Abstract
Injury to the facial nerve can occur after different etiologies and range from simple transection of the branches to varying degrees of segmental loss. Management depends on the extent of injury and options include primary repair for simple transections and using autografts, allografts, or conduits for larger gaps. Tissue engineering plays an important role to create artificial materials that are able to mimic the nerve itself without extra morbidity in the patients. The use of neurotrophic factors or stem cells inside the conduits or around the repair site is being increasingly studied to enhance neural recovery to a greater extent. Preclinical studies remain the hallmark for development of these novel approaches and translation into clinical practice. This review will focus on preclinical models of repair after facial nerve injury to help researchers establish an appropriate model to quantify recovery and analyze functional outcomes. Different bioengineered materials, including conduits and nerve grafts, will be discussed based on the experimental animals that were used and the defects introduced. Future directions to extend the applications of processed nerve allografts, bioengineered conduits, and cues inside the conduits to induce neural recovery after facial nerve injury will be highlighted.
Impact statement
Recovery after facial nerve injury is a complex process, which involves different management options such as primary repair or the use of nerve grafts or conduits. Various tissue-engineered approaches are increasingly studied on preclinical models with limited, but promising, translation to the clinical setting. Herein, preclinical models focusing on different recovery methods after facial nerve injury are comprehensively reviewed based on the experimental animals used. The review provides key insights into current developments and future directions on this highly relevant topic to help researchers further expand the field of tissue engineering and facial nerve recovery.
Keywords: facial nerve repair, preclinical models, allografts, conduits, stem cells, growth factors
Introduction
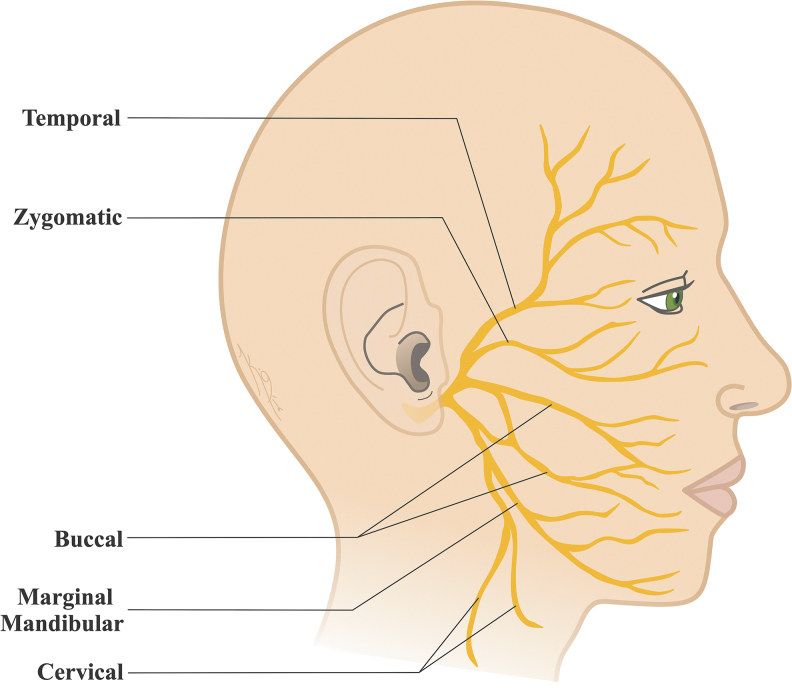
Facial nerve (the seventh cranial nerve VII; CN VII) is associated with motor, sensory, and parasympathetic functions. After exiting the brain stem, the nerve passes through the internal auditory meatus to enter the temporal bone. The nerve gives greater petrosal (parasympathetic), stapedius (branchial motor), and chorda tympani (parasympathetic and taste) branches in the intratemporal portion and exits the temporal bone through the stylomastoid foramen. The nerve branches into the posterior auricular, posterior belly of digastric, and stylohyoid branches from the trunk before entering the parotid gland. The nerve proceeds to form five main motor branches as temporal, zygomatic, buccal, marginal mandibular, and cervical (Fig. 1).1,2 These branches are responsible from the innervation of numerous muscles that work together for the complex functions of facial expressions.
FIG. 1.
Anatomy of the human facial nerve and the five main branches as temporal, zygomatic, buccal, marginal mandibular, and cervical.
Etiologies
Injury to the facial nerve and the resulting facial nerve palsy lead to devastating functional, psychological, and cosmetic challenges.3,4 There are various etiologies of facial nerve palsy, which can be categorized as idiopathic facial paralysis (Bell's palsy), infectious, neoplastic, developmental, metabolic, toxic, traumatic, and iatrogenic.2,5,6 Iatrogenic facial nerve injury occurs most commonly during temporomandibular joint replacement, mastoidectomy, parotidectomy, and rarely during several cosmetic procedures, including face lift.7–9 Iatrogenic injuries range from simple transection of the main trunk or peripheral branches to varying degrees of segmental loss.10
Management options
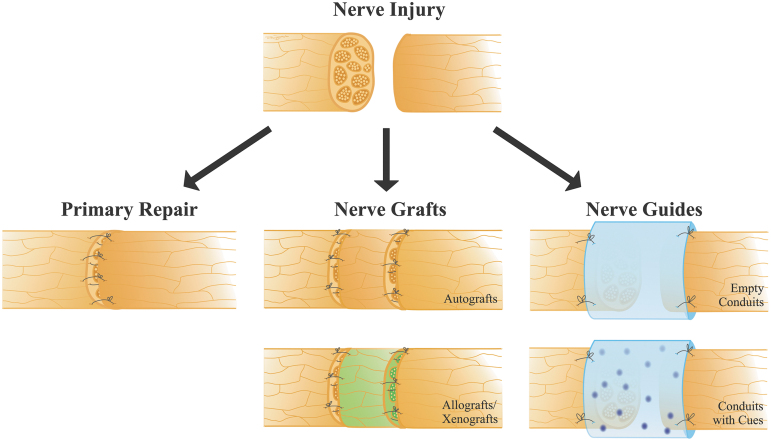
Considering the anatomical and etiological differences, management of the facial nerve injury can be more complex than other peripheral nerves.11,12 The decision for surgical intervention is usually reserved for patients with an anatomical disruption of the nerve, or who are unlikely to make a satisfactory recovery following medical treatment, or chronic complete paralysis.1,2,6,9,10,13 Tension-free primary repair of the divided nerve yields the best results, when possible (Fig. 2). If primary repair is not possible, autografts remain the standard of care for facial nerve defects with gaps. When the proximal and distal ends of the facial nerve are available, graft interposition, commonly using the sensory nerves as donor, such as great auricular or sural nerve, can be performed.14–16 When the proximal nerve end is absent, options for dynamic procedures become much more challenging with poorer outcomes. Reconstruction might require the use of alternative muscles for reanimation or nerve transfers, including hypoglossal, accessory, and masseteric nerves, as well as cross-face nerve grafts.17–23 In cross-face nerve grafting, a facial nerve branch from the healthy side of the face is used to reanimate the paralyzed side of the face by connecting the two with a nerve graft, such as autologous sural nerve. Cross-face nerve grafting using an autologous sural nerve is commonly performed in combination with hypoglossal or masseteric nerve transfer.24–27 However, these procedures result with donor site complications such as scarring and loss of sensation.
FIG. 2.
Different facial nerve repair methods, including primary repair, and the use of nerve grafts or conduits.
Bioengineering approaches to facial nerve repair
Bioengineering plays an important role to create artificial materials that are able to mimic the nerve itself without the need for a donor nerve.28,29 With the concordance to the key design parameters of a useful nerve guide or conduit,30 there are various applications of biomaterials and decellularized allografts to find the best option to enhance recovery compared to the gold standard autografts.31–33 These biomaterials range from simple silicone conduits to more complex and novel technologies, including nanocomposite-coated silk-based34 conduits. In addition, various cues, such as neurotrophic factors,35 stem cells,36 or adipose cells,37 inside the conduits to induce neural recovery are being increasingly studied in preclinical models. While there are limited clinical studies examining facial nerve repair using nerve conduits, early results are promising. For example, a poly(glycolic acid) (PGA) tube was used to repair posttraumatic lesions of the facial nerve in seven patients with gap sizes ranging between 1 and 3 cm.38 Compared to the contralateral side, muscle recovery was >60% in one patient, up to 60% in four patients, and at 30% in two patients. Two additional case reports described PGA/collagen conduits as a promising alternative treatment for facial nerve recovery.39,40 In two patients with a nerve gap of 16 and 20 mm, frontal muscle movement showed recovery at 5 months following repair.39
Allografts, from a different organism within the same species, and xenografts, from a different species, can also be considered options. Processed nerve allografts are another promising alternative treatment for facial nerve repair.41–43 Although their use is more common and established in peripheral nerve repairs, studies are limited on the facial nerve.44 Safa et al. reported two cases of improved meaningful sensory recovery and four cases of improved meaningful motor function using a processed nerve allograft in the head and neck region.42 While no comparison is currently available due to the limited sample size in this region, the initial results are promising. Allografts are also more commonly used in the repair of the trigeminal nerve (e.g., inferior alveolar nerve) to achieve sensory recovery.45–49
This review focuses on preclinical models that study facial nerve repair with bioengineered materials, including conduits and nerve grafts (allograft, xenograft, and autograft).
Overview of Outcome Measurements in Preclinical Models
Preclinical studies remain as the hallmark for the development of novel bioengineering approaches and translation of these developments into the clinical practice. The majority of the studies use rodent models while studying the facial nerve recovery. Table 1 summarizes outcome measures used in these studies. The main focus of this section is the functional outcome measures. Establishing an appropriate model with accurate outcome measures for the aims that are being studied is crucial to quantify recovery of the facial nerve and analyze functional outcomes. Movements of the facial muscles (whisking, eye closure, and ear movements) that are innervated by their corresponding nerve branches are commonly used as functional measures50 and described in detail in the following section. Histological analysis, including nerve sections, retrograde tracing of neuron bodies, and motor endplate reinnervation measures are also briefly described.
Table 1.
Overview of Different Outcome Measures in Rat Studies
| Outcome measures | References | |
|---|---|---|
| Functional | Scoring systems | 62,63,66,67,70–72,78,81,87,106,117 |
| Whisker movements | 58,61,65,83,85,88,90,97,105,110,115,157–159 | |
| Electrophysiological | 58,61,63,66,71–75,77,78,89,90,95–98,103,104,109,110,157–160 | |
| Histological | Toluidine blue staining | 61,65,66,74,76–79,86,87,89,91,95,96,98,103,106,108–110,115,116,158,159 |
| Transmission electron microscopy | 61,62,73–75,77,78,86,87,89,91,96,98,103,104,109,116,157 | |
| Scanning electron microscopy | 75,115 | |
| Immunohistochemical | S-100 antibody stain | 75,76,78,87,89,103,111,158 |
| Neurofilament antibody stain | 58,72,75,78,111 | |
| Tubulin antibody stain | 81,117,158 | |
| Other antibody stains (i.e., Tuj-1, GAFP, NGF, acetylcholine transporter, Synaptophysin, VIP) | 58,66,76,87,89,91,104 | |
| Retrograde neuronal tracing | Fluorescence labeling | 61,65,71,73,74,76,79,81,88,96,98,108,110,115–117 |
| Horseradish peroxidase labeling | 104 | |
Whisking
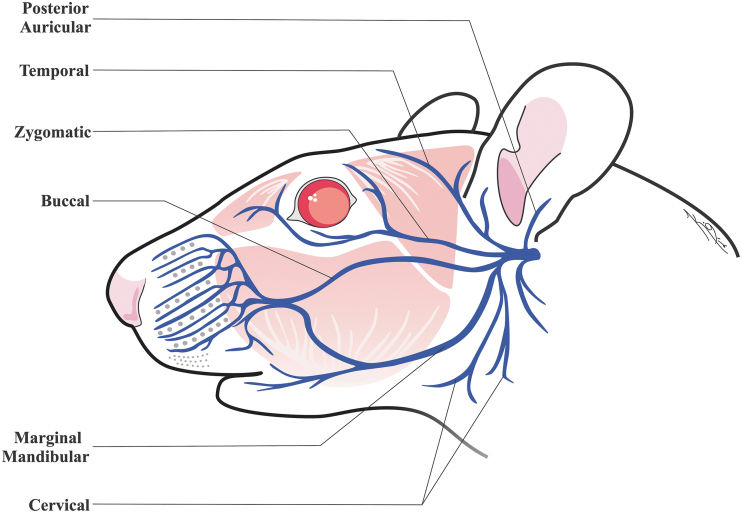
Due to similar facial nerve innervation between humans, rodents, and rabbits, tracking the whisking movements provides a clinically relevant model regarding the degree of compromise of the facial nerve, following an intervention.51–53 The motor activity of protraction and retraction movements of the vibrissae in rodents is controlled by the pilo-erector muscles and innervated mainly by the buccal branch of the facial nerve (Fig. 3).54–56 Whisker movement, symmetry, amplitude, frequency, and other whisking behaviors are indicators of facial nerve functional recovery through innervation of superficial muscles controlling whisking.57 However, the overlap of different efferent motor and autonomic supply to the whiskers should also be considered, while assessing facial nerve regeneration.58–60 In addition, transection and ligation of the ends of the marginal mandibular branch is also used in models focusing on whisking to prevent dual innervation of the buccal and mandibular branches on the pilo-erector muscles.61–63
FIG. 3.
Anatomy of the rat facial nerve.
Whisking measurements in the studies are also used to monitor the progression of recovery over specified time scales. Although the time for the measurement of the functional outcome changes depending on the design, an ideal time to evaluate facial nerve recovery by whisking in rats was found to be within the 4 months after the injury.64 Current whisking protocols range from gross observation, to surgical implantation of restrictors coupled with laser micrometer setups. One method to objectively analyze whisking movement was first described by Heaton et al., for simultaneously monitoring bilateral eyelid and whisker movements in a rat model, where rats were secured in a full body restraint device and surgically fitted with titanium head implants to provide rigid head fixation points.57 However, using surgical implants for rat head fixation raises the possibility for infection, and may affect normal whisking behavior due to the rigid restraining practice. Therefore, to avoid this, other authors have developed parameters to monitor rat health and behavior before continuing with experimentation. Chen et al. allowed 2 weeks for recovery following implantation of the head fixation device, and gradually introduced rats to the restraining apparatus to reduce its impact on the normal whisking behavior of the rats.65 Abbas et al. developed a custom-made restraining device that only secured the body and left the head free to move around.66 To ensure quality and consistency of filming, despite the free range of motion in the rat heads, rats were recorded for a longer duration and the most optimal portions of the recording were chosen for the analysis.
Scoring systems and eye closure
Scoring systems in facial nerve studies often measure similar parameters to those in whisking studies, such as facial symmetry, whisking movement, and whisker symmetry, and create a numeric scale for researchers to score these behaviors.67 In addition, scoring systems normally do not employ a restraining apparatus and instead allow animals to roam free in a cage or transparent box, while being filmed or observed.
Eye closure, which is controlled by the orbicularis oculi muscle and mainly innervated by the zygomatic branch of the facial nerve in rodents, is commonly used together with whisking in qualitative scoring systems.50,57,68,69 Monitoring eye and whisker movement simultaneously results in a more comprehensive outcome measure as the facial nerve innervates a wide range of facial muscles in rodents and other animal models.51 Yasui et al. measured recovery by using a scoring system in a rat model with symmetry of the eyes at rest, eye closure capability, symmetry of the vibrissae at rest, and motion of the vibrissae compared to the untreated side.70 Li et al. utilized gross observation and a five-point scoring system in a rat model involving eye closure, blinking reflex, and whisking.71
Electrophysiology
Electrophysiological measurements are widely utilized to assess facial nerve function in preclinical models to provide insight into the regenerative progress of the impacted nerve. This can be considered a distinct outcome measure compared to other functional measures. One of the major limitations is that it does not address changes that might have occurred with muscle physiology and function due to the loss of innervation during the regenerative period. Therefore, many groups have used a combination of electrophysiological and whisking/scoring systems.66,70,72
Electrophysiology is ideal for identifying specific nerve defects when the impacted nerve is not necessarily known. As the facial nerve has a wide variety of innervated muscles, gross observations of facial dissymmetry and function may not be able to identify the specific branch of the impacted nerve.51–53 Electrical function of specific branches of the facial nerve can be determined using electrophysiology and measuring muscle action potentials.73 Another practical use is the ability to compare latency, amplitude, and duration of compound muscle action potentials between different methods of facial nerve regeneration procedures.74,75
Histological analysis
The majority of the studies use regenerating axon count, myelinated axon count, fiber diameter, axon diameter, myelin thickness, or their variations, to quantify the regenerative capacity of the experimental groups and analyze histological outcomes. A commonly used method is osmium tetroxide-toluidine blue staining, where slides can be viewed both under light microscopy or transmission electron microscopy.76–78 In addition, immunohistochemical staining is used mainly with antibodies against S-100 for Schwann cells, neurofilaments for axon cytoplasm or tubulin.
Retrograde labeling of motor neurons is another method performed to understand the degree of axonal branching both qualitatively and quantitively.50 This allows visualization of target muscle and estimation of motor end-plate reinnervation. Fluorescence staining methods are performed by injecting tracers such as Fluoro-Gold,76,79,80 1,12-dioctadecyl-3,3,3′,3′-tetramethyl indocarbocyanine perchlorate (DiI),77 Fast Blue,81 cholera toxin subunit B conjugate,73 or triple staining82 into the whisker muscles, a few days before the sacrifice. After allowing the agents to travel in a retrograde manner, the labeled motor neurons in the facial nerve nucleus in brainstem sections can be counted under fluorescent microscope. In addition, macroscopic in vivo imaging in Thy1-GFP rats expressing green fluorescent protein in their neural structures can be performed to trace the distal end of the regeneration.79,83
Rat Models of Facial Nerve Defects
Majority of preclinical studies use rat models to study facial nerve repair. Three primary types of nerve injury models are used as nerve crush, transection, and nerve gap.84,85 Facial nerve defect/gap sizes vary between the models, but generally remain <10 mm gap for conduit studies. The vast majority of conduit studies are in the range of 5–8 mm gaps with several nerve transection-only studies, and there are limited applications in larger gaps. Graft studies (allografts and autografts), on the other hand, use models with gaps larger than 10 mm, such as cross-face neurotization.
Permanent conduits
Several studies have used silicone tubes as a vehicle to locally apply and entrap different factors that stimulate nerve regeneration (Table 2). For example, Matsumine et al. delivered fibroblast growth factor (FGF-2) into a 7 mm defect in the facial nerve; the delivery system included FGF-2 embedded in microspheres inside a silicone conduit.86 The FGF-loaded microsphere group facilitated recovery with increased nerve regeneration. Therapeutic potential of adipose stem cells was identified in a model by embedding the cells in a collagen gel that was transplanted into the 7 mm gap in the rat facial nerve, inside a silicone tube.87 Mature adipocyte-derived pluripotent cells demonstrated potential in promotion of maturation of the regenerated nerve in a similar rat model.77 Stromal vascular fraction, a source of adipose stem cells, was infused into silicone tubes and promoted nerve regeneration with better histological and functional outcomes compared to conduit alone.62 Dental pulp cells, as a derivative of neural crest and a source of Schwann and neural progenitor cells, were used to accelerate rat facial nerve recovery, and demonstrated positive effects both histologically76 and functionally63 with silicone conduits in a 7 mm gap. Finally, olfactory ensheathing cells (OEC), as a source of glial cells and trophic factors, were used inside silicone tubes to stimulate axonal growth and sprouting in rat facial nerve injury.88
Table 2.
Overview of Preclinical Models Using Conduits
| References | Model | Gap length (mm) | Branch | Conduit type | Cues being studied inside the conduit |
|---|---|---|---|---|---|
| 159 | Rat | 6 | Buccal branch | NA | PLOD1 depleted adipose-derived stem cells |
| 61 | Rat | 7 | Buccal branch | PGA/collagen | Dedifferentiated fat cells |
| 98 | Rat | Crush injury | Main trunk | PGA/collagen | Interpositional-jump graft |
| 71 | Rat | 5 | Main trunk | PLA/chitosan | Olfactory ensheathing cells |
| 96 | Rat | 7 | Buccal branch | PGA/collagen | Adipose-derived stem cells or stromal vascular fraction |
| 75 | Rat | 7.5 | Buccal branch | Peptide amphiphile nanofiber/collagen | NA |
| 157 | Rat | 8 | Buccal branch | Collagen | Heparin, basic fibroblast growth factor, neural stem/progenitor cells |
| 97 | Rat | 2 | Main trunk | PGA | Neural stem cells originating from the human olfactory mucosa |
| 78 | Rat | 4 | Main trunk | PCL/collagen | Human umbilical cord serum |
| 62 | Rat | 7 | Buccal branch | Silicone | Syngeneic uncultured-stromal-vascular-fraction |
| 74 | Rat | Crush injury | Main trunk | Silicone/collagen | Interpositional-jump graft |
| 77 | Rat | 7 | Buccal branch | Silicone/collagen | Dedifferentiated fat cells |
| 63 | Rat | 7 | Buccal branch | Silicone/collagen | Collagen gel embedded with dental pulp cells |
| 86 | Rat | 7 | Buccal branch | Silicone | Acidic gelatin hydrogel microspheres with basic fibroblast growth factor |
| 87 | Rat | 7 | Buccal branch | Silicone/collagen | Undifferentiated adipose-derived stem cells, differentiated adipose stem cells, Schwann cells |
| 95 | Rat | 5 | Mandibular branch | PGA | Bone marrow stroma mesenchymal stem cell-derived Schwann-like cells |
| 89 | Rat | 7 | Buccal branch | PLA | Type 1 collagen solution |
| 85 | Rat | 2 | Main trunk | Silicone | NA |
| 90 | Rat | 5 | Buccal branch | PLGA | Schwann cells transfected with GDNF |
| 88 | Rat | 5 | Main trunk | Silicone | Olfactory ensheathing cells |
| 76 | Rat | 7 | Buccal branch | Silicone | Collagen gel embedded with dental pulp cells |
| 91 | Rat | 7 | Buccal branch | PLGA | Collagen gel embedded with dental pulp cells |
| 124 | Rabbit | Crush injury | Buccal branch | Collagen | Collagen-binding domain basic fibroblast growth factor |
| 121 | Rabbit | 10 | Buccal branch | Chitosan | Nerve growth factor microspheres |
| 120 | Rabbit | 10 | Buccal branch | Chitosan/collagen | Nerve growth factor, neural stem cells |
| 123 | Rabbit | 6 | Buccal branch | PGA | FK506 dissolved in olive oil |
| 122 | Rabbit | 10 | Buccal branch | Polytetrafluoroethylene/collagen | NA |
| 119 | Rabbit | 8 | Buccal branch | Silicone | Nerve growth factor containing solution |
| 118 | Rabbit | 8 | Buccal branch | Silicone | Nerve growth factor containing solution |
| 142 | Feline | 0 | Main trunk | Collagen | Sterile normal saline |
| 141 | Feline | 5 | Dorsal ramus | Collagen | None |
| 149 | Minipig | 35 | Buccal branch | Collagen/nano-sized b-tricalcium phosphate | Nerve growth factor |
| 148 | Minipig | 35 | Buccal branch | Collagen | Linear ordered collagen scaffold combined with recombinant proteins |
| 147 | Minipig | 10 | Buccal branch | Collagen | Collagen-binding ciliary neurotrophic factor absorbed linear ordered collagen fibers |
GDNF, glial cell line-derived neurotrophic factor; NA, not available; PGA, poly(glycolic acid); PLA, poly(lactic acid); PCL, poly(caprolactone); PLGA, poly(dl-lactide-co-glycolide).
Biodegradable conduits
Biodegradable nerve conduits include type I collagen, PGA, poly(dl-lactide-co-caprolactone), poly(dl-lactide-co-glycolide) (PLGA), poly(lactic acid) (PLA), poly(caprolactone) (PCL), and nonresorbable poly(vinyl alcohol). PLA conduit demonstrated enhanced nerve regeneration than silicone tubes, and comparable results with the gold standard of autologous nerve grafting in a 7 mm gap model.89 PLA/chitosan conduit was also used in combination with OEC to repair a 5 mm defect in the facial nerve, and olfactory mucosal cells were found to be more effective compared with the olfactory bulb.71 PLGA nerve conduit was used in a 5 mm facial nerve defect model, which contained Schwann cells transfected with glial cell line-derived neurotrophic factor (GDNF).90 The GDNF-treated group performed better than nontransfected cells and primary anastomosis. PLGA nerve conduit was used in another study of 7 mm nerve defect in the buccal branch of the facial nerve in combination with dental pulp cells and promoted nerve regeneration.91 PCL/collagen conduit was used in a 4 mm gap model, combined with human umbilical cord serum, and yielded more favorable results compared with the autograft.78
PGA is among the first clinically available and most successful material implanted in humans for peripheral nerves.29,92–94 PGA has been widely studied in rat facial nerve models.28 For example, Costa et al. demonstrated that PGA tubes filled with bone marrow-derived Schwann-like cells were associated with superior nerve regeneration in a 5 mm gap.95 Effects of both adipose stem cells and stromal vascular fraction have also been demonstrated in nerve regeneration with PGA tubes in a 7 mm gap.96 Fujimaki et al. identified that PGA tubes filled with cells cultured from mature adipocytes enhanced regeneration and improved physiological function in a 7 mm gap.61 Human olfactory stem cells were used inside PGA tubes for a 2 mm gap with accelerated nerve regeneration.97 Also, collagen-coated PGA conduit was found to be more effective compared to nerve autograft in hypoglossal-facial nerve connections in an incomplete rat facial nerve paralysis model.98
Three-dimensional printed constructs
A novel approach in tissue engineering and regenerative medicine is three-dimensional (3D) bioprinting of nerve guidance constructs.99 This allows customization of the shape or material of the nerve guides or scaffolds to be used to repair complex defects with bifurcating motor or sensory branches.100 Various cues, including growth factors or stem cells, can be combined with this process to improve physical and chemical properties of the constructs and enhance the customized neuroregenerative functionality.101 In addition, scaffold-free constructs can also be created solely based on stem cell components. For example, Zhang et al. used a scaffold-free bioprinting methodology to create constructs from gingiva-derived mesenchymal stem cell spheroids and transplanted to a 5 mm defect in the buccal branch of the rat facial nerve.102 The functional recovery was better than the simple silicone tubes, yet not as great as the autografts. However, the histological organization of the nerve fascicles and the target muscle recovery achieved through electrophysiology was similar to the autografts, highlighting the promising future applications to promote nerve regeneration.
Nerve grafts
Autografts and allografts have a broad range of applications in both preclinical and clinical studies (Table 3). Sources of grafts include peroneal, median, and sciatic nerves. In a 10 mm gap model of the buccal branch of the facial nerve, three graft types (autogenic peroneal nerve graft, acellular facial nerve graft from another rat, and acellular facial nerve graft from a rabbit) were compared.103 Autografts demonstrated the strongest regenerative capacity, whereas allografts and xenografts had similar effects. In another study, acellularized xenografts from rabbits were used to repair a 6 mm gap in the facial nerve of rats and yielded comparable regenerative capacity with autografts.104
Table 3.
Overview of Preclinical Models Using Grafts
| References | Model | Gap length (mm) | Branch | Graft type | Graft details | Additional treatments | Outcomes |
|---|---|---|---|---|---|---|---|
| 72 | Rat | 5 | Main trunk | Autograft | Motor branch to the quadriceps muscle, sensory femoral cutaneous branch | NA | Similar results between sensory and muscle autografts |
| 65 | Rat | Transection | Main trunk | Venous autograft | Retromandibular vein | NA | Autologous venous ensheathment did not improve recovery |
| 66 | Rat | Cross-face nerve grafting | Main trunk and buccal branch | Autograft | Sciatic nerve | Adipose-derived stem cells | Adipose-derived stem cells enhanced recovery |
| 103 | Rat | 10 | Buccal branch | Allograft, xenograft, autograft | Acellularized facial nerve, acellularized facial nerve from rabbit, peroneal nerve | NA | Autografts performed better than acellular nerve grafts, allografts and xenografts had similar effects |
| 79 | Rat | Cross-face nerve grafting | Marginal mandibular and buccal branches | Autograft | Peroneal nerve | End-to-side coaptation of sensory occipital nerves to the graft | Sensory pathway protection improved recovery |
| 104 | Rat | 6 | Buccal branch | Xenograft, autograft | Acellularized facial nerve from rabbit, facial nerve | NA | Similar results between xenografts and autografts |
| 109 | Rat | 7 | Buccal branch | Autograft | Vascularized and nonvascularized median nerve | NA | Vascularized median nerve graft resulted in improved recovery |
| 105 | Rat | 20 | Marginal mandibular and buccal branches | Autograft | Reversed facial nerve | NA | Recovery after cable grafting is slower, but eventually similar compared to primary neurorrhaphy |
| 108 | Rat | >10 | All major branches | Autograft | Contralateral facial nerve buccal branch | Hypoglossal nerve supercharging | Multiple branches were recovered, axonal supercharge from the hypoglossal nerve improved recovery |
| 160 | Rat | Transection | Not specified | Amniotic membrane | From a donor bank | NA | Amniotic membrane covering improved recovery |
| 81 | Rat | >10 | Marginal mandibular and cervical branches | Arterial allograft | Abdominal aorta and its bifurcation | NA | Y-tube reconstruction decreased axonal branching, but did not improve recovery |
| 116 | Rat | 8 | Buccal branch | Arterial allograft | Acellularized abdominal aorta | Autologous transdifferentiated adipose-derived stem cells | The use of adipose-derived stem cells improved recovery, but was inferior to the autografts. |
| 117 | Rat | >10 | Marginal mandibular branch | Arterial allograft | Abdominal aorta and its bifurcation | NA | Y-tube reconstruction decreased axonal branching, but did not improve recovery |
| 110 | Rat | Cross-face nerve grafting | Bilateral all major branches and main trunk | Allograft | Sciatic and ulnar nerve | NA | End-to-side nerve grafting improved recovery |
| 130 | Rabbit | 20 | Buccal branch | Autograft | Vascularized and nonvascularized central auricular nerve | NA | Vascularized auricular nerve graft resulted in improved recovery |
| 129 | Rabbit | >10 | Buccal branch | Autograft | Predegenerated and normal great auricular nerve | NA | Predegenerated grafts resulted in improved recovery |
| 132 | Rabbit | 10 | Buccal branch | Venous autograft | Ipsilateral facial vein | Transdifferentiated Schwann-like MSC and bone marrow MSC | Transdifferentiated Schwann-like MSC improved recovery |
| 125 | Rabbit | 20 | All major branches and main trunk | Allograft, autograft | Acellularized facial nerve, facial nerve, acellularized peroneal nerve, peroneal nerve | NA | Both facial nerve autografts and allografts performed similar, and better than peroneal nerve grafts |
| 131 | Rabbit | 10 | Buccal branch | Venous autograft | Standard and inside-out ipsilateral facial vein | NA | Inside-out vein graft accelerated recovery |
| 53 | Rabbit | 10 | Intratemporal segment | Autograft | Vascularized and non-vascularized median nerve | NA | Vascularized median nerve graft resulted in improved recovery in a bony recipient bed |
| 153 | Monkey | 30–40 | Middle zygomatic branch | Autograft, allograft | Superficial fibular nerve | Systemic FK506 | Autografts performed better in terms of neuron number and electrophysiological recording, but both allografts and autografts achieved similar functional results |
| 146 | Canine | 20 | Palpebral branch | Autograft | Superficial peroneal nerve | NA | End-to-side coaptation accelerated recovery |
| 145 | Canine | Cross-face nerve grafting | Multiple branches | Autograft | Sural nerve | Pulse generator to continually stimulate the muscle | Improved recovery in stimulated muscles |
MSC, mesenchymal stem cell; NA, not available.
Many of the previously mentioned rat studies used either reverse-polarity autografts or a donor nerve from a distant site as their control groups to compare with conduits. For example, Hohman et al. created a 20 mm gap to compare reverse-polarity autografting with transection and primary repair of the facial nerve.105 Although initially slower, recovery after autografting was similar to the primary repair. In terms of the type of the autograft as sensory or motor branch, histological and physiological outcomes were similar in a 5 mm gap model in the main trunk of the facial nerve.72
Different surgical methods have been tested with autografts.106 End-to-side loop grafting is a technique used to reconstruct multiple branches by allowing the distal stump of the facial nerve to be connected to the single transplanted nerve.107 Matsumine et al. incorporated the concept of double innervation into the end-to-side loop grafting by using a contralateral buccal branch of the facial nerve.108 Li et al. used a predegenerated peroneal nerve graft to connect ipsilateral hypoglossal or accessory nerves with the facial nerve.73 Matsumine et al. showed that vascularized median nerve grafts, where the median nerve was transferred to the defect site with the median artery and vein, in a 7 mm gap model yielded better histological and functional nerve regeneration when compared with nonvascularized graft.109 Adipose-derived stem cells were tested in another study by injecting into the local environment of the cross-face nerve graft and resulted in enhanced recovery.66
End-to-side cross-face nerve grafting has been applied in a 15 mm gap model with improved functional recovery.110 In another study, Placheta et al. used peroneal nerve as a cross-face nerve graft enhanced with end-to-side coaptation of sensory occipital nerves.79 This resulted in improved regeneration and functional outcomes due to the protective effects on chronic denervation. These models are infrequent in rats as they study repairs in longer gaps compared with models that study conduits.
Blood vessels
Autogenous vein and artery grafts are used to provide an enhanced environment for the axonal regeneration, while acting as sturdy barriers against scar ingrowth.111–114 However, in a model of transection of the facial nerve trunk, autologous venous ensheathment failed to improve the quality of regenerated axons in terms of histology and function.65 In another study, decellularized allogenic artery graft was used in a 6 mm gap as a conduit filled with adipose stem cells, where addition of adipose stem cells facilitated the recovery, however, to an extent lower than nerve autografts.115,116 The abdominal aorta and its bifurcations were used as a Y-shaped conduit in a large facial nerve defect, where although the Y-tube improved axonal pathfinding, the functional outcome did not improve.81,117
Rabbit Models of Facial Nerve Defects
Rabbit models are commonly used for larger defects (>10 mm), given that the larger scale of the animal and ample amount of facial nerve length compared with rodents. However, unlike rat models, there are limited numbers of rabbit models of facial nerve repair. The following sections describe some of these studies.
Permanent and biodegradable conduits
Several studies have examined silicone tubes in rabbit facial nerve repair. For example, in an 8 mm nerve gap, silicone tubes were used as conduits that contain nerve growth factor (NGF) solutions.118,119 Conduits with growth factor were associated with fewer collateral nerve sprouts, and the functional outcome was comparable with autologous nerve grafts. Guo et al. combined neural stem cells and NGF in a chitosan conduit and protein sponge to recover a 10 mm defect in the facial nerve, where neural stem cells yield better recovery.120 Liu et al. used NGF releasing microspheres for the local administration in a chitosan conduit.121 Sustained release of NGF from the microspheres significantly improved the outcomes when compared with the injection of NGF into the conduits.
Expanded polytetrafluoroethylene and collagen tubes were used in a 10 mm gap model with similar outcomes with the autologous nerve grafts in the long term.122 Diaz et al. applied FK506 locally at the time of repair within a PGA tubing into a 6 mm defect, where superior results in nerve regeneration were achieved versus interposition autografts.123 In another study, collagen nerve conduits with recombinant proteins were used in a cut or crush model with promising outcomes.124
Nerve grafts
Nerve grafts have various uses in models with longer gaps and different surgical techniques. In a whole facial nerve defect model of 20 mm gap, Hu et al. identified that whole facial nerve allograft was efficient in recovery when the defect was repaired with acellular facial nerve allografts, autologous facial nerve grafts, acellular peroneal nerve allograft, or autologous peroneal nerve grafts.125 Although the difference between acellular and autologous grafts was minimal, facial nerve grafts performed better than peroneal nerve grafts. Another study compared vascularized versus nonvascularized median nerve grafts in a 10 mm nerve gap in the intratemporal part of the facial nerve.126 Vascularized grafts were associated with superior outcomes in a bony recipient bed. Neural cable graft, where the resected segment of 10 mm was rotated 180° (i.e., reverse-polarity autograft), was used with the prefabrication process of antecedent crush injury before grafting.127 Antecedent injury was associated with enhanced innervation of the mimetic muscles. In another model, a 5 mm segment was resected and repaired using sural nerve graft by either classical suturing or a tissue adhesive, where the tissue adhesive was found to be ineffective.128
The auricular nerve of the rabbit that courses along the ear serves as a donor site for autograft harvesting. Predegenerated auricular nerve grafts by crush injury were used in a complete buccal branch defect with comparable outcomes.129 Auricular nerve graft was also used as vascularized or nonvascularized nerve grafts in a 20 mm segmental gap model.130 Vascularized nerve graft was associated with greater numbers of regenerated axons and Schwann cells due to rapid reestablishment of blood circulation in the graft.
Blood vessels
There are a handful of studies that describe the use of autogenous vein grafts in rabbit facial nerve repair. For example, Tang et al. compared autologous vein grafts and vein grafts that are turned inside-out in a 10 mm segmental nerve gap.131 Although both vein grafts were beneficial for nerve regeneration, the inside-out vein grafts were associated with accelerated axonal regeneration. Vein grafts were also used as conduits to deliver stem cells in a 10 mm segmental nerve defect.132
Other Animal Models of Facial Nerve Defects
Murine
There are different surgical models to study the facial nerve injury and repair in mice.133,134 Several models were also used to understand the molecular mechanisms of the facial nerve regeneration after injury.135–140
Feline
Cat models have been used to assess novel nerve guides as the representation of facial muscles in the facial nucleus resemble those in humans and to assess eyelid responses. For example, Kitahara et al. demonstrated that collagen nerve guide in a 5 mm gap in the facial nerve was associated with enhanced recovery.141 Dresner et al. also demonstrated that a collagen conduit resulted enhanced regeneration compared to without conduits, after transection of the main trunk of the facial nerve.142 In another study with a 5 mm defect in the cat facial nerve, alginate was used as a promising material for the repair without suturing.143 The adaptability and plasticity of the facial motor system was also assessed in a study with different surgical approaches.144
Canine
In a study examining effects of continuous electrical stimulation of the denervated muscles, a beneficial effect was demonstrated compared with nonstimulated controls in a canine model of complete facial nerve paralysis that was repaired with cross-facial nerve grafts using two donor sural nerves (20–25 cm in length).145 End-to-side coaptation was found to be effective in producing synchronous blink in a canine facial nerve defect model that was reinnervated with the contralateral side by a peroneal nerve graft.146
Minipig
Minipig models of larger gaps were used with collagen nerve conduits to deliver certain cues to the defects. In an 8 mm nerve gap model, collagen-binding ciliary neurotrophic factor-absorbed fibers were found to have promising results.147 Cui et al. also studied a collagen scaffold combined with recombinant proteins in a 35 mm gap model with favorable outcomes.148 In a similar 35 mm gap model, type 1 collagen and nano-sized beta-tricalcium phosphate conduits were examined and combined with NGF.149 Groups with NGF were found to be associated with promoted nerve regeneration when compared to the groups without NGF.
Ovine
Ovine models were identified as readily available and affordable models for training in head and neck surgery, with similarities in skin, subcutaneous, and bony structures seen in human dissections.150 Glasby et al. studied the repair of 5 cm gaps with freeze-thawed, coaxially aligned skeletal muscle autografts, where muscle grafts were found to be favorable when compared with other surgical techniques.151 Another model studied ciliary neurotrophic factor injection to the muscle and found that it was not effective in the repair of buccal branch of the facial nerve.152
Monkey
In a monkey model, 3–4 cm of the middle zygomatic branch of the facial nerve was removed and repaired with autografts and cold preserved nerve allografts temporarily treated with FK506, and the animals received oral FK506.153 Autografts performed better in terms of neuronal counts and electrophysiological recordings, but both allografts and autografts achieved similar functional results.
Conclusions and Future Prospects
Recovery after facial nerve injury is a complex and multifactorial process. Tissue engineering plays an important role in the recovery to achieve the best possible outcome in challenging clinical scenarios without any extra morbidity in the patients. Results of studies using biomaterials for nerve repair compete, or even enhance recovery compared to the gold standard autografts. The use of cues inside the conduits such as neurotrophic factors or stem cells is an ever-expanding area with exceptional promise to achieve enhanced recovery. However, a consensus on the ideal cell source, neurotrophic factor, and biomaterial is yet to be reached.
Current preclinical models on facial nerve repair are well established with the major focus on rodents. The majority of the studies focusing on bioengineered conduits use relatively smaller nerve gaps, whereas larger gaps in larger animal models are mainly the focus of nerve graft studies (such as cross-face nerve grafting). The effects of conduits on the challenging large gaps need to be further explored. While there are currently limited clinical studies examining facial nerve repair using nerve conduits or processed acellular nerve allografts, early results are promising. Combinatory approaches of stem cells and different treatment modalities with the processed nerve allografts are yet to be applied for the facial nerve repair.154 In addition, further research in the field of 3D bioprinting will allow translation of an individually tailored approach for complex facial nerve injuries. An ideal recovery method could potentially be of major benefit in a wide range of areas concerning with the facial nerve repair, from facial transplantations155,156 to trauma or cancer surgery.
Disclosure Statement
No competing financial interests exist.
Funding information
Research reported in this publication was supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under Award Number U24DE029462 and U24DE026915. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Rosson, G.D., and Redett, R.J.. Facial palsy: anatomy, etiology, grading, and surgical treatment. J Reconstr Microsurg 24, 379, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Gordin, E., Lee, T.S., Ducic, Y., and Arnaoutakis, D.. Facial nerve trauma: evaluation and considerations in management. Craniomaxillofac Trauma Reconstr 8, 1, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bradbury, E.T., Simons, W., and Sanders, R.. Psychological and social factors in reconstructive surgery for hemi-facial palsy. J Plast Reconstr Aesthetic Surg 59, 272, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Coulson, S.E., O'Dwyer, N.J., Adams, R.D., and Croxson, G.R.. Expression of emotion and quality of life after facial nerve paralysis. Otol Neurotol 25, 1014, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Owusu, J.A., Stewart, C.M., and Boahene, K.. Facial nerve paralysis. Med Clin North Am 102, 1135, 2018. [DOI] [PubMed] [Google Scholar]
- 6. Hohman, M.H., and Hadlock, T.A.. Etiology, diagnosis, and management of facial palsy: 2000 patients at a facial nerve center. Laryngoscope 124, E283, 2014. [DOI] [PubMed] [Google Scholar]
- 7. Hohman, M.H., Bhama, P.K., and Hadlock, T.A.. Epidemiology of iatrogenic facial nerve injury: a decade of experience. Laryngoscope 124, 260, 2014. [DOI] [PubMed] [Google Scholar]
- 8. Roostaeian, J., Rohrich, R.J., and Stuzin, J.M.. Anatomical considerations to prevent facial nerve injury. Plast Reconstr Surg 135, 1318, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Condie, D., and Tolkachjov, S.N.. Facial nerve injury and repair. Dermatol Surg 45, 340, 2019. [DOI] [PubMed] [Google Scholar]
- 10. Sahovaler, A., Yeh, D., and Yoo, J.. Primary facial reanimation in head and neck cancer. Oral Oncol 74, 171, 2017. [DOI] [PubMed] [Google Scholar]
- 11. Langhals, N.B., Urbanchek, M.G., Ray, A., and Brenner, M.J.. Update in facial nerve paralysis. Curr Opin Otolaryngol Head Neck Surg 22, 291, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown, S., Isaacson, B., Kutz, W., Barnett, S., and Rozen, S.M.. Facial nerve trauma: clinical evaluation and management strategies. Plast Reconstr Surg 143, 1498, 2019. [DOI] [PubMed] [Google Scholar]
- 13. Razfar, A., Lee, M.K., Massry, G.G., and Azizzadeh, B.. Facial paralysis reconstruction. Otolaryngol Clin North Am 49, 459, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Matejčík, V. Peripheral nerve reconstruction by autograft. Injury 33, 627, 2002. [DOI] [PubMed] [Google Scholar]
- 15. Özmen, Ö.A., Falcioni, M., Lauda, L., and Sanna, M.. Outcomes of facial nerve grafting in 155 cases: predictive value of history and preoperative function. Otol Neurotol 32, 1341, 2011. [DOI] [PubMed] [Google Scholar]
- 16. Hoshal, S.G., Solis, R.N., and Bewley, A.F.. Nerve grafts in head and neck reconstruction. Curr Opin Otolaryngol Head Neck Surg 28, 346, 2020. [DOI] [PubMed] [Google Scholar]
- 17. Volk, G.F., Pantel, M., and Guntinas-Lichius, O.. Modern concepts in facial nerve reconstruction. Head Face Med 6, 1, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jandali, D., and Revenaugh, P.C.. Facial reanimation: an update on nerve transfers in facial paralysis. Curr Opin Otolaryngol Head Neck Surg 27, 231, 2019. [DOI] [PubMed] [Google Scholar]
- 19. Kadakia, S., Helman, S., Saman, M., Cooch, N., and Wood-Smith, D.. Concepts in neural coaptation: using the facial nerve as a paradigm in understanding principles surrounding nerve injury and repair. J Craniofac Surg 26, 1304, 2015. [DOI] [PubMed] [Google Scholar]
- 20. Chuang, D.C.C., Lu, J.C.Y., Chang, T.N.J., and Laurence, V.G.. Comparison of functional results after cross-face nerve graft-, spinal accessory nerve-, and masseter nerve-innervated gracilis for facial paralysis reconstruction: the Chang Gung experience. Ann Plast Surg 81, S21, 2018. [DOI] [PubMed] [Google Scholar]
- 21. Pepper, J.-P. Dual nerve transfer for facial reanimation. JAMA Facial Plast Surg 21, 260, 2019. [DOI] [PubMed] [Google Scholar]
- 22. Hontanilla, B., and Cabello, A.. Spontaneity of smile after facial paralysis rehabilitation when using a non-facial donor nerve. J Craniomaxillofac Surg 44, 1305, 2016. [DOI] [PubMed] [Google Scholar]
- 23. Chen, G., Wang, W., Wang, W., Ding, W., and Yang, X.. Symmetry restoration at rest after masseter-to-facial nerve transfer: is it as efficient as smile reanimation? Plast Reconstr Surg 140, 793, 2017. [DOI] [PubMed] [Google Scholar]
- 24. Terzis, J.K., and Tzafetta, K.. The “babysitter” procedure: minihypoglossal to facial nerve transfer and cross-facial nerve grafting. Plast Reconstr Surg 123, 865, 2009. [DOI] [PubMed] [Google Scholar]
- 25. van Veen, M.M., Dijkstra, P.U., and Werker, P.M.N.. A higher quality of life with cross-face-nerve-grafting as an adjunct to a hypoglossal–facial nerve jump graft in facial palsy treatment. J Plast Reconstr Aesthetic Surg 70, 1666, 2017. [DOI] [PubMed] [Google Scholar]
- 26. Biglioli, F., Colombo, V., Tarabbia, F., et al. Double innervation in free-flap surgery for long-standing facial paralysis. J Plast Reconstr Aesthetic Surg 65, 1343, 2012. [DOI] [PubMed] [Google Scholar]
- 27. Watanabe, Y., Akizuki, T., Ozawa, T., Yoshimura, K., Agawa, K., and Ota, T.. Dual innervation method using one-stage reconstruction with free latissimus dorsi muscle transfer for re-animation of established facial paralysis: simultaneous reinnervation of the ipsilateral masseter motor nerve and the contralateral facial nerve to improve the quality of smile and emotional facial expressions. J Plast Reconstr Aesthetic Surg 62, 1589, 2009. [DOI] [PubMed] [Google Scholar]
- 28. Gaudin, R., Knipfer, C., Henningsen, A., Smeets, R., Heiland, M., and Hadlock, T.. Approaches to peripheral nerve repair: generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. Biomed Res Int 2016, 3856262, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moskow, J., Ferrigno, B., Mistry, N., et al. Review: bioengineering approach for the repair and regeneration of peripheral nerve. Bioact Mater 4, 107, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nectow, A.R., Marra, K.G., and Kaplan, D.L.. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng Part B Rev 18, 40, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bell, J.H.A., and Haycock, J.W.. Next generation nerve guides: materials, fabrication, growth factors, and cell delivery. Tissue Eng Part B Rev 18, 116, 2012. [DOI] [PubMed] [Google Scholar]
- 32. Szynkaruk, M., Kemp, S.W.P., Wood, M.D., Gordon, T., and Borschel, G.H.. Experimental and clinical evidence for use of decellularized nerve allografts in peripheral nerve gap reconstruction. Tissue Eng Part B Rev 19, 83, 2013. [DOI] [PubMed] [Google Scholar]
- 33. Euler De Souza Lucena, E., Guzen, F.P., Lopes De Paiva Cavalcanti, J.R., Galvão Barboza, C.A., Silva Do Nascimento Júnior, E., and Cavalcante, J.D.S.. Experimental considerations concerning the use of stem cells and tissue engineering for facial nerve regeneration: a systematic review. J Oral Maxillofac Surg 72, 1001, 2014. [DOI] [PubMed] [Google Scholar]
- 34. Pillai, M.M., Sathishkumar, G., Houshyar, S., et al. Nanocomposite-coated silk-based artificial conduits: the influence of structures on regeneration of the peripheral nerve. ACS Appl Biol Mater 3, 4454, 2020. [DOI] [PubMed] [Google Scholar]
- 35. Bendella, H., Rink, S., Grosheva, M., Sarikcioglu, L., Gordon, T., and Angelov, D.N.. Putative roles of soluble trophic factors in facial nerve regeneration, target reinnervation, and recovery of vibrissal whisking. Exp Neurol 300, 100, 2018. [DOI] [PubMed] [Google Scholar]
- 36. Wang, T.V., Delaney, S., and Pepper, J.P.. Current state of stem cell-mediated therapies for facial nerve injury. Curr Opin Otolaryngol Head Neck Surg 24, 285, 2016. [DOI] [PubMed] [Google Scholar]
- 37. Kishimoto, N., Honda, Y., Momota, Y., and Tran, S.D.. Dedifferentiated fat (DFAT) cells: a cell source for oral and maxillofacial tissue engineering. Oral Dis 24, 1161, 2018. [DOI] [PubMed] [Google Scholar]
- 38. Navissano, M., Malan, F., Carnino, R., and Battiston, B.. Neurotube® for facial nerve repair. Microsurgery 25, 268, 2005. [DOI] [PubMed] [Google Scholar]
- 39. Nakamura, Y., Takanari, K., Ebisawa, K., Kanbe, M., Nakamura, R., and Kamei, Y.. Repair of temporal branch of the facial nerve with novel polyglycolic acid-collagen tube: a case report of two cases. Nagoya J Med Sci 82, 123, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Inada, Y., Hosoi, H., Yamashita, A., et al. Regeneration of peripheral motor nerve gaps with a polyglycolic acid-collagen tube: technical case report. Neurosurgery 61, E1105, 2007. [DOI] [PubMed] [Google Scholar]
- 41. Gunn, S., Cosetti, M., and Roland, J.T.. Processed allograft: novel use in facial nerve repair after resection of a rare racial nerve paraganglioma. Laryngoscope 120, 2009, 2010. [DOI] [PubMed] [Google Scholar]
- 42. Safa, B., Jain, S., Desai, M.J., et al. Peripheral nerve repair throughout the body with processed nerve allografts: results from a large multicenter study. Microsurgery 40, 527, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hu, M., Xiao, H., Niu, Y., Liu, H., and Zhang, L.. Long-term follow-up of the repair of the multiple-branch facial nerve defect using acellular nerve allograft. J Oral Maxillofac Surg 74, 218.e1, 2016. [DOI] [PubMed] [Google Scholar]
- 44. Brooks, D.N., Weber, R. V., Chao, J.D., et al. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery 32, 1, 2012. [DOI] [PubMed] [Google Scholar]
- 45. Yampolsky, A., Ziccardi, V., and Chuang, S.K.. Efficacy of acellular nerve allografts in trigeminal nerve reconstruction. J Oral Maxillofac Surg 75, 2230, 2017. [DOI] [PubMed] [Google Scholar]
- 46. Salomon, D., Miloro, M., and Kolokythas, A.. Outcomes of immediate allograft reconstruction of long-span defects of the inferior alveolar nerve. J Oral Maxillofac Surg 74, 2507, 2016. [DOI] [PubMed] [Google Scholar]
- 47. Miloro, M., and Zuniga, J.R.. Does immediate inferior alveolar nerve allograft reconstruction result in functional sensory recovery in pediatric patients? J Oral Maxillofac Surg 78, 2073, 2020. [DOI] [PubMed] [Google Scholar]
- 48. Zuniga, J.R. Sensory outcomes after reconstruction of lingual and inferior alveolar nerve discontinuities using processed nerve allograft—a case series. J Oral Maxillofac Surg 73, 734, 2015. [DOI] [PubMed] [Google Scholar]
- 49. Akbari, M., and Miloro, M.. The inferior alveolar nerve: to graft or not to graft in ablative mandibular resection? J Oral Maxillofac Surg 77, 1280, 2019. [DOI] [PubMed] [Google Scholar]
- 50. Chacon, M.A., Echternacht, S.R., and Leckenby, J.I.. Outcome measures of facial nerve regeneration: a review of murine model systems. Ann Anat 227, 151410, 2020. [DOI] [PubMed] [Google Scholar]
- 51. Angelov, D.N., Guntinas-Lichius, O., Wewetzer, K., Neiss, W.F., and Streppel, M.. Axonal branching and recovery of coordinated muscle activity after transection of the facial nerve in adult rats. Adv Anat Embryol Cell Biol Germany 180, 1, 2005. [PubMed] [Google Scholar]
- 52. Guntinas-Lichius, O., Irintchev, A., Streppel, M., et al. Factors limiting motor recovery after facial nerve transection in the rat: combined structural and functional analyses. Eur J Neurosci 21, 391, 2005. [DOI] [PubMed] [Google Scholar]
- 53. Ozcan, G., Shenaq, S., and Spira, M.. Vascularized nerve tube: an experimental alternative for vascularized nerve grafts over short gaps. J Reconstr Microsurg 9, 405, 1993. [DOI] [PubMed] [Google Scholar]
- 54. Berg, R.W., and Kleinfeld, D.. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J Neurophysiol 89, 104, 2003. [DOI] [PubMed] [Google Scholar]
- 55. Bermejo, R., Houben, D., and Zeigler, H.P.. Optoelectronic monitoring of individual whisker movements in rats. J Neurosci Methods 83, 89, 1998. [DOI] [PubMed] [Google Scholar]
- 56. Dörfl, J. The innervation of the mystacial region of the white mouse: a topographical study. J Anat 142, 173, 1985. [PMC free article] [PubMed] [Google Scholar]
- 57. Heaton, J.T., Kowaleski, J.M., Bermejo, R., Zeigler, H.P., Ahlgren, D.J., and Hadlock, T.A.. A system for studying facial nerve function in rats through simultaneous bilateral monitoring of eyelid and whisker movements. J Neurosci Methods 171, 197, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Heaton, J.T., Sheu, S.H., Hohman, M.H., et al. Rat whisker movement after facial nerve lesion: evidence for autonomic contraction of skeletal muscle. Neuroscience 265, 9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Henstrom, D., Hadlock, T., Lindsay, R., et al. The convergence of facial nerve branches providing whisker pad motor supply in rats: implications for facial reanimation study. Muscle Nerve 45, 692, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leckenby, J.I., Chacon, M.A., Rolfe, K., Lichtman, J.W., and Grobbelaar, A.O.. Development of the interscutularis model as an outcome measure for facial nerve surgery. Ann Anat 223, 127, 2019. [DOI] [PubMed] [Google Scholar]
- 61. Fujimaki, H., Matsumine, H., Osaki, H., et al. Dedifferentiated fat cells in polyglycolic acid-collagen nerve conduits promote rat facial nerve regeneration. Regen Ther 11, 240, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matsumine, H., Numakura, K., Climov, M., Watanabe, Y., Giatsidis, G., and Orgill, D.P.. Facial-nerve regeneration ability of a hybrid artificial nerve conduit containing uncultured adipose-derived stromal vascular fraction: an experimental study. Microsurgery 37, 808, 2017. [DOI] [PubMed] [Google Scholar]
- 63. Sasaki, R., Matsumine, H., Watanabe, Y., et al. Electrophysiologic and functional evaluations of regenerated facial nerve defects with a tube containing dental pulp cells in rats. Plast Reconstr Surg 134, 970, 2014. [DOI] [PubMed] [Google Scholar]
- 64. Banks, C.A., Knox, C., Hunter, D.A., Mackinnon, S.E., Hohman, M.H., and Hadlock, T.A.. Long-term functional recovery after facial nerve transection and repair in the rat. J Reconstr Microsurg 31, 210, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen, P., Knox, C.J., Yao, L., Li, C., and Hadlock, T.A.. The effects of venous ensheathment on facial nerve repair in the rat. Laryngoscope 127, 1558, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Abbas, O.L., Borman, H., Uysal, Ç.A., et al. Adipose-derived stem cells enhance axonal regeneration through cross-facial nerve grafting in a rat model of facial paralysis. Plast Reconstr Surg 138, 387, 2016. [DOI] [PubMed] [Google Scholar]
- 67. Saez, D.M., Sasaki, R.T., Martins, D.. de O., Chacur, M., Kerkis, I., and da Silva, M.C.P. Rat facial nerve regeneration with human immature dental pulp stem cells. Cell Transplant 28, 1573, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hadlock, T., Kowaleski, J., Lo, D., et al. Functional assessments of the rodent facial nerve: a synkinesis model. Laryngoscope 118, 1744, 2008. [DOI] [PubMed] [Google Scholar]
- 69. de Faria, S.D., Testa, J.R.G., Borin, A., and Toledo, R.N.. Standardization of techniques used in facial nerve section and facial movement evaluation in rats. Braz J Otorhinolaryngol 72, 341, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yasui, G., Yamamoto, Y., Shichinohe, R., et al. Neuregulin-1 released by biodegradable gelatin hydrogels can accelerate facial nerve regeneration and functional recovery of traumatic facial nerve palsy. J Plast Reconstr Aesthetic Surg 69, 328, 2016. [DOI] [PubMed] [Google Scholar]
- 71. Li, M., Zhu, Q., and Liu, J.. Olfactory ensheathing cells in facial nerve regeneration. Braz J Otorhinolaryngol 86, 525, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ali, S.A., Rosko, A.J., Hanks, J.E., et al. Effect of motor versus sensory nerve autografts on regeneration and functional outcomes of rat facial nerve reconstruction. Sci Rep 9, 1, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li, D., Wan, H., Feng, J., et al. Comparison of hemihypoglossal- and accessory-facial neurorrhaphy for treating facial paralysis in rats. J Neurol Sci 347, 235, 2014. [DOI] [PubMed] [Google Scholar]
- 74. Niimi, Y., Matsumine, H., Takeuchi, Y., et al. Effectively axonal-supercharged interpositional jump-graft with an artificial nerve conduit for rat facial nerve paralysis. Plast Reconstr Surg Glob Open 3, 1, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Greene, J.J., McClendon, M.T., Stephanopoulos, N., Álvarez, Z., Stupp, S.I., and Richter, C.P.. Electrophysiological assessment of a peptide amphiphile nanofiber nerve graft for facial nerve repair. J Tissue Eng Regen Med 12, 1389, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sasaki, R., Aoki, S., Yamato, M., et al. Tubulation with dental pulp cells promotes facial nerve regeneration in rats. Tissue Eng Part A 14, 1141, 2008. [DOI] [PubMed] [Google Scholar]
- 77. Matsumine, H., Takeuchi, Y., Sasaki, R., et al. Adipocyte-derived and dedifferentiated fat cells promoting facial nerve regeneration in a rat model. Plast Reconstr Surg 134, 686, 2014. [DOI] [PubMed] [Google Scholar]
- 78. Jang, C.H., Lee, H., Kim, M.S., and Kim, G.H.. Effect of polycaprolactone/collagen/hUCS microfiber nerve conduit on facial nerve regeneration. Int J Biol Macromol 93, 1575, 2016. [DOI] [PubMed] [Google Scholar]
- 79. Placheta, E., Wood, M.D., Lafontaine, C., et al. Enhancement of facial nerve motoneuron regeneration through cross-face nerve grafts by adding end-to-side sensory axons. Plast Reconstr Surg 135, 460, 2015. [DOI] [PubMed] [Google Scholar]
- 80. Chen, L., Hu, M., Zhang, L., et al. Motor fiber organization in the extratemporal trunk of the facial nerve in rats: a retrograde Fluoro-Gold study. Exp Ther Med 4, 844, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hizay, A., Ozsoy, U., Demirel, B.M., et al. Use of a Y-tube conduit after facial nerve injury reduces collateral axonal branching at the lesion site but neither reduces polyinnervation of motor endplates nor improves functional recovery. Neurosurgery 70, 1544, 2012. [DOI] [PubMed] [Google Scholar]
- 82. Tomov, T.L., Guntinas-Lichius, O., Grosheva, M., et al. An example of neural plasticity evoked by putative behavioral demand and early use of vibrissal hairs after facial nerve transection. Exp Neurol 178, 207, 2002. [DOI] [PubMed] [Google Scholar]
- 83. Placheta, E., Wood, M.D., Lafontaine, C., Frey, M., Gordon, T., and Borschel, G.H.. Macroscopic in vivo imaging of facial nerve regeneration in Thy1-GFP rats. JAMA Facial Plast Surg 17, 8, 2015. [DOI] [PubMed] [Google Scholar]
- 84. Ali, S.A., Stebbins, A.W., Hanks, J.E., et al. Facial nerve surgery in the rat model to study axonal inhibition and regeneration. J Vis Exp 2020, 1, 2020. [DOI] [PubMed] [Google Scholar]
- 85. Hadlock, T.A., Kowaleski, J., Lo, D., MacKinnon, S.E., and Heaton, J.T.. Rodent facial nerve recovery after selected lesions and repair techniques. Plast Reconstr Surg 125, 99, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Matsumine, H., Sasaki, R., Tabata, Y., et al. Facial nerve regeneration using basic fibroblast growth factor-impregnated gelatin microspheres in a rat model. J Tissue Eng Regen Med 10, E559, 2016. [DOI] [PubMed] [Google Scholar]
- 87. Watanabe, Y., Sasaki, R., Matsumine, H., Yamato, M., and Okano, T.. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. J Tissue Eng Regen Med 11, 362, 2017. [DOI] [PubMed] [Google Scholar]
- 88. Guntinas-Lichius, O., Angelov, D.N., Tomov, T.L., Dramiga, J., Neiss, W.F., and Wewetzer, K.. Transplantation of olfactory ensheathing cells stimulates the collateral sprouting from axotomized adult rat facial motoneurons. Exp Neurol 172, 70, 2001. [DOI] [PubMed] [Google Scholar]
- 89. Matsumine, H., Sasaki, R., Yamato, M., Okano, T., and Sakurai, H.. A polylactic acid non-woven nerve conduit for facial nerve regeneration in rats. J Tissue Eng Regen Med 8, 454, 2014. [DOI] [PubMed] [Google Scholar]
- 90. Zhou, L., Du, H.D., Tian, H.. Bin, Li, C., Tian, J., and Jiang, J.J. Experimental study on repair of the facial nerve with Schwann cells transfected with GDNF genes and PLGA conduits. Acta Otolaryngol 128, 1266, 2008. [DOI] [PubMed] [Google Scholar]
- 91. Sasaki, R., Aoki, S., Yamato, M., et al. PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. J Tissue Eng Regen Med 5, 823, 2011. [DOI] [PubMed] [Google Scholar]
- 92. Mackinnon, S.E., and Dellon, A.L.. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast Reconstr Surg 85, 419, 1990. [DOI] [PubMed] [Google Scholar]
- 93. Weber, R.A., Breidenbach, W.C., Brown, R.E., Jabaley, M.E., and Mass, D.P.. A Randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg 106, 1036, 2000. [DOI] [PubMed] [Google Scholar]
- 94. Meek, M.F., and Coert, J.H.. US Food and Drug Administration/Conformit Europe-approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Ann Plast Surg 60, 466, 2008. [PubMed] [Google Scholar]
- 95. Costa, H.J.Z.R., Ferreira Bento, R., Salomone, R., et al. Mesenchymal bone marrow stem cells within polyglycolic acid tube observed in vivo after six weeks enhance facial nerve regeneration. Brain Res 1510, 10, 2013. [DOI] [PubMed] [Google Scholar]
- 96. Shimizu, M., Matsumine, H., Osaki, H., et al. Adipose-derived stem cells and the stromal vascular fraction in polyglycolic acid-collagen nerve conduits promote rat facial nerve regeneration. Wound Repair Regen 26, 446, 2018. [DOI] [PubMed] [Google Scholar]
- 97. Batioglu-Karaaltin, A., Karaaltin, M.V., Oztel, O.N., et al. Human olfactory stem cells for injured facial nerve reconstruction in a rat model. Head Neck 38, E2011, 2016. [DOI] [PubMed] [Google Scholar]
- 98. Niimi, Y., Matsumine, H., Takeuchi, Y., et al. A collagen-coated PGA conduit for interpositional-jump graft with end-to-side neurorrhaphy for treating facial nerve paralysis in rat. Microsurgery 39, 70, 2019. [DOI] [PubMed] [Google Scholar]
- 99. Petcu, E.B., Midha, R., McColl, E., Popa-Wagner, A., Chirila, T.V., and Dalton, P.D.. 3D printing strategies for peripheral nerve regeneration. Biofabrication 10, 032001, 2018. [DOI] [PubMed] [Google Scholar]
- 100. Zhu, W., Tringale, K.R., Woller, S.A., et al. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits. Mater Today 21, 951, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Johnson, B.N., Lancaster, K.Z., Zhen, G., et al. 3D printed anatomical nerve regeneration pathways. Adv Funct Mater 25, 6205, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang, Q., Nguyen, P.D., Shi, S., Burrell, J.C., Cullen, D.K., and Le, A.D.. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci Rep 8, 1, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Huang, H., Xiao, H., Liu, H., Niu, Y., Yan, R., and Hu, M.. A comparative study of acellular nerve xenografts and allografts in repairing rat facial nerve defects. Mol Med Rep 12, 6330, 2015. [DOI] [PubMed] [Google Scholar]
- 104. Zhu, G., and Lou, W.. Regeneration of facial nerve defects with xenogeneic acellular nerve grafts in a rat model. Head Neck 36, 481, 2014. [DOI] [PubMed] [Google Scholar]
- 105. Hohman, M.H., Kleiss, I.J., Knox, C.J., Weinberg, J.S., Heaton, J.T., and Hadlock, T.A.. Functional recovery after facial nerve cable grafting in a rodent model. JAMA Facial Plast Surg 16, 20, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Foecking, E.M., Burgess, B.D., Fridrici, Z.C., Bialek, S.E., Low, C., and Charous, S.J.. Effects of the number of muscle-nerve-muscle grafts on rat facial nerve functional recovery. Ann Otol Rhinol Laryngol 127, 791, 2018. [DOI] [PubMed] [Google Scholar]
- 107. Kakibuchi, M., Tuji, K., Fukuda, K., et al. End-to-side nerve graft for facial nerve reconstruction. Ann Plast Surg 53, 496, 2004. [DOI] [PubMed] [Google Scholar]
- 108. Matsumine, H., Sasaki, R., Takeuchi, Y., et al. Unilateral multiple facial nerve branch reconstruction using “end-to-side loop graft” supercharged by hypoglossal nerve. Plast Reconstr Surg Glob Open 2, 1, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Matsumine, H., Sasaki, R., Takeuchi, Y., et al. Vascularized versus nonvascularized island median nerve grafts in the facial nerve regeneration and functional recovery of rats for facial nerve reconstruction study. J Reconstr Microsurg 30, 127, 2014. [DOI] [PubMed] [Google Scholar]
- 110. Matsuda, K., Kakibuchi, M., Kubo, T., et al. A new model of end-to-side nerve graft for multiple branch reconstruction: end-to-side cross-face nerve graft in rats. J Plast Reconstr Aesthetic Surg 61, 1357, 2008. [DOI] [PubMed] [Google Scholar]
- 111. Galeano, M., Manasseri, B., Risitano, G., et al. A free vein graft cap influences neuroma formation after nerve transection. Microsurgery 29, 568, 2009. [DOI] [PubMed] [Google Scholar]
- 112. Acar, M., Karacalar, A., Ayyildiz, M., et al. The effect of autogenous vein grafts on nerve repair with size discrepancy in rats: an electrophysiological and stereological analysis. Brain Res 1198, 171, 2008. [DOI] [PubMed] [Google Scholar]
- 113. Zhang, F., Blain, B., Beck, J., et al. Autogenous venous graft with one-stage prepared Schwann cells as a conduit for repair of long segmental nerve defects. J Reconstr Microsurg 18, 295, 2002. [DOI] [PubMed] [Google Scholar]
- 114. Barcelos, A.S., Rodrigues, A.C., Silva, M.D.P., and Padovani, C.R.. Inside-out vein graft and inside-out artery graft in rat sciatic nerve repair. Microsurgery 23, 66, 2003. [DOI] [PubMed] [Google Scholar]
- 115. Sun, F., Zhou, K., Mi, W.J., and Qiu, J.H.. Repair of facial nerve defects with decellularized artery allografts containing autologous adipose-derived stem cells in a rat model. Neurosci Lett 499, 104, 2011. [DOI] [PubMed] [Google Scholar]
- 116. Sun, F., Zhou, K., Mi, W.J., and Qiu, J.H.. Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats. Biomaterials 32, 8118, 2011. [DOI] [PubMed] [Google Scholar]
- 117. Ozsoy, U., Demirel, B.M., Hizay, A., et al. Hypoglossal-facial anastomosis (HFA) over a 10 mm gap bridged by a Y-tube-conduit enhances neurite regrowth and reduces collateral axonal branching at the lesion site. Restor Neurol Neurosci 29, 227, 2011. [DOI] [PubMed] [Google Scholar]
- 118. Spector, J.G., Derby, A., Lee, P., and Roufa, D.G.. Comparison of rabbit facial nerve regeneration in nerve growth factor-containing silicone tubes to that in autologous neural grafts. Ann Otol Rhinol Laryngol 104, 875, 1995. [DOI] [PubMed] [Google Scholar]
- 119. Gershon Spector, J. Axonal regeneration in severed peripheral facial nerve of the rabbit: relation of the number of axonal regenerates to behavioral and evoked muscle activity. Ann Otol Rhinol Laryngol 107, 141, 1998. [DOI] [PubMed] [Google Scholar]
- 120. Guo, B.F., and Dong, M.M.. Application of neural stem cells in tissue-engineered artificial nerve. Otolaryngol Head Neck Surg 140, 159, 2009. [DOI] [PubMed] [Google Scholar]
- 121. Liu, H.W., Wen, W.S., Hu, M., et al. Chitosan conduits combined with nerve growth factor microspheres repair facial nerve defects. Neural Regen Res 8, 3139, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Vasconcelos, B.C.E., and Gay-Escoda, C.. Facial nerve repair with expanded polytetrafluoroethylene and collagen conduits: an experimental study in the rabbit. J Oral Maxillofac Surg 58, 1257, 2000. [DOI] [PubMed] [Google Scholar]
- 123. Diaz, L.M., Steele, M.H., Guerra, A.B., et al. The role of topically administered FK506 (Tacrolimus) at the time of facial nerve repair using entubulation neurorrhaphy in a rabbit model. Ann Plast Surg 52, 407, 2004. [DOI] [PubMed] [Google Scholar]
- 124. Wang, P., Zhao, H., Yao, Y., et al. Repair of facial nerve crush injury in rabbits using collagen plus basic fibroblast growth factor. J Biomed Mater Res Part A 108, 1329, 2020. [DOI] [PubMed] [Google Scholar]
- 125. Hu, M., Zhang, L., Niu, Y., Xiao, H., Tang, P., and Wang, Y.. Repair of whole rabbit facial nerve defects using facial nerve allografts. J Oral Maxillofac Surg 68, 2196, 2010. [DOI] [PubMed] [Google Scholar]
- 126. Ozean, G., Shenaq, S., Mirabi, B., and Spira, M.. Nerve regeneration in a bony bed: vascularized versus nonvascularized nerve grafts. Plast Reconstr Surg 91, 1322, 1993. [DOI] [PubMed] [Google Scholar]
- 127. Spector, J.G., Lee, P., and Derby, A.. Rabbit facial nerve regeneration in autologous nerve grafts after antecedent injury. Laryngoscope 110, 660, 2000. [DOI] [PubMed] [Google Scholar]
- 128. Gencer, Z.K., Özkiriş, M., Saydam, L., et al. The comparison of histological results of experimentally created facial nerve defects repaired by 2 different anastomosis techniques: classic suture technique or tissue adhesives for nerve anastomosis? J Craniofac Surg 25, 652, 2014. [DOI] [PubMed] [Google Scholar]
- 129. Izquierdo, J.C., Campos, Á.M., Romero, C.J., and Romero, G.. Predegenerated great auricular nerve graft in facial nerve defects. Otol Neurotol 35, 64, 2014. [DOI] [PubMed] [Google Scholar]
- 130. Zhu, Y., Liu, S., Zhou, S., et al. Vascularized versus nonvascularized facial nerve grafts using a new rabbit model. Plast Reconstr Surg 135, 331e, 2015. [DOI] [PubMed] [Google Scholar]
- 131. Tang, J., Wang, X.M., Hu, J., Luo, E., and Qi, M.C.. Autogenous standard versus inside-out vein graft to repair facial nerve in rabbits. Chin J Traumatol 11, 104, 2008. [DOI] [PubMed] [Google Scholar]
- 132. Wang, X., Luo, E., Li, Y., and Hu, J.. Schwann-like mesenchymal stem cells within vein graft facilitate facial nerve regeneration and remyelination. Brain Res 1383, 71, 2011. [DOI] [PubMed] [Google Scholar]
- 133. Lim, J.H., Boyle, G.M., and Panizza, B.. Novel mouse model for simulating microsurgical tumor excision with facial nerve preservation. Laryngoscope 126, E1, 2016. [DOI] [PubMed] [Google Scholar]
- 134. Wang, H., Fang, F., Yi, J., Xiang, Z., Sun, M., and Jiang, H.. Establishment and assessment of the perinatal mouse facial nerve axotomy model via a subauricular incision approach. Exp Biol Med 237, 1249, 2012. [DOI] [PubMed] [Google Scholar]
- 135. Most, S.P. Facial nerve recovery in bcl2 overexpression mice after crush injury. Arch Facial Plast Surg 6, 82, 2004. [DOI] [PubMed] [Google Scholar]
- 136. Makwana, M., Werner, A., Acosta-Saltos, A., et al. Peripheral facial nerve axotomy in mice causes sprouting of motor axons into perineuronal central white matter: time course and molecular characterization. J Comp Neurol 518, 699, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Guntinas-Lichius, O., Angelov, D.N., Morellini, F., et al. Opposite impacts of tenascin-C and tenascin-R deficiency in mice on the functional outcome of facial nerve repair. Eur J Neurosci 22, 2171, 2005. [DOI] [PubMed] [Google Scholar]
- 138. Hizay, A., Seitz, M., Grosheva, M., et al. FGF-2 is required to prevent astrogliosis in the facial nucleus after facial nerve injury and mechanical stimulation of denervated vibrissal muscles. J Biomed Res 30, 142, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hussain, T., Mastrodimos, M.B., Raju, S.C., et al. Fluorescently labeled peptide increases identification of degenerated facial nerve branches during surgery and improves functional outcome. PLoS One 10, 1, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Olmstead, D.N., Mesnard-Hoaglin, N.A., Batka, R.J., Haulcomb, M.M., Miller, W.M., and Jones, K.J.. Facial nerve axotomy in mice: a model to study motoneuron response to injury. J Vis Exp e52382, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kitahara, A.K., Nishimura, Y., Shimizu, Y., and Endo, K.. Facial nerve repair accomplished by the interposition of a collagen nerve guide. J Neurosurg 93, 113, 2000. [DOI] [PubMed] [Google Scholar]
- 142. Dresner, H.S., King, T.A., Clark, H.B., Juhn, S.K., and Levine, S.C.. Peripheral facial nerve regeneration using collagen conduit entubulation in a cat model. Ann Otol Rhinol Laryngol 115, 631, 2006. [DOI] [PubMed] [Google Scholar]
- 143. Wu, S., Suzuki, Y., Tanihara, M., Ohnishi, K., Endo, K., and Nishimura, Y.. Repair of facial nerve with alginate sponge without suturing: an experimental study in cats. Scand J Plast Reconstr Surg Hand Surg 36, 135, 2002. [DOI] [PubMed] [Google Scholar]
- 144. Gruart, A., Streppel, M., Guntinas-Lichius, O., Angelov, D.N., Neiss, W.F., and Delgado-García, J.M.. Motoneuron adaptability to new motor tasks following two types of facial-facial anastomosis in cats. Brain 126, 115, 2003. [DOI] [PubMed] [Google Scholar]
- 145. Williams, H.B. The value of continuous electrical muscle stimulation using a completely implantable system in the preservation of muscle function following motor nerve injury and repair: an experimental study. Microsurgery 17, 589, 1996. [DOI] [PubMed] [Google Scholar]
- 146. Sundine, M.J., Quan, E.E., Saglam, et al. The use of end-to-side nerve grafts to reinnervate the paralyzed orbicularis oculi muscle. Plast Reconstr Surg 111, 2255, 2003. [DOI] [PubMed] [Google Scholar]
- 147. Lu, C., Meng, D., Cao, J., et al. Collagen scaffolds combined with collagen-binding ciliary neurotrophic factor facilitate facial nerve repair in mini-pigs. J Biomed Mater Res Part A 103, 1669, 2015. [DOI] [PubMed] [Google Scholar]
- 148. Cui, Y., Lu, C., Meng, D., et al. Collagen scaffolds modified with CNTF and bFGF promote facial nerve regeneration in minipigs. Biomaterials 35, 7819, 2014. [DOI] [PubMed] [Google Scholar]
- 149. Zhang, Z., Li, X., Li, Z., et al. Collagen/nano-sized β-tricalcium phosphate conduits combined with collagen filaments and nerve growth factor promote facial nerve regeneration in miniature swine: an in vivo study. Oral Surg Oral Med Oral Pathol Oral Radiol 128, 472, 2019. [DOI] [PubMed] [Google Scholar]
- 150. Ianacone, D.C., Gnadt, B.J., and Isaacson, G.. Ex vivo ovine model for head and neck surgical simulation. Am J Otolaryngol Head Neck Med Surg 37, 272, 2016. [DOI] [PubMed] [Google Scholar]
- 151. Glasby, M.A., Mountain, R.E., and Murray, J.A.. Repair of the facial nerve using freeze-thawed muscle autografts. A surgical model in the sheep. Arch Otolaryngol Head Neck Surg 119, 461, 1993. [DOI] [PubMed] [Google Scholar]
- 152. Al Abri, R., Kolethekkat, A.A., Kelleher, M.O., Myles, L.M., and Glasby, M.A.. Effect of locally administered ciliary neurotrophic factor on the survival of transected and repaired adult sheep facial nerve. Oman Med J 29, 208, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hontanilla, B., Aubá, C., Arcocha, J., and Gorría, O.. Nerve regeneration through nerve autografts and cold preserved allografts using tacrolimus (FK506) in a facial paralysis model: a topographical and neurophysiological study in monkeys. Neurosurgery 58, 768, 2006. [DOI] [PubMed] [Google Scholar]
- 154. Li, W.Y., Jia, H., Wang, Z.D., et al. Combinatory transplantation of mesenchymal stem cells with flavonoid small molecule in acellular nerve graft promotes sciatic nerve regeneration. J Tissue Eng 11, [Epub ahead of print]; DOI: 10.1177/2041731420980136, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Frautschi, R., Rampazzo, A., Bernard, S., Djohan, R., Papay, F., and Gharb, B.B.. Management of the salivary glands and facial nerve in face transplantation. Plast Reconstr Surg 137, 1887, 2016. [DOI] [PubMed] [Google Scholar]
- 156. Audolfsson, T., Rodríguez-Lorenzo, A., Wong, C., et al. Nerve transfers for facial transplantation: a cadaveric study for motor and sensory restoration. Plast Reconstr Surg 131, 1231, 2013. [DOI] [PubMed] [Google Scholar]
- 157. Ma, F., Zhu, T., Xu, F., et al. Neural stem/progenitor cells on collagen with anchored basic fibroblast growth factor as potential natural nerve conduits for facial nerve regeneration. Acta Biomater 50, 188, 2017. [DOI] [PubMed] [Google Scholar]
- 158. Chao, X., Xu, L., Shang, H., et al. The injury of marginal mandibular branch unexpectedly promotes the repair of buccal branch of facial nerve in a rat model. Acta Otolaryngol 136, 956, 2016. [DOI] [PubMed] [Google Scholar]
- 159. Tan, J., Xu, Y., Han, F., and Ye, X.. Genetical modification on adipose-derived stem cells facilitates facial nerve regeneration. Aging (Albany NY) 11, 908, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Karaman, M., Tuncel, A., Sheidaei, S., et al. Amniotic membrane covering for facial nerve repair. Neural Regen Res 8, 975, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]