Abstract
Reduced bioavailability of nonpolar contaminants due to sorption to natural organic matter is an important factor controlling biodegradation of pollutants in the environment. We established enrichment cultures in which solid organic phases were used to reduce phenanthrene bioavailability to different degrees (R. J. Grosser, M. Friedrich, D. M. Ward, and W. P. Inskeep, Appl. Environ. Microbiol. 66:2695–2702, 2000). Bacteria enriched and isolated from contaminated soils under these conditions were analyzed by denaturing gradient gel electrophoresis (DGGE) and sequencing of PCR-amplified 16S ribosomal DNA segments. Compared to DGGE patterns obtained with enrichment cultures containing sand or no sorptive solid phase, different DGGE patterns were obtained with enrichment cultures containing phenanthrene sorbed to beads of Amberlite IRC-50 (AMB), a weak cation-exchange resin, and especially Biobead SM7 (SM7), a polyacrylic resin that sorbed phenanthrene more strongly. SM7 enrichments selected for mycobacterial phenanthrene mineralizers, whereas AMB enrichments selected for a Burkholderia sp. that degrades phenanthrene. Identical mycobacterial and Burkholderia 16S rRNA sequence segments were found in SM7 and AMB enrichment cultures inoculated with contaminated soil from two geographically distant sites. Other closely related Burkholderia sp. populations, some of which utilized phenanthrene, were detected in sand and control enrichment cultures. Our results are consistent with the hypothesis that different phenanthrene-utilizing bacteria inhabiting the same soils may be adapted to different phenanthrene bioavailabilities.
We hypothesize that some contaminant-degrading microorganisms have evolved specialization to low-bioavailability microenvironments that occur due to the propensity of nonpolar contaminants to adsorb strongly to natural organic matter (NOM). Most previously cultivated contaminant-degrading bacteria have been isolated under selection conditions that do not mimic such microenvironments. As a result, they may not exhibit the properties associated with such specialization that may be important for in situ bioremediation. In the accompanying paper (9a), we describe new enrichment strategies that simulated such microenvironments by selecting for microorganisms capable of metabolizing a model nonpolar contaminant. Phenanthrene was presorbed to model organic solids, such as the carboxylic acid cation-exchange resin Amberlite IRC-50 (AMB) (sorption coefficient [log KD] = 2.99 liters kg−1) and the polyacrylate-based resin Biobead SM7 (SM7) (log KD = 3.47 liters kg−1), that reduced its bioavailability to different degrees in the range of bioavailabilities observed with soil NOM (log KD = 2.5 to 3.5 liters kg−1). We used this strategy to enrich phenanthrene-degrading microorganisms from two contaminated soils in order to evaluate whether similar microbial populations were recovered from geographically distant sites when the same selection pressure was used. Phenanthrene degradation was slower in enrichment cultures containing organic solids than in controls containing sand or no sorptive phase. AMB reduced bioavailability to a lesser extent than did SM7. It was found that an isolate from SM7 enrichment cultures exhibited higher relative rates of metabolism of sorbed phenanthrene than did isolates from enrichment cultures without sorptive phases, suggesting that different microbial populations were selected under different phenanthrene bioavailability conditions.
In this study, we determined the compositions of the microbial assemblages that developed in the enrichment cultures by using cultivation-independent molecular tools (i.e., analysis of the 16S ribosomal DNA [rDNA] gene, a universal genetic marker). The application of molecular biology methods to microbial ecology has proven that the naturally occurring rRNA sequences differ from the rRNA sequences of species cultivated from the same habitat (7, 8, 11, 24, 28–30), in part due to a mismatch between adaptations of the native species and the selective nature of the culture methods (23). A cultivation-independent molecular approach for community structure analysis also facilitates detection of microorganisms that are difficult to cultivate or that cannot be cultivated with our current understanding of microbial growth requirements. Consequently, we monitored changes in the compositions of our phenanthrene enrichment cultures by denaturing gradient gel electrophoresis (DGGE) analysis of PCR-amplified 16S rDNA gene segments. DGGE separates double-stranded DNA segments of equal length based on sequence differences (17, 18). Although the segments were only a few hundred nucleotides long and this may have limited resolution of closely related molecules, the resulting DGGE band patterns facilitated detection of differences among the microbial communities in our different enrichment cultures. In addition, individual bands from DGGE profiles can be directly sequenced to identify populations by their 16S rDNA sequences (4, 16). As 16S rRNA gene sequence data do not permit inferences concerning a microbial population's ability to metabolize phenanthrene, we also attempted to cultivate the populations present in the enrichment cultures (9a). Molecular analyses, as well as cultivation, revealed differences in the species compositions of enrichment cultures with different phenanthrene bioavailabilities, especially where bioavailability was most reduced. The ecological relevance of contaminant availability for selection of specialized microbial populations is discussed below.
MATERIALS AND METHODS
Enrichments in the presence of model organic phases.
Microorganisms from hydrocarbon-contaminated coal gasification plant (Dover, Ohio) and creosote-contaminated (Libby, Mont.) soils were enriched on [9-14C]phenanthrene presorbed to model organic phases AMB and SM7, as described in detail in the accompanying paper (9a). Parallel control enrichment cultures contained equivalent amounts of [14C]phenanthrene and either sand or no sorptive phase. Conversion of [14C]phenanthrene to 14CO2 was monitored, and enrichment cultures were transferred when 14CO2 evolution began to reach a plateau. Samples (50 ml) of enrichment cultures were used for cultivation or were frozen, and subsequently they were used for molecular characterization.
DNA extraction.
Frozen samples of enrichment cultures or pure cultures obtained from them (9a) were quickly thawed in a water bath at 30°C and immediately placed on ice. Samples (2 ml) containing model solids and mineral medium were transferred to 2-ml screw-cap tubes. Cells and beads were separated from the medium by centrifugation for 5 min at 14,000 × g. Subsequently, cells were lysed with an FP120 FastPrep cell disruptor (Savant Instruments Inc., Farmingdale, N.Y.). Between 1 and 1.8 g of oven-baked 0.1-mm-diameter zirconium beads, 800 μl of 120 mM sodium phosphate buffer (pH 8.0), and 260 μl of 0.5 M Tris-HCl (pH 8.0)–0.1 M NaCl–10% sodium dodecyl sulfate were added prior to bead beating at 6.5 m s−1 for 45 s. After centrifugation for 5 min at 14,000 × g, 700 μl of supernatant was removed, and the DNA was purified by ammonium acetate precipitation (14), followed by standard isopropanol precipitation (0.7 volume) for 30 min. The DNA was dissolved in 100 μl of distilled H2O and analyzed by standard agarose gel electrophoresis. Samples from earlier transfers containing larger amounts of soil inoculum were subjected to a spin column purification step (Qiamp blood kit; Qiagen Inc., Chatsworth, Calif.) according to the manufacturer's instructions for crude cell lysates.
PCR, DGGE, and sequencing of DGGE bands and pure-culture 16S rRNA genes.
Prior to DGGE analysis, PCR was carried out as described previously (4). Briefly, the 16S rDNA gene was amplified between positions 1055 and 1406 (Escherichia coli numbering), a segment which included some hypervariable regions. It has been shown that the primers which we utilized (primers 1070F and 1392RGC) recover 16S rRNA genes from diverse members of the domain Bacteria under the PCR conditions used in this analysis (32). For pure cultures the almost complete 16S rRNA gene was amplified with primers 27F and 1492R (32). To obtain better band resolution in DGGE gels, 0.75-mm gels (35 to 80% denaturant solution) were employed. For sensitive band detection the gels were stained with SYBR green (Molecular Probes, Eugene, Oreg.) as recommended by the manufacturer and photographed. The photographs of DGGE gels were scanned and converted to negative images. Samples were obtained from individual DGGE bands by removing a small gel core with a sterile 200-μl pipette tip; the core was transferred to a tube containing 150 μl of sterile H2O and incubated overnight at 4°C to allow diffusion of the PCR product out of the gel core. A 0.5- to 1-μl portion of supernatant was used to reamplify the DGGE bands with primers 1070F and 1392RGC, and subsequently the PCR products were reanalyzed by DGGE to verify that bands were pure. Pure DGGE bands and PCR products from pure cultures were sequenced with either an ABI 373A sequencer (Applied Biosystems, Foster City, Calif.) at the Murdock Molecular Biology Facility (University of Montana, Missoula) by using primers 1114F and 1368R, as described elsewhere (5), or an ABI 377 sequencer at Medigenomix Sequencing Service (Martinsried, Germany) by using primers 27F and 1492R. Band sequences were considered unique only if there was unambiguous evidence of sequence difference.
Sequences were compared with sequences in the Ribosomal Database Project (RDP) (http://www.cme.msu.edu/RDP/) 16S rDNA database (release 7.0, 15 July 1998) by using the Similarity_Rank and Check_Chimera software (12) and with GenBank sequences by using BLAST software (2). Our 16S rDNA DGGE band and pure-culture sequences were aligned with closely related 16S rDNA sequences from the RDP and GenBank databases by using the Genetic Data Environment or the ARB software package (version 2.5b; O. Strunk and W. Ludwig, Technische Universität München, Munich, Germany; http://www.biol.chemie.tu-muenchen.de/pub/ARB/), and percent similarity to other sequences was determined.
RESULTS
Stabilization of enrichment culture DGGE patterns.
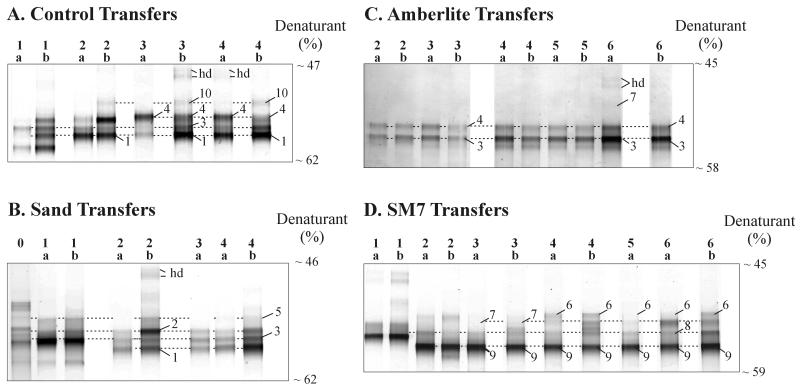
When contaminated soils were analyzed directly by DGGE, they typically produced a smear, which we interpreted as a high level of biodiversity. In contrast, DGGE analysis of enrichment cultures resulted in less complex patterns even after just one transfer, as shown in Fig. 1. Relatively stable DGGE band patterns were observed with subsequent transfers, with only minor band position and intensity differences between duplicates and transfers. This indicated the development of a stable and less diverse set of populations. For instance, for the control enrichment culture (Fig. 1A) the DGGE band patterns changed somewhat in early transfers, but by the third or fourth transfer, four distinct bands (bands 1, 3, 4, and 10) were consistently detected. Similarly, for the sand enrichment culture (Fig. 1B) three distinct bands (bands 1 through 3) were consistently detected after two to four transfers, and a fourth band (band 5) was sometimes observed. For the AMB enrichment culture (Fig. 1C), the patterns were stable after only two transfers, and bands 3 and 4 were consistently detected. For the SM7 enrichment culture (Fig. 1D) the patterns were stable after four to six transfers, and bands 6 through 9 were consistently detected. The labeled bands are those that were actually purified and sequenced; the dashed lines in Fig. 1 indicate the band positions relative to the positions of comigrating bands that were not sequenced and also emphasize that in most cases different band patterns were obtained for early and late transfers. Identical sequences were obtained for comigrating bands after various transfers, increasing our confidence that comigrating bands were likely to have the same sequences. The bands were not numbered consecutively because the numbers reflect the phylogenetic organization of sequences observed in DGGE bands obtained with various enrichment cultures (Table 1) (see below). A few DGGE bands (bands hd) were identified as heteroduplex artifacts based on the fact that reamplification yielded four products, two of which migrated very high in the gels and two of which comigrated with other bands that migrated farther in the gels (4). This was probably the result of a very high template concentration combined with a high sequence similarity of the two bands.
FIG. 1.
DGGE analysis of PCR-amplified 16S rRNA gene segments from replicates (labeled a and b) after sequential transfers (numbers above the lanes) of enrichment cultures inoculated with Dover, Ohio, soil. (A) Control. (B) Sand. (C) AMB. (D) SM7. The band numbers correspond to those in Tables 1 and 2. The dashed lines are included to help visualize comigrating bands. hd, heteroduplex bands.
TABLE 1.
Phylogenetic analysis of 16S rRNA sequences of DGGE bands detected in stable enrichment cultures
| DGGE banda | Closest RDP/GenBank relativeb
|
% Similarity to closest relativec | % Sequence similarity toc:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Phylogenetic group | Strain or species | DGGE band 1 | DGGE band 2 | DGGE band 3 | DGGE band 4 | DGGE band 5 | DGGE band 6 | ||
| 1 | β-Proteobacteria | Burkholderia sp. strain N3P2 | 99.6 | ||||||
| 2 | β-Proteobacteria | B. glathei | 99.4 | 96.7 | |||||
| 3 | β-Proteobacteria | Burkholderia sp. strains N3P2 and N2P5 | 100 | 99.6 | 97.1 | ||||
| 4 | β-Proteobacteria | B. cepacia | 98.1 | 96.3 | 94.7 | 96.7 | |||
| 5 | β-Proteobacteria | R. solanacearum | 99.2 | 92.6 | 93.4 | 93.0 | 94.7 | ||
| 6 | β-Proteobacteria | R. solanacearum | 100 | 91.8 | 93.4 | 92.2 | 93.9 | 99.2 | |
| 7 | β-Proteobacteria | M. methylotrophus | 96.3 | 88.1 | 89.8 | 88.5 | 89.8 | 88.5 | 87.7 |
| 8 | α-Proteobacteria | A. lipoferum | 98.8 | ||||||
| 9 | Gram-positive bacteria | M. gilvumd | 100 | ||||||
| 10 | Chlamydiales | Chlamydia sp. | 96.0 | ||||||
The band numbers correspond to DGGE band numbers in the figures. The GenBank accession numbers for the DGGE band nucleotide sequences are AF247476 to AF247485, respectively.
The GenBank accession numbers for the closest database relatives are as follows: Burkholderia sp. strain N3P2, U37344; Burkholderia sp. strain N2P5, U37342; B. glathei, Y17052; B. cepacia, M22518; R. solanacearum, X67035; M. methylotrophus, L15475, A. lipoferum, Z29619; M. gilvum, X55599; and Chlamydia sp. Y07556.
DGGE band sequences were compared by using E. coli 16S rRNA positions 1115 to 1367.
M. gilvum has a 16S rRNA sequence identical to that of M. chitae and M. smegmatis in the region used for DGGE analysis.
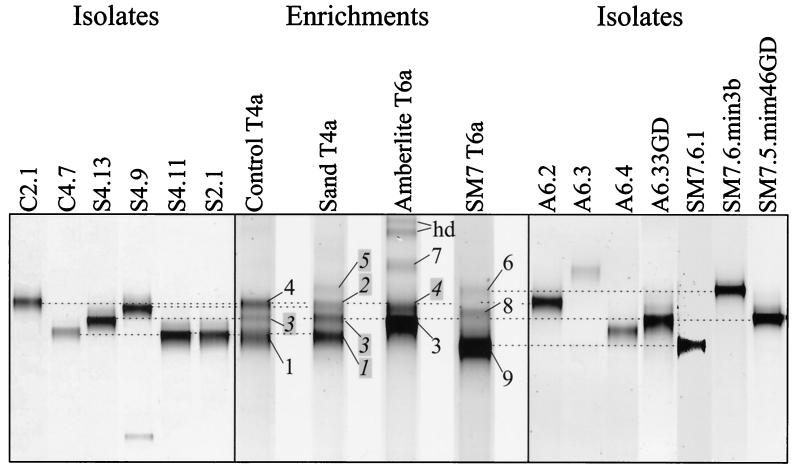
Figure 2 shows a comparison of DGGE patterns of different enrichment cultures after a number of transfers sufficient to achieve stable assemblages. Small differences between comparable lanes in Fig. 1 and Fig. 2 were due to the fact that the data shown in Fig. 2 resulted from independent gel analyses. The band patterns of the control and sand enrichment cultures were similar, though there were differences in both band composition and intensity. Although the AMB enrichment culture appeared to produce bands that comigrated with some of those produced by the control and sand enrichment cultures (e.g., bands 3 and 4), there was an obvious difference in the most intense band (band 3), band 1 was absent, and a unique band (band 7) was detected. The SM7 enrichment culture produced three unique bands (bands 6, 8, and 9) and was most obviously different from the other enrichment cultures.
FIG. 2.
Comparison of the DGGE profiles of PCR-amplified 16S rDNA segments from isolates to those obtained in the enrichment cultures from which the isolates were cultivated. The band numbers correspond to those in Fig. 1 and Tables 1 and 2. The dashed lines indicate possible comigration. The positions of bands whose numbers are highlighted and italicized were inferred based on comigration with bands in earlier transfer preparations that were actually sequenced (Fig. 1).
Sequences of DGGE bands in stabilized enrichment cultures inoculated with Dover soil.
Because all DGGE bands from all enrichment cultures migrated to a narrow section of the denaturing gradient gel (47 to 55% denaturant), even on gels with narrower gradients, it was difficult to determine whether bands actually comigrated. Moreover, different sequences can migrate to the same location on a DGGE gel (see below). Therefore, we sequenced individual bands purified from denaturing gradient gels for a precise determination and comparison of the 16S rRNA genes. For most DGGE bands, the PCR products had to be subjected to multiple purification cycles (extraction from the denaturing gradient gel, PCR, DGGE) to obtain pure bands. We successfully sequenced all DGGE bands that were detected in stabilized enrichment cultures; the sequences were identified in terms of their closest database relatives, and closely related sequences were compared to each other (Table 1).
Bands 1, 2, and 3, which were commonly obtained with the stabilized control, sand, and AMB enrichment cultures (Fig. 2), had sequences that were ≥96.7% similar to each other in the region analyzed (Table 1). These sequences were closely related or identical in this region to the sequences of several members of the β subclass of the class Proteobacteria (β-Proteobacteria), including Burkholderia sp. strains N3P2 and N2P5, which are polycyclic aromatic hydrocarbon (PAH)-mineralizing isolates from creosote-contaminated Norwegian soils and have identical sequences in the region analyzed by DGGE (15), and Burkholderia glathei, an isolate from vertisol microaggregates (1). The sequence of band 4, which was also obtained with control and AMB enrichment cultures, was slightly less closely related to the sequences of bands 1, 2, and 3 and was closely related to the sequence of Burkholderia cepacia. Although there was just one unambiguous base difference between the Burkholderia sp. strain N3P2- and N2P5-like sequences of bands 1 and 3 in the region analyzed (verified by sequences from more than one DGGE band that migrated to the same position [Fig. 1 and 2]), there was a difference in the distribution of these bands among stable enrichment cultures. Band 3 was the most intense band detected in the AMB enrichment culture, whereas band 1 (which was not detected in the AMB enrichment culture) was the most intense band detected in the control and sand enrichment cultures (Fig. 2). The lack of consistent co-occurrence of bands 1 and 3 is important, as it suggests a difference in selective pressure between the AMB and control or sand enrichments for unique Burkholderia sp. populations. The difference cannot be attributed to changes in the expression of different 16S rRNA operons within one organismic population (19), because we analyzed genes and not the 16S rRNA itself (see below).
SM7 enrichments provided the strongest evidence of population selection. The most intense band (band 9) had a sequence identical to those obtained for the gram-positive bacteria Mycobacterium gilvum, Mycobacterium chitae, and Mycobacterium smegmatis. These three species have 16S rRNA sequences that are ≥96.5% similar overall but are identical in the region used for DGGE analysis.
Several other less intense bands were obtained only in AMB and/or SM7 enrichment cultures, providing further evidence of population selection. These bands had sequences closely related to those of other β-Proteobacteria (Ralstonia solanacearum [bands 5 and 6] and Methylophilus methylotrophus [band 7]) and α-Proteobacteria (Azospirillum lipoferum [band 8]). Interestingly, the R. solanacearum-like sequence (band 5) obtained only in sand enrichment cultures was different (two unambiguous base differences) from that obtained in SM7 enrichment cultures (band 6). The control enrichment culture produced one unique band (band 10) (Fig. 1), with a sequence related to that of a Chlamydia sp.
Bacteria isolated from enrichment cultures.
We cultivated bacteria from the various enrichment cultures by using standard techniques (9a) in an attempt to link the 16S rRNA gene segments identified as DGGE bands with the abilities of the populations contributing these genes to metabolize phenanthrene. As shown in Fig. 2, many isolates exhibited DGGE bands that comigrated with bands detected in the enrichment cultures. Table 2 shows the sequence identity between 16S rRNA sequences of isolates and DGGE bands and indicates whether an isolate was capable of phenanthrene degradation. The closest RDP/GenBank relative shown in Table 2 is not always identical to the closest relative based on the corresponding DGGE band in Table 1 because nearly full-length sequence data were used to prepare Table 2. Nearly full-length sequence data also permitted a higher-resolution comparative analysis of closely related strains.
TABLE 2.
Phenanthrene utilization and phylogenetic properties of isolates and correlation with DGGE bands
| Representative isolatea | Phenanthrene useb | Identical DGGE bandc | Closest RDP/GenBank relatived
|
% Similarity to closest relativee | % Sequence similarity toe:
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phylogenetic group | Strain or species | C4.7 | S4.11 | S4.9 | A6.33 GD | S2.1 | ||||
| C4.7 | + | 1 | β-Proteobacteria | B. caryophylli MCII-8 | 98.7 | |||||
| S4.11 | + | 1 | β-Proteobacteria | Burkholderia sp. strain N2P5 | 98.5 | 98.5 | ||||
| S4.9 | − | 2 | β-Proteobacteria | B. glathei | 97.6 | 96.5 | 96.5 | |||
| A6.33GD | + | 3f | β-Proteobacteria | Burkholderia sp. strain N2P5 | 100 | 97.6 | 98.5 | 96.3 | ||
| S2.1 | + | β-Proteobacteria | Burkholderia sp. strain DhA54 | 97.5 | 96.9 | 96.8 | 96.4 | 97.2 | ||
| A6.2 | − | 4g | β-Proteobacteria | B. caryophylli ATCC 25418 | 97.7 | 95.8 | 95.9 | 96.4 | 96.6 | 97.8 |
| SM7.6.min.3b | − | 6 | β-Proteobacteria | R. solanacearum | 100 | |||||
| A6.4 | − | γ-Proteobacteria | F. aurantia | 97.0 | ||||||
| SM7.6.1 | + | 9 | Gram-positive bacteria | Mycobacterium sp. strain HE-5 | 99.1 | |||||
| A6.3 | − | Gram-positive bacteria | B. megaterium | 99.4 | ||||||
C, isolate from a stabilized control enrichment culture; S, isolate from a sand enrichment culture; A, isolate from an AMB enrichment culture; SM7, isolate from an SM7 enrichment culture. The number after an initial letter is the transfer number. The number after the period is the isolate number. GD, isolate obtained from ground beads by using SSE as described in the accompanying paper (9a); min, isolate obtained from an SSE agar plate sprayed with phenanthrene. The GenBank accession numbers for the isolates are as follows: C4.7, AF247493; S4.11, AF247495; S4.9, AF247496; A6.33GD, AF247492; S2.1, AF247494; A6.2, AF247491; SM7.6.min3b, AF247498; A6.4, AF247489; SM7.6.1, AF247497; and A6.3, AF247490.
+, isolate grew on phenanthrene in SSE and/or converted [14C]phenanthrene to 14CO2; −, isolate did not grow on phenanthrene in SSE or convert [14C]phenanthrene to 14CO2.
The band numbers correspond to labeled DGGE band numbers in all figures and Table 1.
GenBank accession numbers for the closest database relatives are as follows: Burkholderia sp. strain N2P5, U37342; Burkholderia sp. strain DhA-54, AJ011508; B. glathei, Y17052; B. caryophylli MCII-8, U91570; B. caryophylli ATCC 25418, X67039; R. solanacearum, X67035; F. aurantia, AJ010481; Mycobacterium sp. strain HE-5, AJ012738; and B. megaterium, D16273.
Determined by using nearly full-length sequences (1413 to 1451 bases).
Based on both comigration and sequence identity data, the most prominent DGGE bands detected in the enrichment cultures were associated with phenanthrene-oxidizing bacterial isolates obtained from high-dilution platings of the enrichment cultures. For instance, a band produced by isolate SM7.6.1, a close relative of Mycobacterium sp. strain HE-5, a bacterium that degrades the heterocyclic xenobiotic compound morpholine (25), corresponded to DGGE band 9, the most prominent band detected in SM7 enrichment cultures. Similarly, AMB isolate A6.33GD, which was identical in the region analyzed to Burkholderia sp. strain N2P5, corresponded to DGGE band 3, the most prominent band detected in the AMB enrichment culture. The situation was more complex with respect to DGGE band 1, the most intense band detected in control and sand enrichment cultures. The DGGE bands of control isolate C4.7 and sand isolate S4.11 both comigrated with and had sequences identical to that of Burkholderia sp. strain N3P2-like band 1. However, these isolates had sequences that were 1.5% different due to 20 unambiguous nucleotide differences outside the region analyzed by DGGE (Table 2). Isolate C4.7 was most closely related to Burkholderia caryophylli MCII-8, whereas isolate S4.11 most closely resembled Burkholderia sp. strain N2P5. Furthermore, the DGGE band of another phenanthrene-degrading Burkholderia sp. strain DhA54-like isolate, S2.1, comigrated with band 1, even though its sequence did not match that of band 1 (Table 2).
Several isolates which were unable to degrade phenanthrene and which were obtained from low-dilution platings had 16S rRNAs corresponding to less intense DGGE bands obtained with enrichment cultures. For instance, the 16S rRNA of B. glathei-like sand isolate S4.9 corresponded to DGGE band 2 detected in sand enrichment cultures, the 16S rRNA of B. caryophylli-like AMB isolate A6.2 corresponded to DGGE band 4 obtained with control and AMB enrichment cultures, and the 16S rRNA of R. solanacearum-like SM7 isolate SM7.6.min.3b corresponded to DGGE band 6 detected in the SM7 enrichment culture. Two AMB isolates that did not degrade phenanthrene, Frateuria aurantia-like isolate A6.4 and Bacillus megaterium-like isolate A6.3, exhibited DGGE band patterns that did not match those of enrichment cultures (Fig. 2).
The six Burkholderia isolates exhibited ≥95.8% similarity in their nearly full-length 16S rRNA sequences (Table 2), despite differences in their abilities to metabolize phenanthrene and in their distribution among the various enrichment cultures.
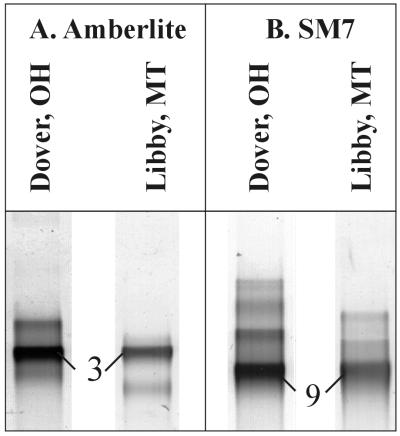
Comparison of enrichment cultures inoculated with Libby and Dover soils.
A DGGE analysis of enrichment cultures inoculated with Libby soil resulted in intense DGGE bands with mobilities similar to those of bands produced by Dover soil enrichment cultures (Fig. 3). AMB enrichment cultures obtained with both soils resulted in intense comigrating bands with identical sequences most closely related to those of Burkholderia sp. strains N3P2 and N2P5 (i.e., identical to the sequence of DGGE band 3). SM7 enrichment cultures obtained with both soils resulted in intense comigrating bands with sequences identical to the sequences of M. smegmatis, M. chitae, and M. gilvum (i.e., identical to the sequence of DGGE band 9).
FIG. 3.
Comparison of DGGE profiles of PCR-amplified 16S rRNA gene segments from stabilized AMB (A) and SM7 (B) enrichment cultures inoculated with soil from Dover, Ohio, or Libby, Mont. The band numbers correspond to those in Tables 1 and 2.
DISCUSSION
In the accompanying paper (9a), we describe model enrichment cultures used to evaluate the selection of phenanthrene-utilizing bacteria under different phenanthrene bioavailability conditions. Model organic solids, specifically AMB, a polystyrene-based weak cation exchanger with carboxylic acid functionality, and SM7, a polyacrylic acid ester, were used to successively reduce bioavailability compared to controls that contained sand or no sorbing phase. Other conditions that might have affected selection (e.g., soil inoculum, medium, and incubation conditions) were held constant. The correspondence between molecular and cultivation methods used to analyze bacteria present in the enrichment cultures was reasonable considering the usual incongruence of these approaches when they are applied to natural samples (29). This correspondence was presumably due to direct plating from enrichment cultures, which must have eliminated competitors present in the soil that might have otherwise dominated our culture collection. The combined molecular and cultivation results suggested that the conditions which we used to reduce phenanthrene availability resulted in selection of phenanthrene-utilizing bacteria different from those found in controls.
The most obvious example of selection occurred in the SM7 treatment, which enriched for a mycobacterial population (DGGE band 9) that was capable of phenanthrene metabolism. In contrast, AMB, sand, and control enrichment cultures selected mostly for Burkholderia sp.-like phenanthrene-utilizing populations. An SM7 mycobacterial isolate (SM7.6.1) representative of DGGE band 9 exhibited 5- to 7.5-fold-greater relative rates of metabolism of phenanthrene bound to SM7 than did Burkholderia sp. isolates C4.7 and S2.1, which were representative of DGGE band 1 and dominated control and sand enrichment cultures (9a). This suggests that enrichment under low-bioavailability conditions selected for isolates that are better able to metabolize phenanthrene when its bioavailability has been reduced by sorption to organic solids.
The 16S rRNA sequences of our mycobacterial isolates from SM7 enrichment cultures were 98.6 and 96.8% similar to the 16S rRNA sequences of PAH-degrading mycobacteria obtained previously from other contaminated soils and sediments, respectively, such as Mycobacterium sp. strain PAH 135 or Mycobacterium sp. strain PYR-1(9). Despite these relatively high levels of similarity, we must leave open the possibility that the phenanthrene-degrading mycobacteria which we selected might be unique species with adaptations to low-bioavailability microenvironments. Differentiation of mycobacterial species by means of comparative 16S rDNA analysis has proven difficult even with identical or nearly identical full-length sequence data (21, 22, 31). We (29) and others (6, 20) have found that populations with closely related or even identical 16S rRNA sequences may be ecologically unique and may actually be unique species (27, 29).
Selection also occurred in the AMB enrichment cultures, which exhibited a level of phenanthrene bioavailability between those of SM7 cultures and control or sand enrichment cultures (9a). The most intense DGGE band produced by AMB enrichment cultures (band 3) represented a Burkholderia sp. strain N3P2- and N2P5-like population. Because of possible PCR biases, band intensity may (3) or may not (5) indicate that a population is dominant. However, the fact that we were able to cultivate a phenanthrene-oxidizing Burkholderia sp. population with the same sequence from high dilutions of AMB enrichment cultures suggests that this population was the dominant phenanthrene-metabolizing population enriched under these conditions. Isolates with this sequence were also recovered from sand and SM7 enrichment cultures but from lower dilutions, consistent with the weaker or undetectable band 3 produced by these enrichment cultures (Fig. 2). The most intense band produced by control and sand enrichment cultures (band 1, whose mobility and sequence were different from those of band 3) was also Burkholderia sp. strain N3P2-like. This band might have indicated that any or all of three different Burkholderia sp. isolates cultivated from control and/or sand enrichment cultures were present. Two of these isolates had sequences that matched that of DGGE band 1, while one sequence that did not match the DGGE band 1 sequence comigrated with band 1. This observation highlights two problems associated with DGGE analysis that may lead to underestimation of genetic diversity: (i) relatively small, identical, conserved sequence domains may be present in molecules with different full-length 16S rRNA gene sequences, and (ii) DGGE bands with different sequences may comigrate. In our study it was necessary to cultivate phenanthrene-degrading bacteria in order to reveal limitations of DGGE.
The partial 16S rRNA sequences of bands 1 and 3 were highly related (Table 1), but the unique mobilities in DGGE and the larger differences in nearly full-length sequences of isolates with bands that matched bands 1 and 3 (Table 2) supported the hypothesis that the populations were different. As mentioned above, ecologically unique populations (i.e., species) may exhibit close phylogenetic relationships. The ecological differences among Burkholderia sp. populations which we observed may be reflected by their differential distributions and abundances under different bioavailability conditions and by the abilities of the populations to metabolize phenanthrene (i.e., isolates with a band that corresponded to band 2 did not oxidize phenanthrene). Burkholderia spp. known for their ability to degrade PAHs have been frequently isolated from soils (15). However, low bioavailability was not considered part of the isolation strategy, and abundance was considered in only a few studies (10). Our evidence of closely related yet ecologically distinct populations forced us to consider the possibility that, like mycobacterial isolates, our Burkholderia sp. isolates, even the ones that were 100% similar in the region analyzed to a previously described isolate (e.g., the nearly full-length sequence of our isolate A6.33GD was 100% similar to the sequence of isolate N2P5 of Mueller et al. [15]), could be unique with regard to utilization of phenanthrene under moderately low-bioavailability conditions.
The use of a small segment of a highly conserved genetic marker may also have limited our ability to observe differences among populations in soils from geographically distant locations. Hence, even though identical DGGE band sequences were detected under the same selection conditions when two distinct soils were used, we cannot eliminate the possibility that such differences might exist and might be detected by using a higher-resolution genetic approach. Mueller et al. (15), for instance, detected minor differences in nearly full-length 16S rRNA sequences of phenanthrene-degrading Burkholderia sp. isolates from different Florida and Norwegian sites; the Norwegian strains formed a separate phylogenetic (possibly geographic) cluster. The DGGE approach which we used did reveal that selection conditions, more than geographic location, controlled the enrichment of either mycobacterial or Burkholderia-like phenanthrene-degrading bacteria, which were obviously present in both Ohio and Montana soils. This suggests that there must be some general adaptive differences between these two very different types of microorganisms that could control their distribution and activity. In our enrichment cultures, selection must have been based on the different properties of SM7 compared to AMB, sand, or no sorptive phase.
The solids used to achieve variation in phenanthrene bioavailability differed not only in their sorption characteristics but also in their surface properties. Hence, we concluded that our results are consistent with selection for reduced bioavailability, as selection controlled by surface properties might also explain our findings. For example, the more hydrophobic surface of SM7 could have favored selection for mycobacteria, which are known for their hydrophobic cell surfaces. A recent observation supports the hypothesis that the basis of selection was reduced bioavailability (26). A phenanthrene-oxidizing bacterium that was selected in the presence of phenanthrene sorbed to SM7 was shown to have a greater propensity to degrade phenanthrene associated with sediments than a phenanthrene-oxidizing bacterium selected in the presence of nonsorbed phenanthrene. However, the isolate's phylogenetic type was not determined. As mentioned above, we found similar evidence of such selection in our companion study (9a). Further work will be necessary to determine whether selection is based on phenanthrene availability and/or surface properties of the model organic phase utilized.
Whether selection is based on reduced bioavailability, surface properties, or both, the ecological significance is that phenanthrene-degrading microorganisms appear to be adapted to different features of the microenvironment. Even in our simple enrichment environments there must have been some niche diversity. For instance, some enrichment cultures contained more than one phenanthrene-degrading population. This might be explained by the simultaneous presence of different types of phenanthrene (e.g., dissolved, solid associated, and perhaps surfactant associated). All enrichment cultures also contained bacteria that do not use phenanthrene, suggesting that the phenanthrene degraders themselves may have increased niche diversity through metabolism of the primary carbon and energy source to other compounds.
In a contaminated soil many more factors must influence the structure of the microbial community responsible for contaminant biodegradation. Microbial populations may, of course, also be specialized with respect to other noncontaminant resources (e.g., oxygen or other nutrient types and concentrations) or other environmental conditions (e.g., temperature, moisture, etc.). However, even when only contaminant partitioning is considered, it is possible to envisage diverse niches. Most hydrocarbon- or creosote-contaminated systems consist of a complex mixture of pollutants at different concentrations rather than a single compound at a single concentration, as in our study. Furthermore, the composition of NOM is more complex and diverse than the uniform model organic phases tested in our study. Different nonionic contaminants may exhibit different degrees of sorption to different solids and, depending on the type and extent of contamination, may also partition into non-aqueous-phase liquids. Such factors constitute the real microenvironmental features that have controlled the evolutionary trajectories of contaminant-degrading bacteria, leading to their present diversity. The existence of different niches in soil could permit the coexistence of different contaminant degraders. The effects of different niches on contaminant distribution and availability could control the relative abundances and distributions of these contaminant degraders, as well as the contributions which they make to contaminant bioremediation. Given the ubiquity of NOM in soils and sediments and its propensity to sorb nonpolar organic solutes, bacteria adapted to degrade NOM-sorbed contaminants may have special relevance. The present study demonstrates the importance of recognizing and understanding microbial adaptations to such conditions if we are to obtain a predictive knowledge of how to use microorganisms to achieve contaminant removal in situ.
ACKNOWLEDGMENTS
This work was supported by awards from the Army Corps of Engineers (DACA39-95-K-0003), the National Science Foundation (DEB-9729857), and the Montana Agriculture Experiment Station (projects 104398 and 911296) to W. P. Inskeep and D. M. Ward, by grants from the German Research Community (DFG) and the Max Planck Society to M. Friedrich and the Center for Biofilm Engineering, which is a National Science Foundation-supported Engineering Research Center (NSF cooperative agreement EEC-890739).
We thank Greg Colores and two anonymous reviewers for their helpful suggestions.
REFERENCES
- 1.Achouak W, Christen R, Barakat M, Martel M H, Heulin T. Burkholderia caribensis sp. nov., an exopolysaccharide-producing bacterium isolated from vertisol microaggregates in Martinique. Int J Syst Bacteriol. 1999;49:787–794. doi: 10.1099/00207713-49-2-787. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Felske A, Akkermans A D L, De Vos W M. Quantification of 16S rRNAs in complex bacterial communities by multiple competitive reverse transcription PCR in temperature gradient gel electrophoresis fingerprints. Appl Environ Microbiol. 1998;64:4581–4587. doi: 10.1128/aem.64.11.4581-4587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferris M J, Muyzer G, Ward D M. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl Environ Microbiol. 1996;62:340–346. doi: 10.1128/aem.62.2.340-346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferris M J, Ward D M. Seasonal distributions of dominant 16S rRNA-defined populations in a hot spring microbial mat examined by denaturing gradient gel electrophoresis. Appl Environ Microbiol. 1997;63:1375–1381. doi: 10.1128/aem.63.4.1375-1381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox G E, Wisotzkey J D, Jurtshuk P. How close is close—16S ribosomal RNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 7.Fuhrman J A, McCallum K, Davis A A. Phylogenetic diversity of subsurface marine microbial communities from the Atlantic and Pacific oceans. Appl Environ Microbiol. 1993;59:1294–1302. doi: 10.1128/aem.59.5.1294-1302.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giovannoni S J, Britschgi T B, Moyer C L, Field K G. Genetic diversity in Sargasso Sea North Atlantic Ocean bacterioplankton. Nature. 1990;345:60–63. doi: 10.1038/345060a0. [DOI] [PubMed] [Google Scholar]
- 9.Govindaswami M, Feldhake D J, Kinkle B K, Mindell D P, Loper J C. Phylogenetic comparison of two polycyclic aromatic hydrocarbon-degrading mycobacteria. Appl Environ Microbiol. 1995;61:3221–3226. doi: 10.1128/aem.61.9.3221-3226.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Grosser R J, Friedrich M, Ward D M, Inskeep W P. Effect of model sorptive phases on phenanthrene degradation: different enrichment conditions influence bioavailability and selection of phenanthrene-degrading isolates. Appl Environ Microbiol. 2000;66:2695–2702. doi: 10.1128/aem.66.7.2695-2702.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaestner M, Breuer-Jammali M, Mahro B. Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons (PAH) Appl Microbiol Biotechnol. 1994;41:267–273. [Google Scholar]
- 11.Liesack W, Stackebrandt E. Occurrence of novel groups of the domain Bacteria as revealed by analysis of genetic material isolated from an Australian terrestrial environment. J Bacteriol. 1992;174:5072–5078. doi: 10.1128/jb.174.15.5072-5078.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maidak B L, Cole J R, Parker C T, Jr, Garrity G M, Larsen N, Li B, Lilburn T G, McCaughey M J, Olsen G J, Overbeek R, Pramanik S, Schmidt T M, Tiedje J M, Woese C R. A new version of the RDP (Ribosomal Database Project) Nucleic Acids Res. 1999;27:171–173. doi: 10.1093/nar/27.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohn W W, Wilson A E, Bicho P, Moore E R B. Physiological and phylogenetic diversity of bacteria growing on resin acids. Syst Appl Microbiol. 1999;22:68–78. doi: 10.1016/S0723-2020(99)80029-0. [DOI] [PubMed] [Google Scholar]
- 14.Moré M I, Herrick J B, Silva M C, Ghiorse W C, Madsen E L. Quantitative cell lysis of indigenous microorganisms and rapid extraction of microbial DNA from sediment. Appl Environ Microbiol. 1994;60:1572–1580. doi: 10.1128/aem.60.5.1572-1580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller J G, Devereux R, Santavy D L, Lantz S E, Willis S G, Pritchard P H. Phylogenetic and physiological comparisons of PAH-degrading bacteria from geographically diverse soils. Antonie Leeuwenhoek. 1997;71:329–343. doi: 10.1023/a:1000277008064. [DOI] [PubMed] [Google Scholar]
- 16.Muyzer G, Hottenträger S, Teske A, Wawer C. Denaturing gradient gel electrophoresis of PCR-amplified 16S rDNA—a new molecular approach to analyse the genetic diversity of mixed microbial communities. In: Akkermans A D L, van Elsas J D, De Bruijn F J, editors. Molecular microbial ecology manual. Dordrecht, The Netherlands: Kluwer; 1996. pp. 3.4.4.1–3.4.4.22. [Google Scholar]
- 17.Muyzer G, Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers R M, Maniatis T, Lerman L S. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:501–527. doi: 10.1016/0076-6879(87)55033-9. [DOI] [PubMed] [Google Scholar]
- 19.Nübel U, Engelen B, Felske A, Snaidr J, Weishuber A, Amann R I, Ludwig W, Backhaus H. Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol. 1996;178:5636–5643. doi: 10.1128/jb.178.19.5636-5643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palys T, Nakamura L K, Cohan F M. Discovery and classification of ecological diversity in the bacterial world: the role of DNA sequence data. Int J Syst Bacteriol. 1997;47:1145–1156. doi: 10.1099/00207713-47-4-1145. [DOI] [PubMed] [Google Scholar]
- 21.Pitulle C, Dorsch M, Kazda J, Wolters J, Stackebrandt E. Phylogeny of rapidly growing members of the genus Mycobacterium. Int J Syst Bacteriol. 1992;42:337–343. doi: 10.1099/00207713-42-3-337. [DOI] [PubMed] [Google Scholar]
- 22.Rogall T, Wolters J, Flohr T, Boettger E C. Towards a phylogeny and definition of species at the molecular level within the genus Mycobacterium. Int J Syst Bacteriol. 1990;40:323–330. doi: 10.1099/00207713-40-4-323. [DOI] [PubMed] [Google Scholar]
- 23.Santegoeds C M, Nold S C, Ward D M. Denaturing gradient gel electrophoresis used to monitor the enrichment culture of aerobic chemoorganotrophic bacteria from a hot spring cyanobacterial mat. Appl Environ Microbiol. 1996;62:3922–3928. doi: 10.1128/aem.62.11.3922-3928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt T M, DeLong E F, Pace N R. Analysis of a marine picoplankton community by 16S ribosomal RNA gene cloning and sequencing. J Bacteriol. 1991;173:4371–4378. doi: 10.1128/jb.173.14.4371-4378.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuffenhauer G, Schrader T, Andreesen J R. Morpholine-induced formation of l-alanine dehydrogenase activity in Mycobacterium strain HE5. Arch Microbiol. 1999;171:417–423. doi: 10.1007/s002030050728. [DOI] [PubMed] [Google Scholar]
- 26.Tang W C, White J C, Alexander M. Utilization of sorbed compounds by microorganisms specifically isolated for that purpose. Appl Microbiol Biotechnol. 1998;49:117–121. doi: 10.1007/s002530051147. [DOI] [PubMed] [Google Scholar]
- 27.Ward D M. A natural species concept for prokaryotes. Curr Opin Microbiol. 1998;1:271–277. doi: 10.1016/s1369-5274(98)80029-5. [DOI] [PubMed] [Google Scholar]
- 28.Ward D M, Bateson M M, Weller R, Ruff-Roberts A L. Ribosomal rRNA analysis of microorganisms as they occur in nature. Adv Microb Ecol. 1992;12:219–286. [Google Scholar]
- 29.Ward D M, Ferris M J, Nold S C, Bateson M M. A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol Mol Biol Rev. 1998;62:1353–1370. doi: 10.1128/mmbr.62.4.1353-1370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward D M, Ferris M J, Nold S C, Bateson M M, Kopczynski E D, Ruff-Roberts A L. Species diversity in hot spring microbial mats as revealed by both molecular and enrichment culture approaches—relationship between biodiversity and community structure. In: Caumete P, Stal L J, editors. Microbial mats—structure, development, and environmental significance. New York, N.Y: Springer; 1994. pp. 33–44. [Google Scholar]
- 31.Wayne L G, Good R C, Boettger E C, Butler R, Dorsch M, Ezaki T, Gross W, Jonas V, Kilburn J, Kirschner P, Krichevsky M I, Ridell M, Shinnick T M, Springer B, Stackebrandt E, Tarnok I, Tarnok Z, Tasaka H, Vincent V, Warren N G, Knott C A, Johnson R. Semantide- and chemotaxonomy-based analyses of some problematic phenotypic clusters of slowly growing mycobacteria, a cooperative study of the International Working Group on Mycobacterial Taxonomy. Int J Syst Bacteriol. 1996;46:280–297. doi: 10.1099/00207713-46-1-280. [DOI] [PubMed] [Google Scholar]
- 32.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]