Abstract
Context
Insulin-like growth factor-1 (IGF-1) is main serum surrogate marker of growth hormone (GH) secretion, used in diagnostics and treatment of GH deficiency (GHD) and acromegaly. Regional, ethnic, racial or nutritional factors obscure cross-population applicability of IGF-1 reference values. Establishment of population- and assay-specific reference values requires sizable representative cohort of healthy subjects.
Subjects and Methods
In representative sample of healthy adult population of Serbia (N=1200, 21-80 years, 1:1 male:female) serum IGF-1 was analyzed by Siemens Immulite 2000 assay under uniform laboratory conditions. Upper and lower limit of reference range (5th - 95th percentile) were calculated for each of the 12 quinquennial age intervals. IGF-1 distribution was normalized and standard deviation score (SDS) calculated by Logarithmic and LMS methods.
Results
IGF-1 and age correlated significantly, with most prominent decline at 21-50 years, followed by a plateau up to age of 70. Gender differences were not significant overall. Plateau in age-related IGF-1 decline was less prominent in women. Correlations of IGF-1 with body mass index (BMI) or waist to hip ratio (WHR) were insignificant. Superior IGF-1 SDS transformation was achieved with LMS method, while logarithmic method was simpler to use.
Conclusions
Normative age-specific serum IGF-1 reference values were established on a representative cohort of healthy adults in Serbia. Our results support recommendations against necessity for gender-specific or BMI- and WHR-specific reference ranges. Population-based data serve to generate IGF-1 SDS, which is valuable in rational application of consensus guidelines, proper longitudinal follow-up, advancement in efficacy and safety and personalization of treatment targets.
Keywords: normative data, Southeast Europe, normal healthy population, SD-score, population-based, IGF-1, Growth hormone
Introduction
Serum insulin-like growth factor 1 (IGF-1) is major biochemical surrogate marker of growth hormone (GH) secretion. This 7650Da monomer forms a tertiary complex with IGF-binding proteins (IGFBP) - mainly IGFBP3, and acid-labile subunit. This prolongs its half-life 100-fold, and limits cross-reactivity with highly similar molecules - insulin or IGF-2 (1). Circulating IGF-1, mainly liver-derived, mediates most GH effects. In GH deficiency (GHD) and acromegaly, IGF-1 analysis is indispensable for diagnosis, and monitoring of treatment efficacy and safety (2-4).
Numerous immunoassays used for measuring serum IGF-1 diverge in performance and results, particularly in the upper reference range limit (5-8). Nichols Advantage was considered golden standard IGF-1 assay, but its use was discontinued in 2006. Compared to other IGF-1 assays, Siemens Immulite 2000 was attributed with a lesser CV, smaller residual dispersion and best correlation with Nichols Advantage (9). IGF-1 immunoassay performance is hampered by multiple preanalytical and analytical challenges, further obstructed by results interpretation dificulties (7, 10). Disagreement in normative IGF-1 levels may alter clinical judgement and impact the treatment outcome (11,12).
Major preanalytical concerns are: age, gender, nutritional status, diet and lifestyle, racial, ethnic or regional differences. Main analytical challenges include: interference from IGFBPs, analytical sensitivity (intra- and inter-assay CV), and calibration standardization. Crucial for interpretation of results are: adequate knowledge of population-specific reference values and proper transformation of non-normally distributed IGF-1 to generate IGF-1 standard deviation score (SDS).
Most cross-sectional studies report of gradual, non-linear IGF-1 decline after puberty until senior age (13-15). Gender-influence on IGF-1 in adults is predominantly characterized as insignificant for clinical interpretation (11,13,16). Consensus on GH and IGF-1 analysis standardization (adopted by the GH Research Society, International Society for IGF Research, Pituitary Society and International Federation of Clinical Chemistry) concludes that age-specific reference values irrespective of gender are sufficient for clinical practice in adults (17,18). Some authors still considered that construction of gender-based reference values was justified (5).
IGF-1 is decreased in the malnourished (BMI < 20 kg/m2) and in extremely obese (BMI > 35-40 kg/m2) (18,19). However, construction of BMI-specific IGF-1 reference values is mostly considered irrelevant for clinical practice (20). Various nutritional and lifestyle factors may affect IGF-1 such as: protein to carbo-hydrate ratio, dairy products consumption, alcohol or coffee intake, smoking, physical activity and micronutrient intake (21). All of these factors vary between populations, possibly influencing IGF-1 reference-range.
Racial, ethnic or regional differences were reported in serum IGF-1 and its age-dependent decline. Large population-based studies in the USA and Republic of South Africa revealed significant racial differences (22,23). Significant inter-regional differences were reported within Turkey and China (14,15).
Certain physiological conditions, comorbidities or concomitant medications may affect serum IGF-1 beyond GH secretion alterations. IGF-1 is elevated in puberty and pregnancy, in malignancies or renal insufficiency (24). IGF-1 is decreased in: diabetes mellitus, liver insufficiency, hypothyroidism, celiac disease, or any sever acute illness. Oral estrogens may decrease, whereas systemic glucocorticoids may elevate circulating IGF-1 (20,25).
IGF-1 is a relatively stable molecule. Serum samples preparation and storage should not cause variability (18,20). Prevention of interference from high affinity IGFBPs is a major challenge to IGF-1 immunoassay analytical reliability. Less than 1% of total circulating IGF-1 is unbound, and its determination is expensive and unnecessary (1,26). Releasing IGF-1 from its tertiary complex with IGFBP3 is a crucial analytical step. Most widely used method combines acidification-based IGF-1 dissociation from IGFBPs, with IGF-2 oversaturation to block IGF-1 binding sites on IGFBPs. Disbalanced IGF-1 to IGFBPs ratio, found in diabetes, hepatic cirrhosis, renal insufficiency or anorexia nervosa, may obscure reliability of this approach (18).
Common IGF-1 calibration standard application is instrumental for inter-laboratory standardization. Consensus statements advise that all IGF-1 assays be calibrated against highly purified standard recombinant IGF-1 preparation 02/254 introduced in 2008 (27). However, the older calibration standard (WHO IRR 87/518) remained in wide practical and research use (5, 15, 28). Sabbah N et al. concluded that the calibration against the new standard only played a minor role in observed intra-cohort IGF-I assays discordances (6).
Converting measured IGF-1 value into a diagnostically relevant information requires reliable and population-specific normative reference values. Serum IGF-1 values are not normally distributed either in the whole population, nor in particular age groups (5,29). IGF-1 standard deviation score (SDS) incorporates measured IGF-1 value and degree and direction of its deviation from age-dependent reference limits (20). Consensus statements provide no specific recommendation on preferred approach to establish reference limits and compute IGF-1 SDS (12,17). Several methods were applied in population IGF-1 investigations. Establishment of 5th and 95th percentile of median as reference borderlines is the simplest approach, but fails to provide IGF-1 SDS calculation. Several population-based IGF-1 studies used logarithmical transformation, with borders of a reference interval set at -2SD and +2SD (16,30,31). Some of the IGF-1 population-based studies used polynomial equation model, robust towards extremes, but lacking SDS calculations (29,32,33). Cole`s LMS method based on Box-Cox transformation is used to optimize population curves in various applications (e.g. growth charts). It was employed in several population-based IGF-1 studies (5,6,14,20,28,29,34). It provides calculation of normalized IGF-1 SDS values for any measured sample, based on parameters of S (generalized coefficient of variation), M (median) and L (skewness) for any given age, calculated from age-related population-specific source data (29). LMS method does not account for kurtosis, possibly affecting its precision at extremes of age-specific IGF-I range (12).
In practice, many laboratories use IGF-1 normative data provided by the assay manufacturer and based on modestly sized samples from a remote source population (11). Due to possible racial, ethnic, regional, dietary or lifestyle influences, reference values of IGF-1 should not be unquestionably imported from one population to another. Population-specific reference values should be established on a sizable cohort of healthy subjects (with at least 100 subjects per age decade), randomly selected from the general population, using an IGF-1 assay validated according to consensus guidelines (13,17,30).
Some authors advocate an “ideal” healthy cohort, while others propose random sample from general working population as sufficient for normative database (5,35). Large and robust sample makes strict exclusion criteria less crucial. Bidlingmaier et al. demonstrated negligible impact of strict exclusion criteria, through meta-analysis of 4 adult cohorts (encompassing 10762 subjects), with various exclusion criteria applied (history of diabetes, malignancies, renal insufficiency, liver disease, BMI deviations) (20).
Subjects and Methods
This non-interventional cross-sectional study was approved by the Ethics Committee of the University Clinical Centre of Serbia (Document Nr. 1734/6 of 17.12.2009) and conducted in 2010-2011 on 1200 healthy adult volunteers, 21 to 80 years old. Ethnic, regional and cultural (dietary or lifestyle) diversity of general population of Serbia was reflected by recruitment throughout the country from populations of diverse occupational and socio-economic backgrounds.
Recruitment extended to healthy blood donors via Blood transfusion Institute of Serbia in Belgrade, and local transfusiology services in Niš, Novi Sad and Kragujevac, to blue-collar workers from different regions of Serbia via regular annual employee medical examinations executed by filed work of Serbian Institute of Occupational Health - Belgrade, and to students of Belgrade University (of various backgrounds of origin, and different study orientations) through annual periodic medical examinations via Institute for Students` healthcare - Belgrade. All participants signed informed consent form to enter the study. Prior or concomitant medical conditions or medications were investigated by short survey. Anthropometric data (body weight and height, waist and hip circumference) was collected from all subjects. Exclusion criteria included: pregnancy, lactation, chronic liver or renal disease, diabetes mellitus, active malignancy, oral contraceptives or systemic glucocorticoids use, and BMI values over 30kg/m2 or under 19kg/m2.
Fasting blood samples were collected at 8-9AM, after a night of normal sleep. All samples (excluding grossly hemolyzed, lipemic or icteric) were stored at -80°C and analyzed, within 2 months of sampling, in single laboratory with highly uniform methodology. Serum IGF-1 was measured batch-wise in the Institute for laboratory medicine – Konzilijum, Belgrade, using Siemens Immulite 2000 - a fully automated, double chemiluminescent immunometric assay with highly specific antibodies, calibrated against WHO IS 87/518 standard. Monoclonal murine anti-IGF-1 antibodies are bound to solid phase and polyclonal rabbit anti-IGF-1 antibodies are conjugated with alkaline phosphatase. IGFBP interference is eliminated through acidification and IGF-2 saturation. LOD was 20ng/mL. Intra and inter-assay CV ˂ 8%.
Subjects were assigned to one of 12 age groups (21-25, 26-30, 31-35, 36-40, 41-45, 46-50, 51-55, 56-60, 61-65, 66-70, 71-75 and 76-80 years). Recruitment by age interval was continued randomly until eligible samples from 50 males and 50 females were included in each group, thus assuring equal gender and age distribution.
Serum IGF-1 concentrations were expressed as median values and percentiles 5th to 95th. IGF-1 SDS values and skewness and kurtosis were expressed as arithmetic mean and standard error of mean (SEM). Normality of distribution was assessed by Kolmogorov-Simirnov test. Genders were compared for IGF-1 differences in overall population and within age groups. Significance of difference was assessed by nonparametric (Mann-Whitney U test, Kruskal-Wallis test) and parametric tests (Student T Test). Correlation of serum IGF-1 with age was tested and dynamics of its age-related change was analyzed. Correlation of serum IGF-1 with nutritional status parameters (BMI, waist circumference - WC and waist-to-hip-ratio - WHR) were tested over age groups. Significance of association was assessed by Spearman’s and Pearson`s correlation analysis. Observed correlations were expressed using linear correlation model and polynomial regression model. Statistical analysis was performed using SPSS software (SPSS for Windows, Release 16.0). P values <0.05 were interpreted as indicating statistical significance and <0.01 - high significance.
Normalization of distribution and SDS calculation were performed by logarithmic method (LogM) and LMS method. The two methods were compared for effectiveness.
In LogM distribution was normalized by logarithmic transformation, using equation
where x expresses individual`s measured IGF-1 value; lnX - arithmetic mean of all log transformed IGF-1 values for given age group; SD lnX – standard deviation of log transformed IGF-1 values for given age group.
In LMS method - IGF-1 SDS was calculated as (for L ≠ 0) where x stands for an individual IGF-1 value, while L, M and S are age-specific coefficients. (For L = 0; Z = ).
L, M and S were generated using software– LMS Chartmaker Light ver. 2.54, by Huiqi Pan, Tim Cole, Copyright 1997-2011, Medical Research Council, UK (http://www.healthforallchildren.com/shop-base/shop/software/lmschartmaker-light/).
RESULTS
Serum IGF-1 values
Median serum IGF-1 (ng/mL) value for each age group, and values of the 5th, 10th, 25th, 75th, 90th and 95th percentile are presented in Table 1 and Figure 1.
Table 1.
IGF-1 values (ng/mL) according to age intervals
| Age (years) | 5th | 10th | 25th | Med | 75th | 90th | 95th |
|---|---|---|---|---|---|---|---|
| 21-25 | 157.80 | 194.00 | 226.00 | 265.00 | 306.00 | 365.00 | 416.40 |
| 26-30 | 127.30 | 146.60 | 182.00 | 222.00 | 259.00 | 320.60 | 347.30 |
| 31-35 | 101.62 | 113.80 | 146.50 | 183.00 | 218.00 | 259.20 | 281.30 |
| 36-40 | 96.46 | 112.00 | 139.00 | 171.00 | 199.00 | 237.80 | 270.40 |
| 41-45 | 72.46 | 91.68 | 119.00 | 148.00 | 175.50 | 215.20 | 236.80 |
| 46-50 | 72.84 | 85.34 | 108.00 | 129.00 | 168.00 | 211.40 | 235.40 |
| 51-55 | 71.99 | 85.81 | 113.00 | 129.50 | 164.75 | 209.70 | 219.30 |
| 56-60 | 59.56 | 72.61 | 95.00 | 130.00 | 168.00 | 207.50 | 232.10 |
| 61-65 | 61.22 | 66.73 | 93.25 | 129.50 | 161.75 | 183.00 | 198.75 |
| 66-70 | 58.95 | 64.40 | 92.00 | 128.00 | 156.50 | 176.20 | 193.70 |
| 71-75 | 65.84 | 73.66 | 92.35 | 123.00 | 145.50 | 186.80 | 202.80 |
| 76-80 | 62.15 | 67.95 | 84.70 | 109.00 | 150.25 | 186.00 | 210.75 |
Figure 1.
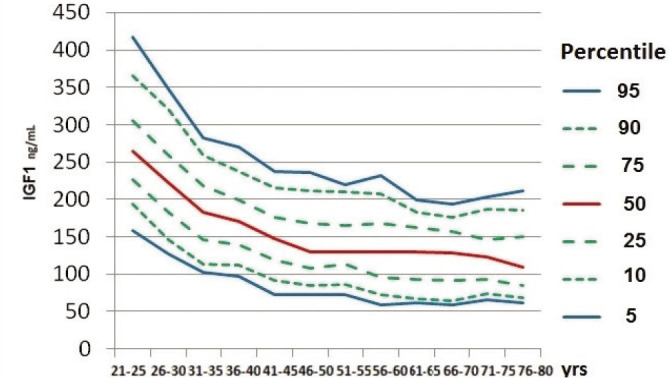
Median and 5th to 95th percentile of serum IGF-1 (ng/mL) relative to age group.
Serum IGF-1 dynamics with age
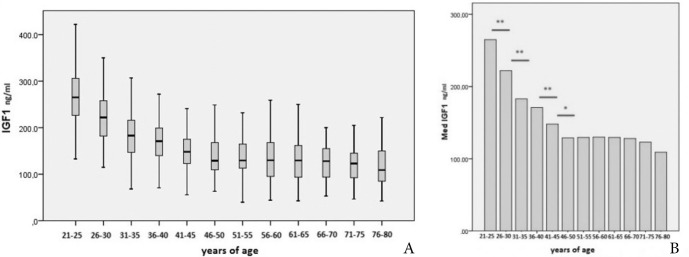
Average serum IGF-1 decreased with age progressively but unevenly and most prominently in the 21-50 years age interval (Fig. 2A). Highly significant difference between bordering age intervals was observed between groups 21-25 vs. 26-30; 26-30 vs. 31-35; and 36-40 vs. 41-54 (p<0.01) while difference between groups 41-45 vs. 46-50 was significant (p<0.05) (Fig. 2B). Average median IGF-1 change between bordering age intervals was 7.7% (0.4-17.6%). The average change for the value of lower reference range limit (5th percentile) was 10.2% (0.3-24.9%). Upper reference range limit value (95th percentile) changed on average by 8.2% (0.6-16.6%).
Figure 2.
A) Age dependent median IGF-1 and B) Significance of median IGF-1 change in consecutive age groups; (* p<0.05) (** p<0.01).
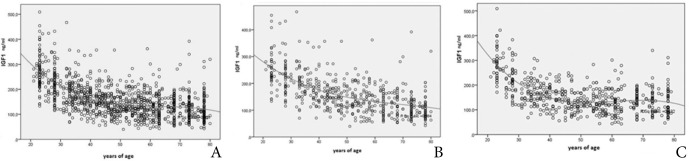
Correlation of serum IGF-1 with age was described by polynomial regression model. Correlation was highly significant in overall sample and for each gender separately. (Table 2, Fig. 3A,B,C) Polynomial regression curve in females exhibited a more uniform, steeper slope (Fig. 3B), while the curve in males formed a plateau in the mid-life interval (Fig. 3C).
Table 2.
Polynomial regression model of serum IGF-1 relation to years of age for the whole sample and for each gender
| R2 cubic | p | ||
|---|---|---|---|
| Overall sample | IGF-1 = 651.037 – 24.285 Y + 0.382 Y 2– 0.02 Y 3 | 0.391 | p < 0.01 |
| Male | IGF-1 = 807.139 – 34.425 Y+ 0.574 Y 2– 0.003 Y 3 | 0.432 | p < 0.01 |
| Female | IGF-1 = 491.078 – 13.862 Y+ 0.184 Y 2 | 0.384 | p < 0.01 |
| Y = years of age |
Figure 3.
Polynomial regression curve of serum IGF-1 (ng/mL) correlation to years of age for the whole sample (A) and for female (B) and male subgroup (C).
The impact of gender on serum IGF-1 values
In 7 age groups there was a significant difference (p<0.05) of mean serum IGF-1 between genders while the difference was non-significant in 5 age sub-groups. Average IGF-1 was higher in males in age groups of 21-25 and 71-80, while higher in females in age intervals 31-46 (Fig. 4).
Figure 4.
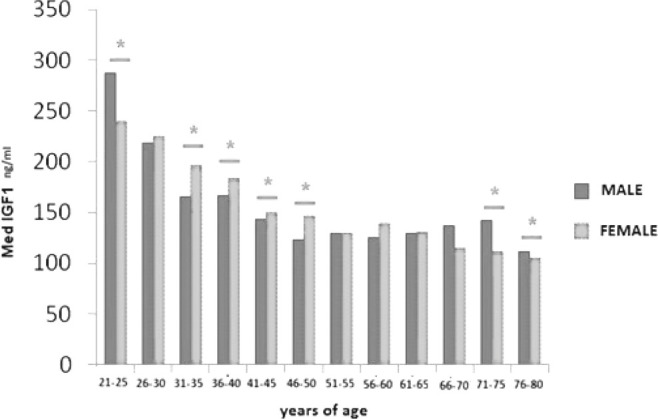
Difference of median IGF-1 for male and female gender over age intervals (* p<0.05).
Correlation of nutritional status parameters and serum IGF-1
Body mass index (BMI)
Correlation of serum IGF-1 with BMI was not significant in either age interval. In overall population, age-corrected BMI did not correlate significantly with serum IGF-1 (Spearman Rho -0.476 p>0.05).
Waist to hip ratio (WHR)
In overall investigated population, WHR (controlled for age) did not correlate significantly with serum IGF-1 (Spearman Rho 0.157 p>0.05). Only in the age interval 31-40, a significant negative correlation of IGF-1 to WHR was observed. (Age 31-35: Spearman Rho -0.423; p<0.01, Age 36-40: Spearman Rho -0.350; p<0.05).
Calculation of IGF-1 standard deviation score (SDS)
Distribution of IGF-1 in the investigated sample was proven by Kolmogorov-Smirnov test as non-gaussian in each 12 age interval groups and in overall sample (K-S = 0.081 p= 0.089).
LMS transformation of IGF-1
Coefficients L, M and S were calculated using LMS Chartmaker software based on individual IGF-1 values and years of age for each subject, and for each year of age in the span of 21 to 80 years (Table 3). The generated coefficients were then used in the IGF-1 SDS equation for each sample.
Table 3.
Skewness (L), Median (M) and Coefficient of variation (S) parameters of IGF-1 curve specific for each year of age (21 to 80) for the sampled reference population.
| Years of Age | L | M | S |
|---|---|---|---|
| 21 | 0.56 | 254.46 | 0.094 |
| 22 | 0.29 | 273.97 | 0.207 |
| 23 | 0.12 | 264.82 | 0.248 |
| 24 | -0.18 | 289.63 | 0.282 |
| 25 | 0.59 | 239.28 | 0.272 |
| 26 | -1.41 | 211.23 | 0.240 |
| 27 | -0.82 | 248.55 | 0.258 |
| 28 | 0.26 | 218.48 | 0.276 |
| 29 | 0.38 | 211.23 | 0.276 |
| 30 | 1.44 | 194.93 | 0.258 |
| 31 | 0.05 | 151.37 | 0.246 |
| 32 | 0.48 | 203.55 | 0.343 |
| 33 | 0.49 | 189.07 | 0.340 |
| 34 | 0.34 | 169.67 | 0.279 |
| 35 | 0.08 | 177.60 | 0.258 |
| 36 | -0.15 | 188.95 | 0.284 |
| 37 | 0.30 | 165.56 | 0.283 |
| 38 | 0.35 | 162.06 | 0.286 |
| 39 | 0.41 | 154.14 | 0.292 |
| 40 | 0.29 | 174.59 | 0.304 |
| 41 | 0.52 | 116.04 | 0.299 |
| 42 | 0.14 | 155.79 | 0.310 |
| 43 | 0.29 | 154.90 | 0.308 |
| 44 | 0.63 | 149.11 | 0.306 |
| 45 | 0.81 | 143.99 | 0.317 |
| 46 | -1.02 | 149.08 | 0.252 |
| 47 | -0.40 | 125.14 | 0.333 |
| 48 | -0.30 | 120.61 | 0.331 |
| 49 | -0.20 | 123.01 | 0.326 |
| 50 | -0.30 | 136.97 | 0.291 |
| 51 | 0.86 | 132.48 | 0.300 |
| 52 | 0.77 | 134.40 | 0.306 |
| 53 | 0.26 | 145.69 | 0.340 |
| 54 | 0.11 | 141.19 | 0.339 |
| 55 | 0.51 | 128.62 | 0.316 |
| 56 | 0.34 | 119.60 | 0.387 |
| 57 | 0.26 | 121.80 | 0.387 |
| 58 | 0.22 | 126.23 | 0.387 |
| 59 | 0.16 | 131.03 | 0.387 |
| 60 | 0.51 | 146.18 | 0.377 |
| 61 | 0.97 | 111.67 | 0.327 |
| 62 | 0.62 | 122.43 | 0.348 |
| 63 | 0.60 | 123.71 | 0.349 |
| 64 | 0.54 | 129.91 | 0.341 |
| 65 | 0.61 | 124.62 | 0.327 |
| 66 | 1.43 | 118.15 | 0.249 |
| 67 | 1.37 | 119.26 | 0.269 |
| 68 | 1.10 | 128.15 | 0.304 |
| 69 | 0.29 | 151.48 | 0.365 |
| 70 | -0.27 | 119.26 | 0.353 |
| 71 | -4.07 | 155.71 | 0.127 |
| 72 | -2.26 | 131.76 | 0.181 |
| 73 | -0.32 | 114.73 | 0.363 |
| 74 | 0.01 | 107.58 | 0.366 |
| 75 | 0.67 | 126.26 | 0.310 |
| 76 | 0.64 | 109.82 | 0.236 |
| 77 | 0.40 | 96.06 | 0.321 |
| 78 | -0.14 | 110.09 | 0.397 |
| 79 | -0.52 | 121.10 | 0.518 |
| 80 | -0.95 | 101.56 | 0.523 |
Comparison of logarithmic and LMS transformation
Both mathematical models (LogM and LMS transformation) achieved IGF-1 distribution normalization and enabled IGF-1 SDS calculation for any sample from investigated cohort or a subsequent subject attributed to the same source population. Correlation of IGF-1 SDS calculated for each subject using LogM vs. LMS method was highly significant for whole sample population (Pearson correlation coefficient 0.963; p<0.01) and for each age group (Pearson correlation coefficient 0.939 to 0.989; all p<0.01).
Efficacy of LogM and LMS methods was compared by analysis of skewness and kurtosis of normal distribution curves for each age interval. LMS-generated curves exhibited significantly lesser average absolute skewness (0.030±0.012 vs. 0.294±0.044; p<0.01) and non-significantly lesser average absolute kurtosis (0.315± 0.055 vs. 0.443± 0.110; p>0.05) as compared to LogM-generated curves.
DISCUSSION
Serum IGF-1 is the most significant surrogate marker of GH secretion, routinely used in diagnostics and management of GH deficiency (GHD) and acromegaly. Relative diurnal stability promotes the use of serum IGF-1 over GH, but important caveats include dependency on age and analytic methodology and population-specificity. Impacts of physiologic conditions, concomitant diseases or medications, dictate caution in interpretation, particularly with single measurements, borderline values or discrepancy from clinical presentation.
Several population-based reports indicate absence of a consistent or significant gender influence on serum IGF-1 (35-38). The impact of nutritional status (assessed as BMI) on IGF-1 appears relevant only upon more extreme deviations thereof (20).
Serum IGF-1 values reported from the same samples diverge significantly when analyzed by different immunoassays (11). For appropriate attribution of IGF-1 change to the effects of treatment, or disease evolution, cautious assay selection is mandated with provision of population-specific normal IGF-1 reference values (30).
Main concerns regarding IGF-1 immunoassay reliability include: interference prevention, analytic sensitivity, adequate calibration standard and establishment of adequate reference values. Diligent evaluation for each new assay is crucial, followed by continuous surveillance over its consistency (26). Establishment of population-specific reference range is essential for reliable use of IGF-1 as a diagnostic laboratory marker. Due to ethnic, racial, regional, dietary and lifestyle differences, IGF-1 reference values cannot be transferred unconditionally from one population to another. Representative sample of population-specific IGF-1 values serves to generate mathematical models for IGF-1 SDS.
Over the last 18 years (2003-2021), 27 studies in 15 countries (and one international) were set on to establish population-specific adult reference values for serum IGF-1, on samples ranging from 272 to 10762 (median - 825.5), with some studies sharing the same cohorts (5,6,8,9,14-16,20,28-33,35-48). Siemens Immulite 2000 was utilized in 8 of the 27 studies, among variety of other IGF-1 immunoassays (5,6,15,28,30,41,43,46). Sample size in some of these studies was below the recommended minimum of 100 subjects per age decade, and some did not provide IGF-1 SDS calculating platforms (13,32).
Aiming to establish the representative adult IGF-1 reference range for Serbia, we included 1200 healthy adults, 21-80 years old, with 50 men and 50 women in each of 12 quinquennial age intervals. To reflect ethnic, cultural and life-style diversity of the population of Serbia, subjects were recruited from various regions, from urban and rural areas, from different professional and economic backgrounds. All samples were collected, stored and analyzed in a highly uniform manner. Siemens Immulite 2000 IGF-1 assay was used for analysis in a single laboratory.
Percentile curves of IGF-1values were constructed, representing the median, 5th, 10th, 25th, 75th, 90th and 95th percentile. The 5th percentile value represented the lower and the 95th percentile the upper limit of normal, for each age interval.
A progressive decrease of serum IGF-1 with age was observed, 15% on average per decade, consistently with the results of previous studies (13). This decrease was most prominent in the interval of 20-46 years of age. A plateau was observed in the range of 46-70 years of age. Similar distribution was observed previously in several large population studies (16,20,22,28,36,43). Dynamics of age related IGF-1 decline (presence of a plateau and the age-interval of its occurrence) may to some extent be assay-dependent (33,46).
There was no significant gender-based difference in serum IGF-1 on the whole sample. The age related IGF-1 decline, observed in both genders, was more uniform in women, while in men, mid-life age plateau was more prominent. A steeper age related IGF-1 decline in women, compared to men was also observable from several previous studies (15,29,31,45).
Many population-based IGF-1 studies reported no significant gender-based differences (35-38). Among those reporting gender dimorphism, there was inconsistency in direction of difference, or in age of its occurrence (5,14-16,28,29,31,33,47). Gender differences may to some extent be assay-dependent (30). We observed IGF-1 differences between genders in some age groups, with higher average IGF-1 in men 21-25 and 70-80 old, while higher average IGF-1 in women 31-46 years old. A similar pattern of higher IGF-1 in young men (25-35 yrs), followed by higher levels in middle-aged women (30-50 yrs), and higher in older men (55-60 yrs), could be observed from several previous studies in various population backgrounds (France, China, Spain, Germany, Turkey, Thailand), on substantial sample sizes (405 to 2791) (5,14-16,28,33,47).
We found no gender-based difference in overall population, and differences in some of age groups were not highly significant, and lacked a uniform direction of gender-dependent IGF-1 difference. We view our results as supportive of the consensus recommendations against necessity for separate gender-specific IGF-1 reference ranges (11,13,17,30,32).
Our study included only subjects with BMI 19-30 kg/m2. After correction for age, neither BMI nor WHR correlated significantly with IGF-1 in the overall sample. There was no correlation with BMI in either of age groups, while a negative correlation of serum IGF-1 with WHR was observed only in the 30-40 years age interval. Specific corrections of reference values for BMI, or WHR are not needed. However, caution is advised with more extreme deviations of these parameters. Most IGF-1 population studies, including the largest one, reported against the relevance of BMI-specific IGF-1 reference values (20).
As demonstrated before and tested in our study, serum IGF-1 values are non-normally distributed, either overall, or in any of age subgroups. Transformation of IGF-1 and calculating IGF-1 SDS is needed for direct interpretation of individual values in respect to age-related reference range. IGF-1 SDS aids in interpreting multicentric data, establishing and implementing consensus recommendations regarding diagnostics and treatment for GHD or acromegaly. IGF-1 SDS facilitates proper longitudinal follow up, and aids in personalization of target treatment goals.
We compared two models for transforming IGF-1 values and calculating IGF-1 SDS – logarithmic (LogM) and LMS. LogM is simpler to use, requiring no specific software. LMS transformation is based on LMS Chartmaker software constructed for percentile curves optimization in various fields. IGF-1 SDS calculated by these two models correlated significantly, overall and in all age subgroups. Average absolute values of skewness (< 0.5) and kurtosis (< 1.0) of curves confirmed satisfactory normalization of distribution for both models. However, for age sub-groups and for the whole sample, the average absolute skewness was significantly (ten-fold) lesser with the LMS method, indicating a transformation closer to optimal. Calculation of L, M and S coefficients for each year of age for the population, based on source reference data, enables simple and precise transformation for each subsequent new sample attributed to same general population (Table 3). Individual IGF-1 SDS calculation formula for each year of age (21 to 80 in our study), adds on precision of LMS over LogM model which provides a single equation for a wider age interval (5 years in our study).
Consensus recommendations regarding diagnostic and therapeutic objectives in GHD and acromegaly should be applied to local practice with caution, if the immunoassays differ or if congruence of reference values is not established (9). Clinicians should expand their interest in the immunoassay used - its limitations, and adequacy of reference range. Laboratories should operate only with IGF-1 assays validated according to consensus guidelines, providing full information on: calibration standard used, cross reactivity, methods of interference elimination, and particularly the normative reference values established by proper methodology and published in peer-reviewed journals. Clinicians and medical biochemists should maintain bidirectional communication aiming for appropriate assay selection and consistency monitoring. Validation of population-specific reference values is necessary, based on substantial randomized samples of healthy subjects. Normative IGF-1 values should be reported as 5th to 95th percentile with a 95% confidence interval, in international mass units and preferably with SDS included after transformation of distribution (17). Clinical endocrinologist must interpret IGF-1 results within the appropriate clinical context. Caution is advised in interpretation of IGF-1 values resulting from different assays, particularly concerning the upper reference range (6).
To the best of our knowledge this is the first population-based IGF-1 reference study in the Southeast Europe region. Its strengths included the sample size (twice the advised minimum per age decade) and representativity (regional, residential, economic diversity), with high methodological and analytical homogeneity. Relative weakness was that the calibrator used (WHO 87/518) is not the latest available. The particular immunoassay was selected as the most commonly used in our clinical practice, and in prior population-based studies.
In conclusion, population-based adult IGF-1 reference values are established for Serbia, based on a large, representative sample and high analytical consistency. Upper and lower limit of normal (corresponding to 5th and 9th percentile) were calculated for each of 12 five-year age intervals 21 to 80 years. Gender-based differences were not significant, but age-related IGF-1 decline was more uniform in women, contrary to prominent mid-life plateau in men. Correlation of IGF-1 with nutritional status was not significant. Superiority of LMS method in computing IGF-1 SDS was demonstrated over the more convenient logarithmic method. In GHD and acromegaly, IGF-1 SDS is valuable for longitudinal follow up, cross-age comparison, improving efficacy and safety, targeting individual treatment goals, and implementing consensus recommendations. Awareness is raised in both clinical endocrinologists and medical biochemists of the importance of acquainting with particular IGF-1 assay used in their practice, committing exclusively to validated assays, preferably with genuine population-specific reference values.
Acknowledgements
This work was supported by the Serbian Ministry of Science (Project Nr. 175033).
Conflict of interest
None of the authors of this manuscript have any conflict of interest to report.
Ethics
Study was approved by the Ethics Committee of University Clinical Centre of Serbia (Document Nr. 1734/6) and informed consent was obtained from all involved subjects.
References
- 1.Frysak Z, Schovanek J, Iacobone M, Karasek D. Insulin-like Growth Factors in a clinical setting: Review of IGF-I. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159(3):347–351. doi: 10.5507/bp.2015.041. [DOI] [PubMed] [Google Scholar]
- 2.Johannsson G, Bidlingmaier M, Biller BMK, Boguszewski M, Casanueva FF, Chanson P, Clayton PE, Choong CS, Clemmons D, Dattani M, Frystyk J, Ho K, Hoffman AR, Horikawa R, Juul A, Kopchick JJ, Luo X, Neggers S, Netchine I, Olsson DS, Radovick S, Rosenfeld R, Ross RJ, Schilbach K, Solberg P, Strasburger C, Trainer P, Yuen KCJ, Wickstrom K, Jorgensen JOL. Growth Hormone Research Society perspective on biomarkers of GH action in children and adults. Endocr Connect. 2018;7(3):R126–R134. doi: 10.1530/EC-18-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho KK. GH Deficiency Consensus Workshop Participants. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology. Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol. 2007;157(6):695–700. doi: 10.1530/EJE-07-0631. [DOI] [PubMed] [Google Scholar]
- 4.Massart C, Poirier JY. Determination of serum insulin-like growth factor-I reference values for the automated chemiluminescent Liaison(R) assay. Clinical utility in the follow-up of patients with treated acromegaly. Clin Chim Acta. 2011;412:398–399. doi: 10.1016/j.cca.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Chanson P, Arnoux A, Mavromati M, Brailly-Tabard S, Massart C, Young J, Piketty ML, Souberbielle JC. VARIETE Investigators. Reference Values for IGF-I Serum Concentrations: Comparison of Six Immunoassays. J Clin Endocrinol Metab. 2016;101(9):3450–3458. doi: 10.1210/jc.2016-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabbah N, Wolf P, Piedvache C, Trabado S, Verdelet T, Cornu C, Souberbielle JC, Chanson P. Reference values for IGF-I serum concentration in an adult population: use of the VARIETE cohort for two new immunoassays. Endocr Connect. 2021;10(9):1027–1034. doi: 10.1530/EC-21-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranke MB, Feldt-Rasmussen U, Bang P, Baxter RC, Camacho-Hübner C, Clemmons DR, Juul A, Orskov H, Strasburger CJ. How should insulin-like growth factor I be measured? A consensus statement. Horm Res. 2001;55(Suppl 2):106–109. doi: 10.1159/000063485. [DOI] [PubMed] [Google Scholar]
- 8.Brugts MP, Ranke MB, Hofland LJ, van der Wansem K, Weber K, Frystyk J, Lamberts SW, Janssen JA. Normal values of circulating insulin-like growth factor-I bioactivity in the healthy population: comparison with five widely used IGF-I immunoassays. J Clin Endocrinol Metab. 2008;93:2539–2545. doi: 10.1210/jc.2007-2454. [DOI] [PubMed] [Google Scholar]
- 9.Krebs A, Wallaschofski H, Spilcke-Liss E, Kohlmann T, Brabant G, Völzke H, Nauck M. Five commercially available insulin-like growth factor I (IGF-I) assays in comparison to the former Nichols Advantage IGF-I in a growth hormone treated population. Clin Chem Lab Med. 2008;46(12):1776–1783. doi: 10.1515/CCLM.2008.349. [DOI] [PubMed] [Google Scholar]
- 10.Blankenstein O, Pedersen BT, Schlumpf M, Andreasen AH, Júlíusson PB. Management and interpretation of heterogeneous observational data: using insulin-like growth factor-I data from the NordiNet® International Outcome Study. Growth Horm IGF Res. 2015;25(1):41–46. doi: 10.1016/j.ghir.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Pokrajac A, Wark G, Ellis AR, Wear J, Wieringa GE, Trainer PJ. Variation in GH and IGF-I assays limits the applicability of international consensus criteria to local practice. Clin Endocrinol (Oxf) 2007;67(1):65–70. doi: 10.1111/j.1365-2265.2007.02836.x. [DOI] [PubMed] [Google Scholar]
- 12.12.Varewijck AJ, van der Lely AJ, Neggers SJCMM, Hofland LJ, Janssen JAMJL. Disagreement in normative IGF-I levels may lead to different clinical interpretations and GH dose adjustments in GH deficiency. Clin Endocrinol (Oxf) 2018;88(3):409–414. doi: 10.1111/cen.13491. [DOI] [PubMed] [Google Scholar]
- 13.Bidlingmaier M. Pitfalls of insulin-like growth factor I assays. Horm Res. 2009;71(Suppl 1):30–33. doi: 10.1159/000178034. [DOI] [PubMed] [Google Scholar]
- 14.Bayram F, Gedik VT, Demir Ö, Kaya A, Gündoğan K, Emral R, Öztürk A, Uysal AR, Çorapçıoğlu D. Epidemiologic survey: reference ranges of serum insulin-like growth factor 1 levels in Caucasian adult population with immunoradiometric assay. Endocrine. 2011;40(2):304–309. doi: 10.1007/s12020-011-9476-z. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H, Xu Y, Gong F, Shan G, Yang H, Xu K, Zhang D, Cheng X, Zhang Z, Chen S, Wang L, Pan H. Reference ranges for serum insulin-like growth factor I (IGF-I) in healthy Chinese adults. PLoS One. 2017;12(10):e0185561. doi: 10.1371/journal.pone.0185561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granada ML, Ulied A, Casanueva FF, Pico A, Lucas T, Torres E, Sanmartí A. Serum IGF-I measured by four different immunoassays in patients with adult GH deficiency or acromegaly and in a control population. Clin Endocrinol (Oxf) 2008;68(6):942–950. doi: 10.1111/j.1365-2265.2007.03120.x. [DOI] [PubMed] [Google Scholar]
- 17.Clemmons DR. Consensus statement on the standardization and evaluation of growth hormone and insulin-like growth factor assays. Clin Chem. 2011;57(4):555–559. doi: 10.1373/clinchem.2010.150631. [DOI] [PubMed] [Google Scholar]
- 18.Junnila RK, Strasburger CJ, Bidlingmaier M. Pitfalls of insulin-like growth factor-1 and growth hormone assays. Endocrinol Metab Clin North Am. 2015;44(1):27–34. doi: 10.1016/j.ecl.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Schneider HJ, Saller B, Klotsche J, März W, Erwa W, Wittchen HU, Stalla GK. Opposite associations of age-dependent insulin-like growth factor-I standard deviation scores with nutritional state in normal weight and obese subjects. Eur J Endocrinol. 2006;154(5):699–706. doi: 10.1530/eje.1.02131. [DOI] [PubMed] [Google Scholar]
- 20.Bidlingmaier M, Friedrich N, Emeny RT, Spranger J, Wolthers OD, Roswall J, Körner A, Obermayer-Pietsch B, Hübener C, Dahlgren J, Frystyk J, Pfeiffer AF, Doering A, Bielohuby M, Wallaschofski H, Arafat AM. Reference intervals for insulin-like growth factor-1 (IGF-1) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99(5):1712–1721. doi: 10.1210/jc.2013-3059. [DOI] [PubMed] [Google Scholar]
- 21.Watling CZ, Kelly RK, Tong TYN, Piernas C, Watts EL, Tin Tin S, Knuppel A, Schmidt JA, Travis RC, Key TJ, Perez-Cornago A. Associations of circulating insulin-like growth factor-I with intake of dietary proteins and other macronutrients. Clin Nutr. 2021;40(7):4685–4693. doi: 10.1016/j.clnu.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berrigan D, Potischman N, Dodd KW, Hursting SD, Lavigne J, Barrett JC, Ballard-Barbash R. Race /ethnic variation in serum levels of IGF-I and IGFBP-3 in US adults. Growth Horm IGF Res. 2009;19(2):146–155. doi: 10.1016/j.ghir.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koegelenberg AS, Smith W, Schutte R, Schutte AE. IGF-1 and NT-proBNP in a black and white population: The SABPA study. Eur J Clin Invest. 2016;46(9):795–803. doi: 10.1111/eci.12663. [DOI] [PubMed] [Google Scholar]
- 24.Bancos I, Algeciras-Schimnich A, Grebe SK, Donato LJ, Nippoldt TB, Erickson D. Evaluation of Variables Influencing the Measurement of Insulin-like Growth Factor-1. Endocr Pract. 2014;20(5):421–426. doi: 10.4158/EP13359.OR. [DOI] [PubMed] [Google Scholar]
- 25.Strasburger CJ, Bidlingmaier M, Wu Z, Morrison KM. Normal values of insulin-like growth factor I and their clinical utility in adults. Horm Res. 2001;55(Suppl 2):100–105. doi: 10.1159/000063484. [DOI] [PubMed] [Google Scholar]
- 26.Laron Z, Bidlingmaier M, Strasburger CJ. Indications limitations and pitfalls in the determination of human growth hormone, IGF-I and their binding proteins. Pediatr Endocrinol Rev. 2007;5(Suppl 1):555–569. [PubMed] [Google Scholar]
- 27.Burns C, Rigsby P, Moore M, Rafferty B. The First International Standard For Insulin-like Growth Factor-1 (IGF-1) for immunoassay: preparation and calibration in an international collaborative study. Growth Horm IGF Res. 2009;19(5):457–462. doi: 10.1016/j.ghir.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Liao ZH, Yin QQ, Wan JX, He W, Ji W, Zhang LY, Li YB. Serum Insulin-like growth factor-1 levels of healthy adults in southern China. Endocr J. 2016;63(12):1081–1086. doi: 10.1507/endocrj.EJ16-0144. [DOI] [PubMed] [Google Scholar]
- 29.Isojima T, Shimatsu A, Yokoya S, Chihara K, Tanaka T, Hizuka N, Teramoto A, Tatsumi KI, Tachibana K, Katsumata N, Horikawa R. Standardized centile curves and reference intervals of serum insulin-like growth factor-I (IGF-I) levels in a normal Japanese population using the LMS method. Endocr J. 2012;59(9):771–780. doi: 10.1507/endocrj.ej12-0110. [DOI] [PubMed] [Google Scholar]
- 30.Rosario PW. Normal values of serum IGF-1 in adults: results from a Brazilian population. Arq Bras Endocrinol Metabol. 2010;54(5):477–481. doi: 10.1590/s0004-27302010000500008. [DOI] [PubMed] [Google Scholar]
- 31.Kim B, Cho Y, Ku CR, Lee SG, Lee KA, Kim JH. Establishment of Reference Intervals for Serum Insulin-Like Growth Factor I in Korean Adult Population. Endocrinol Metab (Seoul) 2020;35(4):960–964. doi: 10.3803/EnM.2020.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brabant G, von zur Muhlen A, Wuster C, Ranke MB, Kratzsch J, Kiess W, Ketelslegers JM, Wilhelmsen L, Hulthen L, Saller B, Mattsson A, Wilde J, Schemer R. Kann P Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: results from a multicenter study. Horm Res. 2003;60:53–60. doi: 10.1159/000071871. [DOI] [PubMed] [Google Scholar]
- 33.Friedrich N, Alte D, Volzke H, Spilcke-Liss E, Ludemann J, Lerch MM, Kohlmann T, Nauck M, Wallaschofski H. Reference ranges of serum IGF-1 and IGFBP-3 levels in a general adult population: results of the Study of Health in Pomerania (SHIP) Growth Horm IGF Res. 2008;18:228–237. doi: 10.1016/j.ghir.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Flegal KM, Cole TJ. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. Natl Health Stat Report. 2013. pp. 1–3. [PubMed]
- 35.Mattsson A, Svensson D, Schuett B, Osterziel KJ, Ranke MB. Multidimensional reference regions for IGF-I, IGFBP-2 and IGFBP-3 concentrations in serum of healthy adults. Growth Horm IGF Res. 2008;18(6):506–516. doi: 10.1016/j.ghir.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Massart C, Poirier JY, Jard C, Pouchard M, Vigier MP. Determination of serum insulin-like growth factor-I reference values for the immunometric Cisbio method on a large number of healthy subjects: clinical utility in the follow-up of patients with treated acromegaly. Clin Chim Acta. 2007;381:176–178. doi: 10.1016/j.cca.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Aimaretti G, Boschetti M, Corneli G, Gasco V, Valle D, Borsotti M, Rossi A, Barreca A, Fazzuoli L, Ferone D, Ghigo E, Minuto F. Normal age-dependent values of serum insulin growth factor-I: results from a healthy Italian population. J Endocrinol Invest. 2008;31:445–449. doi: 10.1007/BF03346389. [DOI] [PubMed] [Google Scholar]
- 38.Guven B, Can M, Mungan G, Acikgoz S. Reference values for serum levels of insulin-like growth factor 1 (IGF-1) and IGF-binding protein 3 (IGFBP-3) in the West Black Sea region of Turkey. Scand J Clin Lab Invest. 2013;73(2):135–140. doi: 10.3109/00365513.2012.755739. [DOI] [PubMed] [Google Scholar]
- 39.Ranke MB, Osterziel KJ, Schweizer R, Schuett B, Weber K, Robbel P, Vornwald A, Blumenstock G, Elmlinger MW. Reference levels of insulin-like growth factor I in the serum of healthy adults: comparison of four immunoassays. Clin Chem Lab Med. 2003;41:1329–1334. doi: 10.1515/CCLM.2003.203. [DOI] [PubMed] [Google Scholar]
- 40.Tiryakioğlu O, Kadiolgu P, Canerolgu NU, Hatemi H. Age dependency of serum insulin-like growth factor (IGF)-1 in healthy Turkish adolescents and adults Indian J Med Sci. 2003;57(12):543–548. [PubMed] [Google Scholar]
- 41.Elmlinger MW, Kühnel W, Weber MM, Ranke MB. Reference ranges for two automated chemiluminescent assays for serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3) Clin Chem Lab Med. 2004;42(6):654–664. doi: 10.1515/CCLM.2004.112. [DOI] [PubMed] [Google Scholar]
- 42.Massart C, Poirier JY. Determination of serum insulin-like growth factor-I reference values for the automated chemiluminescent Liaison® assay. Clinical utility in the follow-up of patients with treated acromegaly. Clin Chim Acta. 2011;412(3-4):398–399. doi: 10.1016/j.cca.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 43.Guitelman M, Smithuis F, Garcia Basavilbaso N, Aranda C, Fabre B, Oneto A. Reference ranges for an automated chemiluminescent assay for serum insulin-like growth factor I (IGF-I) in a large population of healthy adults from Buenos Aires. J Endocrinol Invest. 2015;38(9):951–956. doi: 10.1007/s40618-015-0265-z. [DOI] [PubMed] [Google Scholar]
- 44.Kucera R, Topolcan O, Pecen L, Kinkorova J, Svobodova S, Windrichova J, Fuchsova R. Reference values of IGF1, IGFBP3 and IGF1/IGFBP3 ratio in adult population in the Czech Republic. Clin Chim Acta. 2015;444:271–277. doi: 10.1016/j.cca.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 45.Andreassen M, Nielsen K, Raymond I, Kristensen LO, Faber J. Characteristics and reference ranges of Insulin-Like Growth Factor-I measured with a commercially available immunoassay in 724 healthy adult Caucasians. Scand J Clin Lab Invest. 2009;69:880–885. doi: 10.3109/00365510903165477. [DOI] [PubMed] [Google Scholar]
- 46.Friedrich N, Krebs A, Nauck M, Wallaschofski H. Age-and gender-specific reference ranges for serum insulin-like growth factor I (IGF-I) and IGF-binding protein-3 concentrations on the Immulite 2500: results of the Study of Health in Pomerania (SHIP) Clin Chem Lab Med. 2010;48:115–120. doi: 10.1515/CCLM.2010.009. [DOI] [PubMed] [Google Scholar]
- 47.Plengpanich W, Mangkala J, Buranasukajorn P, Boonruang K, Sunthornyothin S, Suwanwalaikorn S, Khovidhunkit W, Sridama V, Snabboon T. Normal reference range of serum insulin-like growth factor (IGF)-I in healthy Thai adults. J Med Assoc Thai. 2008;91:1681–1684. [PubMed] [Google Scholar]
- 48.Jeong IK, Byun JK, Noh J, Kim SW, Chung YS, Park TS, Kim SW. Reference Ranges of Serum Insulin-Like Growth Factor-I and Insulin-Like Growth Factor Binding Protein-3: Results from a Multicenter Study in Healthy Korean Adults. Endocrinol Metab (Seoul) 2020;35(4):954–959. doi: 10.3803/EnM.2020.785. [DOI] [PMC free article] [PubMed] [Google Scholar]