Abstract
Background
Elaeagnus angustifolia L. is a deciduous tree in the family Elaeagnaceae. It is widely used to study abiotic stress tolerance in plants and to improve desertification-affected land because of its ability to withstand diverse types of environmental stress, such as drought, salt, cold, and wind. However, no studies have examined the mechanisms underlying the resistance of E. angustifolia to environmental stress and its adaptive evolution.
Methods
Here, we used PacBio, Hi-C, resequencing, and RNA-seq to construct the genome and transcriptome of E. angustifolia and explore its adaptive evolution.
Results
The reconstructed genome of E. angustifolia was 526.80 Mb, with a contig N50 of 12.60 Mb and estimated divergence time of 84.24 Mya. Gene family expansion and resequencing analyses showed that the evolution of E. angustifolia was closely related to environmental conditions. After exposure to salt stress, GO pathway analysis showed that new genes identified from the transcriptome were related to ATP-binding, metal ion binding, and nucleic acid binding.
Conclusion
The genome sequence of E. angustifolia could be used for comparative genomic analyses of Elaeagnaceae family members and could help elucidate the mechanisms underlying the response of E. angustifolia to drought, salt, cold, and wind stress. Generally, these results provide new insights that could be used to improve desertification-affected land.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13007-022-00915-w.
Keywords: Elaeagnus angustifolia L., Genome, Evolutionary response mechanism, Desertification-affected land
Background
The world’s population is increasing rapidly and is projected to reach 9.6 billion by 2050 [1]. Hence, global food production will need to increase 38 and 57% by 2025 and 2050, respectively, to maintain the current level of food supply [2]. However, the world’s irrigated land is decreasing by 1–2% annually [3], and soil degradation due to salinization is one of the major causes of this reduction in irrigated land [4]. More than 1125 million hectares of land worldwide are salt-affected, of which approximately 76 million hectares are affected by human-induced salinization and sodification [4]. Salinity stress is a major abiotic stress affecting plant growth and crop productivity [5]. Soil salinization is a major cause of land degradation, and it can make land unsuitable for crop cultivation [6]. In recent years, biological measures have been shown to be some of the most effective approaches for ameliorating salt-affected soil [7].
E. angustifolia L., also known as Russian olive, is a deciduous tree belonging to the family Elaeagnaceae (Fig. 1). It is native to central and western Africa and is distributed in the United States, Canada, the Mediterranean coast, southern Russia, Iran, and India. It is widely distributed in China and occurs in several provinces including Xinjiang, Gansu, Ningxia, and Shandong [8]. The fruit of E. angustifolia is rich in sugars, flavonoids, and other substances that can regulate the circulation of blood and immune function in humans; the branches, leaves, and flowers have anti-aging properties and can be used to treat burns, bronchitis, dyspepsia, and neurasthenia [9]. E. angustifolia is tolerant of drought, salt, cold, and wind stress. It is prolific and highly adaptable, as it can grow in a variety of climates and soils [10]. E. angustifolia can grow and reproduce normally in soil with a salt content of 0.8–1.2% [11]. As a nitrogen-fixing, actinorrhizal plant, E. angustifolia is likely an early successional, pioneer species that can colonize nitrogen-poor soils such as sandy, eroded mineral soils and wetlands [12]. Consequently, E. angustifolia has often been used for the reforestation of arid and salinized zones [13].
Fig. 1.

E. angustifolia in the south campus of Shandong Agricultural University, Tai’an, Shandong, China
Although the development of genome sequencing has aided the domestication and improvement of many species [14], relatively few studies have used genomic tools to study E. angustifolia. Ghodhbane-Gtari et al. [15] reporteds the 11.3-Mbp draft genome sequence of Frankia sp. strain BMG5.11 in E. angustifolia, which had a G + C content of 69.9% and 9926 candidate protein-encoding genes. Lin et al. [16] conducted a genome-wide transcriptome analysis and found that high salt concentration inhibited the growth and photosynthesis of E. angustifolia, which was caused by the down-regulation of genes encoding key enzymes involved in photosynthesis and genes related to important structures in the photosystems and light-harvesting complexes. However, no studies to date have examined the reference genome of E. angustifolia. Furthermore, little is known about the mechanisms of adaptive evolution and transcription of E. angustifolia under salt stress.
Here, we used PacBio, Hi-C assisted assembly, resequencing, and other technologies to explore the adaptive evolution of E. angustifolia. We used the transcriptome to explore the mechanism by which E. angustifolia responds to salt stress. The results of this study provide new insights that could be used to aid the planting of E. angustifolia, increase food income, and promote recovery from global land desertification.
Materials and methods
Sample collection and DNA extraction
Samples from E. angustifolia trees (obtained from Xinjiang Province, NCBI Taxonomic ID, 36777) were collected from the south campus of Shandong Agricultural University, Tai’an, Shandong, China (36.17101°N, 117.16074°E, 134.0 masl) (Fig. 1) for genomic DNA sequencing, Hi-C assisted assembly, and genome evolution and transcriptome analyses. Twelve wild E. angustifolia samples were collected for genome resequencing in Gansu, China (Table 1). After collection, samples were immediately immersed in liquid nitrogen and stored until DNA extraction. DNA was extracted using the Cetyltrimethyl Ammonium Bromide (CTAB) method. The quality of the extracted genomic DNA was assessed using 1% agarose gel electrophoresis [17], and the concentration was quantified using a Qubit fluorimeter (Invitrogen Co., Carlsbad, CA, USA).
Table 1.
Wild E. angustifolia samples used in this study for whole-genome resequencing
| Population | Code ID | Sample ID | Group | Country | Province | City | Coordinates | Altitude (m) |
|---|---|---|---|---|---|---|---|---|
| E. angustifolia | R01 | 1583 | G1 | China | Gansu | Gulang | 37°29ʹ25ʺN; 102°54ʹ37ʺE | 2026.8 |
| R02 | 1619 | G1 | China | Gansu | Jinchang | 38°21ʹ25ʺN; 102°8ʹ24ʺE | 1704.6 | |
| R03 | 1684 | G1 | China | Gansu | Linze | 38°58ʹ1ʺN; 99°54ʹ52ʺE | 1900.3 | |
| R04 | 1697 | G2 | China | Gansu | Jia Yuguan | 39°31ʹ28ʺN; 98°51ʹ8ʺE | 1531.1 | |
| R05 | 1726 | G2 | China | Gansu | Jiuquan | 39°33ʹ37ʺN; 98°51ʹ37ʺE | 1457.4 | |
| R06 | 1758 | G2 | China | Gansu | Jiuquan | 39°41ʹ40ʺN; 98°35ʹ55ʺE | 1433.6 | |
| R07 | 1784 | G2 | China | Gansu | Jiuquan | 39°41ʹ44ʺN; 98°35ʹ57ʺE | 1473.0 | |
| R08 | 1811 | G2 | China | Gansu | Zhangye | 39°41ʹ42ʺN; 99°35ʹ33ʺE | 1450.1 | |
| R09 | 1857 | G3 | China | Gansu | Zhangye | 39°47ʹ14ʺN; 98°14ʹ36ʺE | 1695.0 | |
| R10 | 2063 | G3 | China | Gansu | Dunhuang | 40°7ʹ55ʺN; 94°54ʹ48ʺE | 1756.2 | |
| R11 | 2106 | G3 | China | Gansu | Guazhou | 40°22ʹ3ʺN; 99°54ʹ52ʺE | 1133.0 | |
| R12 | 2216 | G3 | China | Gansu | Lanzhou | 36°29ʹ21ʺN; 103°33ʹ22ʺE | 1902.8 |
The numbers (R01, R02, R03, R04, R05, R06, R07, R08, R09, R10, R11, R12) represent 12 wild E. angustifolia samples. G1, G2, and G3 were short for Group 1, 2, and 3, respectively
Genomic sequencing
The library was constructed after evaluating the quantity and quality of DNA samples. Two 270-bp paired-end libraries were prepared according to the Illumina protocol and sequenced on the Illumina HiSeq 2000 platform (Biomarker Technologies Co., Ltd., Beijing, China). Whole-genome sequencing was performed using the PacBio Sequel sequencer (Biomarker Technologies Co., Ltd., Beijing, China). Twelve 20-kb single-molecule real-time sequencing (SMRT) bell libraries were constructed using a PacBio DNA Template Prep Kit 1.0 (Vazyme Biotech Co., Ltd., Nanjing, Jiangsu, China). The templates were size-selected, and the BluePippin devices (Sage Science, Inc., Annoron, Beijing, China) were used to enrich long snippets (> 10 kb). The PacBio Sequel platform processed a total of 39 single cells per molecule in real time (SMRT).
Genome assembly and genome annotation
A kmer map of k = 19 was constructed using the two 270-bp libraries and used to evaluate genome size, repeat sequence ratio, and heterozygosity. The formula used was G = (N k-mer—Nerror_k-mer)/D, where G is genome size, N k-mer is the number of k-mers, Nerror_k-mer is the number of k-mers with the depth of 1, and D is the k-mer depth.
All low-quality sequences shorter than 500 bp were removed through the PacBio sequencing platform (Biomarker Technologies Co., Ltd., Beijing, China). The results of Canu’s [18] assembly and the wtdbg (https://github.com/ruanjue/wtdbg) assembly were merged using Quickmerge [19]. The homologous contigs of the merged genomes were determined by comparison of the two genome assemblies, and the short-read data were used to error correct the merged genome with Pilon [20].
State programs [21] were used to construct a database of repetitive sequences for the E. angustifolia genome. EVM v1.1.1 [20] software was then used to create a consensus repeat library. Genscan [22] and other programs were used for ab initio gene model prediction. GeMoMa v1.3.1 [23] was used for homology-based gene prediction. Hisat v2.0.4 [24] and Stringtie v1.2.3 [24] were used for assembly based on reference transcripts. TransDecoder v2.0 [25] and GeneMarkS-T v5.1 [26] were used for gene prediction. PASA v2.0.2 [27] was used for the prediction of unigene sequences based on transcriptome data without a reference assembly. Finally, EVM v1.1.1 was used to integrate the prediction results obtained by the above three methods. Based on the Rfam [28] database and miRBase [29] database and Infenal 1.1 [30] for rRNA and microRNA prediction, tRNAscan-SE v1.3.1 [31] was used to identify tRNAs. BLAST v2.2.31 [22] with an E-value cutoff of 1E-5 was used to align the predicted gene sequences with functional databases such as Gene Ontology (GO) [32] and Kyoto Encyclopedia of Genes and Genomes (KEGG) [33].
The Core Eukaryotic Gene Mapping Approach (CEGMA) v2.5 [34] database contains 458 conserved core genes in eukaryotes. CEGMA was used to evaluate the completeness of the final genome assembly. The embryophyta_odb9 database in BUSCO v2 [35] contains 1440 conserved core genes in terrestrial plants. We used BUSCO software to evaluate the completeness of the E. angustifolia genome assembly.
Hi-C analysis and assembly
Formaldehyde was used to fix the fresh E. angustifolia tissue samples. The DNA was digested with the restriction enzyme HindIII; after adding biotin-labeled bases, the repaired DNA was circularized, de-crosslinked, and purified, followed by fragmentation into 300–700-bp fragments. Streptavidin magnetic beads were used to capture the DNA fragments showing interaction relationships for library construction. Qubit2.0 (Life Technologies, CA, USA) and Agilent 2100 (Agilent Technologies) were used to detect the concentration and insert size of the library, and qPCR was used to quantify the effective concentration of the library.
The Illumina high-throughput sequencing platform was used to sequence the Hi-C library to produce a large number of high-quality reads. BWA (version: 0.7.10-r789; alignment mode: aln; other parameters default) [36] was used to compare the sequencing paired-end data with the sequences of the assembled genome. The contigs of the draft genome were split into simulated 500-kb contigs, and LACHESIS software [37] was used to cluster these contigs into groups.
Genome evolution and gene family expansion
We used the single-copy protein sequences of seven other species, Solanum lycopersicum, Arabidopsis thaliana (Linn) Heynh, Populus trichocarpa Torr & Gray, Glycine max (Linn) Merr, Oryza sativa Linn, Amborella trichopoda Baill, and Ziziphus jujuba M, to build phylogenetic trees using PHYML software [38]. The divergence times between E. angustifolia and the seven other sequenced species were estimated using MrBayes60 and the Mcmctree [39] programs implemented in the Phylogenetic Analysis by Maximum Likelihood (PAML) 61 software package. Calibration times were obtained from the TimeTree database (http://www.timetree.org/) with ‘(A. trichopoda, O. sativa) ‘< 199 > 173’, (A. thaliana, P. trichocarpa) ‘< 117 > 98’, (Z. jujuba, E. angustifolia) ‘< 117 > 79’, and (G. max, E. angustifolia) ‘< 113 > 89’. We then calculated the four-fold synonymous third-codon transversion (4DTv) values.
OrthoMCL [40] software was used to cluster the protein sequences of E. angustifolia and seven other sequenced species. CAFE [41] was used to analyze gene family contraction and expansion. Functional enrichment analysis was used to identify the function of genes over-represented in our genome assembly. GO enrichment analysis and KEGG annotation were performed using R scripts [42]. CodeML [43] was used in PAML to detect the rapidly evolving genes in E. angustifolia with a single copy shared by all species.
Genome resequencing
The code IDs of 12 wild E. angustifolia samples were R01–R12 (Table 1). The raw reads obtained by sequencing were filtered, low-quality reads with adapters were removed, and clean reads were obtained for subsequent information analysis. We used single nucleotide polymorphisms (SNPs) and small insertions and deletions (small Indels) to detect differences between our 12 wild E. angustifolia and the reference genome. The demographic history of 12 wild E. angustifolia was inferred using a hidden Markov model approach as implemented in the Pairwise Sequentially Markovian Coalescent (PSMC) model based on the SNP distribution [44]. We scaled results to real time, assuming 2 years per generation and a neutral mutation rate of 7.31 × 10–9 (CI 95% Poisson distribution: 5.20 × 10–9 ~ 8.00 × 10–9) per generation [45]. Genes with SNPs and Indels were analyzed by functional annotation. Polymorphic genes in the 12 wild E. angustifolia samples were compared with BLAST, GO, KEGG, and other functional databases to evaluate their function. MEGA X [46] software was used to construct phylogenetic trees with the neighbor-joining method. Admixture [47] software was used to analyze the group structure of the samples. The number of subgroups (K value) was preset to 1–10 for clustering; the clustering results were cross-validated, and the optimal number of clusters was determined according to the valley value of the cross-validation error rate. We artificially divided the 12 wild E. angustifolia species into three groups (G1–G3) based on factors such as latitude and longitude of the sampling sites (Table 1), and we designated the outgroup Z. jujuba as G0. PCA was performed on 12 wild E. angustifolia species and Z. jujuba.
Salinity experiment
A greenhouse experiment was conducted in 2018 at State Key Laboratory of Crop Biology, Shandong Agriculture University, Tai’an, China. In March 2018, we mixed 200 seeds (collected from the E. angustifolia field in the breeding experimental base of Shandong Agricultural University) and wet sand into woven bags and placed them in an outdoor leeward shelter for lamination. We prepared 45 clay pots with an outer diameter of 29 cm, an inner diameter of 25 cm, and a depth of 18 cm. Each pot contained 15 kg of sterilized soil [48]. Three seeds were planted in each pot after one-third of the seeds had germinated in April 2018.
Irrigation of all pots was carried out for 2 months using normal urban water (salinity of 0.6 dS m−1) at field capacity. When seedlings were well established two months after planting, four concentrations (electrical conductivity of 12 dS m−1, 16 dS m−1, 20 dS m−1, and 24 dS m−1) of NaCl analytical reagent (Keephway Technologies Corporation, Beijing, China) solution were used for the salt stress treatment; 5 pots of E. angustifolia were randomly selected for each treatment following the methods of Zeng et al. [49]. The other 25 pots were watered using normal urban water. Observations of the leaves of the four test groups were made every day to determine the level of salt stress (Fig. 2). Salinity stress continued for 10 days when the salinity toxicity in the leaves was over 20 dS m−1. Salinity treatment was initiated with 25 pots by NaCl analytical reagent (Keephway Technologies Corporation, Beijing, China) solution with 20 dS m−1. We randomly divided 25 pots into 5 treatments (Group 1, 2, 3, 4, 5). The leaves of E. angustifolia in Group 1 were collected immediately (control group including T01&T02&T03); the leaves in Group 2 were collected 1 h after treatment (salinity group including T04&T05&T06); the leaves in Group 3 were collected 6 h after treatment (salinity group including T07&T08&T09); the leaves in Group 4 were collected 12 h after treatment (salinity group including T10&T11&T12); and the leaves in Group 5 were collected 24 h after treatment (salinity group including T13&T14&T15). All fresh leaf samples were immediately dissected and submerged in RNA later® solution and stored in a − 80 °C freezer for RNA extraction. During the experiment, the maximum temperature was 31.0 ± 5.0 °C, and the minimum temperature was 19.5 ± 4.5 °C.
Fig. 2.
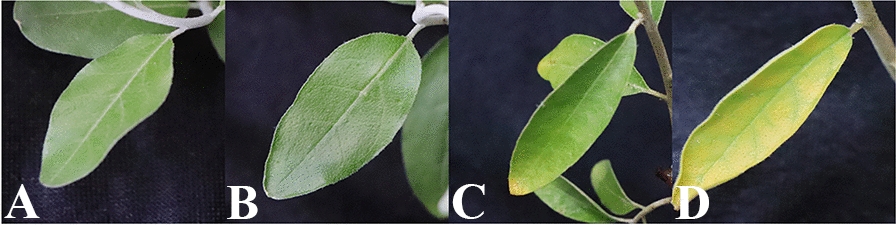
Treatment of E. angustifolia under salt stress for 10 days. The letters in pictures represent the electrical conductivity of different NaCl analytical reagent solutions. A 12 dS m−1; B 16 dS m−1; C 20 dS m−1; D 24 dS m−1
RNA extraction, library construction, and RNA sequencing
The total RNA of each sample was extracted from the leaves of E. angustifolia using the RNA plant Plus Reagent (Vazyme Biotech Co., Ltd., Nanjing, Jiangsu, China). The RNA integrity and concentration were assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA). mRNA was isolated by the NEBNext Poly (A) mRNA Magnetic Isolation Module (NEB, E7490). The cDNA library was constructed using the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, E7530) and NEBNext Multiplex Oligos for Illumina (NEB, E7500) per the manufacturer’s instructions. Briefly, the enriched mRNA was fragmented into approximately 200-nt RNA inserts, which were used to synthesize first-strand cDNA and second-strand cDNA. End-repair/dA-tail and adaptor ligation were performed on the double-stranded cDNA. The suitable fragments were isolated by Agencourt AMPure XP beads (Beckman Coulter, Inc.) and enriched by PCR amplification. Finally, the constructed cDNA libraries of E. angustifolia were sequenced on a flow cell using the Illumina HiSeq™ sequencing platform.
Transcriptome analysis using reference genome-based reads mapping
Low-quality reads, such as those containing adaptors, with unknown nucleotides > 5%, or Q20 < 20% (percentage of sequences with sequencing error rates < 1%), were removed using perl scripts. The clean reads that were filtered from the raw reads were mapped to the E. angustifolia genome (OGSv3.2) using Tophat2 [50]. The aligned records from the aligners in BAM/SAM format were further examined to remove potential duplicate molecules. Gene expression levels were estimated using FPKM values (fragments per kilobase of exon per million fragments mapped) by Cufflinks software [51].
Identification of differentially expressed genes
DESeq2 [52] and Q-value were used to identify differentially expressed genes between Group 1 and Groups 2, 3, 4, and 5. Differences in gene abundance between these samples were calculated based on the ratio of the FPKM values. The false discovery rate (FDR) control method was used to identify the P-value threshold to evaluate the significance of differences. Here, only genes with an absolute value of log2 ratio ≥ 2 and FDR significance score < 0.01 were used in subsequent analyses.
Sequence annotation
Differentially expressed genes were compared against various protein databases by BLASTX, including the National Center for Biotechnology Information (NCBI) non-redundant protein (Nr) database and Swiss-Prot database with a cut-off E-value of 10–5. Furthermore, genes were searched against the NCBI non-redundant nucleotide sequence (Nt) database using BLASTn with a cut-off E-value of 10–5. Genes were retrieved based on the best BLAST hit (highest score) along with their protein functional annotation.
The Nr BLAST results were imported into the Blast2 GO program [53]. GO annotations for the genes were obtained by Blast2GO. In this analysis, all of the annotated genes were mapped to GO terms in the database, and the number of genes associated with each term was counted. A perl script was then used to plot the GO functional classification for the unigenes to visualize the distribution of gene functions. The obtained annotations were enriched and refined using the TopGo (R package). The gene sequences were also aligned to the Clusters of Orthologous Group (COG) database to predict and classify functions [54]. KEGG pathways were assigned to the assembled sequences by perl scripts.
Results and discussion
Genome assembly of E. angustifolia
One band was visible following 1% agarose gel electrophoresis, and the concentration of DNA extracted was approximately 474.3 mg L−1. A total of 5,125,675 subreads were obtained by filtering low-quality data, and a total of 44.27 Gb raw PacBio sequel reads were obtained, with an average length of 8.64 kb (Additional file 1: Table S1). The subread N50 was 12,635 bp, and the average length was 8636 bp (Additional file 1: Table S2). After merging and correcting the assemblies, the final estimated genome size was 526.80 Mb, and the contig N50 was 12.60 Mb (Additional file 1: Table S3).
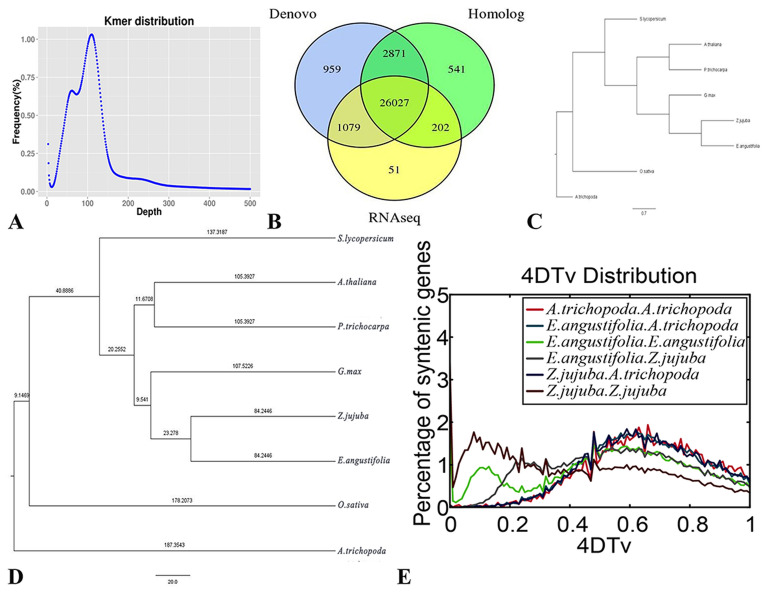
The total number of k-mers was 153,631,375,991, with a k-mers peak at a depth of 111 (Fig. 3A), and the final genome had a heterozygosity estimate of 1.47%. We constructed a specific repeat sequence database for the prediction of repeat sequences for specific species, and the prediction yielded approximately 263.44 Mb of repeats without overlap, accounting for 50.01% of the total length of repeat sequences (Additional file 1: Table S4). A total of 31,730 (Additional file 1: Table S5) protein-coding genes and 127 miRNAs were annotated by integrating different methods (Additional file 1: Table S6). There were 30,771 genes available for transcriptome sequencing, accounting for 96.98% of all genes (Fig. 3B). A total of 96.89% of the genes could be annotated to NR and other databases (Additional file 1: Table S7). Conserved CEGMA analyses indicated that 97.38% of the core protein-coding genes were recovered in our assembled genome (Additional file 1: Table S8). BUSCO revealed 1290 complete gene models out of 1,440 (89.58%); 23 fragmented and 184 complete genes were found in duplicate (Additional file 1: Table S9).
Fig. 3.
Genome assembly and genome evolution. A K-mer distribution of genome sequencing reads of E. angustifolia. K-mers (K = 19) were extracted from the paired-end library with an insert size of 270 bp. The total 23-mer count is 51,064,317,165. The peak 19-mer depth is 111, and the genome size was calculated as 51,064,317,165/111 = 456.24 Mb. B The integrated gene is derived from the distribution map of three prediction methods. C Phylogenetic tree of eight species D Estimation of the divergence time between E. angustifolia and other species. The number in each branch is the divergence time from the present (in million years ago). E Distribution of 4DTV values among Z. jujuba, E. angustifolia, and A. trichopoda genomes
Hi-C assisted assembly
We obtained 39.56 Gb clean reads, with a sequencing coverage of 75 × and Q30 ratio of > 95.46% (Additional file 1: Table S10). The ratio of reads mapped to the assembled genome was 90.68%, and the ratio of unique mapped read pairs was 61.13%, indicating that the Hi-C data were suitable for subsequent analysis (Additional file 1: Table S11). A total of 80.79 M pairs of reads from the genome were obtained in this experimental library. Among them, 72.98 M pairs were valid Hi-C data, accounting for 90.32% of the data from the genome, and the ratio of invalid interaction pairs was 9.68% (Additional file 1: Table S12).
E. angustifolia has 14 chromosomes. The genome was also visualized at the chromosome level. After Hi-C assembly, a total of 510.71 Mb of genomic sequences were mapped to the chromosomes, accounting for 96.94% of the total length of the sequences, and the corresponding number of sequences was 269, accounting for 45.83% of the total number of sequences. Among the sequences located on the chromosomes, the sequence length that could determine the order and direction of chromosomes was 473.91 Mb, accounting for 92.8% of the total length of the sequences located on the chromosomes, and the number of corresponding sequences was 104, accounting for 38.66% of the total number of sequences located on the chromosomes (Table 2).
Table 2.
The genomic sequence of E. angustifolia mapped to the chromosomes
| Group | Sequence count | Sequence length (bp) |
|---|---|---|
| Lachesis group 0 | 28 | 27,993,989 |
| Lachesis group 1 | 41 | 99,096,873 |
| Lachesis group 2 | 23 | 57,474,495 |
| Lachesis group 3 | 12 | 48,501,719 |
| Lachesis group 4 | 28 | 30,089,891 |
| Lachesis group 5 | 7 | 26,462,114 |
| Lachesis group 6 | 28 | 18,656,685 |
| Lachesis group 7 | 10 | 24,577,875 |
| Lachesis group 8 | 8 | 26,266,316 |
| Lachesis group 9 | 8 | 24,034,829 |
| Lachesis group 10 | 9 | 27,579,915 |
| Lachesis group 11 | 19 | 32,396,042 |
| Lachesis group 12 | 26 | 37,035,969 |
| Lachesis group 13 | 22 | 30,539,364 |
| Total sequences clustered (ratio %) | 269 (45.83) | 510,706,076 (96.94) |
| Total sequences ordered and oriented (ratio %) | 104 (38.66) | 473,912,606 (92.8) |
The statistics were all based on sequence lengths of 1 Kb or more
Genome evolution of E. angustifolia
Comparative genome analysis harnesses the power of sequence comparisons within and between species to infer evolutionary history and provide information on the function of specific DNA sequences [55]. The deep-level evolutionary history of E. angustifolia has not yet been clarified. In our study, a phylogenetic tree (Fig. 3C, D) revealed that the origins of A. trichopoda, O. sativa, and S. lycopersicum were dated to 187.35, 178.21, and 137.32 million years ago (Mya), respectively. The origins of A. thaliana in the family Brassicaceae and P. trichocarpa in the family Salicaceae were dated to 137.32 Mya; G. max (107.52 Mya) in the family Leguminosae was in the same branch as Z. jujuba in the family Rhamnaceae and E. angustifolia in the family Elaeagnaceae. The origins of Z. jujuba and E. angustifolia were both dated to 84.24 Mya. These findings are similar to those of Harkess et al. [56].
To further analyze the evolutionary divergence of E. angustifolia and other species, 4DTv rates were calculated (Fig. 3E). The highest 4DTV value of A. trichopoda was 0.68, indicating that no recent genome-wide duplication has occurred. The 4DTV value peak of Z. jujuba was 0.08, and that of E. angustifolia was 0.12, indicating that both Z. jujuba and E. angustifolia have undergone recent genome-wide replication. Both 4DTv values of the autopolyploid of Z. jujuba and E. angustifolia peaked at 0.48, indicating that the time of the polyploid splitting event of Z. jujuba and E. angustifolia was similar to the time before the divergence of Rosaceae and Elaeagnaceae. The orthologs between E. angustifolia and Z. jujuba and between E. angustifolia and A. trichopoda indicated that the 4DTv values peaked at 0.25 and 0.48, respectively, suggesting that the divergence between E. angustifolia and A. trichopoda occurred earlier.
Expanded gene families related to stress adaptation in E. angustifolia
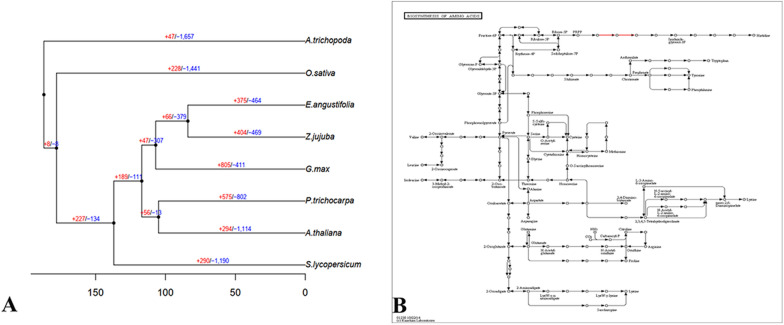
The identification of expanded gene families provides valuable insights into the biological innovation and adaptive evolution of E. angustifolia. A total of 27,553 of 31,730 genes of E. angustifolia could be classified into 13,309 gene families, of which 433 gene families were unique to E. angustifolia (Additional file 1: Table S13). Compared with Z. jujuba, E. angustifolia had fewer expanded gene families (375 vs. 404) and fewer contracted gene families (464 vs. 469) (Fig. 4A, Additional file 2: Table S14). Enrichment analysis of the 839 expanded and contracted gene families revealed that they were enriched in the pathways strictosidine synthase and xylanase inhibitor N-terminal. Strictosidine synthase has been detected in some major crops and is thought to make crops resistant to salt stress [57]. Xylanase inhibitor N-terminal is thought to be related to thermal stability [58]. The amplification of the above taxonomic genes might be related to the environmental conditions experienced by E. angustifolia. High temperature and drought have driven adaptive evolution in E. angustifolia [8] and increased evolutionary differences between E. angustifolia and other species.
Fig. 4.
Expanded gene families. A Expansion (red numbers) and contracted (blue numbers) gene families in different plants; “ + ” represents the number of gene families that have expanded on the node; and “−” represents the number of gene families that have contracted on the node. Black dots indicate a common ancestor. B Biosynthesis of the amino acid regulation pathway in the KEGG analysis of E. angustifolia
The KEGG results revealed a scattered functional distribution of these genes, and 1 variant gene of the 330 genes was related to the biosynthesis of amino acids (Additional file 1: Table S15). Further analysis of this pathway (Fig. 4B) revealed that the expression of the two pathways was increased during the synthesis of PRPP and imidazole-glycerol-3p. Previous studies indicate that both PRPP and imidazole-glycerol-3p are related to defense [59]. The GO (Additional file 1: Table S15) results showed that 28 variant genes were related to catalytic activity, 36 to metabolic process, and 29 to cellular process. Many variant genes were related to response to stimulus, which might indicate previous natural selection on E. angustifolia in response to harsh environments.
Drawing conclusions regarding the reduction in the adaptation of gene families was difficult given that we only analyzed gene families that were partly amplified from E. angustifolia. We identified 80 rapidly evolving genes in E. angustifolia through comparison of genes with 7 other species (Additional file 3: Table S16). The functional predictions showed that several genes were related to pseudouridine-metabolizing bifunctional protein and RNA pseudouridine synthase. Previous research has shown that pseudouridine-metabolizing bifunctional protein is related to human urinary tract pathogenic Escherichia coli, the principal agent of urinary tract infections in humans [60]. Genomic studies of humans and other mammals have shown that there is no gene encoding pseudouridine-metabolizing bifunctional protein, and the ability to metabolize pseudouridine has been lost [60]. This pattern of evolution in E. angustifolia might also be related to human and mammalian activities. The gene encoding carbon catabolite repressor protein 4 is also rapidly evolving in E. angustifolia. The carbon catabolite repressor 4-CCR4 associated factor1 complex is the major enzyme complex responsible for catalyzing mRNA deadenylation. The degradation of messenger RNA caused by poly (A) tail shortening (deadenylation) is a central mechanism for the biological regulation of gene expression. This is a mechanism that plants have evolved to reprogram gene expression to maintain homeostasis in constantly changing environments [61]. Previous studies investigating carbon catabolite repressor 4-CCR4 associated factor1 function in plants have focused on the role of these genes in mediating biotic stress responses, such as resistance to pathogens [62]. This study has shown that the regulation of the gene encoding carbon catabolite repressor protein 4 has evolved rapidly in E. angustifolia to improve its tolerance to abiotic and biotic stress [63]. This is also the first study showing that a gene regulating carbon catabolite repressor protein 4 has been discovered in E. angustifolia. Genes related to biotic and abiotic stress resistance were also among some of the 80 rapidly evolving genes, such as peptidyl-prolyl cis–trans isomerase [64].
Resequencing analysis of 12 wild E. angustifolia samples in Gansu Province, China
The resequencing analysis of 12 wild E. angustifolia samples in Gansu Province, China (Table 1, Additional file 6: Fig. S1) generated 86.58 Gp clean reads, with a Q20 of 96.64% and Q30 of 91.79%. The average coverage depth was 11 ×, and the genome coverage was 92.89% (Additional file 1: Tables S17–S19). In diploid genomes, runs of homozygosity are uninterrupted homozygous segments in the genome [65], and they can be used to quantify the level of inbreeding either in individuals or populations [66]. In this study, homozygous types accounted for most of the other 11 individuals except for R11 (Table 3). This suggests that hybridization might occur between different close relatives in nature, which is consistent with the observation that hybridization is common in plants [67]. We also detected 74,790 CDS and 1,791,719 genome-wide Indels (Additional file 1: Table S20).
Table 3.
Number of SNPs for wild E. angustifolia samples
| Code ID | Sample ID | SNP number | Transition | Transversion | Ti/Tv | Heterozygosity | Homozygosity | Het-ratio (%) |
|---|---|---|---|---|---|---|---|---|
| R01 | 1583 | 3,097,175 | 2,110,179 | 986,996 | 2.13 | 1,412,964 | 1,684,211 | 45.62 |
| R02 | 1619 | 3,223,761 | 2,197,723 | 1,026,038 | 2.14 | 1,492,655 | 1,731,106 | 46.30 |
| R03 | 1684 | 3,094,736 | 2,108,611 | 986,125 | 2.13 | 1,417,978 | 1,676,758 | 45.81 |
| R04 | 1697 | 3,021,352 | 2,057,327 | 964,025 | 2.13 | 1,417,892 | 1,603,460 | 46.92 |
| R05 | 1726 | 3,045,841 | 2,075,585 | 970,256 | 2.13 | 1,256,764 | 1,789,077 | 41.26 |
| R06 | 1758 | 3,193,315 | 2,178,336 | 1,014,979 | 2.14 | 1,442,262 | 1,751,053 | 45.16 |
| R07 | 1784 | 3,225,579 | 2,198,583 | 1,026,996 | 2.14 | 1,405,876 | 1,819,703 | 43.58 |
| R08 | 1811 | 3,108,956 | 2,117,042 | 991,914 | 2.13 | 1,537,532 | 1,571,424 | 49.45 |
| R09 | 1857 | 3,022,369 | 2,058,271 | 964,098 | 2.13 | 1,311,017 | 1,711,352 | 43.37 |
| R10 | 2063 | 3,028,547 | 2,060,171 | 968,376 | 2.12 | 1,379,687 | 1,648,860 | 45.55 |
| R11 | 2106 | 3,223,275 | 2,197,798 | 1,025,477 | 2.14 | 1,617,799 | 1,605,476 | 50.19 |
| R12 | 2216 | 3,115,254 | 2,122,083 | 993,171 | 2.13 | 1,369,366 | 1,745,888 | 43.95 |
In a diploid, if a certain SNP site on homologous chromosomes is the same base, the SNP site is referred to as a homozygous SNP site. If the SNP loci on homologous chromosomes contain different types of bases, the SNP locus is referred to as a heterozygous SNP locus. Greater numbers of homozygous SNPs correspond to greater differences between samples and the reference genome. Greater numbers of heterozygous SNPs correspond to higher degrees of heterozygosity
number number of SNPs detected; Transition number of SNPs of the conversion type; Transversion number of SNPs of transversion type; Ti/Tv the ratio of SNPs of conversion to transversion type; Heterozygosity number of heterozygous SNPs; Homozygosity number of homozygous SNPs; Het-ratio the ratio of heterozygous SNPs
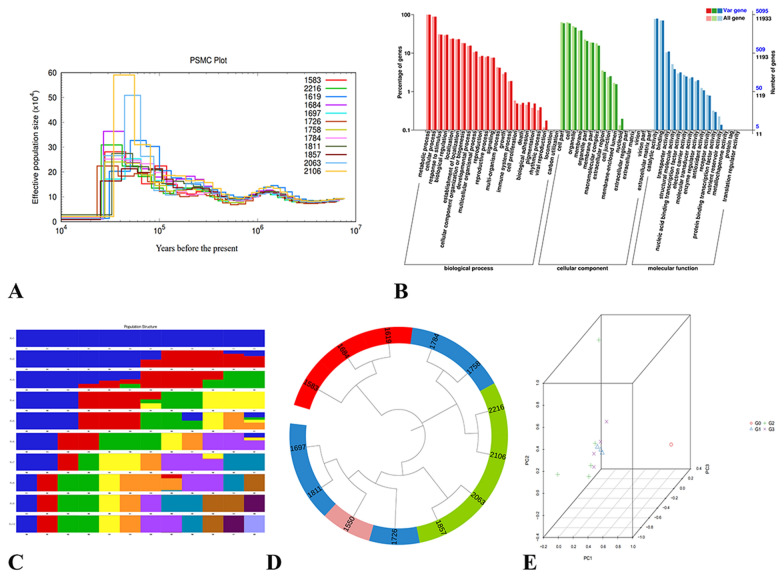
The Pairwise Sequentially Markovian Coalescent (PSMC) model was used to estimate the historical effective population size based on genome-wide data of 12 wild E. angustifolia species (Fig. 5A). In this study, there was no significant difference in the effective population size among 12 wild E. angustifolia species 3.0 Mya, which indicated that there were no evolutionary differences among the 12 E. angustifolia 3.0 Mya. This was also similar to the time of differentiation of A. trichopoda, the sister of all angiosperms [56]. From 3.0 Mya to 350 thousand years ago (Kya), the effective population size of 12 wild E. angustifolia changed slightly. Notably, from 1.5 Mya to 150 Kya, the species occured in Africa and showed no signs of divergence. The effective population size of the genome data of each race in this period was the same [44]. The effective population size of 12 wild E. angustifolia changed greatly from 350 to 23 Kya. The largest change was observed in the effective population size of wild E. angustifolia R11 (2106), especially in the period 55–35 Kya. The effective population size reached a maximum of approximately 60 × 104. At this time, the global temperature continued to rise, which might promote the rapid growth of the population of E. angustifolia [68]. At 23 Kya, the effective population size of 12 wild E. angustifolia decreased drastically, which corresponded to the last glacial period (the duration is approximately 26.5 to 19 Kya). The global temperature continued to decrease, and the glaciers at the poles and mountains began to extend to low latitudes and altitudes [69]. This drastic change in the environment might have led to a reduction in the effective population size of E. angustifolia.
Fig. 5.
Resequencing analysis. A Demographic history of 12 wild E. angustifolia samples. B GO annotation clustering of variant genes in sample R01. C Sample clustering results corresponding to each K value of Admixture. D Phylogenetic trees of 12 wild E. angustifolia samples and outgroup Z. jujuba. The numbers (1583, 1619, 1684, 1697, 1726, 1758, 1784, 1811, 1857, 2063, 2106, 2216) represent the Sample IDs of 12 wild E. angustifolia samples, and the number ‘1550’ represents the outgroup Z. jujuba. The color of G0, G1, G2, and G3 was purple, red, blue, and green. respectively. E Three-way PCA plot of E. angustifolia and Z. jujuba
We used the R01 result to evaluate the function of the mutated genes (Additional file 1: Tables S21, S22, Fig. 5B, C). Substitutions mainly occurred in DNA binding (GO:0003677), Cellular Component: Nucleus (GO:0005634), DNA binding (GO:0003677) related to cytochrome [70], and Cellular Component: Nucleus (GO:0005634) related to defensive T cells [71]. This variation might be related to the environmental conditions experienced by E. angustifolia, including water shortages at high altitude [72].
The results of the phylogenetic tree (Fig. 5D) and PCA (Fig. 5E) were not consistent with expectation, with the exception of G1. This might be related to climate change [73] and landform change [74]. Alternatively, differences in the altitude, latitude, and longitude of the sampling sites might explain this pattern (Table 1); various other factors might cause the results to deviate from expectation.
Genetic mechanisms underlying salt tolerance in E. angustifolia
To investigate the genetic mechanisms underlying salt tolerance in E. angustifolia, we performed a salinity experiment. We obtained 113.85 Gb clean reads. There were a total of 6.40 Gb clean reads for each sample, and the Q30 base percentage was 90.74% and higher (Additional file 1: Table S23). The clean reads of each sample were aligned to the reference genome, and the alignment efficiency ranged from 90.87 to 92.30% (Additional file 1: Table S24). Based on the comparison results, we carried out an alternative splicing prediction analysis and gene structure optimization analysis. We also compared the genome sequencing results of E. angustifolia and identified 4,404 new genes in the transcriptome of E. angustifolia (Additional file 4: Table S25), and we obtained 3953 functional annotations (Additional file 1: Table S26). We analyzed the GO pathways functions of the new genes (Additional file 5: Table S27). The results showed that 3391 genes were involved in the regulation of Molecular Function, 3121 genes were involved in the regulation of Biological Process, and 2613 genes were involved in regulation of Cellular Component. In Molecular Function, 256 genes were involved in regulating ATP binding (GO:0005524), 158 genes were involved in regulating metal ion binding (GO:0046872), and 131 genes were involved in regulating zinc ion binding (GO:0008270), 106 genes were involved in regulating the nucleic acid binding (GO:0003676), and 104 genes were involved in regulating the structural constituent of ribosome (GO:0003735). Previous studies have shown that AtABCG36-overexpressing plants are more resistant to drought and salt stress [75], overexpression of metal ion binding peptides–phytochelatins and metallothionein genes increases tolerance to stress [76], and overexpression of GsZFP1 (zinc ion binding) in alfalfa increases salt tolerance [77]. ‘Nucleic acid binding’ was the most significantly enriched GO term in the MF category for both Supreme and Parish up-regulated genes under salt treatment, suggesting that this process might play an important role in salt tolerance in both cultivars [78]; the structural constituent of ribosome has been shown to play an important role in salinity tolerance [79]. In Biological Process, there were 236 genes involved in the regulation of oxidation–reduction process (GO:0055114), 108 genes involved in the regulation of protein phosphorylation (GO:0006468), and 94 genes involved in the regulation of transcription, DNA-templated (GO:0006355). Previous transcriptome profiling experiments have indicated that oxidation–reduction processes, protein phosphorylation, and DNA-templated are related to salt tolerance in plants [80–83]. Xiong et al. [84] suggested that the homeostasis of the oxidation–reduction process is important for salt tolerance in plants. Hsu et al. [85] identified novel salt stress-responsive protein phosphorylation sites from membrane isolates of abiotic-stressed plants by membrane shaving followed by Zr4 + -IMAC enrichment, and the identified phosphorylation sites play an important role in the salt stress response in plants. Wu et al. [78] showed that genes that were down-regulated under salt treatment are involved in “regulation of transcription, DNA-templated.” In Cellular Component, there were 668 genes involved in the regulation of the integral component of membrane (GO:0016021), 201 genes involved in the regulation of nucleus (GO:0005634), 153 genes in the regulation of membrane (GO:0016020), 116 genes involved in regulating cytoplasm (GO:0005737), and 101 genes involved in regulating plasma membrane (GO:0005886). In conclusion, the GO pathways functions of the new genes in E. angustifolia showed that the functions of the new genes in the Molecular Function and Biological Process categories were mainly involved in the regulation of salt stress.
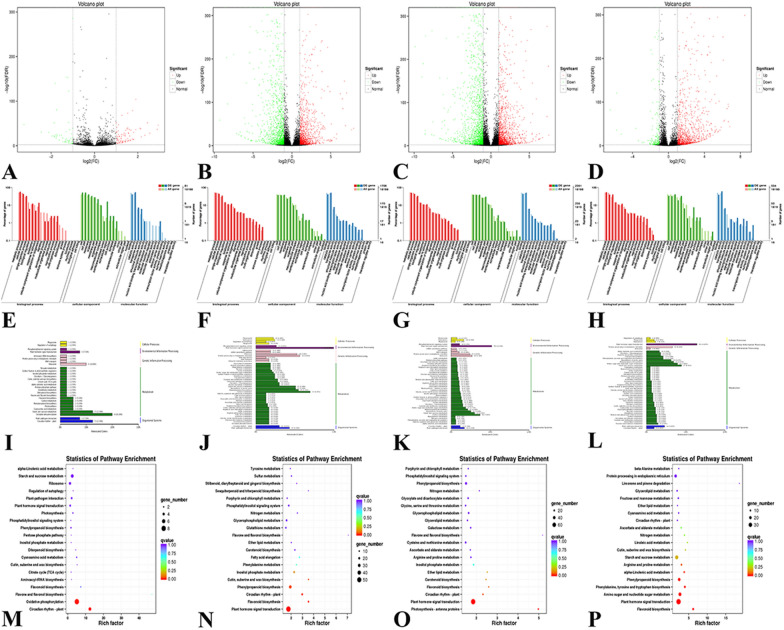
According to Additional file 1: Table S28 and Fig. 6A–D, the number of transcripts with significant expression differences between control Group 1 and experimental Group 2 was 137. The expression of 82 and 55 transcripts was significantly up-regulated and down-regulated, respectively. Similarly, the number of transcripts with significant expression differences between control Group 1 and experimental Group 3 was 2670, of which 1260 were up-regulated and 1410 were down-regulated. The number of transcripts with significant expression differences between control Group 1 and experimental Group 4 was 3619, of which 1668 were up-regulated and 1951 were down-regulated. The number of transcripts with significant expression differences between control Group 1 and experimental Group 5 was 1404, of which 1193 were up-regulated and 211 were down-regulated. A large number of differentially expressed transcripts in the two databases indicate that salt stress induces a large number of gene expression changes in E. angustifolia, which reflects the complexity of the mechanism underlying the response of E. angustifolia to salt stress.
Fig. 6.
Genetic mechanisms. A–D Volcano map of differentially expressed transcripts. A, Group 1 vs. Group 2; B, Group 1 vs. Group 3; C, Group 1 vs. Group 4; D, Group 1 vs. Group 5. E–H GO analysis of the differentially expressed transcripts. E, Group 1 vs. Group 2; F, Group 1 vs. Group 3; G, Group 1 vs. Group 4; H, Group 1 vs. Group 5. I–L KEGG classification of differently-expressed gene transcripts. I, Group 1 vs. Group 2; J, Group 1 vs. Group 3; K, Group 1 vs. Group 4; L, Group 1 vs. Group 5. M–P Enrichment and distribution map of the KEGG pathway of differentially expressed genes. M, Group 1 vs. Group 2; N, Group 1 vs. Group 3; O, Group 1 vs. Group 4; P, Group 1 vs. Group 5
The GO pathway annotations (Fig. 6E–H) of the four experimental groups compared with the control group revealed a pattern consistent with the differential expression among the four experimental groups, and salt stress had a significant effect on proteins in the functional categories Biological Process, Molecular Function, and Cellular Component [86]; the effects on different physiological processes also differed. After salt stress, metabolic process, cellular process, and single-organism process were greatly affected within Biological Process, and binding and catalytic activity were greatly affected within Molecular Function. Salt stress affected the expression of related genes during growth and development as well as the activity of certain proteins [87], which had a greater impact on the growth and development of E. angustifolia.
We also performed KEGG pathway analysis of differentially expressed gene transcripts (Fig. 6I–L). The metabolic process and signal pathways that regulate and change under salt stress in plant bodies could be clearly observed according to the KEGG pathway analysis. Comparison of the experimental group and control group revealed that these changes were mostly concentrated in sugar, amino acid metabolism, and plant hormone signal transduction. Salt stress had obvious effects on Environmental Information Process, Genetic Information Processing, and Metabolism and Organismal Systems. After 1 h of salt stress, there were significant changes in oxidative phosphorylation, ribosome, and starch and sucrose metabolism, and KEGG pathways (Fig. 6M) were enriched in oxidative phosphorylation and circadian rhythm-plant. After 6 h and 12 h of salt stress, there were significant changes in plant hormone signal transduction, protein processing in endoplasmic reticulum, biosynthesis of amino acids, phenylpropanoid biosynthesis, carbon metabolism, and plant-pathogen interaction, and KEGG pathways (Fig. 6N, O) were enriched in plant hormone signal transduction. After 24 h of salt stress, there were significant changes in amino sugar and nucleotide sugar metabolism and other metabolic processes, and KEGG pathways (Fig. 6P) were enriched in plant hormone signal transduction.
Several studies have shown that partial KEGG pathway changes are related to salt resistance. Circadian rhythms synchronize intracellular calcium dynamics and ATP production for growth [88] and have a large effect on plant immunity during plant–pathogen interactions [89]. Oxidative phosphorylation plays a role in plant salt tolerance by coordinating ROS scavenging pathways to regulate intracellular ROS levels, prevent cell damage, and control ROS signal transduction [90]. The significance of compatible solutes such as sugars, amino acids, and tertiary amines in salt stress has been well documented [91]. They not only provide an essential source of energy and nutrients for plants under salt stress but also act as osmotic adjustment substances to balance the osmotic potential appended by high salinity [92]. The accumulation of soluble sugars and sucrose can improve salt tolerance [93]. The loss of integrity of the ribosome through the removal of a putative ribosome maturation factor or a ribosomal protein confers salt tolerance to E. coli cells [94]. The endoplasmic reticulum is associated with salt tolerance in tomato [95]. Metabolic adaptation is crucial for abiotic stress resistance in plants, and the accumulation of specific amino acids has been suggested to increase tolerance to salt [96]. Enrichment of carbon metabolism and the biosynthesis of amino acids suggests that the synthesis of compatible solutes is fortified to alleviate osmotic stress in plant seedlings under salt treatment [26]. Variation in gene expression under salt stress is also regulated by phytohormones [26]. Changes in the transcription level of hormone genes affect drought and salt stress in plants [97]. de Bruxelles et al. [98] showed that hormones are responsible for changes in the expression of salt-induced genes.
Conclusions
In this study, the genome of E. angustifolia L. was obtained using PacBio technology and Hi-C assisted assembly technology. We estimated the origin of E. angustifolia and evaluated its evolutionary relationships with 8 other species. We used comparative genomics to study the adaptive evolution of E. angustifolia in Gansu, China. Genes and pathways of salt resistance of E. angustifolia were identified through transcriptome analysis. Our findings could be used to aid future comparative genomic analyses of E. angustifolia and enhance our understanding of the response of E. angustifolia to drought, salt, cold, and wind stress. Our findings also have implications for the planting of E. angustifolia and recovery from global land desertification. Several limitations of our study require consideration, given that the findings might be affected by the analytical method used, test conditions, and other environmental conditions; follow-up studies are needed to confirm our findings.
Supplementary Information
Additional file 1: Table S1. Filtering raw data of Pac-bio sequencing. Table S2. Length distribution of subreads of Pac-bio sequencing. Table S3. Genome assembly evaluation statistics. Table S4. Repeating sequence statistics. Table S5. Gene information statistics. Table S6. Non-coding RNA information. Table S7. Gene function annotation statistics. Table S8. Statistics of the completeness of the assembled genome. Table S9. Statistics of the BUSCO of the assembled genome. Table S10. Sequencing data volume statistics. Table S11. Clean data and genome alignment results statistics. Table S12. Hi-C sequencing data validation. Table S13. Classification statistics of gene families. Table S15. Function prediction of E. angustifolia. Table S17. Evaluation statistics of sequencing data of wild E. angustifolia samples. Table S18. Comparison results of wild E. angustifolia samples. Table S19. Coverage depth and coverage ratio statistics of wild E. angustifolia samples. Table S20. InDel statistics of whole genome and coding region of wild E. angustifolia samples. Table S21. Statistical table of variation genes of wild E. angustifolia samples. Table S22. Annotated list of some variant genes in sample R01. Table S23. Statistics of transcriptome sequencing data. Table S24. Results of the comparison between the sequencing transcriptome samples and the genome data. Table S26. Statistics of functional annotation results of new genes. Table S28. Statistics on the number of different genes in the transcription.
Additional file 2: Table S14. Functional gene categories enriched for E. angustifolia expansion and contraction families.
Additional file 3: Table S16. 80 rapidly evolving genes in E. angustifolia.
Additional file 4: Table S25. New genes discovered in E. angustifolia partially.
Additional file 5: Table S27. Go pathways functions of new genes.
Additional file 6: Figure S1. 12 wild E. angustifolia samples.
Acknowledgements
Not applicable.
Author contributions
YM and XShen planned and designed the research; YM, XLCui, HW, XQ, YL, YY, XSu, JT and FW performed experiments, conducted fieldwork, analyzed data; YM, FM, ND, DZ, YH, WW, SW, XLChen, ZM, XSC and XShen wrote the manuscript. Every author contributed equally. All authors read and approved the final manuscript.
Funding
This study was supported by Project supported by the National Natural Science Foundation of China (32072520); Fruit innovation team project of Shandong Province (CN) (SDAIT-06-07); Natural Science Foundation of Shandong Province (CN) (ZR2020MC132) and Industrialization project of improved varieties in Shandong Province (CN) (2019LZGC007).
Availability of data and materials
This Whole Genome Shotgun project has been deposited in NCBI. Raw sequencing reads and genome assembly are available at GenBank as BioProject PRJNA647537 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA647537?reviewer=5dse95jg6ae9tao2s0ettacevv). Raw sequencing data (Hi-C, trans data, survey, PB) have been deposited in SRA (Sequence Read Archive) database as SRR12563589–SRR12563593 [99–102]. Resequencing has been deposited in SRA (Sequence Read Archive) database as SRR12578558–SRR12578569 [103]. Transcriptome has been deposited in SRA (Sequence Read Archive) database as SRR12569921–SRR12569935 [104]. Gene annotation data of E. angustifolia genome has been deposited in FigShare (https://doi.org/10.6084/m9.figshare.12957110.v1).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UN News Centre. World population projected to reach 9.6 billion by 2050—UN report. 2013. http://www.un.org/en/development/desa/news/population/2015-report.html. Accessed 30 Jul 2015.
- 2.Wild A. Soils, land and food: managing the land during the twenty-first century. Cambridge: Cambridge University Press; 2003. p. 256. [Google Scholar]
- 3.FAO . World Agricultural Center, FAOSTAT agricultural statistic data-base gateway. Rome: FAO; 2014. [Google Scholar]
- 4.Hossain MS. Present scenario of global salt affected soils, its management and importance of salinity research. Int Res J Biol Sci. 2019;1:1–3. [Google Scholar]
- 5.Gupta B, Huang BR. Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genom. 2014;2014:701596. doi: 10.1155/2014/701596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao Y, Zhao G, Li T, Zhou X, Li J. Soil salinization of cultivated land in Shandong Province, China—dynamics during the past 40 years. Land Degrad Dev. 2019;30:426–436. doi: 10.1002/ldr.3236. [DOI] [Google Scholar]
- 7.Stoto MA. Population health measurement: applying performance measurement concepts in population health settings. EGEMS. 2014;2:1132. doi: 10.13063/2327-9214.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang BS, Qu HY, Ma J, Sun XL, Wang D, Zheng QS. Protective effects of elaeagnus angustifolia leaf extract against myocardial ischemia/reperfusion injury in isolated rat heart. J Chem. 2014;3:693573. doi: 10.1155/2014/693573. [DOI] [Google Scholar]
- 9.Vitas AI, Garcia-Jalon VAEI. Occurrence of Listeria monocytogenes in fresh and processed foods in Navarra (Spain) Int J Food Microbiol. 2004;90:349–356. doi: 10.1016/S0168-1605(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 10.Klich MG. Leaf variations in Elaeagnus angustifolia related to environmental heterogeneity. Environ Exp Bot. 2000;44:171–183. doi: 10.1016/S0098-8472(00)00056-3. [DOI] [PubMed] [Google Scholar]
- 11.Li LX, Zhu TT, Liu J, Zhao C, Li YY, Chen M. An orthogonal test of the effect of NO3−, PO43−, K+, and Ca2+ on the growth and ion absorption of Elaeagnus angustifolia L. seedlings under salt stress. Acta Physiol Plant. 2019;41:179. doi: 10.1007/s11738-019-2969-8. [DOI] [Google Scholar]
- 12.Caru M, Mosquera G, Bravo L, Guevara R, Sepulveda D, Cabello A. Infectivity and effectivity of Frankia strains from the Rhamnaceae family on different actinorhizal plants. Plant Soil. 2003;251:219–225. doi: 10.1023/A:1023060109632. [DOI] [Google Scholar]
- 13.Zhang X, Huang G, Bian X, Zhao Q. Effects of root interaction and nitrogen fertilization on the chlorophyll content, root activity, photosynthetic characteristics of intercropped soybean and microbial quantity in the rhizosphere. Plant Soil Environ. 2013;59:80–8. doi: 10.17221/613/2012-PSE. [DOI] [Google Scholar]
- 14.Zhang X, Liu L, Chen B, Qin Z, Xiao Y, Zhang Y, Yao R, Liu H, Yang H. Progress in understanding the physiological and molecular responses of populus to salt stress. Int J Mol Sci. 2019;20:1312. doi: 10.3390/ijms20061312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghodhbane-Gtari F, Swanson E, Gueddou A, Simpson S, Morris K, Thomas WK, Gtari M, Tisa LS. Draft genome sequence for Frankia sp. strain BMG5.11, a nitrogen-fixing bacterium isolated from Elaeagnus angustifolia. Microbiol Resour Ann. 2020 doi: 10.1128/MRA.00824-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, Li JP, Yuan F, Yang Z, Wang BS, Chen M. Transcriptome profiling of genes involved in photosynthesis in Elaeagnus angustifolia L. under salt stress. Photosynthetica. 2018;56:998–1009. doi: 10.1007/s11099-018-0824-6. [DOI] [Google Scholar]
- 17.Aboul-Maaty NAF, Oraby HAS. Extraction of high-quality genomic DNA from different plant orders applying a modified CTAB-based method. Bull Natl Res Cent. 2019;43:25. doi: 10.1186/s42269-019-0066-1. [DOI] [Google Scholar]
- 18.Wicker T, Sabot F, Hua-Van A, Bennetzen JL, Capy P, Chalhoub B, Flavell A, Leroy P, Morgante M, Panaud O. Aunified classification system for eukaryotic transposable elements. Nat Rev Genet. 2007;8:973–982. doi: 10.1038/nrg2165. [DOI] [PubMed] [Google Scholar]
- 19.Tarailo-Graovac M, Chen N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr Protoc Bioinform. 2009 doi: 10.1002/0471250953.bi0410s25. [DOI] [PubMed] [Google Scholar]
- 20.Haas BJ, Salzberg SL, Zhu W, Pertea M, Allen JE, Orvis J, White O, Buell CR, Wortman JR. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 2008;9:R7. doi: 10.1186/gb-2008-9-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Z, Wang H. LTR_FINDER: an efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res. 2007;35:265–268. doi: 10.1093/nar/gkm286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J Mol Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- 23.Keilwagen J, Hartung F, Grau J. GeMoMa: homology-based gene prediction utilizing intron position conservation and RNA-seq data. Methods Mol Biol. 2019;1962:161–177. doi: 10.1007/978-1-4939-9173-0_9. [DOI] [PubMed] [Google Scholar]
- 24.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JP, Tian SL, Sun XL, Cheng XC, Duan NB, Tao JH, Shen GN. Construction of pseudomolecules for the Chinese chestnut (Castanea mollissima) genome. G3 Genes Genom Genet. 2020;10:3565–74. doi: 10.1534/g3.120.401532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang S, Lomsadze A, Borodovsky M. Identification of protein coding regions in RNA transcripts. Nucleic Acids Res. 2015;43:e78. doi: 10.1093/nar/gkv227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell MA, Haas BJ, Hamilton JP, Mount SM, Buell CR. Comprehensive analysis of alternative splicing in rice and comparative analyses with Arabidopsis. BMC Genom. 2006;7:327. doi: 10.1186/1471-2164-7-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffiths-Jones S, Moxon S, Marshall M, Khanna A, Eddy SR, Bateman A. Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res. 2005;33:121–124. doi: 10.1093/nar/gki081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:140–144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nawrocki EP, Eddy SR. Infernal 1.1: 100-fold faster RNAhomology searches. Bioinformatics. 2013;29:2933–5. doi: 10.1093/bioinformatics/btt509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNAgenes in genomic sequence. Nucleic Acids Res. 1997;25:0955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanco E, Parra G, Guigó R. Using geneid to identify genes. Curr Protoc Bioinform. 2007 doi: 10.1002/0471250953.bi0403s18. [DOI] [PubMed] [Google Scholar]
- 33.Korf I. Gene finding in novel genomes. BMC Bioinform. 2004;5:59. doi: 10.1186/1471-2105-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parra G, Bradnam K, Korf I. CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics. 2007;23:1061–1067. doi: 10.1093/bioinformatics/btm071. [DOI] [PubMed] [Google Scholar]
- 35.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burton JN, Adey A, Patwardhan RP, Qiu R, Kitzman JO, Shendure J. Chromosome-scale scaffolding of de novo genome assemblies based on chromatin interactions. Nat Biotechnol. 2013;31:1119–1125. doi: 10.1038/nbt.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 39.Dekker J, Marti-Renom MA, Mirny LA. Exploring the three-dimensional organization of genomes: interpreting chromatin interaction data. Nat Rev Genet. 2013;14:390–403. doi: 10.1038/nrg3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Stoeckert CJ, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Bie T, Cristianini N, Demuth JP, Hahn MW. CAFE: a computational tool for the study of gene family evolution. Bioinformatics. 2006;22:1269–1271. doi: 10.1093/bioinformatics/btl097. [DOI] [PubMed] [Google Scholar]
- 42.Götz S, Arnold R, Sebastián-León P, Martín-Rodríguez S, Tischler P, Jehl MA, Dopazo J, Rattei T, Conesa A. B2G-FAR, a species-centered GO annotation repository. Bioinformatics. 2011;27:919–924. doi: 10.1093/bioinformatics/btr059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schabauer H, Valle M, Pacher C, Stockinger H, Stamatakis A, Robinson-Rechavi M, Yang ZH, Salamin N. SlimCodeML: an optimized version of CodeML for the branch-site model. Piscataway: 2012 IEEE 26th International Parallel and Distributed Processing Symposium Workshops & PhD Forum; 2012. pp. 706–14. [Google Scholar]
- 44.Li H, Durbin R. Inference of human population history from individual whole-genome sequences. Nature. 2011;475:493–496. doi: 10.1038/nature10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krasovec M, Chester M, Ridout K, Filatov DA. The mutation rate and the age of the sex chromosomes in Silene latifolia. Curr Biol. 2018;28:1832–1838. doi: 10.1016/j.cub.2018.04.069. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1007/s00262-014-1555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang YF, Fu FY, Li JJ, Wang GS, Wu MM, Zhan J, Chen XS, Mao ZQ. Effects of seaweed fertilizer on the growth of Malus hupehensis Rehd. seedlings, soil enzyme activities and fungal communities under replant condition. Eur J Soil Biol. 2016;75:1–7. doi: 10.1016/j.ejsobi.2016.04.003. [DOI] [Google Scholar]
- 49.Zeng L, Tu XL, Dai H, Han FM, Lu BS, Wang MS, Nanaei HA, Tajabadipour A, Mansouri M, Li XL, Ji LL, Irwin DM, Zhou H, Liu M, Zheng HK, Esmailizadeh A, Wu DD. Whole genomes and transcriptomes reveal adaptation and domestication of pistachio. BMC Genome Biol. 2019;20:79. doi: 10.1186/s13059-019-1686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 54.Tatusov RL, Galperin MY, Natale DA, Koonin EV. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28:33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li F, Bian L, Ge J, Han F, Liu Z, Li X, Liu Y, Lin Z, Shi H, Liu C, Chang Q, Lu B, Zhang S, Hu J, Xu D, Shao C, Chen S. Chromosome-level genome assembly of the East Asian common octopus (Octopus sinensis) using PacBio sequencing and Hi-C technology. Mol Ecol Resour. 2020;20:1572–1582. doi: 10.1111/1755-0998. [DOI] [PubMed] [Google Scholar]
- 56.Harkess A, Kochko AD, Chanderbali A, Meyers BC, Walts B, Fogliani B, Guo CC, Zheng CF, de Pamphilis CW, Job C, Sankoff D, Job D, Soltis DE, Paoli ED, Ibarra-Laclette E, Lyons E, Wafula E, Chen F, Li GL, Tang HB, Ma H, Kong HZ, Estill J, Leebens-Mack J, Burnette JM, Talag J, Palmer JD, Harhotl J, Xue JY, Chen JQ, Liu J, Zhai JX, Park JS, Der JP, Acosta JJ, Liu K, Li L, Carretero-Paulet L, Rajjou L, Herrera-Estrella L, Tomsho L, Kirst M, Villegente M, Axtell M, Altman NS, Farrell NP, Soltis PS, Ralph P, Ulvskov P, Bruenn RA, Wing RA, Sederoff R, Detemann RO, Ammi‘Raju’ JSS, Shanid S, Kim ST, Ayyampalayam S, Arikit S, Pissis SP, Chamala S, Wanke S, Schuster SC, Rounsley SD, Wessler SC, Lan TY, Chang TH, Yeh TF, Burtet-Sarramegna V, Poncet V, Albert VA, Chiang V, Barbazuk WB, Mei WB, Yu XX, Zhang XY, Qi XS, Sun YH, Jiao YN. The amborella genome and the evolution of flowering plants. Science. 2013;342:1241089. doi: 10.1126/science.1241089. [DOI] [PubMed] [Google Scholar]
- 57.Aghaei K, Komatsu S. Crop and medicinal plants proteomics in response to salt stress. Front Plant Sci. 2013;4:8. doi: 10.3389/fpls.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin X, Li JF, Wang JQ, Tang CD, Wu MC. Enhanced thermostability of a mesophilic xylanase by N-terminal replacement designed by molecular dynamics simulation. J Sci Food Agr. 2013;93:3016–3023. doi: 10.1002/jsfa.6134. [DOI] [PubMed] [Google Scholar]
- 59.Li XP, Fernández-Ortuño D, Grabke A, Schnabel G. Resistance to fludioxonil in Botrytis cinerea isolates from blackberry and strawberry. Phytopathology. 2014;104:724–732. doi: 10.1094/PHYTO-11-13-0308-R. [DOI] [PubMed] [Google Scholar]
- 60.Preumont A, Snoussi K, Stroobant V, Collet JF, Schaftingen EV. Molecular identification of pseudouridine-metabolizing enzymes. J Biol Chem. 2008;283:25238–25246. doi: 10.1074/jbc.M804122200. [DOI] [PubMed] [Google Scholar]
- 61.Walley JW, Kelley DR, Nestorova G, Hirschberg DL, Dehesh K. Arabidopsis deadenylases AtCAF1a and AtCAF1b play overlapping and distinct roles in mediating environmental stress responses. Plant Physiol. 2010;152:866–875. doi: 10.1104/pp.109.149005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarowar S, Oh HW, Cho HS, Baek KH, Seong ES, Joung YH, Choi GJ, Lee S, Choi D. Capsicum annuum CCR4-associated factor CaCAF1 is necessary for plant development and defence response. Plant J. 2007;51:792–802. doi: 10.1111/j.1365-313X.2007.03174.x. [DOI] [PubMed] [Google Scholar]
- 63.Zhang L, Li MH, Li QQ, Chen CQ, Qu M, Li MY, Wang Y, Shen XH. The catabolite repressor/activator Cra is a bridge connecting carbon metabolism and host colonization in the plant drought resistance-promoting Bacterium Pantoea alhagi LTYR-11Z. Appl Environ Microb. 2018;84:e00054–e118. doi: 10.1128/AEM.00054-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin WL, Bonin M, Boden A, Wieduwild R, Murawala P, Wermke M, Andrade H, Bornhäuser M, Zhang YX. Peptidyl prolyl cis/trans isomerase activity on the cell surface correlates with extracellular matrix development. Commun Biol. 2019;2:58. doi: 10.1038/s42003-019-0315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie R, Shi L, Liu J, Deng T, Wang L, Liu Y, Zhao F. Genome-wide scan for runs of homozygosity identifies candidate genes in three pig breeds. Animals. 2019;9:518. doi: 10.3390/ani9080518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lemes RB, Nunes K, Carnavalli JEP, Kimura L, Mingroni-Netto RC, Meyer D, Otto PA. Inbreeding estimates in human populations: applying new approaches to an admixed Brazilian isolate. PLoS ONE. 2018;13:e0196360. doi: 10.1371/journal.pone.0196360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vigueira CC, Olsen KM, Caicedo AL. The red queen in the corn: agricultural weeds as models of rapid adaptive evolution. Heredity. 2012;110:303–311. doi: 10.1038/hdy.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson RN, O’Meally D, Chen Z, Etherington GJ, Ho SYW, Nash WJ, Grueber CE, Cheng YY, Whittington CM, Dennison S, Peel E, Haerty W, O’Neill RJ, Colgan D, Russell TL, Alquezar-Planas DE, Attenbrow V, Bragg JG, Brandies PA, Chong AYY, Deakin JE, Palma FD, Duda Z, Eldridge MDB, Ewart KM, Hogg CJ, Frankham GJ, Georges A, Gillett AK, Govendir M, Greenwood AD, Hayakawa T, Helgen KM, Hobbs M, Holleley CE, Heider TN, Jones EA, King A, Madden D, Graves JAM, Morris KM, Neaves LE, Patel HR, Polkinghorne A, Renfree MB, Robin C, Salinas R, Tsangaras K, Waters PD, Waters SA, Wright B, Wilkins MR, Timms P, Belov K. Adaptation and conservation insights from the koala genome. Nat Genet. 2018;50:1102–1111. doi: 10.1038/s41588-018-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chase BM, Meadows ME. Late Quaternary dynamics of southern Africa’s winter rainfall zone. Earth Sci Rev. 2007;84:103–138. doi: 10.1016/j.earscirev.2007.06.002. [DOI] [Google Scholar]
- 70.Yang WH, Hammes SR. Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5. J Biol Chem. 2005;280:10196–10201. doi: 10.1074/jbc.M411886200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akeus P, Langenes V, von Mentzer A, Yrlid U, Sjöling Å, Saksena P, Raghavan S, Järbrink M. Altered chemokine production and accumulation of regulatory T cells in intestinal adenomas of APCMin/+ mice. Cancer Immunol Immun. 2014;63:807–819. doi: 10.1007/s00262-014-1555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zalesny RSJ, Stange CM, Rirr BA. Survival, height growth, and phytoextraction potential of hybrid poplar and Russian olive (Elaeagnus angustifolia L.) established on soils varying in salinity in North Dakota, USA. Forests. 2019;10:672. doi: 10.3390/f10080672. [DOI] [Google Scholar]
- 73.Xiang L, Gao X, Peng YH, Liang J. Coupling the occurrence of correlative plant species to predict the habitat suitability for lesser white-fronted goose (Anser erythropus) under climate change: a case study in the middle and lower reaches of the Yangtze River. J Res Ecol. 2020;11:140–149. doi: 10.5814/j.issn.1674-764x.2020.02.002. [DOI] [Google Scholar]
- 74.Rishworth GM, Cawthra HC, Dodd C, Perissinotto R. Peritidal stromatolites as indicators of stepping-stone freshwater resources on the Palaeo-Agulhas Plain landscape. Quat Sci Rev. 2020;235:105704. doi: 10.1016/j.quascirev.2019.03.026. [DOI] [Google Scholar]
- 75.Kim DY, Jin JY, Alejandro S, Martinoia E, Lee Y. Overexpression of AtABCG36 improves drought and salt stress resistance in Arabidopsis. Physiol Plantarum. 2010;139:170–180. doi: 10.1111/j.1399-3054.2010.01353.x. [DOI] [PubMed] [Google Scholar]
- 76.Xu H, Song P, Gu WB, Yang ZR. Effects of heavy metals on production of thiol compounds and antioxidant enzymes in Agaricusbisporus. Ecotox Environ Safe. 2011;74:1685–1692. doi: 10.1016/j.ecoenv.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 77.Tang LL, Cai H, Ji W, Luo X, Wang ZY, Wu J, Wang XD, Cui L, Wang Y, Zhu YM, Bai X. Overexpression of GsZFP1 enhances salt and drought tolerance in transgenic alfalfa (Medicago sativa L.) Plant Physiol Bioch. 2013;71:22–30. doi: 10.1016/j.plaphy.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 78.Wu PP, Cogill S, Qiu YJ, Li ZG, Zhou M, Hu Q, Chang ZH, Noorai RE, Xia XX, Saski C, Raymer P, Luo H. Comparative transcriptome profiling provides insights into plant salt tolerance in seashore paspalum (Paspalum vaginatum) BMC Genom. 2020;21:131. doi: 10.1186/s12864-020-6508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shabala S, Wu H, Bose J. Salt stress sensing and early signalling events in plant roots: current knowledge and hypothesis. Plant Sci. 2015;241:109–119. doi: 10.1016/j.plantsci.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 80.Bushman BS, Amundsen KL, Warnke SE, Robins JG, Johnson PG. Transcriptome profiling of Kentucky bluegrass (Poa pratensis L.) accessions in response to salt stress. BMC Genom. 2016;17:48. doi: 10.1186/s12864-016-2379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang J, Jiang DC, Liu BB, Luo WC, Lu J, Ma T, Wan DS. Transcriptome dynamics of a desert poplar (Populus pruinosa) in response to continuous salinity stress. Plant Cell Rep. 2014;33:1565–1579. doi: 10.1007/s00299-014-1638-z. [DOI] [PubMed] [Google Scholar]
- 82.Zhao S, Zhang Q, Liu M, Zhou H, Ma C, Wang P. Regulation of plant responses to salt stress. Int J Mol Sci. 2021;22:4609. doi: 10.3390/ijms22094609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng Z, He SP, Gong WF, Sun JL, Pan ZE, Xu FF, Lu YL, Du XM. Comprehensive analysis of differentially expressed genes and transcriptional regulation induced by salt stress in two contrasting cotton genotypes. BMC Genom. 2014;15:760. doi: 10.1186/1471-2164-15-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiong HC, Guo HJ, Xie YD, Zhao LS, Gu JY, Zhao SR, Li JH, Liu LX. RNAseq analysis reveals pathways and candidate genes associated with salinity tolerance in a spaceflight-induced wheat mutant. Sci Rep. 2017;7:2731. doi: 10.1038/s41598-017-03024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsu JL, Wang LY, Wang SY, Lin CH, Ho KC, Shi FK, Chang IF. Functional phosphoproteomic profiling of phosphorylation sites in membrane fractions of salt-stressed Arabidopsis thaliana. Proteome Sci. 2009;7:42. doi: 10.1186/1477-5956-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cai GH, Wang G, Wang L, Liu Y, Pan JW, Li DQ. A maize mitogen-activated protein kinase kinase, ZmMKK1, positively regulated the salt and drought tolerance in transgenic Arabidopsis. J Plant Physiol. 2014;171:1003–1016. doi: 10.1016/j.jplph.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 87.Wang C, Lu WJ, He XW, Wang F, Zhou YL, Guo XL, Guo XQ. The cotton mitogen-activated protein kinase kinase 3 functions in drought tolerance by regulating stomatal responses and root growth. Plant Cell Physiol. 2016;57:1629–1642. doi: 10.1093/pcp/pcw090. [DOI] [PubMed] [Google Scholar]
- 88.Yue X, Gao XQ, Zhang XS. Circadian rhythms synchronise intracellular calcium dynamics and ATP production for facilitating Arabidopsis pollen tube growth. Plant Signal Behav. 2015;10:e1017699. doi: 10.1080/15592324.2015.1017699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng DD, Sun XB, Zhao M, Chow WS, Sun GY, Hu YB, Liu MJ, Zhang ZS. Light suppresses bacterial population through the accumulation of hydrogen peroxide in tobacco leaves infected with Pseudomonas syringae pv. tabaci. Front Plant Sci. 2016;7:521. doi: 10.3389/fpls.2016.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jia HH, Hao LL, Guo XL, Liu SC, Yan Y, Guo XQ. A Raf-like MAPKKK gene, GhRaf19, negatively regulates tolerance to drought and salt and positively regulates resistance to cold stress by modulating reactive oxygen species in cotton. Plant Sci. 2016;252:267–281. doi: 10.1016/j.plantsci.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 91.Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 92.Tang XL, Mu XM, Shao HB, Wang HL, Brestic M. Global plant-responding mechanisms to salt stress: physiological and molecular levels and implications in biotechnology. Crit Rev Biotechnol. 2014;32:425–37. doi: 10.3109/07388551.2014.889080. [DOI] [PubMed] [Google Scholar]
- 93.Ashorf M, Akran NA. Improving salinity tolerance of plants through conventional breeding and genetic engineering: an analytical comparison. Biotechnol Adv. 2009;27:744–752. doi: 10.1016/j.biotechadv.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 94.Hase Y, Tarusawa T, Muto A, Himeno H. Impairment of ribosome maturation or function confers salt resistance on Escherichia coli cells. PLoS ONE. 2013;8:e65747. doi: 10.1371/journal.pone.0065747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fu C, Liu XX, Yang WW, Zhao CM, Liu J. Enhanced salt tolerance in tomato plants constitutively expressing heat-shock protein in the endoplasmic reticulum. Genet Mol Res. 2016 doi: 10.4238/gmr.15028301. [DOI] [PubMed] [Google Scholar]
- 96.Hildebrandt TM. Synthesis versus degradation: directions of amino acid metabolism during arabidopsis abiotic stress response. Plant Mol Biol. 2018;98:121–135. doi: 10.1007/s11103-018-0767-0. [DOI] [PubMed] [Google Scholar]
- 97.Yan HR, Jia HH, Chen XB, Hao LL, An HL, Guo XQ. The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stressin transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production. Plant Cell Physiol. 2014;55:2060–2076. doi: 10.1093/pcp/pcu133. [DOI] [PubMed] [Google Scholar]
- 98.de Bruxelles GL, Peacock WJ, Dennis ES, Dolferus R. Abscisic acid lnduces the alcohol dehydrogenase gene in Arabidopsis. Plant Physiol. 1996;111:381–391. doi: 10.1104/pp.111.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.SDAU . Hi-C of Elaeagnus angustifolia L. Bethesda: GenBank; 2020. [Google Scholar]
- 100.SDAU . Trans data of Elaeagnus angustifolia L. Bethesda: GenBank; 2020. [Google Scholar]
- 101.SDAU . Survey of Elaeagnus angustifolia L. Bethesda: GenBank; 2020. [Google Scholar]
- 102.SDAU . PB of Elaeagnus angustifolia L. Bethesda: GenBank; 2020. [Google Scholar]
- 103.SDAU . Resequencing of Elaeagnus angustifolia L. Bethesda: GenBank; 2020. [Google Scholar]
- 104.SDAU . transcriptome of Elaeagnus angustifolia L. Bethesda: GenBank; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Filtering raw data of Pac-bio sequencing. Table S2. Length distribution of subreads of Pac-bio sequencing. Table S3. Genome assembly evaluation statistics. Table S4. Repeating sequence statistics. Table S5. Gene information statistics. Table S6. Non-coding RNA information. Table S7. Gene function annotation statistics. Table S8. Statistics of the completeness of the assembled genome. Table S9. Statistics of the BUSCO of the assembled genome. Table S10. Sequencing data volume statistics. Table S11. Clean data and genome alignment results statistics. Table S12. Hi-C sequencing data validation. Table S13. Classification statistics of gene families. Table S15. Function prediction of E. angustifolia. Table S17. Evaluation statistics of sequencing data of wild E. angustifolia samples. Table S18. Comparison results of wild E. angustifolia samples. Table S19. Coverage depth and coverage ratio statistics of wild E. angustifolia samples. Table S20. InDel statistics of whole genome and coding region of wild E. angustifolia samples. Table S21. Statistical table of variation genes of wild E. angustifolia samples. Table S22. Annotated list of some variant genes in sample R01. Table S23. Statistics of transcriptome sequencing data. Table S24. Results of the comparison between the sequencing transcriptome samples and the genome data. Table S26. Statistics of functional annotation results of new genes. Table S28. Statistics on the number of different genes in the transcription.
Additional file 2: Table S14. Functional gene categories enriched for E. angustifolia expansion and contraction families.
Additional file 3: Table S16. 80 rapidly evolving genes in E. angustifolia.
Additional file 4: Table S25. New genes discovered in E. angustifolia partially.
Additional file 5: Table S27. Go pathways functions of new genes.
Additional file 6: Figure S1. 12 wild E. angustifolia samples.
Data Availability Statement
This Whole Genome Shotgun project has been deposited in NCBI. Raw sequencing reads and genome assembly are available at GenBank as BioProject PRJNA647537 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA647537?reviewer=5dse95jg6ae9tao2s0ettacevv). Raw sequencing data (Hi-C, trans data, survey, PB) have been deposited in SRA (Sequence Read Archive) database as SRR12563589–SRR12563593 [99–102]. Resequencing has been deposited in SRA (Sequence Read Archive) database as SRR12578558–SRR12578569 [103]. Transcriptome has been deposited in SRA (Sequence Read Archive) database as SRR12569921–SRR12569935 [104]. Gene annotation data of E. angustifolia genome has been deposited in FigShare (https://doi.org/10.6084/m9.figshare.12957110.v1).