Abstract
The dorsal hippocampus (DH) is key to the maintenance of cocaine memories through reconsolidation into long-term memory stores after retrieval-induced memory destabilization. Here, we examined the time-dependent role of the cornu ammonis 3 DH subregion (dCA3) in cocaine-memory reconsolidation by utilizing the temporal and spatial specificity of optogenetics. eNpHR3.0-eYFP- or eYFP-expressing male Sprague-Dawley rats were trained to lever press for cocaine infusions in a distinct context and received extinction training in a different context. Rats were then re-exposed to the cocaine-paired context for 15 min to destabilize cocaine memories (memory reactivation) or remained in their home cages (no-reactivation). Optogenetic dCA3 inhibition for one hour immediately after memory reactivation reduced c-Fos expression (index of neuronal activation) in dCA3 stratum pyramidale (SP) glutamatergic and GABAergic neurons and in stratum lucidum (SL) GABAergic neurons during reconsolidation. Furthermore, dCA3 inhibition attenuated drug-seeking behavior (non-reinforced lever presses) selectively in the cocaine-paired context three days later (recall test), relative to no photoinhibition. This behavioral effect was eNpHR3.0-, memory-reactivation, and time-dependent, indicating a memory-reconsolidation deficit. Based on this observation and our previous finding that protein synthesis in the DH is not necessary for cocaine-memory reconsolidation, we postulate that recurrent pyramidal neuronal activity in the dCA3 may maintain labile cocaine memories prior to protein synthesis-dependent reconsolidation elsewhere, and SL/SP interneurons may facilitate this process by limiting extraneous neuronal activity. Interestingly, SL c-Fos expression was reduced at recall concomitant with impairment in cocaine-seeking behavior, suggesting that SL neurons may also facilitate cocaine-memory retrieval by inhibiting non-engram neuronal activity.
Subject terms: Hippocampus, Motivation
Introduction
Cocaine use disorder is characterized by uncontrollable drug craving and high relapse propensity [1, 2]. During chronic drug use, associations are formed between distinct environmental contexts, responses that lead to drug procurement, and the unconditioned motivational effects of cocaine [3]. These context-response-cocaine associations are consolidated, or stored, into long-term memory [4]. Thus, subsequent exposure to drug-predictive environmental contexts (i.e., cocaine-paired contexts) can lead to the retrieval of cocaine memories and to robust motivation to seek cocaine [1, 5]. Importantly, retrieval can also result in the destabilization of cocaine memories [4] (i.e., reactivation). Reactivated memories are susceptible to manipulation [6], and their maintenance requires reconsolidation into long-term memory stores through de novo protein synthesis and glutamatergic synaptic plasticity [7]. Interference with drug-memory reconsolidation transiently reduces craving in human subjects [8, 9] and drug-seeking behavior in animal models of drug relapse [10–12]. Therefore, it is of interest from an anti-relapse treatment perspective to understand the neural substrates recruited for cocaine-memory reconsolidation.
The dorsal hippocampus (DH) is a brain region involved in the reconsolidation of spatial, contextual-fear, and contextual-drug memories [13–16]. Inhibition of mRNA synthesis in the DH after spatial-memory reactivation disrupts memory integrity in rats, as reflected by impaired Morris water maze performance [14]. Similarly, zif268 knockdown in the DH after fear-memory reactivation attenuates conditioned fear-memory strength, as indicated by reduced freezing behavior [15]. We have shown that inhibition of neural conductance or Src-family tyrosine kinase activity in the DH after cocaine-memory reactivation diminishes, whereas anisomycin-induced inhibition of de novo protein synthesis fails to alter, contextual cocaine-memory strength as indicated by attenuated drug context-induced cocaine-seeking behavior [16, 17]. These observations suggest that the DH is not the site of protein synthesis-dependent memory reconsolidation in instrumental models of cocaine relapse. Instead, the DH is requisite for memory reconsolidation in other brain regions.
Associative microcircuits in the cornu ammonis 3 subregion of the DH (dCA3) have been theorized to be critical for short-term memory retrieval, memory consolidation, long-term memory recall [18, 19], and, more recently, memory reconsolidation [20]. Consistent with this, the dCA3 exhibits cAMP response element-binding protein activation at contextual fear-memory recall, and this response is inhibited by treatments that interfere with fear-memory reconsolidation [21]. We have hypothesized that the dCA3 maintains destabilized cocaine memories and, therefore, it is recruited transiently, during the early stages of memory reconsolidation, when most memory traces are still labile. We took advantage of the temporal and spatial precision of optogenetics to inhibit neuronal activity in the dCA3 during early- or later-stage cellular memory reconsolidation, which we operationally defined as the first hour and second hour of memory processing after cocaine-memory reactivation, respectively. We examined the resulting effects on long-term memory strength as indicated by drug context-induced cocaine-seeking behavior three days later. We predicted that dCA3 optogenetic inhibition during early-stage memory reconsolidation would be sufficient to diminish cocaine-memory strength. Furthermore, we explored the possible involvement of the stratum lucidum (SL) and stratum pyramidale (SP) cell layers of the dCA3 to this phenomenon.
Materials and methods
For additional information on Materials and Methods, including food training, surgery, post-operative analgesia, context setup, optogenetic acclimation, perfusion, brain histology, immunohistochemistry, microscopy, image analysis, and data analysis protocols, please see the Supplemental Online Materials.
Animals
Male Sprague-Dawley rats (N = 74; 275–300 g; Envigo, South Kent, WA) were maintained on a reversed light-dark cycle in a climate-controlled vivarium. Rats received water ad libitum and 20–25 g of rat chow per day. Animal-housing and treatment protocols followed the Guide for the Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources on Life Sciences, 2011) and were approved by the Washington State University Institutional Animal Care and Use Committee.
Surgery
Rats were anesthetized using ketamine and xylazine (100.0 mg/kg and 5.0 mg/kg, i.p., respectively). AAV5-hSyn-eNpHR3.0-eYFP-WPRE-PA (5.5x10e12 vm/ml; UNC Vector Core, Chapel Hill, NC) or AAV5-hSyn-eYFP (3.5x10e12 vm/ml; UNC Vector Core) was infused bilaterally into the DH (0.7 μl/hemisphere over 10 min). Optic fibers (200-μm diameter fiber optic cable encased in a 6.4-mm long ceramic ferrule; Thor Labs, Newton, NJ) were implanted into the dCA3. Rats received jugular catheter implantation surgery up to 3 days later, as described previously [22].
Drug self-administration and extinction training
Rats were randomly assigned to receive daily 2-h cocaine self-administration training sessions in one of two environmental contexts (context 1 or 2; see experimental timelines in Figs. 1–5A). Rats were trained to press an “active” lever under a fixed-ratio 1 cocaine-reinforcement schedule (cocaine hydrochloride, 0.5 mg/ml, 50 μl/infusion; NIDA Drug Supply Program, Research Triangle Park, NC) with a 20-sec timeout period. Presses on a second, “inactive”, lever and all lever presses during the timeout period had no programmed consequences. Training continued daily until the rats obtained ≥ 10 infusions/session on 10 days. Rats then received seven extinction training sessions (2 h/day) in the context (1 or 2) that had not been used for cocaine self-administration training. During extinction training, lever presses were not reinforced. After extinction sessions 5-7, rats were acclimated to the optogenetic procedure.
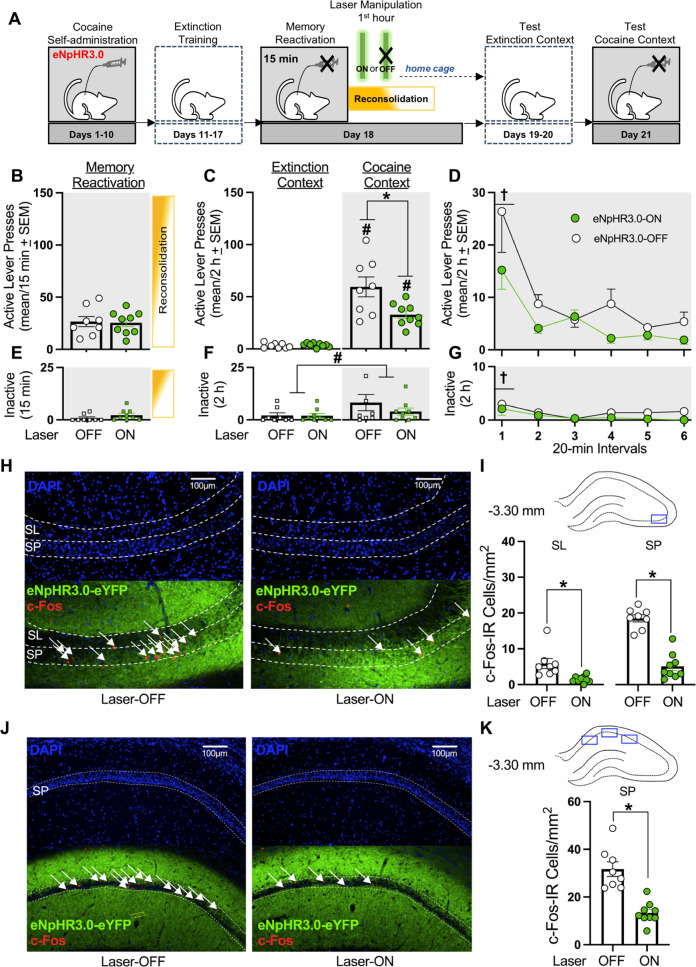
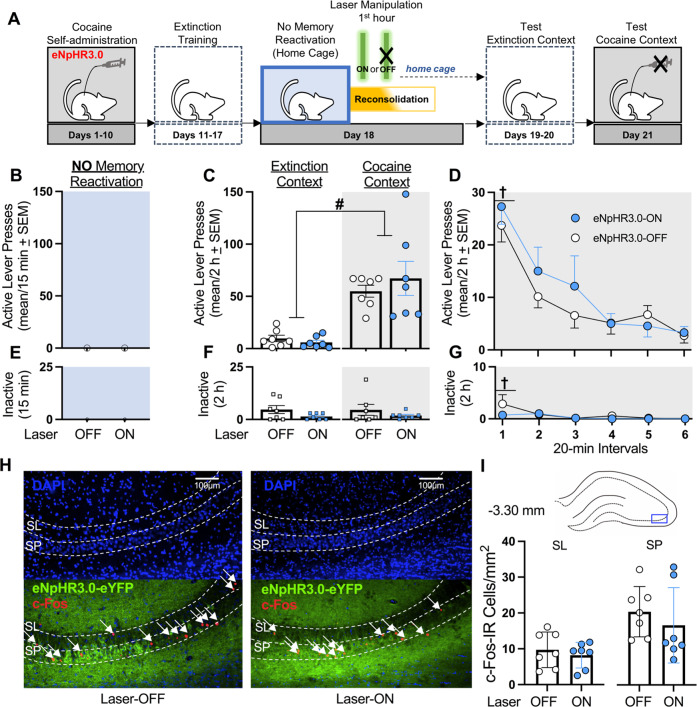
Fig. 1. Optogenetic inhibition of the dCA3 during the first h of cocaine-memory reconsolidation reduces cocaine-memory strength and dCA3 neuronal activation at test.
A Timeline for experiment 1. Following cocaine self-administration and extinction training, eNpHR3.0-expressing rats received a 15-min memory-reactivation session, immediately followed by Laser-ON (n = 9) or Laser-OFF (n = 8) treatment for 1 h. Lastly, rats received test sessions in the extinction and cocaine-paired contexts to assess extinction- and cocaine-memory strength, respectively. B Active-lever presses during the memory-reactivation session (mean/15 min ± SEM). C Active-lever presses upon first post-treatment re-exposure to the extinction and cocaine-paired contexts at test (mean/2 h ± SEM). Symbols: ANOVA #context simple-main effect, p < 0.001; *treatment simple-main effect, p = 0.02. D Time-course of active-lever presses at test in the cocaine-paired context (mean/20-min interval ± SEM). Symbols: ANOVA †time main effect, Tukey’s tests, interval 1 > intervals 2-6, ps < 0.05; *treatment main effect, p = 0.02. E Inactive-lever presses (mean ± SEM) during the memory-reactivation session (mean/15 min ± SEM). F Inactive-lever presses upon first post-treatment re-exposure to the extinction and cocaine-paired contexts at test (mean/2 h ± SEM). Symbol: ANOVA #context main effect, p = 0.03. G Time-course of inactive-lever presses at test in the cocaine-paired context (mean/20-min interval ± SEM). Symbol: ANOVA †time main effect, Tukey’s tests, interval 1 > intervals 2-6, ps < 0.05. H Representative 10x photomicrographs of the dCA3 stratum lucidum (SL) and stratum pyramidale (SP) of rats in the Laser-OFF and Laser-ON groups. Brain tissue was collected immediately after the test in the cocaine-paired context. Images showing DAPI staining (blue) were used to visualize SL and SP boundaries (see also Fig. S1), and corresponding overlay images were used to visualize eNpHR3.0-eYFP expression (green) and c-Fos immunoreactive (IR) cell bodies (red, arrows). I The density of c-Fos-immunoreactive (IR) neurons was quantified in the area indicated by the blue rectangle on the brain schematic. c-Fos-IR cell body density (mean ± SEM) in the SL and SP of rats in the Laser-ON and Laser-OFF groups. Symbols: *t-tests, ps < 0.05. J Representative 10x photomicrographs of the dCA1 SP of rats in the Laser-OFF and Laser-ON groups. Images showing DAPI staining (blue) were used to visualize SP boundaries and corresponding overlay images were used to visualize eNpHR3.0-eYFP expression (green) and c-Fos IR cell bodies (red, arrows). K The density of c-Fos-IR neurons was quantified in the three areas of the dCA1 as indicated by blue rectangles on the brain schematic. Average c-Fos-IR cell body density (mean ± SEM) in the SP of rats in the Laser-ON and Laser-OFF groups. Symbols: *t-test, p < 0.001.
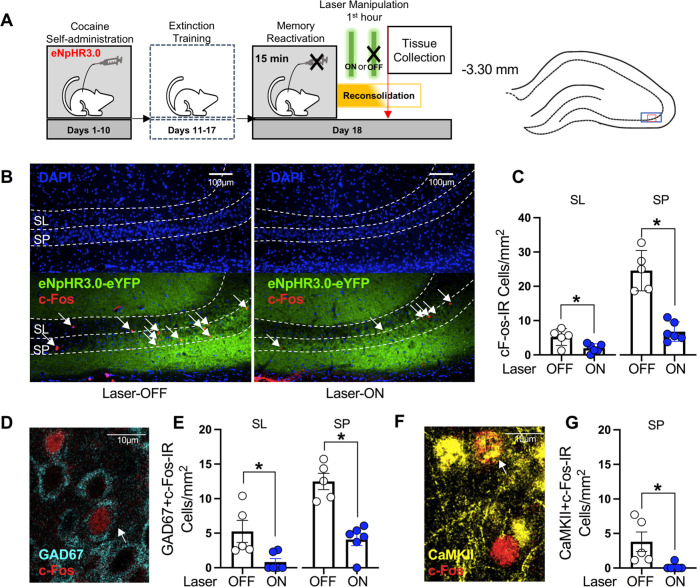
Fig. 5. Laser-light exposure in eNpHR3.0-expressing rats is sufficient to reduce neuronal activity in the dCA3 during reconsolidation.
A Timeline for experiment 5. Following cocaine self-administration and extinction training, eNpHR3.0-expressing rats received a 15-min memory-reactivation session, immediately followed by Laser-ON (n = 6) or Laser-OFF (n = 5) treatment for 1 h. Brain tissue was collected immediately after the optogenetic manipulation. The density of c-Fos-immunoreactive (IR) neurons was quantified in the areas indicated by the blue and red rectangles on the brain schematic on 10x and 40x images, respectively. B Representative 10x photomicrographs of the dCA3 stratum lucidum (SL) and stratum pyramidale (SP) of rats in the Laser-OFF and Laser-ON groups. Images showing DAPI staining (blue) were used to visualize SL and SP boundaries (see also Fig. S1), and corresponding overlay images were used to visualize eNpHR3.0-eYFP expression (green) and c-Fos immunoreactive (IR) cell bodies (red, arrows). C c-Fos-IR cell body density (mean ± SEM) in the SL and SP of rats in the Laser-ON and Laser-OFF groups. Symbols: *t-tests, ps < 0.05. D Representative 40x photomicrograph illustrating a GAD67 + c-Fos-IR cell body (arrow) in the dCA3. E GAD67 + c-Fos-IR cell density (mean ± SEM) in the dCA3 SL and SP of rats in the Laser-ON and Laser-OFF groups. Symbols: *t-tests, ps < 0.05. F Representative 40x photomicrograph illustrating a CaMKII+c-Fos-IR cell body (arrow) in the dCA3. G CaMKII+c-Fos-IR cell density (mean ± SEM) in the dCA3 SP of rats in the Laser-ON and Laser-OFF groups. Symbol: *t-test, p < 0.05.
Memory reactivation
Twenty-four h after the seventh extinction-training session, all rats in experiments 1, 2, 4, and 5 were exposed to the previously cocaine-paired context for 15 min to destabilize cocaine memories and trigger reconsolidation [23]. Lever presses were not reinforced during the session. All rats in experiment 3 remained in their home cages during this time (no-memory reactivation).
In vivo optogenetics
Immediately or 1 h after memory reactivation or no-reactivation, all rats were placed into black metal chambers to which they had been acclimated. Their optic fibers were connected to a 532-nM solid-state laser (Shanghai Laser & Optics Century Company, Shanghai, China) through fiber-optic patch cables (Thor Labs) and a 2 × 1 optical commutator (Doric Lenses, Quebec, Canada). Rats received Laser-ON or Laser-OFF treatment (n = 7–9/group) with treatment assignment balanced based on mean active-lever responding and cocaine intake during the last three drug self-administration sessions. Laser-ON treatment consisted of laser-light stimulation (10 mW, 5 sec on, 5 s off; based on [24, 25]) for 1 h, whereas Laser-OFF treatment involved no laser-light stimulation. Rats in experiment 5 were euthanized immediately after optogenetic treatment.
Testing in the extinction and cocaine-paired contexts
In experiments 1–4, daily 2-h extinction-training sessions resumed 24 h after optogenetic treatment until active-lever responding declined to ≤25 responses/session on two consecutive sessions. Twenty-four h later, the rats were placed into the cocaine-paired context for a 2-h session. During the test sessions, lever presses were not reinforced. Lever responses in the extinction context (first session post treatment) and cocaine-paired context were used to index effects on extinction and cocaine memories, respectively.
Brain Histology and Immunohistochemistry
Rats were overdosed with ketamine and xylazine (300/15 mg/kg, i.p., respectively) then transcardially perfused. In experiments 1–4, brain tissue was collected immediately after the last 2-h test session to visualize virus expression, optic-fiber placement, and c-Fos expression in the SL and SP of dCA3 at test. For experiments 1 and 2, c-Fos expression was also assessed in the dCA1 SP (SL is unique to the dCA3). dCA3 cell layers were visualized using intensity differences in DAPI staining [26] (Fig. S1F). In experiment 5, brain tissue was collected immediately after memory reactivation plus the 1-h optogenetic manipulation to verify that Laser-ON treatment reduced c-Fos expression in the dCA3 during memory reconsolidation and to investigate the cell types affected. c-Fos expression was visualized using standard immunohistochemistry and microscopy protocols. Antibody information is reported in Supplemental Online Materials.
Data analysis
Data from experiments 1–5 were analyzed independently because the experiments were conducted sequentially. Furthermore, differences were detected between the eNpHR3.0-eYFP-expressing and eYFP-expressing groups in training history (i.e., cocaine intake, extinction responding). Data were analyzed using analyses of variance followed by Tukey’s or Bonferroni post-hoc tests or using t tests (where appropriate). Alpha was set at 0.05.
Results
Brain histology
eNpHR3.0-eYFP and eYFP expression was observed in the dCA1-3 in experiments 1–5 (Fig. S1A–E). Optic-fiber tracts were located in the dCA3 for all rats included in data analysis. Eighteen rats (1-3 rats/group) were excluded based on optic-fiber misplacement or unilateral/insufficient virus expression.
Behavioral history
The subsequent treatment groups did not differ significantly in cocaine intake or lever responding during cocaine self-administration and extinction training (Figs. S2A–E) or during the memory reactivation session (Fig. 1B, E–4B, E, S2E) (Table S1), aside from a subsequent treatment group difference in active-lever responding on extinction day 1 in experiment 1 (Fig. S2A, Table S1). Furthermore, optogenetic treatment did not alter the number of extinction test sessions required by treatment groups to reach the extinction criterion (mean ± SEM = 2.30 ± 0.07 sessions).
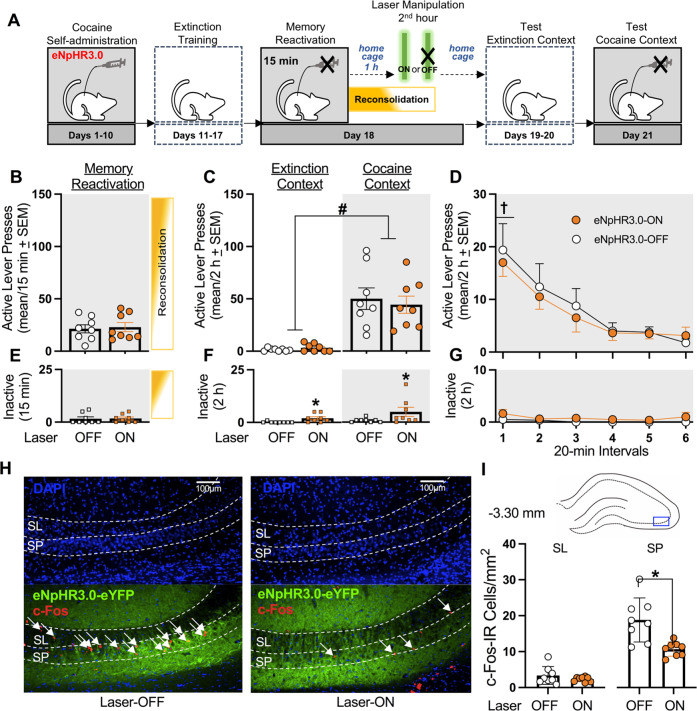
Fig. 4. Optogenetic inhibition of the dCA3 during the second h of memory reconsolidation does not alter cocaine memory strength and reduces dCA3 neuronal activity in a region-specific manner at test.
A Timeline for experiment 4. Following cocaine self-administration and extinction training, eNpHR3.0-expressing rats received a 15-min memory-reactivation session. Rats were returned to their home cages for 1 h and then received Laser-ON (n = 8) or Laser-OFF (n = 8) treatment for 1 h. Lastly, rats received test sessions in the extinction and cocaine-paired contexts to assess extinction- and cocaine-memory strength, respectively. B Active-lever presses during the memory-reactivation session (mean/15 min ± SEM). C Active-lever presses upon first post-treatment re-exposure to the extinction and cocaine-paired contexts at test (mean/2 h ± SEM). Symbol: ANOVA #context main effect, p < 0.001. D Time-course of active-lever presses in the cocaine-paired context at test (mean/20-min interval ± SEM). Symbol: ANOVA †time main effect, Tukey’s test, interval 1 > intervals 3-6, ps < 0.05; *treatment main effect, p = 0.05. E Inactive-lever presses (mean ± SEM) during the memory-reactivation session (mean/15 min ± SEM). F Inactive-lever presses upon first post-treatment re-exposure to the extinction and cocaine-paired contexts at test (mean/2 h ± SEM). Symbols: ANOVA *treatment main effect, p < 0.01. G Time-course of inactive-lever presses in the cocaine-paired context at test (mean/20-min interval ± SEM). H Representative 10x photomicrographs of the dCA3 stratum lucidum (SL) and stratum pyramidale (SP) of rats in the Laser-OFF and Laser-ON groups. Brain tissue was collected immediately after the test session in the cocaine-paired context. Images showing DAPI staining (blue) were used to visualize SL and SP boundaries (see also Fig. S1), and corresponding overlay images were used to visualize eNpHR3.0-eYFP expression (green) and c-Fos immunoreactive (IR) cell bodies (red, arrows). I The density of c-Fos-immunoreactive (IR) neurons was quantified in the area indicated by the blue rectangle on the brain schematic. c-Fos-IR cell body density (mean ± SEM) in the SL and SP of rats in the Laser-ON and Laser-OFF groups. Symbol: *t-test, ps < 0.05.
Optogenetic dCA3 inhibition during the first hour after memory reactivation reduces cocaine-memory strength
Experiment 1 examined whether dCA3 neuronal activity during the first h of cocaine-memory reconsolidation was necessary for cocaine-memory maintenance, as indexed by the magnitude of drug-seeking behavior in the cocaine-paired context at test (Fig. 1A). Optogenetic dCA3 inhibition during the first h after cocaine-memory reactivation reduced active-lever responding at test in a context-specific manner (Fig. 1C; 2 × 2 ANOVA, context x treatment interaction F(1,15) = 8.22, p = 0.01; treatment main F(1,15) = 6.75, p = 0.02; context main F(1,15) = 79.20, p < 0.001). Specifically, three days after Laser-ON (Bonferroni t(8) = 7.0, p < 0.001) or Laser-OFF (Bonferroni t(7) = 6.21, p < 0.001) treatment, cocaine-paired context exposure significantly increased responding at test compared to extinction-context exposure. Furthermore, Laser-ON treatment reduced responding in the cocaine-paired context (Bonferroni t(15) = −2.74, p = 0.02), but not in the extinction context (Bonferroni t(15) = 0.64, p = 0.53), compared to Laser-OFF treatment. Active-lever responding decreased over time in the cocaine-paired context at test, independent of treatment, and Laser-ON treatment reduced active-lever responding compared to Laser-OFF treatment, independent of time (Fig. 1D; 2 ×6 ANOVA, time main F(5,75) = 12.01, p < 0.001, Tukey’s tests, interval 1 > intervals 2–6, ps < 0.05; treatment main F(1,15) = 7.52, p = 0.02; treatment x time interaction F(5,75) = 1.19, p = 0.32).
Cocaine-paired context exposure increased inactive-lever responding at test relative to extinction-context exposure independent of treatment (Fig. 1F; ANOVA, context main F(1,15) = 5.84, p = 0.03; treatment main and treatment x context interaction Fs(1,15) ≤ 1.76, ps ≥ 0.20). Furthermore, inactive-lever responding decreased over time in the cocaine-paired context at test independent of treatment (Fig. 1G; ANOVA, time main F(5,75) = 2.42, p = 0.04, Tukey’s tests, interval 1 > interval 3, ps < 0.05; treatment main and treatment x time interaction Fs(1-5,12-75) ≤ 1.62, ps ≥ 0.22).
In brain tissue collected immediately after the 2-h test session in the cocaine-paired context, c-Fos expression was quantified in the dCA3 SL and SP (Fig. 1H), ventral to the optic fiber tract, and in the dCA1 SP (Fig. 1J). Laser-ON treatment during the first h after cocaine-memory reactivation reduced c-Fos expression in the dCA3 SL (t(15) = 3.20, p < 0.01) and SP (t(15) = 8.45, p < 0.001) (Fig. 1I) and in the dCA1 SP (t(15) = 5.56, p < 0.0001; Fig. 1K) at test, relative to Laser-OFF treatment.
Laser-ON treatment without eNpHR3.0 expression in the dCA3 does not alter cocaine-memory strength
Experiment 2 evaluated whether Laser-ON treatment during the first h of memory reconsolidation would impair cocaine-memory strength in eYFP controls (i.e., without eNpHR3.0) (Fig. 2A) to examine whether the effects observed in experiment 1 reflected AAV- or laser light-induced nonspecific performance deficit [26]. Cocaine-paired context exposure increased active-lever responding relative to extinction-context exposure at test, independent of treatment (Fig. 2C; ANOVA, context main F(1,14) = 60.95, p < 0.001; treatment main and treatment x context interaction Fs(1,14) ≤ 0.51, ps ≥ 0.49). Laser-ON treatment without eNpHR3.0 did not alter responding in either context relative to Laser-OFF treatment. Active-lever responding decreased over time in the cocaine-paired context at test, independent of treatment (Fig. 2D; ANOVA, time main F(5,70) = 18.99, p < 0.001, Tukey’s test interval 1 > intervals 2-6, ps < 0.05; treatment main and treatment x time interaction Fs(1-5,14-70) ≤ 0.34, ps ≥ 0.80).
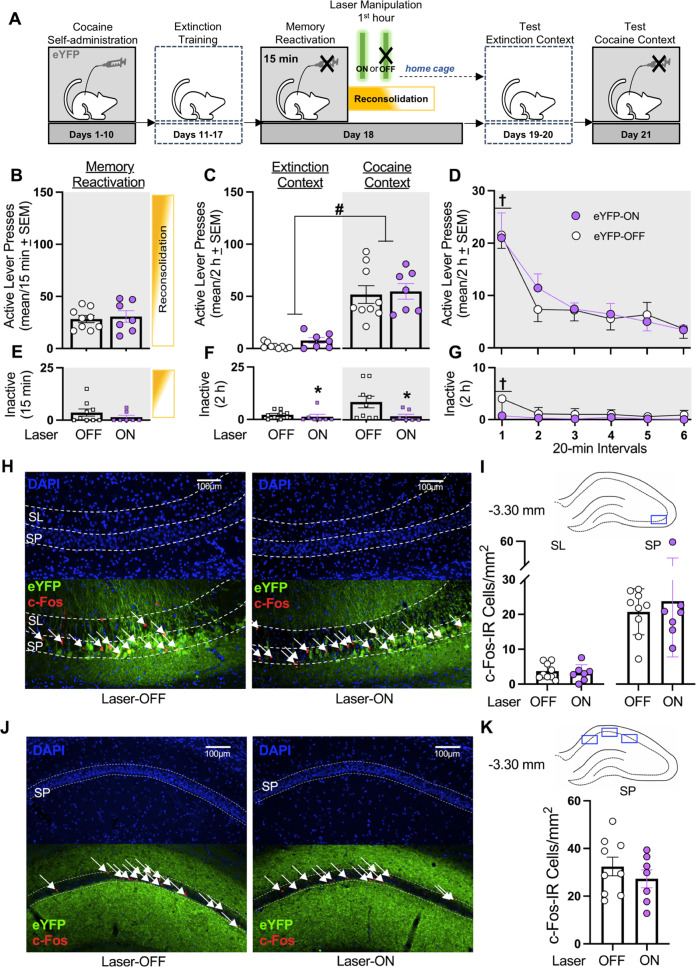
Fig. 2. Laser-ON treatment without eNpHR3.0 expression in the dCA3 does not alter cocaine-memory strength or dCA3 neuronal activity at test.
A Timeline for experiment 2. Following cocaine self-administration and extinction training, eYFP-expressing rats received a 15-min memory-reactivation session, immediately followed by Laser-ON (n = 7) or Laser-OFF (n = 9) treatment for 1 h. Lastly, rats received test sessions in the extinction and cocaine-paired contexts to assess extinction- and cocaine-memory strength, respectively. C Active-lever presses during the memory-reactivation session (mean/15 min ± SEM) and upon first post-treatment re-exposure to the extinction and cocaine-paired contexts at test (mean/2 h ± SEM). Symbols: ANOVA #context main effect, p < 0.001. D Time-course of active-lever presses in the cocaine-paired context at test (mean/20-min interval ± SEM). Symbols: ANOVA †time main effect, Tukey’s test interval 1 > intervals 2-6, ps < 0.05. E Inactive-lever presses (mean ± SEM) during the memory-reactivation session (mean/15 min ± SEM). F Inactive-lever presses upon first post-treatment re-exposure to the extinction and cocaine-paired contexts at test (mean/2 h ± SEM). Symbol: ANOVA #treatment main effect, p = 0.03. G Time-course of inactive-lever presses in the cocaine-paired context at test (mean/20-min interval ± SEM). Symbol: ANOVA †time main effect, Tukey’s test, interval 1 > intervals 2-6, ps < 0.05. H Representative 10x photomicrographs of the dCA3 stratum lucidum (SL) and stratum pyramidale (SP) of rats in the Laser-OFF and Laser-ON groups. Brain tissue was collected immediately after the test in the cocaine-paired context. Images showing DAPI staining (blue) were used to visualize SL and SP boundaries (see also Fig. S1), and corresponding overlay images were used to visualize eYFP expression (green) and c-Fos immunoreactive (IR) cell bodies (red, arrows). I The density of c-Fos-immunoreactive (IR) neurons was quantified in the area corresponding to the blue rectangle on the brain schematic. c-Fos-IR cell body density (mean ± SEM) in the SL and SP of rats in the Laser-ON and Laser-OFF groups. J Representative 10x photomicrographs of the dCA1 SP of rats in the Laser-OFF and Laser-ON groups. Images showing DAPI staining (blue) were used to visualize SP boundaries and corresponding overlay images were used to visualize eNpHR3.0-eYFP expression (green) and c-Fos IR cell bodies (red, arrows). K The density of c-Fos-IR neurons was quantified in the three areas of the dCA1 as indicated by blue rectangles on the brain schematic. Average c-Fos-IR cell body density (mean ± SEM) in the SP of rats in the Laser-ON and Laser-OFF groups. Symbols: *t-test, p < 0.001.
Laser-ON treatment without eNpHR3.0 reduced inactive-lever responding relative to Laser-OFF treatment independent of testing context (Fig. 2F; ANOVA, treatment main F(1,14) = 5.62, p = 0.03; context main and treatment x context interaction Fs(1,14) ≤ 3.39, ps ≥ 0.09) due to unusually high response rate in Laser-OFF group. Time-course analysis confirmed this treatment effect, and it revealed that responding decreased over time in the cocaine-paired context at test independent of treatment type (Fig. 2G; ANOVA treatment main F(1,14) = 4.41, p = 0.05; time main F(5,70) = 4.00, p < 0.01, Tukey’s test, interval 1 > intervals 2-6, ps < 0.05; treatment x time interaction F(5,70) = 2.03, p = 0.09).
eYFP expression was more restricted to dCA3 cell bodies than eNpHR3.0-eYFP expression (Fig. 2H). Laser-ON treatment without eNpHR3.0 did not alter c-Fos expression in the dCA3 SP (t(14) = 0.52, p = 0.61) and SL (t(14) = 0.48, p = 0.64) (Fig. 2I) or in the dCA1 SP (t(14) = 0.93, p = 0.37; Fig. 2J, K) at test, compared to Laser-OFF treatment.
Optogenetic dCA3 inhibition without memory reactivation does not alter cocaine-memory strength
Experiment 3 examined whether optogenetic dCA3 inhibition after home cage stay (i.e., without cocaine-memory reactivation) would impair cocaine-memory strength (Fig. 3A). Without reactivation, long-term memories were expected to be protected from manipulation [27]. Cocaine-paired context exposure increased active-lever responding relative to extinction-context exposure, and Laser-ON treatment immediately after home cage stay did not alter responding in either context, relative to Laser-OFF treatment (Fig. 3C; ANOVA, context main F(1,12) = 35.32, p < 0.001; treatment main and treatment x context interaction Fs(1,12) ≤ 0.81, ps ≥ 0.39). Active-lever responding decreased over time in the cocaine-paired context at test, independent of treatment (Fig. 3D; ANOVA, time main F(5,60) = 24.83, p < 0.0001, Tukey’s tests, interval 1 > intervals 2-6, ps < 0.05; treatment main and treatment x time interaction Fs(1-5,12-60) ≤ 0.86, ps ≥ 0.49).
Fig. 3. Optogenetic inhibition of the dCA3 without memory reactivation fails to alter cocaine-memory strength or dCA3 neuronal activation at test.
A Timeline for experiment 3. Following cocaine self-administration and extinction training, eNpHR3.0-expressing rats remained in their home cages (i.e., no memory reactivation), then received Laser-ON (n = 7) or Laser-OFF (n = 7) treatment for 1 h. Lastly, rats received test sessions in the extinction and cocaine-paired contexts to assess extinction- and cocaine-memory strength, respectively. B Rats did not receive a memory reactivation session. C Active-lever presses upon first post-treatment re-exposure to the extinction and cocaine-paired contexts at test (mean/2 h ± SEM). Symbol: ANOVA #context main effect, p < 0.001. D Time-course of active-lever presses in the cocaine-paired context at test (mean/20-min interval ± SEM). Symbol: ANOVA †time main effect, Tukey’s tests, interval 1 > intervals 2-6, ps < 0.05. E Rats did not receive a memory reactivation session. F Inactive-lever presses (mean ± SEM) upon first post-treatment re-exposure to the extinction and cocaine-paired contexts at test (mean/2 h ± SEM). G Time-course of inactive-lever presses in the cocaine-paired context at test (mean/20-min interval ± SEM). Symbol: ANOVA †time main effect, intervals 1 > intervals 4-6, ps < 0.05. H Representative 10x photomicrographs of the dCA3 stratum lucidum (SL) and stratum pyramidale (SP) of rats in the Laser-OFF and Laser-ON groups. Brain tissue was collected immediately after the test session in the cocaine-paired context. Images showing DAPI staining (blue) were used to visualize SL and SP boundaries (see also Fig. S1), and corresponding overlay images were used to visualize eNpHR3.0-eYFP expression (green) and c-Fos immunoreactive (IR) cell bodies (red, arrows). I The density of c-Fos-immunoreactive (IR) neurons was quantified in the area indicated by the blue rectangle on the brain schematic. c-Fos-IR cell body density (mean ± SEM) in the SL and SP of rats in the Laser-ON and Laser-OFF groups.
Neither testing context nor treatment altered inactive-lever responding (Fig. 3F; ANOVA, all Fs(1,12) ≤ 1.94, ps ≥ 0.76). Inactive-lever responding decreased across time in the cocaine-paired context at test, independent of treatment (Fig. 3G; 2 × 6 ANOVA, time main F(5,60) = 2.88, p = 0.02, Tukey’s tests, interval 1 > intervals 4-6, ps < 0.05; treatment main and treatment x time interaction Fs(1-5,12-60) ≤ 1.10, ps ≥ 0.33).
Laser-ON treatment administered for 1 h after no-memory reactivation did not alter c-Fos expression in the SP (t(12) = 0.79, p = 0.44) or SL (t(12) = 0.65, p = 0.53) at test, compared to Laser-OFF treatment (Fig. 3H–I).
Optogenetic dCA3 Inhibition during the Second Hour after Memory Reactivation Fails to Alter Cocaine-Memory Strength
Experiment 4 examined whether optogenetic dCA3 inhibition during the second h of memory reconsolidation would weaken cocaine-memory strength (Fig. 4A). Cocaine-paired context exposure increased active-lever responding relative to extinction-context exposure, and Laser-ON treatment administered during the second h after memory reactivation failed to alter responding in either context relative to Laser-OFF treatment (Fig. 4C; ANOVA, context main F(1,14) = 40.78, p < 0.001; treatment main and treatment x context interaction Fs(1,14) ≤ 0.87, ps ≥ 0.37). Furthermore, active-lever responding decreased over time in the cocaine-paired context, at test, independent of treatment (Fig. 4D; ANOVA, time main F(5,70) = 13.10, p < 0.001, Tukey’s test, interval 1 > intervals 3-6, ps < 0.05; treatment main and treatment x time interaction Fs(1-5,14-70) ≤ 0.19, ps ≥ 0.67).
Laser-ON treatment in the dCA3 during the second h after memory reactivation increased inactive-lever responding compared to Laser-OFF treatment, independent of testing context (Fig. 4F; ANOVA, treatment main F(1,14) = 9.78, p < 0.01; context main and treatment x context interaction Fs(1,14) ≤ 1.34, ps ≥ 0.27). However, inactive-lever responding in the cocaine-paired context at test did not vary as a function of treatment type or time (Fig. 4G; ANOVA, all Fs(1-5,14-70) ≤ 3.35, ps ≥ 0.09).
Laser-ON treatment administered during the second h after memory reactivation reduced c-Fos expression in the SP (t(14) = 7.62, p < 0.001), but not in the SL (t(14) = 1.94, p = 0.07), at test, compared to Laser-OFF treatment (Fig. 4H, I).
Laser-ON treatment reduces neuronal activation in the dCA3 during cocaine-memory reconsolidation
Experiment 5 examined whether the Laser-ON treatment was sufficient to reduce neuronal activation in the dCA3 SL and SP, and specifically in GAD67-immunoreactive (IR) (GABAergic inhibitory) and CaMKII-IR (excitatory pyramidal) cell populations, during memory reconsolidation (Fig. 5A). Brain tissue was collected immediately after memory reactivation plus 1-h optogenetic treatment. Laser-ON treatment during reduced c-Fos expression in the SL (Fig. 5B, C; t(9) = 2.86, p = 0.02) and SP (Fig. 5D, E; t(9) = 6.61, p < 0.001), relative to Laser-OFF treatment. These effects reflected reductions in GAD67 + c-Fos-IR cell density in the SL (Fig. 5F–G; t(9) = 2.87, p = 0.02) and SP (Fig. 5H-I; t(9) = 5.84, p < 0.001) and CaMKII+c-Fos-IR cell density in the SP (Fig. 5J, K; t(9) = 2.8, p = 0.02).
Discussion
Cellular memory reconsolidation has been theorized to take place over 4–6 h after memory destabilization [27]. However, the time-dependent contributions of brain regions, such as the dCA3, to cocaine-memory reconsolidation within this time window have not been explored, likely because of the lack of spatial and temporal precision afforded by pharmacological manipulations. Here, we report that critical engagement of the dCA3 occurs during the early stages of cocaine-memory reconsolidation.
dCA3 plays a requisite role in early-stage cocaine-memory reconsolidation
eNpHR3.0-mediated optogenetic inhibition of the dCA3 for 1 h, immediately after cocaine-memory reactivation, reduced drug context-induced cocaine-seeking behavior three days later, at test (Fig. 1). This decrease in cocaine-seeking behavior likely indicated interference with cocaine-memory reconsolidation (i.e., memory impairment) or with the re-establishment of memory retrieval links [28, 29] during reconsolidation (i.e., retrieval impairment) as opposed to a protracted deficit in motivation to seek cocaine, given that dCA3 inhibition without memory reactivation did not attenuate cocaine-seeking behavior (Fig. 3). Alternatively, attenuated cocaine-seeking behavior could reflect enhancement in extinction memory consolidation despite the short duration of the non-reinforced memory reactivation session. This possibility is mitigated somewhat by a report that dCA3 lesions after explicit extinction learning fail to alter context-dependent extinction memory recall [30]. Optogenetic dCA3 inhibition during the second h following memory reactivation failed to alter cocaine-seeking behavior (Fig. 4), indicating that neural activity in the dCA3 is no longer required for memory reconsolidation after the first h of information processing. Similarly, laser-light exposure without eNpHR3.0 expression did not alter cocaine-seeking behavior (Fig. 2), further confirming that diminished memory strength following optogenetic inhibition in experiment 1 did not result from heat- or AAV-induced tissue damage. Instead, memory impairment resulted from neuronal inhibition, as indicated by reduced c-Fos expression in dCA3 SL (GAD67-IR) and SP (CaMKII-IR and GAD67-IR) cell populations during memory reconsolidation (Fig. 5). Overall, these findings suggest that the dCA3 is recruited during the reconsolidation of labile cocaine memories in a time-limited fashion and required for the maintenance and/or subsequent recall of these memories.
Our findings expand upon literature indicating that the DH plays a critical role in memory reconsolidation. Studies have shown that pharmacological or chemogenetic inhibition of the DH impairs not only Pavlovian [31] but also instrumental cocaine-memory reconsolidation [16, 17], and our findings suggest that the latter may be mediated at least in part by the dCA3. Conversely, de novo protein synthesis in the DH appears to be required for memory reconsolidation in some, but not all, paradigms. For instance, inhibition of protein synthesis in the DH disrupts spatial [32], object-recognition [33], and morphine-conditioned place preference [34] memory reconsolidation, but it fails to alter contextual fear-memory [35] reconsolidation, as well as cocaine-memory reconsolidation in our instrumental model [16]. Thus, DH engagement in memory reconsolidation probably varies depending on memory age, strength, and type. During the early stages of cocaine-memory reconsolidation, dCA3 neuronal activity may contribute to tagging post-reactivation short-term memories for restabilization [36] or maintaining them before they can be restabilized into long-term memories.
dCA3 SP and SL neurons are components of the neural circuitry of cocaine-memory reconsolidation
Optogenetic dCA3 inhibition diminished SL and SP neuronal activation during cocaine-memory reconsolidation (Fig. 5) and resulted in a cell layer-specific suppression of neuronal activation during the subsequent recall test (Fig. 1–4H, I). The dCA3 SP and SL receive input primarily from dentate gyrus granule cells via mossy fibers [37, 38]. Granule cells can robustly excite SP pyramidal neurons through synapses on apical dendrites proximal to cell bodies but also inhibit them indirectly, by densely innervating SL basket cells that provide feedforward inhibition onto SP pyramidal neurons [38, 39]. In turn, SP pyramidal neurons excite one another, interact with SP interneurons, and stimulate dCA1 pyramidal neurons via the Schaffer collaterals [37, 38]. Correlated activity in recurrent pyramidal neuronal circuits facilitates the (re)establishment of cell ensembles that encode memory traces (i.e., engrams) during associative learning [39] and after memory reactivation [31, 40–42]. The contribution of dCA3 pyramidal neurons to memory reconsolidation has not been explored previously, but studies indicate that glutamatergic pyramidal neurons in the DH are key to memory cocaine- and fear-memory reconsolidation [31, 43]. For instance, CaMKII-IR pyramidal neuronal activation in the DH is necessary for Pavlovian cocaine-memory reconsolidation [27]. NR2A-containing NMDA receptor stimulation in the DH is required for cocaine-memory reconsolidation in instrumental models [29]. Moreover, glutamatergic synaptic plasticity, specifically GluA2-containing AMPA receptor endocytosis is necessary for fear-memory reconsolidation [44]. SP pyramidal neurons and SL/SP GABAergic interneurons likely interact to facilitate cocaine-memory reconsolidation via protein synthesis-independent mechanisms [16]. As noted above, dCA3 pyramidal neurons may tag or maintain labile memories prior to restabilization [36], whereas dCA3 SL/SP GABAergic interneurons may facilitate this process by inhibiting extraneous neuronal activity [39]. The relationship between long-term cocaine-memory strength and dCA3 SL/SP neuronal activation at recall appears to be different. Memory impairment at test was associated with reduced SL and SP c-Fos expression in experiment 1 (Fig. 1H, I), while intact recall was associated with intact SL plus either intact or reduced SP c-Fos expression in experiments 2–4 (Fig. 2–4H–I). This pattern of findings indicates that dCA3 SP pyramidal neuronal mechanisms that result in concomitant c-Fos expression are not sufficient for recall. Intact SL c-Fos expression could result in floor effects in some control experiments, but not in experiment 3. Thus, SL GABAergic interneurons may facilitate recall, possibly by inhibiting non-engram pyramidal neuronal activity [39]. Future research will need to evaluate the causal involvement of specific dCA3 cell populations in these phenomena.
Our current understanding of the larger neural circuitry within which the dCA3 supports cocaine-memory reconsolidation is limited. We have shown previously that interaction between the DH and basolateral amygdala (BLA) is necessary for cocaine-memory reconsolidation [45]. Consistent with this, unilateral inhibition of neuronal activity in the DH combined with inhibition of protein synthesis in the contralateral BLA, a manipulation that functionally disconnects the DH and BLA, during reconsolidation impairs subsequent drug context-induced reinstatement and incubation of cocaine seeking [45]. Since there are no monosynaptic connections between the DH and BLA [46], the dCA3 and BLA must interact through relay brain regions within a more complex neural circuitry. One likely relay region is the dCA1 since dCA1 SP neurons exhibited reduced c-Fos expression during recall following the optogenetic inhibition of dCA3 neurons during reconsolidation (Fig. 1J, K). Furthermore, dCA3 outputs to the dCA1 have been causally implicated in contextual fear-memory reconsolidation [47], and the dCA1 and BLA exhibit synchronized theta activity during fear-memory reconsolidation [48]. In addition, relay regions between the dCA1 or the dCA1-dCA3 circuit and the BLA may include the ventral hippocampus [46, 49, 50], entorhinal cortex [51, 52], perirhinal cortex [46, 53], and septum [54, 55] based on their connectivity with both the BLA and DH and/or their recognized involvement in reconsolidation in other paradigms.
Conclusions
The functional integrity of the dCA3 is required for early-stage memory reconsolidation. Based on extant literature [39, 40] and changes in SP and SL neuronal activation observed after the memory reactivation session and at test, we propose that cocaine-memory reconsolidation and subsequent recall are shaped by interactions between excitatory pyramidal and SL/SP inhibitory interneurons that gate the activity of associative microcircuits. However, to further advance our understanding, studies will need to examine the causal contribution of dCA3 cell types to cocaine-memory reconsolidation. Future research will also need to systematically examine the time-dependent engagement of various brain regions during memory reconsolidation to help develop hypotheses about circuitry dynamics. Lastly, the hippocampal mechanisms of memory reconsolidation will need to be investigated in male and female subjects based on emerging evidence for sex differences in hippocampal physiology [56, 57] and in-memory reconsolidation outside of the DH [58–60].
Supplementary information
Acknowledgements
The authors are grateful for expert technical assistance provided by Justine M. C. Galliou, Peyton J. Krych, Taylor A. Brown, Jaclyn N. Roland-McGowan, and Shayna R. Grogan.
Author contributions
SQ: methodology, validation, investigation, data analysis, visualization, writing original draft. SMT: methodology, investigation, validation, data analysis, writing original draft. RW: investigation, validation. JAH: methodology, validation, investigation. JLR: methodology, investigation, visualization. CKI: methodology, investigation. AAA: methodology, validation, investigation. RJC: investigation. RFL: conceptualization, methodology, data analysis, writing original draft, review and editing, supervision, funding acquisition.
Funding
This research was funded by National Institute on Drug Abuse grants R01 DA025646 (RFL) and F31 DA045430 (JAH), and research funds provided by State of Washington Initiative Measure No. 173 (SMT, SQ). The authors have no conflicts to disclose.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shuyi Qi, Shi Min Tan.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01342-0.
References
- 1.Dackis CA, O’Brien CP. Cocaine dependence: A disease of the brain’s reward centers. J Subst Ab Treat. 2001;21:111–7. doi: 10.1016/S0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 2.Hyman SE. Addiction: A disease of learning and memory. Am J Psych. 2005;162:1414–22. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs RA, Lasseter HC, Ramirez DR, Xie X. Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Disco Today Dis Models. 2009;5:251–8. doi: 10.1016/j.ddmod.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender BN, Torregrossa MM Molecular and circuit mechanisms regulating cocaine memory. Cell Mol Life Sci. 2020;0123456789. [DOI] [PMC free article] [PubMed]
- 5.Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacol (Berl) 1992;107:523–9. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 6.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–75. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 7.Finnie PSB, Nader K. The role of metaplasticity mechanisms in regulating memory destabilization and reconsolidation. Neurosci Biobeh Rev. 2012;36:1667–707. doi: 10.1016/j.neubiorev.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Saladin ME, Gray KM, McRae-Clark AL, Larowe SD, Yeatts SD, Baker NL, et al. A double blind, placebo-controlled study of the effects of post-retrieval propranolol on reconsolidation of memory for craving and cue reactivity in cocaine-dependent humans. Psychopharmacology. 2014;226:721–37. doi: 10.1007/s00213-013-3039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lonergan M, Saumier D, Tremblay J, Kieffer B, Brown TG, Brunet A. Reactivating addiction-related memories under propranolol to reduce craving: A pilot randomized controlled trial. J Beh Ther Exp Psych. 2016;50:245–9. doi: 10.1016/j.jbtep.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56:186–95. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorg BA. Reconsolidation of drug memories. Neurosci Biobeh Rev. 2012;36:1400–17. doi: 10.1016/j.neubiorev.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Exton-McGuinness MTJ, Milton AL. Reconsolidation blockade for the treatment of addiction: Challenges, new targets, and opportunities. Learn Mem. 2018;25:492–500. doi: 10.1101/lm.046771.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JLC. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat Neurosci. 2008;11:1264–6. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- 14.Solstad T, Moser EI, Einevoll GT. Inhibition of mRNA synthesis in the hippocampus impairs consolidation and reconsolidation of spatial memory. Hippocampus. 2006;1031:1026–31. doi: 10.1002/hipo.20244. [DOI] [PubMed] [Google Scholar]
- 15.Lee JLC, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–43. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA, Fuchs RA. Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:1–7. doi: 10.1111/j.1460-9568.2009.06889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells AM, Xie X, Higginbotham JA, Arguello AA, Healey M, Fuchs RA. Contribution of an SFK-mediated signaling pathway in the dorsal hippocampus to cocaine-memory reconsolidation in rats. Neuropsychopharmacology. 2016;41:675–85. doi: 10.1038/npp.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kesner RP. A process analysis of the CA3 subregion of the hippocampus. Front Cell Neurosci. 2013;7:1–17. doi: 10.3389/fncel.2013.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Duigou C, Simonnet J, Teleñczuk MT, Fricker D, Miles R. Recurrent synapses and circuits in the CA3 region of the hippocampus: An associative network. Front Cell Neurosci. 2014;7:1–13. doi: 10.3389/fncel.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lines J, Nation K, Fellous JM. Dorsoventral and proximodistal hippocampal processing account for the influences of sleep and context on memory (re)consolidation: A connectionist model. Comput Intell Neurosci. 2017;2017:8091780. doi: 10.1155/2017/8091780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang B, Zhu H, Zhou Y, Liu X, Ma L. Unconditioned- and conditioned-stimuli induce differential memory reconsolidation and β-AR-dependent CREB activation. Front Neural Circuits. 2017;11:1–10. doi: 10.3389/fncir.2017.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 23.Dȩbiec J, Doyère V, Nader K, LeDoux JE. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc Natl Acad Sci USA. 2006;103:3428–33. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arguello AA, Richardson BD, Hall JL, Wang R, Hodges MA, Mitchell MP, et al. Role of a lateral obital fontal crtex-basolateral amygdala circuit in cue-induced cocaine-seeking behavior. Neuropsychopharmacology. 2017;42:727–35. doi: 10.1038/npp.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calu DJ, Kawa AB, Marchant NJ, Navarre BM, Henderson MJ, Chen B, et al. Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats. J Neurosci. 2013;33:214–26. doi: 10.1523/JNEUROSCI.2016-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–66. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nader K, Hardt O. A single standard for memory: The case for reconsolidation. Nat Rev Neurosci. 2009;10:224–34. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 28.Richards BA, Frankland PW. The persistence and transience of memory. Neuron. 2017;94:1071–84. doi: 10.1016/j.neuron.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 29.Roy DS, Arons A, Mitchell TI, Pignatelli M, Ryan TJ, Tonegawa S. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature. 2016;531:508–12. doi: 10.1038/nature17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji J, Maren S. Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn Mem. 2008;15:244–51. doi: 10.1101/lm.794808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu C, Sun X, Wang Z, Le Q, Liu P, Jiang C, et al. Retrieval-induced upregulation of Tet3 in pyramidal neurons of the dorsal hippocampus mediates cocaine-associated memory reconsolidation. Int J Neuropsychopharmacol. 2018;21:255–66. doi: 10.1093/ijnp/pyx099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossato JI, Bevilaqua LRM, Medina JH, Izquierdo I, Cammarota M. Retrieval induces hippocampal-dependent reconsolidation of spatial memory. Learn Mem. 2006;13:431–40. doi: 10.1101/lm.315206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossato JI, Bevilaqua LR, Myskiw JC, Medina JH, Izquierdo I, Cammarota M. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem. 2007;14:36–46. doi: 10.1101/lm.422607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. J Neurosci. 2006;26:3010–20. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biedenkapp JC, Rudy JW. Context memories and reactivation: Constraints on the reconsolidation hypothesis. Behav Neurosci. 2004;118:956–64. doi: 10.1037/0735-7044.118.5.956. [DOI] [PubMed] [Google Scholar]
- 36.Rabinovich Orlandi I, Fullio CL, Schroeder MN, Giurfa M, Ballarini F, Moncada D. Behavioral tagging underlies memory reconsolidation. PNAS. 2020;117:18029–36. doi: 10.1073/pnas.2009517117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spruston N, Lübke J, Frotscher M. Interneurons in the stratum lucidum of the rat hippocampus: An anatomical and electrophysiological characterization. J Comp Neurol. 1997;385:427–40. doi: 10.1002/(SICI)1096-9861(19970901)385:3<427::AID-CNE7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Le Duigou C, Simonnet J, Teleñczuk MT, Fricker D, Miles R. Recurrent synapses and circuits in the CA3 region of the hippocampus: an associative network. Front Cell Neurosci. 2014;7:262. doi: 10.3389/fncel.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apóstolo N, Smukowski SN, Vanderlinden J, Condomitti G, Rybakin V, Ten Bos J. Synapse type-specific proteomic dissection identifies IgSF8 as a hippocampal CA3 microcircuit organizer. Nat Comm. 2020;11:5171. doi: 10.1038/s41467-020-18956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Kwon JT, Kim HS, Josselyn SA, Han JH. Memory recall and modifications by activating neurons with elevated CREB. Nat Neurosci. 2014;17:65–72. doi: 10.1038/nn.3592. [DOI] [PubMed] [Google Scholar]
- 41.Sekeres MJ, Mercaldo V, Richards B, Sargin D, Mahadevan V, Woodin MA, et al. Increasing CRTC1 function in the dentate gyrus during memory formation or reactivation increases memory strength without compromising memory quality. J Neurosci. 2012;32:17857–68. doi: 10.1523/JNEUROSCI.1419-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yousuf M, Packard PA, Fuentemilla L, Bunzeck N. Functional coupling between CA3 and laterobasal amygdala supports schema dependent memory formation. NeuroImage. 2021;244:118563. doi: 10.1016/j.neuroimage.2021.118563. [DOI] [PubMed] [Google Scholar]
- 43.Wells AM, Xie X, Higginbotham JA, Arguello AA, Healey KL, Blanton M, et al. Contribution of an SFK-mediated signaling pathway in the dorsal hippocampus to cocaine-memory reconsolidation in rats. Neuropsychopharmacology. 2016;41:675–85. doi: 10.1038/npp.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao-Ruiz P, Rotaru DC, Van Der Loo RJ, Mansvelder HD, Stiedl O, Smit AB, et al. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci. 2011;14:1302–8. doi: 10.1038/nn.2907. [DOI] [PubMed] [Google Scholar]
- 45.Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, Fuchs RA. Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem. 2011;18:693–702. doi: 10.1101/lm.2273111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. Ann N. Y. Acad Sci. 2000;911:369–91. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- 47.Lux V, Masseck OA, Herlitze S, Sauvage MM. Optogenetic destabilization of the memory trace in CA1: Insights into reconsolidation and retrieval processes. Cereb Cortex. 2017;27:841–51. doi: 10.1093/cercor/bhv282. [DOI] [PubMed] [Google Scholar]
- 48.Narayanan RT, Seidenbecher T, Sangha S, Stork O, Pape HC. Theta resynchronization during reconsolidation of remote contextual fear memory. NeuroReport. 2007;18:1107–11. doi: 10.1097/WNR.0b013e3282004992. [DOI] [PubMed] [Google Scholar]
- 49.Tao S, Wang Y, Peng J, Zhao Y, He X, Yu X, et al. Whole-brain mapping the direct inputs of dorsal and ventral CA1 projection neurons. Front Neural Circuits. 2021;15:643230. doi: 10.3389/fncir.2021.643230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chia C, Otto T. Hippocampal Arc (Arg3.1) expression is induced by memory recall and required for memory reconsolidation in trace fear conditioning. Neurobiol Learn Mem. 2013;106:48–55. doi: 10.1016/j.nlm.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Baldi E, Bucherelli C. Entorhinal cortex contribution to contextual fear conditioning extinction and reconsolidation in rats. Neurobiol Learn Mem. 2014;110:64–71. doi: 10.1016/j.nlm.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Wahlstrom KL, Alvarez-Dieppa AC, McIntyre CK, LaLumiere RT. The medial entorhinal cortex mediates basolateral amygdala effects on spatial memory and downstream activity-regulated cytoskeletal-associated protein expression. Neuropsychopharmacology. 2021;46:1172–82. doi: 10.1038/s41386-020-00875-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winters BD, Tucci MC, Jacklin DL, Reid JM, Newsome J. On the dynamic nature of the engram: Evidence for circuit-level reorganization of object memory traces following reactivation. J Neurosci. 2011;31:17719–28. doi: 10.1523/JNEUROSCI.2968-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witter MP. Intrinsic and extrinsic wiring of CA3: indications for connectional heterogeneity. Learn Mem. 2007;14:705–13. doi: 10.1101/lm.725207. [DOI] [PubMed] [Google Scholar]
- 55.McGlinchey EM, Aston-Jones G. Dorsal hippocampus drives context-induced cocaine seeking via inputs to lateral septum. Neuropsychopharmacology. 2018;43:987–1000. doi: 10.1038/npp.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferraro A, Wig P, Boscarino J, Reich CG. Sex differences in endocannabinoid modulation of rat CA1 dendritic neurotransmission. Neurobiol Stress. 2020;13:100283. doi: 10.1016/j.ynstr.2020.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McAlinn HR, Reich B, Contoreggi NH, Kamakura RP, Dyer AG, McEwen BS, et al. Sex differences in the subcellular distribution of corticotropin-releasing factor receptor 1 in the rat hippocampus following chronic immobilization stress. Neuroscience. 2018;383:98–113. doi: 10.1016/j.neuroscience.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flint RW, Jr, Valentine S, Papandrea D., Jr Reconsolidation of a long-term spatial memory is impaired by cycloheximide when reactivated with a contextual latent learning trial in male and female rats. Neuroscience. 2007;148:833–44. doi: 10.1016/j.neuroscience.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 59.Higginbotham JA, Jones N, Tan S, Wang R, Presker MJ, Fuchs RA. Endocannabinoid receptor-mediated effects on cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior. Neuropsychopharmacology. 2017;43:S290–S291. [Google Scholar]
- 60.Ritchie JL, Walters JL, Galliou JMC, Christian RJ, Qi S, Savenkova MI, et al. Basolateral amygdala corticotropin-releasing factor receptor type 1 regulates context-cocaine memory strength during reconsolidation in a sex-dependent manner. Neuropharmacology. 2021;200:108819. doi: 10.1016/j.neuropharm.2021.108819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.