Abstract
Although vaccines have been effective against the worldwide pandemic of Coronavirus Disease 19 (COVID-19), some case reports have described autoimmune hepatitis triggered by COVID-19 vaccination. Meanwhile, hepatitis C virus (HCV) is known to be related to autoimmune diseases. Here, we report a case of autoimmune hepatitis with history of HCV treatment triggered by COVID-19 vaccination. An 82-year-old woman was referred to our hospital for severe liver injury. She had received a COVID-19 vaccination 7 days prior. She had a history of HCV treatment with direct-acting antivirals 7 years previously. In her blood data, despite HCV antibody positivity, she was negative for HCV RNA by real-time RT-PCR. Anti-nuclear antibody was positive and IgG was elevated. Interface hepatitis and plasma cell infiltration were confirmed pathologically. She was diagnosed as autoimmune hepatitis and her liver injury quickly improved after initiation of steroid administration. This is a first case report of autoimmune hepatitis with history of HCV treatment triggered by COVID-19 vaccination.
Keywords: COVID-19, Autoimmune hepatitis, Hepatitis C, SARS-CoV-2, Vaccination
Introduction
In 2020–2021, Coronavirus Disease 2019 (COVID-19) spread throughout the world, and people in countries worldwide were recommended to receive COVID-19 vaccination as soon as possible. Although the COVID-19 vaccine was effective and the number of infections in Japan rapidly decreased, severe adverse effects of the vaccine were reported in global phase III clinical trials [1]. Some case reports have described severe liver injury diagnosed as autoimmune hepatitis after COVID-19 vaccination [2–16].
Here, we present the case of an 82-year-old woman who suffered from severe liver injury after her first dose of Pfizer-BioNTech COVID-19 vaccine. She had a history of hepatitis C virus (HCV) treatment with direct-acting antivirals (DAAs). Her clinical course and liver pathology matched the diagnostic criteria for autoimmune hepatitis type 1. Her liver injury improved quickly after steroid administration. This is the first case report of autoimmune hepatitis with history of HCV treatment triggered by COVID-19 vaccination.
Case report
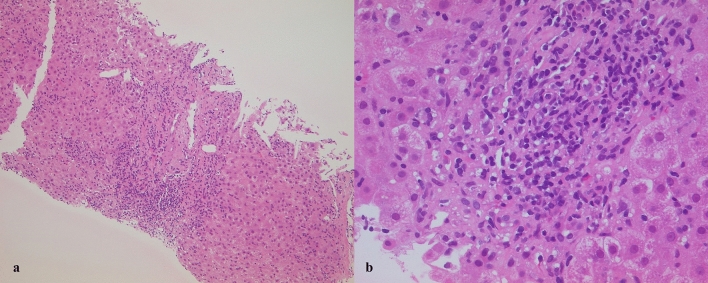
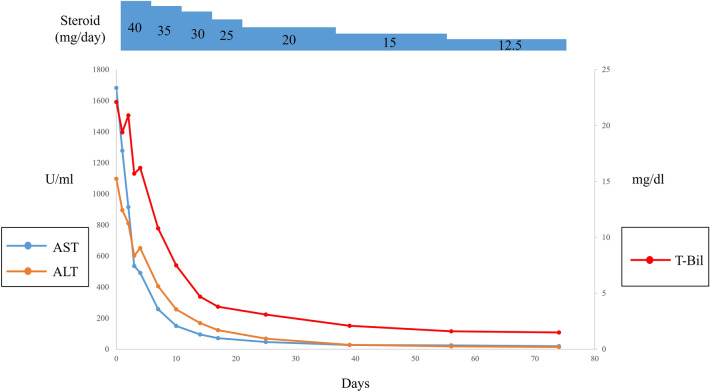
An 82-year-old woman was admitted to our university hospital because of severe liver injury. At 7 days before admission, she had received her first dose of Pfizer-BioNTech COVID-19 vaccine. She lost her appetite on the first day after the vaccination and visited a local clinic at 4 days after the vaccination. At the local clinic, blood examination findings showed severe liver injury and she was referred to our university hospital. On admission, her consciousness was clear and she had only mild fatigue. Her blood data are shown in Table 1. She had a history of HCV treatment with Pegylated Interferon, but the treatment was failed due to side effects. However, 7 years before, she had taken ledipasvir and sofosbuvir for 3 months and sustained viral response was achieved. Although HCV antibody was positive, she was negative for HCV RNA. In her blood data, IgG was elevated and anti-nuclear antibody was positive. (The value of IgG, an antinuclear antibody before DAA treatment were unknown.) Tests for hepatitis A virus antibody (IgM), hepatitis B virus surface antigen, and hepatitis E virus antibody (IgA) were negative. There were no abnormalities in abdominal computed tomography and ultrasound examinations. She had no history of drinking alcohol. We performed a liver biopsy and confirmed plasma cell infiltration and interface hepatitis in the portal area (Fig. 1a, b). In the lobular lesion, massive hepatic necrosis, a reported feature of acute-onset autoimmune hepatitis, [17] was not observed. History of drug administration other than the COVID-19 vaccine was an antihistamine drug for a skin rash that occurred before the COVID-19 vaccination. The antihistamine drug administration was completed before the COVID-19 vaccination. She was diagnosed as autoimmune hepatitis according to the revised diagnostic scoring system for autoimmune hepatitis [18]. Administration of 40 mg/day prednisolone (1 mg/kg/day) was started, and her liver enzyme and total bilirubin levels improved quickly. Her clinical course is shown in Fig. 2. Prednisolone was tapered by 5 mg/day in every week and she discharged on hospital day 14. In the outpatient clinic, her liver enzyme and bilirubin levels remained within the normal ranges and dose of prednisolone was gradually decreased.
Table 1.
Laboratory data on admission
| WBC | 4000/μL |
| Neutrophils | 57.1% |
| Eosinophils | 3.7% |
| Basophils | 1.2% |
| Monocytes | 10.4% |
| Lymphocytes | 27.6% |
| RBC | 384 × 104/μL |
| Hb | 12.2 g/dL |
| Ht | 36.3% |
| Platelets | 12.9 × 104/μL |
| AST | 1682 U/L |
| ALT | 1097 U/L |
| LDH | 421 U/L |
| ALP | 77 U/L |
| GGT | 104 U/L |
| Total bilirubin | 22.1 mg/dL |
| Direct bilirubin | 14.3 mg/dL |
| IgG | 1809 g/L |
| PT-INR | 1.30 |
| Anti-nuclear antibody | 1:40 (homogeneous) |
| Anti‐mitochondria M2 antibody | Negative |
| Hepatitis C antibody | Positive |
| Hepatitis C RNA real-time RT-PCR | Not detected |
| Hepatitis B surface antigen | Negative |
| Hepatitis A IgM antibody | Negative |
| Hepatitis E IgA antibody | Negative |
| Epstein-barr virus viral capsid antigen (VCA) antibody (IgM) | Negative |
| Cytomegalovirus (CMV) IgM antibody | Negative |
Fig. 1.
Hematoxylin–eosin-stained liver biopsy section showing plasma cell infiltration and interface hepatitis in the portal area (a magnification × 100; b magnification ×400)
Fig. 2.
Trends in aspartate aminotransferase, alanine aminotransferase, and total bilirubin following the initiation of steroid administration. AST aspartate aminotransferase, ALT alanine aminotransferase, T-Bil total bilirubin
Discussion
Prior to the COVID-19 pandemic, autoimmune hepatitis triggered by vaccines such as those for influenza [19] or hepatitis A [20] had been reported. The mechanism for the triggering of autoimmune hepatitis after vaccination was presumed to involve autoantibody formation in response to antigenic determinants in the vaccines. COVID-19 vaccines were shown to be effective and spread throughout the world in 2020–2021. However, some case reports described a relationship between COVID-19 vaccines and autoimmune hepatitis [2–16]. There have already been 20 reported cases of autoimmune hepatitis triggered by COVID-19 vaccination (Table 2). In Table 2 cases, 19 out of 20 cases were positive for antinuclear antibodies. The pattern of antinuclear antibodies was mentioned in ten cases (speckled/homogeneous/speckled + homogeneous/speckled + discrete speckled/centromere/granular: 4/2/1/1/1/1). Speckled and homogeneous were the common patterns in past reports. The pattern of antinuclear antibody in our case was homogeneous, similar to past reports. Latency period varied from 4 to 42 days as shown in Table 2. Regarding other vaccines, Sasaki T. reported that in two cases, latency periods were 7 days and 30 days respectively after vaccination against influenza [19]. On the other hand, P-A Berry reported autoimmune hepatitis occurred 10 days after vaccination against hepatitis A [20]. In light of these data, latency period did not differ from COVID-19 vaccines and other vaccines. Autoimmune hepatitis mostly occurred after the first dose of COVID-19 vaccine (15 out of 20), similar to the present case. However, in four cases, autoimmune hepatitis occurred after the second dose of COVID-19 vaccine, so that physicians have to pay attention even if there were no side effect after the first dose of vaccine. As shown in Table 2, the three major COVID-19 vaccines (Pfizer, Moderna, and AstraZeneca) have all been reported to trigger autoimmune hepatitis. Similar to the present case, steroid administration was effective for recovery in 17 out of 19 cases. Two patients died due to autoimmune hepatitis triggered by viral vector vaccine (AstraZeneca). One death case was administered steroid after prolongation of prothrombin time [2]. Another death case had already worsened up to liver failure at the time of hospitalization [15]. Although steroid is effective for autoimmune hepatitis triggered by COVID-19 vaccination, if it progresses to liver failure, the risk of death would increase. Long-term outcome of autoimmune hepatitis triggered by COVID-19 vaccination has not been revealed yet. We think low dose long-term corticosteroid therapy should be continued because autoimmune hepatitis after influenza vaccination needed low-dose 2 years corticosteroid therapy [19].
Table 2.
List of autoimmune hepatitis cases triggered by COVID-19 vaccination
| Age | Gender | Vaccine | Latency period (days) | AST/ ALT (U/L) | T-bil (mg/dL) | Treatment | Outcome | References |
|---|---|---|---|---|---|---|---|---|
| 38 | Female | AstraZeneca | 14 | 1101/1025 | 2.86 | 30 mg prednisolone | Improved | Mohamed Rela [2] |
| 62 | Male | AstraZeneca | 16 | 1361/1094 | 19.2 | 30 mg prednisolone | Death | Mohamed Rela [2] |
| 76 | Female | Moderna | NA | 811/579 | 3.8 | 40 mg prednisolone | Improved | Elise Vuille-Lessard [3] |
| 63 | Male | Moderna | 7 | 1127/1038 | 11.98 | 40 mg prednisolone | Improved | Michele Ghielmetti [4] |
| 41 | Female | Moderna | 7 | 993/1312 | 2.3 | 1 mg/kg prednisolone | Improved | Maria-Carlota Londoño [5] |
| 36 | Male | AstraZeneca | 26 | 633/1774 | 0.99 | 60 mg prednisolone | Improved | Daniel Clayton-Chubb [6] |
| 80 | Female | Pfizer | 7 | 1401/1186 | 10.5 | 1 mg/kg prednisolone | Improved | Alba Rocco [7] |
| 35 | Female | Pfizer | 7 | 754/2001 | 4.8 | 20 mg prednisone | Improved | Fernando Bril [8] |
| 71 | Female | Moderna | 4 | ALT 1067 | 15.79 | 40 mg prednisolone | Improved | Cathy McShane [9] |
| 65 | Female | Moderna | 14 | 1056/1092 | 1.14 | 60 mg prednisolone | Improved | Isabel Garrido [10] |
| 40 | Female | Pfizer | 30 | 4 × upper limit of normal | NA | 40 mg prednisolone | Improved | Panagiota Palla [11] |
| 56 | Female | Moderna | 42 | 1124/1701 | 5.96 | Budesonide | Improved | Chin Kimg Tan [12] |
| 80 | Female | Pfizer | 10 | 995/974 | 9.8 | 0.8 mg/kg prednisone | Improved | Yuji Suzuki [13] |
| 75 | Female | Pfizer | 4 | 1085/820 | 17.7 | 1.0 mg/kg prednisone | Improved | Yuji Suzuki [13] |
| 78 | Female | Pfizer | 7 | 401/542 | 1.3 | 0.6 mg/kg prednisone | Improved | Yuji Suzuki [13] |
| 61 | Female | Pfizer | 14 | 913/455 | 11.8 | 40 mg prednisolone | Improved | Enver Avci [14] |
| 80 | Female | Pfizer | 10 | 583/541 | 4.56 | 1.0 mg/kg steroid | Improved | Domitille Erard [15] |
| 73 | Female | Moderna | 21 | 1163/1027 | 19.53 | 1.0 mg/kg steroid | Improved | Domitille Erard [15] |
| 68 | Female | AstraZeneca | 20 | 2314/2029 | 43.98 | NA | Death | Domitille Erard [15] |
| 79 | Male | AstraZeneca | 15 | 2003/1994 | 11.9 | Hydrocortisone 1000 mg for 3 days, switched to prednisone 50 mg, azathioprine 50 mg | Improved | Laura Camacho-Domínguez [16] |
| 82 | Female | Pfizer | 7 | 1682/1097 | 22.1 | 40 mg prednisolone | Improved | Our case |
NA not available, AST aspartate aminotransferase, ALT alanine aminotransferase, T-bil total bilirubin
Regarding the predisposition to autoimmune disease and vaccination, David C Wraith et al. described in Lancet review article that patients with an autoimmune disease are not at risk of exacerbation after administration of any of the available vaccines [21]. On the other hand, Simonetta Salemi et al. mentioned that mechanism of autoimmunity induced by vaccination had relation to bystander activation and innate immunity [22]. Although mechanism of autoimmunity induced by vaccination has not yet totally been elucidated due to its complexity, we have to pay attention to predisposition to autoimmune disease at the time of vaccination.
HCV infection was also shown to be related to autoimmune diseases, including autoimmune hepatitis [23]. DAAs were recently developed as the mainstream drugs for HCV treatment, and the rate of successful treatment has markedly improved. However, there are some case reports in which autoimmune hepatitis occurred after successful elimination of HCV with DAAs [24] or interferon-based regimens [25]. These reports suggest that even when HCV clearance has been achieved, the risk of autoimmune hepatitis development in patients may remain. Our case had a history of HCV treatment with DAAs 7 years previously and therefore had a risk of autoimmune hepatitis development before COVID-19 vaccination. Regarding the correlation between HCV and COVID-19 vaccines, reactivation of HCV after COVID-19 vaccination was also reported [26]. In our case, although HCV antibody was positive, HCV RNA was negative by real-time RT-PCR, meaning that reactivation of HCV was unlikely.
This is the first report of autoimmune hepatitis with history of HCV treatment triggered by COVID-19 vaccination. Japan is one of the countries with the highest infection rate of HCV in the world. The number of HCV infected people in Japan is reported about 2,000,000 [27]. With regard to autoimmune hepatitis and HCV reactivation, Japanese hepatologists should pay attention to not only patients with current HCV infection, but also patients with past HCV infection upon COVID-19 vaccination.
Acknowledgements
The authors thank Alison Sherwin, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
None.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The authors declare that this study conformed to the Declaration of Helsinki.
Informed consent
Informed consent was obtained from the patient for being included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rela M, Jothimani D, Vij M, et al. Auto-immune hepatitis following COVID vaccination. J Autoimmun. 2021 doi: 10.1016/j.jaut.2021.102688. [DOI] [PubMed] [Google Scholar]
- 3.Vuille-Lessard E, Montani M, Bosch J, et al. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J Autoimmun. 2021 doi: 10.1016/j.jaut.2021.102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghielmetti M, Schaufelberger HD, Mieli-Vergani G, et al. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity? J Autoimmun. 2021 doi: 10.1016/j.jaut.2021.102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Londono MC, Gratacos-Gines J, Saez-Penataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination—still casualty? J Hepatol. 2021;75:1248–1249. doi: 10.1016/j.jhep.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniel C-C, Daniel S, Elliot F, et al. Autoimmune hepatitis developing after the ChAdOx1 nCoV-19(Oxford-AstraZeneca) vaccine. J Hepatol. 2021;75:1249–1250. doi: 10.1016/j.jhep.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocco A, Sgamato C, Compare D, et al. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casuality. J Hepatol. 2021;75:728–729. doi: 10.1016/j.jhep.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bril F, Al Diffalha S, Dean M, et al. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol. 2021;75:222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McShane C, Kiat C, Rigby J, et al. The mRNA COVID-19 vaccine—a rare trigger of autoimmune hepatitis? J Hepatol. 2021;75:1252–1254. doi: 10.1016/j.jhep.2021.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrido I, Lopes S, Simoes MS, et al. Autoimmune hepatitis after COVID-19 vaccine—more than a coincidence. J Autoimmun. 2021 doi: 10.1016/j.jaut.2021.102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palla P, Vergadis C, Sakellariou S, et al. Letter to the editor: autoimmune hepatitis after COVID-19 vaccination. a rare adverse effect? Hepatology. 2022;75:489–490. doi: 10.1002/hep.32156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan CK, Wong YJ, Wang LM, et al. Autoimmune hepatitis following COVID-19 vaccination: true causality or mere association? J Hepatol. 2021;75:1250–1252. doi: 10.1016/j.jhep.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki Y, Kakisaka K, Takikawa Y. Letter to the editor: autoimmune hepatitis after COVID-19 vaccination: need for population-based epidemiological study. Hepatology. 2021 doi: 10.1002/hep.32280. [DOI] [PubMed] [Google Scholar]
- 14.Avci E, Abasiyanik F. Autoimmune hepatitis after SARS-CoV-2 vaccine: new-onset or flare-up? J Autoimmun. 2021 doi: 10.1016/j.jaut.2021.102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erard D, Villeret F, Lavrut PM, et al. Autoimmune hepatitis developing after COVID 19 vaccine: presumed guilty? Clin Res Hepatol Gastroenterol. 2021 doi: 10.1016/j.clinre.2021.101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camacho-Domínguez L, Rodríguez Y, Polo F, et al. COVID-19 vaccine and autoimmunity a new case of autoimmune hepatitis and review of the literature. J Transl. 2022 doi: 10.1016/j.jtauto.2022.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stravitz RT, Lefkowitch JH, Fontana RJ, et al. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology. 2011;53:517–526. doi: 10.1002/hep.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez F, Berg PA, Bianchi FB, et al. International autoimmune hepatitis group report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:928–938. doi: 10.1016/S0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki T, Suzuki Y, Ishida K, et al. Autoimmune hepatitis following influenza virus vaccination: two case reports. Med (Baltimore) 2018 doi: 10.1097/MD.0000000000011621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry P, Smith-Laing G. Hepatitis a vaccine associated with autoimmune hepatitis. World J Gastroenterol. 2007;13:2238–2239. doi: 10.3748/wjg.v13.i15.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David Wraith C, Goldman M, Lambert P-H. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362(1659):1666. doi: 10.1016/S0140-6736(03)14802-7. [DOI] [PubMed] [Google Scholar]
- 22.Salemi S, D’Amelio R. Could autoimmunity be induced by vaccination? Int Rev Immunol. 2010;29(247):269. doi: 10.3109/08830181003746304. [DOI] [PubMed] [Google Scholar]
- 23.Jadali Z, Alavian S-M. Autoimmune diseases co-existing with Hepatitis C virus infection. Iran J Allergy Asthma Immunol. 2010;4:191–206. [PubMed] [Google Scholar]
- 24.Monton C, Mf E, Pascual I. The development of type-1 autoimmune hepatitis after chronic hepatitis C (HCV) clearance by direct-acting antivirals (DAA) Rev Esp Enferm Dig. 2020;112:664–665. doi: 10.17235/reed.2020.6785/2019. [DOI] [PubMed] [Google Scholar]
- 25.Efe C, Heurgué-Berlot A, Ozaslan E, et al. Late autoimmune hepatitis after hepatitis C therapy. Eur J Gastroenterol Hepatol. 2013;25:1308–1311. doi: 10.1097/MEG.0b013e328361c704. [DOI] [PubMed] [Google Scholar]
- 26.Lensen R, Netea MG, Rosendaal FR. Hepatitis C Virus reactivation following COVID-19 vaccination—a case report. Int Med Case Rep J. 2021;14:573–576. doi: 10.2147/IMCRJ.S328482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung H, Ueda T, Kudo M. Changing trends in hepatitis C infection over the past 50 years in Japan. Intervirology. 2010;53:39–43. doi: 10.1159/000252782. [DOI] [PubMed] [Google Scholar]