Abstract
Background:
Evidence from adult psoriasis studies implicates an imbalance between regulatory and effector T cells, particularly TH-17–producing T cells, in the pathogenesis of psoriasis. Little is known about the immunopathology of psoriasis in children.
Objective:
We sought to functionally characterize the inflammatory cell profiles of psoriatic plaques from pediatric patients and compare them with healthy, age-matched controls and adult psoriasis patients.
Methods:
Skin samples from pediatric psoriasis patients and healthy controls were analyzed by multiparameter flow cytometry to determine the dominant immune cell subsets present and cytokines produced.
Results:
Lesional tissue from pediatric psoriasis patients had significantly increased interleukin (IL) 22 derived from CD4+ and CD8+ cells compared with the tissues from healthy pediatric controls and adult psoriasis patients. Tissue from pediatric psoriasis patients had significantly less elevation of IL-17 derived from CD4+ and CD8+ cells compared with the tissue from adult psoriasis patients. In contrast with the lesions from adult patients, lesional skin in pediatric patients with psoriasis did not have increases in regulatory T cells.
Limitations:
This is a pilot study, thus the sample size is small.
Conclusion:
Significant differences in IL-17 and IL-22 expression were observed in the pediatric psoriasis patients compared with pediatric healthy controls and adult psoriasis patients. IL-22 might be relevant in the pathogenesis of pediatric psoriasis and represents a potential treatment target unique to pediatric psoriasis.
Keywords: IL-17, IL-22, immunophenotype, pediatric psoriasis, psoriasis
CAPSULE SUMMARY
Little is known about the pathophysiology of pediatric psoriasis.
Increased expression of interleukin (IL) 22 relative to IL-17 was observed in pediatric compared with adult psoriasis patients.
Elevated expression of IL-22, more so than IL-17, in pediatric compared to adult psoriatic plaques suggests an additional potential treatment target unique to pediatric psoriasis.
Psoriasis is a T-cell–mediated, chronic inflammatory skin condition that affects children and adults. Evidence from studies with adult psoriasis patients implicates an imbalance between regulatory and effector T cells, particularly TH-17–producing T cells, in the pathogenesis of psoriasis.1–3 Pediatric psoriasis often differs from adult psoriasis in presentation, triggers, natural history, and response to therapy, suggesting potential differences in the pathophysiology of the disease processes.4 To date, studies examining the immunology of psoriasis have largely focused on adult patients. In psoriasis patients, innate and adaptive immune responses are thought to stimulate T-cell activation and induce helper T-cell differentiation to produce proinflammatory cytokines, including interferon (IFN) γ, interleukin (IL) 17, IL-22, IL-23, and tumor necrosis factor (TNF) α.5 Serum cytokine levels have been determined to be significantly higher in adult patients with active psoriasis compared with controls.6 Multiple groups have looked at serum cytokine levels in association with clinical disease severity and found that increased levels of cytokines IFN-γ, IL-6, IL-12, IL-17, IL-18, IL-20, IL-22, and IL-23 positively correlated with severity of psoriasis.6,7 Analysis of the cell and cytokine composition in active psoriasis plaques of adult patients has confirmed a change in the composition of skin inflammatory cells, further suggesting that inflammatory cytokines are responsible for driving the disease process. Nograles et al proposed a working model in which normal dermal CD4+ cells that produce IL-17 and IL-22 become pathogenic during inflammation and stimulate the production of chemokines that attract neutrophils to the psoriatic lesions.8 Using a multiparameter flow cytometric approach, we have found that psoriatic lesional skin had increased numbers of regulatory T cells (T regs) and that these cells produced more IL-17 when compared with T regs found in nonlesional skin.2 These findings potentially explain why adult psoriasis responds to targeted biologic therapies against TNF-α and IL-17, among others.
Although there are robust clinical and molecular data to guide treatment innovations for adult psoriasis, psoriasis in children remains understudied. To date in the United States, only 1 systemic therapy is licensed for use by the Food and Drug Administration to treat psoriasis in children. The TNF-α inhibitor etanercept was approved in November 2016 for moderate-to-severe plaque psoriasis in children ≥4 years of age.9 This approval was based largely on data from 2 clinical trials.10,11 Little is known about the immunologic differences between childhood and adult psoriasis that might correlate with the observed clinical differences between the 2 populations. Although the immune composition of the peripheral blood of pediatric psoriasis patients has been recently reported,3 the cutaneous inflammatory cell infiltrates and cytokine profiles of lesional psoriatic skin from children has not been defined. Identifying the specific inflammatory cell and cytokine milieu in children is the next step towards understanding the potential molecular underpinnings of the observed clinical differences and might identify optimal treatment targets. To this end, using multiparameter flow cytometry, we functionally characterized the cell profiles of psoriatic plaques of pediatric patients and compared them with the inflammatory profiles of healthy, age-matched controls and adult psoriasis patients.
METHODS
Participants
Psoriatic skin samples were obtained from a consecutive sample of consenting children who presented to the University of California, San Francisco (UCSF), Pediatric Dermatology Clinic for routine clinical care. Study patients were recruited at the time of clinical visits and from a contact list of patients with psoriasis who indicated willingness to participate in clinical studies. All patients provided written, informed consent, and for minors, dual parental consent and patient assent was obtained before enrollment in the study. Detailed clinical characteristics were recorded by study personnel and included patient demographics, family history, age of onset, comorbidities, morphologic subtype, distribution, and psoriasis body surface area (BSA) of involvement. Disease activity was scored by using clinician judgment and disease severity by using a psoriasis severity grading scale as follows: mild <10% BSA, moderate 10%–20% BSA, severe >20% BSA, very severe >50% BSA. Patients 4–20 years of age with active psoriasis were eligible to be included in the study. Exclusion criteria included use of any systemic or phototherapy within 4 weeks or topical treatment within 2 weeks of enrollment.
Normal skin was obtained from pediatric patients undergoing plastic surgical reconstructive or excisional procedures at UCSF from which benign normal tissue margins were available and would have otherwise been discarded. Adult psoriasis skin from patients 20–76 years of age was obtained from the UCSF General Dermatology clinics. Normal, healthy adult skin was obtained from patients undergoing elective surgery at UCSF and has been detailed elsewhere.2 Site, age, and sex data were available for all normal control tissues in accordance with institutional policies. This study was approved by the UCSF Committee on Human Research (IRB 10–02830, reference 169060).
Skin biopsy and flow cytometry
A 4-mm punch biopsy was obtained from a clinically active psoriasis plaque; control samples were obtained from 4-mm punch biopsies of discarded normal skin from an operating room. The tissue sample was stored at 4°C in a container with sterile gauze and phosphate buffered saline until it was ready to be processed. Following the UCSF Dermatology Cutaneous Immunology Core Facility’s protocol to isolate and functionally characterize the leukocyte population of the skin, the tissue was trimmed to remove subcutaneous fat and hair and the skin was finely minced and mixed with digestion buffer consisting of 0.8 mg/mL collagenase type 4 (4188; Worthington Biochemical Corporation, Lakewood, NJ), 0.02 mg/mL DNAse (DN25–1G; Sigma-Aldrich, St. Louis, MO), 10% fetal bovine serum, 1% HEPES, and 1% penicillin/streptavidin in RPMI medium.2 After overnight incubation in 5% CO2, samples were harvested with wash buffer, double filtered, centrifuged, and counted.2 Cells were then stimulated with Cell Stimulation Cocktail 500X (Tonbo Biosciences, San Diego, CA) and incubated for 4 hours. For flow cytometry analysis, viability stain Ghost Dye Violet 510 (Tonbo Biosciences) and the following antibodies were used: anti-hCD3 PerCP (Biolegend, San Diego, CA), anti-hIL-13 FITC (eBioscience, Waltham, MA), anti-hTNF-α PE-Cy7 (BD Pharmingen, San Jose, CA), anti-hCD4 PE-Texas Red (Invitrogen, Carlsbad, CA), anti-hIL-22 PE (R&D Systems, Minneapolis, MN), anti-hCD45 APC-eFluor 780 (eBioscience), anti-hIFN-γ Alexa Fluor 700 (Biolegend), anti-hIL-17A eFluor 660 (eBioscience), anti-hFoxp3 eFluor 450 (eBioscience), and anti-hCD8a eVolve 605 (eBioscience). The LSRFortessa flow cytometer (BD Biosciences, San Jose, CA) was used for data acquisition and results were analyzed using FlowJo software (Tree Star Inc, Ashland, OR).
Statistical analyses
Means and frequencies of cells and cytokines obtained using the FlowJo software were reported using descriptive statistics. The inflammatory cell and cytokine profiles of pediatric psoriasis patients were compared with that of pediatric healthy controls and adult psoriasis patients separately by using independent 2-sample t tests.
RESULTS
The pediatric psoriasis cohort consisted originally of 12 patients. Two samples were discarded for technical issues related to sample processing, resulting in a final cohort of 10 pediatric psoriasis patients who provided a total of 11 tissue samples. Patient demographics and clinical features of the pediatric psoriasis cohort are summarized in Table I. Eight of 10 patients in this group were boys and their mean age was 12.1 years. Most had mild psoriasis at the time of biopsy, but moderate-to-severe disease was reported by all but 3 pediatric patients at some point during the course of their disease. Two had ongoing moderate and 1 had very severe disease at the time of enrollment. Age at formal diagnosis ranged from 1 to 17 years. Of those who reported a family history, 7/7 had a positive first-degree or second-degree family member with psoriasis. All patients had plaque psoriasis at the time of enrollment, and 4/10 initially had guttate psoriasis. All patients had involvement of the head and neck area and most had involvement of the trunk, extremities, and anogenital area. Previously used therapies included topical steroids (in 100%), calcipotriene (in a few), and systemic therapy including acitretin and phototherapy (4 patients).
Table I.
Pediatric psoriasis cohort characteristics
| Pt | Sex/Age,* years | Initial psoriasis type | Type at time of biopsy | Age at time of biopsy, years | Site of biopsy | Areas of involvement | Severity at time of biopsy | Severity at worst | Prior systemic therapy | Prior photo-therapy | Comorbidities | Family history of psoriasis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/10 | Plaque | Plaque | 12 | Scalp | Head, neck | Mild | Mild | No | No | None | Yes |
| 2 | M/4 | Plaque | Plaque | 12 | Scalp | Head, neck, extremities, trunk | Mild | Severe | Yes | Yes | None | Yes |
| 3 | F/1 | Plaque | Plaque | 11 | R elbow | Head, neck, extremities, trunk, palms, anogenital, nails | Mild | Severe | No | No | None | No |
| 4 | M/10 | Guttate | Plaque | 11 | Lower R leg | Head, neck, extremities, trunk | Moderate | Moderate | No | No | Joint pain | Yes |
| 5 | M/1 | Plaque | Plaque | 7 | Leg | Head, neck, extremities, anogenital, nails | Mild | Moderate | No | No | None | No |
| 6 | M/15 | Guttate | Plaque | 17 | Trunk | Head, neck, extremities, trunk, anogenital | Moderate | Moderate | No | Yes | None | Yes |
| 7 | M/17 | Guttate | Plaque | 19 | Trunk | Head, neck, extremities, trunk, anogenital | Mild | Mild | No | No | None | Yes |
| 8 | M/4 | Plaque | Plaque | 11 | R lower back | Head, neck, extremities, trunk, anogenital, soles | Very severe | Very severe | No | Yes | None | Yes |
| 9 | F/8 | Plaque | Plaque | 17 | Scalp and abdomen | Head, neck, extremities, trunk, anogenital | Mild | Moderate | No | No | None | No |
| 10 | M/2 | Guttate | Plaque | 4 | R arm | Head, neck, extremities, trunk, anogenital | Mild | Mild | No | No | None | Yes |
Areas of involvement include extremities (arms, elbows, legs, and knees) and trunk (abdomen, flanks, and back).
Psoriasis severity is classified as mild (<10% BSA), moderate (10%–20% BSA), severe (>20% BSA), and very severe (>50% BSA).
Patients were classified as having a family history of psoriasis if they had a first-degree or second-degree relative with psoriasis.
BSA, Body surface area; F, female; L, left; M, male; Pt, patient; R, right.
Age at diagnosis.
We comprehensively quantified T-cell subsets and intracellular cytokine production in lesional skin of adult and pediatric psoriasis patients compared with normal healthy control skin (Table II). Our flow cytometric gating strategy and representative intracellular cytokine staining is shown in Fig 1. Consistent with our previous findings,2 when compared with healthy skin from adults, lesional skin of adults with psoriasis has an increased CD4:CD8 ratio and increased IL-17–producing CD4+ T cells (Fig 2, A and B). In contrast, pediatric patients with psoriasis did not have an increased CD4:CD8 ratio when compared with healthy pediatric skin (Fig 2, A). In addition, there was a minimal increase in the IL-17–producing CD4+ T cells in the skin from pediatric patients with psoriasis compared with the skin from controls and a significant reduction compared with the skin from adults with psoriasis (Fig 2, B). CD8+ T cells in both adult and pediatric psoriatic skin produce more IL-17 compared with their respective healthy control skin; however, this increase was smaller in pediatric psoriasis patients (Fig 2, C). Moreover, CD8+ T cells in pediatric psoriatic skin produce significantly less IL-17 compared with these cells in adult psoriatic skin (Fig 2, C). In contrast with adult skin and healthy pediatric skin, CD4+ T cells in pediatric psoriatic skin express high levels of IL-22 (Fig 2, D). An increase in IL-22 expression in pediatric psoriatic skin was also observed with CD8+ T cells (Fig 2, E). Consistent with our previous findings,2 T regs (defined as CD45+CD3+CD4+Foxp3hiCD27hi) were increased in adult psoriatic skin compared with adult control skin (Fig 2, F). This increase was not observed in pediatric psoriatic skin; however, it should be noted that T reg percentages are increased in healthy pediatric skin compared with healthy adult skin, suggesting a difference in baseline percentages of this T-cell subset between adults and children (Fig 2, F). IL-13–expressing CD4+ T cells were reduced in both adult and pediatric psoriatic skin compared with healthy control skin; however, this reduction was attenuated in pediatric psoriatic skin (Fig 2, G).
Table II.
Mean percentages of immune cell types within sampled tissues
| Category | Healthy kids, % | Kids with psoriasis, % | P value† | Adults with psoriasis, % | P value‡ |
|---|---|---|---|---|---|
| CD4:CD8 | 2.9 | 1.79 | .11 | 3.72 | .05 |
| T regs | 32.14 | 30.9 | .82 | 30.74 | .98 |
| IL-17A CD4+ | 8.96 | 13.78 | .07 | 23.46 | .02* |
| IL-17A CD8+ | 2.46 | 11.78 | .02* | 31.23 | .02* |
| IL-22 CD4+ | 3.59 | 11.22 | .008* | 3.71 | .01* |
| IL-22 CD8+ | 1.38 | 8.71 | .004* | 4.76 | .15 |
| IL-13 CD4+ | 6.86 | 2.9 | .04* | 1.07 | .046* |
| IL-13 CD8+ | 1.07 | 1.39 | .59 | 3.97 | .13 |
IL, Interleukin; T regs, regulatory T cells.
Statistically significant at P < .05.
P values of independent t tests comparing mean percentages of healthy kids and kids with psoriasis.
P values of independent t tests comparing mean percentages of kids with psoriasis and adults with psoriasis.
Fig 1.
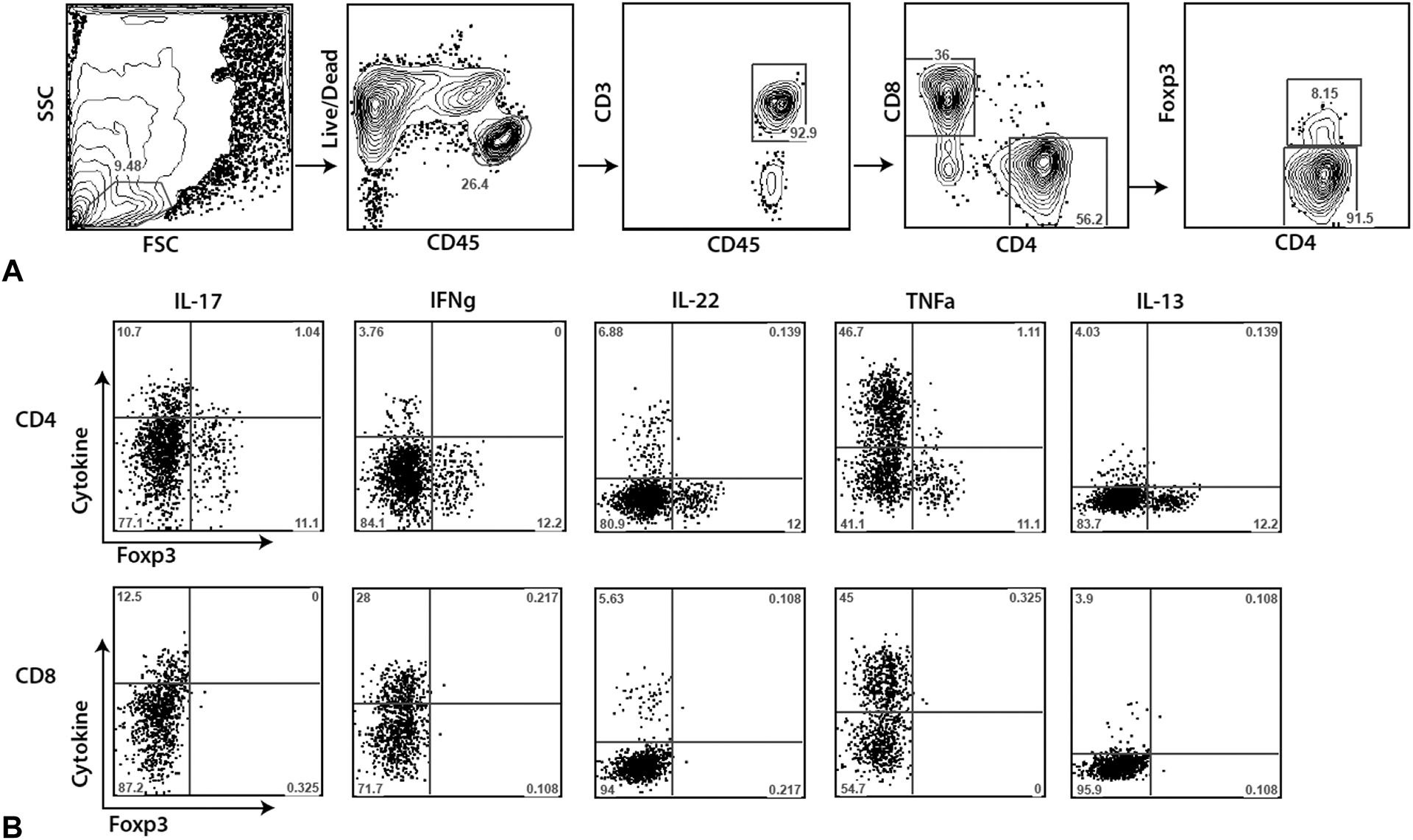
Flow cytometric gating strategy and representative data. Four millimeter skin punch biopsies were obtained from healthy adults, healthy children, adults with psoriasis, and children with psoriasis. Single cell suspensions were prepared and stained for multi-parameter flow cytometry. A, Representative gating strategy for CD4+ T cells, CD8+ T cells, and Tregs. B, Representative flow cytometry staining for intracellular cytokine production from a biopsy taken from lesional skin of a pediatric patient with psoriasis. Cells are pregated as shown in A. Numbers represent percentage of cells that fall within each gate or quadrant. FSC, Forward scatter; IFN-γ, interferon γ; IL, interleukin; SSC, side scatter; TNF-α, tumor necrosis factor α.
Fig 2.
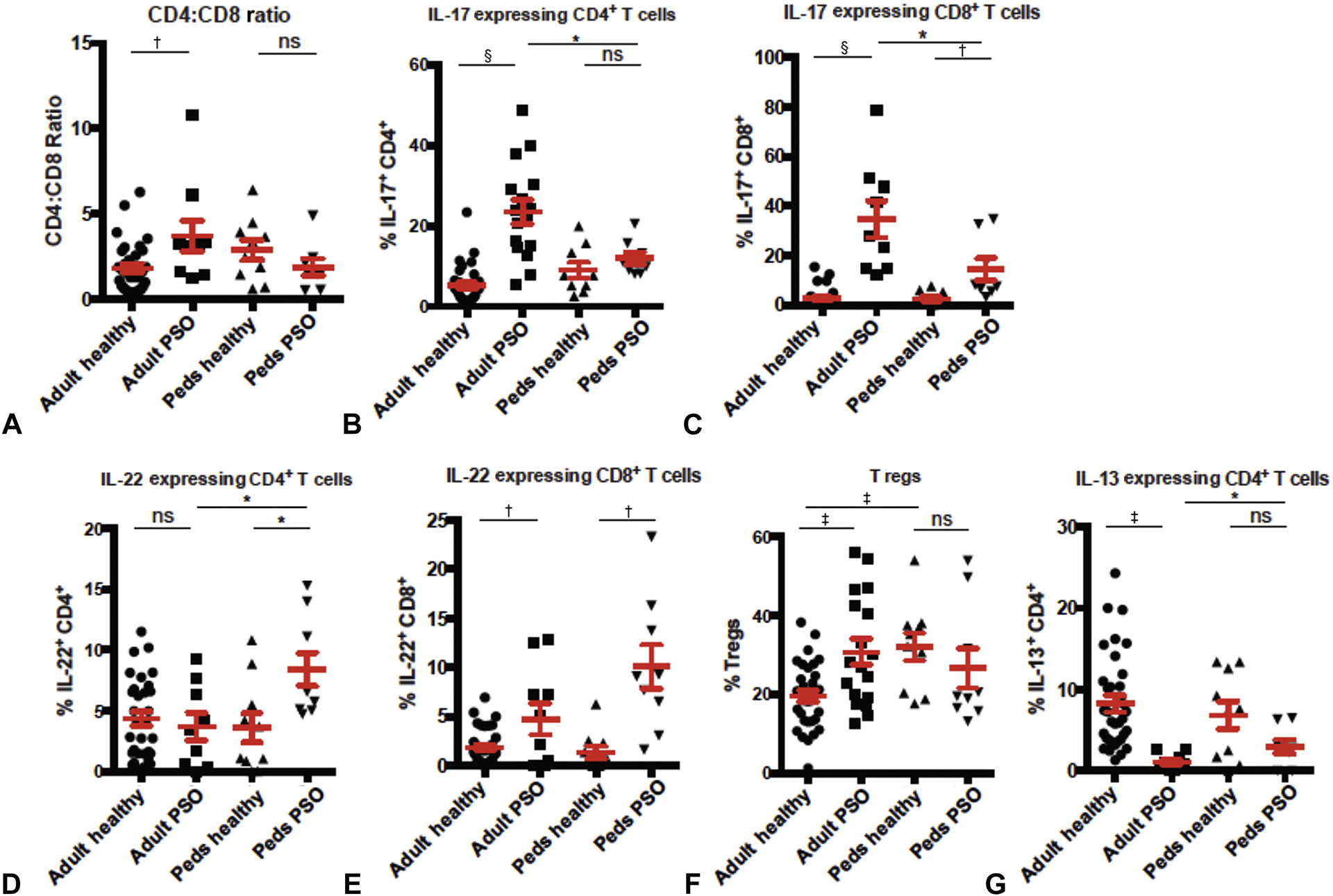
Flow cytometric quantification of T-cell subsets found in pediatric and adult psoriatic skin. Four-millimeter, punch biopsy skin specimens from adult and pediatric psoriatic skin, as well as adult and pediatric healthy skin (from unaffected donors) were enzymatically digested and subjected to multiparameter flow cytometry. A, The CD4:CD8 ratio of skin T cells; cells were pre-gated on live CD45+CD3+ cells. B, Percentage of IL-17–producing CD4+ T cells in skin as detected by intracellular cytokine staining; cells were pre-gated on live CD45+CD3+CD8−CD4+ cells. C, Percentage of IL-17–producing CD8+ T cells in skin as detected by intracellular cytokine staining; cells were pre-gated on live CD45+CD3+CD4−CD8+ cells. D, Percentage of IL-22–producing CD4+ T cells in skin as detected by intracellular cytokine staining; cells were pre-gated on live CD45+CD3+CD8−CD4+ cells. E, Percentage of IL-22–producing CD8+ T cells in skin as detected by intracellular cytokine staining; cells were pre-gated on live CD45+CD3+CD4−CD8+ cells. F, Percentage of T regs; cells were pre-gated on live CD45+CD3+CD8−CD4+Foxp3+CD27+ cells. G, Percentage of IL-13–producing CD4+ T cells in skin as detected by intracellular cytokine staining; cells were pre-gated on live CD45+CD3+CD8−CD4+ cells. Each symbol represents an individual skin biopsy specimen. *P < .05, †P < .005, ‡P < .0005, §P < .00005 by unpaired Student t test. IL, Interleukin; peds, pediatric; PSO, psoriatic; ns, nonsignificant; T regs, regulatory T cells. Bars represent mean values. Whiskers are standard error of the mean.
DISCUSSION
Pediatric psoriasis differs substantially from adult psoriasis in terms of clinical features, genetic risk variants, triggering factors, and disease course.12 The pathophysiology and tissue localized immunopathology of psoriasis in children is understudied compared with adults. We have found that lesional skin of patients with pediatric psoriasis is fundamentally different than that of adult psoriasis with respect to skin-infiltrating immune cell subsets and the cytokines they produce. Most strikingly, pediatric psoriasis is associated with higher levels of IL-22–producing T cells and relatively less IL-17–producing T cells compared with adult psoriasis. Recently, Zhang et al found that increased circulating TH17 and T reg cell frequencies correlated with disease severity in pediatric patients with psoriasis.3 This work supports the importance of IL-17 as a therapeutic target and suggests that pediatric psoriasis might also respond well to anti–IL-17 biologic therapies. We observed significant differences in the levels of IL-22 between study populations, suggesting a potentially important difference in disease immunopathology between children and adults. We identified elevated IL-22 production in pediatric psoriasis plaques compared to healthy pediatric skin as well as adult psoriasis plaques. IL-22 is a strong stimulant of keratinocyte proliferation and has been shown to induce psoriasis-like epidermal changes in animal models.13,14 Nograles et al suggested that IL-22 and IL-17 serve as distinct drivers in psoriasis development, with IL-17 being proinflammatory and IL-22 retarding keratinocyte differentiation.8 Nikamo et al recently reported that a genetic IL-22 variant which promotes epithelial barrier defense is preferentially enriched in and may precipitate the onset of psoriasis at an early age.15 Together with our results showing an enrichment of IL-22–producing T cells in pediatric psoriasis lesions, this suggests that, in part, pediatric psoriasis might stem from an IL-22–related defective differentiation of epidermal cells. Importantly, our data suggest that pediatric psoriasis tissue retains an IL-17 signature. However, it is less pronounced when compared with adults, whereas the IL-22 signature is more pronounced in pediatric versus adult psoriasis tissue. These findings support a potential role for anti–IL-17 therapy and, in addition, suggest that anti–IL-22 therapy might also be efficacious for pediatric psoriasis.
The main limitation of this pilot study is the small sample size. Results need to be confirmed with larger subsets of patients. The small sample size did not allow for comparisons of immunophenotype between pediatric patients of different ages and with different clinical and morphologic features. It would be worthwhile to compare patients who had severe disease at any point in their clinical history with those who have consistently mild disease and to assess differences between those with one consistent clinical phenotype versus those who progressed from initial guttate to plaque morphology. Patients with pediatric onset psoriasis compared with those with adult onset psoriasis have a higher frequency of a positive family history of psoriasis.16–18 It remains unknown whether pediatric patients with a positive family history have a different cytokine profile than pediatric and adult patients without a positive family history.
In summary, significant differences in IL-17 and IL-22 expression were observed in the pediatric psoriasis patients compared with healthy pediatric controls and adult psoriasis patients. Clarifying immunophenotypic differences might further our understanding of the observed clinical differences between children and adults with psoriasis. Targeted therapy against IL-17 exists and warrants trials in psoriatic children. IL-22 might be relevant in the pathogenesis of pediatric psoriasis and biologics are being developed to target this cytokine. Because this represents a potential treatment target unique to pediatric psoriasis, further investigation is necessary. Future studies should assess in situ localization of IL-17 and IL-22–producing T cells in adult versus pediatric skin to discern whether these cells might be occupying distinct anatomic niches and thus may contribute to tissue pathology in different ways. Quantification of serum IL-17 versus IL-22 concentrations in adult and pediatric psoriasis patients will elucidate the relative difference in these cytokines between these patient populations and validate what we have observed at the tissue level.
Funding sources:
Supported by a William Weston pilot grant from the Society for Pediatric Dermatology. Drs Rosenblum and Cordoro are supported by Dermatology Foundation Career Development Awards.
The authors would like to gratefully acknowledge Drs Dana Feigenbaum and Regina Celeste Ahmad for administrative contributions, patient recruitment, and important intellectual contributions; Ms Di Yan for enrolling adult patients; Drs Mio Nakamura and Sahil Sekhon for adult biopsy procurement; the pediatric dermatology faculty at UCSF, including Drs Ilona Frieden, Erin Mathes, Anu Mathur, and Sonal Shah, for referring patients for inclusion; Drs Hillary Copp, William Hoffman, and Jason Pomerantz for supplying normal tissue for comparison; and all of the patients and families for providing clinical information and tissue samples for the study.
Abbreviations used:
- BSA
body surface area
- IFN-γ
interferon γ
- IL
interleukin
- TNF-α:
tumor necrosis factor α
- T regs
regulatory T cells
- UCSF
University of California, San Francisco
Footnotes
Conflicts of interest: None declared.
REFERENCES
- 1.Yoo IS, Lee JH, Song ST, Kim JH, Lee HJ, Kang SW. T-helper 17 cells: the driving force of psoriasis and psoriatic arthritis. Int J Rheum Dis. 2012;15(6):531–537. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez Rodriguez R, Pauli ML, Neuhaus IM, et al. Memory regulatory T cells reside in human skin. J Clin Invest. 2014; 124(3):1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Li Y, Yang X, et al. Characterization of Th17 and FoxP3(+) Treg cells in paediatric psoriasis patients. Scand J Immunol. 2016;83(3):174–180. [DOI] [PubMed] [Google Scholar]
- 4.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009; 361(5):496–509. [DOI] [PubMed] [Google Scholar]
- 5.Michalak-Stoma A, Pietrzak A, Szepietowski JC, Zalewska-Janowska A, Paszkowski T, Chodorowska G. Cytokine network in psoriasis revisited. Eur Cytokine Netw. 2011;22(4): 160–168. [DOI] [PubMed] [Google Scholar]
- 6.Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005(5):273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalak-Stoma A, Bartosińska J, Kowal M, Juszkiewicz-Borowiec M, Gerkowicz A, Chodorowska G. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers. 2013;35(6):625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008; 159(5):1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration. (2016). Drugs@FDA: FDA approved drug products. Etanercept. Retrieved from: http://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=103795. Accessed December 1, 2016. [Google Scholar]
- 10.Paller AS, Siegfried EC, Langley RG, et al. ; Etanercept Pediatric Psoriasis Study Group. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358(3): 241–251. [DOI] [PubMed] [Google Scholar]
- 11.Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74(2): 280–287.e1-3. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Kane S, Chen H, et al. The role of 39 psoriasis risk variants on age of psoriasis onset. ISRN Dermatol. 2013;2013:203941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tokura Y, Mori T, Hino R. Psoriasis and other Th17-mediated skin diseases. J UOEH. 2010;32(4):317–328. [DOI] [PubMed] [Google Scholar]
- 14.Hao JQ. Targeting interleukin-22 in psoriasis. Inflammation. 2014;37(1):94–99. [DOI] [PubMed] [Google Scholar]
- 15.Nikamo P, Cheuk S, Lysell J, et al. Genetic variants of the IL22 promoter associate to onset of psoriasis before puberty and increased IL-22 production in T cells. J Invest Dermatol. 2014; 134(6):1535–1541. [DOI] [PubMed] [Google Scholar]
- 16.Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol. 2000;17(3):174–178. [DOI] [PubMed] [Google Scholar]
- 17.Mercy K, Kwasny M, Cordoro KM, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30(4):424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bronckers IM, Paller AS, van Geel MJ, van de Kerkhof PC, Seyger MM. Psoriasis in children and adolescents: diagnosis, management and comorbidities. Paediatr Drugs. 2015;17(5): 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]


