Abstract
Background
Frailty is increasingly applied as a measure to predict clinical outcomes, but data on the predictive abilities of frailty measures for non-home discharge and functional decline in acutely hospitalized geriatric patients are scarce.
Objectives
The aim of this study was to investigate the predictive ability of the frailty phenotype and a frailty index currently validated as part of the ongoing Swiss Frailty Network and Repository Study based on clinical admission data for non-home discharge and functional decline in acutely hospitalized older patients.
Design
Prospective cohort study.
Setting and Participants
Data were analyzed from 334 consecutive hospitalized patients of a tertiary acute care geriatric inpatient clinic admitted between August 2020 and March 2021.
Measurements
We assessed frailty using 1) the frailty phenotype and 2) the Swiss Frailty Network and Repository Study (SFNR) frailty index based on routinely available clinical admission data. Predictive abilities of both frailty measures were analyzed for the clinical outcomes of non-home discharge and functional decline using multivariate logistic regression models and receiver operating characteristic curves (ROC).
Results
Mean age was 82.8 (SD 7.2) years and 55.4% were women. Overall, 170 (53.1%) were frail based on the frailty phenotype and 220 (65.9%) based on the frailty index. Frail patients based on the frailty phenotype were more likely to be discharged non-home (55 (32.4%) vs. 26 (17.3%); adjusted OR 2.4 (95% CI, 1.4, 5.1)). Similarly, frail patients based on the frailty index were more likely to be discharged non-home compared to non-frail patients (76 (34.6%) vs. 9 (7.9%); adjusted OR, 5.5 (95% CI, 2.6, 11.5)). Both, the frailty phenotype and the frailty index were similarly associated with functional decline (adjusted OR 2.7 (95% CI, 1.5, 4.9); adjusted OR 2.8 (95% CI 1.4, 5.5)). ROC analyses showed best discriminatory accuracy for the frailty index for non-home discharge (area under the curve 0.76).
Conclusions
Frailty using the SFNR-frailty index and the frailty phenotype is a promising measure for prediction of non-home discharge and functional decline in acutely hospitalized geriatric patients. Further study is needed to define the most valid frailty measure.
Electronic Supplementary Material
Supplementary material is available in the online version of this article at 10.14283/jfa.2022.44.
Key words: Frailty syndrome, aged, geriatrics, predictive value of tests, discharge planning, inpatients
Introduction
Frailty is recognized as a common geriatric syndrome associated with adverse clinical outcomes such as functional decline, hospitalization and mortality (1). The assessment of frailty has been shown to be a potentially useful prediction model in different medical settings (2). For example, frailty was shown to be associated with mortality in older patients with coronary artery disease following percutaneous coronary intervention (3). Similarly, disease outcomes were better predicted by frailty than either age or comorbidity among patients admitted to hospital with COVID-19 (4). Various approaches to the assessment of frailty have been described (5). Overall, there are two general concepts of frailty: the physical frailty phenotype and the cumulative deficit frailty (5). While the first concept described by Fried et al (6). defined frailty based on five distinct domains causing vulnerability to adverse outcomes, the second concept of deficit accumulation frailty has been operationalized into a frailty index (7) hypothesizing that the accumulation of health and functional deficits results in a decreased health state (5).
Acutely hospitalized older patients are at particular risk for adverse outcomes, such as functional decline and discharge to higher levels of care (e.g., skilled nursing facility). Consequently, in addition to treating the acute illness, care goals need to also focus on preservation or improvement of functional status to facilitate discharge to home and maintain quality of life of patients (8). Therefore, non-home discharge and functional decline are considered clinically relevant outcomes in the acutely hospitalized geriatric patients (9, 10). To identify patients at particular risk of these outcomes, clinical prediction models would be helpful to identify high risk patients for non-home discharge and functional decline upon admission and guide treatment and early discharge planning.
A promising predictor illustrated by a multitude of studies is frailty (1, 4, 11, 12). Prior studies on the role of frailty in the prediction of adverse outcomes in hospitalized patients established frailty as a risk factor for mortality (13, 14) longer hospital stay (13), and 30-day readmission (12). At the same time, Theou et al. reports in her scoping review, that a minority of studies have addressed the geriatric acute care setting (15). Further, outside the acute care setting, one prior study successfully used a frailty index based on medical records to predict hospitalization and nursing home admissions among primary care patients (16). However, to the best of our knowledge, no prior studies assessed the predictive role of frailty measures with regard to nursing home admission and functional decline in acute care (17).
The goal of this study was to investigate the predictive ability of the clinical frailty phenotype and the SFNR-frailty index under evaluation in a larger research program based on clinical admission data for non-home discharge and functional decline in acutely hospitalized older patients.
Methods
Data were analyzed from 334 consecutive geriatric patients acutely admitted to a tertiary hospital in Bern, Switzerland, between August 2020 and March 2021. Admission criteria was age of 70 years and older, a clinical diagnosis of multimorbidity, and the need for acute inpatient care.
This is a small and preliminary subsample of the ongoing large driver project of the Swiss Frailty and Network Repository (SFNR) coordinated by the Dept. of Ageing Medicine at the University of Zurich. Selected baseline routine admission data including routinely performed baseline assessments were abstracted from patient records for each patients. Baseline assessments were performed upon admission by nurse assistants who were specifically trained for these assessments using standardized assessment tools and standardized questionnaires. In addition, selected clinical outcomes at discharge (i.e., non-home discharge, functional decline) were abstracted from hospital records for each patient. From a methodological perspective, our study is in line with a most recently published article by Oviedo et al. (18) describing sensitivity, specificity, adjusted odds ratios of different frailty measures for clinical outcome measures.
The study protocol was approved by the competent ethics committee of the Canton of Zurich (BASEC-ID 2019-00445).
Frailty measures
We used the frailty phenotype (6) as one of the frailty measures. The frailty phenotype considers five characteristics (i.e., shrinking, low activity, fatigue, slowness, weakness) to define frailty. We used measurement protocols and cut-points for frailty as described by Gagesch et al. (19). Details on coding are summarized in the supplementary information (Table S1). Briefly, standardized questionnaires were used to investigate shrinking (loss of appetite, loose clothing, weight loss in the last 6 months), and low activity prior to admission. Weakness was assessed by grip strength, slowness by gait speed, and fatigue by the 5-item Geriatric depression scale (GDS-5). Patients who were unable to be assessed due to poor health status were assigned one frailty point for each unassessed item. Patients with a score ≥ 3 out of 5 points were considered frail.
The Swiss Frailty and Repository Consortium within the national Swiss Personalized Health Network Program developed a frailty index (FI) (19) based on the published criteria by Searle et al. (20). The validation study of the Swiss Frailty and Repository Study frailty index is ongoing and described elsewhere (19). Notably, the use of the SFNR-frailty index is preliminary in this small pilot-study and its validation is pending (19). A detailed description of the SFNR- frailty index, which consists of 30 health deficits based on the clinically routine admission data, is provided in the supplementary information (Table S2). Table S2 also details questions, assessments, coding and frequencies of each item. The SFNR-frailty index in this preliminary study was calculated for each patient by summing deficit points and dividing the sum by the total number of deficits considered. The denominator was 30 if there were no missing data. If there were missing data, the denominator was reduced by the number of missing deficits (7). Applying the definition by Kerminen et al. (21) and in accordance to a previous study in a similar setting of geriatric inpatients in Switzerland (22), patients with a total FI of ≥0.4 were considered frail. In a sensitivity analysis, we applied the cut-off of ≥0.2 for the frailty index in accordance to the study by Searle et al. (20).
Clinical outcomes
Patients who were discharged to nursing home were classified as having a “non-home discharge”.
Functional decline at discharge was defined by the change in Barthel Index scores between admission and discharge. The Barthel Index is a 10-item ordinal scale to assess activities of daily living (ADL) in geriatric care. Items rate level of dependency in basic self-care activities (e.g., eating, dressing, bathing), sphincter controls, transfer and locomotion. Higher scores reflect a higher degree of functional independence. Functional effectiveness was defined as Barthel gain/((100 points)-(Barthel index at admission)) (22, 23). Functional decline was defined as functional effectiveness <0. This cut-off was used to conservatively separate the group of patients with a functional gain from those with functional decline. Of note, functional decline refers to the change of the overall Barthel index score (ordinal variable) from admission to discharge, whereas the frailty index includes only selected subcomponents of the Barthel, coded as dichotomous (dependent vs. independent) variables as displayed in the supplementary information (Table S2). Therefore, the independence of predictor (frailty index) and outcome (functional decline) is ensured.
Patients who died during the index hospitalization were coded as “non-home discharge” and “functional decline”, respectively. In a sensitivity analysis among surviving patients (n=325), we excluded patients from analyses who died (n=9).
Statistical analyses
Characteristics of the study population are presented by absolute and relative frequencies or by mean and standard deviation (sd) for continuous and categorical variables. Power analysis was based on prior studies expecting a prevalence of non-home discharge to be 20% in older patients after acute geriatric hospitalization. At a two-sided confidence level of 0.05, the sample size of 334 patients yields a precision of +/−4.5% (24). Frailty instruments were correlated by using Spearman correlation coefficients. Predictive capacity (sensitivity, specificity, positive and negative predictive value) and receiver operating characteristic (ROC)-curves of frailty measures were calculated for clinical outcomes. Univariate and multivariate regression models adjusting for age and sex were calculated for each outcome and frailty measure. Sensitivity analyses were performed in subsample of 325 patients surviving hospital stay for both outcomes. Moreover, sensitivity analyses was performed applying a cut-off of ≥0.2 for definition of the frailty index. All analyzes were computed using Stata Version 16.1 (StataCorp LLC, College Station, TX, USA). An adjusted p value of <0.05 was considered statistically significant.
Results
Overall, study patients had a mean age of 82.8 (standard deviation (sd) 7.2) years and 55.4% were women (Table 1). Median length of stay was 12 days (interquartile range (IQR) 12, 15 days).
Table 1.
Clinical characteristics of patients (n=334)
| Demographics | N (%) | Missing, n (%) |
|---|---|---|
| Age, mean (sd) | 82.8 (7.2) | 0 |
| Women, n (%) | 185 (55.4) | 0 |
| Weight, mean (sd) | 70.8 (17.5) | 1 (0.3) |
| Height, mean (sd) | 164.5 (13.5) | 0 |
| BMI, mean (sd) | 26.6 (8.7) | 1 (0.3) |
| Length of stay, median (IQR) | 15 (12, 15) | 0 |
| Summary of admission data included in the SFNR-frailty index | ||
| Total functional score, median (IQR) | 55 (40, 75) | 1 |
| 5-item geriatric depression scale ≥2 points, n (%) | 136 (40.7) | 4 (1.2) |
| Ultrabrief delirium screening score: | ||
| -Question 1 (day of the week) incorrect | 67 (20.1) | 0 |
| -Question 2 (months backwards) incorrect | 135 (40.4) | 0 |
| Hearing impairment, n (%) | 74 (22.2) | 0 |
| Obesity (BMI ≥30kg/m2) | 67 (20.1) | 1 (0.3) |
| Pain, n (%) | 46 (13.8) | 46 (13.8) |
| Falls prior to admission | 203 (60.8) | 1 (0.3) |
| Able to climb one flight of stairs prior to admission | 65 (19.5) | 7 (2.1) |
| Walking distance <200m prior to admission | 51 (15.3) | 2 (0.6) |
| Walking aid prior to admission | 183 (54.8) | 0 |
| Timed up and go test, mean (sd) (sec) | 25.3 (7.5) | 0 |
| Multimorbidity, CIRS deficit score, median (IQR) | 10 (8-11) | 0 |
| Decreased creatinine clearance | 80 (24.0) | 11 (3.3) |
| Hypalbuminemia | 153 (45.8) | 35 (10.5) |
| Abnormal white blood cell count | 29 (8.7) | 7 (2.1) |
| Assessments included in the clinical frailty phenotype | ||
| Shrinking | 198 (59.8) | 3 (0.9) |
| Fatigue | 127 (38.5) | 4 (1.2) |
| Slowness | 228 (70.4)b) | 10 (3.0) |
| Weakness | 207 (63.1)a) | 6 (1.8) |
| Low activity | 67 (20.1) | 1 (0.3) |
| Frailty measures | ||
| Clinical frailty phenotype | 14 (4.2) | |
| Score, median (IQR) | 2 (2,3) | |
| Frail, n (%) | 170 (53.1) | |
| SFNR-frailty index | 0 | |
| Score, median (IQR) | 0.46 (0.33, 0.57) | |
| Frail, n (%) | 220 (65.9) | |
| Clinical outcomes | ||
| Non-home discharge | 85 (25.5) | 0 |
| Functional decline, n (%) | 67 (20.1) | 0 |
Abbreviations: sd, standard deviation; BMI, body mass index; IQR, interquartile range. a) n=51 were unable (too weak due to their underlying health condition) to perform grip strength measurement and were coded as “weak”; b) n=172 were unable (too weak to mobilize due to their underlying health condition) to perform gait speed test and were coded as “slow”
A total of 170 (53.1%) patients were classified frail based on the frailty phenotype, and 220 (65.9%) based on the preliminary use of the SFNR-frailty index (19). Proportions of the key components included in the frailty phenotype and the frailty index are listed in Table 1. Detailed description of the SFNR-frailty index, and histograms displaying the distribution of the SFNR-frailty index related to age by gender are provided in the supplementary material (Figures S1 and S2). The frailty index could be calculated in all patients (n=334), whereas the frailty phenotype could not be assessed in 14 patients (n=320) due to refusal of patients to perform assessments of the frailty phenotype (Table 1). Spearman’s rho coefficient for correlation between the frailty phenotype and the SFNR-frailty index was 0.51 (p<0.01).
Overall, 85 (25.5%) patients were not discharged home and 67 patients (20.1%) experienced functional decline. Nine (2.7%) patients died during the hospital stay. Of the nine patients who died, 7 were frail based on the frailty index and 5 were frail based on the frailty phenotype.
In univariate analyses (Table 2), the frailty phenotype and the SFNR-frailty index were both predictive for non-home discharge and functional decline. Table 2 displays the area under the curve, sensitivity, specificity, positive predictive value, negative predictive value of the frailty measures for the two clinical outcomes. Sensitivity analyses applying a cut-off of ≥0.2 for the frailty index are displayed in the supplementary information (Table S3 and S4).
Table 2.
Predictive and discriminative capacities of the frailty phenotype and the SFNR-frailty index for clinical outcomes (n=334): Univariate analyses and AUC
| Frailty phenotypec) | OR (95%CI)a) | AUC (95% CI)b) | Sensitivity | Specificity | PPV | NPV | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-home discharge | 2.3 (1.3, 3.9)* | 0.65 (0.59, 0.72) | 55/81 | 67.9% | 124/239 | 51.9% | 55/170 | 32.4% | 124/150 | 82.7% |
| Functional decline | 2.6 (1.5, 4.8)* | 0.63 (0.56, 0.70) | 45/63 | 71.4% | 132/257 | 51.4% | 45/170 | 26.5% | 132/150 | 88.0% |
| SFNR-frailty index | ||||||||||
| Non-home discharge | 6.2 (3.0, 12.8)* | 0.76 (0.71, 0.82) | 76/85 | 89.4% | 105/249 | 42.2% | 76/220 | 34.6% | 105/114 | 92.1% |
| Functional decline | 2.8 (1.4, 5.5)* | 0.66 (0.59, 0.74) | 55/67 | 82.0% | 102/267 | 38.2% | 55/220 | 25.0% | 102/114 | 89.5% |
Abbreviations: OR, Odds ratio; CI, confidence interval; AUC, area under the receiver operating curve; PPV, positive predictive value; NPV, negative predictive value; *p-value<0.01 for univariate logistic regression model; a) Odds ratio (95%CI) calculated from univariate logistic regression model; all frailty instruments (dependent variables) included as binary variables (frail vs. non-frail); b) AUC calculated from Receiver operating characteristic curve (ROC); frailty instruments coded as ordinal variables (clinical frailty phenotype) or continuous variable (FI); c) N=320, (n=14 missing)
Overall, 55 (32.4%) frail vs. 26 (17.3%) non-frail patients based on the frailty phenotype had a non-home discharge (adjusted OR 2.4 (95% CI, 1.4, 5.1)), whereas 76 (34.6%) frail vs. 9 (7.9%) non-frail patients based on the SFNR-frailty index had a non-home discharge (adjusted OR, 5.5 (95% CI, 2.6, 11.5)) (Table 3). Concerning functional decline, 45 (26.5%) frail vs. 18 (12%) non-frail patients based on the frailty phenotype (adjusted OR 2.7 (95% CI, 1.5, 4.9), and 55 (25.0%) frail vs. 12 (10.5%) non-frail based on the SFNR-frailty index experienced functional decline (adjusted OR, 2.8, 95% CI 1.4, 5.5). Sensitivity analyses performed in patients surviving the inhospital (n=325) stay are shown in the supplementary material (Tables S5 and S6).
Figure 1 displays the area under the curves of the frailty instruments for the outcomes non-home discharge (Panel A) and functional decline (Panel B).
Figure 1 a.
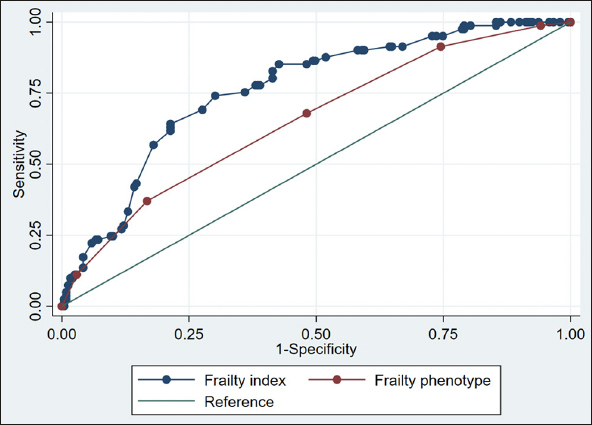
Panel A: Non-home discharge
Figure 1 b.
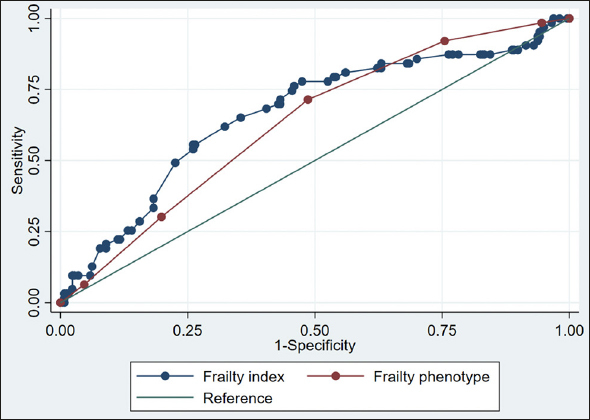
Panel B. Functional decline
Discussion
Frailty was highly prevalent in this population of acutely hospitalized older patients using either frailty measure. We found that both the frailty phenotype and the preliminary use of the SFNR- frailty index based on regular clinical admission data significantly predicted non-home discharge, with a suggestion that the SFNR-frailty index may have a stronger discriminatory capacity for this outcome as compared to the frailty phenotype. Both measures were similarly and moderately predictive for functional decline.
To the best of our knowledge this is the first study evaluating predictive abilities of the frailty phenotype and the SFNR-frailty index based on routine clinical data to predict non-home discharge and functional decline in acutely hospitalized older patients. Prior studies either investigated different outcomes (e.g. mortality, hospitalization) (13) or investigated the same outcomes in other clinical settings (e.g. primary care) (16).
Over half of the acutely hospitalized geriatric patients in our consecutive study sample met criteria for frailty on both of the investigated frailty assessments. We explored to what extent the prevalence of frailty observed in our study is representative of the literature in acute care geriatric patients. Some studies reported very similar prevalence, such as Bieniek et al. who described a prevalence of 54.2% based on the Fried frailty phenotype in geriatric inpatients (25) or Chong et al. described a 50.0% prevalence based on the FRAIL scale (11). However, there are also studies that reported a lower prevalence, such as 19% based on a frailty phenotype defined as self-reported items of weight loss, exhaustion, slowness, weakness, and low physical activity in hospitalized medical patients aged 70 years and older (26, 27). The much lower prevalence may be explained by the fact that included patients were almost all functionally independent compared to our study population consisting of comorbid patient with a limited functional score. Moreover, the frailty phenotype in the study of Feenstra et al (26) was solely based on self-reported items possibly underestimating frailty whereas the frailty phenotype in our study included clinical measures such as gait speed and grip strength.
We also found that both the SFNR-frailty index and frailty phenotype were predictive for non-home discharge, which is in accordance to prior studies, but in other clinical settings (10, 16). In a primary care setting, an electronic frailty index using routine data to identify frailty was predictive for a combined endpoint of mortality, hospitalization and nursing home admission (16). Similarly, Sokas et al. (10) reported an association of patient-reported frailty and non-home discharge among older adults undergoing surgery. Our study adds to the literature, that both frailty tools predict non-home discharge in acute care geriatric patients.
Our relatively small study suggests a possible stronger prediction of the outcome of non-home discharge by the SNFR-frailty index. Similarly, a study assessing biological age measurements found that the frailty index along with other biomarkers (methylation age estimator GrimAge) was best predictive for mortality (28). However, our results needs further validation in a larger study. Importantly, both tools predicted non-home discharge significantly with an odds of 2.4-fold (frailty phenotype) up to 5.5-fold (SFNR-frailty index). A possible explanation for the higher discriminatory accuracy of the still to be further validated SFNR-frailty index in our study compared to the frailty phenotype might be that the SFNR-frailty index includes multimorbidity.
On the other hand, we found similar moderate predictive capacities for the frailty phenotype and the SFNR-frailty index for prediction of functional decline. Thereby, we found a good sensitivity for the frailty measure, but low sensitivities. This finding is in line with a recent study by Oviedo-Briones et al. reporting that a frailty index had a good sensitivity and a low specificity for the prediction of functional decline over one year among older patients in different clinical settings (29).
Limitations
There are several limitations to our study. First, this is a single-site study and thus results may not apply to other settings and inpatients, or the later to be published larger SFNR study findings. Second, the preliminary use of an unvalidated frailty index needs to be interpreted with caution. Clearly, its use still needs refinement and validation, which is the main aim of the ongoing SFNR driver project coordinated by the Dept. of Aging at the University of Zurich. Third, we applied a cut-off of defining frailty based on the not yet validated new SFNR driver project frailty index and the literature-based cut-off of the frailty phenotype. Thus, we cannot exclude that other instruments or other cut-offs may reach different findings. Moreover, in a sensitivity analysis we applied the cut-off of 0.2 for defining frailty based on the frailty index. Forth, we assessed frailty using the frailty phenotype and the SFNR-frailty index. Therefore, our results cannot be extrapolated to other frailty tools such as the frailty scale (30, 31). Fifth, the inclusion of functional status items in the frailty index might lead by design to a better odds of the frailty index on functional outcomes compared to the frailty phenotype. However, the independence of the frailty index as a predictor and the outcome is maintained (the SFNR-frailty index is based on baseline data, whereas the functional outcome is based on outcome data). This methodological approach is also in accordance to another study that investigated the effect of frailty status at baseline on the effect of frailty change from admission to discharge (32). Finally, we investigated only two clinical outcomes, non-home discharge and functional decline. The larger SFNR driver project including five University Hospital sites will extend to additional clinical outcomes including length of stay, mortality and hospital readmission for both instruments.
However, even in this small study of 334 consecutive geriatric acute care patients, both frailty tools predicted both non-home discharge and functional decline. This consistent finding across both frailty tools lends support to the potential validity of the SFNR frailty index and the concept of frailty as a predictor of these two key outcomes for post-acute care planning among geriatric patients.
Conclusions
Our data suggest that assessing frailty either by using the frailty phenotype or the SFNR-frailty index based on clinical admission data is potentially helpful for the prediction of non-home discharge and functional decline in older inpatients. In a next step, as planned in the larger SFNR driver project, these observations need confirmation and the SFNR-frailty index needs proper validation in the larger ongoing SFNR-Study.
Electronic Supplementary Material
Acknowledgements
We thank Karen R. Josephson for language editing of the manuscript, and the SFNR driver project coordination team and network of partners.
Funding
This project was funded as “Driver Project 2017DRI02” (start date 01.03.2018–End Date 28.02.2021; PI Prof. Dr. med. Heike A. Bischoff-Ferrari, DrPH) by the SPHN, an initiative of the Swiss Academy of Medical Sciences (SAMW/ASSM) and in addition includes matching funds by the participating institutions.
Ethical standard
The study protocol was approved by the competent ethics committee of the Canton of Zurich (BASEC-ID 2019-00445).
Footnotes
Conflicts of interest
None declared by the Authors.
How to cite this article: A.K. Stuck, N. Schilling, D. Bertschi, et al. Predictive Abilities of the Frailty Phenotype and the Swiss Frailty Network and Repository Frailty Index for Non-Home Discharge and Functional Decline in Hospitalized Geriatric Patients. J Frailty Aging 2022; 10.14283/jfa.2022.44
References
- 1.Vermeiren S, Vella-Azzopardi R, Beckwee D, et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17(12):1163e1–63 e17. doi: 10.1016/j.jamda.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Tew YY, Chan JH, Keeling P, et al. Predicting readmission and death after hospital discharge: a comparison of conventional frailty measurement with an electronic health record-based score. Age Ageing 2021 doi: 10.1093/ageing/afab043 [DOI] [PMC free article] [PubMed]
- 3.He YY, Chang J, Wang XJ. Frailty as a predictor of all-cause mortality in elderly patients undergoing percutaneous coronary intervention: A systematic review and meta-analysis. Archives of gerontology and geriatrics. 2022;98:104544. doi: 10.1016/j.archger.2021.104544. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. The Lancet Public health. 2020;5(8):e444–e51. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walston J, Bandeen-Roche K, Buta B, et al. Moving frailty toward clinical practice: NIA intramural frailty science symposium summary. J Am Geriatr Soc. 2019;67(8):1559–64. doi: 10.1111/jgs.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 7.Burn R, Hubbard RE, Scrase RJ, et al. A frailty index derived from a standardized comprehensive geriatric assessment predicts mortality and aged residential care admission. BMC Geriatr. 2018;18(1):319. doi: 10.1186/s12877-018-1016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baztan JJ, Suarez-Garcia FM, Lopez-Arrieta J, et al. Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta-analysis. BMJ. 2009;338:b50. doi: 10.1136/bmj.b50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Velilla N, Casas-Herrero A, Zambom-Ferraresi F, et al. Effect of Exercise Intervention on Functional Decline in Very Elderly Patients During Acute Hospitalization: A Randomized Clinical Trial. JAMA Intern Med. 2019;179(1):28–36. doi: 10.1001/jamainternmed.2018.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokas CM, Cowan J, Dalton MK, et al. Association between patient-reported frailty and non-home discharge among older adults undergoing surgery. J Am Geriatr Soc. 2020;68(12):2909–13. doi: 10.1111/jgs.16846. [DOI] [PubMed] [Google Scholar]
- 11.Chong E, Ho E, Baldevarona-Llego J, et al. Frailty in hospitalized older adults: comparing different frailty measures in predicting short- and long-term patient outcomes. J Am Med Dir Assoc. 2018;19(5):450–57.e3. doi: 10.1016/j.jamda.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775–82. doi: 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunha AIL, Veronese N, de Melo Borges S, et al. Frailty as a predictor of adverse outcomes in hospitalized older adults: A systematic review and meta-analysis. Ageing Res Rev. 2019;56:100960. doi: 10.1016/j.arr.2019.100960. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu A, Maeda K, Fujishima I, et al. Hospital Frailty Risk Score predicts adverse events in older patients with vertebral compression fractures: Analysis of data in a nationwide in-patient database in Japan. Geriatr Gerontol Int. 2022;22(3):233–39. doi: 10.1111/ggi.14356. [DOI] [PubMed] [Google Scholar]
- 15.Theou O, Squires E, Mallery K, et al. What do we know about frailty in the acute care setting? A scoping review. BMC Geriatr. 2018;18(1):139. doi: 10.1186/s12877-018-0823-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–60. doi: 10.1093/ageing/afw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Checa-Lopez M, Oviedo-Briones M, Pardo-Gomez A, et al. FRAILTOOLS study protocol: a comprehensive validation of frailty assessment tools to screen and diagnose frailty in different clinical and social settings and to provide instruments for integrated care in older adults. BMC Geriatr. 2019;19(1):86. doi: 10.1186/s12877-019-1042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oviedo-Briones M, Laso AR, Carnicero JA, et al. A comparison of frailty assessment instruments in different clinical and social care settings: the frailtools project. J Am Med Dir Assoc. 2021;22(3):607 e7–07 e12. doi: 10.1016/j.jamda.2020.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Gagesch M, Edler K, Chocano-Bedoya PO, et al. Swiss Frailty Network and Repository: protocol of a Swiss Personalized Health Network’s driver project observational study. BMJ Open. 2021;11(7):e047429. doi: 10.1136/bmjopen-2020-047429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerminen H, Huhtala H, Jantti P, et al. Frailty Index and functional level upon admission predict hospital outcomes: an interRAI-based cohort study of older patients in post-acute care hospitals. BMC Geriatr. 2020;20(1):160. doi: 10.1186/s12877-020-01550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuck AK, Mangold JM, Wittwer R, et al. Ability of 3 Frailty Measures to Predict Short-Term Outcomes in Older Patients Admitted for Post-Acute Inpatient Rehabilitation. J Am Med Dir Assoc 2021 doi: 10.1016/j.jamda.2021.09.029 [published Online First: 2021/10/24] [DOI] [PubMed]
- 23.Koh GC, Chen CH, Petrella R, et al. Rehabilitation impact indices and their independent predictors: a systematic review. BMJ Open. 2013;3(9):e003483. doi: 10.1136/bmjopen-2013-003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Ji X. Sample size estimation in clinical research: from fandomized controlled trials to observational studies. Chest. 2020;158(1S):S12–S20. doi: 10.1016/j.chest.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Bieniek J, Wilczynski K, Szewieczek J. Fried frailty phenotype assessment components as applied to geriatric inpatients. Clin Interv Aging. 2016;11:453–9. doi: 10.2147/CIA.S101369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feenstra M, Oud FMM, Jansen CJ, et al. Reproducibility and responsiveness of the Frailty Index and Frailty Phenotype in older hospitalized patients. BMC Geriatr. 2021;21(1):499. doi: 10.1186/s12877-021-02444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coelho-Junior HJ, Marzetti E, Picca A, et al. Prevalence of Prefrailty and Frailty in South America: A Systematic Review of Observational Studies. J Frailty Aging. 2020;9(4):197–213. doi: 10.14283/jfa.2020.22. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Ploner A, Wang Y, et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. Elife 2020;9 doi: 10.7554/eLife.51507 [published Online First: 20200211] [DOI] [PMC free article] [PubMed]
- 29.Oviedo-Briones M, Rodriguez-Laso A, Carnicero JA, et al. The ability of eight frailty instruments to identify adverse outcomes across different settings: the FRAILTOOLS project. J Cachexia Sarcopenia Muscle 2022 doi: 10.1002/jcsm.12990 [published Online First: 20220415] [DOI] [PMC free article] [PubMed]
- 30.Church S, Rogers E, Rockwood K, et al. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20(1):393. doi: 10.1186/s12877-020-01801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Zepeda MU, Martinez-Velilla N, Kehler DS, et al. The impact of an exercise intervention on frailty levels in hospitalised older adults: secondary analysis of a randomised controlled trial. Age Ageing 2022;51(2) doi: 10.1093/ageing/afac028 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


