy of intravitreal and periocular chemotherapy to induce seed regression.1–3 12 16 17 In contrast, we could not conclusively prove or disprove a significant relationship between the type of vitreous seeds and risk of local treatment failure. This result should be interpreted cautiously since we had limited details of specific treatment modalities, the number of intravitreal injections, the drugs employed or specific focal treatment of subretinal seeds. In addition, some RB eyes may require enucleation for many reasons despite complete regression of seeds. For example, in a recent study, 32% eyes (groups D and E) were treated with secondary enucleation, despite a 100% successful treatment of seeds.12
Limitations of our study include that the data was collected between 2001 and 2013 which does not account for the current use of intra-arterial chemotherapy and intravitreal chemotherapy. In addition, the RB seeding localisation was not separated as vitreous or subretinal, preventing a location-based independent local failure risk assessment. Our retrospective design used locally defined diagnostic and treatment modalities, limiting our ability to analyse specific chemotherapeutic agents. The registry did not collect data on patterns of seed regression or time to regression and hence they could not be analysed in this manuscript.
Strengths of this study include that it used a multicentre, international registry to accrue a large number of patients. There were enough patients to provide statistically significant evidence used to answer an outstanding question on a rare disease. Specifically, it answered a question that may be used to improve the AJCC and UICC staging system for RB.
This study revealed evidence that the distribution of vitreous and subretinal seeding was predictive of risk of need for secondary enucleation or EBRT. Specifically, it revealed that the diffuse seeds carried a higher risk of local treatment failure compared with focal RB seeds. Therefore, we recommend further analysis of the AJCC RB classification to account for distribution of RB seeds.
Footnotes
Twitter: @#paulfingermd, @gchantada, @seeds
Collaborators: American Joint Committee on Cancer Ophthalmic Oncology Task Force members: Paul T. Finger, MD (Chair) (New York Eye Cancer Center, New York); Sarah E. Coupland, MBBS, PhD, FRCPath (Vice Chair) (Royal Liverpool University Hospital, Liverpool, United Kingdom); Daniel M. Albert, MD, MS (Oregon Health and Sciences University, Portland); Anush G. Amiryan, MD, and Svetlana Saakyan, MD (Moscow Helmholtz Research Institute of Eye Disease, Moscow, Russian Federation); Claudia Auw-Hädrich, MD (University of Freiburg, Freiburg, Germany); Diane Baker, CTR (American Joint Committee on Cancer, Chicago, Illinois); Raymond Barnhill, MD, MSc (UCLA Medical Center, Los Angeles); José M. Caminal, MD, PhD (Bellvitge University Hospital, Hospitalet de Llobregat, Barcelona, Spain); William L. Caroll, MD (New York University Cancer Institute–New York University Langone Medical, New York); Nathalie Cassoux, MD, PhD, Laurence G. Desjardins, MD, François Doz, MD, MSc, and Gaelle Pierron, PhD (Institut Curie, Paris, France); Jaume Catalá-Mora, MD (SJD Barcelona Children's Hospital, Hospital Sant Joan de Déu, Barcelona, Spain); Guillermo Chantada, MD (Hospital JP Garrahan, Buenos Aires, Argentina); Patricia Chévez-Barrios, MD (Houston Methodist Hospital, Houston, Texas); R. Max Conway, MD, PhD (Save Sight Institute, Sydney, Australia); Bertil E. Damato, MD, PhD (University of California, San Francisco, San Francisco); Hakan Demirci, MD (Kellogg Eye Center, Ann Arbor, Michigan); Jonathan J. Dutton, MD, PhD (University of North Carolina, Chapel Hill); Bita Esmaeli, MD, Victor G. Prieto, MD, PhD, and Michelle Williams, MD (University of Texas MD Anderson Cancer Center, Houston); Brenda L. Gallie, MD (Hospital for Sick Children, Toronto, Ontario, Canada); Gerardo F. Graue, MD (Instituto de Oftalmología Fundación Conde de Valenciana, Mexico City, Mexico); Hans E. Grossniklaus, MD (Emory Eye Center, Atlanta, Georgia); Steffen Heegaard, MD, PhD (University of Copenhagen, Glostrup Hospital, Copenhagen, Denmark); Leonard M. Holbach, MD (University Erlangen, Nürnberg, Germany); Santosh G. Honavar, MD (Centre For Sight Super-Specialty Eye Hospital, Hyderabad, India); Martine J. Jager, MD, PhD (Leiden University Medical Center, Leiden, the Netherlands); Tero Kivelä, MD, FEBO, and Emma Kujala, MD (Helsinki University Hospital, Helsinki, Finland); Livia Lumbroso-Le Rouic, MD (Institute Claudius Regaud Medical Center, Toulouse, France); Ashwin C. Mallipatna, MBBS, MS, DNB (Hospital for Sick Children, Toronto, Canada); Giulio M. Modorati, MD (San Raffaele Hospital, Milan, Italy); Francis L. Munier, MD (Jules-Gonin Eye Hospital, Lausanne, Switzerland); Timothy G. Murray, MD, MBA (Murray Ocular Oncology and Retina, Coral Gables, Florida); Anna C. Pavlick, MD (Weill Cornell College of Medicine, New York); Jacob Pe’er, MD (Hebrew University, Hadassah Ein Karem Hospital, Jerusalem, Israel); David E. Pelayes, MD (Buenos Aires University, Buenos Aires, Argentina); Manuel Jorge Rodriguez, MD (Mayo Clinic, Jacksonville, Florida); Wolfgang A.G. Sauerwein, MD, PhD (University Hospital Essen, Essen, Germany); Ekaterina Semenova, MD (The New York Eye and Ear Infirmary of Mount Sinai, New York); Stefan Seregard, MD (St Erik’s Eye Hospital, Karolinska Institute, Stockholm, Sweden); Carol Shields, MD (Wills Eye Institute, Philadelphia, Pennsylvania); E. Rand Simpson, MD, FRCS(C) (Mount Sinai Hospital, Toronto, Ontario, Canada); Arun D. Singh, MD (Cole Eye Institute, Cleveland Clinic, Cleveland, Ohio); Shigenobu Suzuki, MD, PhD (National Cancer Center Hospital, Tokyo, Japan); Mary Kay Washington, MD, PhD (Vanderbilt University Medical Center, Nashville, Tennessee); Valerie A. White, MD, MHSc, FRCPC (Vancouver Coastal Health Research Institute, Vancouver, British Columbia, Canada); Mathew W. Wilson, MD (University of Tennessee Health Science Center, Memphis); Christian W. Wittekind, MD (Institut fur Pathologie der Universität, Leipzig, Germany); and Vivian Yin, MPH (Memorial Sloan Kettering Cancer Center, New York, New York).
Contributors: AST: Study conception and design, analysis and interpretation of data, and drafting of manuscript. PTF: Study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, and critical revision. BG: Study conception and design, acquisition of data, analysis and interpretation of data and critical revision. TK: Study conception and design, acquisition of data, analysis and interpretation of data and critical revision. AM: Study conception and design, acquisition of data, analysis and interpretation of data and critical revision. CZ: Study conception and design, acquisition of data and critical revision. JZ: Study conception and design, acquisition of data and critical revision. MW: Study conception and design, acquisition of data and critical revision. RB: Study conception and design, acquisition of data and critical revision. MB: Study conception and design, acquisition of data, and critical revision. JWK: Study conception and design, acquisition of data, and critical revision. JLB: Study conception and design, acquisition of data, and critical revision. RJ: Study conception and design, acquisition of data, and critical revision. VK: Study conception and design, acquisition of data, and critical revision. GS: Study conception and design, acquisition of data, and critical revision. AY: Study conception and design, acquisition of data, and critical revision. VY: Study conception and design, acquisition of data, and critical revision. EK: Study conception and design, acquisition of data, and critical revision. DV: Study conception and design, acquisition of data, and critical revision. AY: Study conception and design, acquisition of data, and critical revision. KPN: Study conception and design, acquisition of data, and critical revision. TLU: Study conception and design, acquisition of data and critical revision. OVY: Study conception and design, acquisition of data and critical revision. VGP: Study conception and design, acquisition of data and critical revision. MAR-O: Study conception and design, acquisition of data, and critical revision. EE-A: Study conception and design, acquisition of data, and critical revision. GLC: Study conception and design, acquisition of data and critical revision. PS: Study conception and design, acquisition of data and critical revision. ACF: Study conception and design, acquisition of data and critical revision. JCY: Study conception and design, acquisition of data and critical revision. WWL: Study conception and design, acquisition of data and critical revision. CPL: Study conception and design, acquisition of data and critical revision. PS: Study conception and design, acquisition of data, and critical revision. SM: Study conception and design, acquisition of data, and critical revision. QBL: Study conception and design, acquisition of data and critical revision. VE: Study conception and design, acquisition of data and critical revision. LAR: Study conception and design, acquisition of data and critical revision. ES: Study conception and design, acquisition of data and critical revision. JC-M: Study conception and design, acquisition of data, and critical revision. MCL: Study conception and design, acquisition of data, and critical revision. EC-B: Study conception and design, acquisition of data and critical revision.
Funding: The Myrna and John Daniels Charitable Trust, the Paul Finger Fund at Princess Margaret Cancer Centre and The Eye Cancer Foundation provided monetary support to the Princess Margaret Cancer Centre’s Internet Technology Programme, which has (in turn) participated in the design, construction and maintenance of this retinoblastoma registry. AST received an ophthalmic oncology fellowship grant to study with PTF (from The Eye Cancer Foundation). TK reported receiving a governmental grant from the Helsinki University Hospital Research Fund. These grants were unrestricted and non-numbered.
Disclaimer: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
The American Joint Committee on Cancer Ophthalmic Oncology Task:
Sarah E Coupland, Daniel M Albert, Anush G Amiryan, Svetlana Saakyan, Claudia Auw-Hädrich, Diane Baker, Raymond Barnhill, José M Caminal, William L Caroll, Nathalie Cassoux, Laurence G Desjardins, François Doz, Gaelle Pierron, Patricia Chévez-Barrios, R Max Conway, Bertil E Damato, Hakan Demirci, Jonathan J Dutton, Bita Esmaeli, Victor G Prieto, Michelle Williams, Brenda L Gallie, Gerardo F Graue, Hans E Grossniklaus, Steffen Heegaard, Leonard M Holbach, Santosh G Honavar, Martine J Jager, Emma Kujala, Livia Lumbroso-Le Rouic, Giulio M Modorati, Francis L Munier, Timothy G Murray, Anna C Pavlick, Jacob Pe’er, David E Pelayes, Manuel Jorge Rodriguez, Wolfgang AG Sauerwein, Stefan Seregard, Carol Shields, E Rand Simpson, Arun D Singh, Shigenobu Suzuki, Mary Kay Washington, Valerie A White, Mathew W Wilson, Christian W Wittekind, and Vivian Yin
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Internal institutional review board approvals were obtained by each centre.
References
- 1. Munier FL. Classification and management of seeds in retinoblastoma. Ellsworth lecture Ghent August 24th 2013. Ophthalmic Genet 2014;35:193–207. 10.3109/13816810.2014.973045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amram AL, Rico G, Kim JW, et al. Vitreous seeds in retinoblastoma: clinicopathologic classification and correlation. Ophthalmology 2017;124:1540–7. 10.1016/j.ophtha.2017.04.015 [DOI] [PubMed] [Google Scholar]
- 3. Yousef YA, Noureldin AM, Sultan I, et al. Intravitreal melphalan chemotherapy for vitreous seeds in retinoblastoma. J Ophthalmol 2020;2020:8628525 10.1155/2020/8628525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kiratli H, Koç I, Öztürk E, et al. Comparison of intravitreal melphalan with and without topotecan in the management of vitreous disease in retinoblastoma. Jpn J Ophthalmol 2020;64:351–8. 10.1007/s10384-020-00743-2 [DOI] [PubMed] [Google Scholar]
- 5. Manjandavida FP, Honavar SG, Reddy VAP, et al. Management and outcome of retinoblastoma with vitreous seeds. Ophthalmology 2014;121:517–24. 10.1016/j.ophtha.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 6. Francis JH, Marr BP, Abramson DH. Classification of vitreous seeds in retinoblastoma: correlations with patient, tumor, and treatment characteristics. Ophthalmology 2016;123:1601–5. 10.1016/j.ophtha.2016.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reese AB, Ellsworth RM. The evaluation and current concept of retinoblastoma therapy. Trans Am Acad Ophthalmol Otolaryngol 1963;67:164–72. [PubMed] [Google Scholar]
- 8. Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am 2005;18:41–53. viii. 10.1016/j.ohc.2004.11.003 [DOI] [PubMed] [Google Scholar]
- 9. Shields CL, Mashayekhi A, Au AK, et al. The International classification of retinoblastoma predicts chemoreduction success. Ophthalmology 2006;113:2276–80. 10.1016/j.ophtha.2006.06.018 [DOI] [PubMed] [Google Scholar]
- 10. Mallipatna A, Gallie BL, Chévez-Barrios P. Retinoblastoma. In: Amin MB, Edge SB, Greene FL, eds. AJCC cancer staging manual. 8th ed. New York, NY: Springer, 2017: 819–31. [Google Scholar]
- 11. Sthapit PR, Rao R, Honavar SG. Periocular topotecan for vitreous seeds in retinoblastoma. Indian J Ophthalmol 2018;66:1833–8. 10.4103/ijo.IJO_737_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berry JL, Bechtold M, Shah S, et al. Not All Seeds Are Created Equal: Seed Classification Is Predictive of Outcomes in Retinoblastoma. Ophthalmology 2017;124:1817–25. 10.1016/j.ophtha.2017.05.034 [DOI] [PubMed] [Google Scholar]
- 13. Tomar AS, Finger PT, Gallie B. A multicenter, international collaborative study for AJCC-Staging of retinoblastoma: metastasis-associated mortality. Ophthalmology 2020. 10.1016/j.ophtha.2020.05.050 [DOI] [PubMed] [Google Scholar]
- 14. Tomar AS, Finger P, Gallie BL. A multicenter, international collaborative study for AJCC-Staging of retinoblastoma: treatment success and globe salvage. Ophthalmology 2020. 10.1016/j.ophtha.2020.05.051 [DOI] [PubMed] [Google Scholar]
- 15. Tomar AS, Finger PT, Gallie B, et al. Global retinoblastoma treatment outcomes: association with national income level. Ophthalmology 2021;128:740-753. 10.1016/j.ophtha.2020.09.032 [DOI] [PubMed] [Google Scholar]
- 16. Kivelä T, Eskelin S, Paloheimo M. Intravitreal methotrexate for retinoblastoma. Ophthalmology 2011;118:1689–1689.e6. 10.1016/j.ophtha.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 17. Schaiquevich P, Buitrago E, Taich P, et al. Pharmacokinetic analysis of melphalan after superselective ophthalmic artery infusion in preclinical models and retinoblastoma patients. Invest Ophthalmol Vis Sci 2012;53:4205–12. 10.1167/iovs.12-9501 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.