Abstract
Bumble bees provide valuable pollination services to many wild and agricultural plants. Populations of some bumble bee species are in decline, prompting the need to better understand bumble bee biology and to develop methodologies for assessing the effects of environmental stressors on these bees. Use of bumble bee microcolonies as an experimental tool is steadily increasing. This review closely examines the microcolony model using peer-reviewed published literature identified by searching three databases through November 2018. Microcolonies have been successfully used for investigating a range of endpoints including behavior, the gut microbiome, nutrition, development, pathogens, chemical biology and pesticides/xenobiotics. Methods for the initiation and monitoring of microcolonies, as well as the recorded variables were catalogued and described. From this information, we identified a series of recommendations for standardizing core elements of microcolony studies. Standardization is critical to establishing the foundation needed to support use of this model for biological response investigations and particularly for supporting use in pesticide risk assessment.
Keywords: bumble bee, hazard assessment, pesticide, methodology, insect behavior, sociobiology
Introduction
Honey bees (Apis mellifera) have long been considered the most important pollinator of many agricultural plants (Allen-Wardell et al. 1998, Kevan 1999, Delaplane and Mayer 2000). The value of these pollination services provided by non-Apis bees is now widely recognized (Klein et al. 2007, Breeze et al. 2011, Garibaldi et al. 2013, Klatt et al. 2014). Bumble bees (Bombus spp.), for example, are important pollinators of many wild plants and provide essential auxiliary and novel crop pollination services valued as high as $963 per hectare (Kleijn et al. 2015).
Disconcertingly, populations of some bees are in decline (Potts et al. 2010, Cameron et al. 2011, Burkle et al. 2013, Koh et al. 2016, Meeus et al. 2018). Many studies have illustrated the effects of environmental stressors on the health and performance of honey bees including pesticides (Johnson et al. 2010, Henry et al. 2012, Whitehorn et al. 2012), pathogens (Cox-Foster et al. 2007), parasites (Le Conte et al. 2010) and poor nutrition (Huang 2012, Di Pasquale et al. 2013). While it is tempting to apply these findings to bumble bees, there are many notable differences between honey bees and bumble bees that complicate extrapolation of honey bee specific data (Stoner 2016, Gradish et al. 2018). For example, bumble bees form annual colonies in contrast to perennial honey bee colonies. These taxa prefer to forage on different plants, have unique parasite and pathogen communities, and can have different responses to pesticide inputs (Thompson and Hunt 1999, Besard et al. 2011).
Recognizing the importance of bumble bees to managed and natural landscapes, there is a pronounced need for bumble bee-specific methodologies to develop a better understanding of their biology and how these bees respond to various stressors. Recently, the Office of Economic Cooperation and Development (OECD) validated two acute toxicity testing protocols for adult bumble bees housed in isolation (OECD 2017b, a). In addition, the International Commission for Plant-Pollinator Relationships (ICPPR) is working to develop a chronic oral toxicity test for bumble bees (OECD 2017b). While these testing formats reduce experimental complexity, they limit investigations to acute exposures in adult bees and ignore important effects related to colony health and production of new progeny. In contrast, microcolonies can facilitate completion of a wide array of investigations and take advantage of bumble bee social plasticity to maintain some elements of colony-level dynamics.
Microcolonies are formed when a group of bumble bee workers are isolated in a queenless environment (Figure 1). Separation from the queen stimulates one of the workers (usually the largest one with the most developed ovaries) to establish dominance and begin laying eggs (Free 1955). These eggs are unfertilized and, due to the haplodiploid reproductive system in bees, result in the production of male offspring (i.e., drones). Using microcolonies, investigators can evaluate the effects of chronic exposures to various factors not only on adult bees but also on brood development. Importantly, these investigations can be conducted under defined laboratory conditions on standardized colonies, and, because large numbers of microcolonies can be maintained simultaneously, experimental sample size and replication capacity are robust. Given these attributes, experimental use of microcolonies has been rapidly increasing (Figure 2). However, there are no published evaluations of microcolony development or performance. Here, we sought to provide a comprehensive review of the microcolony model and discuss how this model has been applied. In addition, we provide suggestions for microcolony protocol standardization for risk assessment that are needed to maximize the utility of this tool across a broad array of investigations.
Figure 1. Microcolony development timeline.
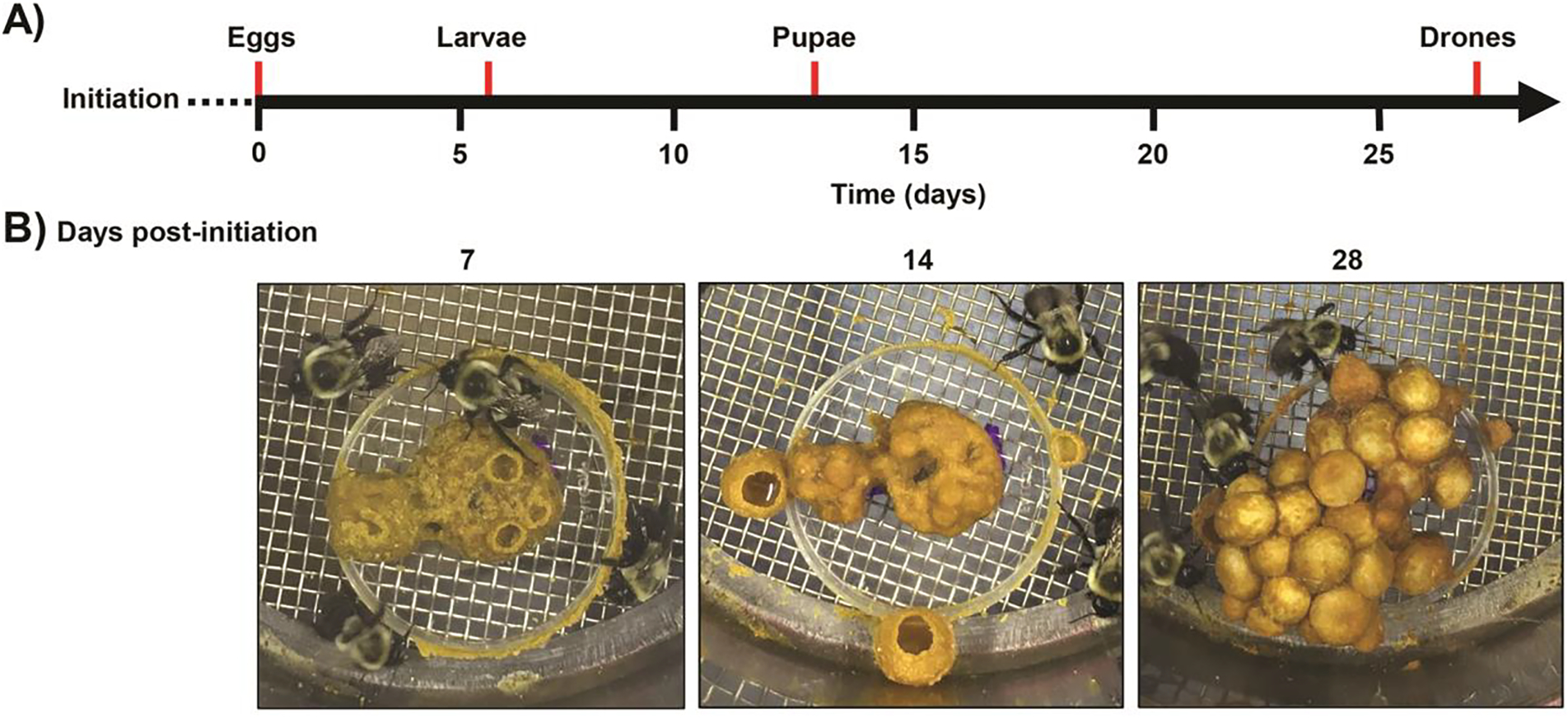
A) Microcolonies were initiated with five callow Bombus impatiens workers and provisioned with a pollen patty (2 g) and syrup to promote nest building. After 7 days, microcolonies were fed fresh pollen paste and syrup every Monday, Wednesday, and Friday. B) Starting from nest initiation, photos show microcolony progression from uncapped egg chambers to pupal cells.
Figure 2. Number of microcolony publications per year.
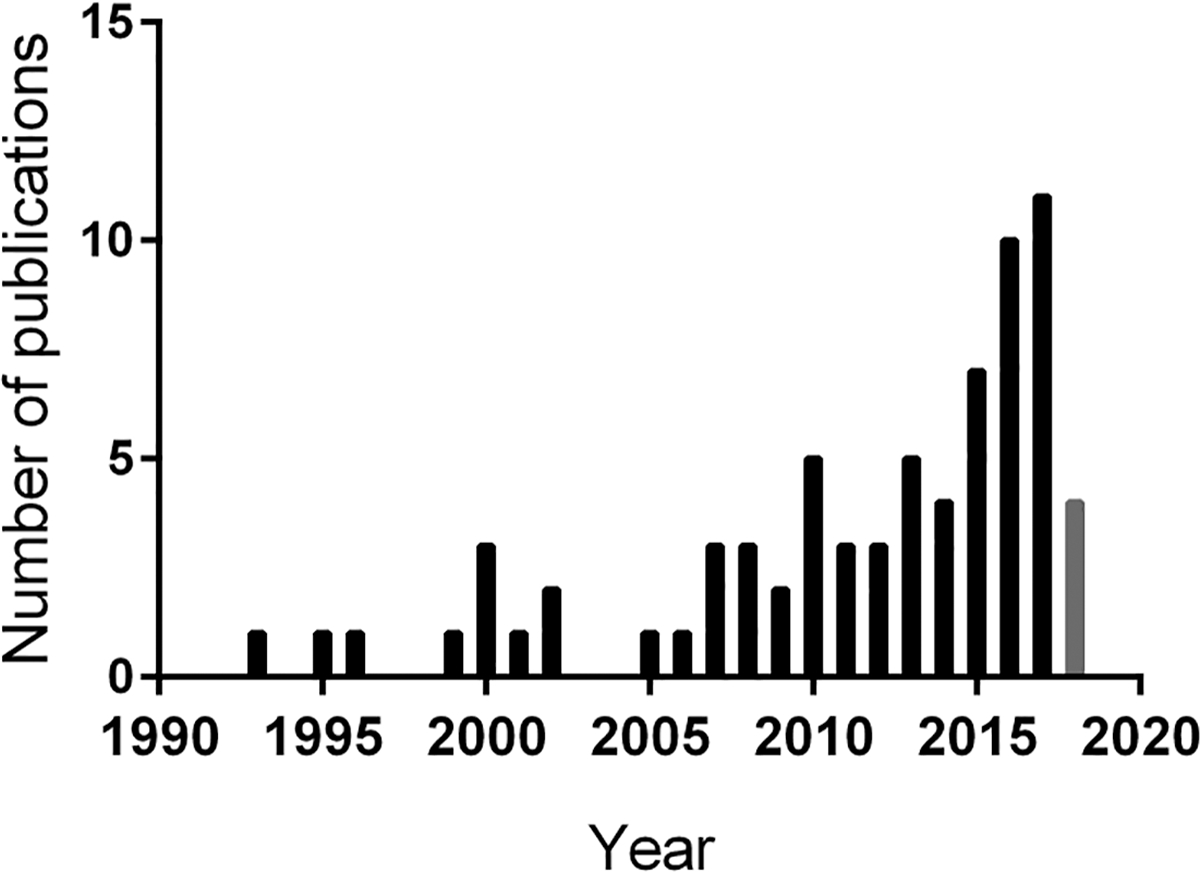
Google Scholar, Web of Science and the Pro Quest Agricultural and Environmental Science Database were searched through November 2018 using a common search string. All results were compiled and organized by year of publication. Given that our search was completed before all publications for 2018 were available, the year 2018 is captured with a grey bar.
Methods
Peer-reviewed research articles were identified by searching Google Scholar, Web of Science, and ProQuest Agricultural and Environmental Science Database for publications through November 2018 using the search string (“Bombus” or “bumblebee” or “bumble-bee” or “bumble bee”) and (“microcolony” or “micro-colony” or “queenless” or “queen-less” or “queenless colony”). An additional 8 articles were identified by reviewing the reference section of these papers. For inclusion in this review, microcolonies had to be queenless, housed in an artificial container that was not the originating colony container, and could not be initiated in the presence of live brood from a queenright colony.
Results and Discussion
A total of 75 peer-reviewed articles were identified. To maximize the utility of this review, microcolony design parameters were extracted from all selected studies (Supp. Table S1). In the sections that follow we catalogued and critically reviewed the approaches applied to establishing microcolonies, the various endpoints assessed in these studies and provide recommendations for methods standardization.
Microcolony composition
Although the basic components of a microcolony (i.e., worker bees, food provisions and the nest chamber) appear straightforward, numerous approaches for establishing microcolonies have been applied (Suppl. Table S1). Since these differences can significantly impact microcolony formation and potentially study outcome, we categorized these design variables and discuss the significance of different configurations.
Bombus species –
The majority of published studies used Bombus terrestris (60/75) to establish microcolonies and a smaller number used Bombus impatiens (13/75) (Suppl. Table S1). Both species are commercially available in their respective continents, providing a reliable source of worker bees for microcolony studies year-round (Velthuis and van Doorn 2006, Winter et al. 2006), B. impatiens is sold in North America (importation of other species is restricted) and B. terrestris is available in most other locations. Seven studies utilized microcolonies composed of wild-caught bees (e.g., B. terrestris (Larrere and Couillaud 1993, Regali and Rasmont 1995, Tasei et al. 2000, Tasei and Aupinel 2008b, Moerman et al. 2016), B. impatiens (Sibbald and Plowright 2014), B. hypnorum (Moerman et al. 2016) and B. pratorum (Free 1955, Moerman et al. 2016).
Age of workers –
Microcolonies can be initiated with either workers of unknown age or newly emerged workers (i.e., callow workers). Using older, non-age matched workers allows for access to more individuals, thereby enabling researchers to setup more microcolonies at one time. However, under this circumstance, worker deaths due to old age may be mistaken for treatment-related effects. Consequently, age-unknown microcolonies are best suited for producing drones for mating or for creating pathogen cultures needed for other purposes. Newly emerged bees are the best choice for studies investigating the effects of experimental treatments. Within the literature, the age of workers used to initiate microcolonies was not always clearly disclosed, confounding study interpretation (Suppl. Table S1). Seeding microcolonies with newly emerged bees creates a uniform age group, promoting experimental reproducibility, and minimizes mortality from aggression between foreign nestmates (Bloch and Hefetz 1999, Doums et al. 2002, Rutrecht et al. 2007). Furthermore, newly emerged bees may carry fewer pathogens than older workers exposed to nestmates longer; but, there may be reductions of gut microbiomes in naïve bees that could have potential downstream effects on worker survival and microcolony development (Meeus et al. 2013, Kwong et al. 2014).
Number and source of bees –
Published studies used anywhere from 1 to 20 worker(s)/microcolony, but use of five workers was most common, followed by three workers (Figure 3; Suppl. Table S1). Seeding microcolonies with more workers dilutes the brood tending responsibilities across more individuals and elevates the microclimate temperature (Plowright and Jay 1968, Cameron 1985, Goulson 2010, Mommaerts et al. 2010b). Under these favorable conditions, more male offspring can ultimately be produced (Gradish et al. 2013). The number of nestmates can also affect worker dominance behavior, ovary development and oocyte length, with some species differences (Cnaani et al. 2007, Amsalem and Hefetz 2011). In a study with B. impatiens, Cnaani et al. (2007) showed that oocytes were smaller in microcolonies containing fewer workers, but this effect was not seen between 2- and 4-member microcolonies in B. terrestris (Cnaani et al. 2002). Understanding these potential sources of variation is important when conducting comparative studies.
Figure 3. Microcolony design parameters.
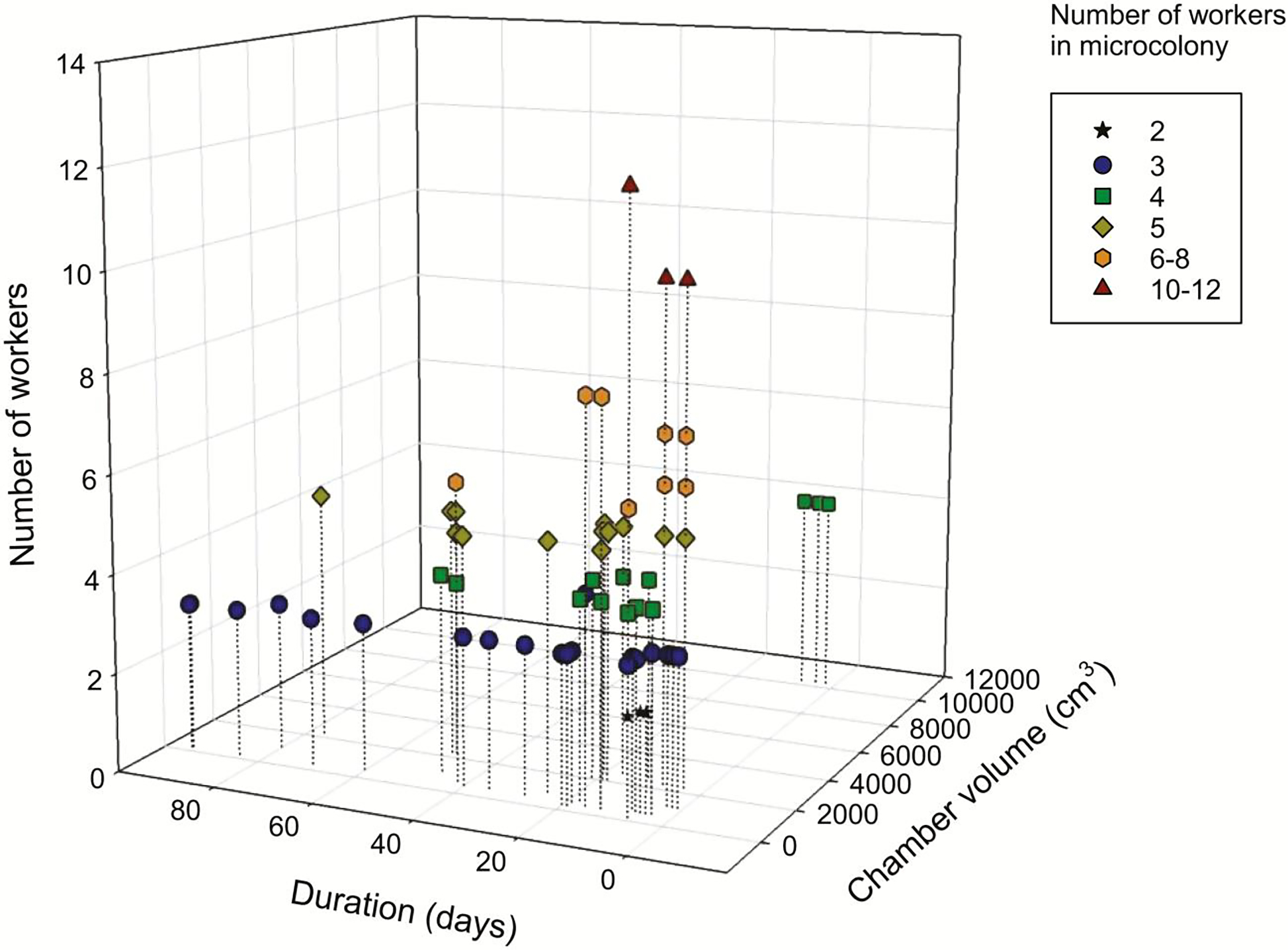
Microcolony design parameters were extracted from research articles published through November 2018 (Table S1) and plotted to show the relationships between the number of workers, microcolony container volume and experimental duration. Only studies reporting data for all three parameters were included (i.e., 52 of the 75 published articles). When a study included microcolonies composed of different numbers of workers, each configuration was counted individually. If study duration indicated a specific number of days after egg laying, we assigned a value of 6 days for egg initiation.
Although B. terrestris and B. impatiens workers can be obtained from commercial vendors (e.g., Koppert and Biobest), it can be challenging to obtain enough newly emerged workers at one time for large experiments. To meet demands, some researchers collected newly emerged workers directly from multiple queenright colonies (Meeus et al. 2013). In other cases, investigators isolated and artificially incubated pupal cocoons by extraction from the queenright colony and allowing the adults to emerge (Billiet et al. 2016). In all cases, the selection, source and handling of newly emerged adult bees may have downstream impacts on microcolony development and progression (Laycock et al. 2012, Laycock et al. 2014, Amsalem et al. 2015).
Artificial microcolony container structure –
Microcolonies can be housed in containers composed of wood, plastic, Styrofoam and metal (Figure 4; Table S1). Compared to wood, plastics offer more versatility allowing the investigator to create a custom nesting habitat. However, plastic can be more expensive than wood and cannot be heat-treated for sterilization. Plastic microcolony chambers can be either purpose-made for bumble bee rearing (i.e., “queen boxes”) or repurposed from common containers (Figure 4A–D). Using disposable plastic “deli containers” can be a relatively inexpensive way to house microcolonies while eliminating pathogen carry over between experiments. Depending on the duration of the experiment, it may be necessary to clean the containers or transfer the microcolony to a new, clean chamber. Some chamber designs incorporate a pass-through bottom to allow bee waste to be removed without causing stress to the bees (Figure 4C–D; (Malone et al. 2007, Gradish et al. 2013, Richardson et al. 2015).
Figure 4. Example microcolony chambers.
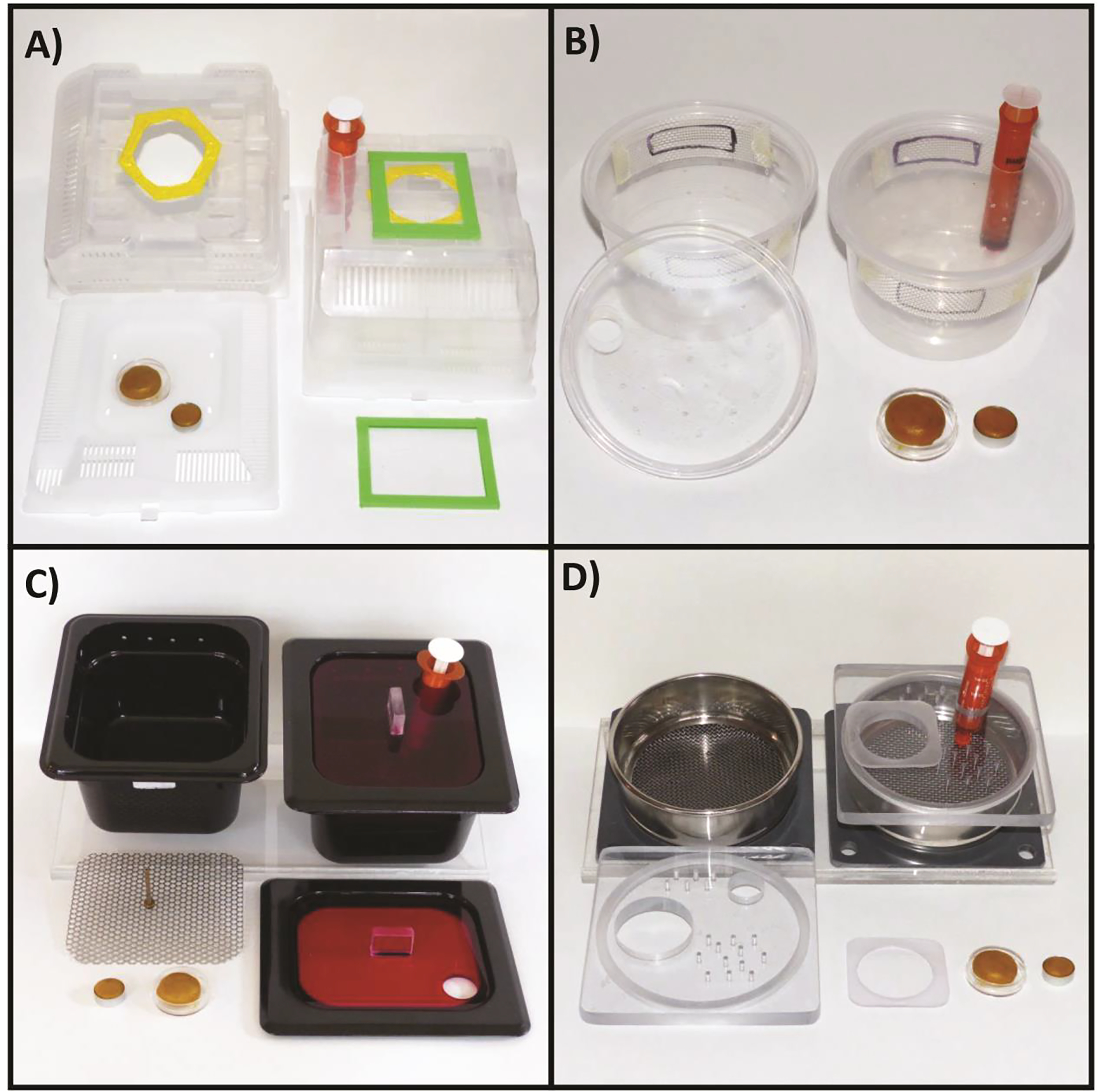
A) “Queen box” (17.8 × 15.2 × 10.1 cm) used by commercial bumble bee vendors (i.e., Koppert, Biobest) with syringe feeder (USDA-ARS Pollinating Insect Research Unit; North Logan, UT). B) Disposable “deli cup” (16 oz, 11.4 cm tall × 7.6 cm diameter) modified with mesh to improve ventilation and to accommodate a syringe feeder (USDA-ARS Pollinating Insect Research Unit; North Logan, UT). C) Food pan (1/4 size, 12.7 × 11.4 × 10.1 cm) modified to include a raised perforated stainless-steel mesh floor to separate bee waste from the nest area, see-through red plexiglass lid, syringe feeder and holes for additional ventilation (US EPA, RTP, NC). D) Stainless steel geology sieve (4 cm tall × 12.7 cm diameter) with pass-through floor, ventilated baseplate, see-through lid and syringe feeder (US EPA, RTP, NC; design adopted from Bayer CropSciences). All container designs are shown with petri dish containing a pollen ball for nest initiation and a separate dish for pollen feeding.
Single chamber designs are the most common, but some study designs incorporated separate feeding and nesting chambers (Mommaerts et al. 2010a, Ruedenauer et al. 2016). When size of chamber was reported, the volume of the nest chamber in reviewed studies ranged from ~127.2 cm3 to 10,304 cm3 (Table S1). Although investigators tend to use larger chambers when seeding microcolonies with more workers (Figure 3; Table S1), the relationship between the number of workers and microcolony container size has not been investigated.
Duration of experiment –
The duration of microcolony investigations is dictated by the purpose of the experiment and limited by worker bee mortality. Study duration ranged from 3 to 100 days with studies evaluating drone production requiring the most time for completion (Table S1).
Sugar source –
Microcolonies must be provisioned with sugar solution ad libitum. Many researchers rely on commercial sugar syrups (e.g., Biogluc®, Attracker, Apiinvert and Invertibee; Table S1). These syrups are convenient, but the formulation may not be disclosed to the investigator. Other researchers make their own sugar solutions that frequently contain only sucrose (Table S1). Occasionally, a mixture of honey and water is used(e.g. Elston et al. 2013, Gradish et al. 2013, Ramanaidu and Cutler 2013), but honey may contain pathogens and pesticides potentially confounding results (Bogdanov 2006, Kujawski et al. 2014). The composition of sugars in the syrup can be important for the establishment of the gut microbiome (Billet et al. 2016). Bees provisioned with syrups containing suboptimal sucrose content (i.e., 15 – 29%) consumed less syrup and experienced higher rates of mortality and infections than those fed syrup containing 30% sucrose (Conroy et al. 2016). High fructose syrups can impact gut microbiome development (Billet et al. 2016). Bombus terrestris microcolonies composed of 10 workers utilized an average of 300–400 μL Biogluc® solution per bee per day (Meeus et al. 2013). For some investigations, such as those evaluating pesticides, evaporation of syrup should be monitored to correct consumption estimates. Development from egg to emerged drone for B. terrestris required on average 128 mg of sugar (Řehoř et al. 2014).
Pollen source –
Since an effective artificial protein source is not available for bumble bee microcolonies, microcolonies must be fed honey bee-collected pollen. The floral composition of the pollen will depend upon the bee foraging environment and, for that reason, exact replication of pollen sources is not possible. Further, long-term storage of pollen has been shown to affect pollen quality (Pernal and Currie 2000). Restricting access to high-quality pollen was shown to increase larval and pupal development times and ultimately impacted drone production (Sutcliffe and Plowright 1990, Regali and Rasmont 1995, Génissel et al. 2002). Bees in microcolonies prefer some species of pollen over others, and pollen choice can have downstream effects on egg deposition, oophagic behavior and drone production (Aupinel et al. 2001, Moerman et al. 2015, Billiet et al. 2016, Ruedenauer et al. 2016, Dance et al. 2017). Pollen quality can also have an undesired effect on bee immune responses (Roger et al. 2017a). Worker B. terrestris pollen needs are estimated to be 25 – 30 mg/day (Meeus et al. 2013).
In some cases, microcolonies were established using sterilized pollen to control the pathogen load. Gamma irradiation (16.9 kGy) has been shown to effectively sterilize pollen (Graystock et al. 2016); however, elimination of microbes in pollen is known to affect the gut microbiome of bees (Meeus et al. 2013). Consequently, the effects of pollen sterilization methods on pollen preference, palatability and microcolony development and productivity require additional investigation.
Nesting material –
Bumble bees combine pollen with wax to produce the scaffold of their nest (Goulson 2010). Pollen composition, which varies greatly, and quantity are known to impact bumble bee colony and offspring size (Moerman et al. 2015). Consequently, the source of pollen, timing of pollen provisioning and the quantity of pollen provided all have the potential to impact microcolony initiation, progression and ultimately productivity. In some studies, microcolonies were provided with artificial nesting material or emerged pupal cells collected from other colonies at the time of nest initiation (e.g. Besard et al. 2011, Munday and Brown 2018). Although these materials appeared to stimulate nest initiation, there is the possibility that providing additional nesting material may introduce pathogens into the microcolony.
Temperature, relative humidity and light regimes –
Microcolonies have been maintained under a variety of environmental conditions including a temperature range of 23 – 30°C, relative humidity range of 20 – 80% and varying amounts of light. Each Bombus species may have an ideal nesting temperature and deviations from that temperature may impact brood development (Yoon et al. 2002, Gurel et al. 2008). Humidity levels can affect foraging rates and pollen collection by B. terrestris species in the wild, with bees collecting more pollen in low humidity environments (Peat and Goulson 2005). Thus, experimental relative humidity may affect microcolony productivity and pesticide exposure levels when delivered in food. Most microcolonies are held in total darkness, but some studies incorporate light:dark cycles (e.g. Génissel et al. 2002, Arnold et al. 2014). Generally, drone offspring masses (larvae, pupae and on the day of adult eclosion) decreased when photoperiods were adjusted to include increasing exposure to light (Amin et al. 2007).
Parameters measured in microcolony experiments
Microcolonies have been used to investigate responses to various treatments including behavior, gut microbiome, nutrition, development, pathogens, chemical biology and pesticides/xenobiotics (Table 1). Depending on the experimental purpose, investigators collected data from various parameters described below. While there are specific data captured under each of these categories, many of these factors are interrelated (Figure 5).
Table 1.
Types of studies performed using microcolonies and number of publications through November 2018.
| Primary study type | Number of publications |
|---|---|
| Behavior | 6 |
| Gut microbiome | 4 |
| Nutrition | 21 |
| Development | 6 |
| Pathogen | 6 |
| Chemical biology | 8 |
| Pesticide/xenobiotic | 24 |
Figure 5. Relationship between microcolony endpoints.
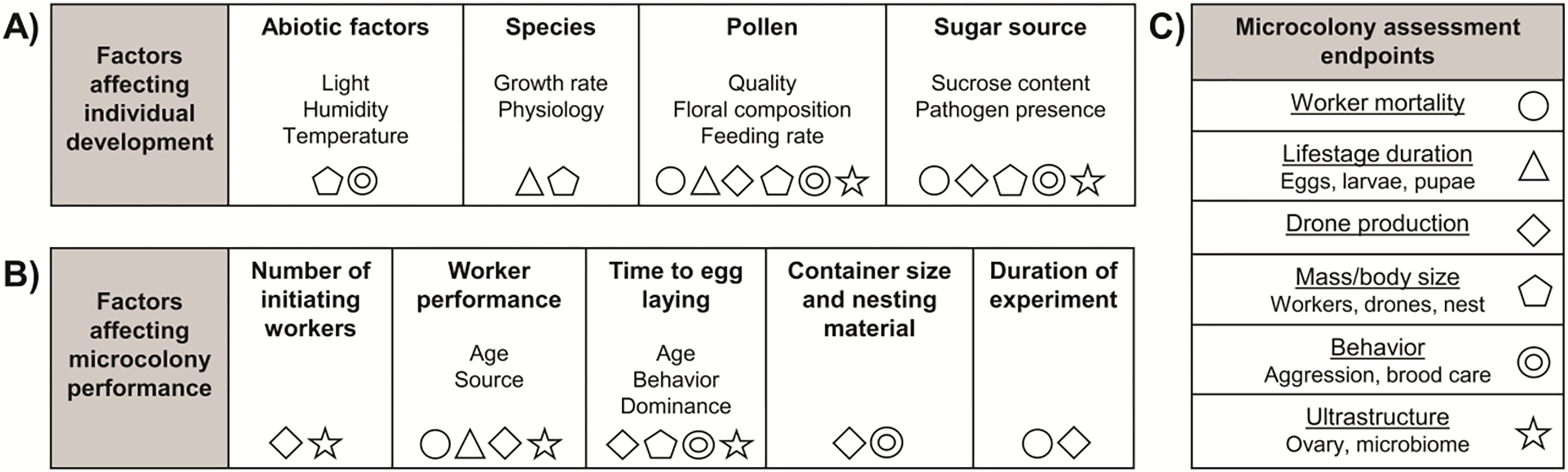
Factors (in bold) known to affect individual bumble bee development (A) and microcolony performance (B) with some examples shown below. Symbols indicate microcolony assessment endpoints (C) impacted by these factors.
Worker mortality –
The number of worker bees remaining after an experimental treatment can be easily quantified, providing information about the effect of experimental treatments on workers (Babendreier et al. 2008, Mommaerts et al. 2010b, Ramanaidu and Cutler 2013, Smagghe et al. 2013, Wang et al. 2016). Remembering that microcolony productivity is influenced by the number of workers (Gradish et al. 2013), loss of workers can impact brood development and ultimately drone production. Consequently, brood effects must ultimately be evaluated in the context of worker bee mortality. For these reasons, monitoring worker mortality is an important core element of microcolony study design.
Duration of lifecycle stages –
The amount of time required to deposit eggs and complete various lifecycle stages (i.e., egg hatching, larval development and pupation) can be monitored in microcolonies (Figure 5). While the number of establishing workers has not been directly shown to be correlated with the time to first oviposition, worker age, and the quality and amount of the pollen are known to impact egg laying activity (Génissel et al. 2002, Gradish et al. 2013, Meeus et al. 2014, Amsalem et al. 2015). In addition, some pesticides, plant toxins and bacteria have been shown to lengthen the time to first oviposition (Ramanaidu and Cutler 2013, Richardson et al. 2015). Prolonging the timeline for microcolony progression can result in increased resource demands and may affect drone production.
Offspring production –
Drone production is a key metric of microcolony productivity and can be easily quantified. Drone production is affected by experimental treatments, including pesticides (Tasei et al. 2000, Mommaerts et al. 2006, Besard et al. 2011, Laycock et al. 2012, Elston et al. 2013, Smagghe et al. 2013, Laycock et al. 2014, Ceuppens et al. 2015), pathogens (Ramanaidu and Cutler 2013, Meeus et al. 2014) and nutritional modifications (Laycock et al. 2012, Moerman et al. 2015, Ruedenauer et al. 2016, Billiet et al. 2017, Dance et al. 2017). The relationship between the number of eggs laid, number of larvae, and number of drones produced is obviously related (Figure 5). However, bumble bee nests are stratified with new structures built directly on top of old structures which hinders our ability to accurately monitor the number of eggs and larvae. One study showed that treatment-related effects on the number of eggs and drones are not always evident when evaluating numbers of larvae and pupae (Moerman et al. 2015). Oophagy and larval ejection may contribute to observed inconsistencies; mortality before the adult stage should be partitioned accordingly between mortality due to treatment effects or due to these behaviors (which may also be treatment induced).
Mass/body size –
It is essential to record initial body weight of the worker bees and to seed microcolonies with bees of similar mass (Roger et al. 2017a, Roger et al. 2017b). Worker size can directly affect the time required to establish dominance in the microcolony and, potentially, the amount of time required for first oviposition (Amsalem and Hefetz 2011). Increased food consumption by larger workers (Peat and Goulson 2005, Couvillon and Dornhaus 2010) may affect consumption/exposure rates in pesticide exposure studies.
Treatment effects may also be detected by weighing the brood. Changes in brood mass will alter the nectar needs of the colony, with heavier brood requiring more sugar (Pendrel and Plowright 1981, Řehoř et al. 2014). Poor quality pollen has been shown to affect larval and occasionally pupal weights (Tasei and Aupinel 2008a, Moerman et al. 2017, Roger et al. 2017a, Roger et al. 2017b, Vanderplanck et al. 2018). Other variables may also influence larval mass, such as light:dark regimes, species of bumble bee used, and relative humidity (Peat and Goulson 2005, Amin et al. 2007, Moerman et al. 2015).
While assessing brood may be difficult and destructive, determining the weight of drones is common and non-destructive. Since the mass of larvae and pupae should correlate with the drone size, collection of larval and pupal masses is not essential for all investigations. Drone size has been shown to be affected by food quality and availability, the presence of antibiotics, toxicants introduced into pollen, light:dark regimen and pathogens (Sutcliffe and Plowright 1988, Amin et al. 2007, Malone et al. 2007, Meeus et al. 2013, Arnold et al. 2014, Meeus et al. 2014, Barbosa et al. 2015, Ceuppens et al. 2015, Dance et al. 2017).
Behavior –
Feeding behavior is integral to microcolony success and, for that reason, quantifying food consumption is common. Pollen and nectar consumption vary between different Bombus species and fluctuate day-to-day (Manson and Thomson 2009, Řehoř et al. 2014, Moerman et al. 2015, Piiroinen et al. 2016, Dance et al. 2017). Larger workers and larger brood require more food (Řehoř et al. 2014, Schaeffer et al. 2017). Experimental treatments may alter food consumption, potentially impacting the size and number of the drones produced (Laycock et al. 2012, Moerman et al. 2015, Ruedenauer et al. 2016). If the experimental design allows for increased foraging movement (i.e., a second chamber), food uptake will be increased to satisfy energetic demands associated with foraging (Ceuppens et al. 2015). It is important to also note that experimental treatments given in sugar syrup may have a delayed effect if existing honeypots are being utilized in a microcolony (Barbosa et al. 2015).
In addition to monitoring feeding activity, investigators can monitor other worker behaviors such as: aggressiveness associated with the establishment of dominance, sluggishness and paralysis (Vandoorn 1989, Wang et al. 2016); nest construction (i.e., honey pots and brood clumps) (Elston et al. 2013); and oophagy and larval ejections (Tasei et al. 2000, Génissel et al. 2002, Munday and Brown 2018, Vanderplanck et al. 2018).
Ultrastructure –
Microcolonies have proven a useful tool for deciphering the role of specific Dufour’s gland signals in reproductive division of labor in queenright colonies (Amsalem and Hefetz 2011, Amsalem et al. 2015) and for investigating treatment effects on worker bee ovarian development and oocyte size (Cnaani et al. 2007, Manson and Thomson 2009, Laycock et al. 2012, Barbosa et al. 2015). Investigators should be aware that Dufour’s gland signals as well as ovarian development can be affected by the group size and age of the microcolony workers, which can impact microcolony initiation and progression (Amsalem and Hefetz 2011, Amsalem et al. 2015).
Gut microbiome—
The bee gut microbiome plays a vital role in metabolism, immune function growth and development (Mockler et al. 2018, Raymann and Moran 2018, Rothman et al. 2019). Studies investigating the impacts of treatments on the composition of the gut microbiome on bee health are becoming increasingly common. For studies of this type, it is important to recognize that the gut microbiome may be affected by social interactions with bees from the maternal colony (Meeus et al. 2013, Kwong et al. 2014, Kwong and Moran 2015, 2016, Billiet et al. 2017) and on the diet fed to the nascent microcolony (Billiet et al. 2017). The gut microbiome may also be affected by exposure to antibiotics and diets high in fructose, as well as the use of sterilized pollen as a food source (Meeus et al. 2013, Billiet et al. 2017), and thus care must be taken to separate microcolony effects from treatment effects.
Recommendations for standardization for pesticide risk assessment
To protect bees, many regulatory authorities require that pesticides be subjected to a risk assessment process to identify potential risks to bees. Traditionally, the honey bee has served as the model organism for this process and results observed in honey bees are assumed to be predictive of outcomes in all other bees (Gradish et al. 2018). Acknowledging differences in life histories, phenology and pesticide sensitivity between Apis and non-Apis bees (Brittain and Potts 2011, Cresswell et al. 2012, Arena and Sgolastra 2014, Stoner 2016, Thompson 2016, Heard et al. 2017), reliance on the honey bee as a surrogate species for pesticide risk assessment has been questioned (Stoner 2016, Rortais et al. 2017), prompting the development of protocols specifically designed for assessing the effects of pesticides on non-Apis bees.
Microcolony protocols can be readily adapted to address a broad range of experimental questions; however, because risk assessment of pesticide impact requires a high level of reproducibility, we recommend adoption of standard protocols. Because we currently do not fully understand the impact of all design parameters on microcolonies, we cannot provide a definitive protocol for all bumble bee species, but we can provide a framework to begin the much-needed work of methods standardization. The following recommendations are derived from a consensus of studies carried out to date using B. terrestris and B. impatiens for the purpose of pesticide risk assessment.
Microcolony chambers –
Microcolony chambers should be well-ventilated and either sanitizable or disposable. The chambers should be maintained in darkness, and if not, the chambers should be made of light-obstructing material. To eliminate the need to clean microcolony chambers during experiments and cause stress to the colony, the chambers should include a pass-through floor to isolate bee waste. Unless the experimental design requires measuring foraging behavior, only one chamber should be used. For microcolonies composed of 5 workers, we suggest chambers like those pictured in Figure 4A – D, but remind the reader that no comparative studies have been conducted to determine the optimal microcolony chamber size.
Microcolony composition –
To maximize study consistency, microcolonies should be established using five newly-emerged workers of similar mass. To account for source colony differences (such as genetics or basal pathogen infections), the workers from multiple colonies should be randomized prior to seeding microcolonies; however, where colony-level differences are of interest, single source microcolonies can be used. Investigators should consider the potential impact of interactions between newly-emerged workers and mature workers occurring in the queenright colony on the establishment of gut microboiota in naïve bumblebees which can lead to downstream effects in microcolony development and progression (Koch and Schmid-Hempel 2011, Kwong and Moran 2016). Because the number of workers in a microcolony impacts microcolony productivity, microcolonies with worker mortality occurring within the first 24 hours of seeding should be discarded and further mortality should be recorded and used to standardize results. Likewise, treatments should not be applied within the first 24 hours of microcolony establishment so that pre-treatment mortality can be detected and to allow the workers to acclimate to the chamber and their nest mates.
Food provisioning–
Microcolonies should be provisioned with fresh sugar syrup ad libitum. Sugar syrup should be composed of ≥30% sucrose content (sucrose should be the dominant sugar). Additives (e.g., vitamins, essential oils) have limited research on effectiveness and should be minimally added and always reported (Gosterit and Oytun Cicek 2017). Fresh sugar syrup should be provided every 2 – 3 days.
Microcolonies should be provisioned with fresh (or fresh-frozen) honey bee-collected pollen ad libitum. At the time of microcolony initiation, the bees must be given a pollen ball (i.e., ground pollen (2.5–4 parts by weight) mixed with sugar syrup (1 part by weight), typically 1 – 2 g, to facilitate nest building (Table S1). After a 1-week period, the bees should be given fresh pollen every 2–3 days. Ideally, pollen species and pre-existing pathogen and pesticide load in the pollen should be captured and the source kept constant through an experiment, if possible. This can be accomplished by thoroughly homogenizing all the pollen prior to the initiation of an experiment. While sterile pollen may be required in some cases, researchers should be aware that sterilization may alter pollen palatability. When applicable, pollen sterilization methods should be reported.
Delivery of test material –
When investigating the effects of pesticides on microcolonies, the test material can be delivered in either the sugar syrup or the pollen. The exposure mechanism should be thoughtfully considered since delivery in the pollen will likely target the developing brood whereas syrup delivery could impact both the brood and the workers. Since microcolony development hinges on the presence of healthy workers, we advise investigators to conduct a range-finding study to identify doses that are not acutely toxic to the workers, unless information on chronic toxicity in adult bumble bees is already available. Also, when conducting pesticide exposure studies, evaporation controls should be incorporated into the study design to correct consumption estimates.
Study duration –
Although the duration of microcolony studies will be determined by the specific needs of the investigator, study duration is ultimately limited by worker bee viability. When focusing on drone production, a minimum of 35 – 40 days from the time of nest initiation is required. Since it is difficult to understand the impact of resultant drones on brood tending, resource utilization and crowding, we recommend removing drones from the microcolony as they emerge unless subsequent drone mortality is a measured parameter. Due to the potential of founding worker mortality in microcolonies we recommend, for pesticide risk assessment, conducting studies for a minimum of 42 days and tracking worker mortality.
Microcolony monitoring –
Although the parameters recorded will be influenced by investigator needs, we recommend collecting a core set of measurements including: 1) initial worker weight, 2) worker mortality, 3) syrup/pollen utilization, 4) days to 1st oviposition, 5) days to drone emergence and 6) the number and weight of drones produced. These measures are needed to establish parameters for microcolony control performance, the baseline for exposure-effects studies. Other measures of microcolony maturation are possible (i.e., eggs hatching, development of larvae, development of pupae); but microcolonies are structurally amorphous with newer structures obstructing older structures hidden beneath. Consequently, attempting to quantify various nest structures may be laborious and of limited utility.
Reporting –
To facilitate data interpretation and to maximize study reproducibility, investigators are strongly encouraged to report the formulation of the syrup used for feeding, the pollen source and age, any pollen sterilization, the exact age and source of the worker bees and environmental conditions (i.e., temperature, relative humidity and light regimen) used. Start date, end date and specific dates of data collection for endpoints should be recorded and reported.
Data extrapolation and application in pesticide risk assessment
Bumble bee species vary in their nesting behaviors (Hobbs et al. 1962, Macfarlane et al. 1994, Knight et al. 2005), basic reproductive parameters (Asada and Ono 2000, Cnaani et al. 2002) and life expectancy (Goldblatt and Fell 1987). Within the same species, pre-experiment commercial-animal husbandry practices and colony quality (e.g., queen status, colony age, colony strength, colony life stage, disease status) vary, potentially impacting data interpretation and study reproducibility. Microcolonies are more easily standardized and provide a mechanism to study treatment effects on brood development while preserving elements of colony structure. This tool also has utility for investigating the effect of different routes of exposure (i.e., pollen vs. nectar) on larval development. Data from well-designed studies comparing outcomes observed in microcolonies and queenright colonies are essential to determining the suitability of microcolonies for hazard assessment.
Use of microcolonies for risk assessment also requires a better understanding of how species selection impacts study outcomes. The overwhelming majority of microcolony studies have been performed using B. terrestris. This species is native to Europe and is not available to North American investigators. Species differences have prompted some investigators to question whether, or not B. terrestris is a suitable surrogate for risk assessment performed on other bumble bee species (Gradish et al. 2013, Moerman et al. 2015). There are only two published studies comparing the sensitivity of different bumble bee species to pesticides (Baron et al. 2017, Wu-Smart and Spivak 2018), but the recent development of acute toxicity tests (OECD 2017b, a) for bumble bees and ongoing development of a chronic toxicity test enhance our ability to make important comparisons between species to inform risk assessment approaches.
Concluding remarks
Microcolonies are a useful tool for studies of bumble bee biology and for assessing the effects of stressors on these bees. Using bumble bee microcolonies, it is possible to conduct chronic exposure studies under defined laboratory conditions with the capacity to evaluate sublethal effects on adults and brood. However, researchers must exercise care by standardizing protocols (especially for risk assessment) as much as possible, fully reporting study design parameters, and collecting data on standardized microcolony endpoints. Additional investigations are needed to better understand the suitability of methods for each bumble bee species. Only then will the true value of this model be understood.
Supplementary Material
Acknowledgments –
The authors thank Drs. R. Koethe and C. Weitekamp for their thoughtful and critical review of this manuscript as well as the many participants of the BOMBUSS 2017 conference for engaging in stimulating discussions regarding the use of bumble bee microcolonies in research. This project was supported in part by an appointment to the Research Participation Program at the Office of Research and Development, U.S. Environmental Protection Agency, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and EPA.
Abbreviations:
- OECD
Office of Economic Cooperation and Development
Footnotes
Disclosure – The authors declare no competing interests.
Disclaimer: This article has been reviewed by the U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency or of the US Federal Government, nor does the mention of trade names or commercial products constitute endorsement or recommendations for use of those products. The authors report no financial or other conflicts of interest. The authors alone are responsible for the content and writing of this article.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
References Cited
- Allen-Wardell G, Bernhardt P, Bitner R, Burquez A, Buchmann S, Cane J, Cox PA, Dalton V, Feinsinger P, et al. 1998. The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conserv. Biol 12: 8–17. [Google Scholar]
- Amin MR, Kwon YJ, and Suh SJ. 2007. Photoperiodic influence on the body mass of bumblebee, Bombus terrestris and its copulation duration. J. Appl. Entomol 131: 537–541. [Google Scholar]
- Amsalem E, and Hefetz A. 2011. The effect of group size on the interplay between dominance and reproduction in Bombus terrestris. Plos One 6: e18238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsalem E, Orlova M, and Grozinger CM. 2015. A conserved class of queen pheromones? Re-evaluating the evidence in bumblebees (Bombus impatiens). Proc. R. Soc. Biol. Sci. Ser. B 282: 20151800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena M, and Sgolastra F. 2014. A meta-analysis comparing the sensitivity of bees to pesticides. Ecotox. 23: 324–334. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Idrovo ME, Peralta, Arias LJ, Lomas, Belmain SR, and Stevenson PC. 2014. Herbivore defence compounds occur in pollen and reduce bumblebee colony fitness. J. Chem. Ecol 40: 878–881. [DOI] [PubMed] [Google Scholar]
- Asada S, and Ono M. 2000. Difference in colony development of two Japanese bumblebees, Bombus hypocrita and B-ignitus (Hymenoptera : Apidae). Appl. Entomol. Zool 35: 597–603. [Google Scholar]
- Aupinel P, Genissel A, Gomond S, Tasei JN, and Poncet J. 2001. Collection of spring pollens by Bombus terrestris queens. Assessment of attractiveness and nutritive value of pollen diets. Proceedings of the Eight International Pollination Symposium Pollination: Integrator of Crops and Native Plant Systems: 101–105. [Google Scholar]
- Babendreier D, Reichhart B, Romeis J, and Bigler F. 2008. Impact of insecticidal proteins expressed in transgenic plants on bumblebee microcolonies. Entomol. Exp. Appl 126: 148–157. [Google Scholar]
- Barbosa WF, De Meyer L, Guedes RN, and Smagghe G. 2015. Lethal and sublethal effects of azadirachtin on the bumblebee Bombus terrestris (Hymenoptera: Apidae). Ecotoxicology 24: 130–142. [DOI] [PubMed] [Google Scholar]
- Baron GL, Raine NE, and Brown MJF. 2017. General and species-specific impacts of a neonicotinoid insecticide on the ovary development and feeding of wild bumblebee queens. Proc. R. Soc. Biol. Sci. Ser. B 284: 20170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besard L, Mommaerts V, Abdu-Alla G, and Smagghe G. 2011. Lethal and sublethal side-effect assessment supports a more benign profile of spinetoram compared with spinosad in the bumblebee Bombus terrestris. Pest Manag. Sci 67: 541–547. [DOI] [PubMed] [Google Scholar]
- Billiet A, Meeus I, Van Nieuwerburgh F, Deforce D, Wackers F, and Smagghe G. 2016. Impact of sugar syrup and pollen diet on the bacterial diversity in the gut of indoor-reared bumblebees (Bombus terrestris). Apidologie 47: 548–560. [Google Scholar]
- Billiet A, Meeus I, Cnockaert M, Vandamme P, Van Oystaeyen A, Wackers F, and Smagghe G. 2017. Effect of oral administration of lactic acid bacteria on colony performance and gut microbiota in indoor-reared bumblebees (Bombus terrestris). Apidologie 48: 41–50. [Google Scholar]
- Bloch G, and Hefetz A. 1999. Reevaluation of the role of mandibular glands in regulation of reproduction in bumblebee colonies. J. Chem. Ecol 25: 881–896. [Google Scholar]
- Bogdanov S 2006. Contaminants of bee products. Apidologie 37: 1–18. [Google Scholar]
- Breeze TD, Bailey APB, Balcombe KG, and Potts SG. 2011. Pollination service in the UK: How important are honeybees? Agric. Ecosyst. Environ 142: 137–143. [Google Scholar]
- Brittain C, and Potts SG. 2011. The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic Appl. Ecol 12: 321–331. [Google Scholar]
- Burkle LA, Marlin JC, and Knight TM. 2013. Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 339: 1611–1615. [DOI] [PubMed] [Google Scholar]
- Cameron SA 1985. Brood care by male bumble bees. Proc. Natl. Acad. Sci. U.S.A 82: 6371–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, and Griswold TL. 2011. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. U.S.A 108: 662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuppens B, Eeraerts M, Vleugels T, Cnops G, Roldan-Ruiz I, and Smagghe G. 2015. Effects of dietary lambda-cyhalothrin exposure on bumblebee survival, reproduction, and foraging behavior in laboratory and greenhouse. J. Pest Sci 88: 777–783. [Google Scholar]
- Cnaani J, Schmid-Hempel R, and Schmidt JO. 2002. Colony development, larval development and worker reproduction in Bombus impatiens Cresson. Insectes Soc. 49: 164–170. [Google Scholar]
- Cnaani J, Wong A, and Thomson JD. 2007. Effect of group size on ovarian development in bumblebee workers (Hymenoptera : apidae : Bombus). Entomol. Gen 29: 305–314. [Google Scholar]
- Couvillon MJ, and Dornhaus A. 2010. Small worker bumble bees (Bombus impatiens) are hardier against starvation than their larger sisters. Insectes Soc. 57: 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-Foster DL, Conlan S, Holmes EC, Palacios G, Evans JD, Moran NA, Quan PL, Briese T, Hornig M, et al. 2007. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318: 283–287. [DOI] [PubMed] [Google Scholar]
- Cresswell JE, Page CJ, Uygun MB, Holmbergh M, Li Y, Wheeler JG, Laycock I, Pook CJ, de Ibarra NH, et al. 2012. Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid). Zool. (Jena) 115: 365–371. [DOI] [PubMed] [Google Scholar]
- Dance C, Botias C, and Goulson D. 2017. The combined effects of a monotonous diet and exposure to thiamethoxam on the performance of bumblebee micro-colonies. Ecotoxicol. Environ. Saf 139: 194–201. [DOI] [PubMed] [Google Scholar]
- Delaplane KS, and Mayer DF. 2000. Crop pollination by bees, CABI Publishing, Wallingford, UK. [Google Scholar]
- Di Pasquale G, Salignon M, Le Conte Y, Belzunces LP, Decourtye A, Kretzschmar A, Suchail S, Brunet JL, and Alaux C. 2013. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? Plos One 8: e72016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doums C, Moret Y, Benelli E, and Schmid-Hempel P. 2002. Senescence of immune defence in Bombus workers. Ecol. Entomol 27: 138–144. [Google Scholar]
- Elston C, Thompson HM, and Walters KFA. 2013. Sub-lethal effects of thiamethoxam, a neonicotinoid pesticide, and propiconazole, a DMI fungicide, on colony initiation in bumblebee (Bombus terrestris) micro-colonies. Apidologie 44: 563–574. [Google Scholar]
- Free JB 1955. The behaviour of egg-laying workers of bumblebee colonies. Br. J. Anim. Beh 3: 147–153. [Google Scholar]
- Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, Cunningham SA, Kremen C, Carvalheiro LG, Harder LD, et al. 2013. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339: 1608–1611. [DOI] [PubMed] [Google Scholar]
- Génissel A, Aupinel P, Bressac C, Tasei JN, and Chevrier C. 2002. Influence of pollen origin on performance of Bombus terrestris micro-colonies. Entomol. Exp. Appl 104: 329–336. [Google Scholar]
- Goldblatt JW, and Fell RD. 1987. Adult longevity of workers of the bumble bees Bombus fervidus (F.) and Bombus pennsylvanicus (De Geer) (Hymenoptera: Apidea). Can. J. Zool 10: 2349–2353. [Google Scholar]
- Gosterit A, and Oytun Cicek G. 2017. Effects of vitamin additive diets on colony foundation success in bumblebee, Bombus terrestris. Scientific Papers: Series D. Animal Science. The International Session of Scientific Communications of the Faculty of Animal Science LX: 240–243. [Google Scholar]
- Goulson D 2010. Bumblebees: behaviour, ecology, and conservation, Oxford University Press, Oxford, UK. [Google Scholar]
- Gradish AE, Scott-Dupree CD, McFarlane AD, and Frewin AJ. 2013. Too much work, not enough tarsi: Group size influences Bombus impatiens (Hymenoptera: Apidae) worker reproduction with implications for sublethal pesticide toxicity assessments. J. Econ. Entomol 106: 552–557. [DOI] [PubMed] [Google Scholar]
- Gradish AE, van der Steen J, Scott-Dupree CD, Cabrera AR, Cutler GC, Goulson D, Klein O, Lehmann DM, Luckmann J, et al. 2018. Comparison of pesticide exposure in honey bees (Hymenoptera: Apidae) and bumble bees (Hymenoptera: Apidae): Implications for risk assessments. Environ. Entomol 48: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graystock P, Jones J, Pamminger T, Parkinson J, Norman V, Blane E, Rothstein L, Wäckers F, Goulson D, et al. 2016. Hygienic food to reduce pathogen risk to bumblebees. J. Invertebr. Pathol 136: 68–73. [DOI] [PubMed] [Google Scholar]
- Gurel F, Gosterit A, and Eren O. 2008. Life-cycle and foraging patterns of native Bombus terrestris (L.) (Hymenoptera, Apidae) in the Mediterranean region. Insectes Soc 55: 123–128. [Google Scholar]
- Heard MS, Baas J, Dorne JL, Lahive E, Robinson AG, Rortais A, Spurgeon DJ, Svendsen C, and Hesketh H. 2017. Comparative toxicity of pesticides and environmental contaminants in bees: Are honey bees a useful proxy for wild bee species? Sci. Total Environ 578: 357–365. [DOI] [PubMed] [Google Scholar]
- Henry M, Beguin M, Requier F, Rollin O, Odoux JF, Aupinel P, Aptel J, Tchamitchian S, and Decourtye A. 2012. A common pesticide decreases foraging success and survival in honey bees. Science 336: 348–350. [DOI] [PubMed] [Google Scholar]
- Hobbs GA, Nummi WO, and Virostek JF. 1962. Managing colonies of bumble bees (Hymenoptera: Apidae) for pollination purposes. Can. J. Zool 94: 1121–1132. [Google Scholar]
- Huang Z 2012. Pollen nutrition affects honey bee stress resistance. Terrestrial Arthropod Reviews 5: 175–189. [Google Scholar]
- Johnson RM, Ellis MD, Mullin CA, and Frazier M. 2010. Pesticides and honey bee toxicity–USA. Apidologie 41: 312–331. [Google Scholar]
- Kevan PG 1999. Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agric. Ecosyst. Environ 74: 373–393. [Google Scholar]
- Klatt BK, Holzschuh A, Westphal C, Clough Y, Smit I, Pawelzik E, and Tscharntke T. 2014. Bee pollination improves crop quality, shelf life and commercial value. Proc. R. Soc. Biol. Sci. Ser. B 281: 20132440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijn D, Winfree R, Bartomeus I, Carvalheiro LG, Henry M, Isaacs R, Klein AM, Kremen C, M’Gonigle LK, et al. 2015. Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nature Commun 6: 7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AM, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, and Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. Biol. Sci. Ser. B 274: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight ME, Martin AP, Bishop S, Osborne JL, Hale RJ, Sanderson RA, and Goulson D. 2005. An interspecific comparison of foraging range and nest density of four bumblebee (Bombus) species. Mol. Ecol 14: 1811–1820. [DOI] [PubMed] [Google Scholar]
- Koch H, and Schmid-Hempel P. 2011. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proceedings of the National Academy of Sciences 108: 19288–19292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh I, Lonsdorf EV, Williams NM, Brittain C, Isaacs R, Gibbs J, and Ricketts TH. 2016. Modeling the status, trends, and impacts of wild bee abundance in the United States. Proc. Natl. Acad. Sci. U.S.A 113: 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawski MW, Bargańska Ż, Marciniak K, Miedzianowska E, Kujawski JK, Ślebioda M, and Namieśnik J. 2014. Determining pesticide contamination in honey by LC-ESI-MS/MS–Comparison of pesticide recoveries of two liquid–liquid extraction based approaches. LWT-Food Science and Technology 56: 517–523. [Google Scholar]
- Kwong WK, and Moran NA. 2015. Evolution of host specialization in gut microbes: The bee gut as a model. Gut Microbes 6: 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong WK, and Moran NA. 2016. Gut microbial communities of social bees. Nat. Rev. Microbiol 14: 374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong WK, Engel P, Koch H, and Moran NA. 2014. Genomics and host specialization of honey bee and bumble bee gut symbionts. Proc. Natl. Acad. Sci. U.S.A 111: 11509–11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrere M, and Couillaud F. 1993. Role of Juvenile Hormone biosynthesis in dominance status and reproduction of the bumblebee Bombus terrestris. Behav. Ecol. Sociobiol 33: 335–338. [Google Scholar]
- Laycock I, Lenthall KM, Barratt AT, and Cresswell JE. 2012. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 21: 1937–1945. [DOI] [PubMed] [Google Scholar]
- Laycock I, Cotterell KC, O’Shea-Wheller TA, and Cresswell JE. 2014. Effects of the neonicotinoid pesticide thiamethoxam at field-realistic levels on microcolonies of Bombus terrestris worker bumble bees. Ecotoxicol. and Environ. Saf 100: 153–158. [DOI] [PubMed] [Google Scholar]
- Le Conte Y, Ellis M, and Ritter W. 2010. Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie 41: 353–363. [Google Scholar]
- Macfarlane RP, Patten KD, Royce LA, Wyatt BK, and Mayer DF. 1994. Management potential of sixteen North American bumble bee species. Melanderia 50: 1–2. [Google Scholar]
- Malone LA, Scott-Dupree CD, Todd JH, and Ramankutty P. 2007. No sub-lethal toxicity to bumblebees, Bombus terrestris, exposed to Bt-corn pollen, captan and novaluron. N. Z. J. Crop and Hortic. Sci 35: 435–439. [Google Scholar]
- Manson JS, and Thomson JD. 2009. Post-ingestive effects of nectar alkaloids depend on dominance status of bumblebees. Ecol. Entomol 34: 421–426. [Google Scholar]
- Meeus I, Pisman M, Smagghe G, and Piot N. 2018. Interaction effects of different drivers of wild bee decline and their influence on host-pathogen dynamics. Current Opinion in Insect Science 26: 136–141. [DOI] [PubMed] [Google Scholar]
- Meeus I, de Miranda JR, de Graaf DC, Wäckers F, and Smagghe G. 2014. Effect of oral infection with Kashmir bee virus and Israeli acute paralysis virus on bumblebee (Bombus terrestris) reproductive success. J. Invertebr. Pathol 121: 64–69. [DOI] [PubMed] [Google Scholar]
- Meeus I, Mommaerts V, Billiet A, Mosallanejad H, Van de Wiele T, Wackers F, and Smagghe G. 2013. Assessment of mutualism between Bombus terrestris and its microbiota by use of microcolonies. Apidologie 44: 708–719. [Google Scholar]
- Mockler BK, Kwong WK, Moran NA, and Koch H. 2018. Microbiome structure influences infection by the parasite Crithidia bombi in bumble bees. Appl. Environ. Microbiol 84: e02335–02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman R, Nathalie R, De Jonghe R, Michez D, and Vanderplanck M. 2016. Interspecific variation in bumblebee performance on pollen diet: New insights for mitigation strategies. Plos One 11: e0168462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman R, Vanderplanck M, Fournier D, Jacquemart A-L, and Michez D. 2017. Pollen nutrients better explain bumblebee colony development than pollen diversity. Insect conservation and diversity 10: 171–179. [Google Scholar]
- Moerman R, Vanderplanck M, Roger N, Decleves S, Wathelet B, Rasmont P, Fournier D, and Michez D. 2015. Growth rate of bumblebee larvae is related to pollen amino acids. J. Econ. Entomol 109: 25–30. [DOI] [PubMed] [Google Scholar]
- Mommaerts V, Sterk G, and Smagghe G. 2006. Hazards and uptake of chitin synthesis inhibitors in bumblebees Bombus terrestris. Pest Manag. Sci 62: 752–758. [DOI] [PubMed] [Google Scholar]
- Mommaerts V, Jans K, and Smagghe G. 2010a. Impact of Bacillus thuringiensis strains on survival, reproduction and foraging behaviour in bumblebees (Bombus terrestris). Pest Manag. Sci 66: 520–525. [DOI] [PubMed] [Google Scholar]
- Mommaerts V, Reynders S, Boulet J, Besard L, Sterk G, and Smagghe G. 2010b. Risk assessment for side-effects of neonicotinoids against bumblebees with and without impairing foraging behavior. Ecotoxicology 19: 207–215. [DOI] [PubMed] [Google Scholar]
- Munday Z, and Brown MJF. 2018. Bring out your dead: quantifying corpse removal in Bombus terrestris, an annual eusocial insect. Anim. Behav 138: 51–57. [Google Scholar]
- OECD. 2017a. Test No. 246: Bumblebee, Acute Contact Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2, No. 246., OECD Publishing, Paris, France. [Google Scholar]
- OECD. 2017b. Test No. 247: Bumblebee, Acute Oral Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2, No. 247., OECD Publishing, Paris, France. [Google Scholar]
- Peat J, and Goulson D. 2005. Effects of experience and weather on foraging efficiency and pollen versus nectar collection in the bumblebee, Bombus terrestris. Behav. Ecol. Sociobiol 58: 152–156. [Google Scholar]
- Pendrel BA, and Plowright RC. 1981. Larval feeding by adult bumble bee workers (Hymenoptera, Apidae). Behav. Ecol. Sociobiol 8: 71–76. [Google Scholar]
- Pernal SF, and Currie RW. 2000. Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie 31: 387–409. [Google Scholar]
- Piiroinen S, Botias C, Nicholls E, and Goulson D. 2016. No effect of low-level chronic neonicotinoid exposure on bumblebee learning and fecundity. Peerj 4: e1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RC, and Jay SC. 1968. Caste differentiation in bumblebees (Bombus Latr.: Hym.) I.—The determination of female size. Insectes Soc. 15: 171–192. [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, and Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol 25: 345–353. [DOI] [PubMed] [Google Scholar]
- Ramanaidu K, and Cutler GC. 2013. Different toxic and hormetic responses of Bombus impatiens to Beauveria bassiana, Bacillus subtilis and spirotetramat. Pest Manag. Sci 69: 949–954. [DOI] [PubMed] [Google Scholar]
- Raymann K, and Moran NA. 2018. The role of the gut microbiome in health and disease of adult honey bee workers. Current Opinion in Insect Science 26: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regali A, and Rasmont P. 1995. New bioassays to evaluate diet in Bombus terrestris (L.) (Hymenoptera, Apidae) Apidologie 26: 273–281. [Google Scholar]
- Řehoř I, Macháčková L, Bučánková A, Stanislava M. j., Kateřina Ä. e., and Straka J. 2014. Measuring the sugar consumption of larvae in bumblebee micro-colonies: a promising new method for tracking food economics in bees. Apidologie 45: 116–128. [Google Scholar]
- Richardson LL, Adler LS, Leonard AS, Andicoechea J, Regan KH, Anthony WE, Manson JS, and Irwin RE. 2015. Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc. R. Soc. Biol. Sci. Ser. B 282: 20142471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger N, Michez D, Wattiez R, Sheridan C, and Vanderplanck M. 2017a. Diet effects on bumblebee health. J. Insect Physiol 96: 128–133. [DOI] [PubMed] [Google Scholar]
- Roger N, Moerman R, Carvalheiro LG, Aguirre-Guitierrez J, Jacquemart AL, Kleijn D, Lognay G, Moquet L, Quinet M, et al. 2017b. Impact of pollen resources drift on common bumblebees in NW Europe. Global Change Biology 23: 68–76. [DOI] [PubMed] [Google Scholar]
- Rortais A, Arnold G, Dorne JL, More SJ, Sperandio G, Streissl F, Szentes C, and Verdonck F. 2017. Risk assessment of pesticides and other stressors in bees: Principles, data gaps and perspectives from the European Food Safety Authority. Sci. Total Environ 587–588: 524–537. [DOI] [PubMed] [Google Scholar]
- Rothman JA, Leger L, Graystock P, Russell K, and McFrederick QS. 2019. The bumble bee microbiome increases survival of bees exposed to selenate toxicity. Environmental Microbiology. [DOI] [PubMed] [Google Scholar]
- Ruedenauer FA, Spaethe J, and Leonhardt SD. 2016. Hungry for quality--individual bumblebees forage flexibly to collect high-quality pollen. Behav. Ecol. Sociobiol 70: 1209–1217. [Google Scholar]
- Rutrecht ST, Klee J, and Brown MJF. 2007. Horizontal transmission success of Nosema bombi to its adult bumble bee hosts: effects of dosage, spore source and host age. Parasitology 134: 1719–1726. [DOI] [PubMed] [Google Scholar]
- Schaeffer RN, Mei YZ, Andicoechea J, Manson JS, and Irwin RE. 2017. Consequences of a nectar yeast for pollinator preference and performance. Funct. Ecol 31: 613–621. [Google Scholar]
- Sibbald ED, and Plowright CMS. 2014. Social interactions and their connection to aggression and ovarian development in orphaned worker bumblebees (Bombus impatiens). Behav. Process 103: 150–155. [DOI] [PubMed] [Google Scholar]
- Smagghe G, Deknopper J, Meeus I, and Mommaerts V. 2013. Dietary chlorantraniliprole suppresses reproduction in worker bumblebees. Pest Manag. Sci 69: 787–791. [DOI] [PubMed] [Google Scholar]
- Stoner KA 2016. Current pesticide risk assessment protocols do not adequantely address differences between honey bees (Apis mellifera) and bumble bees (Bombus spp.). Front. Environ. Sci 4: 1–8. [Google Scholar]
- Sutcliffe G, and Plowright R. 1988. The effects of food supply on adult size in the bumble bee Bombus terricola Kirby (Hymenoptera: Apidae). Can. Entomol 120: 1051–1058. [Google Scholar]
- Sutcliffe GH, and Plowright RC. 1990. The effects of pollen availability on development time in the bumble bee Bombus terricola K (Hymenoptera, Apidae). Can. J. Zool 68: 1120–1123. [Google Scholar]
- Tasei J-N, and Aupinel P. 2008a. Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: Apidae). Apidologie 39: 397–409. [Google Scholar]
- Tasei J.-N. l., and Aupinel P. 2008b. Validation of a method using queenless bombus terrestris micro-colonies for testing the nutritive value of commercial pollen mixes by comparison with queenright colonies. J. Econ. Entomol 101: 1737–1742. [DOI] [PubMed] [Google Scholar]
- Tasei JN, Lerin J, and Ripault G. 2000. Sub-lethal effects of imidacloprid on bumblebees, Bombus terrestris (Hymenoptera : Apidae), during a laboratory feeding test. Pest Manag. Sci 56: 784–788. [Google Scholar]
- Thompson H 2016. Extrapolation of acute toxicity across bee species. Integr Environ Assess Manag 12: 622–626. [DOI] [PubMed] [Google Scholar]
- Thompson HM, and Hunt LV. 1999. Extrapolating from honeybees to bumblebees in pesticide risk assessment. Ecotoxicology 8: 147–166. [Google Scholar]
- Vanderplanck M, Decleves S, Roger N, Decroo C, Caulier G, Glauser G, Gerbaux P, Lognay G, Richel A, et al. 2018. Is non-host pollen suitable for generalist bumblebees? Insect Sci. 25: 259–272. [DOI] [PubMed] [Google Scholar]
- Vandoorn A 1989. Factors influencing dominance behavior in queenless bumblebee workers (Bombus terrestris). Physiol. Entomol 14: 211–221. [Google Scholar]
- Velthuis HHW, and van Doorn A. 2006. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 37: 421–451. [Google Scholar]
- Wang HD, Meeus I, and Smagghe G. 2016. Israeli acute paralysis virus associated paralysis symptoms, viral tissue distribution and Dicer-2 induction in bumblebee workers (Bombus terrestris). J. Gen. Virol 97: 1981–1989. [DOI] [PubMed] [Google Scholar]
- Whitehorn PR, O’Connor S, Wackers FL, and Goulson D. 2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336: 351–352. [DOI] [PubMed] [Google Scholar]
- Winter K, Adams L, Thorp R, Inouye D, Day L, Ascher J, and Buchmann S. 2006. Importation of non-native bumble bees into North America: Potential consequences of using Bombus terrestris and other non-native bumble bees for greenhouse crop pollination in Canada, Mexico, and the United States. North American Pollinator Protection Campaign (NAPPC), San Fransisco, CA. [Google Scholar]
- Wu-Smart J, and Spivak M. 2018. Effects of neonicotinoid imidacloprid exposure on bumble bee (Hymenoptera: Apidae) queen survival and nest initiation. Environ. Entomol 47: 55–62. [DOI] [PubMed] [Google Scholar]
- Yoon HJ, Kim SE, and Kim YS. 2002. Temperature and humidity favorable for colony development of the indoor-reared bumblebee, Bombus ignitus. Appl. Entomol. Zool 37: 419–423. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


