Abstract
Decorin (Dcn), a small leucine-rich proteoglycan, is involved in the regulation of corneal wound healing. Epidermal growth factor receptor (EGFR) plays a critical role in corneal fibroblasts proliferation, migration and extracellular matrix (ECM) modulation upon injury or infection. The present study aimed to investigate the mechanistic role of Dcn in EGFR internalization to the regulation of corneal stromal fibroblasts (CSFs) migration, a key step in the corneal wound healing. Human corneal stromal fibroblasts (hCSF) cultures were generated from donor corneas. At 70% confluence, cells were switched to serum-free conditions for 48 h and then treated with decorin (250 nM) in the presence or absence of EGF (100 ng/ml) for various time points (10–60 min). Cell lysates were subjected to proteome array analysis screening for 42 different phosphorylated human receptor tyrosine kinases (RTKs), immunocytochemistry, and western blots to analyze EGFR phosphorylation. The scratch-wound assay was performed to evaluate the effects of decorin on EGF-mediated hCSF migration. Dcn caused a rapid EGFR phosphorylation within 10 min of exposure in RTK blot defining its role as a biological ligand for EGFR in hCSFs. Prolonged exposure to Dcn caused complete disappearance of EGFR and inhibition of the hCSF migration in the scratch wound assay suggesting Dcn binding to EGFR causes EGFR down-regulation. Immunostaining studies indicated that Dcn-treatment to hCSFs internalizes Dcn-EGFR complex, which does not require tyrosine kinase activity when treated with the AG1478 inhibitor and co-localizes the complex to the perinuclear region. Next, we found that Dcn-EGFR complex does not follow canonical early endosome internalization as revealed by the EEA1 antibody instead binds to the CD63 antibody directed for degradation by the late endosome. We also found that Dcn regulates the EGFR recycling by preventing its binding to Rab11, a specific antibody for recycling endosome. Further, hCSFs-pretreated with pharmacological inhibitors, methyl-β-cyclodextrin and chlorpromazine and supplemented with Dcn suggested EGFR trafficking via the caveolae-mediated pathway. These results suggest that Dcn acts as a biological ligand for EGFR and modulates hCSF migration via EGFR down-regulation, thus playing a vital role in corneal wound healing.
Keywords: Decorin, Epidermal growth factor receptor, Caveolae, Endocytosis, Corneal wound healing
1. Introduction
Decorin (Dcn) is a proteoglycan containing leucine repeats with glycosaminoglycan chain. It is involved in a wide range of biological processes including angiogenesis, wound healing, and modulation of various growth factors and receptors (Iozzo et al., 1999; Grant et al., 2002; Mohan et al., 2011a). Decorin is a natural inhibitor of TGF-β and shows anti-fibrotic effects via regulation of cell migration, proliferation, and differentiation. (Yamaguchi et al., 1990; Border et al., 1992; Baghy et al., 2012; Mohan et al., 2011a; Neill et al., 2012). Decorin has been shown to regulate signaling cascades controlling cellular proliferation and migration through receptor tyrosine kinases (RTKs) like epidermal growth factor receptor (EGFR) (Zhu et al., 2005; Lee et al., 2015; Schaefer et al., 2017) in many non-ocular tissues and organs.
EGFR is a member of the Erb family that contains transmembrane receptor and intrinsic tyrosine kinase activity. It regulates cellular proliferation, adhesion, and migration (Voldborg et al., 1997; Yarden, 2001). EGFR signaling is initiated upon ligand binding and proceeds through receptor dimerization followed by autophosphorylation on tyrosine residues of the cytoplasmic tail and recruitment of downstream signaling such as RAS/MAP, PI3K/AKT and STAT pathways (Novak et al., 2001; Prenzel et al., 2001; Yarden, 2001; Jorissen et al., 2003). These signaling cascades are regulated by endocytic trafficking and recycling of the EGFR involved for cellular migration in many systems including cancer cells and fibroblast cells (Yarden, 2001). Conventionally, EGF activates EGFR signaling, which leads to internalization of the EGF receptor and trafficking to the early endosomal compartment and some part in recycling endosome mediated by clathrin-dependent and independent pathways, and after internalization, the intracellular route of receptors regulates signal transduction (Zhu et al., 2005; Sigismund et al., 2005). The receptor binding plays a vital role in EGFR signaling termination via lysosome or propagation via recycling endosome.
Corneal keratocytes residing in stroma are quiescent cells which primarily facilitate stromal wound repair and maintain corneal transparency (West-Mays and Dwivedi, 2006; Meek, 2009; Hassell and Birk, 2010). Following injury or infection, these cells acquire repair phenotypes by undergoing migration, proliferation, and differentiation to myofibroblasts during wound healing (Wilson et al., 2001; Hassell and Birk, 2010). In our previous studies, we found that Dcn inhibits corneal stromal fibroblasts (CSFs) differentiation, fibrosis, and neovascularization in human cornea in vitro and rabbit cornea in vivo (Mohan et al., 2011a, 2011b; 2011c; Donnelly et al., 2014). Several non-ocular studies have indicated Dcn can act as a biological ligand for EGFR and cause cell cycle arrest by activating a cascade of signaling involving phosphorylation of mitogen-activated protein (MAP) kinase (Iozzo et al., 1999; Santra et al., 2002). Literature suggests that Dcn is involved directly in the control of cell growth since elevated Dcn levels were shown in cell growth arrest and quiescence (Iozzo et al., 1999; Nash et al., 2002; Iozzo and Schaefer, 2010). However, the fate of decorin-EGFR complex in corneal fibroblast during wound healing environment is still unknown. In non-ocular cells activation of EGFR by Dcn was described to follow non-clathrin-dependent endocytosis via caveolar pathways to late endosome for final degradation and thus terminating the EGFR signaling (Zhu et al., 2005). This research report prompted us to postulate that Dcn-mediated EGFR signaling may be regulating keratocyte function and stromal wound healing in the cornea. In this study, we explored Dcn-induced internalization of the EGFR and its intracellular fate in corneal fibroblasts during corneal wound healing using an in vitro model.
2. Materials and methods
2.1. Human corneal stromal fibroblast (hCSF) primary cultures
All experiments on the human cornea and hCSF were carried-out following the tenets of the Declaration of Helsinki and guidelines of the Institutional Review Board of the University of Missouri. Thirty healthy human corneas from male and female donors (23–78 years of age) were procured from the Saving Sight, Kansas City, Missouri, USA to generate primary hCSF cultures as described previously (Sharma et al., 2009). In brief, corneal buttons were washed with sterile minimal essential medium (MEM; Gibco, Grand Island, NY). The epithelial and endothelium layers were gently scraped with a #64 surgical blade. The bare stromal tissue was cut into small pieces, placed into a culture dish containing MEM medium supplemented with 10% fetal bovine serum (FBS), and incubated in a humidified 5% CO2 incubator at 37 °C for 4–6 weeks to yield hCSF primary cultures. The generated primary cultures from donor corneas were harvested, pooled, and used for the study.
2.2. Treatments of recombinant proteins and pharmacological inhibitors
Human CSFs were seeded at a density of 7.5 × 104 in 6-well culture dish in MEM medium and treated with either 250 nM recombinant human decorin (rhDcn; PeproTech, Rocky Hills, NJ) or 100 ng/ml recombinant human EGF (rhEGF; R&D Systems, Minneapolis, MN, USA) or the combination of Dcn (250 nM) and EGF (100 ng/ml) for 15, 30, or 60 min. The cells were pretreated with AG1478 (Cayman chemicals company 10010244), a specific EGFR kinase inhibitor at 5 μM for 60 min then incubated with EGF or Dcn. hCSF cells were preincubated with 2.5 mM methyl-β-cyclodextrin (Sigma, St. Louis; M7439) or 7.5 μM chlorpromazine (Tokyo Chemical Industry, C281) for 30 min and then treated with EGF or Dcn. At selected time points, cultures were either fixed with fresh 4% paraformaldehyde solution for immunocytochemistry or used for preparing protein lysate with RIPA buffer for western blot analysis. Each experiment was performed in triplicate.
2.3. Human phospho-receptor tyrosine kinase (RTK) array
A total of 42 RTKs were examined using a Proteome Profiler Human Phospho-RTK Array Kit (ARY001, R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. hCSFs were seeded at an initial density of 7.5 × 104 per well in 6-well culture dish using MEM medium supplemented with 10% FBS. At 60% confluence, cultures were grown under serum-free conditions for 12–14 h, then incubated in the presence or absence of rhDcn (250 nM) for 10min, washed with PBS, and solubilized in lysis buffer containing protease inhibitors (aprotinin, leupeptin and pepstatin; 10 μg/ml each). The protein concentration of samples was quantified by Bradford assay. A 200 μg of each sample was solubilized in Array Buffer, applied on the nitrocellulose membrane in multi-dish, and incubated overnight at 4 °C. Array membrane was washed twice with wash buffer provided in the kit, and probed with anti-phospho-tyrosine horseradish peroxidase (HRP) antibody, incubated 2 h at room temperature, rinsed twice with wash buffer, and subjected to chemiluminescence. Samples were loaded in duplicate in RTK array. The array assay was performed thrice, and samples were loaded in duplicate each time.
2.4. Western blot
Human CSFs were seeded at an initial density of 60 × 104 in 60 mm culture dish using MEM medium supplemented with 10% FBS and switched to serum-free medium at 50% confluence. At 70% confluence, cultures were incubated in ± rhDcn (250 nM) for 0, 15, 30, or 60 min. Cells were lysed and protein content was quantified using the Bradford assay. Samples were resolved on 4–12% SDS-PAGE on NuPAGE Novex Bis-Tris mini gels (Invitrogen, Carlsbad, CA), transferred onto polyvinylidene fluoride membrane (0.45 μm pore size), and incubated with EGFR and β-actin antibodies (Cat # sc-03-G and sc-47778; Santa Cruz Biotechnology, Santa Cruz). Protein levels were visualized using appropriate HRP-conjugated secondary antibodies (Santa Cruz) and ECL detecting system (Millipore). Image Studio software (Version 5.2; Lincoln, NE) and NIH Image J software were used for the quantification of bands in western blots.
2.5. Scratch wound assay
The effects of Dcn on hCSF's migration were examined using an in vitro standard wound scratch assay as reported previously (Anumanthan et. al., 2018). Human CSFs at a density of 7.5 × 104 were seeded in a 6-well plate using MEM media containing 10% FBS. Once hCSFs reached 70% confluence, an identical scratch was created on each well with a p200 pipet tip and cultures were grown in serum-free conditions in ± rhEGF (100 ng/ml) and ± rhDcn (250 nM) or combination of both for 24 h. The changes in cellular migration were recorded with a phase-contrast microscope equipped with a Leica LASV3.8 imaging system (Leica Microsystems, Bannockburn, IL) 24 h after making scratch-wound in all treatment groups. The data presented in manuscript is an average of three experiments.
2.6. Immunocytochemistry
Human CSFs were grown in 6-well dish at 70% confluence, preincubated with ± recombinant human rhDcn (250 nM) and ± rhEGF (100 ng/ml) for 30 min, fixed with fresh 4% paraformaldehyde for 30 min, and washed with PBS. Cells were blocked for 1 h at room temperature with 5% normal donkey serum (Jackson Immuno Research Laboratories, West Grove, PA) and 0.1% Tween-20 (Sigma-Aldrich, St Louis, MO), followed by a 90-min application of mouse monoclonal EGFR antibody (1:50 dilution; Cat # sc-03-G), EEA1 (1:100 dilution; Cat # sc-13730, Santa Cruz), CD63 (1:100 dilution; Cat # sc-59284, Santa Cruz), Rab11 (1:200 dilution; Cat # sc-6565, Santa Cruz). Cells were washed three times and incubated in Alexa Fluor 488 donkey antimouse antibody (1:1000 dilution; Cat # A21202, Invitrogen) and Alexa Fluor 594 donkey anti-goat antibody (1:1000 dilution; Cat # A11058, Invitrogen) for 1 h at room temperature. Double immunostaining was performed by sequential incubations with antibodies directed for two different species. The cells were washed three times in PBS, mounted in Vectashield containing 4′-6-diamidino-2-phenylindole (DAPI; Cat # H1200, Vector Laboratories, Burlington, CA), and digital images were captured using a Leica DM 4000B fluorescent microscope (Leica, Bannockburn, IL). The immuno-stained cells were quantified by counting EGFR+, Dcn+, EEA1+, CD63+, Rab11 + and DAPI + cells in non-overlapping ten randomly selected areas at 200× magnification under a Leica DM 4000B fluorescent microscope (Leica). The primary antibody alone, secondary antibody alone, or irrelevant isotype-matched antibody alone and pre-absorption with blocking antigen were used as negative controls for all immunostaining experiments.
2.7. Statistical analysis
The GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA) was used for statistical analysis. Studen's t-test, Wilcoxon rank-sum test and One-way analysis of variance (ANOVA) were used to determine statistical significance. The results are expressed as the mean ± standard error of the mean (SEM). The value of p < 0.05 was considered to be statistically significant.
3. Results
3.1. Decorin phosphorylates EGFR in hCSF cells
A commercial array kit comprising of 42 different phosphorylated human RTKs was used to test the response of Dcn to various phosphorylated human receptor tyrosine kinases. As evident from Fig. 1, rhDcn (250 nM) treatment to hCSF cultures showed a significant increase in the expression of phosphorylated EGFR (Fig. 1B; p < 0.001) compared to the vehicle-treated control (Fig. 1A). An average of three experiments demonstrating increase in the expression of phosphorylated EGFR after decorin treatment compared to control (vehicle) in hCSF is shown in Fig. 1C. We did not detect phosphorylation of other receptor tyrosine kinases under tested experimental conditions.
Fig. 1. Decorin increases phosphorylated EGFR in hCSF cells.
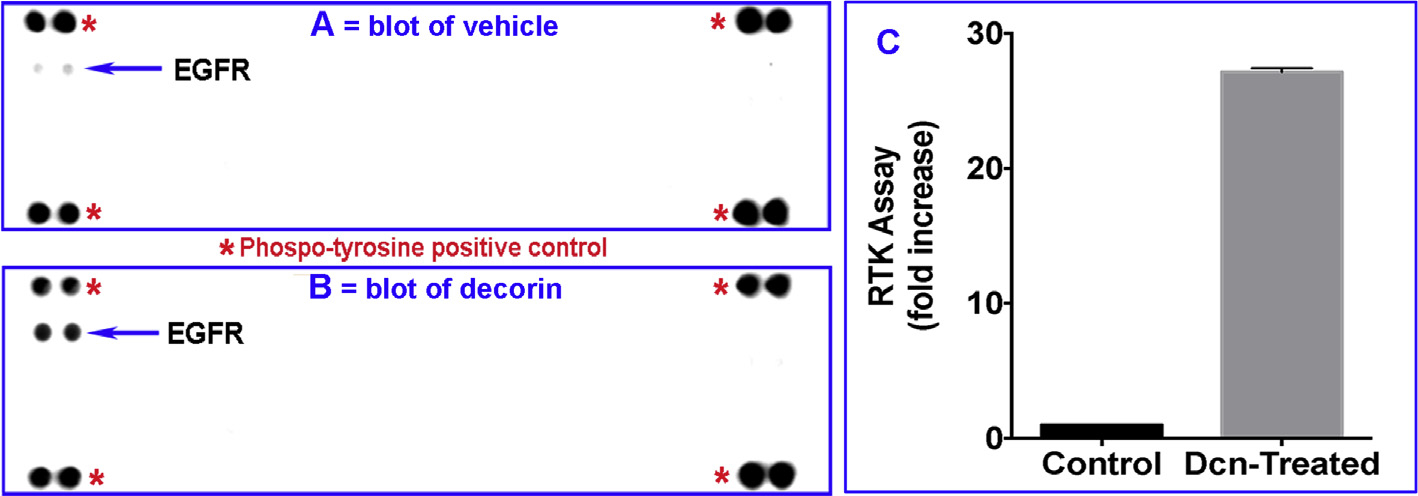
Proteome Profiler Human Phospho-receptor tyrosine kinase (RTK) kit was used to test 42 phosphorylated RTKs in hCSF cells under serum-free conditions treated with ± rhDcn (250 nM) for 10 min. EGFR band was observed in the Dcn-treated culture (B) shown by arrow compared to the control (vehicle only) group (A). Panel C shows quantification data of three assays. Data were presented as mean ± SEM. * represents the reference spots on the array blot as described in the RTK kit.
3.2. Prolonged exposure of hCSF cells to decorin downregulates EGFR expression
Next, we tested a time-dependent regulation of EGFR expression in hCSF upon exposure to decorin. hCSFs cultured in the serum-free medium were incubated with rhDcn (250 nM) or rhEGF (100 ng/ml) for 15, 30, or 60 min hCSF lysates were collected for western blot and probed with EGFR and beat actin antibodies. We found that Dcn treatment caused a significant increase in EGFR expression within 15 min (p < 0.05), which was found significantly decreased in 30 min (p < 0.001) and completely disappeared in 60 min compared to basal EGFR level at 0 min (Fig. 2A). On the contrary, hCSF cultures incubated with EGF showed constant EGFR expression at different time-points tested up to 60 min (Fig. 2B). The quantification of EGFR levels after rhDcn or rhEGF treatments in hCSFs from three experiments has been provided in Fig. 2C.
Fig. 2. Prolonged exposure to decorin decreases EGFR expression.
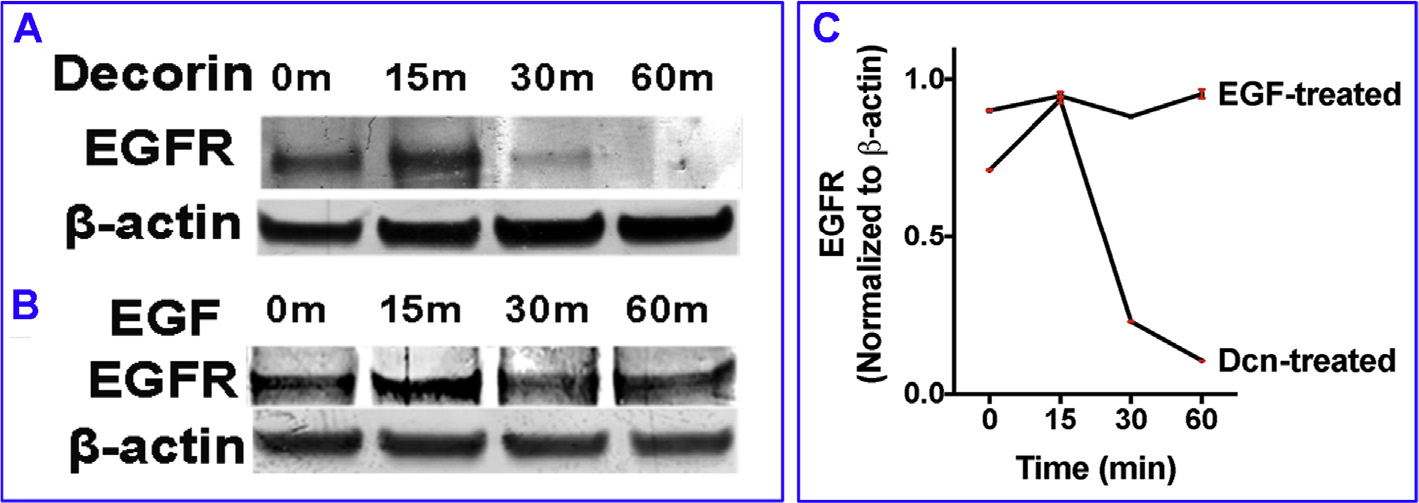
Human CSF cultures were incubated in rhDcn (250 nM) for 15, 30, or 60 min. EGFR expression was increased at 15 min timepoint, but decreases at 30 min and completely disappeared at 60 min time point (A). On the other hand, hCSF cultures incubated in rhEGF (100 ng/ml) showed significantly increased expression of EGFR throughout the duration of the experiment (B). Panel C shows quantification data of three experiments. Data were presented as mean ± SEM.
3.3. Decorin impedes hCSF cell migration
To know whether decorin-induced downregulation of EGFR inhibits hCSF migration, we performed in vitro scratch wound assay on hCSFs grown in the presence or absence of rhEGF (100 ng/ml) alone, or in combination with rhDcn (250 nM) or EGF antibody in a serum-free media (Fig. 3). Treatment of rhEGF significantly enhanced hCSF migration (91% ± 7.2%, p < 0.001) (Fig. 3B) in wounded area compared to the vehicle-control (Fig. 3A) at 24 h. A significant reduction in EGF-mediated hCSF migration (89% ± 9.4%, p < 0.001) (Fig. 3D) by rhDcn treatment demonstrated its role in suppressing hCSF migration. The inhibitory effects of decorin were remarkably high and were comparable to that of the EGF antibody treatment (92% ± 8.1%, p < 0.001) (Fig. 3C).
Fig. 3. Decorin impedes EGF-mediated migration of hCSFs.
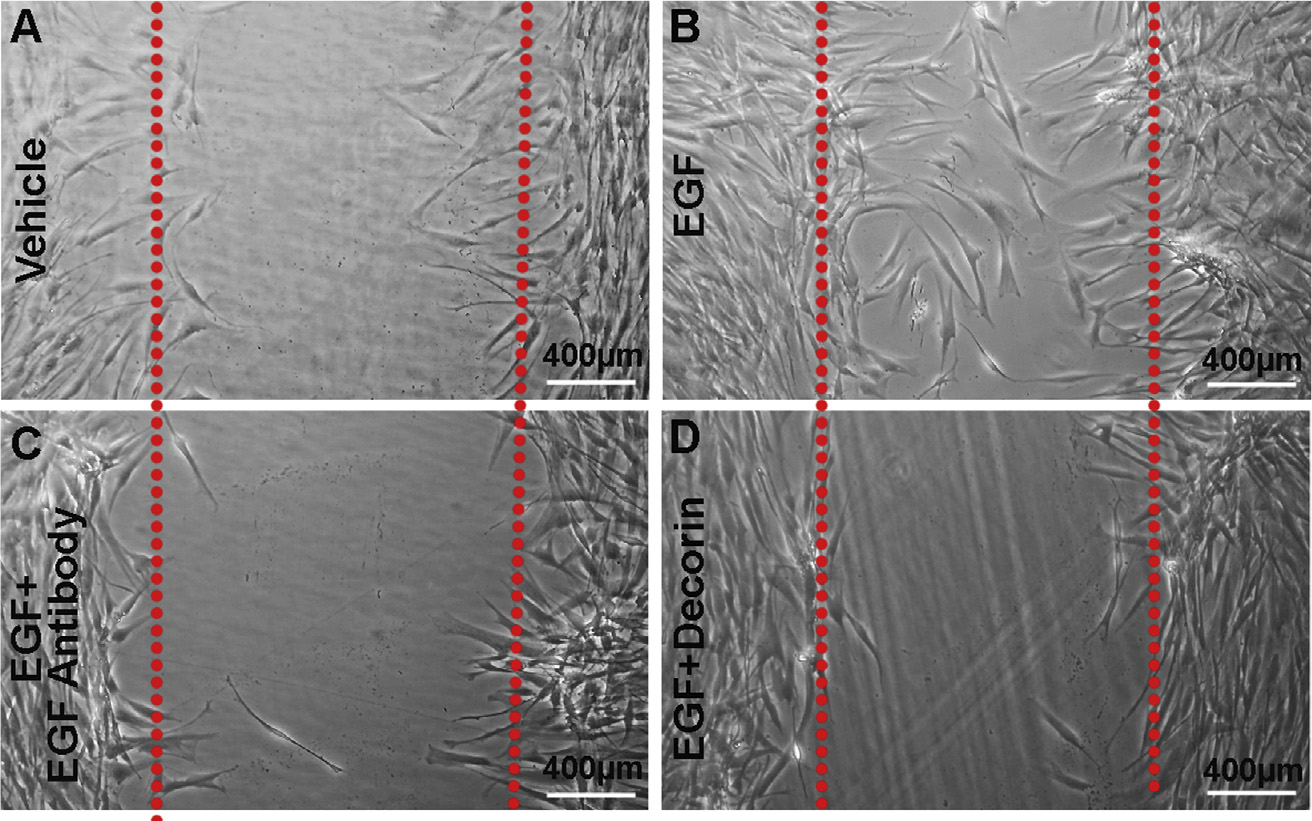
In vitro wound scratch assay was performed in hCSF cultures with ± rhEGF and/or rhDcn. In this assay, EGF (100 ng/ml) profoundly stimulated hCSF migration (B; 91% ± 7.2%, p < 0.001) than vehicle (A). The Dcn (250 nM) treatment significantly reduced EGF-mediated hCSF migration (D; 89% ± 9.4%, p < 0.001). The inhibitory effects of decorin were similar to the effects observed with the EGF antibody (C; 92% ± 8.2%, p < 0.001). Data were presented as mean ± SEM.
3.4. Decorin induces internalization of EGFR
To explore the possibility of Dcn regulating the localization of EGFR, we incubated the hCSF cells with rhDcn (250 nM) for 30 min and performed double immunofluorescence with EGFR and Dcn antibodies. We observed large amounts of Dcn-EGFR internalized within the cytoplasm and in perinuclear region (Fig. 4B) in cultures treated with decorin. Conversely, we noted EGFR expression localized solely on the plasma membrane in non-Dcn treated (vehicle-treated) cultures (Fig. 4A). This observation provided evidence that decorin induces EGFR internalization into human corneal fibroblasts in vitro.
Fig. 4. Decorin induces internalization of EGFR and co-localizes with decorin to the perinuclear region.
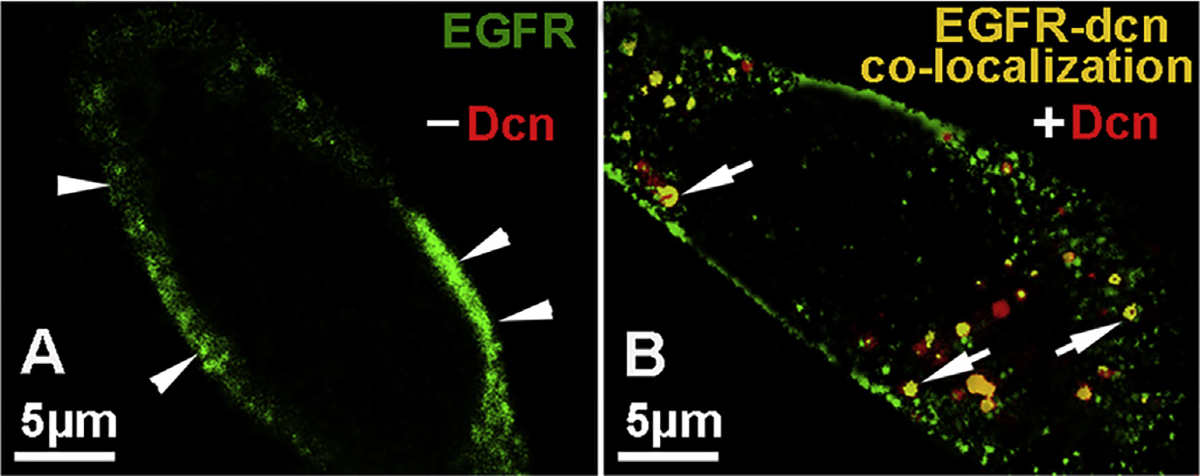
EGFR was localized on the plasma membrane (green, arrowhead) in the non-treated hCSF cells (A). Incubation of hCSF cultures for 1 h with rhDcn (250 nM) translocated EGFR to the perinuclear region (B) as evident from the co-localized double EGFR-Dcn stained cells (yellow; arrows) which was absent in vehicle-treated (-dcn) cultures (A). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Decorin-induced internalization of EGFR does not require tyrosine kinase activity
Next, we investigated whether Dcn-induced internalization of EGFR in hCSF involves tyrosine kinase activity. Human CSFs exposed to rhDcn or rhEGF in ± AG1478 (30 μM for 16 h), a specific inhibitor for tyrosine kinase activity, were subjected to double immunofluorescence. The Dcn-exposed hCSFs grown in −AG1478 (Fig. 5A) showed Dcn-EGFR complex localized in the perinuclear region similar to + AG1478 (Fig. 5C). On the contrary, the EGF-treated hCSFs grown in +AG1478 did not show co-localized EGF-EGFR complex in the perinuclear region and instead showed only at the plasma membrane (Fig. 5D) whereas hCSF grown in −AG1478 showed EGF-EGFR complex in the perinuclear region (Fig. 5B). This data suggested that Dcn-induced EGFR internalization did not depend on tyrosine kinase activity. The vehicle-treated cultures showed EGFR staining on the plasma membrane (Fig. 4A).
Fig. 5. Decorin-induced internalization of EGFR does not require tyrosine kinase activity.
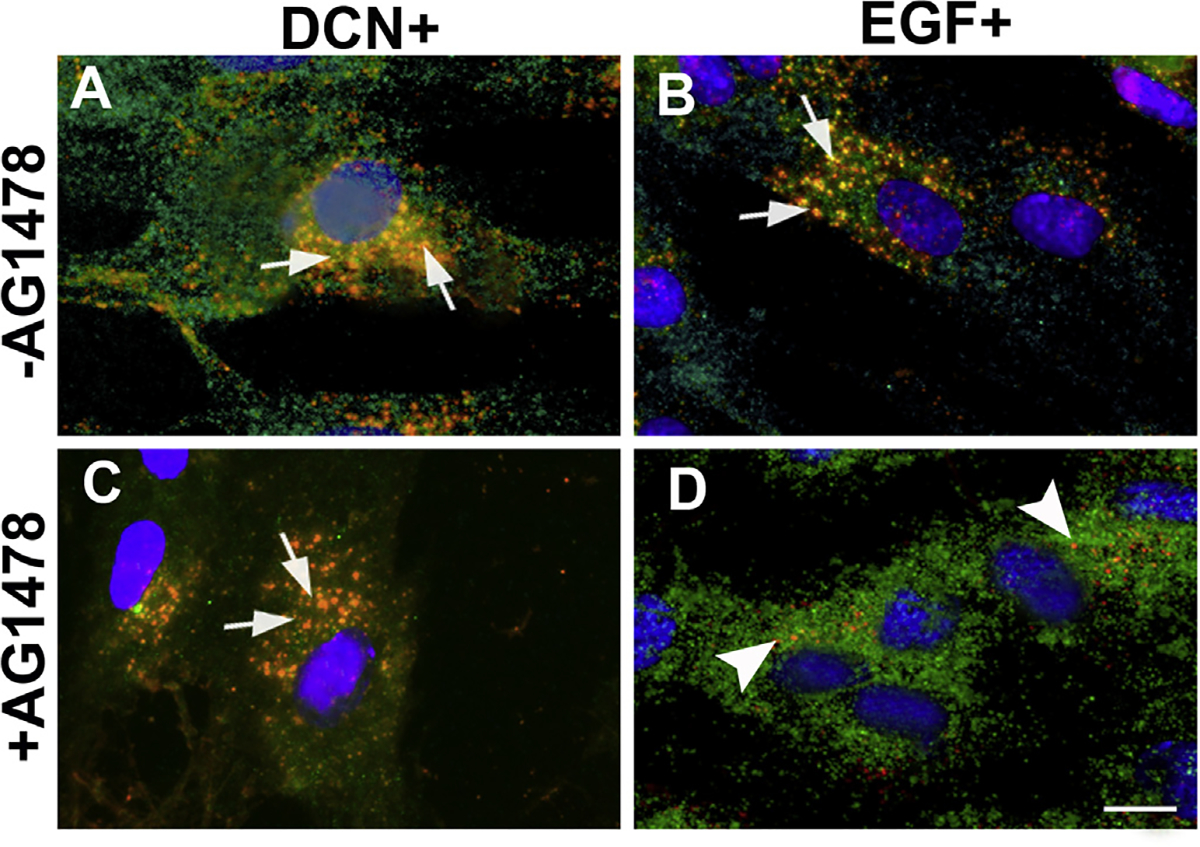
Confocal microscopy of hCSF cells pretreated with AG1478 (a specific tyrosine kinase activity inhibitor at 5 μM for 60 min) and incubated with rhDcn (250 nM) do not inhibit internalization of EGFR and colocalize with Dcn (yellow; arrows) in the perinuclear space (C) similar to the non-treated cells (A). On the contrary, rhEGF-treatment (100 ng/ml) internalizes of EGFR was inhibited by AG1478 and EGFR expression (D) was seen only at the cell surface (green, arrowheads) while non-treated cells show internalization of EGFR + EGF (yellow; arrows). Merged image shows co-localization of EGFR + EGF observed near peri-nuclear region (B). Bar = 25 μM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.6. Decorin-mediated EGFR internalization does not involve early endosome
To evaluate further regulation of EGFR, we questioned whether Dcn-EGFR complex internalization and degradation involve formation of early endosomes? To answer this, we double-immunoassayed hCSF cells with EEA1 (early endosome marker) and EGFR specific antibodies. Dcn-treated cells showed minimal co-localization of EEA1 with EGFR (Fig. 6A–C) compared to EGF-treated cells where EGFR was co-localized with EEA1 (Fig. 6E–G). This data suggests that Dcn-EGFR complex directly goes to caveosome (Fig. 6D) whereas EGF-EGFR complex goes primarily to early endosome (Fig. 6H). No immunofluorescence was observed in negative controls (data not shown).
Fig. 6. Decorin-mediated EGFR internalization does not involve early endosome.
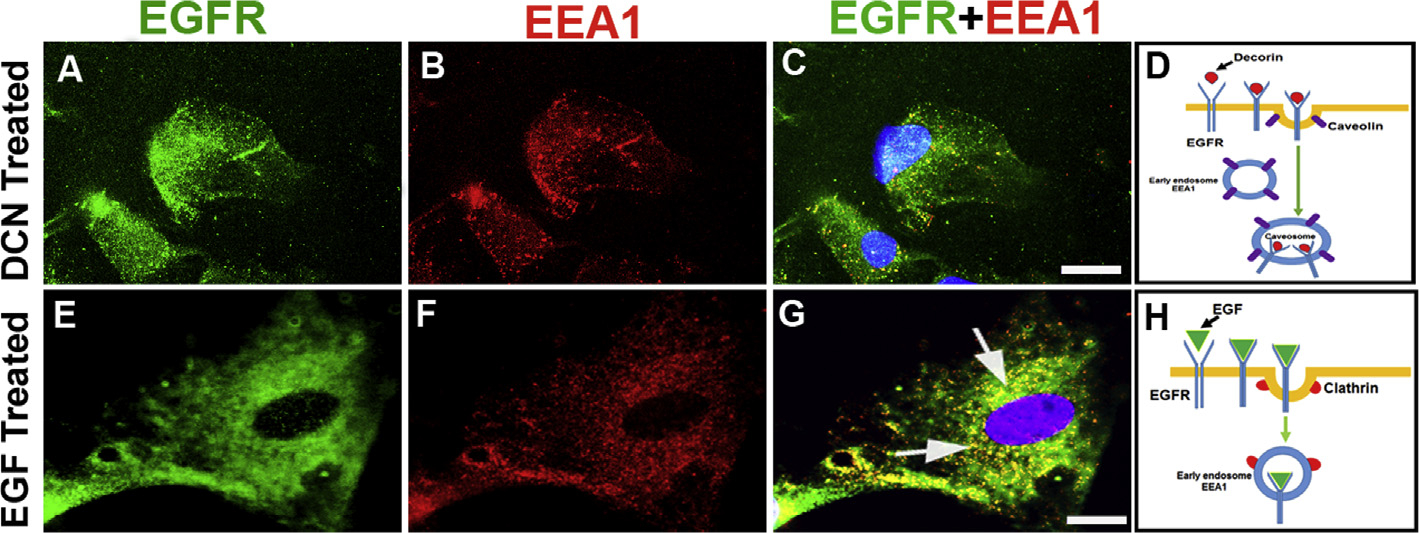
Human CSFs were incubated with rhDcn (250 nM, A-D) or rhEGF (100 ng/ml, E-H). Double immunostaining of hCSFs with EGFR (green, A and E) and early endosome marker, EEA1, (red, B and F) showed EGFR and EEA1 co-localization vesicle only with rhEGF treatment (G, arrows). On the other hand, rhDcn-treatment to hCSF cells showed insignificant co-localization of EGFR and EEA1 (C). Schematic diagram shows internalization does not target early endosome (EEA1) upon decorin treatment (D) but it targets EEA1 when treated with EGF (H). Bar = 10 μM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.7. Decorin-EGFR complex is processed by late endosome for degradation
Next, we looked at the fate of Dcn-EGFR complex by late endosome. We treated hCSF cells with rhDcn or rhEGF and double immunostained with EGFR and CD63, a late endosome marker. Both, Dcn (Fig. 7A–C) or EGF (Fig. 7E–G), treatment showed co-localization of CD63 with EGFR. This confirms that the disappearance of EGFR at 60 min time-point (Fig. 2A) in Dcn-incubated hCSFs is due to degradation of the Dcn-EGFR complex via late endosome. The schematic diagrams show how Dcn- (Fig. 7D) and EGF- (Fig. 7H) induced EGFR-complexes are fed into late endosome for degradation. No immunofluorescence was observed in negative controls (data not shown).
Fig. 7. Decorin-EGFR complex degradation involves late endosome.
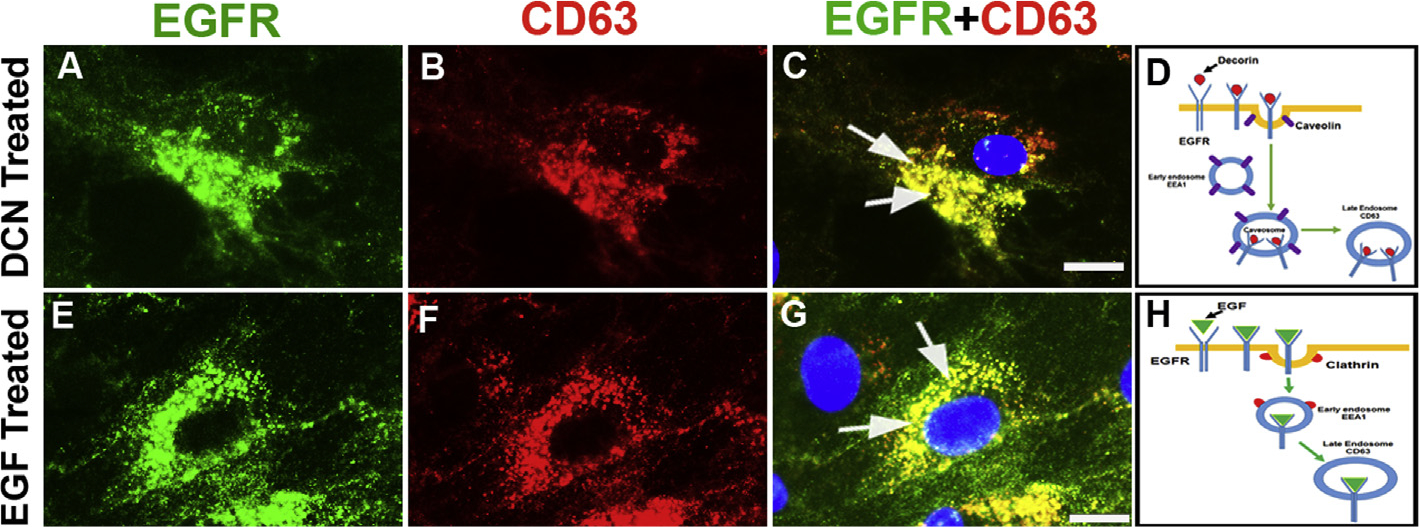
Human CSFs incubated with rhDcn (A–D) or rhEGF (E–H) and double-immunostained with EGFR (green) and late endosome marker, CD63, (red). Decorin treatment showed high co-localization of EGFR and CD63 in an overlay image (C; yellow, arrows) compared to the rhEGF (G, yellow, arrows). The EGFR staining (green) is shown in panels A and E, and CD63 staining (red) is shown in panels B and F. Schematic diagram shows EGFR internalization target late endosome upon decorin treatment (D) compared to EGF treatment (H). Bar = 10 μM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.8. Decorin prevents endosome-mediated recycling of EGFR
We found that Dcn regulates degradation of EGFR in a time-dependent manner whereas EGF does not affect EGFR levels significantly (Fig. 2). This may be due to the recycling of the EGFR. To test this postulate, we incubated hCSFs with Dcn or EGF and subjected cultures to double immunofluorescence with antibodies specific for EGFR and Rab11 (a recycling endosome specific marker). We found that EGFR in Dcn-incubated hCSFs did not co-localize with Rab11 (Fig. 8A–C) but EGFR in EGF-incubated cells co-localized with Rab11 (Fig. 8E–G). This data confirms that Dcn-induced internalization does not take part in endosome recycling of EGFR (Fig. 8D) whereas EGF-induced internalization of EGFR goes to the endosome for the recycling of the receptor (Fig. 8H). This might explain the constant expression of EGFR observed in hCSFs after treatment of EGF (Fig. 2B). No immunofluorescence was observed in negative controls (data not shown).
Fig. 8. Decorin prevents recycling of EGFR.
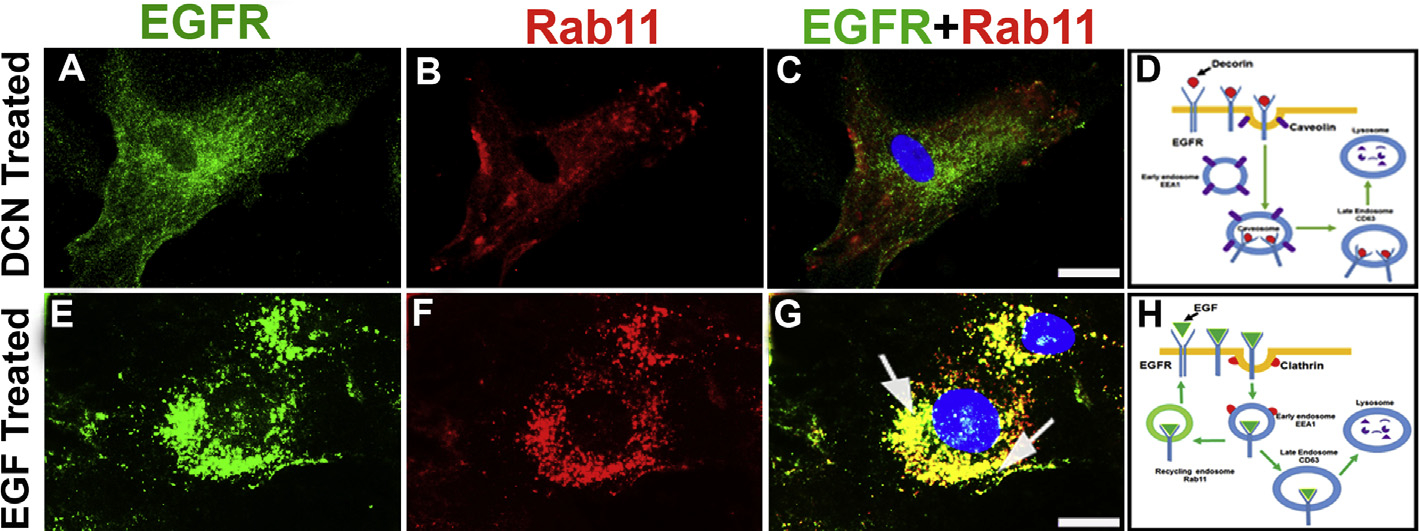
Human CSF cells incubated with rhDcn (A–D) or rhEGF (E–H) and double immunostained with EGFR (green) and recycling endosome marker, Rab11 (red). The overlay images of double-immunostaining showed their co-localization with rhEGF treatment (G; yellow; arrows), which was absent in the rhDcn-treated hCSF cells (D) indicating attenuation of EGFR recycling. Schematic diagram shows internalization does not target late endosome (Rab11) upon decorin treatment (D) while it targets Rab11 when treated with EGF (H). Bar = 10 μM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.9. Decorin-induced EGFR internalization is driven by caveolae-mediated endocytosis
We used caveolae- and clathrin-specific inhibitors to further decipher the mechanism involved in the Dcn-mediated internalization of EGFR. Methyl-β-cyclodextrin (MβCD) is a cyclic heptasaccharide that depletes cholesterol from the plasma membrane and inhibit caveolae formation. Human CSFs-treated with Dcn and MβCD (2.5 mM) for 30 min prevented the internalization of EGFR (Fig. 9B) compared to the perinuclear localization of the Dcn-EGFR complex in the vehicle-treated (Fig. 9A) or EGF-treated (Fig. 9E) cells. Chlorpromazine is a cationic amphiphilic drug, which blocks the receptor recycling by preventing the adapter protein AP-2 of clathrin assembly on clathrin-coated pits. Human CSFs incubated with EGF and Chlorpromazine (7.5 μM) for 30 min blocked the EGFR internalization (Fig. 9F) remarkably compared to the vehicle control (Fig. 9D). Conversely, Dcn-treated hCSFs under similar experimental conditions showed co-localization of Dcn-EGFR complexes near perinuclear region (Fig. 9C) suggesting internalization of the EGFR occurs via caveolae-mediated endocytosis. No immunofluorescence was observed in negative controls (data not shown).
Fig. 9. Decorin-mediated internalization of EGFR ensues via caveolar-mediated endocytosis.
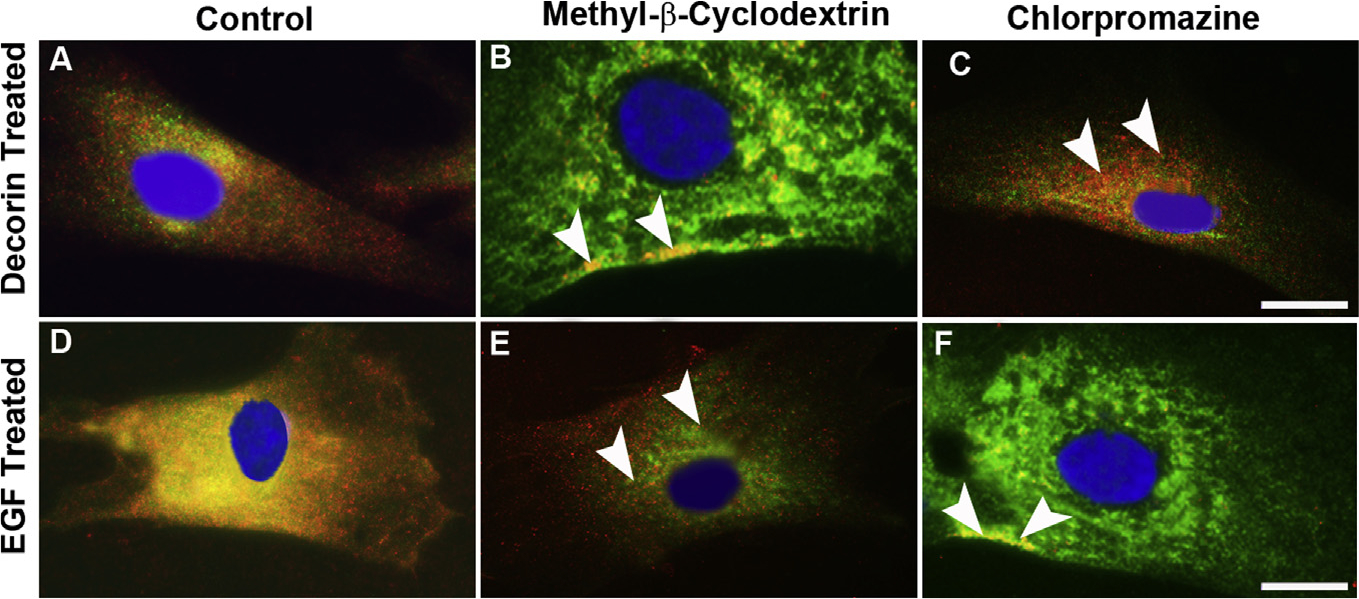
Confocal photomicrograph showing inhibition of caveolae formation in hCSF cells using methyl-β-cyclodextrin (MβCD) that depletes cholesterol from the plasma membrane, rhDcn-induced internalization of EGFR was completely blocked (B) and EGFR was localized only in the plasma membrane (arrowheads). Contrary to this, rhEGF-induced internalization of EGFR was not affected (E, arrowheads) when treated with MβCD. When clathrin assembly was inhibited by chlorpromazine, a cationic amphiphilic drug that preferentially blocks the receptor recycling by preventing the adapter protein AP-2 of clathrin assembly on clathrin-coated pits, rhDcn-induced internalization of EGFR was observed (C, arrowheads) while rhEGF-induced internalization of EGFR was disrupted (F, arrowheads). The vehicle-treated (-dcn) showed no EGFR internalization as shown in Fig. 4A. Bar = 5 μM.
4. Discussion
Corneal wound healing is a highly sophisticated and orchestrated mechanism involving cellular transdifferentiation of quiescent corneal keratocytes to activated motile corneal myofibroblasts (Wilson et al., 2001; Hassell and Birk, 2010; Chaurasia et al., 2015; Ljubimov and Saghizadeh, 2015). At the active wound site, epithelial-stromal interactions cause cellular proliferation, migration and extracellular matrix (ECM) remodeling governed by a variety of growth factors including transforming growth factor beta (TGFβ), epidermal growth factor (EGF) and its family members (Wilson et al., 2001; Tandon et al., 2010; Mohan et al., 2011a). In the present study, we found that decorin (Dcn) treatment to hCSF cells in the human phospho-receptor tyrosine kinase (RTK) array showed significantly high levels of phosphorylated EGFR compared to the vehicle-treated controls (Fig. 1). EGFR phosphorylation is one of the critical steps in the corneal wound healing, and impaired EGF signaling that has been found to be associated with sight-threatening complications, such as stromal opacity, surface irregularity, and microbial keratitis. In fact, topical application of EGF has been shown to be an effective therapy for corneal erosions, suggesting EGFR agonists for the treatment of corneal disorders (Nakamura et al., 2001).
Dcn plays a predominant role in the ECM homeostasis that regulates the structure, tissue organization, and surface properties of collagen in many tissues including the cornea (Iozzo and Schaefer, 2010; Neill et al., 2012). The targeted deletion of Dcn in mice resulted in abnormal collagen fibrillogenesis, reduced tensile strength, and fragility of the skin (Danielson et al., 1997). In the cornea, Dcn acts as an ECM stabilizer and regulates collagen fibrillogenesis, a prerequisite for the corneal transparency (Hassell and Birk, 2010; Mohan et al., 2011a). During wound healing, keratocytes acquire a fibroblast phenotype, activates EGFR signaling to initiate cellular migration, proliferation, and differentiation. Dcn is rarely expressed in the actively proliferating cells but how EGFR signaling interacts with Dcn during corneal wound healing has not yet been studied. In the present study, Dcn-treatment to hCSFs showed complete loss of EGFR expression in a time-dependent manner (Fig. 2). These results corroborated with the scratch wound assay where Dcn prevented the migration of hCSFs similar to observations made with the addition of EGF antibody (Fig. 3). On the contrary, EGF-treatment to hCSFs showed constant expression of EGFR protein and EGFR-mediated cellular migration and proliferation of hCSFs in the scratch wound assay (Fig. 3).
Dcn has been described to act as a signaling molecule driving multiple signaling pathways for its ability to bind several growth factors for the regulation of growth, differentiation and tissue organization (Zhu et al., 2005; Lee et al., 2015; Schaefer et al., 2017). In our previous report, we demonstrated that Dcn gene delivery inhibits TGFβ signal transduction, prevents myofibroblast formation, thus inhibits fibrosis in the cornea (Mohan et al., 2011b). Recently, Dcn has been shown to compete and regulate EGFR signaling cascade to regulated cellular migration and proliferation in the quiescent cells (Nishimura et al., 2008; Adam et al., 2012). Dcn levels were found to be suppressed within the actively proliferating and transformed cells derived from the malignant tumors. It has been well established that Dcn plays a critical role in tumorigenesis. Decorin binds to EGFR with low-to-mid nanomolar range (Kd approx. 30–90 nM) to i) phosphorylate EGFR to activate caspase-3 initiating apoptotic pathways, ii) control intracellular Ca2+ mobilization, and iii) regulate availability of EGFR by directing its fate to lysosomal degradation, highlights its therapeutic potential in the treatment of cancer. In the cornea, Dcn is mostly localized and expressed in the stroma but how it regulates the availability of the EGFR is still unknown. We found that Dcn supplementation to the hCSFs co-localizes Dcn with EGFR and translocates the Dcn-EGFR complex to the perinuclear space of the cell compared to the vehicle where EGFR was solely present on the plasma membrane (Fig. 4). Thus, Dcn shifts the ECM equilibrium towards the inhibition of EGFR signaling via internalization of the Dcn-EGFR complex. In fact, abnormally low levels of Dcn have been observed in many cancers where the lack of Dcn causes activation of EGFR signaling resulting in the abnormal migration and proliferation of tumor cells (Reed et al., 2002; Xu et al., 2016).
Ligand-activated EGFR signaling occurs mostly via plasma membrane, but EGFR internalization by the early endosome constitutes a vital step in enabling the signaling cascades from the intracellular sites (Zhu et al., 2005). Since the EGFR signaling involves receptor tyrosine kinase, we tested whether Dcn-mediated EGFR internalization requires its tyrosine kinase activity. We pre-incubated hCSF cells with AG1478, a highly specific inhibitor of EGFR kinase prior to the treatment with Dcn and found that Dcn-EGFR complex was still located in the perinuclear area whereas EGF-treated hCSFs completely blocked in the EGF-EGFR complex (Fig. 5). Similar observations have been made in A431 (Zhu et al., 2005) and Chinese hamster ovary cells (Schmidt et al., 2003) treated with AG1478, suggesting tyrosine kinase activity was not essential for the Dcn-induced internalization of EGFR.
Regulation of endocytic trafficking of activated RTKs has been considered as the primary pathway for the EGFR-mediated signal attenuation. It has been reported that EGF binding to the EGFR internalizes the complex into the early endosome and causes either recycling of the EGFR or leads to degradation (Burke et al., 2001). In hCSF, stained with EAA1, an early endosome marker resulted in the colocalization of EEA1 with EGFR upon EGF-treatment whereas Dcn supplementation does not have any effect on the EGFR expression in EEA1-treated cells (Fig. 6). These results suggest that the early endosome does not process Dcn-induced internalization of EGFR instead followed a more specialized compartment, known as “caveosome” whereas EGF led to the recycling of the EGFR via clathrin-mediated entry into the early endosome. Next, we studied whether Dcn-EGFR and EGF-EGFR complexes enter late endosomes using CD63, a specific late endosome marker (Fig. 7). hCSF cells treated with Dcn showed co-localization of Dcn-EGFR complex suggesting its entry into the late endosome destined for lysosomal degradation. This agrees with our results depicting the disappearance of EGFR expression within 30–60 min of Dcn-treatment in a western blot analysis (Fig. 2). As observed earlier, we also found the marginal entry of EGF-EGFR complex into the late endosome (Sorkin and Goh, 2008).
Previous studies have suggested that EGF-induced EGFR internalization involves early endosome, which can either return EGFR to the plasma membrane by recycling endosomes or captured by the endosomal sorting complex for transport (Tomas et al., 2014; Bakker et al., 2017). The Rab11 family of proteins regulates EGFR trafficking from the early endosome to the endocytic recycling compartment (ERC) by recycling the EGFR. We found colocalization of Rab11 and EGFR upon treatment of EGF using double immunofluorescence (Fig. 8). Our western blot data obtained from the EGF-treated hCSFs also showed a similar phenomenon where EGFR was continuously expressed throughout the experiment (Fig. 2). This is because of the fact that some part of EGFR during the internalization of early endosome connecting through Rab domain recycled to the plasma membrane (Fig. 8). In contrast, Dcn-treatment does not co-localize EGFR with Rab11 in hCSFs. This supports our earlier data suggesting Dcn prevents EGFR from entering the early endosome and forces its entry to late endosome directed towards caveolar-mediated endocytosis.
Dcn exhibits its anti-proliferative and anti-oncogenic properties by down-regulating the tyrosine kinase activity of EGFR in a variety of tumor cells. Under physiological conditions, EGF directs EGFR recycling via early endosome in a clathrin-dependent mechanism. In contrast, Dcn has been reported to inhibit EGFR signaling via EGFR dimerization, internalization, and caveolar-mediated degradation in transformed cells and tumor xenografts (Grant et al., 2002; Reed et al., 2002). We used methyl-β-cyclodextrin (MβCD), a water-soluble cyclic heptasaccharide that depletes cholesterol vital for the caveolae endocytosis function. Addition of Dcn to the hCSFs pre-treated with MβCD inhibited the internalization of EGFR, thus prevented the Dcn-induced EGFR degradation (Fig. 9). On the other hand, hCSF pre-treated with chlorpromazine, a cationic amphiphilic drug that prevents clathrin-mediated endocytosis and blocks receptor recycling caused no damage to the Dcn-induced internalization of EGFR supporting the caveolae-dependent EGFR degradation (Fig. 9).
In conclusion, this study for the first time shows that decorin acts as a biological ligand for EGFR and regulates corneal stromal fibroblast migration which is a prerequisite for corneal wound healing by redirecting EGFR internalization and degradation during active wound healing state via caveolae-mediated endocytosis (Fig. 10).
Fig. 10. Schematic representation describing the internalization of EGFR by decorin and EGF induction.
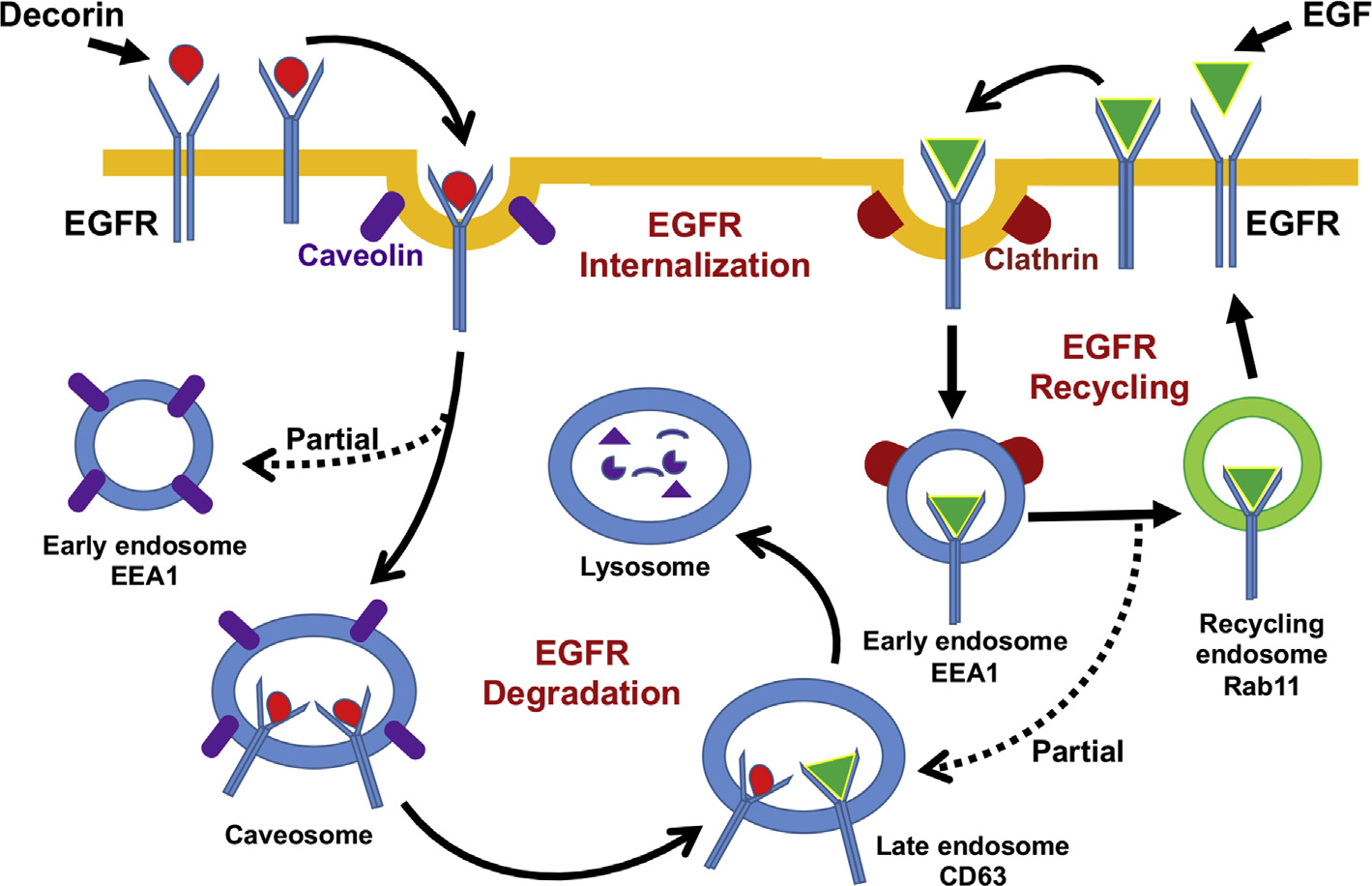
Decorin is taken up by the EGFR and the Dcn-EGFR complex is internalized and transferred to the perinuclear vesicles, commonly known as caveosome bypassing the early endosome intake. At this stage, Dcn-EGFR complexes are sent to late endosome (CD63) for final degradation. Conversely, EGF-induces stepwise EGFR endocytosis primarily in early endosome (EEA1), where some part of the EGF-EGFR complex enters the recycling endosome (Rab11) and some portion enters into the late endosome (CD63) for final degradation.
Acknowledgements
This work was primarily supported by the NIH/NEI R01EY017294 grant (RRM), and partial support from the Veterans Health Affairs Merit 1I01BX00357 grant (RRM), and Ruth M. Kraeuchi Missouri Endowed Chair Ophthalmology University of Missouri Fund (RRM). Thanks are due to Dr. Govindaraj Anumanthan his help in immunofluorescence.
References
- Adam M, Urbanski HF, Garyfallou VT, Welsch U, Köhn FM, Schwarzer JU, Mayerhofer A, 2012. High levels of the extracellular matrix proteoglycan decorin are associated with inhibition of testicular function. Int. J. Androl. 35, 550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumanthan G, Gupta S, Fink MK, Tripathi R, Hesemann NP, Bowles DK, McDaniel LM, Muhammad M, Mohan RR, 2018. KCa3.1 ion channel: a novel therapeutic target for corneal fibrosis. PLoS One 13, e0192145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghy K, Iozzo RV, Kovalszky I, 2012. Decorin-TGFβ axis in hepatic fibrosis and cirrhosis. J. Histochem. Cytochem. 60, 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Spits M, Neefjes J, Berlin I, 2017. The EGFR odyssey-from activation to destruction in space and time. J. Cell Sci. 130, 4087–4096. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E, 1992. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 360, 361–364. [DOI] [PubMed] [Google Scholar]
- Burke P, Schooler K, Wiley HS, 2001. Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol. Biol. Cell 12, 1897–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Lim RR, Lakshminarayanan R, Mohan RR, 2015. Nanomedicine approaches for corneal diseases. J. Funct. Biomater. 6, 277–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV, 1997. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 136, 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly KS, Giuliano EA, Sharma A, Tandon A, Rodier JT, Mohan RR, 2014. Decorin-PEI nanoconstruct attenuates equine corneal fibroblast differentiation. Vet. Ophthalmol. 17, 162–169. [DOI] [PubMed] [Google Scholar]
- Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV, 2002. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene 21, 4765–4777. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Birk DE, 2010. The molecular basis of corneal transparency. Exp. Eye Res. 91, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I, 1999. Decorin is a biological ligand for the epidermal growth factor receptor. J. Biol. Chem. 274, 4489–4492. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Schaefer L, 2010. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 277, 3864–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW, 2003. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp. Cell Res. 284, 31–53. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Ahn HM, Roh H, Na Y, Choi IK, Lee JH, Kim YO, Lew DH, Yun CO, 2015. Decorin-expressing adenovirus decreases collagen synthesis and upregulates MMP expression in keloid fibroblasts and keloid spheroids. Exp. Dermatol. 24, 591–597. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M, 2015. Progress in corneal wound healing. Prog. Retin. Eye Res. 49, 17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM, 2009. Corneal collagen-its role in maintaining corneal shape and transparency. Biophys. Rev. 1, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tovey JC, Gupta R, Sharma A, Tandon A, 2011a. Decorin biology, expression, function and therapy in the cornea. Curr. Mol. Med. 11, 110–128. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Tandon A, Sharma A, Cowden JW, Tovey JC, 2011b. Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Invest. Ophthalmol. Vis. Sci. 52, 4833–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tovey JCK, Sharma A, Schultz GS, Cowden JW, Tandon A, 2011c. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PLoS One 6, e26432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Sotozono C, Kinoshita S, 2001. The epidermal growth factor receptor (EGFR): role in corneal wound healing and homeostasis. Exp. Eye Res. 72, 511–517. [DOI] [PubMed] [Google Scholar]
- Nash MA, Deavers MT, Freedman RS, 2002. The expression of decorin in human ovarian tumors. Clin. Canc. Res. 6, 1754–1760. [PubMed] [Google Scholar]
- Neill T, Schaefer L, Iozzo RV, 2012. Decorin: a guardian from the matrix. Am. J. Pathol. 181, 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Nozu K, Kishioka Y, Wakamatsu J, Hattori A, 2008. Decorin expression in quiescent myogenic cells. Biochem. Biophys. Res. Commun. 370, 383–387. [DOI] [PubMed] [Google Scholar]
- Novak U, Walker F, Kaye A, 2001. Expression of EGFR-family proteins in the brain:role in development, health and disease. J. Clin. Neurosci. 8, 106–111. [DOI] [PubMed] [Google Scholar]
- Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A, 2001. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr. Relat. Canc. 8, 11–31. [DOI] [PubMed] [Google Scholar]
- Reed CC, Gauldie J, Iozzo RV, 2002. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene 21, 3688–3695. [DOI] [PubMed] [Google Scholar]
- Santra M, Reed CC, Iozzo RV, 2002. Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope. J. Biol. Chem. 38, 35671–35681. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Tredup C, Gubbiotti MA, Iozzo RV, 2017. Proteoglycan neofunctions :regulation of inflammation and autophagy in cancer biology. FEBS J. 284, 10–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MH, Furnari FB, Cavenee WK, Bögler O, 2003. Epidermal growth factor receptor signaling intensity determines intracellular protein interactions, ubiquitination, and internalization. Proc. Natl. Acad. Sci. U. S. A 100, 6505–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Mehan MM, Sinha S, Cowden JW, Mohan RR, 2009. Trichostatin a inhibits corneal haze in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 50, 2695–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S, 2005. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. U. S. A 102, 2760–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, Goh LK, 2008. Endocytosis and intracellular trafficking of ErbBs. Exp. Cell Res. 314, 3093–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon A, Tovey JC, Sharma A, Gupta R, Mohan RR, 2010. Aug. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr. Mol. Med. 10 (6), 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas A, Futter CE, Eden ER, 2014. EGF receptor trafficking: consequences for-signaling and cancer. Trends Cell Biol. 24, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voldborg BR, Damstrup L, Spang-Thomsen M, Poulsen HS, 1997. Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann. Oncol. 8, 1197–1206. [DOI] [PubMed] [Google Scholar]
- West-Mays JA, Dwivedi DJ, 2006. The keratocyte: corneal stromal cell with variable repair phenotypes. Int. Biochem. Cell. Biol. 38, 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Mohan RR, Ambrósio R Jr., Hong J, Lee J, 2001. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin. Eye Res. 20, 625–637. [DOI] [PubMed] [Google Scholar]
- Xu Y, Xia Q, Rao Q, Shi S, Shi Q, Ma H, Lu Z, Chen H, Zhou X, 2016. DCN deficiency promotes renal cell carcinoma growth and metastasis through downregulation of P21and E-cadherin. Tumour Biol. 37, 5171–5183. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, Ruoslahti E, 1990. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 346, 281–284. [DOI] [PubMed] [Google Scholar]
- Yarden Y, 2001. The EGFR family and its ligands in human cancer. Signaling mechanisms and therapeutic opportunities. Eur. J. Cancer 37 (Suppl. 4), S3–S8. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Goldoni S, Bix G, Owens RT, McQuillan DJ, Reed CC, Iozzo RV, 2005. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J. Biol. Chem. 280, 32468–32479. [DOI] [PubMed] [Google Scholar]


