Abstract
Bacteraemia has attracted great attention owing to its serious outcomes, including deterioration of the primary disease, infection, severe sepsis, overwhelming septic shock or even death. Candidemia, secondary to bacteraemia, is frequently seen in hospitalised patients, especially in those with weak immune systems, and may lead to lethal outcomes and a poor prognosis. Moreover, higher morbidity and mortality associated with candidemia. Owing to the complexity of patient conditions, the occurrence of candidemia is increasing. Candidemia-related studies are relatively challenging. Because candidemia is associated with increasing mortality related to invasive infection of organs, its pathogenesis warrants further investigation. We collected the relevant clinical data of 367 patients with concomitant candidemia and bacteraemia in the first hospital of China Medical University from January 2013 to January 2018. We analysed the available information and attempted to obtain the undisclosed information. Subsequently, we used machine learning to screen for regulators such as prognostic factors related to death. Of the 367 patients, 231 (62.9%) were men, and the median age of all patients was 61 years old (range, 52–71 years), with 133 (36.2%) patients aged >65 years. In addition, 249 patients had hypoproteinaemia, and 169 patients were admitted to the intensive care unit (ICU) during hospitalisation. The most common fungi and bacteria associated with tumour development and Candida infection were Candida parapsilosis and Acinetobacter baumannii, respectively. We used machine learning to screen for death-related prognostic factors in patients with candidemia and bacteraemia mainly based on integrated information. The results showed that serum creatinine level, endotoxic shock, length of stay in ICU, age, leukocyte count, total parenteral nutrition, total bilirubin level, length of stay in the hospital, PCT level and lymphocyte count were identified as the main prognostic factors. These findings will greatly help clinicians treat patients with candidemia and bacteraemia.
Keywords: Candidemia, Bacteriaemia, Epidemiology, Prognosis, Machine learning
Introduction
Candidemia and bacteraemia frequently occur in hospitalised or critical care patients with unfavourable prognosis and high mortality (Raoult & Richet, 2011; Bloos et al., 2013; Heimann et al., 2015; Kullberg & Arendrup, 2015; Logan, Martin-Loeches & Bicanic, 2020). Candidemia is diagnosed in >250,000 people annually worldwide and causes >50,000 deaths (Arendrup, 2010). According to a population-based study from the United States of America (USA) and a database-based systematic analysis from Europe, bacteraemia is the fourth leading cause of mortality (following cardiac diseases, combined lung and larynx cancers and cerebrovascular diseases) (Jensen et al., 2011; Sogaard et al., 2011). The mortality rate of candidemia was 29% according to a population-based study from the USA, 31% according to a study from Spain, 54% according to a multi-centre study from Brazil and 60% according to a survey conducted in South Africa (Colombo et al., 2006; Cleveland et al., 2012; Kreusch & Karstaedt, 2013; Puig-Asensio et al., 2014). In addition, on the basis of a study from the University of Pennsylvania, catheter-associated bacteraemia was discovered to be the 12th leading cause of death in USA (Umscheid et al., 2011). Furthermore, the distribution and prevalence of different Candida species differ according to regional discrepancies and patient populations (Pappas et al., 2018). Among fungal infections, Candida albicans infection is the most prevalent infection leading to death; however, the incidence of non-albicans candidemia has increased over the past decades worldwide (Pfaller et al., 2011; Castanheira et al., 2016; Lamoth et al., 2018). Moreover, the number of cases of Candida albicans infection dramatically decreased during the past decade in the USA and is less than half of that reported previously (Lockhart et al., 2012; Matsumoto et al., 2014; Pfaller, Jones & Castanheira, 2014). Because candidemia is frequently reported and Candida is the third most common causative agent of infection in intensive care units (ICUs) worldwide (17%), medical support and intensive treatment are challenging (Vincent et al., 2009; Colombo et al., 2014; Lortholary et al., 2014; Chakrabarti et al., 2015). Bloodstream infections often lead to severe diseases with high morbidity and mortality and can be acute or chronic (Opota et al., 2015). Candidemia and bacteraemia are associated with a heavy socioeconomic burden and widespread prevalence (Martinez & Wolk, 2016). Studies reporting on the epidemiological features and risk factors of candidemia and bacteraemia are limited; therefore, further investigation and integration are required.
Machine learning is a rapidly developing technique that is widely used for analysing medical information and making clinical decisions. Programming algorithms through machine learning can define rules based on extensive data and interpret unknown relationships between factors (Jordan & Mitchell, 2015; Peiffer-Smadja et al., 2020). Random forest (RF), logistic regression (LR) and support vector machine (SVM) are widely used classification tools in bioinformatics and medical fields. RF is a supervised tree-based ensemble machine learning methodology, whereas SVM is a nonparametric, supervised and kernel-based statistical learning approach (Saberioon et al., 2018). The relationship between one or more independent factors and a binary dependent variable can be estimated using logistic regression (Schober & Vetter, 2021). Epidemiological characteristics and risk factors can be processed, analysed and predicted by using machine learning. Therefore, we used machine learning methods in this retrospective study to analyse the clinical information and find out risk factors for patients with candidemia and bacteraemia.
Methods
Human ethics
This study was conducted in accordance with the declaration of Helsinki. It was approved by The Human Ethics Review Committee of the First Hospital of China Medical University (number: 2021-260). The ethics review board of the First Hospital of China Medical University exempted the acquisition of informed consent because it was a retrospective study. During data collection and preparation of the manuscript, all patients’ information was considered to be confidential.
Patient selection
Patients were selected and data were collected as described in our previous study (Li et al., 2020). Specially, all data on Candida recovered from the blood of patients with invasive candidal infection were acquired (2008 version of EORTC/MSG criteria). We used the date when the first positive result of blood culture was obtained as the onset of candidemia and bacteraemia. The data set captured relevant information from the selected patients, including patient clinical features, risk factors for candidemia and bacteraemia, treatment and survival status at discharge, haematological diagnoses, Candida and bacterial test results, antifungal therapy and so on. The hospitalisation of each patient was regarded as an event. It should be noted that it was considered to be a new event once the patient was re-hospitalised and received new treatments.
Definition
Persistent candidemia was defined as a condition in which the blood culture tests yielded positive results with the same Candida species after 7 days of initiating appropriate therapy.
Microbiological test
The collected blood samples were cultured for 5 days, then we selected the positive blood samples and transferred them to blood AGAR plates. Fungal isolates and bacterial isolates were cultured at 35 °C for 48-72 h subsequently. We carried out gram staining and microscopic examination simultaneously. Strain identification was performed on a VITEK two Compact (Bio-Merieux SA, Marcy l’etoile, France), including fungal isolates and bacterial isolates. Drug susceptibility tests were performed followed the reagent instructions and the “national clinical test operating procedures” using the ATB FUNGUS three (Bio-Merieux SA, Marcy l’etoile, France). The ATB Fungus 3 yeast-like fungi was applied into a drug susceptibility test box, and the minimum inhibitory concentration (MIC) value was determined according to the CLSI m27-a3 and m27-s4 antifungal susceptibility test standards. Candida ATCC6258 and Candida albicans ATCC90028 were used as the quality control strains.
Machine learning
We pre-processed the data, removed missing cases (missing data with 50% features) and filled the mean value of missing data. We performed five-fold cross-validation to analyze these data. The predictive power of the model was measured using the average area under the curve (AUC) of the receiver operating characteristic curve based on five-fold cross-validation process. In addition, random forest, logistic regression and support vector machine were used to develop the final prediction model. We applied the model of random forest to identify important features.
Statistical analysis
Statistical analysis was performed using the SPSS 20.0 software. Non-normally distributed quantitative data were expressed as median and quartile ranges [M(P25, P75)] and the intergroup comparisons were performed using the Mann–Whitney test. Qualitative data were described by relative numbers and the intergroup comparisons were made using chi-square test.
Results
Clinical features of patients
A total of 367 patients with candidemia and bacteraemia were included in this study, 231 (62.9%) were male and 136 (37.1%) were female. The median age of all patients with candidemia was 61 years (range, 52–71 years), with 133 (36.2%) patients aged >65 years. Among all patients, 169 (46.0%) stayed in ICU during hospitalisation, 358 (97.5%) stayed in the hospital for >10 days and 224 (61.0%) had multiple hospitalisations within the past 2 years. Diseases found in patients were hypoproteinaemia (249 patients), solid tumours (182 patients), haematological malignancies (10 patients), diabetes (50 patients), renal failure (37 patients) and pancreatitis (37 patients). Other common relevant conditions contributing to candidemia included the long-term use of broad-spectrum antibiotics (196/367, 53.4%), recent surgery (<2 weeks ago) (198/367, 54.0%), invasive mechanical ventilation (143/367, 39.0%), urinary catheter insertion (285/367, 77.7%), gastric tube insertion (213/367, 58.0%), central venous catheter insertion (229/367, 62.4%), drainage catheter insertion (245/367, 66.8%) and total parenteral nutrition (298/367, 81.2%). In addition, 146 patients had persistent invasive mycosis. Detailed information is provided in Table S1.
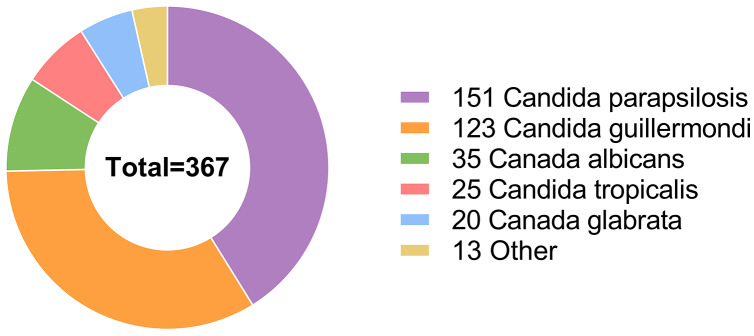
Candida parapsilosis was the most common causative agent affecting 151 patients. Candida guilliermondi was the causative agent in 123 patients, whereas other Candida species caused infections in a relatively small proportion of patients, including Candida albicans (35/367 patients), Candida tropicalis (25/367 patients), Candida glabrata (20/367 patients), Cryptococcus neoformans (9/367 patients), Cadida lusitaniae (2/367 patients), Cadida krusei (1/367 patients) and Streptomyces (1/367 patients) (Fig. 1).
Figure 1. Among the 367 hospitalized patients, the most frequent infection agent was Candida parapsilosis, followed by Candida guilliermondii, Candida albicans, Candida tropicalis, Candida glabrata, and some other Candida species.
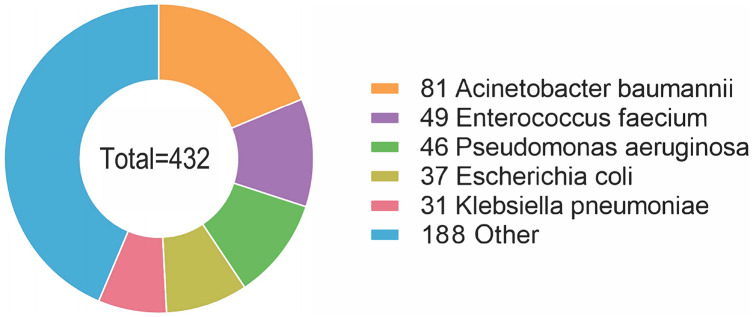
Furthermore, 38 species of bacteria were isolated, with 16 (16/38, 42.1%) Gram-positive species and 22 (22/38, 57.9%) Gram-negative species. A total of 176 (176/367, 48.0%) patients were infected with Gram-positive bacteria, and 256 (256/367, 69.8%) patients were infected with Gram-negative bacteria. The most common species of bacteria was Acinetobacter baumannii, affecting 81 patients (81/367, 22.1%). Enterococcus faecium and Pseudomonas aeruginosa were also common species, affecting 49 (13.4%) and 46 (12.5%) patients, respectively, followed by Escherichia coli (37/367, 10.1%), Klebsiella pneumoniae (31/367, 8.4%), Staphylococcus aureus (29/367, 7.9%), Staphylococcus epidermidis (28/367, 7.6%), Staphylococcus hominis (21/367, 5.7%), Stenotrophomonas maltophilia (14/367, 3.8%), Staphylococcus haemolyticus (12/367, 3.3%), Burkholderia cepacia (10/367, 2.7%) and some other species of bacteria (Fig. 2). Detailed information is provided in Table S2.
Figure 2. The most common species of bacteria was Acinetobacter baumannii, followed by Enterococcus faecium, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and some other species of bacteria.
In vitro antifungal susceptibility test
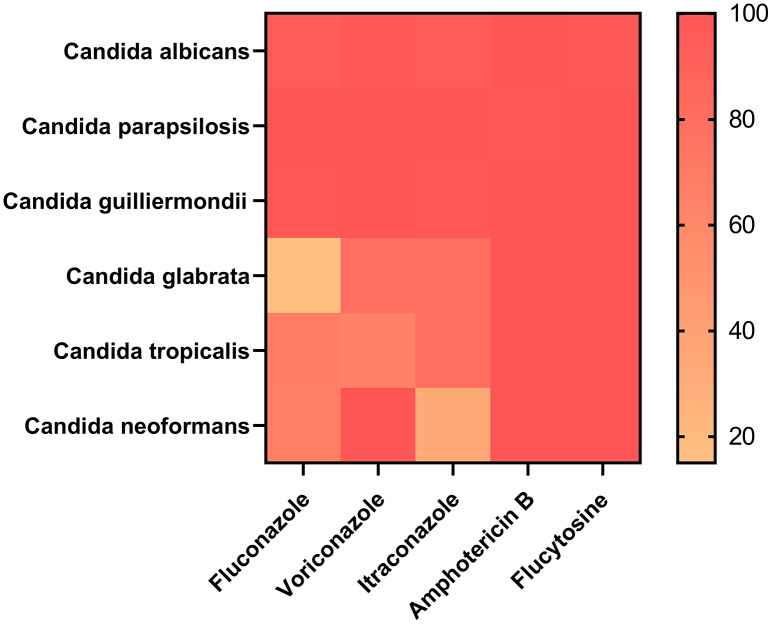
Of the 367 patients, 353 patients with information on drug sensitivity were selected. Approximately 20 patients were resistant to at least one drug. Amphotericin B exhibited strong efficiency because all 353 patients were sensitive to it. A total of 352 patients were sensitive to flucytosine, with only one patient being resistant to it, which showed that amphotericin B and flucytosine may be potential drugs for treating Candida infection. In addition, 339 and 335 patients were sensitive to voriconazole and itraconazole, respectively. Moreover, 17 (17/20, 85%) patients with Candida glabrata infection showed strong dose-dependent drug sensitivity to fluconazole, which was not observed in any other groups, suggesting that fluconazole is a candidate drug for the treatment of Candida glabrata infection. However, 6 (6/25, 24%) patients with Candida tropicalis infection were resistant to fluconazole and voriconazole. Detailed information is provided in Fig. 3 and Table S3.
Figure 3. Amphotericin B and flucytosine are more effective drugs for the treatment of Candida infection.
Risk factors for Candida albicans and non- Candida albicans infections
Clinical information and statistical data are presented in Table 1. Approximately 52.11% of patients with non-Candida albicans infection and 31.42% of patients with Candida albicans infection had solid tumours. Furthermore, it was observed that a relatively large proportion (84.64%) of patients with non-Candida albicans infection was administered total parenteral nutrition; however, only 48.57% of patients with Candida albicans infection were administered total parenteral nutrition. In addition, 31.13% of patients with Candida albicans infection and 56.33% of patients with non-Candida albicans infection underwent a recent surgery (within 2 weeks). Urinary, central venous and drainage catheters were used in 79.22%, 64.16% and 71.39% of patients with non-Candida albicans infection, respectively; and 62.86%, 45.71% and 22.86% of patients with Candida albicans infection, respectively. As a result, patients with the catheters usage were more susceptible to non-Candida albicans infection. Furthermore, higher percentage of patients with Candida albicans infection (22.86%) were predisposed to endotoxic shock than that of patients with non-Candida albicans infection (10.54%).
Table 1. Risk factors for Candida albicans and non-Candida albicans infections.
| Candida albicans (%) (n = 35) | non-Candida albicans (%) (n = 332) | Statistic | P value | |
|---|---|---|---|---|
| Male | 20 (57.14%) | 211 (63.55%) | 0.747 | 0.455 |
| Age (years)a | 62.00 (45.50, 72.50) | 61.00 (52.00, 71.00) | −0.152 | 0.879 |
| Length of stay (days)a | 33.00 (21.50, 67.00) | 32.00 (23.00, 51.00) | 0.101 | 0.920 |
| Length of stay in ICUa | 3.00 (0.00, 20.50) | 0.00 (0.00, 11.25) | 1.452 | 0.147 |
| Solid tumor | 11 (31.42%) | 173 (52.11%) | 2.327 | 0.020 |
| Diabetes | 4 (11.43%) | 46 (13.86%) | 0.398 | 0.691 |
| Pancreatitisb | 3 (8.57%) | 34 (10.24%) | – | 1 |
| Total parenteral nutrition | 17 (48.57%) | 281 (84.64%) | 5.194 | <0.001 |
| Renal failure | 6 (17.14%) | 31 (9.34%) | – | 0.145 |
| Recent surgery (within 2 weeks) | 11 (31.13%) | 187 (56.33%) | 2.811 | 0.005 |
| Use immunosuppressantsb | 3 (8.57%) | 24 (7.23%) | – | 0.733 |
| ICU | 20 (57.14%) | 149 (44.88%) | 1.384 | 0.166 |
| Hypoproteinemia | 25 (71.43%) | 224 (67.47%) | 0.477 | 0.633 |
| Invasive mechanical ventilation | 17 (48.57%) | 126 (37.95%) | 1.225 | 0.221 |
| Urinary catheter | 22 (62.86%) | 263 (79.22%) | 2.210 | 0.027 |
| Gastric tube | 17 (48.57%) | 196 (59.04%) | 1.193 | 0.233 |
| Central venous catheter | 16 (45.71%) | 213 (64.16%) | 2.142 | 0.032 |
| Drainage catheter | 8 (22.86%) | 237 (71.39%) | 5.797 | <0.001 |
| Endotoxic shockb | 8 (22.86%) | 35 (10.54%) | – | 0.048 |
| Multiple hospitalizations within 2 years (>2 times) | 22 (62.86%) | 202 (60.84%) | 0.232 | 0.816 |
| Persistent fungal infection | 14 (40.00%) | 132 (39.76%) | 0.028 | 0.978 |
| Serum albumin levela (g/l) | 26.20 (21.50, 31.25) | 27.70 (23.90, 31.05) | −1.182 | 0.237 |
| Serum creatinine levela (μmol/L) | 67.00 (49.50, 92.25) | 59.00 (45.00, 79.00) | 1.725 | 0.085 |
| Leukocyte counta (109/l) | 8.84 (6.94, 12.64) | 6.46 (4.39, 9.80) | 3.171 | 0.002 |
| Total bilirubin levela (μmol/l) | 14.30 (8.95, 21.40) | 13.25 (7.65, 24.00) | 0.476 | 0.634 |
| Neutrophil counta (109/l) | 6.75 (4.63, 9.42) | 5.13 (3.47, 8.06) | 2.030 | 0.042 |
| Lymphocyte counta (109/l) | 0.75 (0.56, 1.14) | 0.69 (0.45, 1.00) | 1.375 | 0.169 |
| CRPa (mg/l) | 106.00 (71.53, 166.50) | 86.30 (47.35, 129.50) | 1.520 | 0.128 |
| PCTa (ng/ml) | 1.25 (0.52, 3.05) | 0.50 (0.24, 1.39) | 2.706 | 0.007 |
Note:
Described by median and quartile, and the statistic was the Z value; other items were described as numbers (n - %) and the statistic was the χ2 value.
Fisher χ2 value.
In patients with concomitant candidemia and bacteraemia, procalcitonin (PCT) levels, leukocyte and neutrophil counts were elevated, especially in patients with Candida albicans infection. Detailed information is provided in Table 1.
Analysis of risk factors in patients with persistent and non-persistent Candida infections
The clinical information and statistical data of patients with persistent and non-persistent Candida infection are provided in Table 2. Persistent Candida infection was associated with prolonged hospital and ICU stays, total parenteral nutrition, recent surgery (within the past 2 weeks) and central venous catheter insertion. In addition, leukocyte, neutrophil and lymphocyte counts were significantly elevated in patients with persistent Candida infection, with statistically significant differences. Detailed information is provided in Table 2.
Table 2. Risk factors in patients with persistent and non-persistent candidal infections.
| Persistent candidal infection (%) (n = 146) | non-Persistent candidal infection (%) (n = 114) | Statistic | P value | |
|---|---|---|---|---|
| Male | 92 (63.01%) | 73 (64.04%) | 0.170 | 0.865 |
| Age (years)a | 62.00 (52.00, 72.75) | 60.00 (51.00, 68.50) | 1.297 | 0.195 |
| Length of stay (days)a | 40.50 (27.25, 66.75) | 32.00 (24.00, 48.50) | 3.241 | 0.001 |
| Length of stay in ICUa | 7.00 (0.00, 30.00) | 0.00 (0.00, 8.00) | 3.777 | <0.001 |
| Solid tumor | 62 (42.47%) | 59 (51.75%) | 1.490 | 0.136 |
| Diabetesb | 24 (16.44%) | 14 (12.28) | – | 0.381 |
| Pancreatitisb | 16 (10.96%) | 12 (10.53%) | – | 1 |
| Total parenteral nutrition | 113 (77.40%) | 100 (87.71%) | 2.146 | 0.032 |
| Renal failureb | 22 (15.07%) | 8 (7.02%) | – | 0.051 |
| Recent surgery (within 2 weeks) | 65 (44.52%) | 67 (58.77%) | 2.281 | 0.023 |
| Use immunosuppressants within the past 30 daysb | 11 (7.53%) | 10 (8.77%) | – | 0.820 |
| Stay in ICU during hospitalization | 86 (58.90%) | 48 (42.11%) | 2.689 | 0.007 |
| Hypoproteinemia | 108 (73.97%) | 77 (67.54%) | 1.135 | 0.256 |
| Invasive mechanical ventilation | 74 (50.68%) | 46 (40.35%) | 1.659 | 0.097 |
| Urinary catheter | 115 (78.77%) | 86 (75.44%) | 0.636 | 0.525 |
| Gastric tube | 89 (60.96%) | 66 (57.89%) | 0.500 | 0.617 |
| Central venous catheter | 107 (73.29%) | 66 (57.89%) | 2.610 | 0.009 |
| Drainage catheter | 98 (67.12%) | 80 (70.18%) | 0.526 | 0.599 |
| Endotoxic shockb | 24 (16.44%) | 12 (10.53%) | – | 0.207 |
| Multiple hospitalizations within 2 years (>2 times) | 99 (67.81%) | 70 (61.40%) | 1.074 | 0.283 |
| Serum albumin levela (g/l) | 27.05 (23.10, 30.08) | 27.85 (24.30, 32.25) | −1.453 | 0.146 |
| Serum creatinine levela (μmol/L) | 59.00 (42.00, 85.50) | 59.00 (47.00, 77.00) | −0.148 | 0.882 |
| Leukocyte counta (109/l) | 7.50 (5.37, 10.87) | 5.86 (3.96, 8.80) | 3.507 | <0.001 |
| Total bilirubin levela | 13.75 (9.25, 29.20) | 14.00 (7.40, 22.55) | 0.808 | 0.419 |
| Neutrophil counta (109/l) | 6.28 (4.22, 8.67) | 4.44 (2.89, 7.30) | 3.515 | <0.001 |
| Lymphocyte counta (109/l) | 0.77 (0.57, 1.10) | 0.58 (0.42, 0.83) | 3.378 | <0.001 |
| CRPa (mg/ml) | 92.00 (48.83, 130.50) | 87.90 (58.18, 130.00) | −0.165 | 0.869 |
| PCTa (ng/ml) | 0.54 (0.26, 1.19) | 0.48 (0.26, 1.48) | 0.164 | 0.870 |
Note:
Described by median and quartile, and the statistic was the Z value; other items were described as numbers (n - %) and the statistic was the χ2 value.
Fisher χ2 value.
Analysis of risk factors in patients with single and multiple fungal infections
Of the 367 patients with candidemia and bacteraemia, 59 (16.1%) patients had multiple fungal infections, whereas 308 (83.9%) patients had a single fungal infection. Clinical information and statistical data are presented in Table 3.
Table 3. Analysis of risk factors in patients with single fungal infection and multiple fungal infections.
| single fungal infection (n = 308) | multiple fungal infection (n = 59) | Statistic | P value | |
|---|---|---|---|---|
| Male | 200 (64.94%) | 31 (52.54%) | 1.806 | 0.071 |
| Age (years)a | 61.00 (51.00, 69.00) | 65.00 (53.00, 75.00) | −1.980 | 0.048 |
| Length of stay (days)a | 30.00 (22.00, 47.00) | 55.00 (33.50, 95.00) | −5.193 | <0.001 |
| Length of stay in ICUa | 0.00 (0.00, 8.00) | 19.00 (0.00, 56.00) | −6.045 | <0.001 |
| Solid tumor | 165 (53.57%) | 16 (27.12%) | 3.723 | <0.001 |
| Diabetes | 32 (10.39%) | 18 (30.51%) | 4.127 | <0.001 |
| Pancreatitisb | 33 (10.71%) | 4 (6.78%) | – | 0.481 |
| Total parenteral nutrition | 249 (80.84%) | 49 (83.05%) | 0.397 | 0.691 |
| Renal failure | 29 (9.42%) | 8 (13.56%) | – | 0.346 |
| Recent surgery (within 2 weeks) | 183 (59.42%) | 15 (25.42%) | 4.799 | <0.001 |
| Use immunosuppressants within the past 30 daysb | 26 (8.44%) | 1 (1.69%) | – | 0.098 |
| Stay in ICU during hospitalization | 125 (40.58%) | 42 (71.19%) | 4.324 | <0.001 |
| Hypoproteinemia | 209 (67.86%) | 40 (67.80%) | 0.009 | 0.993 |
| Invasive mechanical ventilation | 105 (34.09%) | 38 (64.41%) | 4.374 | <0.001 |
| Urinary catheter | 236 (76.62%) | 49 (83.05%) | 1.086 | 0.278 |
| Gastric tube | 174 (56.49%) | 39 (66.10%) | 1.370 | 0.171 |
| Central venous catheter | 182 (59.09%) | 47 (79.66%) | 2.988 | 0.003 |
| Drainage catheter | 203 (65.91%) | 42 (71.19%) | 0.788 | 0.431 |
| Endotoxic shock | 27 (8.77%) | 16 (27.12%) | 4.015 | <0.001 |
| Multiple hospitalizations within 2 years (>2 times) | 180 (58.44%) | 44 (74.58%) | 2.328 | 0.020 |
| Persistent fungal infection | 107 (34.74%) | 39 (66.10%) | 4.509 | <0.001 |
| Serum albumin levela (g/l) | 27.50 (23.50, 31.05) | 28.00 (24.65, 31.15) | −0.940 | 0.347 |
| Serum creatinine levela (μmol/L) | 59.00 (46.00, 78.00) | 63.00 (36.00, 91.00) | −0.035 | 0.972 |
| Leukocyte counta (109/l) | 6.56 (4.40, 10.05) | 8.22 (5.18, 10.55) | −1.624 | 0.105 |
| Total bilirubin levela (μmol/l) | 13.30 (7.48, 22.13) | 13.80 (9.55, 26.85) | −0.558 | 0.577 |
| Neutrophil counta (109/l) | 5.17 (3.47, 8.09) | 6.43 (3.93, 8.41) | −1.153 | 0.249 |
| Lymphocyte counta (109/l) | 0.64 (0.44, 0.95) | 0.95 (0.73, 1.26) | −4.245 | <0.001 |
| CRPa (mg/ml) | 87.40 (49.70, 135.00) | 90.45 (34.38, 118.25) | 0.421 | 0.674 |
| PCTa (ng/ml) | 0.51 (0.26, 1.86) | 0.56 (0.27, 0.99) | 0.701 | 0.483 |
Note:
Described by median and quartile, and the statistic was the Z value; other items were described as numbers (n - %) and the statistic was the χ2 value.
Fisher χ2 value.
Patients with multiple fungal infections were older (65 vs 61 years, respectively, based on the median age) and had longer hospital stays (55 vs 30 days, respectively, based on the median) or ICU stays (19 vs 0 days, respectively, based on the median) than patients with a single fungal infection. Furthermore, patients with multiple fungal infections had a higher requirement for invasive mechanical ventilation (64.41%) and central venous catheter (79.66%) compared with patients with a single fungal infection (34.09% and 59.09%, respectively). In addition, patients with multiple fungal infections had a higher predisposition to diabetes (30.51%), endotoxic shock (27.12%) and persistent fungal infection (66.10%) compared with patients with a single fungal infection (10.39%, 8.77% and 34.74%, respectively). Patients with multiple fungal infections had multiple hospitalisations within the past 2 years (>2 times) (74.58%), whereas the number of patients with a single fungal infection with multiple hospitalisations was smaller (58.44%). In addition, 53.57% of patients with a single fungal infection and 27.12% of patients with multiple fungal infections had solid tumours, whereas 59.42% of patients with a single fungal infection and 25.42% of patients with multiple fungal infections had recent surgery (within the past 2 weeks). Furthermore, the lymphocyte count was significantly lower in patients with a single fungal infection (0.64 × 109/L, based on the median) than in patients with multiple fungal infections (0.95 × 109/L, based on the median), with statistically significant differences. Detailed information is provided in Table 3.
Prediction of risk factors related to death using machine learning
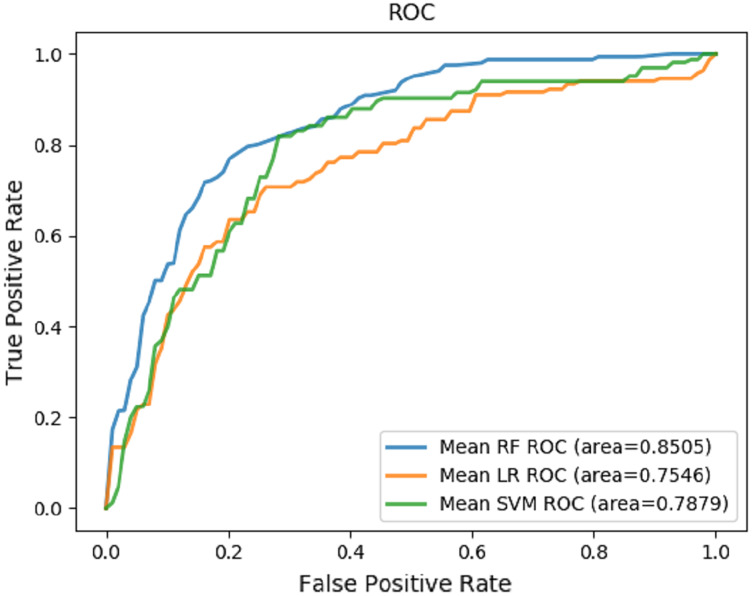
Random forest, logistic regression and support vector machine were used to predict death and evaluate performance (Table 4). The receiver operating characteristic (ROC) curves were generated based on our datasets (Fig. 4). The random forest played an important role in classification and regression and showed excellent performance. It was used to interpret different characteristics of patients and predict the risk factors for candidemia and bacteraemia. The results revealed that serum creatinine level, endotoxic shock, length of stay in ICU, age, leukocyte count, total parenteral nutrition, total bilirubin level, length of stay in the hospital, PCT level and lymphocyte count were the most important prognostic factors for concomitant candidemia and bacteraemia. Detailed information is provided in Table 5. The RF model showed satisfactory performance with these 10 characteristics in our datasets, with an AUC value of 0.8505 (Fig. 4).
Table 4. Performance of the machine-learning algorithms.
| Model | F1 score | Accuracy | Precision | Recall | AUC |
|---|---|---|---|---|---|
| Random forest (RF) | 0.5787 (+/− 0.1283) | 0.7836 (+/− 0.0363) | 0.6824 (+/− 0.1007) | 0.5904 (+/− 0.1452) | 0.8505 (+/− 0.1062) |
| Logistic regression (LR) | 0.5730 (+/− 0.1100) | 0.7589 (+/− 0.0910) | 0.6419 (+/− 0.1221) | 0.5848 (+/− 0.1189) | 0.7546 (+/− 0.0559) |
| Support vector machine (SVM) | 0.4630 (+/− 0.0587) | 0.7726 (+/− 0.0372) | 0.4549 (+/− 0.1531) | 0.5065 (+/− 0.0162) | 0.7879 (+/− 0.0822) |
Figure 4. Prediction mode.
The ROCs of the prediction of risk factors for death using machine learning mode.
Table 5. Feature importance rank.
| Risk factor variables | Importance rank |
|---|---|
| Serum creatinine level (g/L) | 0.066185 |
| Endotoxic shock | 0.063646 |
| Length of stay in ICU (days) | 0.057055 |
| Age | 0.054846 |
| Leukocyte count (109/L) | 0.050269 |
| Total parenteral nutrition | 0.045256 |
| Total bilirubin level (umol/L) | 0.042580 |
| Length of stay in the hospital (days) | 0.037216 |
| PCT level (ng/mL) | 0.037109 |
| Lymphocyte count (109/L) | 0.036857 |
Discussion
To the best of our knowledge, researches on concomitant candidemia and bacteraemia are limited. We collected the detailed clinical information of 367 patients with candidemia and bacteraemia from January 2013 to January 2018 in a provincial medical centre in the northeast of China.
Among all the selected patients in this study, 169 (46.0%) patients stayed in the ICU during hospitalisation, 358 (97.5%) patients stayed in the hospital for >10 days and 224 (61.0%) patients had multiple hospitalisations within the past 2 years. Most patients had diseases including hypoproteinaemia (249/367, 67.8%) and solid tumours (182/367, 49.6%). These conditions may be associated with weak immunity and long-term hospitalisation; moreover, Candida colonisation may have worsened the condition of these patients (Nami et al., 2019). Some recent studies have reported features similar to those of the abovementioned conditions (Keighley et al., 2019; Koehler et al., 2019). In addition, urinary catheter insertion (285/367, 77.7%), gastric tube insertion (213/367, 58.0%), central venous catheter insertion (229/367, 62.4%), drainage catheter insertion (245/367, 66.8%) and total parenteral nutrition (298/367, 81.2%) were identified as high-risk factors for candidemia and bacteraemia. As shown in previous studies, invasive medical support may be associated with the deteriorating state of patients with candidemia and bacteraemia (Ang et al., 1993; Fisher et al., 2011; Janum & Afshari, 2016; Ala-Houhala & Anttila, 2020).
Furthermore, Candida parapsilosis was identified as the most common fungi affecting patients (151/367, 41.1%), followed by Candida guilliermondi (123/367, 33.5%), Candida albicans (35/367, 9.5%), Candida tropicalis (25/367, 6.8%), Candida glabrata (20/367, 5.4%), Cryptococcus neoformans (9/367, 2.5%), Candida lusitaniae (2/367, 0.5%), Candida krusei (1/367, 0.3%) and Streptomyces (1/367, 0.3%). In addition, 38 bacterial species were isolated, with 16 (16/38, 42.1%) Gram-positive species and 22 (22/38, 57.9%) Gram-negative species. The most common bacterial species was Acinetobacter baumannii, affecting 81 (22.1%) patients. Enterococcus faecium and Pseudomonas aeruginosa were also common species, affecting 49 (13.4%) and 46 (12.5%) patients, respectively, followed by Escherichia coli (37/367, 10.1%), Klebsiella pneumoniae (31/367, 8.4%), Staphylococcus aureus (29/367, 7.9%) and Staphylococcus epidermidis (28/367, 7.6%). This study showed that the Candida infection pattern was different from that found in other regions, which can be further investigated (Arendrup et al., 2013; Asmundsdottir, Erlendsdottir & Gottfredsson, 2013; Ulu Kilic et al., 2017; Giacobbe et al., 2020).
Based on the results of machine learning in this study, we found that the most important predictors of death in patients with concomitant candidemia and bacteraemia included serum creatinine level, endotoxic shock, length of stay in ICU, age, leukocyte count, total parenteral nutrition, total bilirubin level, length of stay in the hospital, PCT level and lymphocyte count. Studies have reported that serum creatinine levels were increased in Candida infection, which may lead to renal dysfunction and increase infection-related mortality (Wong et al., 1982; Bellomo et al., 2017; Ronco, Bellomo & Kellum, 2019; Arase et al., 2020). Endotoxic shock was associated with Candida infection and was one of the main causes of morbidity and mortality worldwide; however, different features might be exhibited according to different species and the immune status of patients (Duggan et al., 2015; Eggimann et al., 2015; Poissy et al., 2020). Prolonged ICU stays could lead to a higher risk of complications and death based on the duration of intensive care (Ruping, Vehreschild & Cornely, 2008; Vincent et al., 2009). Age was also an important factor; elderly patients had low immunity, chronic diseases and multi-organ failure, making them susceptible to invasive Candida infections such as candidemia (Pluim et al., 2012; Mundula et al., 2019). The leukocyte count was associated with mortality in patients with Candida infections including candidemia (Riley & Rupert, 2015). In addition, total parenteral nutrition could increase the risk of complications and death (Ruiz-Ruigómez et al., 2018; Logan, Martin-Loeches & Bicanic, 2020; Poissy et al., 2020). The total bilirubin level was associated with mortality and factors such as age and primary diseases (Yang et al., 2017; Novák et al., 2020). The length of stay in the hospital could also serve as a prognostic indicator and could be influenced by conditions such as individual differences, primary diseases and different treatment strategies. Moreover, the longer the patients stayed in the hospital, the higher the hospitalisation costed (Lü et al., 2018; Zhang et al., 2020b). PCT is a biomarker for infections and can also serve as a promising prognostic indicator in patients with Candida and bacterial infections. Some studies have attempted to differentiate between candidemia and bacteraemia based on PCT levels; however, the differentiation remains challenging (Cortegiani et al., 2019; Honore et al., 2020). Besides, the lymphocyte count was a prognostic indicator of infection and was associated with mortality (Hatinguais, Willment & Brown, 2020; Zhang et al., 2020a).
However, this study has some limitations. First, the data was collected from a single-centre medical database; therefore, the results may be affected by geographical differences, specific management of hospitals and regional policies. Second, limited samples and regional differences may influence the outcomes of machine learning. Therefore, multi-centre studies should be conducted to further explore the epidemiological features and prospective risk factors.
Conclusions
In this study, the most common Candida and bacterial species found in patients with concomitant candidemia and bacteraemia in the First Affiliated Hospital of China Medical University were Candida parapsilosis and Acinetobacter baumannii, respectively. Serum creatinine level, endotoxic shock, length of stay in ICU, age, leukocyte count, total parenteral nutrition, total bilirubin level, length of stay in the hospital, PCT level and lymphocyte count were identified as the main prognostic factors of death. So far as is known, the types of Candida infection and bacterial infection are highly regional. There are few studies on candidemia and bacteremia in China, and the studies in this field in Northeast China remain lacking. Our research fills the gap in this part.
Supplemental Information
Acknowledgments
The authors thank the patients, their families, and all investigators who participated in the study.
Funding Statement
This work was funded by the National Science and Technology Major Projects of China, Grant/Award Number (2018ZX10101003 and 2018ZX10712001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Yali Gao conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Mingsui Tang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Yaling Li conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Xueli Niu analyzed the data, prepared figures and/or tables, and approved the final draft.
Jingyi Li analyzed the data, prepared figures and/or tables, and approved the final draft.
Chang Fu analyzed the data, prepared figures and/or tables, and approved the final draft.
Zihan Wang analyzed the data, prepared figures and/or tables, and approved the final draft.
Jiayi Liu analyzed the data, prepared figures and/or tables, and approved the final draft.
Bing Song conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Hongduo Chen conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Xinghua Gao conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Xiuhao Guan conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
This study was approved by The Human Ethics Review Committee of the First Hospital of China Medical University (No. 2021-260).
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.
References
- Ala-Houhala & Anttila (2020).Ala-Houhala M, Anttila VJ. Persistent vs non-persistent candidaemia in adult patients in 2007–2016: a retrospective cohort study. Mycoses. 2020;63(6):617–624. doi: 10.1111/myc.13085. [DOI] [PubMed] [Google Scholar]
- Ang et al. (1993).Ang BSP, Telenti A, King B, Steckelberg JM, Wilson WR. Candidemia from a urinary tract source: microbiological aspects and clinical significance. Clinical Infectious Diseases. 1993;17(4):662–666. doi: 10.1093/clinids/17.4.662. [DOI] [PubMed] [Google Scholar]
- Arase et al. (2020).Arase H, Yamada S, Hiyamuta H, Taniguchi M, Tokumoto M, Tsuruya K, Nakano T, Kitazono T. Modified creatinine index and risk for long-term infection-related mortality in hemodialysis patients: ten-year outcomes of the Q-Cohort Study. Scientific Reports. 2020;10(1):1241. doi: 10.1038/s41598-020-58181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendrup (2010).Arendrup MC. Epidemiology of invasive candidiasis. Current Opinion in Critical Care. 2010;16(5):445–452. doi: 10.1097/MCC.0b013e32833e84d2. [DOI] [PubMed] [Google Scholar]
- Arendrup et al. (2013).Arendrup MC, Dzajic E, Jensen RH, Johansen HK, Kjaldgaard P, Knudsen JD, Kristensen L, Leitz C, Lemming LE, Nielsen L, Olesen B, Rosenvinge FS, Røder BL, Schønheyder HC. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clinical Microbiology and Infection. 2013;19(8):E343–E353. doi: 10.1111/1469-0691.12212. [DOI] [PubMed] [Google Scholar]
- Asmundsdottir, Erlendsdottir & Gottfredsson (2013).Asmundsdottir LR, Erlendsdottir H, Gottfredsson M. Nationwide study of candidemia, antifungal use, and antifungal drug resistance in Iceland, 2000 to 2011. Journal of Clinical Microbiology. 2013;51(3):841–848. doi: 10.1128/JCM.02566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo et al. (2017).Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, Bagshaw SM, Glassford NJ, Lankadeva Y, Vaara ST, Schneider A. Acute kidney injury in sepsis. Intensive Care Medicine. 2017;43(6):816–828. doi: 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]
- Bloos et al. (2013).Bloos F, Bayer O, Sachse S, Straube E, Reinhart K, Kortgen A. Attributable costs of patients with candidemia and potential implications of polymerase chain reaction-based pathogen detection on antifungal therapy in patients with sepsis. Journal of Critical Care. 2013;28(1):2–8. doi: 10.1016/j.jcrc.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Castanheira et al. (2016).Castanheira M, Messer SA, Rhomberg PR, Pfaller MA. Antifungal susceptibility patterns of a global collection of fungal isolates: results of the SENTRY Antifungal Surveillance Program (2013) Diagnostic Microbiology and Infectious Disease. 2016;85(2):200–204. doi: 10.1016/j.diagmicrobio.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Chakrabarti et al. (2015).Chakrabarti A, Sood P, Rudramurthy SM, Chen S, Kaur H, Capoor M, Chhina D, Rao R, Eshwara VK, Xess I, Kindo AJ, Umabala P, Savio J, Patel A, Ray U, Mohan S, Iyer R, Chander J, Arora A, Sardana R, Roy I, Appalaraju B, Sharma A, Shetty A, Khanna N, Marak R, Biswas S, Das S, Harish BN, Joshi S, Mendiratta D. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Medicine. 2015;41(2):285–295. doi: 10.1007/s00134-014-3603-2. [DOI] [PubMed] [Google Scholar]
- Cleveland et al. (2012).Cleveland AA, Farley MM, Harrison LH, Stein B, Hollick R, Lockhart SR, Magill SS, Derado G, Park BJ, Chiller TM. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clinical Infectious Diseases. 2012;55(10):1352–1361. doi: 10.1093/cid/cis697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo et al. (2014).Colombo AL, Guimarães T, Sukienik T, Pasqualotto AC, Andreotti R, Queiroz-Telles F, Nouér SA, Nucci M. Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: an analysis of five multicenter studies sequentially conducted over a 9-year period. Intensive Care Medicine. 2014;40(10):1489–1498. doi: 10.1007/s00134-014-3400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo et al. (2006).Colombo AL, Nucci M, Park BJ, Nouér SA, Arthington-Skaggs B, da Matta DA, Warnock D, Morgan J. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. Journal of Clinical Microbiology. 2006;44(8):2816–2823. doi: 10.1128/JCM.00773-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortegiani et al. (2019).Cortegiani A, Misseri G, Ippolito M, Bassetti M, Giarratano A, Martin-Loeches I, Einav S. Procalcitonin levels in candidemia versus bacteremia: a systematic review. Critical Care. 2019;23(1):190. doi: 10.1186/s13054-019-2481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan et al. (2015).Duggan S, Leonhardt I, Hünniger K, Kurzai O. Host response to Candida albicans bloodstream infection and sepsis. Virulence. 2015;6:316–326. doi: 10.4161/21505594.2014.988096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggimann et al. (2015).Eggimann P, Que Y-A, Revelly J-P, Pagani J-L. Preventing invasive candida infections. Where could we do better? Journal of Hospital Infection. 2015;89(4):302–308. doi: 10.1016/j.jhin.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Fisher et al. (2011).Fisher JF, Kavanagh K, Sobel JD, Kauffman CA, Newman CA. Candida urinary tract infection: pathogenesis. Clinical Infectious Diseases. 2011;52(Supplement 6):S437–S451. doi: 10.1093/cid/cir110. [DOI] [PubMed] [Google Scholar]
- Giacobbe et al. (2020).Giacobbe DR, Maraolo AE, Simeon V, Magnè F, Pace MC, Gentile I, Chiodini P, Viscoli C, Sanguinetti M, Mikulska M, Fiore M, Bassetti M. Changes in the relative prevalence of candidaemia due to non-albicans Candida species in adult in-patients: a systematic review, meta-analysis and meta-regression. Mycoses. 2020;63(4):334–342. doi: 10.1111/myc.13054. [DOI] [PubMed] [Google Scholar]
- Hatinguais, Willment & Brown (2020).Hatinguais R, Willment JA, Brown GD. PAMPs of the fungal cell wall and mammalian PRRs. Current Topics in Microbiology and Immunology. 2020;425:187–223. doi: 10.1007/978-3-030-49928-0. [DOI] [PubMed] [Google Scholar]
- Heimann et al. (2015).Heimann SM, Cornely OA, Wisplinghoff H, Kochanek M, Stippel D, Padosch SA, Langebartels G, Reuter H, Reiner M, Vierzig A, Seifert H, Vehreschild MJGT, Glossmann J, Franke B, Vehreschild JJ. Candidemia in the intensive care unit: analysis of direct treatment costs and clinical outcome in patients treated with echinocandins or fluconazole. European Journal of Clinical Microbiology & Infectious Diseases. 2015;34(2):331–338. doi: 10.1007/s10096-014-2230-8. [DOI] [PubMed] [Google Scholar]
- Honore et al. (2020).Honore PM, David C, Attou R, Redant S, Gallerani A, De Bels D. Biomarkers to delineate bacteremia from candidemia remain a challenging issue. Critical Care. 2020;24(1):20. doi: 10.1186/s13054-019-2720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janum & Afshari (2016).Janum S, Afshari A. Central venous catheter (CVC) removal for patients of all ages with candidaemia. Cochrane Database of Systematic Reviews. 2016;7(12):CD011195. doi: 10.1002/14651858.CD011195.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen et al. (2011).Jensen US, Knudsen JD, Wehberg S, Gregson DB, Laupland KB. Risk factors for recurrence and death after bacteraemia: a population-based study. Clinical Microbiology and Infection. 2011;17(8):1148–1154. doi: 10.1111/j.1469-0691.2011.03587.x. [DOI] [PubMed] [Google Scholar]
- Jordan & Mitchell (2015).Jordan MI, Mitchell TM. Machine learning: trends, perspectives, and prospects. Science. 2015;349(6245):255–260. doi: 10.1126/science.aaa8415. [DOI] [PubMed] [Google Scholar]
- Keighley et al. (2019).Keighley C, Chen SC-A, Marriott D, Pope A, Chapman B, Kennedy K, Bak N, Underwood N, Wilson HL, McDonald K, Darvall J, Halliday C, Kidd S, Nguyen Q, Hajkowicz K, Sorrell TC, Van Hal S, Slavin MA. Candidaemia and a risk predictive model for overall mortality: a prospective multicentre study. BMC Infectious Diseases. 2019;19(1):445. doi: 10.1186/s12879-019-4065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler et al. (2019).Koehler P, Stecher M, Cornely OA, Koehler D, Vehreschild MJGT, Bohlius J, Wisplinghoff H, Vehreschild JJ. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clinical Microbiology and Infection. 2019;25(10):1200–1212. doi: 10.1016/j.cmi.2019.04.024. [DOI] [PubMed] [Google Scholar]
- Kreusch & Karstaedt (2013).Kreusch A, Karstaedt AS. Candidemia among adults in Soweto, South Africa, 1990–2007. International Journal of Infectious Diseases. 2013;17(8):e621–e623. doi: 10.1016/j.ijid.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Kullberg & Arendrup (2015).Kullberg BJ, Arendrup MC. Invasive candidiasis. New England Journal of Medicine. 2015;373(15):1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- Lamoth et al. (2018).Lamoth F, Lockhart SR, Berkow EL, Calandra T. Changes in the epidemiological landscape of invasive candidiasis. Journal of Antimicrobial Chemotherapy. 2018;73(suppl_1):i4–i13. doi: 10.1093/jac/dkx444. [DOI] [PubMed] [Google Scholar]
- Li et al. (2020).Li Y, Gao Y, Niu X, Wu Y, Du Y, Yang Y, Qi R, Chen H, Gao X, Song B, Guan X. A 5-year review of invasive fungal infection at an academic medical center. Frontiers in Cellular and Infection Microbiology. 2020;10:553648. doi: 10.3389/fcimb.2020.553648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart et al. (2012).Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, Baughman W, Stein B, Hollick R, Park BJ, Chiller T. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. Journal of Clinical Microbiology. 2012;50(11):3435–3442. doi: 10.1128/JCM.01283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, Martin-Loeches & Bicanic (2020).Logan C, Martin-Loeches I, Bicanic T. Invasive candidiasis in critical care: challenges and future directions. Intensive Care Medicine. 2020;46(11):2001–2014. doi: 10.1007/s00134-020-06240-x. [DOI] [PubMed] [Google Scholar]
- Lortholary et al. (2014).Lortholary O, Renaudat C, Sitbon K, Madec Y, Denoeud-Ndam L, Wolff M, Fontanet A, Bretagne S, Dromer F. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010) Intensive Care Medicine. 2014;40(9):1303–1312. doi: 10.1007/s00134-014-3408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al. (2018).Lu Y, Cai MH, Cheng J, Zou K, Xiang Q, Wu JY, Wei DQ, Zhou ZH, Wang H, Wang C, Chen J. A multi-center nested case-control study on hospitalization costs and length of stay due to healthcare-associated infection. Antimicrobial Resistance & Infection Control. 2018;7(1):99. doi: 10.1186/s13756-018-0386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez & Wolk (2016).Martinez RM, Wolk DM. Bloodstream infections. Microbiology Spectrum. 2016;4(4):1–34. doi: 10.1128/microbiolspec.DMIH2-0031-2016. [DOI] [PubMed] [Google Scholar]
- Matsumoto et al. (2014).Matsumoto E, Boyken L, Tendolkar S, McDanel J, Castanheira M, Pfaller M, Diekema D. Candidemia surveillance in Iowa: emergence of echinocandin resistance. Diagnostic Microbiology and Infectious Disease. 2014;79(2):205–208. doi: 10.1016/j.diagmicrobio.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Mundula et al. (2019).Mundula T, Ricci F, Barbetta B, Baccini M, Amedei A. Effect of probiotics on oral candidiasis: a systematic review and meta-analysis. Nutrients. 2019;11(10):2449. doi: 10.3390/nu11102449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nami et al. (2019).Nami S, Aghebati-Maleki A, Morovati H, Aghebati-Maleki L. Current antifungal drugs and immunotherapeutic approaches as promising strategies to treatment of fungal diseases. Biomedicine & Pharmacotherapy. 2019;110(Suppl. 2):857–868. doi: 10.1016/j.biopha.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Novák et al. (2020).Novák P, Jackson AO, Zhao G-J, Yin K. Bilirubin in metabolic syndrome and associated inflammatory diseases: new perspectives. Life Sciences. 2020;257:118032. doi: 10.1016/j.lfs.2020.118032. [DOI] [PubMed] [Google Scholar]
- Opota et al. (2015).Opota O, Croxatto A, Prod’hom G, Greub G. Blood culture-based diagnosis of bacteraemia: state of the art. Clinical Microbiology and Infection. 2015;21(4):313–322. doi: 10.1016/j.cmi.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Pappas et al. (2018).Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nature Reviews Disease Primers. 2018;4(1):18026. doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- Peiffer-Smadja et al. (2020).Peiffer-Smadja N, Rawson TM, Ahmad R, Buchard A, Georgiou P, Lescure F-X, Birgand G, Holmes AH. Machine learning for clinical decision support in infectious diseases: a narrative review of current applications. Clinical Microbiology and Infection. 2020;26(5):584–595. doi: 10.1016/j.cmi.2019.09.009. [DOI] [PubMed] [Google Scholar]
- Pfaller, Jones & Castanheira (2014).Pfaller MA, Jones RN, Castanheira M. Regional data analysis of Candida non-albicans strains collected in United States medical sites over a 6-year period, 2006–2011. Mycoses. 2014;57(10):602–611. doi: 10.1111/myc.12206. [DOI] [PubMed] [Google Scholar]
- Pfaller et al. (2011).Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009) Journal of Clinical Microbiology. 2011;49(1):396–399. doi: 10.1128/JCM.01398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluim et al. (2012).Pluim T, Halasa N, Phillips SE, Fleming G. The morbidity and mortality of patients with fungal infections before and during extracorporeal membrane oxygenation support. Pediatric Critical Care Medicine. 2012;13(5):e288. doi: 10.1097/PCC.0b013e31824fbaf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poissy et al. (2020).Poissy J, Damonti L, Bignon A, Khanna N, Von Kietzell M, Boggian K, Neofytos D, Vuotto F, Coiteux V, Artru F, Zimmerli S, Pagani J-L, Calandra T, Sendid B, Poulain D, van Delden C, Lamoth F, Marchetti O, Bochud P-Y. Risk factors for candidemia: a prospective matched case-control study. Critical Care. 2020;24(1):109. doi: 10.1186/s13054-020-2766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Asensio et al. (2014).Puig-Asensio M, Padilla B, Garnacho-Montero J, Zaragoza O, Aguado JM, Zaragoza R, Montejo M, Muñoz P, Ruiz-Camps I, Cuenca-Estrella M, Almirante B. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clinical Microbiology and Infection. 2014;20(4):O245–O254. doi: 10.1111/1469-0691.12380. [DOI] [PubMed] [Google Scholar]
- Raoult & Richet (2011).Raoult D, Richet H. Nosocomial bacteraemia: the most neglected causes of death in Europe? Clinical Microbiology and Infection. 2011;17(8):1123. doi: 10.1111/j.1469-0691.2011.03583.x. [DOI] [PubMed] [Google Scholar]
- Riley & Rupert (2015).Riley LK, Rupert J. Evaluation of patients with leukocytosis. American Family Physician. 2015;92:1004–1011. [PubMed] [Google Scholar]
- Ronco, Bellomo & Kellum (2019).Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949–1964. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ruigómez et al. (2018).Ruiz-Ruigómez M, Dueñas C, Hernandez C, Vinuesa D, Coronado-Álvarez NM, Portillo-Tuñón V, Cardozo C, Muñoz-Medina L, Cabo-Magadán R, Luna JD, Mensa J, Parra-Ruiz J. Clinical predictors of candidemia in medical non-neutropenic, non-ICU patients. The CaMed score. International Journal of Clinical Practice. 2018;72(12):e13275. doi: 10.1111/ijcp.13275. [DOI] [PubMed] [Google Scholar]
- Ruping, Vehreschild & Cornely (2008).Ruping MJ, Vehreschild JJ, Cornely OA. Patients at high risk of invasive fungal infections: when and how to treat. Drugs. 2008;68(14):1941–1962. doi: 10.2165/00003495-200868140-00002. [DOI] [PubMed] [Google Scholar]
- Saberioon et al. (2018).Saberioon M, Císař P, Labbé L, Souček P, Pelissier P, Kerneis T. Comparative performance analysis of support vector machine, random forest, logistic regression and k-nearest neighbours in rainbow trout (Oncorhynchus Mykiss) classification using image-based features. Sensors. 2018;18(4):1027. doi: 10.3390/s18041027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober & Vetter (2021).Schober P, Vetter TR. Logistic regression in medical research. Anesthesia and Analgesia. 2021;132(2):365–366. doi: 10.1213/ANE.0000000000005247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard et al. (2011).Sogaard M, Norgaard M, Dethlefsen C, Schonheyder HC. Temporal changes in the incidence and 30-day mortality associated with bacteremia in hospitalized patients from 1992 through 2006: a population-based cohort study. Clinical Infectious Diseases. 2011;52(1):61–69. doi: 10.1093/cid/ciq069. [DOI] [PubMed] [Google Scholar]
- Ulu Kilic et al. (2017).Ulu Kilic A, Alp E, Cevahir F, Ture Z, Yozgat N. Epidemiology and cost implications of candidemia, a 6-year analysis from a developing country. Mycoses. 2017;60(3):198–203. doi: 10.1111/myc.12582. [DOI] [PubMed] [Google Scholar]
- Umscheid et al. (2011).Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infection Control & Hospital Epidemiology. 2011;32(2):101–114. doi: 10.1086/657912. [DOI] [PubMed] [Google Scholar]
- Vincent et al. (2009).Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- Wong et al. (1982).Wong B, Bernard EM, Gold JWM, Fong D, Armstrong D. The arabinitol appearance rate in laboratory animals and humans: estimation from the arabinitol/creatine ratio and relevance to the diagnosis of candidiasis. Journal of Infectious Diseases. 1982;146(3):353–359. doi: 10.1093/infdis/146.3.353. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2017).Yang T‐L, Lin Y‐C, Lin Y‐C, Huang C‐Y, Chen H‐H, Wu M‐S. Total bilirubin in prognosis for mortality in end-stage renal disease patients on peritoneal dialysis therapy. Journal of the American Heart Association. 2017;6(12):104. doi: 10.1161/JAHA.117.007507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2020a).Zhang J, Cui N, Wang H, Han W, Li Y, Xiao M, Liu D. Invasive fungal disease in critically Ill patients at high risk: usefulness of lymphocyte subtyping. Journal of Intensive Care Medicine. 2020a;35(9):909–918. doi: 10.1177/0885066618800690. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2020b).Zhang Y, Du M, Johnston JM, Andres EB, Suo J, Yao H, Huo R, Liu Y, Fu Q. Estimating length of stay and inpatient charges attributable to hospital-acquired bloodstream infections. Antimicrobial Resistance & Infection Control. 2020b;9(1):137. doi: 10.1186/s13756-020-00796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.