Abstract
The sorption of organic contaminants by natural organic matter (NOM) often limits substrate bioavailability and is an important factor affecting microbial degradation rates in soils and sediments. We hypothesized that reduced substrate bioavailability might influence which microbial assemblages are responsible for contaminant degradation under enrichment culture conditions. Our primary goal was to characterize enrichments in which different model organic solid phases were used to establish a range of phenanthrene bioavailabilities for soil microorganisms. Phenanthrene sorption coefficients (expressed as log KD values) ranged from 3.0 liters kg−1 for Amberlite carboxylic acid cation-exchange resin (AMB) to 3.5 liters kg−1 for Biobeads polyacrylic resin (SM7) and 4.2 liters kg−1 for Biobeads divinyl benzene resin (SM2). Enrichment cultures were established for control (no sorptive phase), sand, AMB, SM7, and SM2 treatments by using two contaminated soils (from Dover, Ohio, and Libby, Mont.) as the initial inocula. The effects of sorption by model phases on the degradation of phenanthrene were evaluated for numerous transfers in order to obtain stable microbial assemblages representative of sorptive and nonsorptive enrichment cultures and to eliminate the effects of the NOM present in the initial inoculum. Phenanthrene degradation rates were similar for each soil inoculum and ranged from 4 to 5 μmol day−1 for control and sand treatments to approximately 0.4 μmol day−1 in the presence of the SM7 sorptive phase. The rates of phenanthrene degradation in the highly sorptive SM2 enrichment culture were insignificant; consequently, stable microbial populations could not be obtained. Bacterial isolates obtained from serial dilutions of enrichment culture samples exhibited significant differences in rates of phenanthrene degradation performed in the presence of SM7, suggesting that enrichments performed in the presence of a sorptive phase selected for different microbial assemblages than control treatments containing solid phase phenanthrene.
Sorption of nonpolar contaminants by natural organic matter (NOM) is often characterized by a rapid step involving association with labile domains near the particle surface, followed by a slower step controlled by diffusion through intraparticle pores to hydrophobic regions (nonlabile domains) within the solid phase matrix (3, 17, 27). Retarded intraparticle diffusion of sorbed molecules results in significant desorption hysteresis (3, 7, 27) and is thought to be the rate-limiting step for biodegradation in soils and sediments. Many studies performed during the last decade support the hypothesis that rates of desorption or mass transfer from sorbed domains control rates of contaminant biodegradation (3, 5, 17, 27). Several studies support the conclusion that contaminants partitioned in the NOM phase are not directly available for degradation by microorganisms (4–6, 8, 13, 33). However, this general conclusion should be tempered by recent evidence that there is significant diversity in the abilities of microorganisms to degrade contaminants partitioned in or sorbed by NOM (4, 11, 31).
For example, two naphthalene-degrading isolates have been shown to exhibit significant variation in rates of degradation of sorbed naphthalene (11). One bacterial isolate, Alcaligenes sp. strain NP-Alk, did not enhance desorption of bound naphthalene, whereas Pseudomonas putida ATCC 17484 promoted naphthalene desorption and may have utilized bound naphthalene. Additional studies using naphthalene sorbed to XAD-2 (cross-linked polystyrene), a modified 2:1 layer silicate clay, and Tenax (2,6-diphenyl-p-phenylene oxide) confirmed that the P. putida strain had a greater ability to mineralize sorbed naphthalene than Alcaligenes sp. strain NP-Alk (6, 11). The possible physiological explanations for such diversity include variations in attachment to NOM surfaces (12, 16, 24), production of biosurfactants (14, 25), motility, rates of membrane transport, and the metabolic pathways used to mineralize phenanthrene (30). Although the majority of studies showing variations in responses to sorbed substrates have been done with cultivated microorganisms, they suggest that microenvironmental gradients in contaminant bioavailability play a role in the selection of specialized contaminant-degrading microorganisms. Many phenotypically different polycyclic aromatic hydrocarbon (PAH)-degrading isolates have been cultured in the laboratory by using classical enrichment approaches (2, 9, 18, 30) where the contaminant was provided as the sole carbon source, often in crystalline form at concentrations greater than the aqueous solubility limit. However, given the importance of sorption in controlling PAH bioavailability, it is questionable whether microbial isolates cultivated by using this approach are important in soil and sediment environments. Enrichment strategies in which contaminants are sorbed to organic solid phases may provide a more relevant selection environment and may eventually lead to a better understanding of what microorganisms and microbial processes are involved in the degradation of contaminants present primarily as a sorbed phase.
Model organic phases have been used in several recent studies to evaluate the effects of sorption and mass transfer rates on the microbial degradation of organic contaminants. Scow and Alexander (28) showed that the degradation of phenol and glutamate by a Pseudomonas sp. was controlled by diffusion from interparticle pore spaces in clay and polyacrylamide gel exclusion beads. Harms and Zehnder (13) used porous Teflon beads to study the degradation of sorbed 3-chlorodibenzofuran by a Sphingomonas sp. and found that attached cells degraded the bound chemical more rapidly than could be accounted for by the rates of desorption into the aqueous phase. Calvillo and Alexander (4) used polyacrylic porous resins to study the microbial degradation of sorbed biphenyl and found that biphenyl sorbed on polyacrylic beads was mineralized at a rate higher than the rate of abiotic desorption. However, until the recent work by Tang et al. (31), model solid phases had not been employed to cultivate microorganisms specifically adapted to low-bioavailability environments. In the study by Tang et al., a bacterium isolated by enrichment on phenanthrene sorbed to polyacrylic porous resin degraded sorbed phenanthrene more rapidly than did an isolate cultivated by using the traditional approach of providing contaminants in soluble or crystalline form. Although that study did not focus on microbial community structure, the results demonstrate the importance of enrichment strategies designed to more closely simulate the conditions in soil and/or sediment environments.
In this paper, we describe the use of model solid phases in enrichment experiments designed to evaluate degradation of a representative PAH (phenanthrene) and microbial selection for a range of contaminant bioavailabilities. In this study, we (i) determined the sorption-desorption characteristics of model solid phases used in enrichment cultures, (ii) evaluated the effects of phenanthrene sorption to model solid phases on the degradation kinetics in enrichment cultures by using two contaminated soils as initial inocula, and (iii) found that bacterial isolates enriched under reduced phenanthrene bioavailability conditions exhibit higher rates of phenanthrene degradation in a sorptive environment than isolates enriched in the presence of solid phase phenanthrene. In the accompanying paper (6a), we present the results of molecular analyses in which we examined the nature of microbial communities and isolates enriched under these conditions in order to corroborate that sorption-limited bioavailability plays an important role in the selection of contaminant-degrading microorganisms.
MATERIALS AND METHODS
Soils.
Two soils from geographically distant sites were used as inocula for batch enrichments. One soil was obtained from an abandoned coal gasification site in Dover, Ohio, and the other was obtained from a wood treatment facility in Libby, Mont. (Table 1). Both soils exhibited significant levels of total petroleum hydrocarbons and PAHs (determined by Environmental Protection Agency methods 8440 and 8275A, respectively [22, 29]), suggesting that prior enrichment of PAH-degrading microorganisms had occurred. The potential of these soils to mineralize phenanthrene was verified by performing serum bottle radiorespirometry as described by Knaebel and Vestal (20). The soils were wetted to approximate field capacity by using sterile deionized distilled water (dH2O), and then 0.1-ml aliquots of [9-14C]phenanthrene (total [9-14C]phenanthrene added = 0.007 mg kg−1) dissolved in acetone were added, followed by brief mixing. The [9-14C]phenanthrene (>98% pure) was obtained from Sigma Chemical Co. (St. Louis, Mo.) and had a specific activity of 59.5 mCi mmol−1. Evolution of 14CO2, which was trapped on paper wicks saturated with 0.5 M NaOH and quantified by liquid scintillation counting, showed that indigenous microbial populations were capable of degrading approximately 45% of the freshly added phenanthrene (Table 1). Consequently, the soils contained active phenanthrene-degrading microbial populations.
TABLE 1.
Physical and chemical characteristics of soils used as inocula in phenanthrene enrichment experiments
| Soil | % Organic C | % Sand | % Silt | % Clay | % of phenanthrene degradeda | Concn of PAHs (mg kg−1)
|
Total petroleum hydrocarbon (mg kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Naphthalene | Phenanthrene | Anthracene | Pyrene | Fluorene | Benz[a]- anthracene | Chrysene | Benzo[a]- pyrene | |||||||
| Dover, Ohio | 3.7 | 71 | 19 | 4 | 44.1 ± 2.4 | NDb | 60 | ND | 110 | 110 | 75 | 80 | 120 | 4,200 |
| Libby, Mont. | 3.9 | 46 | 35 | 11 | 45.9 ± 9.1 | ND | 85 | 150 | 210 | 50 | 65 | 75 | 22 | 14,000 |
Extent of phenanthrene degradation in a batch soil environment after 30 days of incubation.
ND, not detected.
Model organic phases.
Model solid phases (Table 2) with different polarities, C/O ratios, and surface functional groups were selected to provide a range of phenanthrene sorption in enrichment cultures. The geometric surface areas of the solids were calculated based on mean particle size and ranged from 0.01 to 0.05 m2 g−1. The surface area of the nonporous polymethylmethacrylate (PMMA) beads determined by using BET-N2(g) isotherms (15) was 10-fold greater than the geometric surface area, indicating moderate surface roughness. The other solids used (Amberlite IRC-50 [AMB], Biobeads SM7, and Biobeads SM2) are porous resins that have considerably larger total surface areas. The surface area of AMB determined by using BET-N2(g) isotherms while the solid was in the dry state may not reflect contributions from the internal surface area. Regardless, the internal surfaces of AMB, SM7, and SM2 are not accessible to microorganisms because the pore diameters are <0.01 μm. The AMB weak cation-exchange phase with carboxylic acid functionality was chosen as a model phase to represent the importance of carboxylic acid functional groups in humic substances.
TABLE 2.
Properties of model solid phases used in enrichment environments to obtain a range of phenanthrene bioavailabilities
| Solid phase | Chemical nature | Particle size (μm) | Avg pore diam (Å) | Surface area (m2 g−1) | Carbon content (%) | Phenanthrene log KD (liters kg−1) | Source |
|---|---|---|---|---|---|---|---|
| Sand | SiO2 | 75–600 | Nonporous | 0.01a | <0.01 | <0.01 | Fluka Chemical |
| PMMA | Polymethyl methacrylate | <100 | Nonporous | 0.2b | ∼50 | 2.30 | ICI Acrylics |
| AMB | Carboxylic acid | 330–500 | <100 | 2.2b | ∼50 | 2.99 | Supelco |
| SM7 | Acrylic ester | 300–1,180 | 90 | 450c | ∼50 | 3.47 | Bio-Rad |
| SM2 | Divinyl benzene | 300–1,180 | 90 | 300c | 92 | 4.16 | Bio-Rad |
Sorption isotherms describing the extent of phenanthrene sorbed to each model solid phase were determined with 25-ml glass centrifuge tubes containing 0.5 g of solid and a 7.5-ml total volume containing phenanthrene at initial concentrations of 0.01, 0.05, 0.1, 0.5, and 1.0 mg liter−1. The background solution (pH 7.0) contained the following dissolved ions at concentrations intended to represent the concentrations of constituents typically found in soil solutions (1): Ca (4 mM), Mg (2 mM), Na (2.5 mM), K (0.5 mM), NH4 (2.5 mM), Fe (0.02 mM), SO4 (5 mM), NO3 (3.5 mM), PO4 (0.005 mM), and Cl (4 mM). This minimal salts solution (designated SSE) had an ionic strength of approximately 0.029 M and was identical to the solution used in the desorption and degradation experiments described below. All treatments were performed in triplicate, and all preparations contained sufficient [9-14C]phenanthrene for analysis of supernatants by scintillation counting. An equilibration time of 48 h was chosen based on preliminary kinetic experiments demonstrating constancy of the aqueous-phase phenanthrene concentration within several hours. The amount of phenanthrene sorbed was calculated by determining the difference between the initial and equilibrium concentrations of aqueous-phase [14C]phenanthrene.
All desorption experiments and the subsequent degradation experiments described below were conducted by using model solid phases preloaded with phenanthrene. Approximately 30 g of each solid was washed with dH2O three times to remove fine particles and dried overnight at 50°C. Each solid was preequilibrated for 12 h in dH2O-methanol (1:1, vol/vol), and then the suspensions were spiked approximately 30 times at 15-min intervals with 50-μl acetone aliquots, each containing 5.0 mg of phenanthrene plus 0.022 μCi of [9-14C]phenanthrene. Phenanthrene additions were made sequentially to ensure that phenanthrene concentrations remained below the solubility limit during sorption to model solid phases. The suspensions were then shaken overnight, after which the solid phases were washed with dH2O. All supernatants were analyzed for aqueous-phase [14C]phenanthrene by scintillation counting. The solids were then dried at 50°C for 12 h, and triplicate subsamples were combusted with a Harvey biological oxidizer to quantify and confirm the amount of sorbed phenanthrene. Mass balance calculations based on direct determination of sorbed phenanthrene contents were in agreement with the amounts of phenanthrene added to the solids. The sorbed phenanthrene concentrations achieved with this protocol were approximately 2.5 mg g−1 for AMB and 5.0 mg g−1 for SM2 and SM7.
The extent of phenanthrene desorption in the absence of microbial activity was determined by using preloaded solid phases in three different solvent systems: SSE, dH2O-methanol (1:1, vol/vol), and 1% Tween 80 (a surfactant known to enhance the apparent solubility of phenanthrene at concentrations above the critical micelle concentration) (21). Triplicate 0.25-g samples of preloaded solid phases were added to 25-ml glass test tubes, followed by addition of 10 ml of each solvent phase. The tubes were shaken on a reciprocating shaker for 28 days; on six sampling dates, the supernatant was removed and replaced with fresh solution. All supernatants were analyzed for [14C]phenanthrene by scintillation counting, and the cumulative release of phenanthrene was plotted as the percentage of phenanthrene desorbed as a function of time.
Phenanthrene degradation in the presence of model solid phases.
Initial degradation experiments were conducted by using several phenanthrene-degrading isolates obtained from previous work in our laboratories. The purpose of this initial screening was to verify reduction in phenanthrene bioavailability in the presence of model solid phases and to demonstrate potential differences in the ability of microbial isolates to degrade phenanthrene in different sorptive environments. Individual isolates were obtained from several contaminated soils by using enrichment techniques (9) and phenanthrene spray-over plates (19). The additional soils utilized for bacterial isolation are not described in detail here but were either from abandoned coal gasification sites (Dover, Ohio) or creosote-contaminated sites that had previous hydrocarbon contamination (Gulf Breeze, Fla.). Phenanthrene degradation by nearly 30 isolates was confirmed by serum bottle radiorespirometry (20). Each isolate was grown in liquid culture (SSE) for 1 to 2 weeks by using solid phase phenanthrene as the sole C source. All cultures were brought to a constant optical density at 500 nm of 0.1 absorbance unit, and then 10-ml portions were inoculated into serum bottles in the absence (control) and presence of AMB, SM7, or SM2 solid phase containing sorbed [14C]phenanthrene. The initial cell densities, based on correlation of optical densities with CFU, ranged from 2 × 107 to 9 × 107 CFU ml−1.
Enrichment culture using model sorptive phases.
The primary objectives of enrichment experiments were to determine the effects of phenanthrene sorption on phenanthrene bioavailability, as shown by reduced degradation rates, and to select for stable microbial populations that were potentially adapted to low-bioavailability environments. Duplicate batch enrichment cultures were initiated by using 1 g of soil (from Dover, Ohio, or Libby, Mont.) as inocula in 250-ml culture flasks containing 100 ml of SSE either in the presence or in the absence of model sorptive phases; this was followed by incubation at 25 ± 2°C with continuous aeration on an oscillating shaker (130 rpm). The amount of sorptive phase added to each enrichment culture (2 g of AMB, 1 g of SM2, or 1 g of SM7) was determined based on a target equivalent mass of 5.0 mg of phenanthrene (4.7 mg of C) per flask containing 0.022 μCi of [14C]phenanthrene. Controls without sorptive phases also contained 5.0 mg of phenanthrene per flask, which was added as an acetone solution; when the acetone evaporated, the solid phase phenanthrene precipitated (water solubility of phenanthrene = 1.8 mg liter−1 [22]). Phenanthrene degradation was monitored by frequent analysis (every 2 days) of 14CO2, expressed as the percentage of added [14C]phenanthrene evolved over time. A 10-ml aliquot of each enrichment suspension (containing both solid and aqueous phases) was transferred to an identical fresh enrichment vessel just as the rate of phenanthrene degradation approached zero. The enrichment cultures were maintained for 5 to 12 transfers to determine the consistency of phenanthrene degradation rates and the stabilities of microbial populations selected under such conditions and to reduce any confounding effects of NOM or DNA added with the initial soil inoculum. At each transfer time, subsamples were removed and used to isolate phenanthrene-degrading microorganisms. Additional subsamples were frozen at −70°C for use in molecular analyses of microbial communities (6a).
Isolation of phenanthrene-degrading bacteria.
Samples from enrichment cultures were used to isolate pure cultures of microorganisms, which were characterized for their abilities to degrade phenanthrene. Samples were taken from all enrichment cultures that were actively degrading phenanthrene, 10-fold serial dilutions in sterile 0.85% NaCl were prepared, and 100-μl portions of each dilution were spread on SSE plates or on yeast extract-peptone-glucose (YEPG) medium (1, 9). In some cases, AMB and SM7 beads were harvested by centrifugation, washed with SSE, and then treated in a sterile tissue homogenizer with SSE at the maximum speed. Dilutions of the supernatants were spread on agar plates so that bacteria attached to the bead surfaces could potentially form colonies on the agar medium. Phenanthrene was sprayed onto the agar plate surfaces, resulting in an opaque layer (19). Morphologically distinct colonies that were obtained with higher dilutions and formed clearing zones were considered potential phenanthrene-mineralizing organisms and were purified further by passages on either YEPG or SSE agar plates. The purity of >40 strains was checked microscopically and by molecular analysis by using denaturing gradient gel electrophoresis (DGGE) (6a). Bacterial isolates growing either on SSE agar plates or on YEPG agar plates were examined for their ability to degrade [14C]phenanthrene by using serum bottle radiorespirometry as described above. The phenanthrene-degrading isolates were associated primarily with three DGGE bands that were the predominant bands produced by the different enrichment cultures (6a). Phenanthrene degradation rates in the presence and in the absence of sorptive phases were evaluated in greater detail with three isolates that produced the dominant DGGE bands observed with SM7 enrichment (isolate SM7.6.1), control (isolate C4.7), and sand enrichment (isolate S2.1) cultures. Each isolate was pregrown in SSE by using solid-phase phenanthrene as the sole C source for 1 to 2 weeks. The optical density at 500 nm of each culture was adjusted to a constant value of 0.12 absorbance unit, and then 10-ml portions were inoculated into 250-ml culture flasks containing 90 ml of fresh SSE and 5 mg of phenanthrene (0.022 μCi of [14C]phenanthrene) as described above for control and sand, AMB, and SM7 enrichment cultures. The initial cell densities, based on correlation of optical density values with numbers of CFU, ranged from 1 × 107 to 2 × 107 CFU ml−1. Triplicate flasks kept under constant aeration were monitored for evolution of 14CO2, which was used as a measure of phenanthrene degradation over time.
RESULTS
Model solid-phase properties: sorption and desorption.
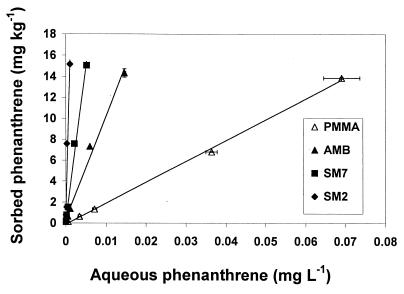
Sorption isotherms describing the partitioning of phenanthrene for each model solid phase were highly linear over a range of equilibrium phenanthrene concentrations (Fig. 1) and were used to calculate sorption coefficients (log KD), which ranged from 2.3 liters kg−1 for PMMA to 4.2 liters kg−1 for the nonpolar SM2 phase (Table 2). The log KD values for soils vary depending on the organic C content but often range from 2.5 to 3.5 liters kg−1 (23). The sand used in these experiments showed no appreciable sorption of phenanthrene (data not shown) and provided a nonsorptive control surface for microbial attachment. The results of sorption experiments suggest that the model solid phases which we used were suitable for achieving a range of phenanthrene bioavailability in enrichment experiments designed to select for microorganisms specifically adapted to low-bioavailability conditions. Preliminary experiments using PMMA as a model sorptive phase showed little reduction in phenanthrene degradation rate relative to controls when solid-phase phenanthrene was used. These results were consistent with the fact that the nonporous PMMA solid used in these experiments exhibited a lower KD value than other model sorptive phases and the fact that the majority of phenanthrene bound by PMMA was not subject to significant limitations in mass transfer to degrading microorganisms, perhaps due to the lack of microporosity. Consequently, for the purpose of focusing on sorptive phases exhibiting reduced phenanthrene bioavailability, PMMA was not used in any further experiments described here.
FIG. 1.
Isotherms showing the sorption of phenanthrene by model organic solids, including PMMA, AMB (a porous weak cation exchanger with COOH functionality), SM7 (porous acrylic ester beads), and SM2 (porous divinyl benzene beads). The isotherm slopes correspond to calculated log KD values of 2.3, 3.0, 3.5, and 4.2 liters kg−1 for PMMA, AMB, SM7, and SM2, respectively. Error bars indicate standard deviations for both x and y axis variables; where error bars are absent, they fall within the symbols.
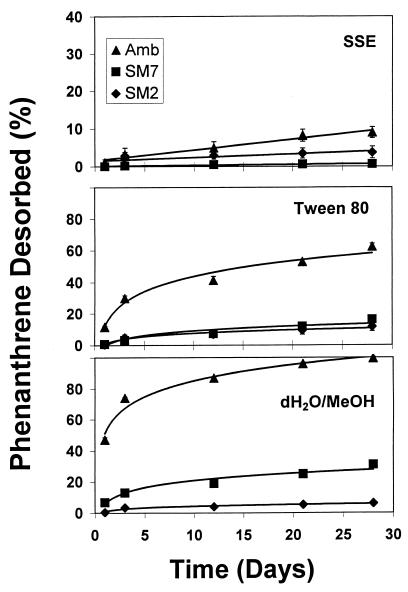
Desorption of phenanthrene from preloaded solid phases was measured by using repeated extraction steps over a 28-day period with SSE, a 1% Tween 80 solution, or dH2O-methanol (1:1). The purpose of these experiments was to evaluate the extent to which phenanthrene desorbed in the absence of microbial growth over a time period similar to that used in the enrichment experiments. When SSE was used, less than 10% of the sorbed phenanthrene desorbed from AMB and less than 3% desorbed from the more nonpolar SM7 and SM2 phases (Fig. 2). The amount of phenanthrene desorbed after five extractions with SSE would not support the extent of phenanthrene degradation observed in enrichment experiments (see below). Significantly larger amounts of phenanthrene were desorbed from AMB in the presence of 1% Tween 80, indicating that surfactant micelles were capable of solubilizing a fraction of the sorbed phenanthrene (Fig. 2). However, the cumulative amount of phenanthrene desorbed from SM7 and SM2 was still less than 10 to 12% of the total amount of sorbed phenanthrene. Finally, 100% of AMB-sorbed phenanthrene was desorbed with the dH2O-methanol (1:1) solution, while only 10 and 25% of the phenanthrene were desorbed from SM2 and SM7 solid phases, respectively (Fig. 2). With each extracting solution, the amount of phenanthrene desorbed generally increased as the polarity of the solid phase increased (AMB > SM7 > SM2). The results of the desorption experiments are consistent with the expected relationships between sorption coefficients and desorption kinetic parameters of nonpolar organic compounds (3), and they also show the range of phenanthrene bioavailability for the solid phases used.
FIG. 2.
Cumulative desorption of phenanthrene from preloaded model solids in three different extracting solutions: SSE, 1% Tween 80, and dH2O-methanol (1:1, vol/vol). The value for each time point represents desorption into fresh extracting solution. Error bars represent standard deviations; where error bars are absent, they fall within the symbols.
Phenanthrene degradation: initial characterization of enrichment conditions.
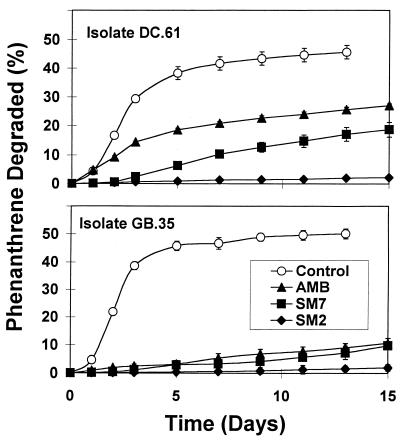
The phenanthrene degradation experiments conducted with known phenanthrene-degrading bacterial isolates produced two important results. First, the maximum phenanthrene degradation rates and extents of degradation for nearly all of the isolates decreased in the following order: control ≈ sand > AMB > SM7 > SM2. The degradation rates were highest in control vessels containing no sorptive phase and were nearly zero in the presence of SM2 (Fig. 3). This order is consistent with the results of sorption and desorption experiments, in which phenanthrene sorption rates increased and desorption rates decreased as the nonpolarity of the sorptive phase increased.
FIG. 3.
Phenanthrene degradation in the absence (control) and presence of sorbing solid phases by two bacterial isolates (GB.35, DC.61) obtained from soils by using phenanthrene spray-over plates. Control, no sorbed-phase phenanthrene present; AMB, phenanthrene sorbed to AMB; SM7, phenanthrene sorbed to SM7; SM2, phenanthrene sorbed to SM2.
Second, individual isolates exhibited significant variation in their ability to degrade phenanthrene sorbed to solid phases. Of nearly 30 phenanthrene-degrading isolates examined, several mineralized less than 10% of the added phenanthrene when it was presorbed to SM7 and AMB. An example of this behavior was observed with an isolate (GB.35) obtained from a creosote-contaminated soil (Gulf Breeze, Fla.), where phenanthrene degradation was negligible in the presence of AMB, SM7, or SM2 (Fig. 3). Other phenanthrene-degrading microbial isolates (e.g., DC.61 from Dover, Ohio) exhibited different capabilities for degrading phenanthrene sorbed to AMB and SM7, as shown in Fig. 3. However, all isolates screened degraded little or none (<5%) of the phenanthrene sorbed to SM2, the most nonpolar phase used in our experiments. These results suggest that by employing different solid phases with different polarities, it may be possible to structure enrichment conditions to select for contaminant-degrading microorganisms with different capabilities for degrading a sorbed contaminant and to isolate microorganisms specifically adapted to low-bioavailability conditions.
Phenanthrene degradation in enrichment cultures.
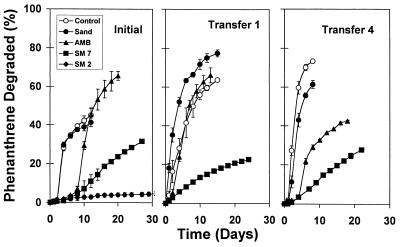
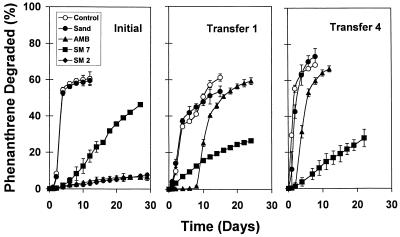
We performed enrichment experiments to simultaneously evaluate phenanthrene degradation kinetics and microbial community structure for a range of phenanthrene bioavailabilities. Our primary goal was to identify microorganisms selected in the enrichment cultures by using both cultivation-dependent and cultivation-independent methods (6a). Consequently, repeated transfers to fresh media were made in an effort to (i) minimize carryover of DNA and NOM associated with the initial soil inoculum and (ii) develop steady-state microbial assemblages exhibiting consistent phenanthrene degradation kinetics over time. Phenanthrene degradation rates in initial enrichment cultures (1% Dover soil inoculum) followed trends similar to those observed in individual microbial isolate experiments, where rates of degradation were inversely correlated with the phenanthrene sorption coefficients of the solids used in the enrichment cultures (Fig. 4). After subsequent transfers into fresh enrichment media, the preparations continued to exhibit consistent trends in degradation rates as a function of phenanthrene bioavailability (i.e., the order of the degradation rates was as follows: control ≈ sand > AMB > SM7). Lag phases of 2 to 10 days were observed in the initial enrichment cultures containing AMB and SM7 when the Dover soil was used, but lag phases were generally nonexistent after subsequent transfers (Fig. 4), suggesting that these enrichment cultures selected for stable phenanthrene-degrading microbial assemblages. In the SM2 enrichment cultures, less than 5% of the phenanthrene added was degraded during the initial enrichment period, and less than 1% was degraded after subsequent transfers. These enrichment culture experiments were not continued due to insufficient phenanthrene degradation and due to a lack of sufficient biomass to permit molecular analysis of microbial populations.
FIG. 4.
Degradation of phenanthrene obtained with a Dover soil inoculum in batch enrichment cultures grown in the absence (control) and in the presence of sand, AMB, SM7, and SM2 sorptive phases. Results are shown for selected transfers and indicate the development of consistent patterns for the phenanthrene degradation rate as a function of time and as a function of solid-phase polarity. Error bars represent one standard deviation.
The effects of sorptive phases on phenanthrene degradation rates when Libby soil was used were consistent with the results obtained with Dover soil. Initially, the phenanthrene degradation rates were higher in SM7 than in AMB enrichment cultures (Fig. 5). An apparent lag phase extending 8 days into the first transfer was observed prior to rapid degradation of phenanthrene in the AMB enrichment culture. Although subsequent transfers continued to show short lag phases for AMB enrichment cultures, by the fourth transfer the phenanthrene degradation rates were consistently following the order control ≈ sand > AMB > SM7, which was similar to the order observed with the Dover soil. Maximum phenanthrene degradation rates averaged for several transfers (zero to four transfers) revealed consistent and statistically significantly different trends for enrichment conditions for both the Dover and Libby soils (Table 3).
FIG. 5.
Degradation of phenanthrene obtained with a Libby soil inoculum in batch enrichment cultures grown in the absence (control) and presence of different phenanthrene sorptive phases. Results are shown for selected transfers and indicate the development of consistent patterns for the phenanthrene degradation rate as a function of time and as a function of solid-phase polarity. Error bars represent one standard deviation.
TABLE 3.
Maximum phenanthrene degradation rates in enrichment cultures inoculated with two contaminated soils (from Dover, Ohio, and Libby, Mont.), in which a constant mass of phenanthrene was present either in the absence of sorptive phases (control and sand) or in the presence of sorptive phases (AMB, SM7, and SM2)
| Enrichment condition | Maximum phenanthrene degradation rate (μmol day−1)
|
|
|---|---|---|
| Dover soil inoculum | Libby soil inoculum | |
| Control | 3.9 (1.2)a | 5.6 (2.2) |
| Sand | 3.7 (0.3) | 4.9 (1.8) |
| AMB | 1.8 (0.4) | 2.6 (0.7) |
| SM7 | 0.34 (0.09) | 0.37 (0.16) |
| SM2 | 0.06 (0.01)b | 0.07 (0.01)b |
The values in parentheses are standard deviations of means for five transfers, all of which were done in duplicate.
The values for SM2 were based only on the initial mineralization rates and are included to show that no significant phenanthrene degradation occurred over a 30-day period.
Phenanthrene degradation by isolates obtained from enrichment cultures with different bioavailabilities.
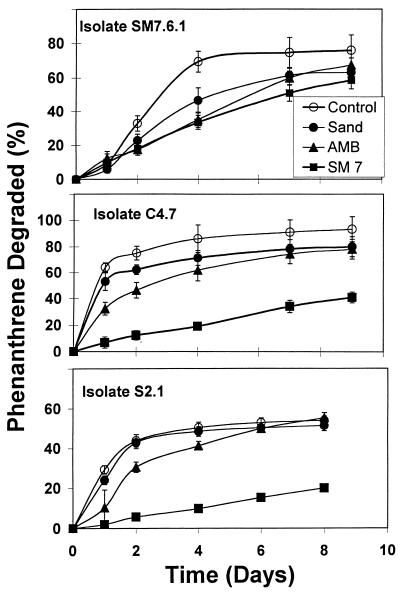
More than 40 phenanthrene-degrading bacterial isolates were cultivated from the model enrichment cultures in an effort to identify important species present in all treatments. A subset of these isolates representing two of the primary populations associated with control and SM7 enrichment cultures (6a) was used to test the hypothesis that organisms selected under sorptive conditions (such as SM7 cultures) may differ in the ability to degrade sorbed-phase phenanthrene from organisms isolated under nonsorptive conditions. A comparison of representative isolates obtained from control (strain C4.7), sand (strain S2.1), and SM7 (strain SM7.6.1) enrichment cultures (Fig. 6) showed that isolates obtained from nonsorptive enrichment cultures degraded phenanthrene more slowly in the presence of SM7 than did isolates obtained from SM7 enrichment cultures. The maximum phenanthrene degradation rates in control enrichment cultures containing solid-phase phenanthrene were 8.8 μmol day−1 for isolate C4.7, 3.2 μmol day−1 for isolate S2.1, and 2.4 μmol day−1 for isolate SM7.6.1 (Fig. 6). These results show that isolates SM7.6.1 and S2.1 exhibited slower growth kinetics in the absence of AMB or SM7 than isolate C4.7. In the presence of the SM7 sorptive phase, the maximum phenanthrene degradation rates declined for all isolates. However, the reductions in degradation rates caused by reduced phenanthrene bioavailability varied considerably among isolates. This was best illustrated by normalizing for each isolate the maximum phenanthrene degradation rate in the presence of SM7 to that in the control environment. When this was done, the comparison should have factored out simple differences in microbial growth rates among isolates when no constraints on phenanthrene bioavailability were present. Sorption of phenanthrene to SM7 reduced the degradation rate by a factor of 2 for isolate SM7.6.1. In contrast, the sorptive phase reduced the rates of phenanthrene degradation approximately 10- to 15-fold for isolates C4.7 and S2.1. These results indicate that sorption of phenanthrene by organic phases constitutes an important environmental constraint for certain microorganisms, providing a basis for possible selection of different species in heterogeneous soil and sediment microenvironments. Although we have not examined the underlying mechanisms responsible for the differences among the isolates, we hypothesize that isolates such as SM7.6.1 are better adapted to sorptive environments than isolates C4.7 and S2.1.
FIG. 6.
Phenanthrene degradation in the absence (control) and presence of sand, AMB, and SM7 solid phases by Dover soil isolates obtained from either SM7 (SM7.6.1), control (C4.7), or sand (S2.1) enrichment cultures. Error bars represent one standard deviation.
DISCUSSION
Past research focusing on the effects of sorption on degradation of organic contaminants has generally been conducted by using microorganisms isolated under nonsorptive enrichment conditions (4, 10, 11, 26, 30, 33). It has often been concluded that a sorbed contaminant is not directly available for microbial degradation and that desorption into the aqueous phase is necessary for microbial uptake and subsequent degradation. It is thought that in such situations the contaminant desorption rate is the rate-limiting step for microbial degradation. However, recent work suggests that microorganisms vary in their abilities to degrade substrates bound to NOM or nonpolar solid phases (4, 10, 31). Consequently, conclusions concerning rates of microbial utilization of a sorbed substrate are likely dependent on the specific microbial isolate. Moreover, enrichments using sorptive phases to select for microorganisms specifically adapted to low-bioavailability conditions may be useful for identifying species that are important in soil and sediment environments and for elucidating mechanisms important for accessing sorbed-phase contaminants.
In the present work, we have shown that phenanthrene-degrading microbial isolates obtained by using canonical enrichment approaches (i.e., phenanthrene spray-over plates [19]) vary in their abilities to degrade phenanthrene sorbed by organic solid phases intended to simulate NOM. Some isolates obtained by using nonsorbed phenanthrene as the sole carbon source did not degrade appreciable amounts of phenanthrene sorbed to AMB (a weak cation-exchange phase with carboxylic acid functionality) or SM7 (acrylic ester functionality). For isolates capable of degrading phenanthrene in the presence of sorbing phases, degradation rates were generally inversely related to phenanthrene sorption. These results are consistent with those reported by others (4, 11, 13, 33) showing that sorption of nonpolar compounds to model solid phases reduced the degradation rates. Recently, Tang et al. (31) utilized sorptive environments to select microorganisms with enhanced abilities to mineralize sorbed PAHs.
In this paper and the accompanying paper (6a) we describe enrichment experiments designed to evaluate the relationships among phenanthrene sorption, phenanthrene degradation, and microbial selection. Phenanthrene degradation rates were monitored under four different enrichment conditions for numerous transfers following initial inoculation with two hydrocarbon-contaminated soils. Microbial populations selected in these enrichment cultures exhibited consistent patterns of phenanthrene degradation kinetics after several transfers to fresh media. An inverse relationship between the phenanthrene degradation rate and phenanthrene sorption to model solid phases was observed in enrichment cultures prepared with both contaminated soils (Fig. 4 and 5). In contrast to the degradation rates in the presence of SM2, the degradation rates in the presence of AMB and SM7 were much higher than would have been predicted from the rates of phenanthrene desorption in the absence of microorganisms (compare the percentage desorbed as shown in Fig. 2 to the amount mineralized as shown in Fig. 4 and 5). Weissenfels et al. (33) observed no degradation of anthracene sorbed on a resin similar to SM2 (XAD-2) by an acclimated mixed bacterial culture known to degrade several PAHs. Potential mechanisms of microbially facilitated desorption in SM7 and AMB environments include solubilization of the contaminant via production of biosurfactants and development of steep concentration gradients between the solid phase and interfacial phenanthrene (10, 12, 13, 32). It has also been suggested that microorganisms capable of attachment exhibit higher rates of degradation of a sorbed substrate (10, 12, 13). An examination of selected solid phases by scanning electron microscopy confirmed the presence of attached microorganisms in both AMB and SM7 enrichment cultures, suggesting that at least one member of each enriched culture was present on bead surfaces. However, further work will be necessary to evaluate the mechanisms responsible for the different degradation behaviors of isolates obtained from enrichment cultures in the presence and in the absence of sorptive phases and to determine whether other factors, such as surface characteristics of the solid phases, may play a role in the selection of phenanthrene-degrading microorganisms.
A subset of microbial isolates obtained from the enrichment cultures was used in experiments to compare the rates of phenanthrene degradation by individual isolates for the same range of phenanthrene bioavailabilities. We observed that one of the primary isolate types (6a) that originated from the SM7 enrichment cultures (SM7.6.1) degraded phenanthrene more rapidly in the presence of SM7 than isolates obtained from control enrichment cultures containing no sorptive phase (C4.7, S2.1). Our results are consistent with those of Tang et al. (31) showing that sorption of phenanthrene has an important effect on selection of microbial species with greater adaptation to low-bioavailability environments. In addition, our work shows that the range of phenanthrene bioavailabilities obtained by using solid phases with different sorptive properties provides a useful approach for determining the effects of contaminant sorption on microbial selection and for cultivating and characterizing microorganisms that may have more relevance in natural environments. Furthermore, in the accompanying paper (6a) we describe molecular analyses that were used to corroborate the finding that different microbial assemblages were obtained from enrichment cultures with different degrees of phenanthrene sorption.
ACKNOWLEDGMENTS
We appreciate support from the Army Corps of Engineers Waterways Experiment Station (contract DACA39-95-K-0003), the National Science Foundation (grant DEB-9729857), the German Research Community (DFG), the Max Planck Society, and the Montana Agricultural Experiment Station (projects 104398 and 911296).
We thank Ron Doughten for assistance with the sorption isotherm experiments, Kim Anderson for technical support, and Eric Kern, Greg Colores, and anonymous reviewers for constructive comments.
REFERENCES
- 1.Angle J S, McGrath S P, Chaney R L. New culture medium containing ionic concentrations of nutrients similar to concentrations found in the soil solution. Appl Environ Microbiol. 1991;57:3674–3676. doi: 10.1128/aem.57.12.3674-3676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boldrin B, Tiehm A, Fritzsche C. Degradation of phenanthrene, fluorene, fluoranthrene, and pyrene by a Mycobacterium sp. Appl Environ Microbiol. 1993;59:1927–1930. doi: 10.1128/aem.59.6.1927-1930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brusseau M L, Jessup R E, Rao P S C. Nonequilibrium sorption of organic chemicals: elucidation of rate-limiting processes. Environ Sci Technol. 1991;25:134–142. [Google Scholar]
- 4.Calvillo Y M, Alexander M. Mechanism of microbial utilization of biphenyl sorbed to polyacrylic beads. Appl Microbiol Biotechnol. 1996;45:383–390. doi: 10.1007/s002530050700. [DOI] [PubMed] [Google Scholar]
- 5.Chung G-Y, McCoy B J, Scow K M. Criteria to assess when biodegradation is kinetically limited by intraparticle diffusion and sorption. Biotechnol Bioeng. 1993;41:625–632. doi: 10.1002/bit.260410605. [DOI] [PubMed] [Google Scholar]
- 6.Crocker F H, Guerin W F, Boyd S A. Bioavailability of naphthalene sorbed to cationic surfactant-modified smectite clay. Environ Sci Technol. 1995;29:2953–2958. doi: 10.1021/es00012a010. [DOI] [PubMed] [Google Scholar]
- 6a.Friedrich M, Grosser R G, Kern E A, Inskeep W P, Ward D M. Effect of model sorptive phases on phenanthrene degradation: molecular analysis of enrichments and isolates suggests selection based on bioavailability. Appl Environ Microbiol. 2000;66:2703–2710. doi: 10.1128/aem.66.7.2703-2710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu G, Kan A T, Tomson M. Adsorption and desorption hysteresis of PAHs in surface sediment. Environ Toxicol Chem. 1994;13:1559–1567. [Google Scholar]
- 8.Gordon A S, Millero F J. Adsorption mediated decrease in the biodegradation rate of organic compounds. Microb Ecol. 1985;11:289–298. doi: 10.1007/BF02016813. [DOI] [PubMed] [Google Scholar]
- 9.Grosser R J, Warshawsky D, Vestal J R. Indigenous and enhanced mineralization of pyrene, benzo(a)pyrene, and carbazole in soils. Appl Environ Microbiol. 1991;57:3462–3469. doi: 10.1128/aem.57.12.3462-3469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerin W F, Boyd S A. Differential bioavailability of soil-sorbed naphthalene to two bacterial species. Appl Environ Microbiol. 1992;58:1142–1152. doi: 10.1128/aem.58.4.1142-1152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerin W F, Boyd S A. Bioavailability of naphthalene associated with natural and synthetic sorbents. Water Res. 1997;51:1504–1512. [Google Scholar]
- 12.Harms H, Zehnder A J B. Influence of substrate diffusion on degradation of dibenzofuran and 3-chlorodibenzofuran by attached and suspended bacteria. Appl Environ Microbiol. 1994;60:2736–2745. doi: 10.1128/aem.60.8.2736-2745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harms H, Zehnder A J B. Bioavailability of sorbed 3-chlorodibenzofuran. Appl Environ Microbiol. 1995;61:27–33. doi: 10.1128/aem.61.1.27-33.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman D C, Lenhard R J, Miller R M. Formation and removal of hydrocarbon residual in porous media: effects of attached bacteria and biosurfactants. Environ Sci Technol. 1997;31:1290–1294. [Google Scholar]
- 15.Hiemenz P C. Principles of colloid and surface chemistry. New York, N.Y: Marcel Dekker; 1977. [Google Scholar]
- 16.Holm P E, Nielsen P H, Albrechtsen H-J, Christensen T H. Importance of unattached bacteria and bacteria attached to sediment in determining potentials for degradation of xenobiotic organic contaminants in an aerobic aquifer. Appl Environ Microbiol. 1992;58:3020–3026. doi: 10.1128/aem.58.9.3020-3026.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karickhoff S W, Brown D S, Scott T A. Sorption of hydrophobic pollutants on natural sediments. Water Res. 1979;13:241–248. [Google Scholar]
- 18.Keuth S, Rehm H-J. Biodegradation of phenanthrene by Arthrobacter polychromogenes isolated from a contaminated soil. Appl Microbiol Biotechnol. 1991;34:804–808. [Google Scholar]
- 19.Kiyohara H, Nagao K, Yana K. Rapid screen for bacteria degrading water-insoluble, solid hydrocarbons on agar plates. Appl Environ Microbiol. 1982;43:454–457. doi: 10.1128/aem.43.2.454-457.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knaebel D B, Vestal J R. A comparison of double vial to serum bottle radiorespirometry to measure microbial mineralization in soils. J Microbiol Methods. 1988;7:309–317. [Google Scholar]
- 21.Ko S-O, Schlautman M A, Carraway E R. Effects of solution chemistry on the partitioning of phenanthrene to sorbed surfactants. Environ Sci Technol. 1998;32:3542–3548. [Google Scholar]
- 22.Lopez-Avila V, Dodhiwala N S, Benedicto J, Young R. SFE/IR for the determination of petroleum hydrocarbons in soils and sediments. Document 600/X-92-046. Las Vegas, Nev: US EPA Environmental Monitoring Systems Laboratory; 1992. [Google Scholar]
- 23.Mackay D, Shiu W Y, Ma K C. Illustrated handbook of physical-chemical properties and environmental fate for organic chemicals. II. Chelsea, Mich: Lewis Publishers; 1992. [Google Scholar]
- 24.Neu T R. Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol Rev. 1996;60:151–166. doi: 10.1128/mr.60.1.151-166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noordman W H, Ji W, Brusseau M L, Janssen D B. Effects of rhamnolipid biosurfactants on removal of phenanthrene from soil. Environ Sci Technol. 1998;32:1806–1812. [Google Scholar]
- 26.Ogram A V, Jessup R E, Ou L T, Rao P S C. Effects of sorption on biological degradation rates of (2,4-dichlorophenoxy)acetic acid in soils. Appl Environ Microbiol. 1985;49:582–587. doi: 10.1128/aem.49.3.582-587.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pignatello J J, Xing B. Mechanisms of slow sorption of organic chemicals to natural particles. Environ Sci Technol. 1995;30:1–11. [Google Scholar]
- 28.Scow K M, Alexander M. Effect of diffusion on the kinetics of biodegradation: experimental results with synthetic aggregates. Soil Sci Soc Am J. 1992;56:128–134. [Google Scholar]
- 29.Snelling R, King D, Belair B. Analysis of PAHs in soils and sludges using thermal extraction-GC-MS. Application note 228-228. Wilmington, Del: Hewlett-Packard Co.; 1993. [Google Scholar]
- 30.Stringfellow W T, Aitken M D. Comparative physiology of phenanthrene degradation by two dissimilar pseudomonads isolated from a creosote-contaminated soil. Can J Microbiol. 1994;40:432–438. doi: 10.1139/m94-071. [DOI] [PubMed] [Google Scholar]
- 31.Tang W-C, White J C, Alexander M. Utilization of sorbed compounds by microorganisms specifically isolated for that purpose. Appl Microbiol Biotechnol. 1998;49:117–121. doi: 10.1007/s002530051147. [DOI] [PubMed] [Google Scholar]
- 32.Verstraete W, Devliegher W. Formation of non-bioavailable organic residues in soil: perspectives for site remediation. Biodegradation. 1996;7:471–485. [Google Scholar]
- 33.Weissenfels W D, Klewer H-J, Langhoff J. Adsorption of polycyclic aromatic hydrocarbons (PAHs) by soil particles: influence on biodegradability and biotoxicity. Appl Microbiol Biotechnol. 1992;36:689–696. doi: 10.1007/BF00183251. [DOI] [PubMed] [Google Scholar]