Abstract
Aim
The rapid outbreak of the coronavirus disease 2019 (COVID-19) pandemic posed challenges across different medical fields, especially reproductive health, and gave rise to concerns regarding the effects of SARS-CoV-2 on male infertility, owing to the fact that the male reproductive system indicated to be extremely vulnerable to SARS-CoV-2 infection. Only a small number of studies have investigated the effects of SARS-CoV-2 on male reproduction, but the results are not consistent. So, we performed this meta-analysis to draw a clearer picture and evaluate the impacts of COVID-19 on male reproductive system.
Method
We searched Embase, Web of Science, PubMed, and Google Scholar databases to identify the potentially relevant studies. Standardized mean difference (SMD) with 95% confidence interval (CI) was applied to assess the relationship. Heterogeneity testing, sensitivity analysis, and publication bias testing were also performed.
Results
A total of twelve studies including 7 case control investigations and 5 retrospective cohort studies were found relevant and chosen for our research. Our result showed that different sperm parameters including semen volume [SMD = − 0.27 (− 0.46, − 1.48) (p = 0.00)], sperm concentration [SMD = − 0.41 (− 0.67, − 0.15) (p = 0.002)], sperm count [SMD = − 0.30 (− 0.44, − 0.17) (p = 0.00)], sperm motility [SMD = − 0.66 (− 0.98, − 0.33) (p = 0.00)], and progressive motility [SMD = − 0.35 (− 0.61, − 0.08) (p = 0.01)] were negatively influenced by SARS-CoV-2 infection. However, sperm concentration (p = 0.07) and progressive motility (p = 0.61) were not found to be significantly associated with SARS-CoV-2 infection in case control studies. No publication bias was detected.
Conclusion
The present study revealed the vulnerability of semen quality to SARS-CoV-2 infection. Our data showed a strong association of different sperm parameters with SARS-CoV-2 infection. The results suggested that SARS-CoV-2 infection in patients may negatively influence their fertility potential in a short-term period, but more studies are needed to decide about the long-term effects.
Keywords: SARS-CoV-2 infection, COVID-19, Semen parameters, Male reproduction, Male infertility
Introduction
Infertility is a reproductive system disorder [1, 2] and about 20% of reproductive-aged couples suffer from infertility [3, 4]. Male factors constitute 40 to 50% of all infertility cases [5]. Pathological agents, especially viruses, play a distinctive role in infertility [6, 7]. Viruses as a major cause of fertility problems are able to potentially interfere with reproductive function in men [8]. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was found to be the causing viral pathogen of COVID-19 and strongly capable of affecting human health [9]. It was first discovered in December 2019 in Wuhan, China. This new viral strain leads to life-threatening complications including fever, nasal congestion, asthenia, anosmia, ageusia, and dyspnea in human and has shown to spread quickly [10], so shortly after its appearance, in February 2020, the World Health Organization (WHO) announced the global pandemic [11]. It became crucially important to identify different routes of COVID-19 transmission to reduce its high rates of spread. Close contact and respiratory droplets were two main transmission routes [12]. Then, traces of SARS-CoV-2 were found in feces and urine [13] and made scientists curious about the possibility of sexual transmission of SARS-CoV-2 and also possible negative impacts of this deadly virus on fertility potential. In addition, multiple studies showed that the expression level of angiotensin-converting enzyme 2 (ACE2), which is considered to be the major receptor molecule of SARS-CoV-2 for binding and entry into host cells [14], was the highest in testes, making the male reproductive system extremely vulnerable to SARS-CoV-2 infection. Therefore, researchers tried more to focus on the impacts of COVID-19 on male reproduction. However, several investigations were done; to date, results are controversial. Thus, in this study, we aimed to further clarify the impacts of SARS-CoV-2 on male reproduction. We accomplished this comprehensive meta-analysis to evaluate the effects of SARS-CoV-2 on different semen parameters including sperm count, sperm concentration, volume, motility, and progressive motility. To the best of our knowledge, this is the first meta-analysis to investigate the role of this pathogen on male fertility potential.
Methods
Search strategy
We searched Embase, Web of Science, PubMed, and Google Scholar databases to find potentially relevant papers concerning the effects of SARS-CoV-2 infection on male reproduction and semen parameters published up to February 2022. We applied no restrictions on date, language, or geographical location. Only human studies were included. We used keywords that were a combination of SARS-CoV-2 infection or COVID-19 and words such as semen, sperm, seminal fluid, semen parameters, male infertility, male fertility, male reproductive system, male reproduction, sperm abnormalities, sperm quality, sperm parameters, sperm concentration, sperm count, sperm volume, motility, immotile, progressive motility (refers to sperm that are swimming in a mostly straight line or large circles), morphology, sperm viability, apoptosis, sperm integrity, and sperm damage. Two authors were in charge of screening the titles and abstracts to find potentially relevant articles. Then, the full text was screened to collect data.
In the present study, all review and screening processes were done according to the PRISMA guidelines for reporting systematic reviews (http://www.prisma-statement.org/). We reached consensus on which reports to include by discussion or by asking a third reviewer to adjudicate.
Types of studies
Eligible studies that were chosen to be included in this research were those studying the impacts of SARS-CoV-2 infection on male reproduction and different semen parameters. Only full articles (cohort or case-controlled study) were selected to be included in this meta-analysis. Abstracts, comments, reviews, and editorials were not included. Duplicated hits and studies with low-quality or insufficient data were excluded. The reference list of identified articles was searched for possible relative papers too. If applicable, the corresponding authors of relevant ongoing studies were contacted for information regarding unpublished data.
Data collection
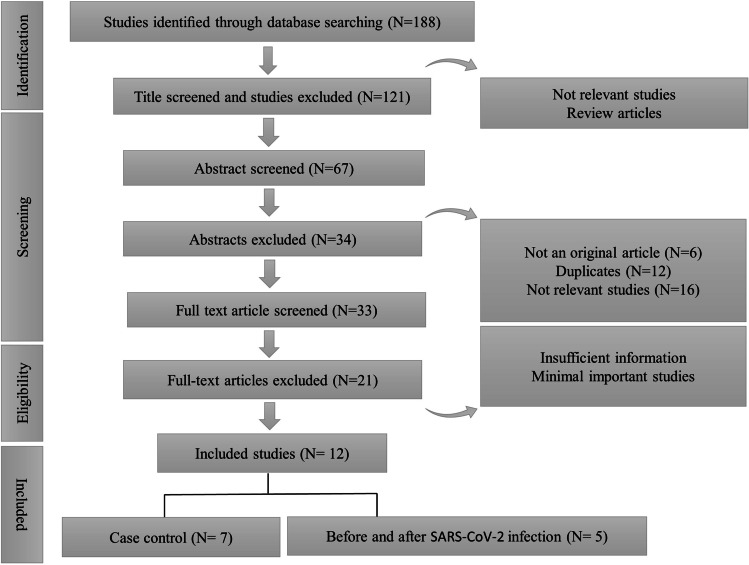
For data collection, data extraction forms were used. The data that were collected from each article were as follows: first author, year of publication, the month of publication, country of origin, city of origin, the design of the study, the total number of subjects, number of infected cases, number of controls, the age of subjects, the smoking status, the weight of participants, the effect on different semen parameters, the detection method of SARS-CoV-2 infection, the tissue used for detection, and the presence of SARS-CoV-2 in semen. For each parameter, the number of individuals (COVID + /COVID −) or the mean difference along with the relevant standard deviation (SD) was imported. In case the mean differences or SDs were not available, the mean differences and SDs were calculated using available data or the authors of the study were connected to obtain the needed data. Eventually, after evaluating the data of 24 articles, a total of 9 studies were selected for this meta-analysis. The data were extracted by three authors independently. Disagreements between reviewers were discussed to obtain consensus. The process of study selection is demonstrated in Fig. 1.
Fig. 1.
The flow chart for the search methodology
Assessment of study quality
The quality of the included studies was assessed using the Newcastle–Ottawa scale [15]. The scale consists of nine items covering three dimensions: (i) selection, scoring a maximum of 4; (ii) comparability, maximum score 2; and (iii) exposure (case control)/outcome (cohort), maximum score 3, with a maximum total of nine representing high quality.
Statistical analysis
All data were analyzed using Stata® 13.0 (StataCorp LP., College Station, TX, USA). When p > 0.1 and I2 < 50%, the fixed effects model was used; otherwise, the random effects model was used. In addition, publication bias was detected by Begg’s and Egger’s test. For all of the statistical analyses, a value of p < 0.05 was regarded as statistically significant and all of the tests were two-sided.
Results
Characteristics of the included studies
Of the 118 articles that were identified to be related to our study, 67 articles were chosen to go through data collection after title screening. Thirty-four articles were removed in abstract screening and 33 articles underwent full-text screening. From 33 articles, only 12 papers met our inclusion criteria and had sufficient data for the analysis. The studies that did not meet our inclusion criteria had insufficient data in results or just evaluated the data in patients and did not compare it with controls or COVID-free individuals. Some did not have the adequate quality to be included in the analysis or their methodology was not clearly described. In some articles, patients suffered from multiple genital infections that would have questioned their results. The articles chosen for this meta-analysis were divided into two different groups:
A group of 7 articles studying the effect of SARS-CoV-2 infection on various sperm parameters in case and control subjects.
A group of 5 retrospective cohort articles that studying the impacts of SARS-CoV-2 infection on different sperm parameters among subjects before and after COVID-19 infection.
Table 1 shows a summary of the data extracted from each article. All the study subjects involved were treated in hospital-based settings.
Table 1.
Characteristics of included studies concerning the effect of SARS-CoV-2 on semen parameters
| Ref | Country | Date | Specimen | Presence of SARS-CoV − 2 in semen | Population | Outcome | Newcastle–Ottawa scale |
|---|---|---|---|---|---|---|---|
| [18] | Duesseldorf, Germany | August 2020 |
Blood Pharyngeal swab Semen |
+ |
14 mild 14 moderates 14 controls |
Decreased sperm concentration, count, and motility | 8 |
| [23] | Hefei, China | March 2021 |
Blood Throat swabs or respiratory specimens Semen |
_ |
29 mild 10 moderates 2 severe 50 controls |
Decreased sperm concentration, count, and motility | 9 |
| [22] | Istanbul, Turkey | October 2020 |
Blood Nasopharyngeal and oropharyngeal Semen |
+ |
10 patients 10 controls |
Decreased sperm morphology | 7 |
| [24] | Wuhan, China | November 2020 |
Blood Pharyngeal swab Semen Urine |
+ |
11 mild 31 moderates 32 severe 145controls |
Decreased sperm concentration, count, and motility | 7 |
| [21] | Wuhan, China | October 2020 |
Throat swab Semen |
+ |
9 mild 14 moderates 22 controls |
Decreased sperm concentration, impairment of spermatogenesis | 7 |
| [30] | Miami, USA | July 2021 |
Nasal or pharyngeal swab Semen |
+ |
30 patients 30 controls |
Decreased sperm concentration and sperm count | 6 |
| [16] | Qom, Iran | June 2021 |
Nasopharyngeal swab Blood Semen |
- |
60 patients 40 controls |
Decreased sperm concentration, motility, vitality, and normal form | 6 |
| [31] | Ankara, Turkey | July 2021 |
Blood Nasopharyngeal swab Semen |
- | 21 patients | Decreased sperm count, concentration, morphology, motility, and semen volume | 5 |
| [19] | Istanbul, Turkey | June 2021 |
Nasopharyngeal swabs Semen |
- | 24 mild | Decreased sperm count and concentration | 6 |
| [20] | Karaman, Turkey | April 2021 |
Oropharyngeal and nasopharyngeal swabs Semen |
- |
26 mild 43 moderates |
Decreased sperm count, concentration, motility, and semen volume | 7 |
| [32] | Konya, Turkey | October 2021 | Semen | - |
39 mild 43 moderates |
Decreased sperm count, morphology, and concentration | 5 |
| [17] | Shiraz, Iran | October 2021 |
Nasopharyngeal swabs Blood Semen |
- | 200 patients | Decreased sperm concentration, morphology, and motility | 6 |
Meta-analysis outcomes
This investigation evaluated the effects of SARS-CoV-2 infection on semen volume, sperm count, sperm concentration, sperm motility, and progressive motility.
-
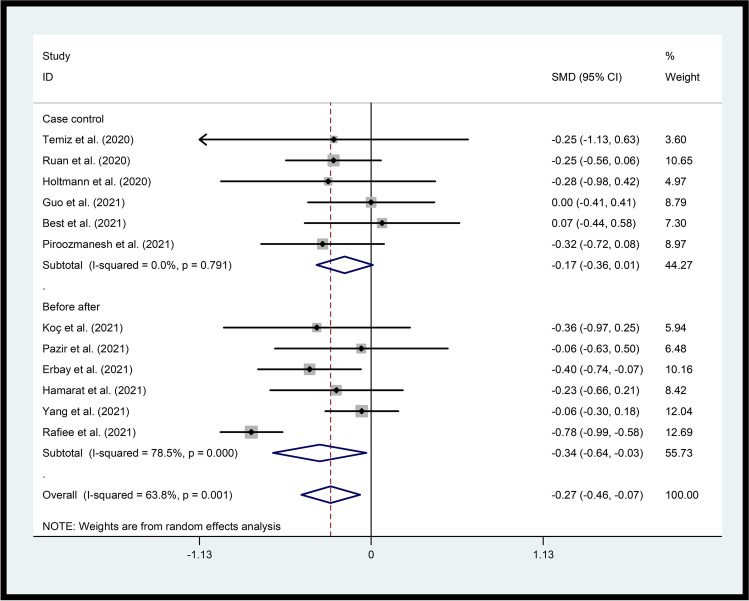
A.Sperm volumeOur results revealed a significant association between SARS-CoV-2 infection and decreased semen volume in both case control (p=0.05) and before after studies (p=0.03). The overall effect also showed that SARS-CoV-2 infection was negatively related to semen volume in patients (p=0.007), with a SMD (95% CI) = −0.27 (−0.46, −1.48) and p=0.00 for the overall effect, and p=0.001 and I2 = 63.8% for heterogeneity (Fig. 2).
-
B.Sperm concentrationThe data from seven studies that investigated the sperm concentration among patients and healthy individuals showed lower sperm concentrations in COVID-19 patients compared with controls, but it was not significant (p=0.07). In contrast, cases with COVID-19 had significantly decreased sperm concentrations than before the infection (p=0.02). For heterogeneity, p=0.00 and I2 = 81.3% and for overall effect, p=0.002 with a SMD (95% CI) = −0.41 (−0.67, −0.15) (Fig. 3).
-
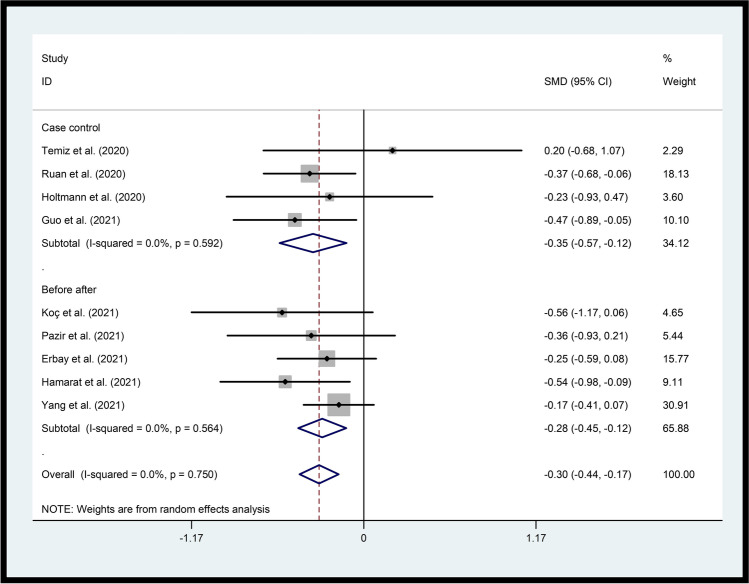
C.Sperm countPatients with SARS-CoV-2 infection indicated significantly reduced sperm counts in comparison to healthy subjects (p=0.003) and before SARS-CoV-2 infection (p=0.001). For overall effect, p=0.00 [SMD (95% CI) = −0.30 (−0.44, −0.17)] and for heterogeneity, p=0.75 and I2 = 0.0% (Fig. 4).
-
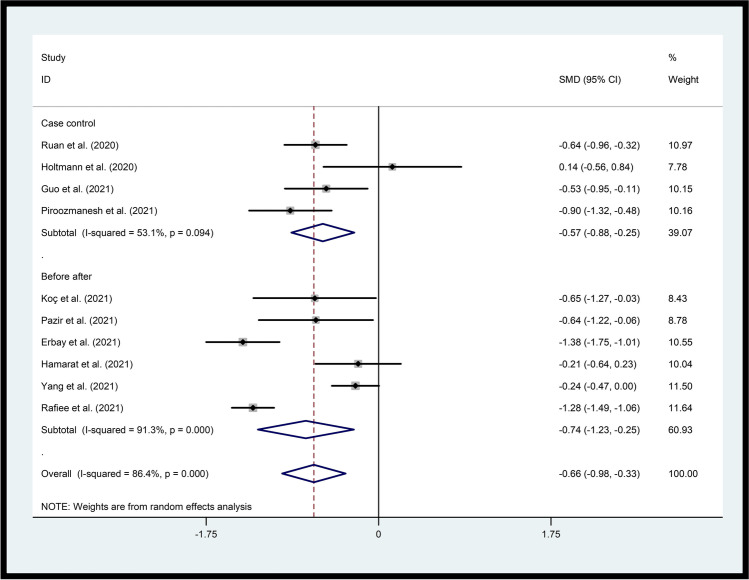
D.Sperm motilityReduced sperm motility was observed in both groups. The data suggested that motility of the sperm was significantly lower in patients compared to healthy subjects (p=0.001) and before SARS-CoV-2 infection (p=0.003). For overall effect, p=0.00 [SMD (95% CI) = −0.66 (−0.98, −0.33)] and for heterogeneity, p=0.00 and I2 = 84.6% (Fig. 5).
-
E.
Progressive motility
As opposed to case control studies where progressive motility showed no correlation to disease occurrence (p=0.61), individuals who experienced COVID-19 had significantly reduced rates of progressive motility compared to their condition before SARS-CoV-2 infection (p=0.00). For heterogeneity, p=0.001 and I2 = 70.1% and for overall effect, p=0.01 with a SMD (95% CI) = −0.35 (−0.61, −0.08) (Fig. 6).
Fig. 2.
Forest plots of the effect of SARS-CoV-2 infection on semen volume. The solid line on the forest plot is the point of no effect (OR = 1), and the dashed line represents the overall pooled estimate. The gray squares and horizontal lines represent the odds ratios of each study and their 95% confidence intervals
Fig. 3.
Forest plots of the effect of SARS-CoV-2 infection on sperm concentration
Fig. 4.
Forest plots of the effect of SARS-CoV-2 infection on sperm count
Fig. 5.
Forest plots of the effect of SARS-CoV-2 infection on sperm motility
Fig. 6.
Forest plots of the effect of SARS-CoV-2 infection on progressive motility
Sensitivity analysis
Sensitivity analysis was done in our study to see if any of the included studies affected our results. The data showed that no single study altered our results, indicating that our findings were statistically stable and reliable.
Publication bias
For publication bias, Egger’s regression test was performed. The results showed no publication bias [semen volume (p = 0.14), sperm concentration (p = 0.31), sperm count (p = 0.63), sperm motility (p = 0.67), and progressive motility (p = 0.38)].
Discussion
Our data indicated that SARS-CoV-2 infection could lead to significant impairments of male reproductive function through exerting negative influences on different semen parameters. The data of this research demonstrated that semen volume, sperm concentration, sperm count, and sperm motility were negatively under the influence of SARS-CoV-2 infection and resulted in deterioration of mentioned parameters in COVID-19 patients. To the best of our knowledge, this is the first study to investigate the effects of SARS-CoV-2 on semen quality.
The negative effects of SARS-CoV-2 infection on semen parameters and male fertility repetitively confirmed in different studies [16, 17]. The first study to evaluate the effects of COVID-19 on sperm parameters belonged to Holtmann and colleagues in Germany [18]. They discovered that a mild COVID-19 infection most likely does not affect testis and epididymis function, whereas semen parameters did appear impaired after a moderate infection. However, their sample size was quite limited and is the major weakness of their research. Their patient group included only a limited number of patients in mild and moderate conditions and they only had two patients in critical condition. Pazir et al. showed different results. Their observations suggested that sperm motility and total motile sperm count were significantly reduced in cases with a history of mild COVID-19 [19]. Likewise, Erby and authors in an investigation on 69 recovered patients declared short-term negative impacts of SARS-CoV-2 on spermatogenesis [20].
Another study in China on a group of 46 cases and controls showed that spermatogenesis could be severely impaired due to SARS-CoV-2 infection as a result of an elevated immune response in testis. They also observed that COVID-19 led to autoimmune orchitis in some patients [21]. In contrast to these studies, a case control study in Turkey reported that SARS-CoV-2 has no specific deteriorative effect on male sexual and reproductive health in a short-time period [22].
Interestingly, Guo and co-workers reported that the effects of SARS-CoV-2 infection on sperm quality could be adverse but potentially reversible [23]. They showed that the total sperm count, sperm concentration, and percentages of motile and progressively motile spermatozoa in the patients were significantly lower at first sampling, while sperm vitality and morphology were not affected. However, the former parameters enhanced after 3 weeks, and the percentage of morphologically abnormal sperm was reduced at the second sampling compared with those at first sampling, for which they did not provide any explanation and they conclude that the after-effects of COVID-19 may last for one spermatogenic cycle that is about 74 days. An explanation for their results can be seen in another research in Turkey where decreased morphology rated in patients was observed in comparison to controls and the authors suggested the fever to be the blame for this impairment [22].
There is only one study regarding the long-term effects of SARS-CoV-2 on semen parameters [24]. In this investigation, patients ≥ 90 days into their recovery still had lower total sperm counts.
It should be noted that impairment of semen parameters may not be due to a direct effect of the SARS-CoV-2 virus. There are the impacts of stress and other psychopathological factors on reproduction [25, 26]. Fever can also lead to reduced sperm number and quality, morphology, and motility in particular [27]. There are likely to be additional factors that contribute to long-COVID sequelae but whose identity is currently unknown.
There are a number of strengths and limitations that should be mentioned. Our meta-analysis included 12 studies of 973 men which enabled a much greater possibility of drawing relatively accurate conclusions. Our inclusion of studies from countries on different continents enhanced robustness and reliability of the data. Finally, the results from the quality appraisal indicated that the methodological quality of the included studies was generally good.
Several limitations need to be considered in the interpretation of our results.
First, not considering some potential confounders which may influence the quality of semen and sperm and subsequently the fertility potential together with virus infection is another major weakness in studies thus far. For example, sociodemographic factors like drug use and tobacco and alcohol consumption, that might be risk factors for decreased semen quality, were not reported in these studies [28, 29].
Second, even though the age of patients is an important factor in assessing the risk, the information regarding the age of patients was not reported in all the studies included in this meta-analysis Therefore, we were unable to measure the impact of age on the incidence rates of the included complications.
Third, the duration of disease and the time between positive COVID-19 test and semen collection vary from one study to another. However, the time between last positive RT-PCR test and semen samples collection was not less than 70 days in COVID-free patients which is the necessary time for after-effects of COVID-19 to wear off as the duration of one spermatogenic cycle is about 74 days. The sampling process in patients during their disease course was also when their blood or pharyngeal swab RT-PCR test was positive and had the related symptoms. Almost in all studies, this time was 14–40 days from their positive results. Finally, the severity of the disease was not indicated in several studies.
In conclusion, the present study revealed the vulnerability of semen quality to SARS-CoV-2 infection. While the long-term impacts of SARS-CoV-2 on male reproduction should be interpreted cautiously, our data showed negative impact of COVID-19 on various parameters of sperm quality. Further efforts should be made to study the long-time effects of COVID-19 infection on male reproduction. Lack of data regarding the influence of SARS-CoV-2 on reproductive function in men needs more attention from researchers in different countries. We suggest investigating the short- and long-term effects of SARS-CoV-2 on male reproduction in different regions. Studying the effects of SARS-CoV-2 infection on male fertility potential in patients from different age groups, with different severity of the disease, and different stages would significantly assist researchers to further reveal the role of this virus in male reproduction.
Acknowledgements
We would like to thank M. Motamedifar and S. Ghadimi for their help.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marziye Farsimadan, Email: mary.farsimadan@gmail.com.
Giandomenico Roviello, Email: giandomenicoroviello@hotmail.it.
References
- 1.Siahpoosh Z, et al. KISS1R polymorphism rs587777844 (Tyr313His) is linked to female infertility. Br J Biomed Sci. 2021;78(2):98–100. doi: 10.1080/09674845.2020.1856496. [DOI] [PubMed] [Google Scholar]
- 2.Li, R., et al., Genetic variants miR-126, miR-146a, miR-196a2, and miR-499 in polycystic ovary syndrome. British Journal of Biomedical Science, 2022: p. 7. [DOI] [PMC free article] [PubMed]
- 3.Farsimadan, M., et al., MicroRNA variants in endometriosis and its severity. Br J Biomed Sci, 2021: p. 1–5. [DOI] [PubMed]
- 4.Farsimadan, M., et al., Association analysis of KISS1 polymorphisms and haplotypes with polycystic ovary syndrome. Br J Biomed Sci, 2021: p. 1–5. [DOI] [PubMed]
- 5.Farsimadan, M. and M. Motamedifar, Bacterial infection of the male reproductive system causing infertility. J Reproductive Immunology, 2020: p. 103183. [DOI] [PubMed]
- 6.Farsimadan M, et al. The effects of hepatitis B virus infection on natural and IVF pregnancy: a meta-analysis study. J Viral Hepatitis. 2021;28(9):1234–1245. doi: 10.1111/jvh.13565. [DOI] [PubMed] [Google Scholar]
- 7.Farsimadan MM. Motamedifar, The effects of human immunodeficiency virus, human papillomavirus, herpes simplex virus-1 and -2, human herpesvirus-6 and -8, cytomegalovirus, and hepatitis B and C virus on female fertility and pregnancy. Br J Biomed Sci. 2021;78(1):1–11. doi: 10.1080/09674845.2020.1803540. [DOI] [PubMed] [Google Scholar]
- 8.Hezavehei M, et al. Possible male reproduction complications after coronavirus pandemic. Cell Journal (Yakhteh) 2021;23(4):382. doi: 10.22074/cellj.2021.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoine D, et al. Rapid, point-of-care scFv-SERS assay for femtogram level detection of SARS-CoV-2. ACS sensors. 2022;7(3):866–873. doi: 10.1021/acssensors.1c02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farsimadan, M. M. Motamedifar, SARS-CoV-2 effects on male reproduction: should men be worried?? Human Fertility, 2021: p. 1–11. [DOI] [PubMed]
- 11.Jebril, N., World Health Organization declared a pandemic public health menace: a systematic review of the coronavirus disease COVID-19. Available at SSRN. 2019;3566298:2020. [Google Scholar]
- 12.Bao L, et al. Transmission of severe acute respiratory syndrome coronavirus 2 via close contact and respiratory droplets among human angiotensin-converting enzyme 2 mice. J Infect Dis. 2020;222(4):551–555. doi: 10.1093/infdis/jiaa281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones DL, et al. Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Sci Total Environ. 2020;749:141364. doi: 10.1016/j.scitotenv.2020.141364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baig MS, et al. Identification of a potential peptide inhibitor of SARS-CoV-2 targeting its entry into the host cells. Drugs R&D. 2020;20(3):161–169. doi: 10.1007/s40268-020-00312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa Scale comparing reviewers’ to authors’ assessments. BMC Med Res Methodology. 2014;14(1):45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piroozmanesh H, et al. The effect of COVID-19 infection on sperm quality and male fertility. Jentashapir J Cellular Molecular Biol. 2021;12:2. doi: 10.5812/jjcmb.115390. [DOI] [Google Scholar]
- 17.Rafiee BSMB. Tabei, The effect of N-acetyl cysteine consumption on men with abnormal sperm parameters due to positive history of COVID-19 in the last three months. Archivio Italiano di Urologia e Andrologia. 2021;93(4):465–467. doi: 10.4081/aiua.2021.4.465. [DOI] [PubMed] [Google Scholar]
- 18.Holtmann N, et al. Assessment of SARS-CoV-2 in human semen—a cohort study. Fertil Steril. 2020;114(2):233–238. doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pazir Y, et al. Impaired semen parameters in patients with confirmed SARS-CoV-2 infection: a prospective cohort study. Andrologia. 2021;53(9):e14157. doi: 10.1111/and.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erbay, G., et al., Short-term effects of COVID-19 on semen parameters: a multicenter study of 69 cases. Andrology, 2021. [DOI] [PMC free article] [PubMed]
- 21.Li H, et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine. 2020;28:100604. doi: 10.1016/j.eclinm.2020.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Temiz MZ, et al. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: a cross-sectional, pilot study. Andrologia. 2021;53(2):e13912. doi: 10.1111/and.13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo T-H, et al. Semen parameters in men recovered from COVID-19. Asian J Androl. 2021;23(5):479. doi: 10.4103/aja.aja_31_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruan Y, et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: a perspective and urogenital evaluation. Andrology. 2021;9(1):99–106. doi: 10.1111/andr.12939. [DOI] [PubMed] [Google Scholar]
- 25.Li T, et al. Chronic stress impairs male spermatogenesis function and Nectin-3 protein expression in the testis. Physiol Res. 2020;69(2):297–306. doi: 10.33549/physiolres.934287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordkap L, et al. Impact of psychological stress measured in three different scales on testis function: a cross-sectional study of 1362 young men. Andrology. 2020;8(6):1674–1686. doi: 10.1111/andr.12835. [DOI] [PubMed] [Google Scholar]
- 27.Sergerie M, et al. High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertility and sterility. 2007;88(4):970–e1-970. e7. doi: 10.1016/j.fertnstert.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 28.Sansone A, et al. Smoke, alcohol and drug addiction and male fertility. Reprod Biol Endocrinol. 2018;16(1):1–11. doi: 10.1186/s12958-017-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durairajanayagam D. Lifestyle causes of male infertility. Arab J Urol. 2018;16(1):10–20. doi: 10.1016/j.aju.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Best, JC., et al., Evaluation of SARS-CoV-2 in human semen and effect on total sperm number: a prospective observational study. The world journal of men’s health, 2021. 39. [DOI] [PMC free article] [PubMed]
- 31.Koç E, Keseroğlu BB. Does COVID-19 worsen the semen parameters? Early results of a tertiary healthcare center. Urol Int. 2021;105(9–10):743–748. doi: 10.1159/000517276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamarat MB, et al. Effect of SARS-CoV-2 infection on semen parameters. Canadian Urological Association J. 2022;16:3. doi: 10.5489/cuaj.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]