Abstract
Premature ventricular contractions (PVCs) during recovery of exercise stress testing are associated with increased cardiovascular mortality, but the cause remains unknown. We aimed to evaluate the association of PVCs during recovery with echocardiographic abnormalities, and their combined prognostic performance. Echocardiographic abnormalities [reduced left ventricular (LV) ejection fraction, valvular heart disease, LV dilatation, LV hypertrophy, or increased filling pressures] and PVCs during recovery were identified among patients having undergone both echocardiography and exercise stress test. Among included patients (n = 3106, age 59 ± 16 years, 55% males), PVCs during recovery were found in 1327 (43%) patients, among which the prevalence of echocardiographic abnormalities was increased (58% vs. 43%, p < 0.001). Overall, PVCs during recovery were associated with increased cardiovascular mortality (219 total events, 7.9 [5.4–11.1] years follow-up; adjusted hazard ratio (HR [95% confidence interval]) 1.6 [1.2–2.1], p < 0.001). When analyzed in combination with either presence or absence of echocardiographic abnormalities, PVCs during recovery were associated with increased risk when such were present (HR 3.3 [1.9–5.5], p < 0.001) but not when absent (HR 1.5 [0.8–2.8], p = 0.22), in reference to those with neither. Our findings provide mechanistic insights to the increased CV risk reported in patients with PVCs during recovery.
Subject terms: Cardiology, Medical research, Risk factors
Introduction
Exercise-induced premature ventricular contractions (PVCs) are common findings in exercise stress testing and have been described to associate with increased risk of cardiovascular (CV) mortality, especially when occurring during the recovery phase1–11. This has been observed in patients across a wide range of clinical risk profiles, from presumably healthy, asymptomatic individuals to patients with established CV disease1–4,6,7,11–13. This suggests that the etiology of PVCs during recovery is multifactorial although the cause remains to be elucidated. PVCs during recovery after exercise have been suggested to be caused by imbalances in autonomic tone5,6,14, or to be related to myocardial ischemia12. Despite the extensive data on prognostic information from exercise-induced PVCs, data on structural heart disease in patients with PVCs during the recovery phase are limited and restricted to assessment of left ventricular ejection fraction (LVEF)1,15. Therefore, we aimed to investigate the association of PVCs during recovery after bicycle exercise stress with abnormalities on echocardiographic examination, and to evaluate the prognostic value of the combination of PVCs during recovery and abnormal findings on echocardiography.
Methods
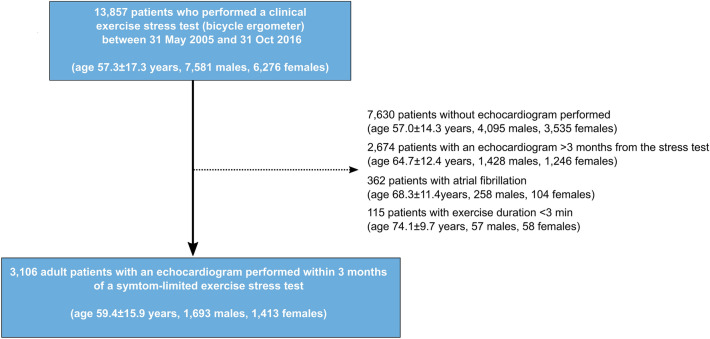
We performed a retrospective cohort study including patients aged 18 years or older who had performed a clinical bicycle exercise stress test and a resting transthoracic echocardiography within 90 days of the exercise stress test at the Department of Clinical Physiology at Kalmar County Hospital, Sweden, between 31 May 2005 and 31 Oct 2016. The exercise stress test database has been described in detail elsewhere16–20. We excluded those who had an exercise time less than 3 min or had atrial fibrillation, considering the challenge of distinguishing aberrantly conducted supraventricular complexes from PVCs. Full inclusion and exclusion criteria are shown in Fig. 1. The database has been cross-linked with comorbidity and hospital admission data in the Swedish National Patient Registry, and with the Swedish National Causes of Death Registry to obtain survival status of all patients (up to 31 Dec 2019)21. The underlying cause of death, e.g. CV death, can be identified in the National Causes of Death Registry and is based on the death certificate, usually completed by the patient’s regular physician or the physician who last saw the patient. A detailed description on how the underlying cause of death is identified has been described previously22. In brief, it is defined as the disease (or injury) which initiated the train of events leading directly to death. For this study, any underlying cause of death within the CV disease International Classification of Diseases (ICD)-10 chapter (Ix) has been considered as CV death. List of definitions of comorbid conditions including ICD-10 codes can be found in Supplementary Table 1.
Figure 1.
Flow chart of patient inclusion and exclusion.
Exercise stress testing
Exercise stress testing was performed according to a nationally standardized protocol on an electronically braked, regularly calibrated bicycle ergometer (Rodby Inc, Karlskoga, Sweden) and commenced at a starting load of 40–100 W for males and 30–50 W for females, with an incremental increase of 10–20 W/min23. A 12-lead ECG was recorded at rest, during exercise and the recovery phase which lasted at least 4 min (CASE 12; Marquette Electronics Inc., Milwaukee, WI, USA and CASE v 6.51, GE Healthcare, Milwaukee, WI, USA). Every 2 min during exercise, the patients were queried regarding rating of perceived exertion (RPE), and systolic blood pressure was measured. The test was terminated at the patient’s will or if any termination criteria were fulfilled (severe chest pain, ST-depression ≥ 0.4 mV, a drop in systolic blood pressure or occurrence of severe arrhythmia (ventricular tachycardia [VT] defined as at least 3 PVCs in a row, PVCs in increasing frequency or complexity, supraventricular tachycardia > 200/min, or AV block II or III occurring during exercise)23. At termination of exercise, the patient directly returned to supine position.
ECG abnormalities at rest before, after, and during stress were noted by the attending physician at the time of the test and were not re-interpreted for the purpose of this study. Peak workload was related to nationally standardized reference values (% of predicted Wmax)19. The amplitude and slope of the ST segment during the exercise stress test were measured 60 ms following the J-point (ST60). For this study, ST depression was defined as horizontal or down-sloping ST depression ≥ 0.1 mV in V5 during exercise or during the recovery phase. Heart rate recovery was defined as the difference in heart rate between the maximal heart rate and the heart rate 1 min after cessation of exercise24,25. For patients who had performed more than one test, only the most recent test was included.
Frequency of PVCs in the recovery phase
PVC frequency during recovery was graded at the time of the stress test according to clinical routine as either < 1 PVC/min, 1–5 PVCs/min, 5–10/min, or > 10/min during the first 4 min of recovery. Studies have applied different definitions of frequent PVCs1–7,9,10, ranging from the use of the median frequency in the dataset (frequent = above the median)1,6,10, to 10% of all depolarizations within a specific time frame4,7,9, or a fixed value, e.g. > 10/min3. Thus, any cutoff would be arbitrary and previous studies have shown that cardiovascular risk may be similar for patients with frequent PVCs compared to those with infrequent PVCs6. This suggests that the prognostic significance lies in the presence of PVCs during recovery rather than the quantity. Consequently, we chose to primarily divide our study population into two groups based on < 1 or ≥ 1 PVC/min during the first 4 min of recovery. For readability, these patients will subsequently be referred to as having no PVCs during recovery, although they, by definition, may have had 0–3 PVCs during the first 4 min of recovery. As a supplemental analysis, we also present the results based on the grading used in the clinical reports, i.e., < 1 PVC/min, 1–5 PVCs/min, 5–10/min, or > 10/min.
Echocardiography
Standard echocardiographic examinations were acquired during clinical routine by skilled ultrasound technicians under physician supervision. LV ejection fraction (EF) was reported either based on the Simpson biplane method, from M-mode data, or by visual estimation. Data on grade of aortic, mitral or tricuspid regurgitation (0–3: none, mild, moderate, severe) were extracted from the echocardiographic reports. Moderate aortic stenosis was defined as aortic valve (AV) maximal velocity ≥ 3.1–4.0 m/s or AV mean gradient 20–40 mmHg, and severe aortic stenosis as AV maximal velocity ≥ 4.0 m/s or AV mean gradient ≥ 40 mmHg26.
LV mass was calculated according to the Cube formula (LV mass (g) = 0.8 × 1.04 ([IVS + LVID + PW]3 – LVID3) + 0.6)27. Left-sided cardiac dimensions (LV diameter, LA diameter, LV mass) were indexed to body-surface area (BSA) and were considered abnormal if they exceeded established sex-based reference values (LV diameter: ≥ 31 mm/m2 (males), ≥ 32 mm/m2 (females); LA diameter: ≥ 23 mm/m2 (both sexes); LV mass: > 115 g/m2 (males), > 95 g/m2 (females))27. Similarly, LVEF below the sex-based reference value was considered to be reduced (< 52% (males), < 54% (females))27.
Significant valvular heart disease was defined as at least moderate aortic/mitral/tricuspid regurgitation or at least moderate aortic stenosis. Increased right ventricle to right atrial (RV–RA) pressure gradient was defined as a tricuspid regurgitant velocity exceeding 2.8 m/s. Increased LV filling pressures were defined as E/e′ ≥ 15 and a dilated LA, or E/e′ ≥ 15 and an increased RV–RA pressure gradient. An echocardiographic abnormality was considered present if any of the following criteria were met: (a) reduced LVEF, (b) significant valvular heart disease, (c) LV dilatation, (d) increased LV filling pressures or (e) LV hypertrophy. Reasons for referral/primary referral questions are presented as supplemental material.
Statistical analysis
Continuous variables were described as mean ± standard deviation [SD], or as median [interquartile range]. Proportional differences between groups were assessed using the χ2 test. Comparison of group means was performed using Student’s t test or analysis of variance (ANOVA). In order to account for baseline differences in age and sex, the relation between the frequency of PVCs in the stress test recovery phase and subsequent findings on echocardiography was described not only as proportions, but also using odds ratios (OR) with 95% confidence intervals (CI) from multivariable logistic regression.
Time-to-event analysis was performed using Kaplan–Meier analysis with censoring at study end (31 Dec 2019). The association between PVCs during recovery and CV mortality was analyzed using multivariable Cox proportional hazard regression models; unadjusted and adjusted for age, sex, hypertension, heart failure, ischemic heart disease, diabetes mellitus, body mass index, peak workload, maximal heart rate, maximal systolic blood pressure, heart rate recovery, ST depression, cardiovascular medications, and echocardiographic abnormalities. Further, the combination of PVCs during recovery and presence/absence of echocardiographic abnormalities were analyzed unadjusted and adjusted for age, sex, baseline comorbidities (hypertension, heart failure, ischemic heart disease, diabetes mellitus, body mass index), peak workload, maximal heart rate, maximal systolic blood pressure, heart rate recovery, and ST depression, and cardiovascular medications. The choice of confounding variables was based on subject matter knowledge and directed acyclic graphs.
In order to evaluate which echocardiographic abnormalities that were predictive of CV mortality in patients with PVCs during recovery, each single echocardiographic parameter included in the definition of ‘echocardiographic abnormalities’ (increased E/e ratio, LV dilatation, increased LV mass, increased LA diameter, reduced LVEF, increased RV-RA pressure gradient, moderate/severe aortic regurgitation, moderate/severe aortic stenosis, moderate/severe mitral regurgitation, moderate/severe tricuspid regurgitation) was evaluated regarding its independent relation to CV death, both in univariable and multivariable analysis, instead of analyzing the association between any echocardiographic abnormalities as a group. Furthermore, the combination of PVCs during recovery and echocardiographic abnormalities were separately evaluated for its association with incident acute coronary syndrome (ACS; acute myocardial infarction or unstable angina) during follow-up and within one year. Hazard ratios (HR) with 95% CI were presented. The assumption of proportional hazards was confirmed using Schoenfeld’s residuals. Possible multicollinearity was evaluated by variance inflation factor. The following sensitivity analyses were performed in relation to the Cox regression analysis: (1) excluding patients with PVCs at rest according to the clinical resting ECG report, since PVCs during the recovery phase could reflect PVCs at rest although unrelated to exercise, and (2) including only patients who underwent echocardiography more than 3 months after the exercise stress test, since there may be a selection bias when only those that were referred for both exercise stress test and echocardiography at almost the same time were included, and (3) stratifying patients based on the grading of PVC frequency used in the clinical reports (< 1 PVC/min, 1–5/min, ≥ 5–10, > 10/min), and (4) stratifying the analysis by recency (echocardiogram performed before or after the year 2010), since technical advances may have had an impact of the results. The results of the sensitivity analyses are presented as supplemental material. We also reported the results for all-cause mortality since this outcome is likely to be less dependent on the consistency in diagnostic reporting.
The likelihood of PVCs during recovery was assessed by univariable and multivariable logistic regression analysis. Age, male sex, relevant comorbidities (diabetes, hypertension, ischemic heart disease), echocardiographic variables (at least moderate valvular heart disease, LVEF, increased E/e′, increased LV mass, LV and LA dilatation) and exercise variables assumed to be related to parasympathetic tone (resting heart rate, difference in heart rate from supine rest to sitting, heart rate recovery, % of predicted maximal heart rate) were included in univariable analysis and, if the p value was less than 0.1, subsequently in the multivariable analysis.
Statistical significance was accepted at the level of p < 0.05 (two-sided). Statistical analysis was performed using R v. 3.5.3 (R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (https://www.R-project.org/).
Ethical approval
The study complies with the Declaration of Helsinki and was approved by the Regional Ethical Review Board in Linköping (Dnr 2012/379-31; 2018/141-31 and 2020/00352). Informed consent was waived by the Ethical Review Board in Linköping.
Results
In total, 3106 patients were included (age 59 ± 16 years, 54.5% males) and followed for a median 7.9 years [IQR 5.4–11.1, range 0–15], range 0.0–14.6 years.
PVCs during the recovery phase were present in 1327 (42.7%) patients. During follow-up, 219 (7.1%) patients died from a CV cause (10.4% of patients with vs. 4.6% without PVCs during recovery phase, p < 0.001). Details on cause of death beyond the first categorical level (I: Diseases of the circulatory system) are presented in Supplementary Table 2. Baseline characteristics based on the presence of PVCs during the recovery phase are presented in Table 1. Besides older age and a larger proportion of male subjects among those with than without PVCs during recovery, there were no major differences in comorbidities between groups. Peak workload was lower in patients with PVCs, but the difference in mean peak workload as % of predicted was not clinically relevant. A larger proportion of tests were terminated by the physician due to arrhythmia among those with PVCs during recovery.
Table 1.
Baseline characteristics including exercise stress test characteristics stratified by frequency of premature ventricular contractions during the recovery phase.
| PVC frequency during recovery phase | ||
|---|---|---|
| < 1/min | ≥ 1/min | |
| Number of patients | 1779 | 1327 |
| Age, years | 55.6 ± 17.0 | 65.6 ± 13.3 |
| Male sex, n (%) | 901 (50.6) | 792 (59.7) |
| Hypertension, n (%) | 265 (14.9) | 216 (16.3) |
| Diabetes, n (%) | 149 (8.4) | 133 (10.0) |
| Ischemic heart disease, n (%) | 164 (9.2) | 133 (10.0) |
| Of which, myocardial infarction, n (%) | 82 (4.6) | 46 (3.5) |
| Cerebrovascular disease, n (%) | 32 (1.8) | 36 (2.7) |
| COPD, n (%) | 22 (1.2) | 40 (3.0) |
| Clinical HF classification | ||
| No HF, n (%) | 1651 (92.8) | 1168 (88.0) |
| HFpEF, n (%) | 33 (1.9) | 39 (2.9) |
| HFmrEF, n (%) | 43 (2.4) | 63 (4.7) |
| HFrEF, n (%) | 52 (2.9) | 57 (4.3) |
| Medications | ||
| ACE inhibitor, n (%) | 288 (16.2) | 289 (21.8) |
| Betablocker, n (%) | 486 (27.3) | 419 (31.6) |
| Loop diuretics, n (%) | 136 (7.6) | 126 (9.5) |
| Calcium antagonists, n (%) | 219 (12.3) | 194 (14.6) |
| Thiazide diuretics, n (%) | 92 (5.2) | 90 (6.8) |
| Anti-thrombotic, n (%) | 355 (20.0) | 372 (28.0) |
| Nitrates, n (%) | 203 (11.4) | 197 (14.8) |
| Anti-arrhythmic, n (%) | 4 (0.2) | 1 (0.1) |
| Anti-coagulant, n (%) | 46 (2.6) | 62 (4.7) |
| Exercise stress test variables | ||
| Peak workload, W | 156 ± 62 | 144 ± 53 |
| Peak workload, % of predicted | 86 ± 19 | 84 ± 18 |
| PVCs during exercise, n (%) | ||
| 1–5/min | 346 (19.4) | 654 (49.3) |
| 5–10/min | 17 (1.0) | 159 (12.0) |
| > 10/min | 2 (0.1) | 18 (1.4) |
| Test terminated due to arrhythmia, n (%) | 3 (0.2) | 35 (2.6) |
| Resting heart rate, beats/min | 73 ± 14 | 72 ± 13 |
| Maximum heart rate, beats/min | 148 ± 26 | 143 ± 25 |
| Heart rate recovery, beats/min | 31 ± 15 | 28 ± 15 |
| Maximum SBP, mmHg | 189 ± 30 | 192 ± 30 |
| RPE (6–20), units | 17 ± 1 | 17 ± 1 |
| ST depression, n (%) | 151 (8.5) | 212 (16.0) |
Data presented as mean ± standard deviation or n (%).
ACE angiotensin converting enzyme, COPD chronic obstructive pulmonary disease, HF heart failure, HFmrEF heart failure with moderately reduced ejection fraction, HFpEF heart failure with preserved ejection fraction, HFrEF heart failure with reduced ejection fraction, PVC premature ventricular contractions, SBP systolic blood pressure, W watts.
An echocardiographic examination was performed within 1 day of the exercise stress test in 74.6% of patients, median [interquartile range] 0 [0–2] days. An increased prevalence of echocardiographic abnormalities was found for patients with PVCs during the recovery phase (57.6% vs 42.9%, p < 0.001 (Table 2). Mean LVEF was only slightly lower in patients with PVCs during the recovery phase (62% vs 64%, p < 0.001), but the proportion of patients with reduced LVEF was higher in patients with PVCs during recovery compared to those without PVCs (14% vs. 9%, p < 0.001). PVCs during recovery was associated with increased odds of having echocardiographic abnormalities, even after adjustment for age and sex (OR 1.4 [1.2–1.6], p < 0.001).
Table 2.
Echocardiographic outcomes stratified by occurrence of premature ventricular contractions during the recovery phase.
| PVC frequency during recovery phase | |||
|---|---|---|---|
| < 1/min | ≥ 1/min | ||
| Number of patients | 1779 | 1327 | p |
| LV diameter, indexed to BSA, mm/m2 | 47 ± 5 | 49 ± 6 | < 0.001 |
| LA diameter, indexed to BSA, mm/m2 | 38 ± 6 | 40 ± 6 | < 0.001 |
| LVEF, % | 64 ± 11 | 62 ± 11 | 0.001 |
| Septal wall thickness, mm | 10.7 ± 2.2 | 11.3 ± 2.2 | < 0.001 |
| Posterior wall thickness, mm | 10.1 ± 1.9 | 10.6 ± 1.8 | < 0.001 |
| LV mass, indexed to BSA, g/m2 | 95 ± 25 | 105 ± 29 | < 0.001 |
| E/A ratio | 1.2 ± 0.5 | 1.1 ± 0.4 | < 0.001 |
| E/e′ | 10.3 ± 4.3 | 11.2 ± 4.4 | < 0.001 |
| AV Vmax, m/s | 1.5 ± 0.5 | 1.6 ± 0.7 | < 0.001 |
| VTILVOT, cm | 21.4 ± 4.1 | 20.9 ± 4.2 | 0.001 |
| Moderate/severe AS, n (%) | 49 (2.8) | 55 (4.1) | 0.042 |
| Moderate/severe AR, n (%) | 43 (2.4) | 49 (3.7) | 0.049 |
| Moderate/severe MR, n (%) | 96 (5.4) | 135 (10.2) | < 0.001 |
| Moderate/severe TR, n (%) | 121 (6.8) | 144 (10.9) | < 0.001 |
| TR velocity, m/s | 2.3 ± 0.3 | 2.4 ± 0.3 | < 0.001 |
| Reduced LVEF, n (%) | 163 (9.2) | 191 (14.4) | < 0.001 |
| LV dilatation, n (%) | 42 (2.4) | 80 (6.0) | < 0.001 |
| Increased LA diameter, n (%) | 266 (15.0) | 289 (21.8) | < 0.001 |
| Increased LV mass, n (%) | 439 (24.7) | 453 (34.1) | < 0.001 |
| Increased RV-RA pressure gradient, n (%) | 62 (3.5) | 80 (6.5) | 0.001 |
| Increased filling pressuresa, n (%) | 63 (3.5) | 87 (6.6) | < 0.001 |
| Significant valve disease, n (%) | 244 (13.7) | 289 (21.8) | < 0.001 |
| Any echocardiographic abnormality, n (%) | 781 (43.9) | 763 (57.5) | < 0.001 |
Data presented as mean ± standard deviation or n (%).
Data on LVEF, LV diameter, LA diameter and E/e′ were available in all 3106 cases, data on BSA-indexed value for LV diameter, LA diameter and LV were available in 3046 cases, data on RV/RA pressure gradient were available in 1949 cases.
AR aortic regurgitation, AS aortic stenosis, BSA body-surface area, LA left atrial, LV left ventricular, LVEF left ventricular ejection fraction, MR mitral regurgitation, PVC premature ventricular contractions, TR tricuspid regurgitation.
aIncreased LV filling pressures were defined as E/e′ ≥ 15 and a dilated LA, or E/e′ ≥ 15 and an increased RV–RA pressure gradient.
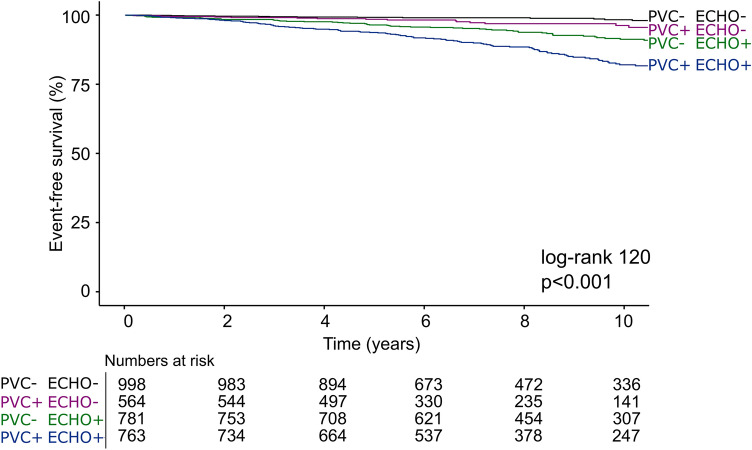
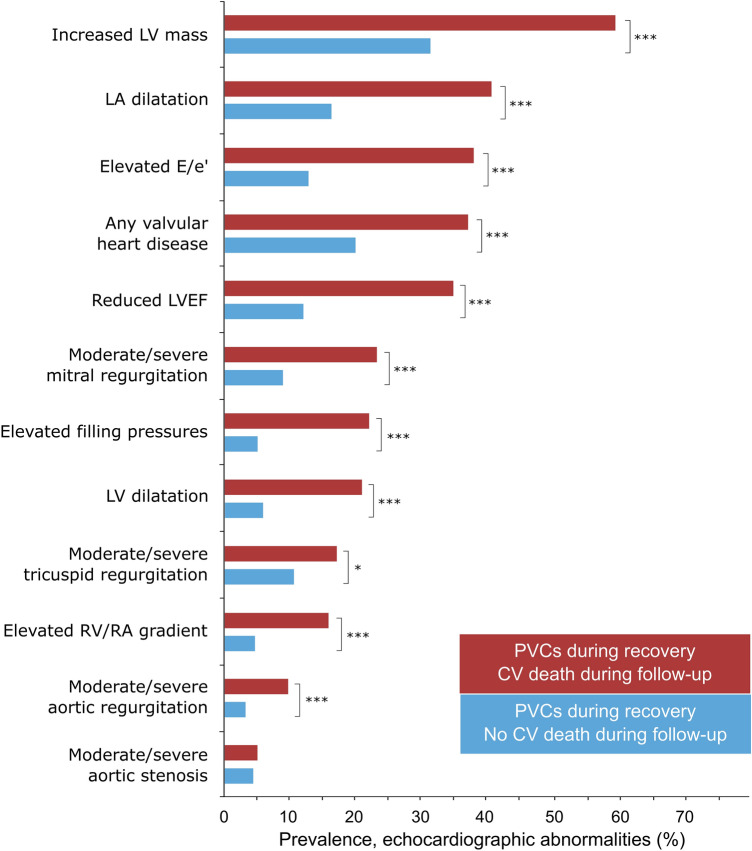
PVCs during recovery were associated with increased CV mortality (unadjusted HR 2.6 [2.0–3.4]), also after adjustment for age, sex, clinical variables, exercise stress testing variables and echocardiographic abnormalities (1.6 [1.2–2.1]). However, when analyzed in combination with either presence or absence of echocardiographic abnormalities, PVCs during recovery were only associated with increased risk of CV death when echocardiographic abnormalities were present (Table 3, Figs. 2, 3). The risk for patients with PVCs during recovery but no echocardiographic abnormalities was not increased relative to those with < 1 PVC/min PVCs during recovery and absent echocardiographic abnormalities. The proportion of echocardiographic abnormalities in patients with PVCs during recovery stratified by CV death during follow-up is presented in Fig. 4. Among those with PVCs during recovery who died from a CV cause, the most common echocardiographic abnormality was increased LV mass, followed by LA dilatation, elevated E/e′, any valvular heart disease, and reduced LVEF. In patients with PVC during recovery, increased LV mass, increased E/e′ ratio, LV dilatation were associated with CV mortality in the multivariable analysis, whereas LVEF, LA dilatation, increased RV/RA gradient and valvular heart disease were not (Table 4).
Table 3.
Hazard ratios [95% CI] for cardiovascular mortality based on combination of absence/presence of PVCs during recovery, and presence or absence of significant echocardiographic abnormalities, n = 3,106 (219 events).
| Unadjusted | Adjusted for age, sex, clinical and exercise variablesa | |
|---|---|---|
| PVC−/ECHO− | 1.0 | 1.0 |
| PVC+/ECHO− | 2.8 [1.5–5.2] | 1.5 [0.8–2.8] |
| PVC−/ECHO+ | 4.4 [2.5–7.4] | 2.0 [1.2–3.4] |
| PVC+/ECHO+ | 9.1 [5.5–15.2] | 3.3 [1.9–5.5] |
ECHO− no significant abnormality on echocardiography, ECHO+ significant abnormality on echocardiography, PVC premature ventricular contractions, PVC− < 1 PVCs/min during recovery, PVC+ ≥ 1 PVCs/min during recovery.
aHypertension, diabetes, ischemic heart disease, heart failure, body-mass index, peak workload, maximal heart rate, heart rate recovery, maximal systolic blood pressure, ST depression, use of either betablocker, angiotension-converting enzyme inhibitor, angiotension II blockers, loop diuretics, antihrombotics, anti-coagulants, or calcium channel blockers. Coefficients of confounders are presented in Supplements Table C.
Figure 2.
Time-to-event analysis for the combination of PVCs during recovery and abnormalities on echocardiography among 3106 patients experiencing 219 cardiovascular mortality events during 7.9 [5.4–11.1] years of follow-up. ECHO− no significant abnormality on echocardiography, ECHO+ significant abnormality on echocardiography, PVC premature ventricular contractions, PVC− < 1 PVC/min during recovery, PVC+ ≥ 1 PVC/min during recovery. An echocardiographic abnormality was defined as either: reduced left ventricular ejection fraction, at least moderate valvular heart disease, left ventricular dilatation, increased left ventricular mass, or increased left ventricular filling pressures.
Figure 3.
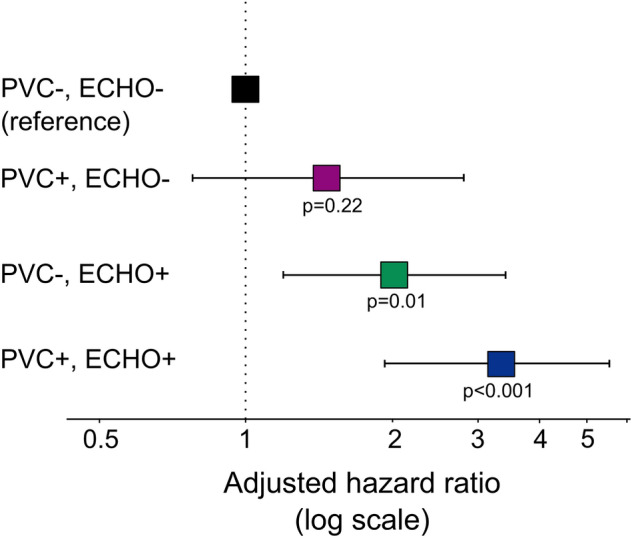
Forest plot showing hazard ratios for cardiovascular death with 95% confidence limits (adjusted for age, sex, hypertension, heart failure, ischemic heart disease, diabetes mellitus, body mass index, peak workload, maximal heart rate, maximal systolic blood pressure, heart rate recovery, ST depression, and cardiovascular medications) based on combinations of presence/absence of PVC during recovery and echocardiographic abnormalities. ECHO− no significant abnormality on echocardiography, ECHO+ significant abnormality on echocardiography, PVC premature ventricular contractions, PVC− < 1 PVC/min during recovery, PVC+ ≥ 1 PVC/min during recovery. An echocardiographic abnormality was defined as either: reduced left ventricular ejection fraction, at least moderate valvular heart disease, left ventricular dilatation, increased left ventricular mass or increased filling pressures defined as E/e′ ≥ 15 and either dilated LA or increased RV–RA pressure gradient.
Figure 4.
Prevalence of echocardiographic abnormalities in patients with PVC during recovery (n = 1327) stratified by cardiovascular (CV) death during follow-up (CV death: n = 138 (red bars); no death: 1189 (light blue bars). ***< 0.001, *< 0.05. Values for echocardiographic measurements within each group can be found in Table 2. LV left ventricular, LVEF left ventricular ejection fraction, LA left atrial, RV right ventricle, RA right atrium. Echocardiographic abnormalities were more common in patients with PVC during recovery and an adverse outcome.
Table 4.
Hazard ratio [95% confidence interval] for cardiovascular mortality based on presence or absence of echocardiographic abnormalities in patients with PVCs during recovery, n = 1327 (138 events).
| Univariable analysis | Multivariable analysisa | Wald | p | |
|---|---|---|---|---|
| Increased E/e′ ratio | 3.7 [2.6–5.3] | 2.0 [1.4–3.0] | 3.5 | < 0.01 |
| LV dilatation | 3.6 [2.3–5.6] | 2.2 [1.2–3.8] | 2.6 | 0.01 |
| Increased LV mass | 2.6 [1.8–3.6] | 1.7 [1.1–2.5] | 2.6 | 0.01 |
| Reduced LVEF | 3.3 [2.2–4.8] | 1.5 [1.0–2.5] | 1.8 | 0.07 |
| Increased RV/RA gradient | 3.4 [2.2–5.3] | 1.4 [0.9–2.6] | 1.8 | 0.08 |
| Moderate/severe aortic regurgitation | 2.4 [1.4–4.4] | 1.4 [0.8–2.6] | 1.1 | 0.27 |
| Moderate/severe mitral regurgitation | 2.6 [1.7–3.8] | 1.1 [0.7–1.9] | 0.6 | 0.52 |
| Increased LA diameter | 2.6 [1.8–3.6] | 1.0 [0.7–1.5] | 0.0 | 0.98 |
| Moderate/severe tricuspid regurgitation | 1.7 [1.1–2.7] | 0.9 [0.5–1.5] | − 1.2 | 0.22 |
| Moderate/severe aortic stenosis | 1.1 [0.5–2.4] | 0.6 [0.3–1.4] | − 1.1 | 0.29 |
LA left atrial, LV left ventricle/ventricular, LVEF LV ejection fraction.
aHypertension, diabetes, ischemic heart disease, heart failure, body-mass index, use of either betablocker, angiotension-converting enzyme inhibitor, angiotension II blockers, loop diuretics, antihrombotics, anti-coagulants, or calcium channel blockers.
In multivariable analysis, age, male sex, LV dilatation, increased LV mass, and maximal heart rate (in % of predicted) were the only independent predictors of PVCs during the recovery phase. Beyond maximal heart rate, indicators of abnormal autonomic tone, such as heart rate recovery, were not associated with increased frequency of PVCs during the recovery phase in multivariable analysis (Supplementary Table 3).
During follow-up, 285 patients (9.2%) were diagnosed with acute coronary syndrome (10.6% of patients with PVCs during recovery vs. 8.2% of patients without PVCs during recovery, p = 0.03). In patients with no prior diagnosis of ACS (n = 2954), PVCs during recovery was associated with increased risk of incident ACS both in the absence of concomitant echocardiographic abnormalities (HR 1.7 [1.1–2.6]), and in combination with echocardiographic abnormalities (HR 2.9 [2.0–4.2]). However, for ACS within 1 year, PVCs during recovery were only associated with increased risk in the presence of concomitant echocardiographic abnormalities (HR 3.3 [1.4–8.0]), but not for those with PVCs without echocardiographic abnormalities (HR 0.8 [0.2–3.0]), in reference to patients with neither.
Discussion
The main finding of the current study was that exercise-induced PVCs during the recovery phase were associated with increased CV mortality, and that this was only apparent when present together with echocardiographic abnormalities. These findings have two important implications. Firstly, they provide insights which may aid in the understanding of the well-known increased long-term CV risk in patients with exercise-induced PVCs during recovery. Secondly, the occurrence of PVCs during recovery implies a high diagnostic yield of subsequent echocardiography, therefore warranting further evaluation in search of structural heart disease.
Although patients with PVCs during the recovery phase had a negligible difference in mean LVEF compared to those without PVCs, the proportion of patients with reduced LVEF was markedly higher. Previous reports from cardiac imaging in the setting of exercise-induced PVCs have been restricted to the assessment of LVEF1. Frolkis, et al., studied the prognostic value of PVCs during the recovery phase in almost 30,000 patients without a history of heart failure, valvular heart disease, or arrhythmia, and found it to be a stronger predictor of all-cause death than PVCs during exercise. In 6421 of these patients, LVEF was assessed either by echocardiography or by contrast ventriculography within 3 months following the stress test. That study had similar findings as the current study, albeit with a higher prevalence of reduced LVEF than in the current study, both among patients with PVCs during recovery and in those without (28% vs. 13%). Our study provides additional insights that echocardiographic findings beyond LVEF are associated—and even more strongly so—with a worsened prognosis in patients with PVCs during the recovery phase1–6. This study adds to the current literature by showing that PVCs during recovery are associated with a higher rate of structural heart disease including LV hypertrophy, LV dilatation, increased LV filling pressures, and valvular heart disease. Moreover, several of these findings were more common in those with PVCs during recovery who died during follow-up, compared to those who survived, increased LV mass, elevated E/e′ ratio and LV dilatation, in particular. The possibility that the increased risk among those with PVCs is mediated by LV hypertrophy and/or mechanical stress is theoretically appealing since structural and electrical cardiac remodeling are known to be closely related28,29. LV hypertrophy has been described to increase repolarization heterogeneity, to disturb calcium ion concentration, and to cause both ion channel and gap junction remodeling, leading to increased susceptibility to develop ventricular arrythmias28,30. Also, this is in agreement with previous findings of increased CV risk among patients with PVCs at rest in combination with structural heart disease31–33. From our results, however, it is not possible to conclude whether it is the increased risk of arrythmia associated with structural heart disease, or if PVCs is a marker of significant structural heart disease, that would explain the increased CV risk.
Interestingly, we found no association between exercise variables assumed to be related to autonomic tone and the occurrence of PVCs during the recovery phase, after adjusting for age, sex and echocardiographic findings. Autonomic abnormalities have been suggested as a plausible cause of the increased CV mortality that has been observed both in low- and high-risk patients5,6,14. However, the results of the current study do not support such an association.
The findings of the current study are based on data from patients who performed both an exercise stress test and an echocardiogram based on clinical referral. This induces selection bias, and groups of patients that are not referred for an echocardiogram may have a different proportion of abnormal findings. However, in a sensitivity analysis for those who performed an echocardiogram unrelated to the exercise test, results were highly similar (Supplementary Table 6), which strengthens the generalizability of our results. A detailed description of the clinical and exercise characteristics in patients who did not perform any echocardiogram can be found in Supplementary Table 10.
Furthermore, we lack information on coronary anatomy as well as future investigations for coronary artery disease. Instead, incident ACS diagnoses were explored. We also lack information on differentiated CV causes of death, e.g. sudden cardiac death. However, very few patients underwent cardiac resuscitation and/or defibrillation during follow-up (11 out of 3106). Also, the effect of different medications on the outcome cannot be reliably assessed, since medications were known only at the start of the observation, and since prescriptions may be biased towards higher-risk individuals.
The use of registry data for cause of death is highly dependent on the quality of the records. Agreement for diagnoses between hospital records and registry data is high on the first categorical level, as applied in this study, but decreases with increasing level of detail22. Also, similar results were obtained when using all-cause mortality as an outcome.
This is a registry-based study including several thousands of patients and for practical reasons the analysis relied on clinical reports with tabulated values, instead of raw ECG or imaging data. We have no information on the absolute number of PVCs but relied on the grading in the clinical report (< 1/min, 1–5/min, 5–10/min, ≥ 10/min) and patients referred to in text as having no PVCs (< 1/min) may thus have had up to 3 PVCs during the first 4 min of recovery. We believe that equating 0–3 to none, in general, would be in agreement with most clinical test result interpretations.
Further, the classifications used relied on the number of PVCs only, i.e. not taking into account the complexity of ventricular arrythmia such as couplets, bigeminy or VT. The number of tests prematurely terminated due to severe arrhythmia among those with no or low-grade PVCs was small, indicating that this likely did not have had a substantial impact on the findings. Furthermore, measurements of RV and RA dimensions were not available and rare diagnoses such as arrhythmogenic right ventricular cardiomyopathy were therefore not evaluated. Nonetheless, they are expected to constitute a very small number of patients34. Despite the above-mentioned limitations, this large registry is unique in combining results of exercise stress testing and echocardiography, and although causal inferences cannot be made, we consider our findings to provide important, new insights to the previously established increased long-term CV risk reported in patients with PVCs during recovery.
In conclusion, PVCs during the recovery phase of exercise stress testing were associated with increased risk of abnormal findings on echocardiography. Importantly, increased CV mortality was observed only for subjects with PVCs who had concomitant echocardiographic abnormalities. Our findings provide mechanistic insights to the increased CV risk reported in patients with PVCs during recovery.
Supplementary Information
Author contributions
T.L. and M.U. contributed to the conception or design of the work. L.B., M.E., T.L. and K.H. contributed to the acquisition of data. L.B. and M.E. curated the data. All authors contributed to analysis, and interpretation of data for the work. T.L. drafted the manuscript. All authors critically revised the manuscript, gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
Open access funding provided by Lund University. This work was supported by The Swedish Heart–Lung Foundation [Grant no 20200553 to TL]; the Swedish Cardiac Society, the Royal Swedish Academy of Sciences [Grant no LM2019-0013 to TL]; Women and Health Foundation [TL], Region Kronoberg [Grant no 8301 to TL]; The Swedish Heart and Lung Association [Grant no LKH1387 to TL]; Swedish Association of Clinical Physiology [TL]; the Scandinavian Society of Clinical Physiology & Nuclear Medicine [TL); the Swedish Research Council [Dnr: 2019-02081 to ME]; unrestricted ALF grants from county council of Östergötland [KH]; New South Wales Health, Heart Research Australia [MU]; and the University of Sydney [TL, MU].
Data availability
The data that support the findings of this study are available from the corresponding author, [TL], upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-14535-w.
References
- 1.Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N. Engl. J. Med. 2003;348(9):781–790. doi: 10.1056/NEJMoa022353. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill JO, Young JB, Pothier CE, Lauer MS. Severe frequent ventricular ectopy after exercise as a predictor of death in patients with heart failure. J. Am. Coll. Cardiol. 2004;44(4):820–826. doi: 10.1016/j.jacc.2004.02.063. [DOI] [PubMed] [Google Scholar]
- 3.Refaat MM, Gharios C, Moorthy MV, Abdulhai F, Blumenthal RS, Jaffa MA, Mora S. Exercise-induced ventricular ectopy and cardiovascular mortality in asymptomatic individuals. J. Am. Coll. Cardiol. 2021;78(23):2267–2277. doi: 10.1016/j.jacc.2021.09.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jouven X, Zureik M, Desnos M, Courbon D, Ducimetière P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N. Engl. J. Med. 2000;343(12):826–833. doi: 10.1056/NEJM200009213431201. [DOI] [PubMed] [Google Scholar]
- 5.Lee V, Perera D, Lambiase P. Prognostic significance of exercise-induced premature ventricular complexes: A systematic review and meta-analysis of observational studies. Heart Asia. 2017;9(1):14–24. doi: 10.1136/heartasia-2016-010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewey FE, Kapoor JR, Williams RS, Lipinski MJ, Ashley EA, Hadley D, Myers J, Froelicher VF. Ventricular arrhythmias during clinical treadmill testing and prognosis. Arch. Intern. Med. 2008;168(2):225–234. doi: 10.1001/archinte.168.2.225. [DOI] [PubMed] [Google Scholar]
- 7.Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, Blumenthal RS. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: A 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290(12):1600–1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 8.Beckerman J, Mathur A, Stahr S, Myers J, Chun S, Froelicher V. Exercise-induced ventricular arrhythmias and cardiovascular death. Ann. Noninvasive Electrocardiol. 2005;10(1):47–52. doi: 10.1111/j.1542-474X.2005.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Partington S, Myers J, Cho S, Froelicher V, Chun S. Prevalence and prognostic value of exercise-induced ventricular arrhythmias. Am. Heart J. 2003;145(1):139–146. doi: 10.1067/mhj.2003.60. [DOI] [PubMed] [Google Scholar]
- 10.Morshedi-Meibodi A, Evans JC, Levy D, Larson MG, Vasan RS. Clinical correlates and prognostic significance of exercise-induced ventricular premature beats in the community: The Framingham Heart Study. Circulation. 2004;109(20):2417–2422. doi: 10.1161/01.CIR.0000129762.41889.41. [DOI] [PubMed] [Google Scholar]
- 11.Henry RL, Kennedy GT, Crawford MH. Prognostic value of exercise-induced ventricular ectopic activity for mortality after acute myocardial infarction. Am. J. Cardiol. 1987;59(15):1251–1255. doi: 10.1016/0002-9149(87)90899-X. [DOI] [PubMed] [Google Scholar]
- 12.Dzikowicz DJ, Carey MG. Exercise-induced premature ventricular contractions are associated with myocardial ischemia among asymptomatic adult male firefighters: Implications for enhanced risk stratification. Biol. Res. Nurs. 2020;22(3):369–377. doi: 10.1177/1099800420921944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Kwon M, Chang J, Harris D, Gerson MC, Hwang SS, Oh SW. Meta-analysis of prognostic implications of exercise-induced ventricular premature complexes in the general population. Am. J. Cardiol. 2016;118(5):725–732. doi: 10.1016/j.amjcard.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Frigy A, Csiki E, Caraşca C, Szabó IA, Moga VD. Autonomic influences related to frequent ventricular premature beats in patients without structural heart disease. Medicine (Baltimore) 2018;97(28):e11489. doi: 10.1097/MD.0000000000011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saha SA. Premature ventricular complexes during exercise in asymptomatic adults: Do they deserve a closer look? J. Am. Coll. Cardiol. 2021;78(23):2278–2280. doi: 10.1016/j.jacc.2021.09.1365. [DOI] [PubMed] [Google Scholar]
- 16.Lindow T, Brudin L, Elmberg V, Ekstrom M. Long-term follow-up of patients undergoing standardized bicycle exercise stress testing: New recommendations for grading of exercise capacity are clinically relevant. Clin. Physiol. Funct. Imaging. 2019;40:83–90. doi: 10.1111/cpf.12606. [DOI] [PubMed] [Google Scholar]
- 17.Lindow T, Ekström M, Brudin L, Carlén A, Elmberg V, Hedman K. Typical angina during exercise stress testing improves the prediction of future acute coronary syndrome. Clin. Physiol. Funct. Imaging. 2021;41(3):281–291. doi: 10.1111/cpf.12695. [DOI] [PubMed] [Google Scholar]
- 18.Hedman K, Lindow T, Elmberg V, Brudin L, Ekström M. Age- and gender-specific upper limits and reference equations for workload-indexed systolic blood pressure response during bicycle ergometry. Eur. J. Prev. Cardiol. 2020;28:1360–1369. doi: 10.1177/2047487320909667. [DOI] [PubMed] [Google Scholar]
- 19.Brudin L, Jorfeldt L, Pahlm O. Comparison of two commonly used reference materials for exercise bicycle tests with a Swedish clinical database of patients with normal outcome. Clin. Physiol. Funct. Imaging. 2014;34(4):297–307. doi: 10.1111/cpf.12097. [DOI] [PubMed] [Google Scholar]
- 20.Hedman K, Lindow T, Cauwenberghs N, Carlén A, Elmberg V, Brudin L, Ekström M. Peak exercise SBP and future risk of cardiovascular disease and mortality. J. Hypertens. 2022;40(2):300–309. doi: 10.1097/HJH.0000000000003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H, Feychting M, Ljung R. The Swedish cause of death register. Eur. J. Epidemiol. 2017;32(9):765–773. doi: 10.1007/s10654-017-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eriksson A, Stenlund H, Ahlm K, Boman K, Bygren LO, Johansson LA, Olofsson BO, Wall S, Weinehall L. Accuracy of death certificates of cardiovascular disease in a community intervention in Sweden. Scand. J. Public Health. 2013;41(8):883–889. doi: 10.1177/1403494813499653. [DOI] [PubMed] [Google Scholar]
- 23.Jorfeldt, L. & Pahlm, O. Kliniska arbetsprov: metoder för diagnos och prognos. 1. uppl. ed: Studentlitteratur (2013).
- 24.Sipila K, Tikkakoski A, Alanko S, Haarala A, Hernesniemi J, Lyytikainen LP, Viik J, Lehtimaki T, Nieminen T, Nikus K, Kahonen M. Combination of low blood pressure response, low exercise capacity and slow heart rate recovery during an exercise test significantly increases mortality risk. Ann. Med. 2019;51(7–8):390–396. doi: 10.1080/07853890.2019.1684550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999;341(18):1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017;38(36):2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 27.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2015;16(3):233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 28.Cutler MJ, Rosenbaum DS, Dunlap ME. Structural and electrical remodeling as therapeutic targets in heart failure. J. Electrocardiol. 2007;40(6 Suppl):S1–7. doi: 10.1016/j.jelectrocard.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 29.Hilfiker-Kleiner D, Landmesser U, Drexler H. Molecular mechanisms in heart failure: Focus on cardiac hypertrophy, inflammation, angiogenesis, and apoptosis. J. Am. Coll. Cardiol. 2006;48(9 Supplement):A56–A66. doi: 10.1016/j.jacc.2006.07.007. [DOI] [Google Scholar]
- 30.Hill JA. Electrical remodeling in cardiac hypertrophy. Trends Cardiovasc. Med. 2003;13(8):316–322. doi: 10.1016/j.tcm.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Ventricular ectopy as a predictor of heart failure and death. J. Am. Coll. Cardiol. 2015;66(2):101–109. doi: 10.1016/j.jacc.2015.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sassone B, Muser D, Casella M, Luzi M, Virzì S, Balla C, Nucifora G, Task Force on I, Task Force on Ablation of Ventricular Tachycardia of the Italian Association of A, Cardiac P Detection of concealed structural heart disease by imaging in patients with apparently idiopathic premature ventricular complexes: A review of current literature. Clin. Cardiol. 2019;42(12):1162–1169. doi: 10.1002/clc.23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bigger JT, Jr, Fleiss JL, Kleiger R, Miller JP, Rolnitzky LM. The relationships among ventricular arrhythmias, left ventricular dysfunction, and mortality in the 2 years after myocardial infarction. Circulation. 1984;69(2):250–258. doi: 10.1161/01.CIR.69.2.250. [DOI] [PubMed] [Google Scholar]
- 34.Corrado D, Basso C, Judge DP. Arrhythmogenic cardiomyopathy. Circ. Res. 2017;121(7):784–802. doi: 10.1161/CIRCRESAHA.117.309345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [TL], upon reasonable request.