Abstract
Objective
Intestinal protozoa Blastocystis hominis and Cryptosporidium spp. are two influential factors in intestinal complications and malignancies. In present study, we estimated the pooled prevalence and odds ratio (OR) of the two parasites in colorectal cancer (CRC) patients and their possible association with the deadly disease.
Method
Our systematic search was conducted for published researches between January 1, 2000 and April 30, 2022 by using four international databases include Scopus, PubMed, and Web of Science as well as Google scholar search engine. The random- and fixed-effects models were used to estimate the pooled prevalence, OR, and 95% confidence interval (CI) by comprehensive meta-analysis (V2.2, Bio stat) software. Inclusion and exclusion criteria were applied.
Results
Thirteen papers (seven case–control and six cross-sectional studies) for B. hominis/CRC and six papers (two case–control and four cross-sectional studies) for Cryptosporidium spp./CRC were eligible to include in data synthesis. Pooled prevalence of B. hominis and Cryptosporidium spp. in CRC patients was calculated to be 26.8% (95% CI 19.4–35.7%) and 12.7% (95% CI 6.8–22.5%), respectively. Based on case–control studies, significant difference was found between case and controls in both protozoa (B. hominis OR 2.10; 95% CI 1.39–3.18% vs. Cryptosporidium spp. OR 5.06; 95% CI 1.8–13.6%). Considering the Blastocystis subtypes, ST1 (5/6; 83.33% studies) and ST3 (5/6; 83.33% studies) had the highest number of reports in CRC patients. Regarding the Cryptosporidium species, only C. parvum and C. hominis were reported.
Conclusion
Given the significant prevalence of both parasites in CRC patients and their statistically significant association, there is a need to pay more attention to these two intestinal parasites in under treatment patients.
Keywords: Blastocystis hominis, Cryptosporidium spp., Colorectal cancer, Meta-analysis
Introduction
According to the latest figures from the World Health Organization (WHO), 12 million cancerous case and 4 million deaths from cancer have been reported worldwide, of these, colorectal cancer (CRC) rank third in terms of morbidity (~ 2 million) and second in mortality rate (~ 1 million deaths) [1]. Chronic infections and inflammation along with unhealthy diet, stressful lifestyle, cell damages, constant exposure to radiation and harmful chemicals are risk factors for development of cancers [2, 3]. Infectious agents including parasites account for approximately 16% of cancers [4]. As infectious factors, the Blastocystis hominis and Cryptosporidium spp. are ubiquitous opportunistic protozoa isolated from the human host gastrointestinal tract [5, 6]. These prevalent enteric parasites may cause serious challenges in people undergoing colorectal cancer (CRC) chemotherapy (immunocompromised) due to their location in gastrointestinal tract [4, 7]. Among the Cryptosporidium species, Cryptosporidium parvum (C. parvum) and Cryptosporidium hominis (C. hominis) are responsible for over 90% of all human cases [8]. So far, out of 22 identified B. hominis subtypes (ST1-ST22), ten subtypes have been isolated from humans (ST1-9 and ST12), which ST3 is more prevalent [9]. Both parasites have zoonotic potential and transmission routes are oral-fecal alongside the contaminated water and food sources as well as close animal contact [10, 11]. Although the pathogenesis of B. hominis and Cryptosporidium spp. have not been clearly established, they have been frequently reported in individuals with gastrointestinal complications including diarrhea, abdominal cramps, etc. [12, 13]. B. hominis and Cryptosporidium spp. has occasionally been controversially found in healthy people as well as people with gastrointestinal symptoms, the risk of being opportunistic in people undergoing chemotherapy cannot be ignored [14, 15]. Up to now, numerous studies have been conducted on the pathogenic power and possible association of both protozoa with non-communicable diseases such as irritable bowel syndrome (IBS), Crohn's disease, and gastrointestinal cancers [16–19]. In the latter, scattered studies have shed new light on the potential virulence role and prevalence of parasites in CRC. The present systematic review and meta-analysis was designed and performed with the aim of aggregating the available data and providing a comprehensive and statistically documented picture of the pooled prevalence and odds ratio (OR) of B. hominis and Cryptosporidium spp. in CRC patients and their possible association with the deadly disease.
Materials and methods
Search strategy
We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines for the design, analysis and interpretation of the present study [20]. To evaluate the prevalence and OR of B. hominis and Cryptosporidium spp. in the CRC patients, a search was performed on the related literatures in four international databases, including Scopus, PubMed, and Web of Science as well as Google Scholar scientific search engine between January 1, 2000 and April 30, 2022. The searching process was accomplished using combinations of the following search keywords, including: “Blastocystis”, “Cryptosporidium” AND “Colorectal cancer”, “Gastrointestibal cancer” in English language.
Inclusion criteria
The following inclusion criteria were applied in the current review: (1) the peer-reviewed original research papers and short reports; (2) case–control and cross-sectional studies that estimated the prevalence of B. hominis and Cryptosporidium spp. in CRC patients; and (3) studies published with full text or abstracts in English which published online up to April 30, 2022.
Exclusion criteria
The exclusion criteria were included: (1) All types of review studies, editorials, letters and case reports; (2) those articles that were not available in English language; and (3) researches that report the prevalence of both parasites in cancers other than CRC, as well as studies with confusing and/or unclear data.
Study selection and data extraction
The primary screening of eligible studies based on inclusion criteria in mentioned databases was the responsibility of two expert researchers (AT and SB). Additionally, the references of the eligible papers were carefully hand-checked to find relevant articles that were not retrieved in the database searching. After removing duplicate and irrelevant records, and ensuring the existence of extractable data, studies information was extract by the ER and AB. Extracted items included first author name, year of publication, study design, geographical location of study, total CRC patients sample size and the number of isolated B. homins and Cryptosporidium spp. Finally, the extracted data was double checked by AA and the controversial issues were resolved by AT.
Quality assessment
To assess the quality of included case–control studies, we used the Newcastle–Ottawa Scale (NOS), as suggested by the Cochrane collaboration [21]. In this 9-star scale, a study could be awarded a maximum of one star for each numbered item within the selection and exposure categories. Also, a maximum of two stars could be given for comparability. Papers with a total score of 0–3, 4–6 or 7–9 points were categorized as poor, moderate or of high quality, respectively. The Joanna Briggs Institute (JBI) checklist also was used for quality assessment of the included cross-sectional records which have contains ten questions with four options including, yes, no, unclear, and not applicable [22]. The papers with a total score of 4–6 and 7–10 points were classified as the moderate and high quality, respectively. We have decided to include (4–10 points) and exclude (≤ 3 points) the researches.
Data synthesis and statistical analysis
Data was analyzed using comprehensive meta-analysis software version 2. To assessment the association between B. homins and Cryptosporidium spp. with CRC, an OR and pooled prevalence using random- and fixed-effects models and corresponding 95% confidence intervals (CI) were calculated for each study. In order to assess heterogeneity of studies, I2 value was considered that the value > 50% indicates a statistical significant heterogeneity. Eggers regression (Qualitative method) was applied to assess the possibility of publication bias during the analysis. P-value < 0.05 considered statistically significant.
Results
Study characteristics
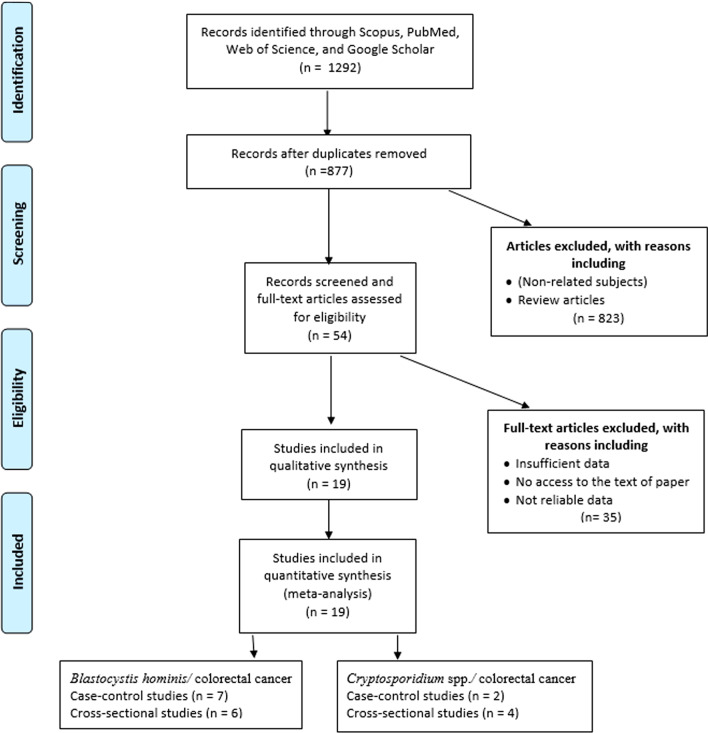
As shown in Fig. 1, a total of 1292 studies were identified by the initial search in the major databases. Finally, after removing duplicate and papers with non-related subjects, thirteen papers (seven case–control [4, 23–28] and six cross-sectional studies [29–34]) for B. hominis/CRC and six papers (two case–control [6, 8] and four cross-sectional studies [35–38]) for Cryptosporidium spp./CRC were eligible to include in data synthesis. These studies were conducted in ten different countries from four in Poland (one study for B. hominis and three studies for Cryptosporidium spp.), three in Iran (only B. hominis), two in China (one study for B. hominis and one study for Cryptosporidium spp.), two in Iraq (only B. hominis), two in Malaysia (only B. hominis), two in Saudi Arabia (only B. hominis), one in Turkey (only B. hominis), one in Egypt (only B. hominis), one in Tunisia (only Cryptosporidium spp.), and one in United States (only Cryptosporidium spp.). Further data are shown in Tables 1 and 2. The results of quality assessment according to NOS and JBI for eligible studies are depicted in Tables 1 and 2. The included articles in the present meta-analysis showed an acceptable quality.
Fig. 1.
PRISMA flow diagram of the search strategy and study selection process
Table 1.
Summary of the included studies reporting prevalence of Blastocystis hominis in CRC patients
| References | Country | Study design | Diagnostic methods | Statistical significant difference | Total case | Positive case | Blastocystis subtypes in case group | Total control | Positive control | Blastocystis subtypes in control group | QA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ali et al. [23] | Egypt | CC* | Microscopic (modified trichrome stain), culture, and PCR | No significant differences (p value = 0.101) | 100 | 52 | ST1 (1), ST2 (2), ST3 (4), and ST7 (3) | 100 | 42 | ST1 (4), ST2 (2), and ST3 (4) | 10 |
| Mahmoudvand et al. [24] | Iran | CC | Microscopic (modified trichrome stain) and PCR | Significant differences (p value < 0.001) | 67 | 16 | 67 | 6 | 9 | ||
| Hawash et al. [25] | Saudi Arabia | CC | Microscopic | Significant differences (p value < 0.05) | 75 | 20 | 25 | 2 | 9 | ||
| Sulzyc Bielicka et al. [4] | Poland | CC | Microscopic and PCR | Significant differences (p value = 0.00409) | 107 | 13 | ST1 (2), ST2 (1), and ST3 (9) | 124 | 3 | ST1 (1) and ST3 (1) | 10 |
| Al-Dabbagh and Al-Mukhtar [26] | Iraq | CC | Microscopic (formalin saline) and ELISA (B. hominis antigen) | No significant differences (p value = 0.6956) | 40 | 15 | 80 | 33 | 9 | ||
| Mohamed et al. [27] | Saudi Arabia | CC | Microscopic, culture, and PCR | Significant differences (p value < 0.05) | 74 | 22 | ST1 (12) and ST5 (10) | 80 | 12 | ST1 (2), ST2 (7), and ST5 (3) | 10 |
| Kumarasamy et al. [28] | Malaysia | CC | Microscopic (formal ether concentration), culture, and PCR | Significant differences (p value < 0.05) | 204 | 43 | ST1 (9), ST2 (1), ST3 (26), ST1 + 2 (2), ST2 + 3 (4), and ST5 (1) | 221 | 22 | ST1 (6), ST2 (2), ST3 (7), ST2 + 3 (4), and ST5 (3) | 10 |
| Asghari et al. [29] | Iran | CS* | Microscopic (modified trichrome stain), culture, and PCR | 4 | 2 | ST3 (2) | 5 | ||||
| Majeed et al. [30] | Iraq | CS | Microscopic | 116 | 51 | 7 | |||||
| Esteghamati et al. [31] | Iran | CS | Microscopic and PCR | 39 | 11 | 6 | |||||
| Zhang et al. [32] | China | CS | Microscopic and PCR | 49 | 4 | 6 | |||||
| Yersal et al. [33] | Turkey | CS | Microscopic, culture, and PCR | 66 | 5 | ST1 (3) and ST3 (2) | 7 | ||||
| Chandramathi et al. [34] | Malaysia | CS | Microscopic and culture | 15 | 7 | 5 |
*CC: Case–control
*CS: Cross-sectional
QA: Quality assessment
Table 2.
Summary of the included studies reporting prevalence of Cryptosporidium spp. in CRC patients
| References | Country | Study design | Diagnostic methods | Statistical significant difference | Total case | Positive case | Cryptosporidium species-subtypes in case group | Total control | Positive control | QA |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhang et al. [6] | China | CC* | PCR | Significant differences (p value < 0.001) | 116 | 20 | C. parvum-IIaA15G2R1 (10), C. parvum-IIaA15G2R2 (9), and C. parvum-IIaA13G2R2 (1) | 141 | 0 | 10 |
| Sulzyc Bielicka et al. [7] | Poland | CC | Immunoenzymatic test | Significant differences (p value = 0.015) | 108 | 14 | 125 | 5 | 10 | |
| Essid et al. [35] | Tunisia | CS* | Modified Ziehl Neelsen stain and PCR | 15 | 5 | C. hominis-IaA27G1R1 (2), C. parvum-IIaA15G2R1 (1), and C. parvum-IIcA5G3 (2) | 7 | |||
| Shebl et al. [36] | United States | CS | Microscopic | 320 | 7 | 7 | ||||
| Sulzyc Bielicka et al. [37] | Poland | CS | Immunoenzymatic test | 87 | 11 | 6 | ||||
| Sulzyc Bielicka et al. [38] | Poland | CS | Enzyme immunoassay | 55 | 10 | 6 |
*CC: Case–control
*CS: Cross-sectional
QA: Quality assessment
B. hominis and CRC
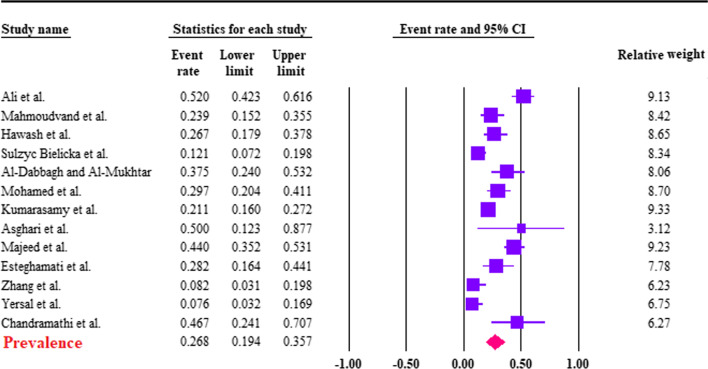
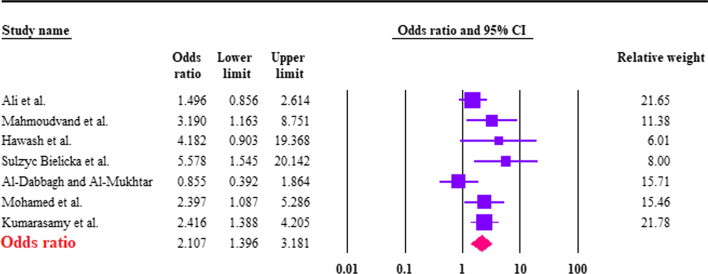
Based on the random-effects model, the pooled prevalence of B. hominis in CRC patients was calculated to be 26.8% (95% CI 19.4–35.7%). The heterogeneity was substantial (I2 = 85.2%; τ2 = 0.45; ρ = 0.00). Forest plot diagram is presented in Fig. 2. According to the seven studies with a case–control design, the pooled prevalence of B. hominis in case 27.7%; (95% CI 18.8–38.8%; I2 = 86.8%) was higher than controls 14.4% (95% CI 6.7–28.5%; I2 = 92.7%), a significant difference was found between case and controls (OR 2.10; 95% CI 1.39–3.18; I2: 42.4%) (Fig. 3). Six studies had extractable data regarding the Blastocystis subtypes. In this regard, ST1 (5/6; 83.33% studies) and ST3 (5/6; 83.33% studies) had the highest number of reports in CRC patients (Table 1).
Fig. 2.
Forest plot of prevalence of Blastocystis hominis in CRC patients, estimated with random-effects model
Fig. 3.
Forest plot of the association between Blastocystis hominis and being CRC patients, estimated with random effects model, showing the odds ratio (OR) and 95% confidence interval (CI)
Cryptosporidium spp. and CRC
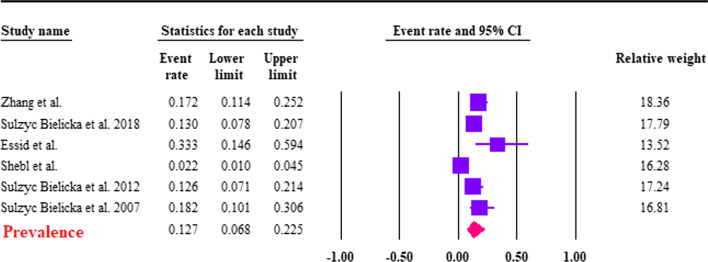
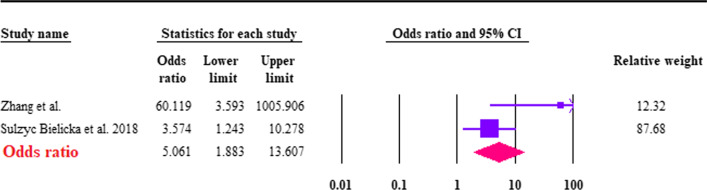
The estimation of the pooled prevalence of Cryptosporidium spp. was 12.7% (95% CI 6.8–22.5%; I2 = 84.7%) among CRC patients (Fig. 4). Based on the two case–control studies, we found that the pooled prevalence of Cryptosporidium spp. was significantly higher in CRC patients 15.3% (95% CI 11.1–20.7%; I2 = 80.2%) compared to controls 1.7% (95% CI 0.2–15%; I2 = 88.1%), the difference between the case and control groups was significant (OR 5.06; 95% CI 1.8–13.6; I2: 70.4%) (Fig. 5). Cryptosporidium species were identified in only two studies. Among them, only C. parvum and C. hominis were reported; the type of their subtype is shown in Table 2.
Fig. 4.
Forest plot of prevalence of Cryptosporidium spp. in CRC patients, estimated with random-effects model
Fig. 5.
Forest plot of the association between Cryptosporidium spp. and being CRC patients, estimated with fixed effects model, showing the odds ratio (OR) and 95% confidence interval (CI)
Publication bias
Considering the B. hominis and CRC studies, detecting publication bias using the Eggers regression revealed that publication bias in case–control studies was not statistically significant (p value < 0.05). Due to the fact that only two case–control studies were performed on Cryptosporidium spp. and CRC, publication bias is not applicable.
Discussion
Over the past decade, there has been considerable evidence of parasitic infections, such as Cryptosporidium spp. and B. hominis, with various types of cancer [39, 40]. Cryptosporidium spp. and B. hominis have been suggested as important intestinal parasites in CRC patients and the severe form of these diseases occurs most frequently in such patients [8, 41]. In this regard, the current study is a systematic review and meta-analysis to address the pooled prevalence and ORs of Cryptosporidium spp. and B. hominis infections in CRC patients compared with non-cancer individuals. This meta-analysis revealed a positive association between Cryptosporidium spp. and B. hominis infections with CRC. Among the seven case–control studies regarding the B. hominis, five reported a significant and two reported a non-significant difference related with B. hominis infection in the case group compared to the control group (Table 1). Both case control studies on Cryptosporidium spp./CRC showed a significant prevalence of this protozoan in the case group compared to the control group (Table 2). High heterogeneity (I2) was observed in this meta-analysis. Several sources of heterogeneity have been reported in the literature which include study design, detection method, geographical distribution, sample size, and high or low prevalence in some studies (different weights of each study) [42, 43].
Considering B. hominis, some studies conducted on in vitro and in vivo, and epidemiological studies from human populations have revealed an association between B. hominis and CRC [27, 41]. In this regard, in vitro studies have shown severe cytopathic and immunological effects by the solubilized antigen of B. hominis in human colorectal cancer cell line [41]. The findings of these studies suggest that B. hominis infection may increase the proliferative, invasive, and metastatic properties of CRC cells [41]. Another in vitro study indicated that the five subtypes of B. hominis significantly increased the proliferation of human CRC cell line HCT116, particularly ST3 [44]. The present systematic review has shown that ST1 and ST3 is more common in CRC patients than other subtypes. It is suggested that in the future studies, in order to deeper understanding the mechanism of these subtypes in CRC development, further cellular studies should be performed focusing on these subtypes in CRC patients.
Regarding the Cryptosporidium spp., several experimental and epidemiological studies have shown the potential role of cryptosporidiosis and CRC progression [19, 38, 45]. It has been suggested that C. parvum is one of the pathogen agents that may trigger intestinal dysplasia [45]. However, the pathophysiological mechanisms of Cryptosporidium spp. infection are multifactorial and not completely specified. An experimental study revealed that C. parvum is able to modulate host-cell cytoskeleton and intracellular signals, which may explain the transformed phenotype of the infected epithelial cells [46]. Moreover, our findings showed that several C. parvum (IIa and IIc) and C. hominis (Ia) subtypes were present in CRC patients (Table 2). Therefore, it is suggested to evaluate the progression of CRC in laboratory and human studies by considering these species/subtypes.
Some limitations of this systematic review and meta-analysis, which may affect the results, are listed as follows: (1) lack of access to the full text of some articles, (2) low sample size of some studies, (3) geographical dispersion of studies, (4) different diagnostic methods and (5) lack of some variables such as age and gender. Also, the state of immunosuppression of the patients and the time of the evolution of the CRC may be another source of heterogeneity which was not considered in the present study.
In conclusion, the present meta-analysis demonstrates that CRC may be related with elevated risks of Cryptosporidium spp. and B. hominis infections. However, further studies should be performed to investigate the impact of Cryptosporidium spp. and B. hominis infections in the onset or development of CRC in the future.
Acknowledgements
We thank the scientists and personnel of the Medical Parasitology Department in Jahrom University of Medical Sciences, Jahrom, for their collaboration.
Author contributions
All authors contributed to study design. AT, AA, and SB contributed to study implementation. AT conducted the analysis and interpretation of data. AT, SB, ER, and AB collaborated in manuscript writing and revision and approved the final version of the article. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in public, commercial, or not-for-profit sectors.
Availability of data and materials
The data used to support the findings of this study are included within the article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing of interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ali Taghipour, Email: alitaghipor71@yahoo.com.
Esmail Rayatdoost, Email: e.rayat.dost@gmail.com.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022 doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Khatami A, Nahand JS, Kiani SJ, Khoshmirsafa M, Moghoofei M, Khanaliha K, Tavakoli A, Emtiazi N, Bokharaei-Salim F: Human papilloma virus (HPV) and prostate cancer (PCa): The potential role of HPV gene expression and selected cellular MiRNAs in PCa development. Microb Pathog. 2022:105503. [DOI] [PubMed]
- 3.Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529(7584):43–47. doi: 10.1038/nature16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sulżyc-Bielicka V, Kołodziejczyk L, Adamska M, Skotarczak B, Jaczewska S, Safranow K, Bielicki P, Kładny J, Bielicki D. Colorectal cancer and Blastocystis sp. infection. Parasites Vectors. 2021;14(1):1–9. doi: 10.1186/s13071-021-04681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abedi SH, Fazlzadeh A, Mollalo A, Sartip B, Mahjour S, Bahadory S, Taghipour A, Rostami A: The neglected role of Blastocystis sp. and Giardia lamblia in development of irritable bowel syndrome: a systematic review and meta-analysis. Microb Pathog. 2021:105215. [DOI] [PubMed]
- 6.Zhang N, Yu X, Zhang H, Cui L, Li X, Zhang X, Gong P, Li J, Li Z, Wang X. Prevalence and genotyping of Cryptosporidium parvum in gastrointestinal cancer patients. J Cancer. 2020;11(11):3334. doi: 10.7150/jca.42393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasir KA, Hama AA, Ali SI: Prevalence of Cryptosporidiosis among cancer patients in Sulaimani province/Iraq. Int J Psychosoc Rehabil. 2020;24(09).
- 8.Sulżyc-Bielicka V, Kołodziejczyk L, Jaczewska S, Bielicki D, Safranow K, Bielicki P, Kładny J, Rogowski W. Colorectal cancer and Cryptosporidium spp infection. PLoS ONE. 2018;13(4):e0195834. doi: 10.1371/journal.pone.0195834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauff-Adedotun AA, Mohd Zain SN, Farah Haziqah MT. Current status of Blastocystis sp in animals from Southeast Asia: a review. Parasitol Res. 2020;119(11):3559–3570. doi: 10.1007/s00436-020-06828-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng L, Chai Y, Zhou Z, Liu H, Zhong Z, Hu Y, Fu H, Yue C, Peng G. Epidemiology of Blastocystis sp. infection in China: a systematic review. Parasite 2019;26. [DOI] [PMC free article] [PubMed]
- 11.Feltus DC, Giddings CW, Schneck BL, Monson T, Warshauer D, McEvoy JM. Evidence supporting zoonotic transmission of Cryptosporidium spp. in Wisconsin. J Clin Microbiol. 2006;44(12):4303–4308. doi: 10.1128/JCM.01067-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajdušek O, Ditrich O, Šlapeta J. Molecular identification of Cryptosporidium spp. in animal and human hosts from the Czech Republic. Vet Parasitol. 2004;122(3):183–192. doi: 10.1016/j.vetpar.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Kumarasamy V, Anbazhagan D, Subramaniyan V, Vellasamy S. Blastocystis sp., parasite associated with gastrointestinal disorders: an overview of its pathogenesis, immune modulation and therapeutic strategies. Curr Pharmaceut Des. 2018;24(27):3172–3175. doi: 10.2174/1381612824666180807101536. [DOI] [PubMed] [Google Scholar]
- 14.Roberts T, Stark D, Harkness J, Ellis J. Update on the pathogenic potential and treatment options for Blastocystis sp. Gut pathogens. 2014;6(1):1–9. doi: 10.1186/1757-4749-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Certad G, Viscogliosi E, Chabé M, Cacciò SM. Pathogenic mechanisms of Cryptosporidium and Giardia. Trends Parasitol. 2017;33(7):561–576. doi: 10.1016/j.pt.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Scanlan PD, Stensvold CR. Blastocystis: getting to grips with our guileful guest. Trends Parasitol. 2013;29(11):523–529. doi: 10.1016/j.pt.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Scanlan PD. Blastocystis: past pitfalls and future perspectives. Trends Parasitol. 2012;28(8):327–334. doi: 10.1016/j.pt.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Kumarasamy V, Kuppusamy UR, Jayalakshmi P, Samudi C, Ragavan ND, Kumar S. Exacerbation of colon carcinogenesis by Blastocystis sp. PLoS ONE. 2017;12(8):e0183097. doi: 10.1371/journal.pone.0183097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawant M, Baydoun M, Creusy C, Chabé M, Viscogliosi E, Certad G, Benamrouz-Vanneste S. Cryptosporidium and colon cancer: Cause or consequence? Microorganisms. 2020;8(11):1665. doi: 10.3390/microorganisms8111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 22.Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 23.Ali SH, Ismail MA, El-Badry AA, Abu-Sarea EY, Dewidar AM, Hamdy DA: An association between Blastocystis subtypes and colorectal cancer patients: a significant different profile from non-cancer individuals. Acta Parasitol. 2022:1–12. [DOI] [PMC free article] [PubMed]
- 24.Mahmoudvand H, Sepahvand A, Badparva E, Khatami M, Moayyedkazemi A. Possible association and risk factors of Blastocystis infection and colorectal cancers in Western Iran. Arch Clin Infect Dis. 2021.
- 25.Hawash YA, Ismail KA, Saber T, Eed EM, Khalifa AS, Alsharif KF, Alghamdi SA, Dahlawi HA, Alsanie W, Khalifa AM. Predominance of infection with Blastocystis hominis in patients with colorectal cancer and its association with high mucin content, infiltration of inflammatory cells and elevated serum tumor necrosis factor α. Infect Dis Clin Pract. 2021;29(1):e32–e38. doi: 10.1097/IPC.0000000000000931. [DOI] [Google Scholar]
- 26.Al-Dabbagh LKA, Al-Mukhtar AM. Infections with Blastocystis hominis in patients with colorectal cancer in Mosul city, Iraq. Int J Enhanced Res Sci Technol Eng. 2017;6(9):1–4. doi: 10.15623/ijret.2017.0609001. [DOI] [Google Scholar]
- 27.Mohamed AM, Ahmed MA, Ahmed SA, Al-Semany SA, Alghamdi SS, Zaglool DA. Predominance and association risk of Blastocystis hominis subtype I in colorectal cancer: a case control study. Infect Agents Cancer. 2017;12(1):1–8. doi: 10.1186/s13027-017-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumarasamy V, Roslani AC, Rani KU, Govind SK. Advantage of using colonic washouts for Blastocystis detection in colorectal cancer patients. Parasit Vectors. 2014;7(1):1–5. doi: 10.1186/1756-3305-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asghari A, Zare M, Hatam G, Shahabi S, Gholizadeh F, Motazedian M. Molecular identification and subtypes distribution of Blastocystis sp isolated from children and adolescent with cancer in Iran: evaluation of possible risk factors and clinical features. Acta Parasitol. 2020;65(2):462–473. doi: 10.2478/s11686-020-00186-2. [DOI] [PubMed] [Google Scholar]
- 30.Majeed GH, Mohammed NS, Muhsen SS. The synergistic effect of Blastocystis hominis and H pylori in Iraqi colorectal cancer patients. J Pharmaceut Sci Res. 2019;11(2):523–526. [Google Scholar]
- 31.Esteghamati A, Khanaliha K, Bokharaei-Salim F, Sayyahfar S, Ghaderipour M. Prevalence of intestinal parasitic infection in cancer, organ transplant and primary immunodeficiency patients in Tehran, Iran. Asian Pacific J Cancer Prev APJCP. 2019;20(2):495. doi: 10.31557/APJCP.2019.20.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Ren G, Zhao W, Yang Z, Shen Y, Sun Y, Liu A, Cao J. Genotyping of Enterocytozoon bieneusi and subtyping of Blastocystis in cancer patients: relationship to diarrhea and assessment of zoonotic transmission. Front Microbiol. 1835;2017:8. doi: 10.3389/fmicb.2017.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yersal O, Malatyali E, Ertabaklar H, Oktay E, Barutca S, Ertug S. Blastocystis subtypes in cancer patients: Analysis of possible risk factors and clinical characteristics. Parasitol Int. 2016;65(6):792–796. doi: 10.1016/j.parint.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Chandramathi S, Suresh K, Anita ZB, Kuppusamy UR. Infections of Blastocystis hominis and microsporidia in cancer patients: are they opportunistic? Trans R Soc Trop Med Hyg. 2012;106(4):267–269. doi: 10.1016/j.trstmh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Essid R, Menotti J, Hanen C, Aoun K, Bouratbine A. Genetic diversity of Cryptosporidium isolates from human populations in an urban area of Northern Tunisia. Infect Genet Evol. 2018;58:237–242. doi: 10.1016/j.meegid.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Shebl FM, Engels EA, Goedert JJ. Opportunistic intestinal infections and risk of colorectal cancer among people with AIDS. AIDS Res Hum Retroviruses. 2012;28(9):994–999. doi: 10.1089/aid.2011.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sulżyc-Bielicka V, Kołodziejczyk L, Jaczewska S, Bielicki D, Kładny J, Safranow K. Prevalence of Cryptosporidium sp. in patients with colorectal cancer. Pol Przegl Chir. 2012;84(7):348–351. doi: 10.2478/v10035-012-0058-4. [DOI] [PubMed] [Google Scholar]
- 38.Sulżyc-Bielicka V, Kuźna-Grygiel W, Kołodziejczyk L, Bielicki D, Kładny J, Stępień-Korzonek M, Telatyńska-Śmieszek B. Cryptosporidiosis in patients with colorectal cancer. J Parasitol. 2007;93(3):722–724. doi: 10.1645/GE-1025R1.1. [DOI] [PubMed] [Google Scholar]
- 39.Dheilly NM, Ewald PW, Brindley PJ, Fichorova RN, Thomas F. Parasite-microbe-host interactions and cancer risk. PLoS Pathog. 2019;15(8):e1007912. doi: 10.1371/journal.ppat.1007912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ujvari B, Beckmann C, Biro PA, Arnal A, Tasiemski A, Massol F, Salzet M, Mery F, Boidin-Wichlacz C, Misse D. Cancer and life-history traits: lessons from host–parasite interactions. Parasitology. 2016;143(5):533–541. doi: 10.1017/S0031182016000147. [DOI] [PubMed] [Google Scholar]
- 41.Kumarasamy V, Atroosh WM, Anbazhagan D, Abdalla MMI, Azzani M. Association of Blastocystis hominis with colorectal cancer: a systematic review of in vitro and in vivo evidences. World J Gastrointest Oncol. 2022;14(3):734. doi: 10.4251/wjgo.v14.i3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khademvatan S, Majidiani H, Khalkhali H, Taghipour A, Asadi N, Yousefi E. Prevalence of fasciolosis in livestock and humans: a systematic review and meta-analysis in Iran. Comp Immunol Microbiol Infect Dis. 2019;65:116–123. doi: 10.1016/j.cimid.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Taghipour A, Olfatifar M, Javanmard E, Norouzi M, Mirjalali H, Zali MR. The neglected role of Enterobius vermicularis in appendicitis: a systematic review and meta-analysis. PLoS ONE. 2020;15(4):e0232143. doi: 10.1371/journal.pone.0232143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumarasamy V, Kuppusamy UR, Samudi C, Kumar S. Blastocystis sp. subtype 3 triggers higher proliferation of human colorectal cancer cells, HCT116. Parasitol Res. 2013;112(10):3551–3555. doi: 10.1007/s00436-013-3538-5. [DOI] [PubMed] [Google Scholar]
- 45.Abdou AG, Harba NM, Afifi AF, Elnaidany NF. Assessment of Cryptosporidium parvum infection in immunocompetent and immunocompromised mice and its role in triggering intestinal dysplasia. Int J Infect Dis. 2013;17(8):e593–e600. doi: 10.1016/j.ijid.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 46.Benamrouz S, Conseil V, Chabé M, Praet M, Audebert C, Blervaque R, Guyot K, Gazzola S, Mouray A, Chassat T. Cryptosporidium parvum-induced ileo-caecal adenocarcinoma and Wnt signaling in a mouse model. Dis Model Mech. 2014;7(6):693–700. doi: 10.1242/dmm.013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.