Abstract
Purpose
To detect antibody responses to inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine in patients undergoing hemodialysis and to investigate vaccine-related adverse events.
Patients and Methods
A total of 120 hemodialysis (HD) patients and 24 healthy controls (HCs) who had not been previously infected with SARS-CoV-2 and had received their first dose of the inactivated vaccine (CoronaVac; Sinovac Biotech Ltd) were recruited for this study. All participants were scheduled to receive a second dose of inactivated SARS-CoV-2 vaccine. Serum-specific immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies against the SARS-CoV-2 were detected at least 14 days after the second dose of vaccine using a commercial kit. Positive and negative results were defined as a sample/cutoff (S/CO) ratio≥1.00 and <1.00, respectively. Vaccination-related adverse events were assessed using a standardized questionnaire.
Results
There were no significant differences regarding the seroprevalences of IgG and IgM antibodies against SARS-CoV-2 and the self-reported vaccination-related adverse events between HD patients and HCs. The analysis results for HD patients suggest that 82 (68.3%) and 27 (22.5%) tested positive for IgG and IgM, respectively. The levels of IgG were higher than IgM levels (P<0.0001). In addition, the IgG-positive group had significantly higher serum albumin levels than the IgG-negative group (P<0.05). Only mild vaccine-related adverse events were observed in two patients (1.66%) and in one healthy individual (4.2%).
Conclusion
The seroprevalences of IgG and IgM antibodies against SARS-CoV-2 and vaccination-related adverse effects are similar between HD and HCs. The inactivated SARS-CoV-2 vaccine is effective and safe in inducing near-term immunity in hemodialysis patients.
Keywords: adverse events, COVID-19, inactivated SARS-CoV-2 vaccine, IgM and IgG antibody
Introduction
Since the end of 2019, an outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a global pandemic of coronavirus disease 2019 (COVID-19). The rapid spread of SARS-CoV-2 and the high mortality rate associated with this infection brought a great burden to people worldwide.1 As of 19 January 2022, more than 330 million people have been infected with SARS-CoV-2 and more than 5.55 million have died due to COVID-19 (https://covid19.who.int/). Currently, there is a lack of clinically targeted drugs for the targeted treatment of the SARS-CoV-2.2,3 Apart from implementing measures of personal protection and maintaining social distance, vaccination is an effective method for preventing infection and protecting susceptible populations. Through orderly vaccination, herd immunity can be gradually established in the population, thereby effectively blocking the transmission and prevalence of SARS-CoV-2.4,5
Hemodialysis (HD) patients are a high-risk group for SARS-CoV-2 infection. Studies have shown that HD patients have a significantly increased risk of SARS-CoV-2 infection and mortality compared with the general population.6,7 This may be related to the fact that HD patients suffer from complex underlying diseases, more comorbidities, poorer nutrition and low immune function. The invasive procedures frequently performed in these patients and a centrally closed HD environment increase the risk of infection in HD patients. Data from the US National Dialysis Platform survey showed a mortality rate of 24.9% among dialysis patients after infection with COVID-19.8 Therefore, blocking SARS-CoV-2 infection by vaccination with the SARS-CoV-2 vaccine is crucial for HD patients.
Recently, numerous studies have shown that a SARS-CoV-2 messenger RNA (mRNA) vaccine is highly immunogenic (seroconversion rate: >95%) in patients undergoing HD.9,10 However, there are insufficient data on the effectiveness of inactivated vaccines in HD patients. This study evaluated the efficacy and safety of an inactivated vaccines (CoronaVac; Sinovac Biotech Ltd., Beijing China) in HD patients. Moreover, factors that influence the immunogenicity of the vaccine and adverse events (AEs) were investigated.
Materials and Methods
Study Population
A total of 120 HD patients who had received the first dose of inactivated vaccine (Vero cells, 2 doses, Sinovac Biotech Ltd.) were recruited from the Department of Nephrology at the Second Hospital of Anhui Medical University (Hefei, China) between June and September 2021. The HD patients were at least 18 years old and on hemodialysis for at least 3 months. The exclusion criteria were as follows: 1) previous known COVID-19 infection in the past; 2) inability to respond to the questionnaire; 3) any kind of immunosuppressive treatments in the previous 12 months; 4) unwillingness to participate in the clinical study; and 5) participation in other clinical trials. Twenty-four healthy volunteers who have received the first dose of the same vaccine were enrolled as healthy controls (HCs). All subjects will receive a second 3µg dose of SARS-CoV-2 inactivated vaccine as scheduled.
The study protocol was approved by the ethics committee of the Second Hospital of Anhui Medical University (approval number: YX2021-107), and all participants were enrolled after providing written informed consent. The study complied with the tenets of the Declaration of Helsinki.
Serological Assessment
Fasting venous blood (3–5 mL) was collected from all subjects ≥14 days after the second dose of the vaccine. The blood samples were centrifuged at 3000 r/min for 10 min prior to testing. The levels of serum immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies against the SARS-CoV-2 were detected using SARS-CoV-2 IgG and IgM chemiluminescent immunoassay microparticles (CMU0302/CMU0402, 100T/kit; Autobio Diagnostics Co., Ltd., Zhengzhou, China). These microparticles are coated by anti-human IgG or IgM antibodies, and enzyme conjugate is prepared with HPR-labeled SARS-CoV-2 antigen. The solid phase secondary antibody-IgG or antibody-IgM antibody-enzyme-labeled antigen complex is generated by immunological reactions. The complex catalyzes substrate, resulting in a chemiluminescent reaction, which is proportional to the amount of IgG or IgM antibodies against SARS-CoV-2.
All serum samples were tested. The sensitivity and specificity of kits were 89% and 100% for IgG, and 90% and 100% for IgM, respectively. The cut-off coefficient was 0.2 for IgG, and 0.1 for IgM. The cut-off value of the kit (cut-off value) was calculated by average relative luminescence unit (RLU) of positive control well × cut-off coefficient. The value of S/CO was used to determine if the sample is positive for IgG or IgM antibodies against SARS-CoV-2, with the use of the equation (RLU of tested samples/cut off), if the value of S/CO ≥ 1.00, the result is positive. Otherwise, the result is negative.
AEs
AEs occurring within 7 days after the second dose of the vaccine were recorded using a standardized questionnaire.11,12 These included local (pain, swelling, bruising) and systemic (fever, headache, fatigue, myalgia, arthralgia, dizziness, vomiting) events. AEs in subjects were graded using a Grade 0–4 scale (Grade 0: no event; Grade 1: mild, not interfering with daily activities; Grade 2: moderate, interference with activities of daily living; Grade 3: severe, interruption of usual activities of daily living; and Grade 4: hospitalization).
Statistical Analysis
Quantitative variables were expressed as the mean and standard deviation (SD) or median and interquartile range (IQR). Categorical variables were expressed as numbers and percentages. All statistical analyses were performed using the SPSS version 23.0 (IBM Corp., Armonk, NY, USA) software, and all figures were created using the Prism version 8.3.1 (GraphPad Inc., San Diego, CA, USA) software. P-values < 0.05 statistically significant differences.
Results
Basic Characteristics of Vaccinated Population
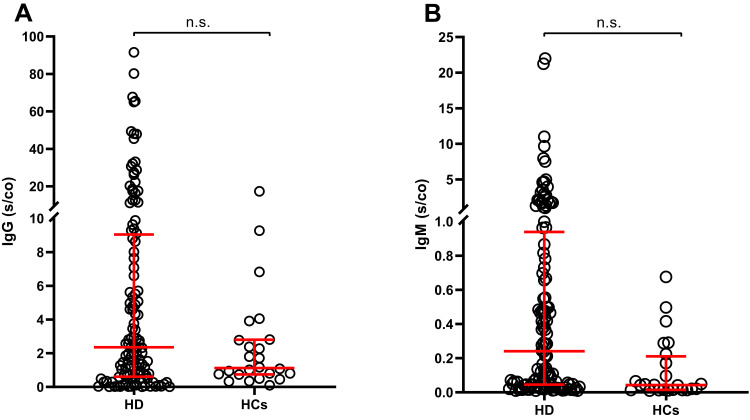
A total of 120 HD patients and 24 HCs were recruited for this study (Table 1). Although mismatched in age and sex distribution, we did not observe differences in specific IgG and IgM antibody levels between the two groups (P = 0.058 and P = 0.084 respectively; Figure 1). In addition, there was no difference in the incidence of vaccination-related AEs between the two groups of subjects (P = 0.434). The above results suggest that inactivated vaccine against COVID-19 appears to be immunogenic and well-tolerated in HD patients.
Table 1.
Basic Characteristics and Vaccination Information of the Two Groups
| Characteristics | HD (n=120) | HCs (n=24) | P |
|---|---|---|---|
| Male n (%) | 79 (65.8) | 2 (8.3) | <0.001 |
| Age (years) | 54.28 (12.94) | 42.83 (6.54) | <0.001 |
| BMI (kg/m2) | 21.58 (3.09) | 21.25 (2.52) | 0.396 |
| IgG (S/CO) | 2.36 (0.61–9.05) | 1.12 (0.74–2.80) | 0.058 |
| IgM (S/CO) | 0.24 (0.04–0.94) | 0.04 (0.01–0.21) | 0.084 |
| Interval time (days) | 22 (20–25) | 17 (14–23) | 0.212 |
| Advers events n (%) | 2 (1.66) | 1 (4.2) | 0.434 |
Notes: Data are presented as the mean (standard deviation) or median (interquartile range). Categorical variables were expressed as numbers and percentages.
Abbreviations: HD, hemodialysis; HCs, healthy controls; BMI, body mass index.
Figure 1.
Comparison of IgG (A) and IgM (B) antibody levels in the two groups (n=144).
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; HD, hemodialysis; HCs, healthy controls; n.s., non-statistically significant.
Among the 120 HD patients, etiologies mainly included glomerulonephritis (n=51; 42.5%), hypertensive nephropathy (n=24; 20.0%), diabetic nephropathy (n=22; 18.3%), polycystic kidney (n=6; 5.0%), obstructive nephropathy (n=4; 3.3%), and other causes of renal failure (n=13; 10.8%).
Levels of SARS-CoV-2-Specific IgG and IgM Antibodies in HD Patients
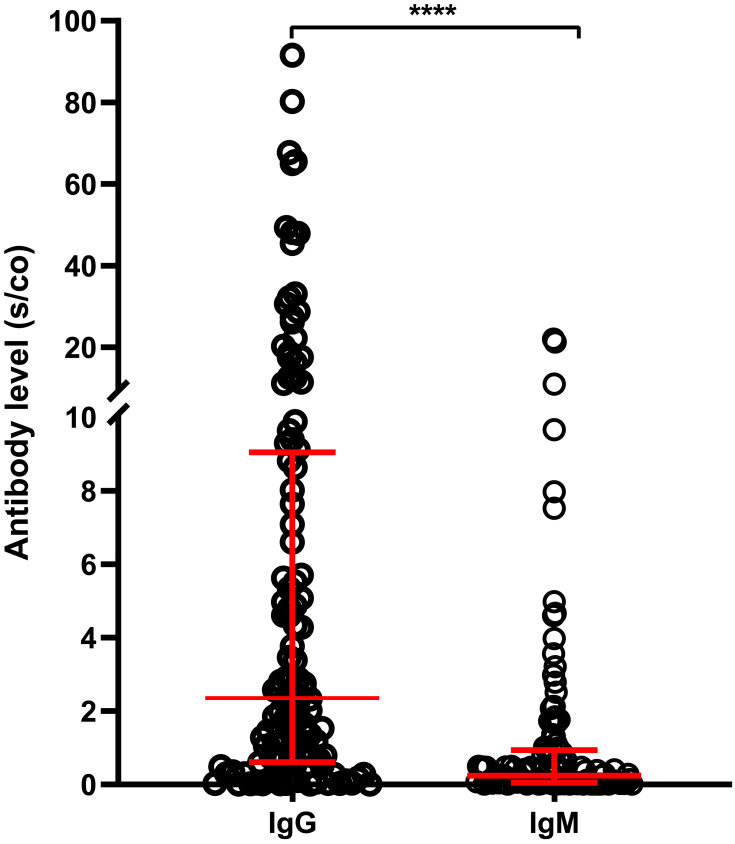
Of the 120 HD patients, 82 (68.3%) and 27 (22.5%) tested positive for IgG and IgM, respectively. The levels of IgG antibody levels were higher than those of IgM (median S/CO:2.36 vs 0.24, respectively; P < 0.0001, Figure 2). In addition, there were no differences between IgG-positive and-negative groups in terms of gender, age, HD duration, body mass index, hemoglobin, Kt/V, and interval time prior to blood collection. However, the IgG-positive group had higher serum albumin levels than the IgG-negative group (P<0.05) (Table 2).
Figure 2.
Comparison of SARS-CoV-2-specific IgG and IgM antibody levels in HD patients after completion of vaccination (n=120). ****P<0.0001.
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; SARS-CoV-2, severe acute respiratory syndrome-coronavirus 2, HD, hemodialysis.
Table 2.
The General Information and Laboratory Parameters of the HD Patients (n=120)
| Characteristics | Total n=120 | IgG | P | IgM | P | ||
|---|---|---|---|---|---|---|---|
| (+), n=82 | (-), n=38 | (+), n=27 | (-), n=93 | ||||
| Male n (%) | 79 (65.8) | 50 (41.7) | 29 (24.2) | 0.099 | 16 (13.3) | 63 (52.5) | 0.413 |
| Age (years) | 54.28 (12.94) | 54.28 (12.94) | 54.28 (12.94) | 0.256 | 52.07 (12.61) | 52.07(12.61) | 0.316 |
| HD duration (months) | 59.07 (51.35) | 60.21 (50.07) | 56.63 (54.61) | 0.929 | 61.52 (49.09) | 58.37(52.22) | 0.774 |
| BMI (kg/m2) | 21.58 (3.09) | 21.48 (3.19) | 21.80 (2.88) | 0.426 | 21.00 (3.01) | 21.75(3.11) | 0.272 |
| Hemoglobin (g/L) | 113.02(14.15) | 113.45(12.33) | 112.08(17.61) | 0.828 | 113.67(12.03) | 112.83(14.77) | 0.788 |
| Albumin (g/L) | 39.52 (3.81) | 40.26 (2.33) | 37.92 (5.65) | 0.027 | 40.56 (1.85) | 39.21 (4.17) | 0.107 |
| Kt/V | 1.43 (0.28) | 1.43 (0.27) | 1.42 (0.29) | 0.770 | 1.42 (0.20) | 1.43 (0.30) | 0.832 |
| Interval time (days) | 22(20–25) | 23(20–25) | 22(20–28) | 0.876 | 23(18–25) | 22(20–25.5) | 0.343 |
Notes: Data are presented as the mean (standard deviation) or median (interquartile range). Categorical variables were expressed as numbers and percentages.
Abbreviations: BMI, body mass index; IgG, immunoglobulin G; IgM, immunoglobulin M; (+), postive; (-), negative.
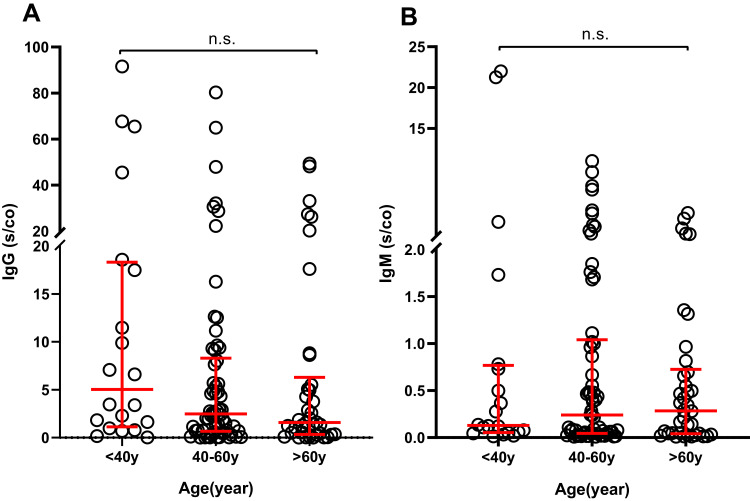
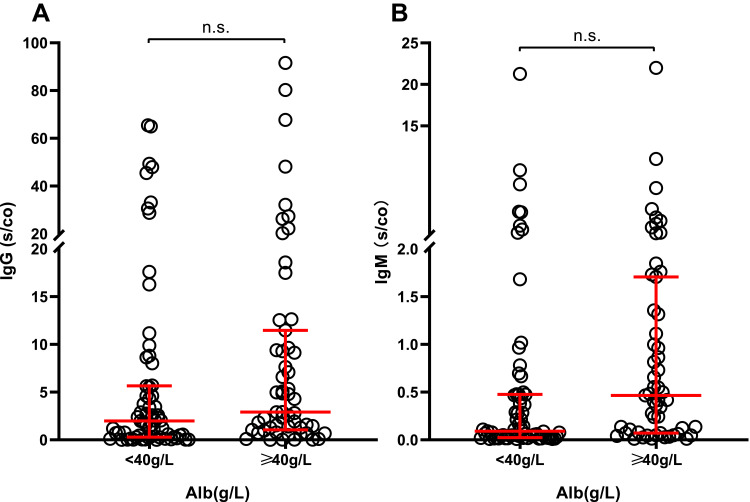
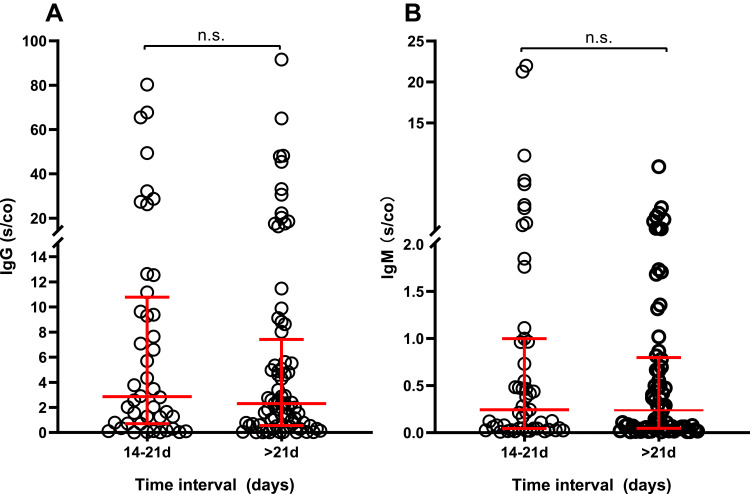
To investigate the factors that may influence vaccine-induced immunogenicity, we grouped subjects according to age (<40years, 40–60years, >60years), albumin levels (<40g/L, ≥40g/L) and interval time before antibodies detection (14–21days, ≥21days) to perform independent comparisons of the levels of IgG and IgM antibodies. This analysis did not reveal significant differences in the levels of IgG or IgM antibodies between the groups (P>0.05) (Figures 2–5).
Figure 3.
IgG (A) and IgM (B) levels in HD patients grouped by age (n=120).
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; HD, hemodialysis; n.s., non-statistically significant.
Figure 4.
IgG (A) and IgM (B) levels in HD patients grouped by the albumin level (n=120).
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; HD, hemodialysis; n.s., non-statistically significant.
Figure 5.
IgG (A) and IgM (B) levels in HD patients grouped by interval time prior to antibody detection (n=120).
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; HD, hemodialysis; n.s., non-statistically significant.
Reported AEs
Using the questionnaire, all subjects were asked to report local and systemic AEs that occurred within 7 days after the administration of the second dose of the vaccine (Table 3). Only two (1.66%) vaccine-related AEs were reported in 120 HD patients, namely local skin swelling (n=1; 0.83%) and fever (n=1; 0.83%). In addition, there was only one case (4.2%) of localized skin swelling in HCs. All observed AEs were mild and there was no requirement for hospitalization due to severe AEs.
Table 3.
Vaccine-Related Adverse Events in HD Patients (n=120)
| Adverse Events | N | % | Grade |
|---|---|---|---|
| Total | 2 | 1.66 | / |
| Local reaction | |||
| Swelling | 1 | 0.83 | 1 |
| System reaction | |||
| Fever | 1 | 0.83 | 1 |
Abbreviations: HD, hemodialysis; /, no corresponding information.
Discussion
Vaccination is an important strategy for controlling viral infections, especially in the absence of approved drugs designed to combat emerging viruses. It has been determined that herd immunity protection can be achieved when 47–85% of the population is infected or vaccinated with an effective vaccine against coronavirus.13,14 Coronavirus infection can trigger a strong B-cell response, with specific IgM, IgG and IgA antibodies detectable in patients within days.15 Antibodies, which primarily bind to the viral external spike glycoprotein and the internal nucleocapsid proteins, can effectively block the binding of the virus to the host cell surface receptor angiotensin converting enzyme 2 (ACE2).16 Despite the high efficacy of most currently approved vaccines, particularly in limiting the risk of clinical deterioration,17 a growing body of data suggests considerable heterogeneity in the immune response triggered by vaccination in different individuals.18–21 Patients undergoing hemodialysis are at a high risk of SARS-CoV-2 infection and death due to COVID-19. Therefore, the vaccination of this group of patients is a priority. However, because they were excluded from the pivotal study and had a weak immune response, it remains unknown whether these patients can be protected after the standard two-dose vaccination with an inactivated vaccine.
In this study, we sought to assess the immunogenicity of the inactivated SARS-CoV-2 vaccine (CoronaVac) in producing antibody responses in hemodialysis patients and healthy controls. This was achieved by measuring the levels of IgG and IgM after the completion of a two-dose immunization program. The results showed that the seroprevalences of both IgG and IgM antibodies against SARS-CoV-2 had no significant differences between the two groups. We conclude that vaccination with two-dose of the inactivated vaccine against SARS-CoV-2 can elicit a humoral response in the hemodialysis population. This is consistent with the results of previous studies on hemodialysis patients. Results from a prospective national cohort study of 10.2 million people who completed the vaccination in Chile indicated that the effectiveness rate of the CoronaVac vaccine was 65.9%.22 Although there are some reports of slightly lower antibody levels in HD patients, the persistence of IgG antibodies in most HD patients in response to the COVID-19 vaccine is encouraging.22–25 For seronegative subjects, a third dose may be a reasonable solution. A recent strategy developed in China involves a booster dose for HD patients who have already received the inactivated vaccine.
The present study showed that IgG plays an important role in the humoral response against SARS-CoV-2 virus. It is generally accepted that IgM appears early in viral infection (approximately 1 week after infection) and is a diagnostic indicator of acute-phase infection; however, these antibodies are found at relatively low concentrations, with short maintenance time and low affinity. IgG appears approximately 3 weeks after viral sensitization and is present in the blood for a longer period of time than IgM. It is established that IgG levels are essential for ensuring the prevention of viral diseases.26 Moreover, IgG has a longer maintenance time than IgM and represents the class of antibodies that are primarily evaluated in clinical and research settings to determine long-term protection or immunity. It has been reported that, in response to a two-dose vaccination with a mRNA vaccine (BNT162b2), subjects developed a weaker IgM antibody response and a stronger IgG response.27 Therefore, the seropositive IgG antibody response after immunization may be an alternative biomarker reflecting vaccine efficacy.
In the present study, we investigated possible factors affecting the immune response. We compared the levels of IgG and IgM antibodies after grouping patients according to age, albumin level and time between the completion of vaccination completion and testing. The results showed these factors were not associated with the levels of IgM and IgG antibodies. Most current studies have indicated that older age is a negative predictor of immune response after vaccination.24 The development of immune memory decreases with age because aging T cells favor short-term inflammatory effects over memory or follicular helper T cells. Indeed, elderly patients undergoing HD typically have a lower response to vaccines versus younger patients due to immune aging. In future studies, the sample size should be increased to analyze the effect of aging on vaccine immunogenicity. Our data showed that only patients in the IgG antibody-positive group had increased albumin levels. When patients were grouped according to the levels of albumin, we found that albumin was not associated with the levels of IgG antibodies. Agur et al demonstrated that lower serum albumin and higher intravenous iron dose were negative predictors of antibody response.28 In the present study, the incidence of AEs was 1.67%, and there was no occurrence of severe AEs. This is consistent with the findings of previous studies and indicates a reassuring safety profile of inactivated vaccines. Our findings might reassure patients who remain hesitant about COVID-19 vaccinations, and help physicians in guiding their patients toward accepting such vaccines.
Nevertheless, this study has some limitations. The research was limited to a short-term study of hemodialysis patients and focused on observing the early immune response after vaccination. The present findings reflect only a certain degree of immunity after immunization with the vaccine against SARS-CoV-2 and do not reflect the dynamic changes of serum-specific antibodies in the vaccinated patients. Moreover, we were unable to observe a sufficient number of negative samples due to the small sample size and short observation period in this study. Thus, we cannot determine the causes of negative antibody production or further investigate the immunization methods in this population. Finally, apart from the humoral response, we did not assess other aspects of the immune response, such as cellular immunity. However, with the development of the vaccine against SARS-CoV-2 and the extension of the observation period, subsequent research will focus on investigating the sustained protective effect of serum-specific antibodies in vaccinated patients. Furthermore, future approaches to improving the response of patients undergoing HD to vaccination should be explored, such as increasing the vaccine dose, using adjuvants, using heterologous primary immunizations, or increasing the number of immunizations.
Conclusion
The present findings demonstrate that an inactivated vaccine against SARS-CoV-2 may induce IgG antibody responses in hemodialysis patients, with a favorable safety profile. Further investigation is warranted to assess the long-term kinetics of vaccination responses in hemodialysis patients.
Disclosure
Wen-Man Zhao, Rui Shi, Peng Wang, and Jun He are co-first authors for this study. The authors report no conflicts of interest in this work.
References
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmelz K, Bowles S. Overcoming COVID-19 vaccination resistance when alternative policies affect the dynamics of conformism, social norms, and crowding out. Proc Natl Acad Sci U S A. 2021;118(25):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wouters O, Shadlen K, Salcher-Konrad M, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397(10278):1023–1034. doi: 10.1016/S0140-6736(21)00306-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson N, Nelveg-Kristensen K, Freese Ballegaard E, et al. Increased vulnerability to COVID-19 in chronic kidney disease. J Intern Med. 2021;290(1):166–178. doi: 10.1111/joim.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry B, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52(6):1193–1194. doi: 10.1007/s11255-020-02451-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu Caroline M, Weiner Daniel E, Aweh G, et al. COVID-19 among US dialysis patients: risk factors and outcomes from a national dialysis provider. Am J Kidney Dis. 2021;77(5):748–756.e1. doi: 10.1053/j.ajkd.2021.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longlune N, Nogier MB, Miedougé M, et al. High immunogenicity of a messenger RNA-based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol Dial Transplant. 2021;36(9):1704–1709. doi: 10.1093/ndt/gfab193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grupper A, Sharon N, Finn T, et al. Humoral response to the pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16(7):1037–1042. doi: 10.2215/CJN.03500321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popmihajlov Z, Pang L, Brown E, et al. A post hoc analysis utilizing the FDA toxicity grading scale to assess injection site adverse events following immunization with the live attenuated Zoster Vaccine (ZVL). Hum Vaccin Immunother. 2018;14(12):2916–2920. doi: 10.1080/21645515.2018.1502517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon B, Rubey H, Treipl A, et al. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared to healthy controls. Nephrol Dial Transplant. 2021;36:1709–1716. doi: 10.1093/ndt/gfab179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randolph H, Barreiro L. Herd immunity: understanding COVID-19. Immunity. 2020;52(5):737–741. doi: 10.1016/j.immuni.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwok K, Lai F, Wei W, Wong S, Tang J. Herd immunity - estimating the level required to halt the COVID-19 epidemics in affected countries. J Infection. 2020;80(6):e32–e33. doi: 10.1016/j.jinf.2020.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Guo X, Xin Q, et al. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis. 2020;71(10):2688–2694. doi: 10.1093/cid/ciaa721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doroftei B, Ciobica A, Ilie O, Maftei R, Ilea C. Mini-review discussing the reliability and efficiency of COVID-19 vaccines. Diagnostics. 2021;11(4):579. doi: 10.3390/diagnostics11040579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beilhack G, Monteforte R, Frommlet F, Gaggl M, Strassl R, Vychytil A. Antibody response and safety after mRNA-1273 SARS-CoV-2 vaccination in peritoneal dialysis patients - the Vienna cohort. Front Immunol. 2021;12:780594. doi: 10.3389/fimmu.2021.780594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terpos E, Trougakos I, Apostolakou F, et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am J Hematol. 2021;96(7):E257–E259. doi: 10.1002/ajh.26185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu Jabal K, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6). doi: 10.2807/1560-7917.ES.2021.26.6.2100096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yau K, Abe K, Naimark D, et al. Evaluation of the SARS-CoV-2 antibody response to the BNT162b2 vaccine in patients undergoing hemodialysis. JAMA netw open. 2021;4(9):e2123622. doi: 10.1001/jamanetworkopen.2021.23622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jara A, Undurraga E, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boongird S, Chuengsaman P, Setthaudom C, et al. Short-term immunogenicity profiles and predictors for suboptimal immune responses in patients with end-stage kidney disease immunized with inactivated SARS-CoV-2 vaccine. Infect Dis Ther. 2022;11(1):351–365. doi: 10.1007/s40121-021-00574-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yen J, Wang I, Yen T. COVID-19 vaccination & dialysis patients: why the variable response. QJM. 2021;114(7):440–444. doi: 10.1093/qjmed/hcab171 [DOI] [PubMed] [Google Scholar]
- 25.Speer C, Goth D, Benning L, et al. Early humoral responses of hemodialysis patients after COVID-19 vaccination with BNT162b2. Clin J Am Soc Nephrol. 2021;16(7):1073–1082. doi: 10.2215/CJN.03700321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murin C, Wilson I, Ward A. Antibody responses to viral infections: a structural perspective across three different enveloped viruses. Nature Microbiol. 2019;4(5):734–747. doi: 10.1038/s41564-019-0392-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jalkanen P, Kolehmainen P, Häkkinen H, et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat commun. 2021;12(1):3991. doi: 10.1038/s41467-021-24285-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agur T, Ben-Dor N, Goldman S, et al. Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients - a prospective cohort study. Nephrol Dial Transpl. 2021;36:1347–1349. doi: 10.1093/ndt/gfab155 [DOI] [PMC free article] [PubMed] [Google Scholar]