Abstract
Glucagon-like peptide-1 (GLP-1) is expressed in retinal neurons, but its role in the retina is largely unknown. Here, we demonstrated that GLP-1 or the GLP-1 receptor (GLP-1R; a G protein-coupled receptor) agonist exendin-4 suppressed γ-aminobutyric acid receptor (GABAR)-mediated currents through GLP-1Rs in isolated rat retinal ganglion cells (GCs). Pre-incubation with the stimulatory G protein (Gs) inhibitor NF 449 abolished the exendin-4 effect. The exendin-4-induced suppression was mimicked by perfusion with 8-Br-cAMP (a cAMP analog), but was eliminated by the protein kinase A (PKA) inhibitor Rp-cAMP/KT-5720. The exendin-4 effect was accompanied by an increase in [Ca2+]i of GCs through the IP3-sensitive pathway and was blocked in Ca2+-free solution. Furthermore, when the activity of calmodulin (CaM) and CaM-dependent protein kinase II (CaMKII) was inhibited, the exendin-4 effect was eliminated. Consistent with this, exendin-4 suppressed GABAR-mediated light-evoked inhibitory postsynaptic currents in GCs in rat retinal slices. These results suggest that exendin-4-induced suppression may be mediated by a distinct Gs/cAMP-PKA/IP3/Ca2+/CaM/CaMKII signaling pathway, following the activation of GLP-1Rs.
Supplementary Information
The online version of this article (10.1007/s12264-022-00826-9) contains supplementary material, which is available to authorized users.
Keywords: Glucagon-like peptide-1, Exendin-4, GABA current, Retinal ganglion cells, Neuromodulation
Introduction
Glucagon-like-peptide-1 (GLP-1) is a metabolic hormone secreted by intestinal endocrine L-cells and stimulates insulin secretion in a glucose-dependent manner [1]. GLP-1 is also produced in the brain, particularly from preproglucagon neurons, which are distributed in the solitary tract of the brain stem, and it functions as a neuropeptide [2–5]. This peptide has been implicated in modulating neuronal cell differentiation, neurite outgrowth, and performing neuroprotective functions through the activation of GLP-1 receptors (GLP-1Rs), class B sub-group G protein-coupled receptors [2, 6].
GLP-1 expression has been found in the vertebrate retina [7–9]. Specifically, GLP-1R immunoreactivity has been observed in the ganglion cell layer (GCL) in the human and rat retina [7, 10–12], and sparse staining has also been detected in the inner and outer nuclear layers of the human retina. More recently, systemic or topical administration (eye drops) of GLP-1 and GLP-1R agonists has been reported to prevent electroretinogram abnormalities and neurodegeneration of the retina in rat and mouse models of diabetes [7, 10]. However, whether GLP-1 modulates retinal information processing is still largely unknown.
In the retina, ganglion cells (GCs) are the sole output neurons that integrate inhibitory signals from amacrine cells and excitatory signals from bipolar cells and transmit the processed information to higher centers [13]. GABA receptors (GABARs) and glycine receptors mediate the inhibitory signals of amacrine cells onto GCs. It has been shown that GLP-1/the GLP-1R agonist exendin-4 increases GABAAR-mediated tonic currents through GLP-1R activation in rat hippocampal CA3 pyramidal neurons [14], but no data concerning the regulation of GABARs of retinal neurons by GLP-1/GLP-1R agonists are now available. In the present work, we used whole-cell patch-clamp recording techniques to explore whether GLP-1 or exendin-4 modulates GABARs of rat retinal GCs. We first demonstrated that GLP-1 or the GLP-1R agonist exendin-4 suppressed GABAR-mediated currents (GABA currents) in isolated rat retinal GCs through GLP-1R activation. By pharmacological approaches, we further showed that a distinct Gs/cAMP-protein kinase A (PKA)/inositol 1,4,5-trisphosphate (IP3)/Ca2+/calmodulin (CaM)/CaM-dependent protein kinase II (CaMKII) signaling pathway is responsible for the exendin-4 effect on GCs. Consistent with this, we also showed that exendin-4 suppressed GABAR-mediated the light-evoked inhibitory postsynaptic currents (L-IPSCs) of GCs via GLP-1Rs.
Materials and Methods
Animals
Male Sprague–Dawley rats (15–20 days of age) were used in this study. All procedures were approved by the Animal Care and Use Committee of the Shanghai Medical College of Fudan University and were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All participants signed the written informed consent.
Retrograde Labeling of GCs
The detailed procedure were based on previous work [15, 16]. GCs were retrogradely labeled by 20% rhodamine-labeled fluorescent latex microspheres (LumaFluor, Durham, USA).
Preparation of Isolated GCs
As previously reported [16], GCs were isolated by enzymatic digestion of retinal tissue with papain and mechanical separation. Rhodamine-labeled GCs (15–25 μm in diameter) were used for electrophysiological recording within 2–3 h after separation.
Whole-cell Patch-clamp Recording
Whole-cell membrane currents were recorded from rhodamine-labeled GCs using a patch amplifier (EPC9/2, HEKA Elektronik, Lambrecht, Germany) with Pulsefit 8.80 software (HEKA Elektronik). The sampling rate was set at 5 kHz and with a high-pass filter at 2 kHz .The recording pipettes (6–8 MΩ) were filled with internal solution containing (in mmol/L) CsCl 120, CaCl2 1, MgCl2 2, EGTA 10, HEPES 10, ATP-Mg 2, GTP-Na 0.4, NaCl 5, and phosphocreatine 10; adjusted to pH 7.25 with CsOH and osmolality to 280–300 mOsm/L with sucrose, as in our previous paper [17]. The GCs were bathed in Ringer’s that contained (in mmol/L) NaCl 145, KCl 5, CaCl2 2, MgCl2 1, HEPES 10, and glucose 16; pH adjusted to 7.4 with NaOH [18]. The cells were clamped at −60 mV and GABA (30 μmol/L; 5 s) was administered every 2 min to induce currents. All recordings were captured at 20–25°C.
We recorded GABAR-mediated L-IPSCs in retinal slices that were prepared as described in detail previously [19]. The slices were transferred to a recording chamber and superfused constantly with carbogen (95% O2/5% CO2)-bubbled Ames medium (Sigma-Aldrich, Inc., St. Louis, USA). The pipette solution consisted of the following (in mmol/L): TEA-Cl 10, CsCH3SO3 120, HEPES 10, EGTA 1, CaCl2 0.1, phosphocreatine 12, GTP-Na 0.5, ATP-Mg 3; pH adjusted to 7.25 with CsOH. In the GCL, we distinguished GCs from displaced amacrine cells based on cell body diameter and physiological criteria [20–22]. The GABAR-mediated L-IPSCs of GCs were recorded with a patch amplifier (MultiClamp 700B, Molecular Devices, Novato, USA). The cells were clamped at 0 mV. A mixture of synaptic blockers that contained tetrodotoxin (TTX; 1 μmol/L) and strychnine (1 μmol/L) was added to the Ames medium to block voltage-gated Na+ channels and glycine receptors, respectively. An LED (λ = 470 nm) was used to generate full-field light stimuli (0.3−0.5 μW/cm2, 3 s, at 60-s intervals); it was controlled by Polylite software (Mightex Systems, Pleasanton, USA) and transmitted to the retina.
Ca2+ Imaging
We used the membrane permeability indicator Fura-2AM (Dojindo, Kumamoto, Japan) to assess changes in the intracellular Ca2+ concentration ([Ca2+]i) of isolated GCs as described previously [15]. Fluorescence images were captured on an Olympus inverted microscope equipped with a digital CCD camera (Hamamatsu Photonics, Shizuoka, Japan). We used high-speed continuous scanning monochromatic light sources (Till Photonics, Grafeling, Germany) to excite at 340 nm and 380 nm. Fluorescence intensities at 340 nm and 380 nm (F340 and F380) were measured every 1–10 s, and images were acquired using C-imaging systems (Hamamatsu Photonic). The [Ca2+]i of the cell was proportional to the ratio of fluorescence intensity between the two images. Before an experiment, we measured the background fluorescence level and subtracted it from the obtained data.
Chemicals
GLP-1, exendin-4, and exendin(9-39) were from AnaSpec (Fremont, USA); U73122, ryanodine, and W-7 hydrochloride were from Tocris Bioscience (Ellisville, USA). All other chemicals were from Sigma-Aldrich. The RSC-200 (Bio-Logic, Claix, France), a fast solution exchanger based on a stepping motor, was used for solution transport, with a solution exchange time being ~5 ms.
Statistical Analysis
Data were analyzed using Pulsefit (HEKA Elektronik, Lambrecht/Pfalz, Germany), Igor 4.0 (WaveMetrics, Lake Oswego, USA), and SigmaPlot 12.0 (Systat Software, Inc., San Jose, USA). Data are shown as the mean ± SEM. Significant differences were identified by either paired Student’s t-test (for paired data) or one-way ANOVA with post hoc Tukey’s test (for multiple comparisons). For all analyses, P <0.05 was considered statistically significant.
Results
GABAR-mediated Currents in Rat GCs
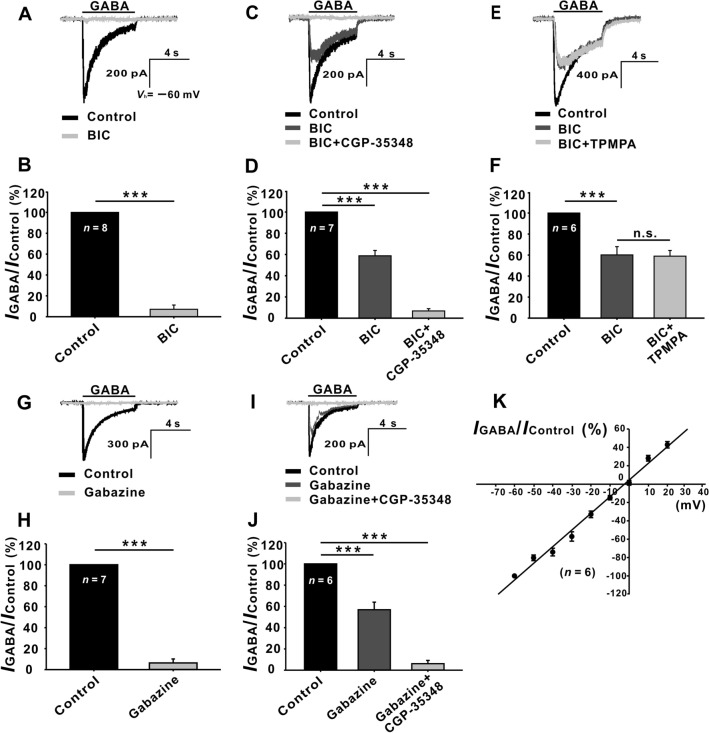
The currents induced by GABA were recorded from rhodamine-labeled rat retinal GCs with relatively large somata (>15 μm in diameter) in normal Ringer’s. In some GCs, the currents induced by GABA (30 μmol/L) were almost completely suppressed (7.09% ± 4.12% of control, P <0.001, n = 8) by 10 μmol/L bicuculline (BIC), an antagonist of GABAA receptors (Fig. 1A, B), which suggests that the GABA currents of these cells are exclusively mediated by GABAA receptors. However, in the remaining GCs, the GABA-induced currents were reduced to 58.76% ± 5.02% of control (P <0.001, n = 7) (Fig. 1C, D) in the presence of 10 μmol/L BIC, and the remaining currents were almost eliminated by perfusing CGP-35348 (100 μmol/L), an antagonist of GABAB receptors (6.79% ± 2.14% of control, P <0.001, n = 7) (Fig. 1D). Moreover, in the cells in which BIC only partially suppressed the GABA currents (60.09% ± 7.86% of control, P <0.001, n = 6) (Fig. 1E, F), co-application of BIC and the GABAC receptor antagonist TPMPA (10 μmol/L) did not further suppress the GABA currents (58.94% ± 5.43% of control, P <0.001,n = 6). Similar results were obtained with the application of gabazine, another GABAA receptor antagonist. That is, in some GCs, addition of 10 μmol/L gabazine almost completely suppressed the GABA currents (6.34% ± 3.79% of control, P <0.001,n = 7) (Fig. 1G, H), while in the remaining GCs, 10 μmol/L gabazine suppressed the GABA currents to 56.89% ± 7.12% of control (P <0.001,n = 6) (Fig. 1I, J), and co-application of 100 μmol/L CGP-35348 almost eliminated the currents (6.12% ± 3.05% of control, P <0.001, n = 6) (Fig. 1I, J). The current-voltage relationship of the GABAA receptor-mediated currents was linear, with a reversal potential of −3.1 ± 2.3 mV (n = 6, Fig. 1K), which was very close to the ECl- (−4.3 mV) calculated according to the Nernst equation. These results indicated that the GABA currents of these GCs are mediated by both GABAA and GABAB receptors. All these data suggest that GCs express functional GABAA and GABAB receptors, but not GABAc receptors, and this is consistent with the results of in situ hybridization and immunohistochemistry showing that GCs express GABAA and GABAB receptors [23–27].
Fig. 1.
Characterization of GABA-induced currents in isolated rat retinal GCs. A Representative recordings showing that the current induced in a GC by 30 μmol/L GABA is almost completely suppressed by 10 μmol/L bicuculline (BIC). The cell is clamped at Vh = −60 mV and 30 μmol/L GABA is repetitively applied for 5 s at intervals of 2 min. C Representative recordings from another GC, showing that the GABA-induced current is partially attenuated by BIC, and the remaining current is completely eliminated by co-application of 100 μmol/L CGP-35348. E Current traces of a GC showing that the GABA-induced current is partially suppressed by BIC, and the remaining current is not changed by co-application of 10 μmol/L TPMPA. G Representative recordings showing that the GABA-induced current of a GC is almost completely suppressed by 10 μmol/L gabazine. I Representative recordings of another GC showing that the GABA-induced current is partially attenuated by gabazine, and the remaining current is completely eliminated by co-application of 100 μmol/L CGP-35348. B, D, F, H, J Bar charts showing statistical analysis of the above data. ***P <0.001, n.s., P >0.05, paired Student’s t-test. The data are presented as the mean ± SEM in all figures. The data for each cell are normalized to the current amplitude of the cell in normal Ringer’s (control) and then averaged. K Average current-voltage relationship of GABAA receptor-mediated currents from six GCs. Current responses for each cell at different holding potentials are normalized to the response obtained at –60 mV. Cell numbers (n) are marked inside the bars, and the cell numbers in different bars in the same subgraph are the same. It is also the case for other figures.
Exendin-4 Suppresses GABA Currents in GCs
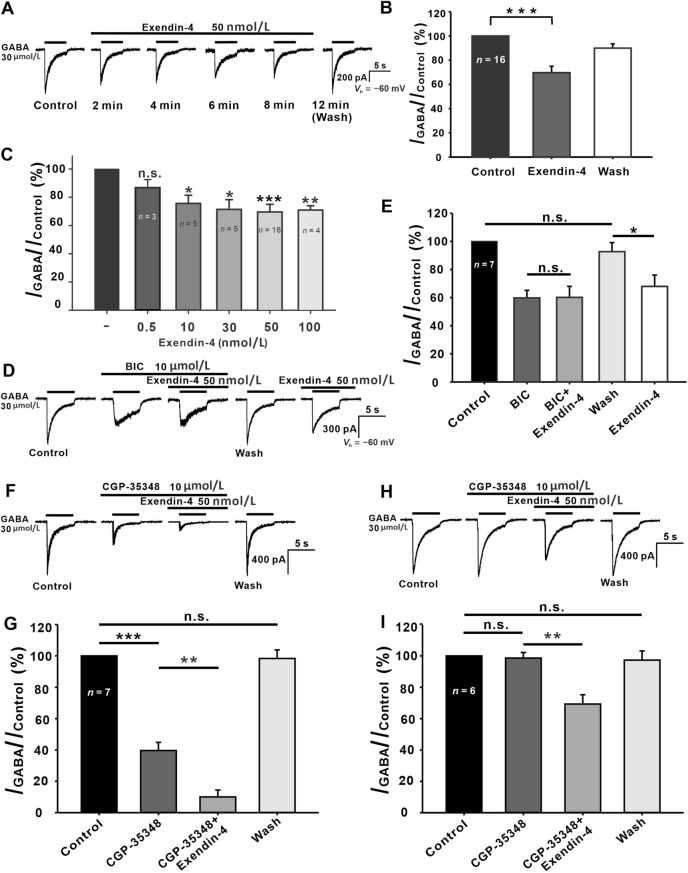
The endogenous GLP-1 is highly sensitive to degradation by dipeptidyl peptidase IV (DPP-IV) [28–32]; for this reason, we investigated the effects of the protease-resistant long-acting GLP-1R agonist exendin-4 [28, 33, 34] on the GABA currents of GCs (Fig. 2A). No detectable current in GCs was elicited by perfusion of 50 nmol/L exendin-4 (data not shown). After applying exendin-4 for ~2 min, the amplitude of peak current decreased and tended to stabilize in ~6 min (Fig. 2A). The current returned to the control level following a 4-min washout. Suppression by exendin-4 of GABA currents was recorded in most of the tested GCs (16 out of 18, 88.9%). On average, the current amplitudes of these cells after 6 min incubation with 50 nmol/L exendin-4 were decreased to 69.08% ± 5.71% of control (P <0.001, n = 16, Fig. 2B). In the other two cells, exendin-4 had no effect on the GABA currents (2/18, 11.1%). When the significance test was performed on the whole data set (18 cells), exendin-4 inhibited the GABA currents to a smaller extent (76.96% ± 4.41% of control), but the difference was still statistically significant (P <0.01 vs control).
Fig. 2.
Effects of exendin-4 on GABA currents in isolated rat GCs. A Representative recordings showing the effect of 50 nmol/L exendin-4 on GABA currents in a GC. Drug application is indicated by the horizontal lines above the current traces and the times at which the current traces were recorded are marked below (min). B Bar chart summarizing the effects of exendin-4 on GABA current amplitude. C Exendin-4 suppresses GABA currents in a dose-dependent manner. D Representative recordings of a GC showing that GABA currents are largely suppressed by 10 μmol/L BIC. The remaining current (GABAB receptor-mediated current) is not further suppressed by 50 nmol/L exendin-4. After the response returns to the control level on washout, exendin-4 addition suppresses the GABA current. E Bar chart summarizing the effects of BIC and exendin-4 on the GABA currents. F Representative recordings of a GC showing that GABA current is largely suppressed by 100 μmol/L CGP-35348. The remaining current (GABAA receptor-mediated current) is further suppressed by exendin-4. G Bar chart summarizing the effects of CGP-35348 and exendin-4 on the GABA current. H Representative recordings of another GC showing that the peak amplitude of GABA current is hardly changed by CGP-35348, and then exendin-4 addition still suppresses the GABA current. I Bar chart summarizing the effects of CGP-35348 and exendin-4 on GABA currents. *P <0.05, **P <0.01, ***P <0.001, n.s., P >0.05, one-way ANOVA with post hoc Tukey’s test.
We further investigated the concentration-dependence of the exendin-4 effects on GABA currents. The GABA current amplitude recorded after 6 min incubation with exendin-4 was normalized to the level recorded before the incubation (control). As shown in Fig. 2C, 0.5 nmol/L exendin-4 did not significantly suppress the GABA currents (89.81% ± 6.26% of control, P >0.05, n = 3), but when the exendin-4 concentration was increased to 10, 30, 50, and 100 nmol/L, the GABA currents were suppressed to 74.35% ± 6.79% (P <0.05, n = 5), 71.92% ± 8.34% (P <0.05, n = 5), 69.08% ± 5.71% (P <0.001, n = 16), and 70.66% ± 3.24% (P <0.01, n = 4) of control, respectively. Based on these data, we chose 50 nmol/L exendin-4 for subsequent experiments, unless otherwise specified.
Since GABA currents were mediated by both GABAA and GABAB receptors in ~62% of GCs, whether exendin-4 regulates GABAA and GABAB currents differentially was further investigated. Application of BIC (10 μmol/L) greatly inhibited the GABAA current (the initial transient component in response to GABA) in some GCs and isolated the GABAB current (the fairly sustained current) (Fig. 2D). The peak amplitudes of GABAB currents were hardly changed by exendin-4 (59.76% ± 5.42% of control for BIC vs 60.14% ± 7.86% of control for BIC + exendin-4, P >0.05,n = 7) (Fig. 2D, E). The response returned to the control level after 6 min washout, and then perfusion of exendin-4 still suppressed the GABA currents to 67.89% ± 8.14% of control (P <0.05 vs control). Furthermore, in the GCs that expressed both GABAA and GABAB receptors, perfusion with 100 μmol/L CGP-35348 greatly inhibited the GABAB currents (39.63% ± 5.23% of control, P <0.001, n = 7) (Fig. 2F, G), and the remaining GABAA currents were further suppressed by co-application of exendin-4 (39.63% ± 5.23% of control for CGP-35348 vs 10.05% ± 4.39% of control for CGP-35348 + exendin-4, P <0.01) (Fig. 2F, G). In other GCs that only expressed GABAA receptors, perfusion with 100 μmol/L CGP-35348 did not change the GABA currents, and addition of exendin-4 significantly inhibited them (98.63% ± 3.57% of control for CGP-35348 vs 69.31% ± 5.94% of control for CGP-35348 + exendin-4, P <0.01, n = 6) (Fig. 2H, I). All these results indicate that exendin-4 suppresses the GABAA receptor-, but not the GABAB receptor-mediated currents of GCs.
GLP-1R Mediates Exendin-4-/GLP-1-induced Suppression of GABA Currents in GCs
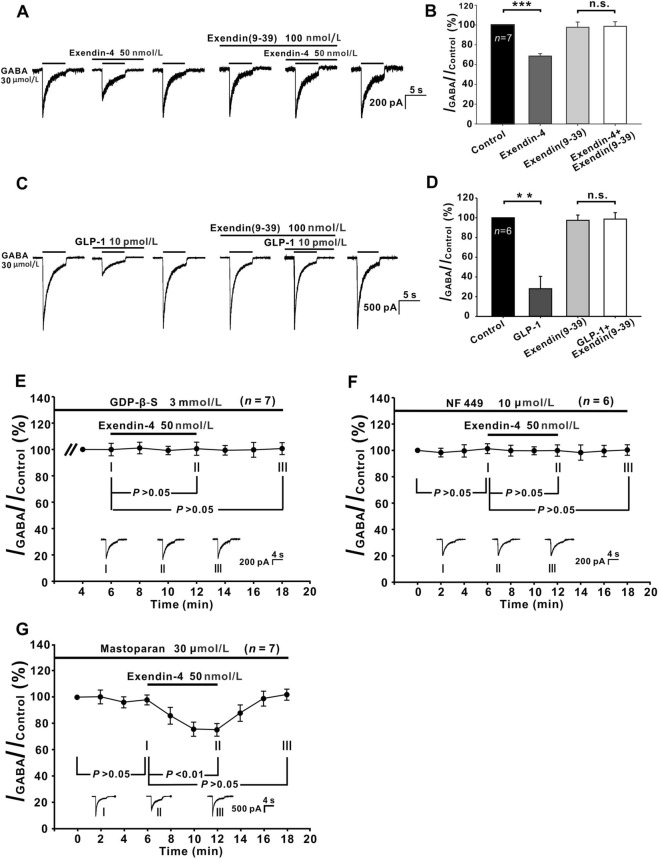
It has been shown that GLP-1R is expressed on neurons of the GCL in the rat retina [10, 11], so we further investigated whether GLP-1R mediated the exendin-4-induced inhibition of GABA currents in GCs. A representative result is shown in Fig. 3A. As a general rule, we tested the effect of exendin-4 on a GC before an experiment to ensure that its GABA current was indeed inhibited by exendin-4. When the current returned to the control level after washout, 100 nmol/L exendin(9-39), a competitive GLP-1R antagonist [35], was perfused for 6 min. Exendin(9-39) alone had no effect on the GABA currents (97.7% ± 5.94% of the control, P >0.05 vs control, n = 7) (Fig. 3A, B), and in the presence of exendin(9-39), addition of exendin-4 for 6 min did not affect the currents [98.63% ± 5.71% of control, P >0.05 vs exendin(9-39)] (Fig. 3B).
Fig. 3.
Exendin-4-/GLP-1-induced suppression of GABA currents in GCs is mediated by Gs-linked GLP-1R. A Representative current traces from a GC showing the effects of exendin-4 (50 nmol/L) on the GABA current amplitude in Ringer’s, and in the presence of 100 nmol/L exendin(9-39). B Bar chart summarizing the changes in GABA current amplitudes caused by exendin-4 alone and in the presence of exendin(9-39). C Current traces of another GC showing the effects of GLP-1 (10 pmol/L) on the GABA current amplitude in Ringer’s, and in the presence of 100 nmol/L exendin(9-39). D Bar chart summarizing the changes in GABA current amplitudes caused by GLP-1 alone and in the presence of exendin(9-39). **P <0.01, ***P <0.001. E Changes in GABA currents caused by exendin-4 plotted as a function of time during internal infusion of 3 mmol/L GDP-β-S. Note that during the infusion, the addition of exendin-4 does not change the current amplitudes. F Averaged time course of the effect of exendin-4 on GABA currents in NF 449-treated GCs. Exendin-4 does not change the currents in the NF 449-treated GCs. G Effects of exendin-4 on GABA currents plotted as a function of time during internal infusion of 30 μmol/L mastoparan. Mastoparan per se has no effect on the GABA currents of GCs, and co-application of exendin-4 persists in significantly suppressing the GABA currents. The waveforms shown below the data lines are the current responses recorded at the times indicated by I, II, and III shown in E, F, and G. **P <0.01, ***P <0.001, n.s., P >0.05, one-way ANOVA with post hoc Tukey’s test.
We also determined whether GLP-1 had effects similar to exendin-4. Since the maximal concentration of GLP-1 in human postprandial plasma is <40 pmol/L [1], we chose 10 pmol/L GLP-1 for extracellular perfusion. As shown in Fig. 3C and D, application of GLP-1 for 6 min significantly suppressed the GABA currents of GCs to 28.11% ± 12.50% of control (P <0.01, n = 6), and the extent of suppression was larger than that of exendin-4 (69.08% ± 5.71% of control). The currents returned to the control level following a 2-min washout. Moreover, the GLP-1-induced suppression was also abolished by exendin(9-39). These results indicate that both exendin-4 and GLP-1 suppress GABA currents by activating GLP-1Rs.
Exendin-4 has been widely used in the treatment of diabetes because its plasma half-life (120 min) is much longer than GLP-1 (1.5 min) [36, 37]. Therefore, we selected exendin-4 for further mechanism study. Because GLP-1R is a G-protein-coupled receptor [38], to determine whether G-protein is associated with the suppression of GABA currents by exendin-4, we added GDP-β-S, a nonhydrolyzable G-protein inhibitor, into patch pipettes. After whole-cell recording from a GC, we waited 4 min to allow GDP-β-S to diffuse throughout the cell. When the GABA currents of GCs reached a stable level at ~6 min after membrane rupture (control), application of exendin-4 for 6 min failed to suppress the currents (99.35% ± 3.64% of control, P >0.05, n = 7) (Fig. 3E). Since GLP-1R can be coupled to Gs or Gi/o [39–41], we further investigated which subtype(s) of G-proteins may be associated with the exendin-4 effect. Cell suspensions were preincubated with 10 μmol/L NF 449, a Gs antagonist, for at least 30 min before recording and then exposure to exendin-4 for 6 min failed to change the GABA currents of these cells (99.87% ± 4.31% of control) (P >0.05, n = 6) (Fig. 3F). Furthermore, internal infusion of 30 μmol/L mastoparan, a peptide activator of Gi and Go, for 6 min did not change the GABA currents of GCs (control), and in the presence of mastoparan, applying exendin-4 for 6 min still suppressed the GABA currents to 76.85% ± 4.54% of control (P <0.01, n = 7) ( Fig. 3G). These results suggest that GLP-1R is coupled to Gs in rat GCs.
cAMP-PKA Signaling Pathway Mediates Exendin-4-induced Suppression of GABA Currents
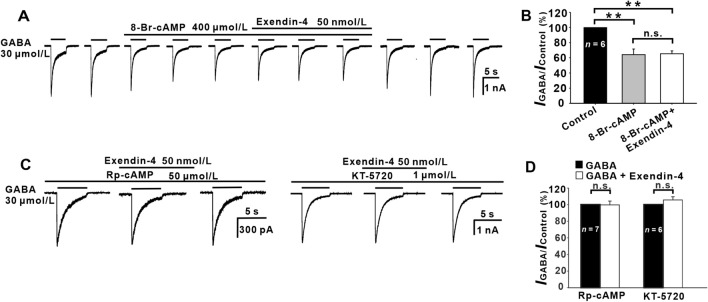
Following activation of GLP-1Rs, the main intracellular signaling pathway stimulates Gs, which in turn activates adenylate cyclase, resulting in increased intracellular cAMP levels and activation of PKA [42, 43]. To investigate whether this pathway is involved, we studied the effects of extracellular perfusion of 8-Br-cAMP, a membrane-permeable cAMP analog, on the GABA currents of GCs. Fig. 4A shows that GABA currents of a GC were gradually suppressed by applying 8-Br-cAMP (400 μmol/L) and reached a stable level 6 min (control) after 8-Br-cAMP application. Co-application of exendin-4 did not change the suppression of the peak currents. Average data showed that the peak current amplitude was suppressed to 64.18% ± 7.42% of control (P < 0.01,n = 6) (Fig. 4B) by 8-Br-cAMP and applying exendin-4 did not cause further suppression (65.32% ± 3.83% of control, P < 0.01 vs control and P > 0.05 vs 8-Br-cAMP) (Fig. 4B). The action of 8-Br-cAMP was reversible. Furthermore, after intracellular application of the PKA inhibitor Rp-cAMP (50 μmol/L), exendin-4 addition no longer changed the currents (98.57% ± 5.01% of control, P > 0.05,n = 7) (Fig. 4C, D). In addition, when another PKA inhibitor, KT-5720, was intracellularly applied to GCs, additional exendin-4 also failed to affect the GABA currents (102.86% ± 4.34% of control, P >0.05, n = 6) (Fig. 4C, D).
Fig. 4.
Involvement of the cAMP-PKA pathway in the exendin-4-induced suppression of GABA currents. A Representative recordings of a GC showing that perfusion with 400 μmol/L 8-Br-cAMP reduces the GABA currents. In the presence of 8-Br-cAMP, exendin-4 no longer suppresses the currents. B Bar chart summarizing the effects of 8-Br-cAMP and exendin-4 on the GABA current amplitudes. **P <0.01. C Representative recordings from two GCs, showing that internal infusion of 50 μmol/L Rp-cAMP (left) or 1 μmol/L KT-5720 (right) eliminates the exendin-4-induced reduction of the GABA currents. D Bar chart summarizing the effects of Rp-cAMP and KT-5720 on GABA currents. P <0.01, n.s., P >0.05, one-way ANOVA with post hoc Tukey’s test.
Involvement of Ca2+ and CaM in Exendin-4-induced Suppression of GABA Currents
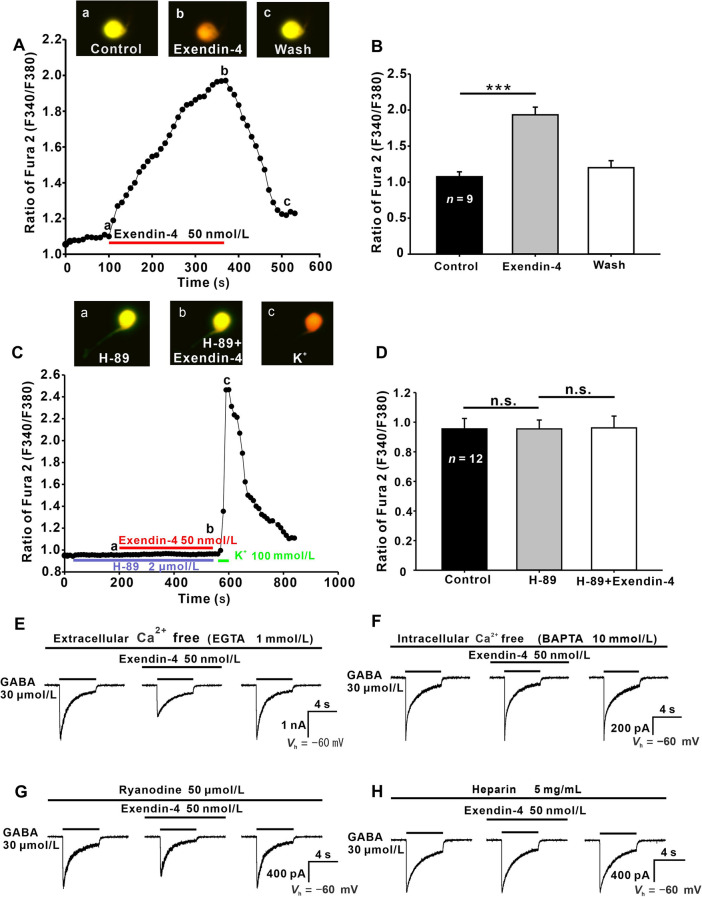
Activation of PKA causes an increase in [Ca2+]i by regulating Ca2+ release from intracellular stores [44, 45]. Moreover, there is much evidence showing that GABAR activity is modulated by [Ca2+]i [46–51]. We used Ca2+ imaging with Fura-2 to test whether exendin-4 could change [Ca2+]i in dissociated GCs. As illustrated by an example in Fig. 5A, the application of 50 nmol/L exendin-4 caused a gradual increase in [Ca2+]i of the GC in a reversible manner, the F340/F380 ratio reaching a maximum in 4 min. Exendin-4-induced changes in [Ca2+]i of the GC soma are shown in three CCD images (Fig. 5A a−c). In the nine GCs tested, the mean peak ratio of Fura-2 (F340/F380) after exendin-4 perfusion was 1.93 ± 0.11, which was significantly higher than that in Ringer’s (1.08 ± 0.07, P <0.001) (Fig. 5B).
Fig. 5.
Intracellular Ca2+ relevance of exendin-4-induced suppression of GABA currents in GCs. A Fura-2 Ca2+ recordings from a GC, represented by the F340/F380 ratio, showing the addition of 50 nmol/L exendin-4 dramatically increases [Ca2+]i in a reversible manner. Three CCD images of a GC loaded with Fura-2 were captured at times indicated by a, b, and c in the data line. B Bar chart summarizing the changes in [Ca2+]i induced by exendin-4 in GCs. ***P <0.001. C A continuous recording of [Ca2+]i in a GC showing that 50 nmol/L exendin-4 fails to change [Ca2+]i in the presence of 2 μmol/L H-89, but a brief application of 100 mmol/L KCl induces a dramatic increase in [Ca2+]i. Three CCD images of the GC loaded with Fura-2 AM were captured at the times indicated by a, b, and c in the data line. D Bar chart summarizing the changes in [Ca2+]i induced by H-89 and exendin-4. E Representative recordings from a GC showing that eliminating extracellular Ca2+ with 1 mmol/L EGTA does not affect the action of exendin-4 in reducing GABA currents. F Representative recordings from a GC showing that eliminating intracellular Ca2+ with the internal infusion of 10 mmol/L BAPTA completely blocks the exendin-4-induced suppression of GABA currents. G Depleting ryanodine-sensitive intracellular stores with ryanodine (50 μmol/L) infusion does not affect the exendin-4-induced suppression of GABA currents. H Internal infusion of heparin (5 mg/mL) completely blocks the actions of exendin-4. ***P <0.001, n.s., P >0.05, one-way ANOVA with post hoc Tukey’s test.
To investigate whether the increase of [Ca2+]i in the GCs is related to the PKA activation induced by exendin-4, the changes of [Ca2+]i were measured by Ca2+ imaging when PKA activity was inhibited by H-89. There was no change in [Ca2+]i in GCs, either when perfused H-89 alone (0.95 ± 0.06 vs 0.96 ± 0.07 for control, n = 12) or along with exendin-4 (0.96 ± 0.08 vs 0.96 ± 0.07 for control, n = 12) (all P >0.05) (Fig. 5C, D). The data indicate that the exendin-4-induced [Ca2+]i elevation may be a result of PKA activation.
There are two sources for increased [Ca2+]i: the influx of extracellular Ca2+ through voltage-gated Ca2+ channels on the plasma membrane, and intracellular Ca2+ pools. When GCs were perfused by Ca2+-free solution containing the Ca2+ chelator EGTA (1 mmol/L) [18], exendin-4 still decreased the GABA currents to 67.33% ± 5.16% of control (P <0.001, n = 6) (Fig. 5E), indicating that the exendin-4 effect was not associated with changes in extracellular Ca2+ levels under our experimental conditions. In contrast, when GCs were intracellularly dialyzed with Ca2+-free solution containing the Ca2+ chelator BAPTA (10 mmol/L) [52], exendin-4 application did not inhibit the GABA currents (99.04% ± 4.01% of control, P >0.05, n = 5) (Fig. 5F), suggesting that intracellular Ca2+ is indeed associated with the exendin-4 effect.
IP3- and/or ryanodine-sensitive pathways mediate the release of Ca2+ from intracellular Ca2+ pools. After intracellular infusion of 50 μm ryanodine to deplete the ryanodine-sensitive Ca2+ sites [53], exendin-4 continued to significantly suppressed the GABA currents of GCs (70.67% ± 4.14% with ryanodine alone, P <0.001, n = 7) ( Fig. 5G). In contrast, during internal infusion of heparin (5 mg/mL), an IP3 receptor antagonist, additional exendin-4 did not further suppressed the GABA currents of GCs (96.65% ± 2.13% of heparin alone, P >0.05, n = 5) (Fig. 5H).
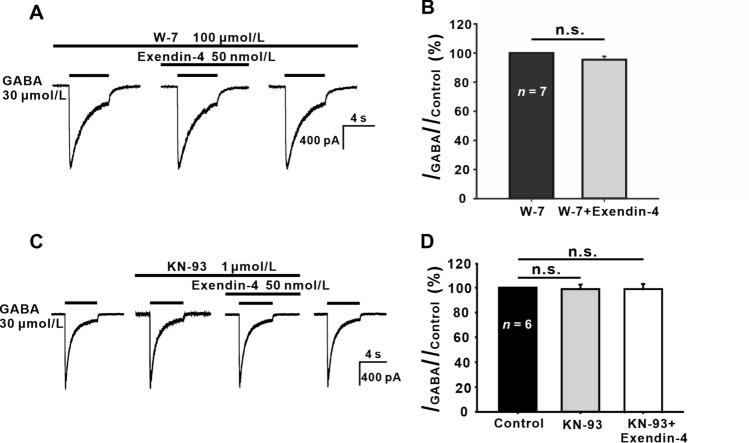
Ca2+/CaMKII has been found to play prominent roles in orexin A-mediated inhibition of GABAA receptor [54]. We further examined the effect of the CaM blocker W-7 [55], and found during intracellular infusion of 100 μmol/L W-7 (control), exendin-4 did not change the GABA currents of GCs (95.47% ± 3.67% of control, P >0.05, n = 7) (Fig. 6A, B). Moreover, after perfusion of KN-93, a CaMKII inhibitor, the addition of exendin-4 failed to suppress the GABA currents (97.90% ± 5.01% of control, P >0.05, n = 6) (Fig. 6C, D). These results suggest that CaM/CaMKII is involved in the exendin-4 effect on GABA currents.
Fig. 6.
Involvement of CaM and CaMKII in the exendin-4-induced suppression of GABA currents. A, C Representative recordings from two GCs, showing that exendin-4 (50 nmol/L) no longer suppresses the GABA currents during the internal infusion of 100 μmol/L W-7 (A) or extracellular perfusion with 1 μmol/L KN-93 (C). B, D Bar charts summarizing the results for the effects of W-7 (B) and KN-93 (D). n.s., not significant, paired Student’s t-test.
PI/PC-PLC-Independent Effects of Exendin-4
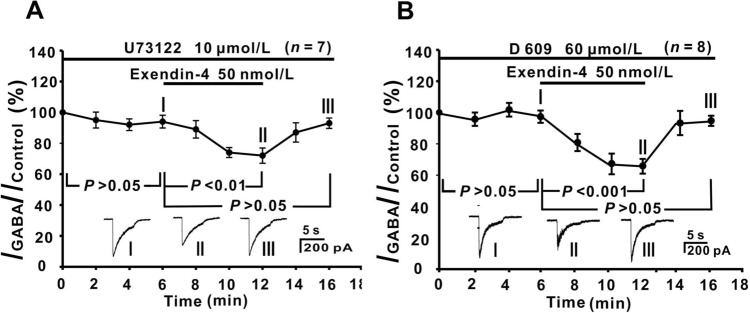
While GLP-1 has been shown to regulate the phosphatidylinositol-phospholipase C (PI-PLC) or phosphatidylcholine-phospholipase C (PC-PLC) activity in mouse pancreatic β cells [56], experiments with the PI-PLC inhibitor U73122 or with the PC-PLC inhibitor D609, did not support the involvement of these two pathways in the exendin-4 effects on GCs. As shown in Fig. 7A and B, internal infusion of 10 μmol/L U73122 or 60 μmol/L D609 for 6 min did not change GABA currents of GCs (control), then addition of exendin-4 for 6 min still suppressed the GABA currents (73.28% ± 5.09% of control, P <0.01 for U73122, n = 7; 67.41% ± 5.43% of control, P <0.001 for D609, n = 8).
Fig. 7.
No involvement of the PI-/PC-PLC signaling pathway in the action of exendin-4. A, B Plots of the average peak current amplitudes as a function of time, showing that exendin-4-induced suppression of GABA currents is still seen in the presence of 10 μmol/L U73122 (A) or 60 μmol/L D 609 (B). The waveforms shown below the data lines are the current responses recorded at the times indicated by I, II, and III in A and B. Note that exendin-4 has similar suppressive effects on the GABA currents compared with normal Ringer’s. P <0.01, P <0.001, P >0.05, one-way ANOVA with post hoc Tukey’s test.
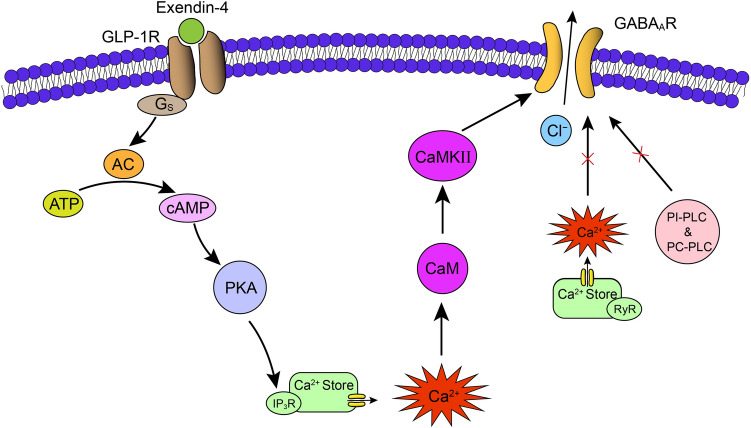
Taken together, the putative signaling pathway mediating the effects of exendin-4 on GABA currents of rat GCs is summarized in the schematic diagram in Fig. 8.
Fig. 8.
Schematic showing the putative signaling pathway that mediates the suppression of GABA currents by exendin-4 in rat retinal GCs. By activating Gs-coupled GLP-1Rs, exendin-4 suppresses GABA currents via a distinct intracellular cAMP-PKA/IP3/Ca2+/CaM/CaMKII signaling pathway. Note that neither PI-PLC nor PC-PLC is involved in the effect. AC, adenylate cyclase; IP3R, IP3 receptor; CaM, calmodulin; CaMKII, calmodulin-dependent protein kinase II; RyR, ryanodine receptor; PI-PLC, phosphatidylinositol-phospholipase C; PC-PLC, phosphatidylcholine-phospholipase C.
Exendin-4 Suppresses GABAR-mediated L-IPSCs in GCs of Rat Retinal Slices
To further verify the effect of exendin-4 on GABARs on GCs, we examined the effects of exendin-4 on GABAR-mediated L-IPSCs in rat retinal slice preparations. During perfusion with TTX and strychnine (see Methods for details), the GABAR-mediated L-EPSCs of GCs were recorded. As shown in Fig. S1A and S1B, bath application of 100 nmol/L exendin-4 significantly suppressed the GABAR-mediated L-IPSC to 68.38% ± 4.54% of control (P <0.001, n = 8), and the response returned to the control level after washout (91.02% ± 7.94% of control, P >0.05). We also determined whether GLP-1R mediated the exendin-4 effect on GABAR-mediated L-IPSCs in GCs. Perfusion with 100 μmol/L exendin(9-39) did not change the L-IPSCs (95.09% ± 3.57%, P >0.05, n = 10) (Fig. S1C, D), and the average current peak amplitude after co-application of exendin-4 for 6 min was 95.53% ± 2.76% of that obtained prior to exendin-4 perfusion (P >0.05, n = 10).
Discussion
GLP-1/exendin-4 Suppresses GABA Currents of Rat GCs by Activating GLP-1R
Although GLP-1 and GLP-1R agonists have been reported to regulate AMPA receptors and GABAA receptors in central neurons [14, 57–60], no data concerning the regulation of ligand-gated channels of retinal neurons by GLP-1/GLP-1R agonists were available. A major finding in the present work was that GLP-1/exendin-4 significantly suppressed GABA currents in rat GCs by activating GLP-1R. This result concurs with the report that GLP-1R-immunoreactivity occurs in the neurons of the rat GCL [10–12]. In plasm, the maximal concentration of GLP-1 after a meal is reported to be <40 pmol/L [1, 14]. Interestingly, in our experiments, 10 pmol/L GLP-1 (normal physiological postprandial concentration) suppressed GABA currents (28.11% ± 12.50% of control) more strongly than 50 nmol/L exendin-4 (69.08% ± 5.71% of control). Different from our results, GLP-1/exendin-4 increases GABAAR-mediated tonic currents and GABAAR-mediated spontaneous IPSC frequency through GLP-1R activation in rat hippocampal CA3 pyramidal neurons [14]. These results suggest that the modulation by GLP-1/exendin-4 of GABA receptors may vary depending on the cell type.
GLP-1 is a hormone released by intestinal L-cells [1]. In the brain, GLP-1 is synthesized by preproglucagon neurons, which are distributed in the nucleus of the solitary tract of the brain stem [2–5]. Real-time quantitative RT-PCR analysis has revealed that GLP-1 mRNA is present in the human retina [7]. Moreover, immunostaining of GLP-1 has been reported in neurons in the GCL of human and mouse retinas [7, 8]. These data suggest that GLP-1 may be also synthesized and released from GCs and/or displaced amacrine cells in the GCL. Previous studies have shown that GLP-1 can cross the blood-brain barrier [43, 61], but whether it can cross the blood-retina barrier remains unknown.
Intracellular Mechanisms Underlying Exendin-4-induced Suppression of GABA Currents
GLP-1R has been reported to primarily act through Gs [2, 43] and has also been shown to couple with the inhibitory Gi/o proteins [40]. Using selective pharmacological inhibitors, we found that Gs, but not Gi/o, were involved in the exendin-4-induced suppression of GABA currents (Fig. 3 E–G). Actually, studies on rat dorsal root ganglion neurons have shown that activation of Gs decreases GABA-induced currents [62].
Even though the PLC/PKC signaling pathway has been shown to be involved in GLP-1-induced insulin secretion in mouse pancreatic β cells [56], this signaling pathway was unlikely involved in the effect of exendin-4 on GABA currents in GCs, due to the persistence of this effect when PI-PLC or PC-PLC was blocked (Fig. 7). In contrast, our evidence suggested that the cAMP-PKA signaling pathway mediates the exendin-4-induced inhibition in GCs. The inhibitory effect of exendin-4 on GABA current was mimicked by perfusion with the cAMP analog 8-Br-cAMP. Moreover, the exendin-4-induced inhibition of GABA currents was eliminated when PKA was inhibited by KT-5720 or Rp-cAMP (Fig. 4). These results suggest that activating Gs-linked GLP-1R stimulates adenylyl cyclase and increases the cAMP level, which in turn activates PKA. cAMP and PKA are important signaling molecules responsible for GLP-1R-mediated effects, which have been shown in neurons and non-neuronal cells [63–70].
Furthermore, the exendin-4 effect on GABA currents in GCs was Ca2+-dependent. Ca2+ imaging showed that exendin-4 significantly increased [Ca2+]i in GCs (Fig. 5), consistent with the results reported in cultured hippocampal neurons showing that acute application of GLP-1 induces a transient elevation of [Ca2+]i [58]. GLP-1 has been shown to regulate voltage-gated Ca2+ channels. For example, GLP-1 enhances L-type Ca2+ currents through activation of the cAMP-dependent PKA pathway in canine cardiomyocytes [71], whereas when rat hippocampal neurons are pre-incubated with GLP-1 for 24 h, Ca2+ currents are significantly decreased [58]. However, when we chelated extracellular Ca2+ with EGTA, exendin-4 still suppressed the GABA currents in GCs, suggesting that Ca2+ entry through voltage-gated channels was not be involved in the action of exendin-4 under our experimental conditions. In contrast, when [Ca2+]i was greatly reduced by internal infusion of Ca2+-free solution, exendin-4 no longer suppressed GABA currents (Fig. 5F). Moreover, the exendin-4 effect on GABA currents was blocked when IP3 receptors were blocked by heparin. These data indicate that the Ca2+ release from IP3 receptor-mediated intracellular pools is associated with the exendin-4 effect on GCs. Elevated [Ca2+]i in rat GCs may be a result of exendin-4-induced PKA activation, because our experiments demonstrated that exendin-4 no longer caused changes in [Ca2+]i when PKA activity was blocked by H-89 (Fig. 5C, D). Actually, PKA activation has been shown to lead to Ca2+ release from intracellular storage in heart cells and hepatocytes through the ryanodine and/or IP3 receptor pathway [44, 45, 72].
Many cellular Ca2+-stimulated signaling cascades utilize the intermediate, CaM. The binding of Ca2+ alters the conformation of CaM and increases its affinity to many CaM-binding proteins, including CaMKII [73]. In GCs, the CaM/CaMKII pathway may mediate the exendin-4 effect, because the inhibitory effect by exendin-4 on GABA currents did not occur when CaM and CaMKII were blocked by W-7 and KN-93, respectively (Fig. 6). Consistent with our results, that CaM mediates the suppression of GABAA currents induced by elevated levels of [Ca2+]i has been reported in turtle retinal GCs [49] and rat rod bipolar cells [52], while that Ca2+/CaMKII mediates orexin-A-induced inhibition of GABAA currents has been reported in HEK293 cells [54].
GABA is one of the main inhibitory neurotransmitters and is predominantly released by wide-field GABAergic amacrine cells in the inner retina. It has been reported that GABAergic amacrine cells contribute to the organization of the GC receptive field [74–76]. The receptive field surround of GCs may be generated by feedforward inhibition directly onto GCs [75] or presynaptically, by GABAergic feedback inhibition from amacrine cells onto bipolar cell axons [77, 78]. Application of GABA suppresses both the spontaneous and light-evoked activity of all GCs and the GABAAR antagonist bicuculline potentiates the spontaneous and light-evoked activity of ON-type GCs [79], which suggests that GABA directly inhibits GCs. Light stimulation-induced GABAR-mediated responses on most GCs in this work indicated that these cells receive direct inhibitory inputs from GABAergic amacrine cells. Suppression by exendin-4 of the GABA responses of GCs suggests that exendin-4 weakens inhibitory inputs mediated by GABA, which modulates the receptive field properties of GCs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Xiong-Li Yang for his generous support of this project. This work was supported by the National Natural Science Foundation of China (81790640, 32070989, 31872766, 31571075, 82070993, and 31571072), the Ministry of Science and Technology of China (2011CB504602 and 2015AA020512), Shanghai Municipal Science and Technology Major Project (2018SHZDZX01), ZJLab, Shanghai Center for Brain Science and Brain-Inspired Technology, and Sanming Project of Medicine in Shenzhen (SZSM202011015).
Conflict of interest
The authors claim that there are no conflicts of interest.
References
- 1.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007, 87: 1409–1439. [DOI] [PubMed]
- 2.Salcedo I, Tweedie D, Li YZ, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: An emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol 2012, 166: 1586–1599. [DOI] [PMC free article] [PubMed]
- 3.Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C, Cardoso S, et al. Crosstalk between diabetes and brain: Glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration. Biochim Biophys Acta BBA Mol Basis Dis 2013, 1832: 527–541. [DOI] [PubMed]
- 4.Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab 2015, 4: 718–731. [DOI] [PMC free article] [PubMed]
- 5.Trapp S, Richards JE. The gut hormone glucagon-like peptide-1 produced in brain: Is this physiologically relevant? Curr Opin Pharmacol 2013, 13: 964–969. [DOI] [PMC free article] [PubMed]
- 6.Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology 1997, 138: 4445–4455. [DOI] [PubMed]
- 7.Hernández C, Bogdanov P, Corraliza L, García-Ramírez M, Solà-Adell C, Arranz JA, et al. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes 2016, 65: 172–187. [DOI] [PubMed]
- 8.Shu XS, Zhang YL, Li MQ, Huang XY, Yang YF, Zeng JH, et al. Topical ocular administration of the GLP-1 receptor agonist liraglutide arrests hyperphosphorylated tau-triggered diabetic retinal neurodegeneration via activation of GLP-1R/Akt/GSK3β signaling. Neuropharmacology 2019, 153: 1–12. [DOI] [PubMed]
- 9.Fischer AJ, Stanke JJ, Ghai K, Scott M, Omar G. Development of bullwhip neurons in the embryonic chicken retina. J Comp Neurol 2007, 503: 538–549. [DOI] [PubMed]
- 10.Zhang Y, Wang QP, Zhang JF, Lei X, Xu GT, Ye W. Protection of exendin-4 analogue in early experimental diabetic retinopathy. Graefe's Arch Clin Exp Ophthalmol 2009, 247: 699–706. [DOI] [PubMed]
- 11.Zhang Y, Zhang JF, Wang QP, Lei X, Chu Q, Xu GT, et al. Intravitreal injection of exendin-4 analogue protects retinal cells in early diabetic rats. Invest Ophthalmol Vis Sci 2011, 52: 278–285. [DOI] [PubMed]
- 12.Fan YC, Liu K, Wang QP, Ruan YY, Zhang Y, Ye W. Exendin-4 protects retinal cells from early diabetes in Goto-Kakizaki rats by increasing the Bcl-2/Bax and Bcl-xL/Bax ratios and reducing reactive gliosis. Mol Vis 2014, 20: 1557–1568. [PMC free article] [PubMed]
- 13.Li LZ, Yin N, Li XY, Miao YY, Cheng S, Li F, et al. Rac1 modulates excitatory synaptic transmission in mouse retinal ganglion cells. Neurosci Bull 2019, 35: 673–687. [DOI] [PMC free article] [PubMed]
- 14.Korol SV, Jin Z, Babateen O, Birnir B. GLP-1 and exendin-4 transiently enhance GABAA receptor-mediated synaptic and tonic currents in rat hippocampal CA3 pyramidal neurons. Diabetes 2015, 64: 79–89. [DOI] [PubMed]
- 15.Chen L, Yu YC, Zhao JW, Yang XL. Inwardly rectifying potassium channels in rat retinal ganglion cells. Eur J Neurosci 2004, 20: 956–964. [DOI] [PubMed]
- 16.Deng QQ, Sheng WL, Zhang G, Weng SJ, Yang XL, Zhong YM. Signalling mechanism for somatostatin receptor 5-mediated suppression of AMPA responses in rat retinal ganglion cells. Neuropharmacology 2016, 107: 215–226. [DOI] [PubMed]
- 17.Zhang G, Wu XH, Xu GZ, Weng SJ, Yang XL, Zhong YM. Orexin-B modulates synaptic transmission of rod bipolar cells in rat retina. Neuropharmacology 2018, 133: 38–50. [DOI] [PubMed]
- 18.Zheng C, Deng QQ, Liu LL, Wang MY, Zhang G, Sheng WL, et al. Orexin-A differentially modulates AMPA-preferring responses of ganglion cells and amacrine cells in rat retina. Neuropharmacology 2015, 93: 80–93. [DOI] [PubMed]
- 19.Zhang PP, Zhang G, Zhou W, Weng SJ, Yang XL, Zhong YM. Signaling mechanism for modulation by ATP of glycine receptors on rat retinal ganglion cells. Sci Rep 2016, 6: 28938. [DOI] [PMC free article] [PubMed]
- 20.Chen L, Yang XL. Hyperpolarization-activated cation current is involved in modulation of the excitability of rat retinal ganglion cells by dopamine. Neuroscience 2007, 150: 299–308. [DOI] [PubMed]
- 21.Liu F, Weng SJ, Yang XL, Zhong YM. Orexin-A potentiates L-type calcium/barium currents in rat retinal ganglion cells. Neuroscience 2015, 305: 225–237. [DOI] [PubMed]
- 22.Zhang XJ, Liu LL, Wu Y, Jiang SX, Zhong YM, Yang XL. σ receptor 1 is preferentially involved in modulation of N-methyl-D-aspartate receptor-mediated light-evoked excitatory postsynaptic currents in rat retinal ganglion cells. Neurosignals 2011, 19: 110–116. [DOI] [PubMed]
- 23.Brecha NC, Sternini C, Humphrey MF. Cellular distribution of L-glutamate decarboxylase (GAD) andγ-aminobutyric acidA (GABAA) receptor mRNAs in the retina. Cell Mol Neurobiol 1991, 11: 497–509. [DOI] [PMC free article] [PubMed]
- 24.Greferath U, Grünert U, Müller F, Wässle H. Localization of GABAA receptors in the rabbit retina. Cell Tissue Res 1994, 276: 295–307. [DOI] [PubMed]
- 25.Yeh HH, Grigorenko EV, Veruki ML. Correlation between a bicuculline-resistant response to GABA and GABAA receptor rho 1 subunit expression in single rat retinal bipolar cells. Vis Neurosci 1996, 13: 283–292. [DOI] [PubMed]
- 26.Koulen P, Sassoè-Pognetto M, Grünert U, Wässle H. Selective clustering of GABAA and glycine receptors in the mammalian retina. J Neurosci 1996, 16: 2127–2140. [DOI] [PMC free article] [PubMed]
- 27.Koulen P, Malitschek B, Kuhn R, Bettler B, Wässle H, Brandstätter JH. Presynaptic and postsynaptic localization of GABAB receptors in neurons of the rat retina. Eur J Neurosci 1998, 10: 1446–1456. [DOI] [PubMed]
- 28.Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, et al. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem 1993, 268: 19650–19655. [PubMed]
- 29.Thorens B, Porret A, Bühler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor: Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes 1993, 42: 1678–1682. [DOI] [PubMed]
- 30.Mentlein R. Dipeptidyl-peptidase IV (CD26)-role in the inactivation of regulatory peptides. Regul Pept 1999, 85: 9–24. [DOI] [PubMed]
- 31.Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci 2003, 23: 2939–2946. [DOI] [PMC free article] [PubMed]
- 32.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 2002, 110: 43–52. [DOI] [PMC free article] [PubMed]
- 33.Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 1992, 267: 7402–7405. [PubMed]
- 34.Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): A potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 2004, 117: 77–88. [DOI] [PubMed]
- 35.Roed SN, Wismann P, Underwood CR, Kulahin N, Iversen H, Cappelen KA, et al. Real-time trafficking and signaling of the glucagon-like peptide-1 receptor. Mol Cell Endocrinol 2014, 382: 938–949. [DOI] [PubMed]
- 36.Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang DL, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: A randomised, open-label, non-inferiority study. Lancet 2008, 372: 1240–1250. [DOI] [PubMed]
- 37.Greig NH, Holloway HW, de Ore KA, Jani D, Wang Y, Zhou J, et al. Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia 1999, 42: 45–50. [DOI] [PubMed]
- 38.Mayo KE, Miller LJ, Bataille D, Dalle S, Göke B, Thorens B, et al. International union of pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev 2003, 55: 167–194. [DOI] [PubMed]
- 39.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A 1987, 84: 3434–3438. [DOI] [PMC free article] [PubMed]
- 40.Hällbrink M, Holmqvist T, Olsson M, Ostenson CG, Efendic S, Langel U. Different domains in the third intracellular loop of the GLP-1 receptor are responsible for Galpha(s) and Galpha(i)/Galpha(o) activation. Biochim Biophys Acta 2001, 1546: 79–86. [DOI] [PubMed]
- 41.Zhang Y, Sun B, Feng D, Hu H, Chu M, Qu Q, et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 2017, 546: 248–253. [DOI] [PMC free article] [PubMed]
- 42.Hölscher C. The role of GLP-1 in neuronal activity and neurodegeneration. Vitam Horm 2010, 84: 331–354. [DOI] [PubMed]
- 43.Koshal P, Jamwal S, Kumar P. Glucagon-like Peptide-1 (GLP-1) and neurotransmitters signaling in epilepsy: An insight review. Neuropharmacology 2018, 136: 271–279. [DOI] [PubMed]
- 44.Bird GS, Burgess GM, Putney JW Jr. Sulfhydryl reagents and cAMP-dependent kinase increase the sensitivity of the inositol 1, 4, 5-trisphosphate receptor in hepatocytes. J Biol Chem 1993, 268: 17917–17923. [PubMed]
- 45.Wang Y, Li G, Goode J, Paz JC, Ouyang K, Screaton R, et al. Inositol-1, 4, 5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature 2012, 485: 128–132. [DOI] [PMC free article] [PubMed]
- 46.Chen QX, Wong RK. Suppression of GABAA receptor responses by NMDA application in hippocampal neurones acutely isolated from the adult guinea-pig. J Physiol 1995, 482 ( Pt 2): 353–362. [DOI] [PMC free article] [PubMed]
- 47.Jones MV, Westbrook GL. Shaping of IPSCs by endogenous calcineurin activity. J Neurosci 1997, 17: 7626–7633. [DOI] [PMC free article] [PubMed]
- 48.Aguayo LG, Espinoza F, Kunos G, Satin LS. Effects of intracellular calcium on GABAA receptors in mouse cortical neurons. Pflügers Arch 1998, 435: 382–387. [DOI] [PubMed]
- 49.Akopian A, Gabriel R, Witkovsky P. Calcium released from intracellular stores inhibits GABAA-mediated currents in ganglion cells of the turtle retina. J Neurophysiol 1998, 80: 1105–1115. [DOI] [PubMed]
- 50.Lu YF, Hawkins RD. Ryanodine receptors contribute to cGMP-induced late-phase LTP and CREB phosphorylation in the hippocampus. J Neurophysiol 2002, 88: 1270–1278. [DOI] [PubMed]
- 51.Hoffpauir BK, Gleason EL. Activation of mGluR5 modulates GABAA receptor function in retinal amacrine cells. J Neurophysiol 2002, 88: 1766–1776. [DOI] [PubMed]
- 52.Yu YC, Cao LH, Yang XL. Modulation by brain natriuretic peptide of GABA receptors on rat retinal ON-type bipolar cells. J Neurosci 2006, 26: 696–707. [DOI] [PMC free article] [PubMed]
- 53.Buck E, Zimanyi I, Abramson JJ, Pessah IN. Ryanodine stabilizes multiple conformational states of the skeletal muscle calcium release channel. J Biol Chem 1992, 267: 23560–23567. [PubMed]
- 54.Sachidanandan D, Reddy HP, Mani A, Hyde GJ, Bera AK. The neuropeptide orexin-A inhibits the GABAA receptor by PKC and Ca2+/CaMKII-dependent phosphorylation of its β1 subunit. J Mol Neurosci 2017, 61: 459–467. [DOI] [PubMed]
- 55.Osawa M, Kuwamoto S, Izumi Y, Yap KL, Ikura M, Shibanuma T, et al. Evidence for calmodulin inter-domain compaction in solution induced by W-7 binding. FEBS Lett 1999, 442: 173–177. [DOI] [PubMed]
- 56.Shigeto M, Ramracheya R, Tarasov AI, Cha CY, Chibalina MV, Hastoy B, et al. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J Clin Invest 2015, 125: 4714–4728. [DOI] [PMC free article] [PubMed]
- 57.Babateen O, Korol SV, Jin Z, Bhandage AK, Ahemaiti A, Birnir B. Liraglutide modulates GABAergic signaling in rat hippocampal CA3 pyramidal neurons predominantly by presynaptic mechanism. BMC Pharmacol Toxicol 2017, 18: 83. [DOI] [PMC free article] [PubMed]
- 58.Gilman CP, Perry T, Furukawa K, Grieg NH, Egan JM, Mattson MP. Glucagon-like peptide 1 modulates calcium responses to glutamate and membrane depolarization in hippocampal neurons. J Neurochem 2003, 87: 1137–1144. [DOI] [PubMed]
- 59.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, et al. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab 2013, 305: E1367–E1374. [DOI] [PMC free article] [PubMed]
- 60.Liu J, Conde K, Zhang P, Lilascharoen V, Xu ZH, Lim BK, et al. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron 2017, 96: 897–909.e5. [DOI] [PMC free article] [PubMed]
- 61.Orskov C, Poulsen SS, Moller M, Holst JJ. Glucagon-like peptide I receptors in the subfornical organ and the area postrema are accessible to circulating glucagon-like peptide I. Diabetes 1996, 45: 832–835. [DOI] [PubMed]
- 62.Huang CS, Narahashi T. The role of G proteins in the activity and mercury modulation of GABA-induced currents in rat neurons. Neuropharmacology 1997, 36: 1623–1630. [DOI] [PubMed]
- 63.Green BD, Gault VA, Flatt PR, Harriott P, Greer B, O'Harte FPM. Comparative effects of GLP-1 and GIP on cAMP production, insulin secretion, and in vivo antidiabetic actions following substitution of Ala8/Ala2 with 2-aminobutyric acid. Arch Biochem Biophys 2004, 428: 136–143. [DOI] [PubMed]
- 64.Holz GG, Leech CA, Heller RS, Castonguay M, Habener JF. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic beta-cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7-37). J Biol Chem 1999, 274: 14147–14156. [DOI] [PMC free article] [PubMed]
- 65.Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 2012, 13: 33. [DOI] [PMC free article] [PubMed]
- 66.Perry T, Lahiri DK, Chen DM, Zhou J, Shaw KTY, Egan JM, et al. A novel neurotrophic property of glucagon-like peptide 1: A promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther 2002, 300: 958–966. [DOI] [PubMed]
- 67.Perry TA, Greig NH. A new Alzheimer's disease interventive strategy: GLP-1. Curr Drug Targets 2004, 5: 565–571. [DOI] [PubMed]
- 68.Sharma MK, Jalewa J, Hölscher C. Neuroprotective and anti-apoptotic effects of liraglutide on SH-SY5Y cells exposed to methylglyoxal stress. J Neurochem 2014, 128: 459–471. [DOI] [PubMed]
- 69.Wang MD, Huang Y, Zhang GP, Mao L, Xia YP, Mei YW, et al. Exendin-4 improved rat cortical neuron survival under oxygen/glucose deprivation through PKA pathway. Neuroscience 2012, 226: 388–396. [DOI] [PubMed]
- 70.Zhang HN, Liu YH, Guan SY, Qu D, Wang L, Wang XS, et al. An orally active allosteric GLP-1 receptor agonist is neuroprotective in cellular and rodent models of stroke. PLoS One 2016, 11: e0148827. [DOI] [PMC free article] [PubMed]
- 71.Xiao YF, Nikolskaya A, Jaye DA, Sigg DC. Glucagon-like peptide-1 enhances cardiac L-type Ca2+ currents via activation of the cAMP-dependent protein kinase A pathway. Cardiovasc Diabetol 2011, 10: 6. [DOI] [PMC free article] [PubMed]
- 72.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor). Cell 2000, 101: 365–376. [DOI] [PubMed]
- 73.Brzozowski JS, Skelding KA. The multi-functional calcium/calmodulin stimulated protein kinase (CaMK) family: Emerging targets for anti-cancer therapeutic intervention. Pharmaceuticals (Basel) 2019, 12: E8. [DOI] [PMC free article] [PubMed]
- 74.Cook PB, McReynolds JS. Lateral inhibition in the inner retina is important for spatial tuning of ganglion cells. Nat Neurosci 1998, 1: 714–719. [DOI] [PubMed]
- 75.Flores-Herr N, Protti DA, Wässle H. Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. J Neurosci 2001, 21: 4852–4863. [DOI] [PMC free article] [PubMed]
- 76.Buldyrev I, Taylor WR. Inhibitory mechanisms that generate centre and surround properties in ON and OFF brisk-sustained ganglion cells in the rabbit retina. J Physiol 2013, 591: 303–325. [DOI] [PMC free article] [PubMed]
- 77.Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol 2000, 83: 1817–1829. [DOI] [PubMed]
- 78.Matsui K, Hasegawa J, Tachibana M. Modulation of excitatory synaptic transmission by GABAC receptor-mediated feedback in the mouse inner retina. J Neurophysiol 2001, 86: 2285–2298. [DOI] [PubMed]
- 79.Bolz J, Frumkes T, Voigt T, Wässle H. Action and localization of gamma-aminobutyric acid in the cat retina. J Physiol 1985, 362: 369–393. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.