Abstract
The latency between acquisition of an initiating somatic driver mutation by a single-cell and clinical presentation with cancer is largely unknown. We describe a remarkable case of monozygotic twins presenting with CALR mutation-positive myeloproliferative neoplasms (MPNs) (aged 37 and 38 years), with a clinical phenotype of primary myelofibrosis. The CALR mutation was absent in T cells and dermal fibroblasts, confirming somatic acquisition. Whole-genome sequencing lineage tracing revealed a common clonal origin of the CALR-mutant MPN clone, which occurred in utero followed by twin-to-twin transplacental transmission and subsequent similar disease latency. Index sorting and single-colony genotyping revealed phenotypic hematopoietic stem cells (HSCs) as the likely MPN-propagating cell. Furthermore, neonatal blood spot analysis confirmed in utero origin of the JAK2V617F mutation in a patient presenting with polycythemia vera (aged 34 years). These findings provide a unique window into the prolonged evolutionary dynamics of MPNs and fitness advantage exerted by MPN-associated driver mutations in HSCs.
Subject terms: Myeloproliferative disease, Myeloproliferative disease, Translational research, Cancer genomics
Lineage tracing analyses of cells from two monozygotic twins presenting with myelofibrosis in adulthood provide evidence of in utero transplacental transmission of the tumorigenic clone.
Main
BCR-ABL1− MPNs are a heterogeneous group of myeloid neoplasms characterized by the excessive production of mature myeloid cells, typically driven by somatic mutations affecting the MPL/JAK/STAT signaling pathway1. Primary myelofibrosis (PMF) is a subtype of MPN associated with bone marrow (BM) fibrosis, disease-associated symptoms, splenomegaly and abnormal blood counts. MPN-associated mutations are thought to originate in a single hematopoietic stem/progenitor cell (HSPC), driving a subsequent clonal expansion, eventually culminating in overt MPN after an unknown period of time. Emerging evidence from whole-genome sequencing (WGS) studies suggest that the latency between acquisition of an initiating driver mutation and disease presentation can be prolonged, even occurring over decades, with considerable tumor-to-tumor heterogeneity2–4. Studies of the increased risk of myeloid neoplasms (including MPNs) following radiation exposure raise the possibility of a short disease latency, suggesting that the peak risk occurs as early as 2–3 years following radiation exposure and falls sharply thereafter5. However, MPN-associated driver mutations are much more prevalent in individuals with normal blood counts than in disease6, so-called clonal hematopoiesis7, suggesting that the fitness advantage imposed by an MPN-driver mutation and disease latency might vary considerably from person to person.
Studies of the shared clonal origin of childhood leukemia in monochorionic twins have provided unique insights into the developmental timing, evolutionary trajectories and disease propagating cells in these diseases8. However, such cases were hitherto considered to be unique to blood cancers of childhood. We describe a remarkable case of monozygotic twins presenting with CALR mutation-positive9,10 PMF, which originated in utero and presented with overt disease after an almost identical and prolonged latency of over 35 years in both twins.
Results
Clinical findings
Twin A presented at the age of 37 years with symptomatic splenomegaly, measuring 21 cm on computed tomography imaging. Blood parameters were hemoglobin 138 g per liter, white cell count 5.9 ×109 per liter and platelets 183 ×109 per liter. Blood film examination revealed a leukoerythroblastic picture (Extended Data Fig. 1a,b). The BM showed typical features of PMF with distorted intertrabecular spaces, prominent dilated sinuses and evidence of heavily disrupted hematopoiesis with moderate numbers of highly atypical, small to medium-sized megakaryocytes forming small clusters, and World Health Organization MF-3 fibrosis (Extended Data Fig. 1c,d). Genetic testing revealed presence of a type 1 CALR mutation (NM_004343.4[CALR]:c.1092_1143del52 p.L367fs*46), a highly recurrent mutation in MPN9,10. Targeted next-generation sequencing revealed the presence of a frameshift TET2 mutation (NM_001127208.2[TET2]:c.509del p.N170Tfs*13). The overall clinical presentation, genetic and morphological features were in keeping with a diagnosis of PMF. Due to disease-associated symptoms, twin A commenced treatment with the JAK1/2 inhibitor ruxolitinib with good clinical response. He remains on a stable dose of ruxolitinib (10 mg twice per day), with no disease-associated symptoms and reduction in spleen size to 17.6 cm on the most recent imaging. Blood parameters at the time of sampling for our studies were hemoglobin 138 g per liter, white cell count 7.3 × 109 per liter and platelets 62 ×109 per liter.
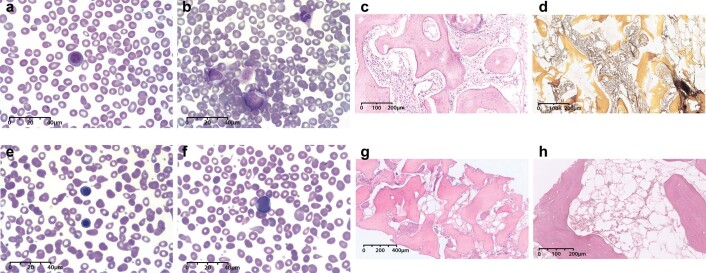
Extended Data Fig. 1. Blood film and bone marrow examination findings.
a,b, Leucoerythroblastic blood film of twin A (May-Grunwald-Giemsa stain; 100x). c, Bone marrow trephine examination from twin A at diagnosis (hematoxylin and eosin stain). Reticulin stain (d) showed diffuse fiber network with scattered coarse fibers (WHO MF-3) (reticulin stain). e,f, Leucoerythroblastic blood film of twin B (May-Grunwald-Giemsa stain; 100x). g, Bone marrow trephine examination from twin B (hematoxylin and eosin stain). Reticulin stain (h) revealed evidence of patchy, irregular fibrosis and a hypocellular marrow (reticulin stain).
Twin A’s identical twin brother, twin B, presented 1 year later at the age of 38 years with marked splenomegaly, measuring 20 cm below the costal margin, and associated early satiety. Blood parameters were normal apart from borderline leukopenia (white cell count, 3.88 × 109 per liter). Blood film examination revealed the presence of a leukoerythroblastic picture (Extended Data Fig. 1e,f). BM biopsy showed a hypocellular marrow with markedly abnormal bony trabeculae with irregular outlines and areas of notable thickening, alongside areas of increased reticulin fibers (Extended Data Fig. 1g,h). Genetic testing revealed presence of the same 52-bp deletion type 1 CALR mutation as seen in twin A. Targeted next-generation sequencing revealed no additional mutations in twin B. Taking clinical, genetic and morphological features into account, a diagnosis of PMF was made. He remains well, with no remarkable disease-associated symptoms, and to date, he has required no treatment.
There was no history of transfusion for either twin or other family history of MPN or other blood cancer. Information regarding whether the twins shared a monochorionic placenta was not available. The twins lived in the same household until the age of 25 years. Both are nonsmokers who do not drink alcohol, and they both have a normal body mass index and no autoimmune or other chronic medical conditions apart from MPN. They report no known exposure to ionizing radiation or occupational and chemical exposure.
In utero origin of MPN
As the concordant disease in both twins might have occurred due to germline CALR mutation, we carried out mutational analysis of CALR in purified T cells (Extended Data Fig. 2) and myeloid cells for each twin and skin fibroblasts from twin B (Fig. 1a) by fragment analysis. The CALR mutation was present in myeloid cells, but only at a very low level in T cells from both twins. The CALR mutation was not detected in skin fibroblasts from twin B. These findings confirm that the CALR mutation was not present in the germline and was somatically acquired.
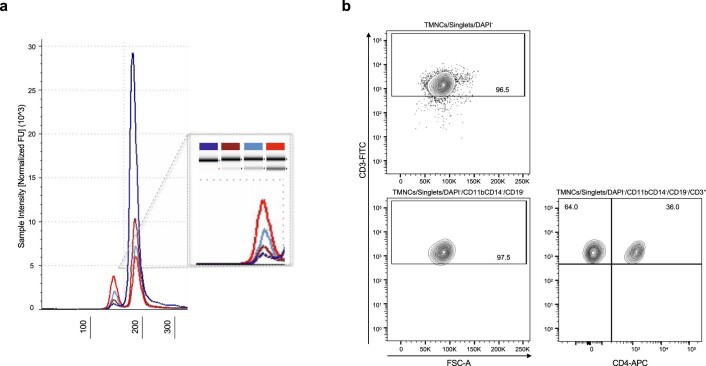
Extended Data Fig. 2. Cell-lineage tracing of the CALR mutation.
a, Comparison of different sources of germline DNA for twin A. Automated electrophoresis for double-filtration CD3-enriched cell DNA is shown in dark blue, nail DNA in brown, single-filtration CD3-enriched cell DNA in light blue, while CD34-enriched cell DNA is shown in red. CALRdel52bp VAF was calculated 1.8%, 9.0%, 10.8%, and 37.1%, respectively. b, T cell purity assessment of the double-filtration CD3-enriched population by flow cytometry.
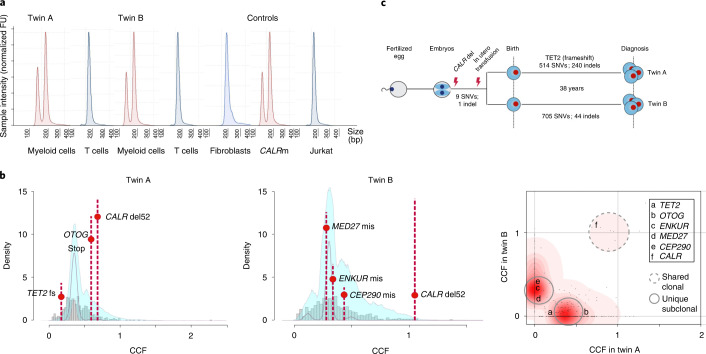
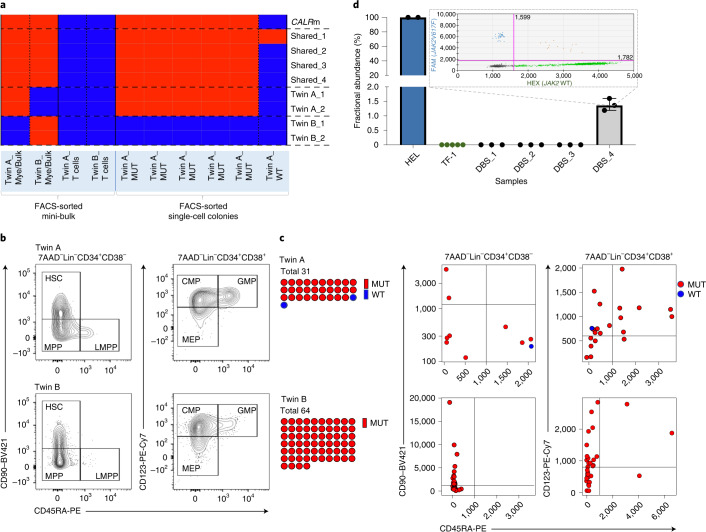
Fig. 1. Genetic lineage tracing confirms a common in utero clonal origin of CALR mutation.
a, Cell-lineage-specific CALR mutational analysis. T cell DNA electropherograms (blue) have a single peak at 207 bp, whereas DNA extracted from myeloid cells (red) shows two peaks, one at 207 bp and another one corresponding to the 52-bp deletion fragment (CALRdel52bp variant allele frequency (VAF) was calculated 28.4%, 32.6% and 43.7 for twin A, twin B and CALR type 1 myelofibrosis (CALRm) control, respectively). The absence of CALR 52-bp deletion in germline DNA was confirmed by analysis of dermal fibroblast DNA from twin B. DNA from a patient with known CALR type 1 myelofibrosis and DNA from Jurkat cells were used as positive and negative controls, respectively. Rearranged electropherograms from parallel experiments, all scaled to sample. FU, fluorescence units. b, Statistical modeling of the distribution of subclonal and clonal mutations by Dirichlet-process clustering. The histogram of mutations is represented with gray bars, with the fitted distribution as a gray line; 95% posterior confidence intervals for the fitted distribution are also shown (pale blue area). In the rightmost panel, a two-dimensional density plot shows Dirichlet clustering of the fraction of cancer cells (CCF) within each twin for all somatic mutations detected (black dots). Higher posterior probability of a cluster is indicated by increasing intensity of red. The cluster indicated around (1,1) corresponds to mutations present in all cells in both twins; clusters along the axes correspond to twin-specific clones. A subset of nonsilent coding mutations found in TET2, OTOG, ENKUR, MED27, CEP290 and CALR are highlighted. c, Schematic representation of the shared in utero clonal origin and number of high-confidence shared variants and separate postnatal clonal evolution with total number of SNV and indels for each twin shown. fs, frameshift; mis, missense.
We next reasoned that the somatic CALR mutation could either have been independently acquired by both twins or that the MPN was transmitted by intraplacental twin-to-twin transfer. To examine these two possibilities, we carried out lineage tracing of the MPN clone by WGS of peripheral blood granulocytes (derived from the MPN clone) and T cells (germline control) from both twins. If the MPN cells in both twins had a shared clonal origin, then we would expect to find a number of shared somatic variants in the granulocytes of both twins. We identified 514 and 705 somatic single-nucleotide variants (SNVs), 240 and 44 somatic indels and 5 structural variants (SVs) in twin A and twin B, respectively (Fig. 1b,c, Extended Data Fig. 3 and Supplementary Tables 1–3)11–16. The mean genome-wide substitution rate was 0.24 mutations per megabase, which represents a low mutational load, in line with the silent mutational landscape of MPNs17. In addition to CALR p.L367fs*46, which was the only shared mutation affecting a coding region, nine high-confidence SNVs were also detected in both twins, strongly supporting the in utero origin of the MPN (Fig. 1b,c and Supplementary Table 3).
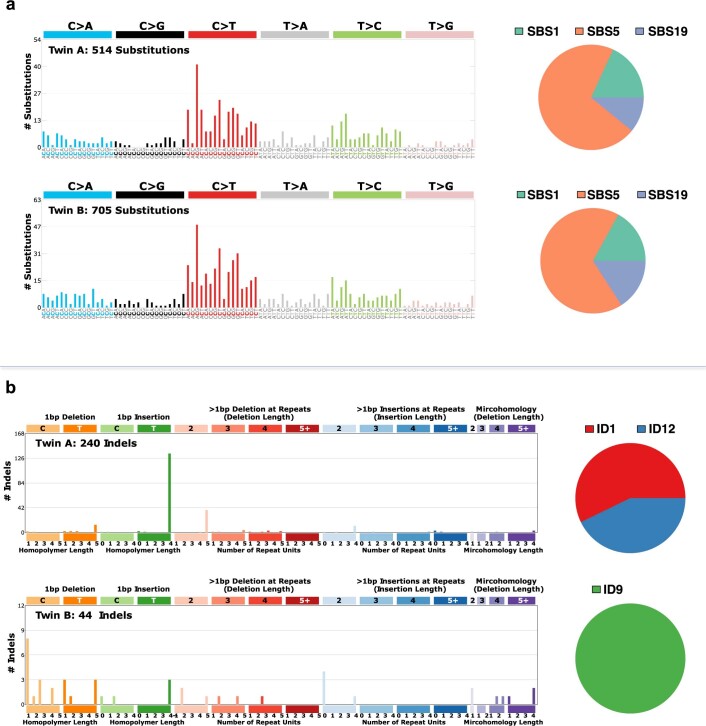
Extended Data Fig. 3. (a) Somatic point substitution and (b) indel spectra with corresponding mutational signatures for each of the twins.
We identified 514 and 705 somatic single-nucleotide variants (SNVs), 240 and 44 somatic indels, and 5 structural variants unique to twin A and twin B, respectively. Analysis of the somatic point substitution signatures revealed shared signatures with near identical contributions in twin A and B. Indel signatures differed markedly between the twins; ID1 and ID12 accounted for respectively 57% and 43% of indels in twin A, while all indels in twin B were attributable to ID9.
No significant difference in overall somatic mutation burden was observed between the twins, although SNV and indel burdens differed when considered separately. Nonsilent somatic coding mutations exclusive to twin A included a frameshift indel not previously reported in TET2, an epigenetic regulator frequently mutated in hematological cancers, and a stop codon in OTOG associated with nonsyndromic hearing loss18, whereas twin B carried missense mutations in MED27, ENKUR and CEP290 (Fig. 1b and Supplementary Table 4)19. The ENKUR variant is reported as a driver in the COSMIC (Catalogue of Somatic Mutations in Cancer) database20, but it is not associated with hematological cancer. Analysis of the mutational signatures by nonnegative matrix factorization21 revealed the presence of signatures 1, 5 and 19, all previously reported in MPNs, with near-identical contributions in twin A and twin B (Extended Data Fig. 3a)3. However, indel signatures differed markedly between the twins; ID1 and ID12 accounted for respectively 57% and 43% of indels in twin A, whereas all indels in twin B were attributable to ID9 (Extended Data Fig. 3b)20. This finding supports that the mutational processes were distinct, even though disease latency was almost identical in both twins. To assess clonal architecture, we clustered mutations based on cancer cell fraction (CCF), determined by adjusting the variant allele frequencies of SNVs for copy-number status and sample purity estimates (Fig. 1b and Extended Data Fig. 4)22. A number of studies have used mutational processes as a molecular clock to carry out chronological time estimates of disease latency in cancer3,23. The current study provided a unique opportunity to orthogonally validate such an approach for a blood cancer with a relatively silent mutational landscape such as MPN. We derived an estimate for the time to the most recent common ancestor of MPN cells in both twins (that is, the time of twin-to-twin transmission of the CALR-mutant clone) from WGS-identified SNV data arising from a clock-like mutational process. Time to the most recent common ancestor was consistent with an in utero origin of MPN (Extended Data Fig. 5 and Supplementary Table 5).
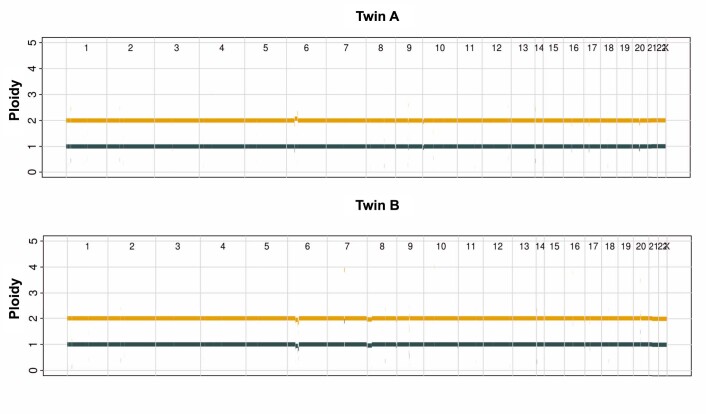
Extended Data Fig. 4. Genome-wide copy number profiles of twin A and twin B.
Copy number profiles of twin A and twin B estimated using the Battenberg algorithm. The gold line corresponds to total copy number, the dark blue line corresponds to copy number of the minor allele. Average ploidy was estimated to be 1.98 and 2.03 for twin A and twin B, respectively. Aberrant cell fraction (cellularity) was estimated as 100% and 92% for twin A and twin B, respectively.
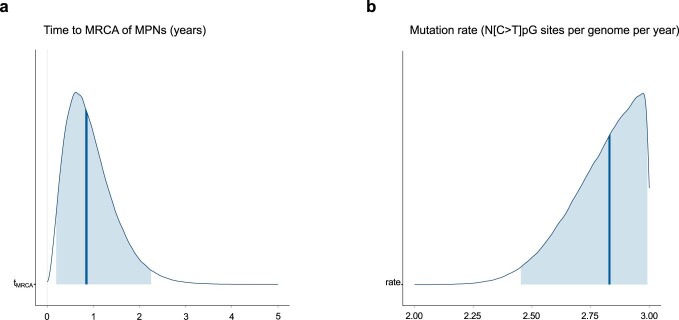
Extended Data Fig. 5. Posterior interval estimates from Markov chain Monte Carlo draws for (a) time to the most recent common ancestor (MRCA) of myeloproliferative neoplasms in twin A and twin B and (b) mutation rate.
Posterior distributions with median (thick dark blue line) and 95% confidence interval (shaded blue area) for (a) MRCA timing and (b) mutation rate estimated using the numbers of somatic cytosine-to-thymine single-nucleotide variants (SNVs) at CpG sites (N[C>T]pG) in each twin. a. Time to MPN origin is estimated as years post-zygote (horizontal axis). b. Mutation rate is estimated as number of N[C>T]pG sites per genome per year (horizontal axis). N[C>T]pG mutation rate is truncated at an upper bound of 3 N[C>T]pG per genome per year; this bound was based on the highest N[C>T]pG rate reported in any Myeloid-MPN case in Gerstung et al.3.
Phenotypic and genetic analysis of HSPCs
Studies in monozygotic twins have provided unique insights into the cancer-propagating cells in childhood leukemia8. Some evidence suggests that the cell of origin in MPNs is the phenotypic HSC1. Twin-to-twin transfusion of the MPN clone provided an opportunity to study the cell-of-origin in MPN. If the CALR mutation originally occurred in an HSC, then the CALR mutation should be present in HSCs from both twins. We performed index sorting of single Lin−CD34+ HSPCs into individual wells for colony-forming assay, recording the surface phenotype for CD90, CD45RA and CD123. Resulting colonies were picked, and DNA was whole-genome amplified. We first confirmed that a number of the shared somatic mutations present in both twins were also present in the colony-derived material for twin A and bulk material for both twins. As expected, for the four shared mutations analyzed, they were all present in both twins, whereas somatic mutations exclusive to each twin were only detected in twin A and twin B, respectively (Fig. 2a and Supplementary Table 6). The CALR mutation was present in all colonies from twin A with shared mutations. One wild-type CALR colony from twin A showed presence of one of the shared mutations, supporting that this mutation occurred before the CALR mutation.
Fig. 2. Single-cell-derived colony CALR genotyping and neonatal blood spot analysis.
a, Orthogonal validation of the WGS results at the single-cell level. Somatic variants shared between the twins and somatic variants unique for twin A or twin B were assessed in DNA from fluorescence-activated cell sorting (FACS)-sorted single-HSPC colonies (from twin A) or mini-bulk populations using Sanger sequencing. The samples with presence of the studied variant are shown in red, whereas absence of the variant is shown in blue. Details of the tested variants are shown in Supplementary Table 6. b, Flow cytometry profiles of HSPCs for each of the twins. c, CALR genotyping of single-HSPC-derived colonies, integrated with index sorting data. d, Results of ddPCR analysis for the detection and quantification of JAK2V617F in DNA extracted from neonatal dried blood spots from patients diagnosed with JAK2-mutant myeloproliferative neoplasms as adults. In one out of three patients studied (DBS_4) JAK2V617F was detected in neonatal blood with a fractional abundance of 1.38% (three technical replicates in one experiment, independently validated by nested PCR). FAM channel-positive events on the y axis correspond to JAK2V617F positivity, and HEX channel-positive events on the x axis correspond to JAK2 WT events. JAK2V617F-positive DNA from HEL cells, and JAK2 wild-type DNA from TF-1 cells and a dried blood spot from a patient with systemic mastocytosis (DBS_1), were used as positive and negative controls, respectively. All results were independently validated by a nested PCR method. Bars and error bars show median and 95% confidence interval, respectively. Relevant clinical information for the patients studied is provided in Supplementary Table 7. CMP, common myeloid progenitor (Lin−CD34+CD38+CD123+CD45RA−); GMP, granulocyte macrophage progenitor (Lin−CD34+CD38+CD123+CD45RA+); HSC, Lin−CD34+CD38−CD90+CD45RA−; LMPP, lymphoid-primed multipotent progenitor (Lin−CD34+CD38−CD90−CD45RA+); MEP, megakaryocyte-erythroid progenitor (Lin−CD34+CD38+CD123−CD45RA−); MPP, multipotent progenitor (Lin−CD34+CD38−CD90−CD45RA−); MUT, mutated; Mye, myeloid; PE-Cy7, PE-cyanine7; WT, wild-type.
Analysis of 95 colonies demonstrated high clonal dominance of CALR-mutant cells, with 29 of 31 colonies in twin A and 64 of 64 in twin B showing presence of the CALR mutation. The two CALR mutation-negative colonies in twin A likely represent residual nonclonal hematopoiesis, as they were negative for additional shared variants between the twins. The index sorting data (Fig. 2b) allowed colonies to be traced back to canonical HSPC hierarchies, revealing presence of CALR mutation-positive phenotypic HSCs (Lin−CD34+CD90+CD45RA−) in both twins (Fig. 2c), supporting that the HSC is the propagating cell for the MPN clone.
Neonatal blood spot analysis in JAK2V617F-mutant MPN
To determine whether JAK2 mutation-positive MPN might also originate in utero yet present clinically decades later, we carried out JAK2V617F mutation analysis of neonatal blood spots stored from patients subsequently diagnosed with MPN as adults. We were able to identify three JAK2V617F mutation-positive MPN patients whose Guthrie cards were available (Supplementary Table 7). JAK2V617F mutation detection and quantification was performed by droplet digital polymerase chain reaction (ddPCR). The analysis revealed presence of the JAK2V617F mutation in the neonatal blood spot of one of the three patients studied, with a variant allele frequency of 1.38% (Fig. 2d). This patient presented with JAK2V617F mutation-positive polycythemia vera at age 34 years. These findings support that an in utero origin of the MPN clone, presenting after a prolonged disease latency, may be a widespread phenomenon in MPN, warranting further studies.
Discussion
Emerging evidence from WGS studies of solid tumors supports that the latency between acquisition of an initiating driver mutation and presentation with overt cancer can be prolonged, with important implications for early diagnosis and intervention2,3. However, these studies are based on multiple-site biopsies of tumors2 or analysis of tumors with high genetic heterogeneity and require certain assumptions to be made about linearity of mutational processes in tumors2,3. Transplacental transmission of hematological neoplasms between twins has provided a unique opportunity to study disease latency and genetic evolution of blood cancers but was hitherto considered to be a phenomenon occurring exclusively during childhood, including pediatric MPN24,25. We describe transplacental transmission of a CALR-mutant clone between a pair of monozygotic twins, which resulted in the development of overt myelofibrosis after a period of almost four decades. This remarkable case provides definitive evidence of a very early developmental origin of CALR-mutant blood cancer, presenting in adults as overt disease decades later. Single-colony-derived WGS data support that a prolonged disease latency might also be a widespread phenomenon in JAK2V617F-mutated MPNs4,26 and that JAK2 mutations might even occur in utero in some patients26. We also carried out neonatal blood spot analysis, which confirmed the in utero origin of JAK2V617F mutation in a patient presenting with polycythemia vera at an age of 34 years. Our findings definitively confirm long disease latency and in utero origin can occur for both JAK2 and CALR mutation-driven MPN. Furthermore, the unique ability to follow the same clone in the two siblings revealed a strikingly similar and prolonged disease latency for CALR-mutation-positive MPN. This finding is compatible with the fitness advantage exerted by the clone being established early and not being contingent upon subsequent clonal evolution. However, it also remains possible that the TET2 or other putative driver mutations identified in the twins collectively resulted in the same latency in the two twins. Moreover, the similar disease latency in both twins suggests that its primary determinants are cell-intrinsic rather than extrinsic factors, although environmental exposures in the twins would be largely shared in childhood. Notably, the similar disease latency might reflect the identical germline genetics, which may influence HSC response to MPN-associated mutations27. Our study also provided additional evidence that the phenotypic HSC is the key propagating population for MPN1. Of note, overexpression of fetal-associated genes in HSPCs has been demonstrated to promote the development of MPN in model systems, suggesting that fetal hematopoiesis might be permissive for the acquisition of MPN-associated mutations28.
A Danish cohort study previously reported concordance for MPN in 15% of monozygotic twins;29 although the authors concluded that this might reflect genetic predisposition to MPN, it may be that these cases were due to an in utero origin and transplacental transmission, as concordant cases were not observed in dizygotic twins. Furthermore, concordance of clonal hematopoiesis with identical somatic mutations in elderly twins has also been reported, suggesting that clones that arise in utero might even persist lifelong until old age without causing disease30,31. Whether an in utero versus postnatal acquisition of an MPN-driver mutation might influence the subsequent phenotype is unknown. Larger studies of blood cancers in monozygotic twins and of neonatal blood spots in sporadic MPNs are warranted, as this will provide a unique window into the evolution of MPNs and fitness advantage exerted by MPN-associated driver mutations. The long disease latency in combination with a better understanding of the evolutionary dynamics in MPN and availability of robust polygenic risk scores27 might open up opportunities for early intervention. Although currently the only curative treatment for MPN remains BM transplantation, there is accumulating evidence that certain treatments such as interferon or targeted inhibitors can induce molecular remissions, and it is also possible that the fetal origin in some cases of MPN might also present unanticipated therapeutic vulnerabilities, paving the way for disease-modifying early intervention strategies in MPN32.
Methods
Cell isolation
Granulocyte enrichment and isolation was carried out by Ficoll density gradient centrifugation (Ficoll-Paque PLUS; GE Healthcare Bio-Sciences AB), and subsequent red blood cell lysis of the pellet, using the QIAGEN RBC Lysis Solution (QIAGEN). Granulocytes were also sorted in mini-bulk by fluorescence-activated cell sorting (FACS) and whole-genome amplified, as described below. The antibody panel that was used is shown in Supplementary Table 8 and granulocytes were defined as 7AAD−CD3−CD71−CD19−CD11bCD14+CD33+. HSPC and T cell enrichment was done with magnetic-activated cell sorting of total mononuclear cells, using the CD34 MicroBead Kit UltraPure, human and CD3 MicroBeads (double-columnenrichment to increase purity of T cells) human kits, respectively (Miltenyi Biotec). The purity of the final T cell (CD3-enriched) population was assessed by flow cytometry analysis (Supplementary Fig. 1). The antibody panel that was used is shown in Supplementary Table 8, and T cells were defined as DAPI−CD11bCD14−CD19−CD3+. T cell purity was greater than 96% (Extended Data Fig. 2b).
Nail DNA from nail clippings of twin A and neonatal dried blood spot DNA were extracted using the QIAamp DNA Micro kit (QIAGEN) according to the manufacturer’s instructions.
Dermal fibroblast cell cultures were set up following a skin biopsy from the proximal thigh in twin B. Cells were cultured in Gibco AmnioMAX C-100 Complete Medium (Thermo Fisher Scientific) and Chang medium D (FUJIFILM Irvine Scientific) without additives. They were cultured at 37 °C, 5% CO2, and subcultured when confluent. At 22 days in culture, cells were removed from the subcultures in Chang medium using trypsin, and DNA was extracted. DNA from the cells in the AmnioMAX medium was extracted at day 27.
Cell lines
A Jurkat cell line (CVCL_0065) was used as the CALRdel52bp-negative control. TF-1 (CVCL_0559) and HEL (CVCL_0001) cell lines were used as JAK2V617F positive and negative controls, respectively. All cell lines were purchased from American Type Culture Collection (catalog numbers TIB-152, CRL-2003, and TIB-180 for Jurkat, TF-1 and HEL, respectively). Cells were cultured according to American Type Culture Collection recommendations, and DNA was extracted using the DNeasy Blood & Tissue kit (QIAGEN).
Microscopy
Peripheral blood microscopy was done using the Olympus BX60 microscope with the Olympus UPlanFl 40×/0.75 Infinity/0.17 and Olympus UPlanSApo 100×/1.40 Oil Immersion Infinity/0.17/FN26.5 objectives (Olympus). Imaging was performed with the INFINITY3S-1UR camera and use of the Infinity ANALYZE software, release 6.5 (Lumenera). Tissue microscopy was performed using a Nikon Eclipse E400 microscope (40×/20× objectives) (Nikon) on routinely prepared 4-μm sections cut from formalin-fixed paraffin-embedded blocks. Microscope fields were selected from whole-slide scanned images using the NanoZoomer 2.0-HT scanner in 40× mode and NDP.view2 viewer software v2.9.25 (Hamamatsu Photonics). Image analysis was done using Adobe Photoshop v21.2 (Adobe) and was limited to white balance of complete image and addition of scale bars.
Flow cytometry and cell sorting
Flow cytometry and cell sorting was performed using a BD FACSAria Fusion Cell Sorter (Becton, Dickinson and Company) with FACSDIVA software v8.0.1. Sorting of single cells into 96-well plates was performed using the automated cell deposition unit. Verification of the single-cell sorting mode was established by sorting single fluorescent beads (Alignflow Flow Cytometry Alignment Beads; Thermo Fisher Scientific) into a flat-bottomed 96-well tissue culture plate. A fluorescence microscope was used to visualize the beads to verify that no wells contained more than one fluorescent bead and that they were centrally positioned in the wells. Further flow cytometry analysis was performed using FlowJo software v10.7 (Becton, Dickinson and Company).
Cryopreserved peripheral blood mononuclear cells were thawed and processed for flow cytometry analysis and cell sorting as previously described33. Staining panel details for granulocyte, T cell and HSPC analysis and sorting are shown in Supplementary Table 8, and the gating strategy is provided in Supplementary Fig. 1.
Single-cell cloning assay
Single HSPCs (7AAD−, Lin−, CD34+) were sorted into 96-well plates with 50 μl of MethoCult H4435 Enriched (STEMCELL Technologies). The mean fluorescence intensities of CD34, CD38, CD90, CD45RA and CD123 were also recorded for each individual HSPC sorted using the index sorting feature. Colony output was assessed on day 14 under direct light microscopy, and individual colonies were picked. All colonies were flash-frozen and stored at −80oC for subsequent whole-genome amplification. Index-sorting analysis was performed by in-house bioinformatics pipelines using R studio v3.6.3.
Whole-genome amplification
Whole-genome amplification for each sorted population was performed using the REPLI-g Mini/Midi Kits (QIAGEN).
PCR
Twin studies
PCR target regions and corresponding primer set sequences for the lineage-specific CALR genotyping, and validation of the targeted sequencing and WGS results at the single-cell level are shown in Supplementary Tables 6 and 9. All PCRs were performed using the KAPA2G Robust HotStart ReadyMix PCR Kit (Merck) with the following conditions: 12.5 μl KAPA2G Robust HotStart ReadyMix (2×), primer set at 0.5 μM each, template DNA as required and PCR-grade water up to 25 μl; and the following cycling protocol: initial denaturation for 3 min at 95 °C, 35 cycles of denaturation for 15 s at 95 °C, annealing for 15 s at 60 °C, extension for 15 s at 72 °C and final extension for 3 min at 72 °C. All PCR amplicons were purified using either Agencourt AMPure XP Magnetic Beads (Beckman Coulter) or the Monarch DNA Gel Extraction Kit (New England Biolabs).
Neonatal dried blood spot studies
Neonatal dried blood spot mutational analysis was performed with use of ddPCR in a two-step PCR. JAK2 exon 14 (chr9(GRCh38):5073730–5073813) was amplified in the first reaction, and the amplicon was then tested for presence of the NM_004972.3[JAK2]:c.1849G > T p.V617F mutation using a ddPCR assay. The same primers were used for both reactions. Primer and probe details are provided in Supplementary Table 9. The first round of PCR was performed using the KAPA HiFi HotStart ReadyMix PCR Kit (Merck) with the following conditions: 10 μl KAPA HiFi HotStart ReadyMix (2×), primers at 0.3 μM, 3 μl template DNA and PCR-grade water up to 20 μl; and the following cycling protocol: initial denaturation for 3 min at 95 °C, 20 cycles of denaturation for 20 s at 98 °C, annealing for 15 s at 62.5 °C, extension for 30 seconds at 72 °C and final extension for 1 min at 72 °C. ddPCR reactions were performed using the Bio-Rad QX200 AutoDG Droplet Digital PCR System (Bio-Rad Laboratories). Sample preparation was done with Bio-Rad’s Supermix for Probes (no dUTP) with the following conditions: 11 μl of the supermix (2×), primers at 900 nM each, probes at 250 nM each, 5 ng preamplified DNA template in a 1:100,000 dilution and PCR-grade water up to 22 μl. The amplification was performed using the Bio-Rad C1000 Touch Thermal Cycler with the following cycling protocol: initial enzyme activation for 10 min at 95 °C, 43 cycles of denaturation for 30 s at 94 °C, annealing/extension for 1 min at 57 °C and enzyme deactivation for 10 min at 98 °C (ramp rate 2 °C/s at all steps). DNA extracted from the Guthrie card of a patient with JAK2 wild-type systemic mastocytosis (DBS_1) and TF-1 cells were used as negative controls, whereas HEL cell DNA was used as the positive control, with multiple replicates per run. Presence of JAK2V617F in one of the samples was independently validated by nested PCR. ddPCR data analysis was done using QX Manager Software, Standard Edition, v1.2 (Bio-Rad Laboratories).
Gel electrophoresis
Initial assessment of DNA fragment size was performed using gel electrophoresis on a 3% ethidium bromide-containing (10−4 g per liter) agarose gel in TAE buffer (Tris acetate-EDTA, Tris acetate 40 mM and 1 mM EDTA, pH 8.3) at 150 mV. Approximate DNA fragment size was determined using a 50-bp ladder (PCRBIO Ladder III, PCR Biosystems).
Automated electrophoresis
Separation, identification and quantitation of the type 1 CALR mutation within the different cell populations examined was performed with use of the Agilent Fragment Analyzer automated fluorescence-based capillary electrophoresis and the DNF-905 dsDNA kit of 35–500 bp sizing range and/or the Agilent 2200 TapeStation automated electrophoresis system, using the D1000 ScreenTape System and the A.0202 SR1 software version (Agilent Technologies).
Sanger DNA sequencing
Sanger DNA sequencing was performed using the Applied Biosystems 3730 DNA Analyzer (Thermo Fisher Scientific) with use of the BigDye Terminator v3.1 chemistry. Results were visualized and annotated using the SnapGene Viewer software, v5.3 (GSL Biotech) and the Heatmapper visualizer34.
Next-generation sequencing
Next-generation sequencing of granulocyte DNA from each twin was performed using an adapted method based on the previously described method by Silveira et al.35. The genes and transcript accessions covered by the panel are shown in Supplementary Table 10.
Notably, the targeted panel analysis for twin A showed presence of two TET2 mutations at chr4(GRCh38):105234449 (NM_001127208.2[TET2]:c.509del p.N170TfsTer13) and chr4(GRCh38):105259633 (NM_001127208.2[TET2]:c.3818 G > A p.C1273Y), with VAFs 9.21% and 8.63%, respectively. Both mutations were validated by Sanger sequencing both in bulk populations and in single-HSPC colonies (data not shown). The frameshift mutation was also detected in the WGS data, but the substitution was supported from a single caller only and therefore did not meet the variant calling criteria set in the study (as described below). This is a missense mutation that is not annotated in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) database, but it appears in COSMIC as pathogenic (https://cancer.sanger.ac.uk/cosmic/mutation/overview?id=145018270), with a CADD score of 27.2 (ref. 36), whereas the SIFT37 and PolyPhen38 tools predict its effect to be deleterious and probably damaging, respectively. However, because it was not included in the high-confidence set for WGS data, it was excluded from the downstream analysis.
WGS
Paired-end sequencing of matched tumor and germline DNA samples for each twin was performed by Novogene, and 350-bp insert libraries were prepared for all samples and run on the NovaSeq 6000 System (Illumina) to generate 150-bp reads, aiming for 30× sequencing coverage.
Whole-genome data processing and alignment
Whole-genome sequence FASTQ files were processed and aligned as follows. The quality of short insert paired-end reads was assessed by FASTQC v0.11.9 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Sequencing reads were aligned to the human reference genome (GRCh38.p13) using the BWA-MEM39 (v0.7.17) aligner in default mode. Duplicates were marked using Picard tools v2.3.0 (https://broadinstitute.github.io/picard/); indels were realigned and base quality scores were recalibrated according to Genome Analysis Toolkit best practice to generate analysis-ready BAM files. The resulting sequencing coverage ranged from 36.5× to 42.4×.
Variant calling
We reported somatic SNVs and indels that overlapped across three different software packages: MuTect2 (Genome Analysis Toolkit v4.1.2.0), strelka2 (v2.8.4) and Octopus (v0.6.3-beta)11–13. Random forest filtering was applied to both somatic and germline calls generated using the Octopus algorithm13. Somatic variants notated as ‘somatic’ and ‘PASS’ in the VCF files generated by Octopus and strelka2 and “PASS” in the VCF files generated by MuTect2 were retained and constitute the high-confidence call set. Variants were classified as shared or unique based on overlap between the twin call sets.
Copy-number estimation
The Battenberg package (v.2.2.8; https://github.com/Wedge-lab/battenberg) was implemented in R 3.3.0 to estimate tumor cellularity and detect clonal and subclonal copy-number aberrations (CNAs) for each sample40 (Extended Data Fig. 4). An approach similar to the R-only WGS pipeline described at https://github.com/Wedge-lab/battenberg/blob/master/inst/example/battenberg_wgs.R was used, including the preprocessing, allele-counting and phasing steps and using genome assembly hg38. Replication timing correction was not implemented and SV breakpoint information was not included. Default parameters were used, but the PHASING_GAMMA parameter was increased to 2 to correct for overfitting. To run Battenberg in a way that was compatible with the hg38 genome build, Impute2 inputs were converted to hg37 using liftOver41. Heterozygous SNP positions were then phased using Beagle 5 (ref. 41) with use of the 1000 Genomes genotypes as a reference panel and the output was converted to hg38. The ‘ntot’ values for nearly all CNAs identified by Battenberg were approximately 2, suggesting that these were likely to be homozygous SNPs rather than somatic CNAs. To confirm whether these were heterozygous SNPs in the normal, distributions of allele frequencies of all SNPs within these CNAs in both the tumor and normal samples were inspected; copy-number segments were excluded where these distributions did not correspond to ‘real’ somatic CNAs.
Mutation clustering
Copy-number changes called by Battenberg (‘Copy-number estimation’) along with VAFs for each mutation were used to calculate CCF and prepared as input for DPClust22, a Bayesian Dirichlet algorithm available at https://github.com/Wedge-lab/dpclust. Before running DPClust, we used the vafCorrect realignment tool (https://github.com/cancerit/vafCorrect) to calculate VAF values for all somatic variant calls (‘Variant calling’) from tumor and germline BAM files directly42. The vafCorrect read mapping and base quality thresholds were set to 30. Variants with VAF > 0.05 in either germline sample or VAF < 0.1 in a tumor sample were flagged and removed prior to mutation clustering.
For each twin, we inferred the number of subclones and the fraction of tumor cells within each subclone using DPClust (v2.2.2) implemented in R 3.4.0 to cluster mutations according to their CCF. A pipeline similar to that described at https://github.com/Wedge-lab/dpclust/blob/master/inst/example/dpclust_pipeline.R was used with 1,000 iterations to run the Markov chain Monte Carlo chain (no.iters) and discarding 200 iterations as burn-in (no.iters.burn.in or burnin). Clusters that included less than 1% total mutations were removed.
DPClust was also used to cluster mutations according to their CCFs across samples from both twins simultaneously (Supplementary Table 11). DPClust input files for multidimensional DPClust contained all mutations across both samples. Variant sites with total coverage below 20 reads or with a mutation multiplicity estimate (no.chrs.bearing.mut) of zero in either sample were flagged and removed prior to multidimensional clustering. Depth at variant sites was determined using vafCorrect.
To perform statistical tests on individual mutations and establish confidence intervals for CCF estimates, the binomial probability function pbinom was used in R. This returns the likelihood pbinom(x, y, z) of observing x or fewer mutant reads from a depth of y given an expected VAF of z.
SV analysis
SVs were called using Manta14 (v1.6.0; https://github.com/Illumina/manta) and LUMPY15 (v0.2.13; https://github.com/arq5x/lumpy-sv). The LUMPY express wrapper was used to run LUMPY jointly on tumor-normal pairs. SV breakpoints identified by the algorithm were then genotyped using SVTyper16 (v0.7.1; https://github.com/hall-lab/svtyper), run as a command line python script, and variants with any alt (AO) evidence in the normal were removed. In Manta, tumor-normal analysis workflows were configured using the configManta.py script with default settings; SVs were then filtered to include only calls marked as “PASS”. For each twin, high-confidence somatic calls were derived by requiring either both callers to support an SV or one caller with additional support from a nearby CNV changepoint. In comparing alignments between Manta and LUMPY, a bidirectional ‘slop’ of 200 bp was used to account for possible imprecision in some reads. SVs were annotated using the online AnnotSV interface v3.0.2 (https://lbgi.fr/AnnotSV/runjob) using default settings43.
Extraction of mutational signatures
Single-base substitution, doublet-base substitution and indel signatures were extracted by nonnegative matrix factorization, as implemented in SigProfilerMatrixGenerator v1.1.23 (https://github.com/AlexandrovLab/SigProfilerMatrixGenerator) using Python 3.8.3 (ref. 21). SigProfilerExtractor (v1.1.0; https://github.com/AlexandrovLab/SigProfilerMatrixExtractor) was used to determine the proportion of mutations in each sample attributable to specific mutational signatures within the COSMIC database of signatures (COSMIC v3.1, available at https://cancer.sanger.ac.uk/cosmic/signatures).
Putative driver mutation analyses
To identify nonsilent coding mutations of interest, we considered 82 genes with relevant biological evidence44–46 (Supplementary Table 12) as well as genes from the COSMIC Cancer Gene Census list (August 2020, http://cancer.sanger.ac.uk/census). Somatic variants were annotated using Ensembl Variant Effect Predictor release 103 (ref. 19). Variants were considered pathogenic if their clinical significance was pathogenic, their impact was high or their CADD score was ≥30 (ref. 36).
Bayesian estimates of myeloproliferative neoplasm developmental timing
An estimate for the time to the most recent common ancestor (MRCA) of MPN cells in both twins, tMRCA (that is the time at which in utero transfusion took place), was derived using a Poisson Bayesian model. Known data used in the model included the ages of both twins at sampling (39 years 11 months 26 days for twin A, 38 years 5 months 14 days for twin B) and mutation count data generated in this study. Using the pigeonhole principle, it was inferred that mutations from clusters 1 and 2 with CCF > 0.5 occurred on the same individual cell lineages in twin B and twin A, respectively (Supplementary Table 11). Mutations shared by the twins and occurring on the same cell lineage were assigned to cluster 3 (Supplementary Table 11). An initial model was built using these lineage-restricted counts (Supplementary Table 5). The mutation rate and the number of SNVs (somatic variation data obtained from twin A and twin B) was modeled as mutations per genome per year. The prior on the mutation rate was exponential with mean of 10.6 clonal substitutions per genome per year; this estimate was based on reported clonal point mutation counts and donor ages in patients with MPN within the Pan-Cancer Analysis of Whole Genomes (PCAWG; see Gerstung et al., clonal counts from source data for Fig. 2 and donor ages Extended Data Fig. 8b)3. Pre-MRCA mutation count data (SNVs shared by the twins and occurring on the same cell lineage) had observed values of 7. Post-MRCA mutation count data (lineage-restricted SNVs unique to each twin) had observed values of 91 and 90 for twin A and twin B, respectively. An upper bound on the rate parameter of the Poisson process (mutations per genome per year) was set based on the highest reported clonal substitution rate in a patient with MPN in PCAWG.
Results presented in the main text derive from a model using C>T somatic mutations in a NpCpG context (N[C>T]pG) (Supplementary Table 5a), as these mutations are hypothesized to arise in a clock-like manner as a result of spontaneous deamination of 5-methylcytosine and can be used been to estimate MRCA timing in cancers3,23. N[C>T]pG mutation counts were extracted using the R/Bioconductor package MutationalPatterns (v1.12.0) implemented in R 3.6.0 (ref. 47). An important assumption of this model is that the N[C>T]pG mutation rate has remained constant over time in the MPN lineages in both twins and that MPN lineages in twin A and B shared a common ancestor in the past. A further limitation of this model is that N[C>T]pG counts estimated from bulk data will not necessarily correspond to those arising on a single-cell lineage; 89 and 114 C>T somatic mutations in an NpCpG context were identified uniquely in twin A and twin B, respectively. Two shared N[C>T]pG somatic mutations were identified from the set of all shared mutations listed in Supplementary Table 3. Mutation counts were based on vafCorrect genotyping data after filtering (‘Mutation clustering’). The prior on the mutation rate was exponential with a mean of 1.71 N[C>T]pG substitutions per genome per year; the mean estimated rate was based on reported N[C>T]pG counts and donor ages in patients with MPN within PCAWG (see Gerstung et al., Extended Data Fig. 8b)3. 40 weeks of gestation time was also added to donor ages when generating mutation rate estimates from PCAWG data. An upper bound on the rate parameter of the Poisson process (mutations per genome per year) was set based on the highest reported N[C>T]pG rate in a patient with myeloid-MPN in PCAWG. The prior on tMRCA (that is, the span of time after zygote over which shared N[C>T]pG mutations arose) was defined as uniform between the time of fertilization and the age of earliest presentation in the twins. t[1] and t[2] are time intervals during which subsequent (post-tMRCA) N[C>T]pG mutations arose in twin A and twin B, respectively. The prior on these intervals was defined as uniform between tMRCA and the time of sampling (approximately 40 years of age).
Mutation counts were modeled using a Poisson likelihood with rates given by the product of the mutation rate and the relevant time interval (that is, the time interval from fertilization up to tMRCA for pre-MRCA count data and the time interval between tMRCA up to the time of sampling for post-MRCA count data). The No-U-Turn sampler, implemented in Stan using the rstan package (https://CRAN.R-project.org/package=rstan; v2.21.2, GitRev: 2e1f913d3ca3), was used to draw samples from the model’s posterior distribution through Markov chain Monte Carlo sampling using five chains, each with 100,000 iterations, of which the first 20,000 are warmup48. Log marginal likelihoods and error measures for marginal likelihood estimates were computed via bridge sampling using the ‘bridgesampling’ package in R49. Stan model outputs, likelihood estimates and error measures are reported in Supplementary Table 5. A model using lineage-restricted or clonal N[C>T]pG mutations could not be implemented, as the mutation count data were too sparse.
Informed consent
All procedures followed in the present study were performed in accordance with the ethical standards of the current revision of the Declaration of Helsinki. All patients provided written informed consent. Regarding twin A, samples were obtained from the center in which he is followed up (Christian Medical College, Vellore, India) for diagnostic purposes. Other patients were enrolled to The INForMeD Study (REC reference 16/LO/1376). All research analyses were conducted according to The INForMeD Study (REC reference 16/LO/1376) and ANNB_NBS_027 study (Public Health England Antenatal and Newborn screening research advisory committee, 13 March 2020).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41591-022-01793-4.
Supplementary information
Supplementary Figure 1 and Tables 1–12.
Excel workbook with multiple tabs containing Supplementary Tables 1–12 (one table per tab). The tables have also been provided in the Supplementary Information PDF file.
Acknowledgements
This work was funded by a Hellenic Society of Haematology Foundation Fellowship and a Cancer Research UK Oxford Centre Clinical Research Training Fellowship (C130623/A249471) to N.S., a Junior Research Fellowship (Trinity College, University of Oxford) to M.N.L. and a Medical Research Council (MRC) Senior Clinical Fellowship (MR/L006340/1), a Cancer Research UK Senior Cancer Research Fellowship (C42639/A26988) and the MRC Molecular Haematology Unit core award (MC_UU_12009/5) to A.J.M. We acknowledge the flow cytometry facility at the MRC Weatherall Institute of Molecular Medicine (WIMM), which is supported by the MRC Human Immunology Unit, the MRC Molecular Haematology Unit (MC_UU_12009), the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC), the Kay Kendall Leukaemia Fund (KKL1057), the John Fell Fund (131/030 and 101/517), the EPA fund (CF182 and CF170) and the MRC WIMM Strategic Alliance awards G0902418 and MC_UU_12025. We also acknowledge the contribution of the WIMM Sequencing Facility, supported by the MRC Human Immunology Unit and by the EPA fund (CF268). The computational aspects of this research were supported by the Wellcome Trust Core Award 203141/Z/16/Z and the NIHR Oxford BRC to D.C.W. and A.J.M. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Extended data
Author contributions
All authors made important contributions to the work that is reported and accept responsibility for the aspects of the work with which they were involved. Furthermore, all authors agree with the content of the manuscript as a whole. N.S. provided clinical care, performed and analyzed experiments and wrote the paper. M.N.L. conducted all bioinformatic analyses. C.S.K. and E.L. provided protocols and assisted with experiments. N.B. provided clinical care. D.R. performed the pathology evaluation. S.-A.C. assisted with flow cytometry and cell sorting. A.H. and K.H. performed the myeloid-gene panel analysis. V.M. and B.G. provided clinical care. A.R. and B.P. reviewed the data and provided conceptual advice. D.C.W. and A.J.M. jointly supervised the work. D.C.W. supervised bioinformatic analysis. A.J.M. conceived and supervised the project, provided clinical care and wrote the paper.
Peer review
Peer review information
Nature Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. Editor recognition statement Anna Maria Ranzoni was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Data availability
Sequence data have been deposited at the European Genome-phenome Archive, which is hosted by the European Bioinformatics Institute and the Centre for Genomic Regulation, under accession number EGAS00001005744. Data on all somatic SNVs, indels and structural rearrangements for twin A and twin B are available in Supplementary Tables 1 and 2. Source data for the Bayesian timing model are described in Supplementary Table 5. COSMIC Mutational Signatures v3.1 and Gene Curation data can be accessed at https://cancer.sanger.ac.uk/cosmic/. Source data described in Gerstung et al.3 and used in this study can be accessed via the ICGC Data Portal at https://dcc.icgc.org/pcawg. Clinical details of the monozygotic twins are reported in Results (‘Clinical findings’ section). Clinical details of the JAK2V617F-mutant MPN cohort are described in Results (‘Neonatal blood spot analysis in JAK2V617F-mutant MPN’ section) plus Supplementary Table 7.
Code availability
Any bespoke code used in this paper can be found online at https://github.com/Wedge-lab/InUtero_MPN.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nikolaos Sousos, Máire Ní Leathlobhair.
Contributor Information
David C. Wedge, Email: David.Wedge@manchester.ac.uk
Adam J. Mead, Email: adam.mead@imm.ox.ac.uk
Extended data
is available for this paper at 10.1038/s41591-022-01793-4.
Supplementary information
The online version contains supplementary material available at 10.1038/s41591-022-01793-4.
References
- 1.Mead AJ, Mullally A. Myeloproliferative neoplasm stem cells. Blood. 2017;129:1607–1616. doi: 10.1182/blood-2016-10-696005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell TJ, et al. Timing the landmark events in the evolution of clear cell renal cell cancer: TRACERx Renal. Cell. 2018;173:611–623. doi: 10.1016/j.cell.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstung M, et al. The evolutionary history of 2,658 cancers. Nature. 2020;578:122–128. doi: 10.1038/s41586-019-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Egeren D, et al. Reconstructing the lineage histories and differentiation trajectories of individual cancer cells in myeloproliferative Neoplasms. Cell Stem Cell. 2021;28:514–523.e519. doi: 10.1016/j.stem.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molenaar RJ, et al. Risk of developing chronic myeloid neoplasms in well-differentiated thyroid cancer patients treated with radioactive iodine. Leukemia. 2018;32:952–959. doi: 10.1038/leu.2017.323. [DOI] [PubMed] [Google Scholar]
- 6.Cordua S, et al. Prevalence and phenotypes of JAK2 V617F and calreticulin mutations in a Danish general population. Blood. 2019;134:469–479. doi: 10.1182/blood.2019001113. [DOI] [PubMed] [Google Scholar]
- 7.Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019;366:eaan4673. doi: 10.1126/science.aan4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong D, et al. Initiating and cancer-propagating cells in TEL-AML1-associated childhood leukemia. Science. 2008;319:336–339. doi: 10.1126/science.1150648. [DOI] [PubMed] [Google Scholar]
- 9.Nangalia J, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl. J. Med. 2013;369:2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klampfl T, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N. Engl. J. Med. 2013;369:2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin, D., et al. Calling somatic SNVs and Indels with Mutect2. Preprint at bioRxiv10.1101/861054 (2019).
- 12.Kim S, et al. Strelka2: fast and accurate calling of germline and somatic variants. Nat. Methods. 2018;15:591–594. doi: 10.1038/s41592-018-0051-x. [DOI] [PubMed] [Google Scholar]
- 13.Cooke DP, Wedge DC, Lunter G. A unified haplotype-based method for accurate and comprehensive variant calling. Nat. Biotechnol. 2021;39:885–892. doi: 10.1038/s41587-021-00861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics. 2016;32:1220–1222. doi: 10.1093/bioinformatics/btv710. [DOI] [PubMed] [Google Scholar]
- 15.Layer RM, Chiang C, Quinlan AR, Hall IM. LUMPY: a probabilistic framework for structural variant discovery. Genome Biol. 2014;15:R84. doi: 10.1186/gb-2014-15-6-r84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang C, et al. SpeedSeq: ultra-fast personal genome analysis and interpretation. Nat. Methods. 2015;12:966–968. doi: 10.1038/nmeth.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danial-Farran N, et al. Genetics of hearing loss in the Arab population of Northern Israel. Eur. J. Hum. Genet. 2018;26:1840–1847. doi: 10.1038/s41431-018-0218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaren W, et al. The Ensembl variant effect predictor. Genome Biol. 2016;17:122. doi: 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forbes SA, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:D805–D811. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergstrom EN, et al. SigProfilerMatrixGenerator: a tool for visualizing and exploring patterns of small mutational events. BMC Genomics. 2019;20:685. doi: 10.1186/s12864-019-6041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolli N, et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat. Commun. 2014;5:2997. doi: 10.1038/ncomms3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rustad EH, et al. Timing the initiation of multiple myeloma. Nat. Commun. 2020;11:1917. doi: 10.1038/s41467-020-15740-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greaves M, Hughes W. Cancer cell transmission via the placenta. Evol. Med. Public Health. 2018;2018:106–115. doi: 10.1093/emph/eoy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valdés-Mas R, et al. Transplacental transfer of essential thrombocythemia in monozygotic twins. Blood. 2016;128:1894–1896. doi: 10.1182/blood-2016-06-724252. [DOI] [PubMed] [Google Scholar]
- 26.Williams N, et al. Life histories of myeloproliferative neoplasms inferred from phylogenies. Nature. 2022;602:162–168. doi: 10.1038/s41586-021-04312-6. [DOI] [PubMed] [Google Scholar]
- 27.Bao EL, et al. Inherited myeloproliferative neoplasm risk affects haematopoietic stem cells. Nature. 2020;586:769–775. doi: 10.1038/s41586-020-2786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda K, et al. Hmga2 collaborates with JAK2V617F in the development of myeloproliferative neoplasms. Blood Adv. 2017;1:1001–1015. doi: 10.1182/bloodadvances.2017004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen MA, et al. Myeloproliferative neoplasms in Danish twins. Acta Haematol. 2018;139:195–198. doi: 10.1159/000488384. [DOI] [PubMed] [Google Scholar]
- 30.Fabre MA, et al. Concordance for clonal hematopoiesis is limited in elderly twins. Blood. 2020;135:269–273. doi: 10.1182/blood.2019001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen JW, et al. Clonal hematopoiesis in elderly twins: concordance, discordance, and mortality. Blood. 2020;135:261–268. doi: 10.1182/blood.2019001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abu-Zeinah G, et al. Interferon-alpha for treating polycythemia vera yields improved myelofibrosis-free and overall survival. Leukemia. 2021;35:2592–2601. doi: 10.1038/s41375-021-01183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Meira A, et al. Unravelling intratumoral heterogeneity through high-sensitivity single-cell mutational analysis and parallel RNA Sequencing. Mol. Cell. 2019;73:1292–1305. doi: 10.1016/j.molcel.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babicki S, et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 2016;44:W147–W153. doi: 10.1093/nar/gkw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silveira DRA, et al. Integrating clinical features with genetic factors enhances survival prediction for adults with acute myeloid leukemia. Blood Adv. 2020;4:2339–2350. doi: 10.1182/bloodadvances.2019001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019;47:D886–d894. doi: 10.1093/nar/gky1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaser R, Adusumalli S, Leng SN, Sikic M, Ng PC. SIFT missense predictions for genomes. Nat. Protoc. 2016;11:1–9. doi: 10.1038/nprot.2015.123. [DOI] [PubMed] [Google Scholar]
- 38.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013;Chapter 7:Unit7.20. doi: 10.1002/0471142905.hg0720s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nik-Zainal S, et al. The life history of 21 breast cancers. Cell. 2012;149:994–1007. doi: 10.1016/j.cell.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhn RM, Haussler D, Kent WJ. The UCSC genome browser and associated tools. Brief Bioinform. 2013;14:144–161. doi: 10.1093/bib/bbs038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yates LR, et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell. 2017;32:169–184.e167. doi: 10.1016/j.ccell.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geoffroy V, et al. AnnotSV: an integrated tool for structural variations annotation. Bioinformatics. 2018;34:3572–3574. doi: 10.1093/bioinformatics/bty304. [DOI] [PubMed] [Google Scholar]
- 44.Papaemmanuil E, et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016;374:2209–2221. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nangalia J, Green AR. Myeloproliferative neoplasms: from origins to outcomes. Hematol. Am. Soc. Hematol. Educ. Program. 2017;2017:470–479. doi: 10.1182/asheducation-2017.1.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pich, O., Reyes-Salazar, I., Gonzalez-Perez, A. & Lopez-Bigas, N. Discovering the drivers of clonal hematopoiesis. Preprint at bioRxiv, 10.1101/2020.10.22.350140 (2020). [DOI] [PMC free article] [PubMed]
- 47.Blokzijl F, Janssen R, van Boxtel R, Cuppen E. MutationalPatterns: comprehensive genome-wide analysis of mutational processes. Genome Med. 2018;10:33. doi: 10.1186/s13073-018-0539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carpenter B, et al. Stan: a probabilistic programming language. J. Stat. Softw. 2017;76:1–32. doi: 10.18637/jss.v076.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gronau QF, Singmann H, Wagenmakers E-J. bridgesampling: an R package for estimating normalizing constants. J. Stat. Softw. 2020;92:1–29. doi: 10.18637/jss.v092.i10. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 and Tables 1–12.
Excel workbook with multiple tabs containing Supplementary Tables 1–12 (one table per tab). The tables have also been provided in the Supplementary Information PDF file.
Data Availability Statement
Sequence data have been deposited at the European Genome-phenome Archive, which is hosted by the European Bioinformatics Institute and the Centre for Genomic Regulation, under accession number EGAS00001005744. Data on all somatic SNVs, indels and structural rearrangements for twin A and twin B are available in Supplementary Tables 1 and 2. Source data for the Bayesian timing model are described in Supplementary Table 5. COSMIC Mutational Signatures v3.1 and Gene Curation data can be accessed at https://cancer.sanger.ac.uk/cosmic/. Source data described in Gerstung et al.3 and used in this study can be accessed via the ICGC Data Portal at https://dcc.icgc.org/pcawg. Clinical details of the monozygotic twins are reported in Results (‘Clinical findings’ section). Clinical details of the JAK2V617F-mutant MPN cohort are described in Results (‘Neonatal blood spot analysis in JAK2V617F-mutant MPN’ section) plus Supplementary Table 7.
Any bespoke code used in this paper can be found online at https://github.com/Wedge-lab/InUtero_MPN.