Abstract
Purpose
The purpose of this study was to investigate the effects of lysozyme, an antimicrobial enzyme found in tears that protects the eye against pathogens, on pseudotyped severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection through corneal epithelial cells.
Methods
The expression of the angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease (TMPRSS2) in human corneal epithelial cells (HCECs) was measured by RT-PCR and Western blotting. The altered expression of the pro-inflammatory molecules induced by spike protein and lysozyme was analyzed by RT-PCR. Cell toxicity was tested by CCK8 assay. The cell entry of SAR-CoV-2 in HCECs and primary rabbit corneal epithelial cells (RbCECs) was detected by luciferase assay.
Results
ACE2 and TMPRSS2 were highly expressed in HCECs. The spike proteins of SARS-CoV-2 stimulated a robust inflammatory response in HCECs, characterized by increased secretion of pro-inflammatory molecules, including IL-6, TNF-α, iNOS, and MCP-1, and pretreatment with lysozyme in HCECs markedly decreased the production of proinflammatory molecules induced by spike proteins. In addition, the inflammatory cytokine TNF-α enhanced the entry of SARS-CoV-2 into HCECs, which can be mitigated by pretreatment with lysozyme.
Conclusions
In this study, we analyzed the susceptibility of human corneal epithelial cells to SARS-CoV-2 infection and suggested the protective effects of lysozyme on SARS-CoV-2 infection.
Keywords: lysozyme, coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), human corneal epithelial cell (HCEC), inflammation
Coronavirus disease 2019 (COVID-19) is a global health emergency1,2 that is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 As of January 2022, the virus resulted in over 318 million confirmed cases of COVID-19 worldwide (World Health Organization [WHO], accessed January 16, 2022). The clinical manifestations of COVID-19 are extremely broad, ranging from mild symptoms to respiratory and multi-organ failure.3,4 The most common symptoms in patients with COVID-19 include fever, cough, shortness of breath, expectoration, diarrhea, alteration in taste, and conjunctivitis.5
The causative agent of the COVID-19 pandemic is SARS-CoV-2, which is transmitted primarily by respiratory droplets through close interaction with an infected person.6 Recent studies have reported that this novel coronavirus may infect patients through ocular surface tissue, and viral RNA can be detected in tears and conjunctival secretions.7,8 These results suggest that the eye can be severely affected by COVID-19 infection as the respiratory system. Therefore, it is of importance to identify the ocular target of SARS-CoV-2 infection and to explore protective strategies against SARS-CoV-2 infection via the ocular routes.
The lysozyme, a 14kDa cationic protein, was first discovered by Alexander Fleming in 1922.9 Lysozyme has been identified in various biological fluids, such as nasal secretions, tears, and sputum.10,11 Lysozyme is a potent antimicrobial enzyme that hydrolyzes bacterial cell wall peptidoglycan.12,13 It has antiviral activity against herpes simplex, herpes zoster, and HIV.14,15 In the eyes, several studies have revealed the antimicrobial properties and immune modulate properties.16,17 In addition, the tears of patients with dry eye and herpes simplex keratitis showed lower levels of lysozyme.18,19 Therefore, we speculate that lysozyme might provide protection against the infection of SARS-CoV-2 through the cornea, which is yet to be determined.
In this study, we analyzed the susceptibility of human corneal epithelial cells (HCECs) to SARS-CoV-2 infection and investigated the potential protective effects of lysozyme on SARS-CoV-2 infection.
Materials and Methods
Cell Culture
Normal HCECs were obtained from Prof. Zhao Shaozhen (Tianjin Medical University, China). The HCECs were kept in Dulbecco's modified Eagle's medium (DMEM)/F12 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 6% fetal bovine serum (FBS), 1% penicillin-streptomycin, 0.1% recombinant human epithelial growth factor, and 0.35% insulin. HCECs were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air and grown to 80% to 90% confluence, and passages 4 to 6 were used for the experiment. Primary rabbit corneal epithelial cells (RbCECs) and their specific media were purchased from China Center for Type Culture Collection (Wuhan, China). RbCECs were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air and grown to 80% to 90% confluence, and passages 1 to 4 were used for the experiment.
The full name of spike protein used to treat cells in vitro was SARS-CoV-2 Spike S1 + S2 ECD-His Recombinant Protein, which was bought from Sino Biological company (40589-V08B1, Sino Biological, Beijing, China; available at: https://www.sinobiological.com/recombinant-proteins/2019-ncov-cov-spike-40589-v08b1). In some experiments, HCECs were allowed to attach overnight, then washed and starved overnight prior to treatment with or without spike protein (1 µg/mL) for 24 hours. Lysozyme was kindly provided by Xingqi Pharmaceutical Co., Ltd. (Shenyang, China).
Western Blot Analysis
HCECs or RbCECs were lysed in ice-cold RIPA buffer containing protease and phosphatase inhibitor cocktails (APExBIO, Houston, TX, USA). The extracted proteins were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes (Millipore, MA, USA). After blocking with 5% skim milk, the membrane was incubated at 4°C overnight with the following primary antibodies: angiotensin-converting enzyme 2 (ACE2; 1:1000; Cell Signalling Technology, Danvers, MA, USA), transmembrane serine protease (TMPRSS2; 1:1000; Abcam, Cambridge, UK), Flag-Tag (1:1000; Multiscience, Hangzhou, China), β-tubulin (1:5000, UM4001, utibody), and GAPDH (1:5000, UM4002, utibody). The membrane was washed and incubated with horseradish peroxidase-linked secondary antibodies for 1 hour at 37°C. GAPDH was used as an endogenous control, and protein bands were quantified using ImageJ software.
RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
To evaluate the protective effect of lysozyme on HCECs, HCECs were pretreated with 10 mg/mL of lysozyme 2 hours before, or simultaneously with spike protein stimulation. After exposure to spike protein for 24 hours, RNA from HCECs was extracted using TRIzol reagent (Sigma-Aldrich, St. Louis, MO, USA), following the manufacturer's protocol. RNA concentrations were measured using a NanoDrop One C spectrophotometer (Thermo Fisher Scientific, Santa Clara, CA, USA). The quality of the extracted RNA was detected using a UV spectrophotometer and was further confirmed to be of good quality, with A260/280 between 1.8 and 2.0 and A260/230 between 2.0 and 2.2. Total RNA (1000 ng) was used to synthesize cDNA using the TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgene, Beijing, China). Real-time quantitative polymerase chain reaction (qRT-PCR) was performed in triplicate in a total volume of 10 µL containing 5 µL perfect start green qPCR supermix (Transgene, Beijing, China), 1 µL PCR primers (at a final concentration of 0.3 mM), 0.5 µL cDNA template, and 2.5 µL of nuclease-free water. Amplifications were performed using the Bio-Rad System (CFX Connect; BioRad, Hercules, CA, USA) with the following cycling conditions: 95°C for 30 seconds, 40 cycles of 95°C for 5 seconds, and 60°C for 30 seconds. The expression levels of GAPDH were used as an endogenous control. The sequences of the RT-PCR primers are listed in the Table.
Table.
Primer Sequences Used for Real-Time RT-PCR
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| ACE2 | GGGATCAGAGATCGGAAGAAGAAA | AGGAGGTCTGAACATCATCAGTG |
| TMPRSS2 | AATCGGTGTGTTCGCCTCTAC | CGTAGTTCTCGTTCCAGTCGT |
| TNF-α | GAGGCCAAGCCCTGGTATG | CGGGCCGATTGATCTCAGC |
| IL-6 | CCACTCACCTCTTCAGAACG | CATCTTTGGAAGGTTCAGGTTG |
| MCP-1 | GATCTCAGTGCAGAGGCTCG | TTTGCTTGTCCAGGTGGTCC |
| iNOS | TTCTGTGCTGTCCCAGTGAG | TGAAGAAAACCCCTTGTGCT |
| IL-8 | AAGAAACCACCGGAAGGAAC | ACTCCTTGGCAAAACTGCAC |
| IL-1β | ATGATGGCTTATTACAGTGGCAA | GTCGGAGATTCGTAGCTGGA |
CCK 8
Cell activity and lysozyme concentration were determined using the CCK-8 assay. Cells were incubated in 96-well transparent plates and treated with lysozyme at different concentrations (2, 5, 10, and 20 mg/mL groups) for 24 hours at the time of 70% to 80% fusion on the second day. After 24 hours, 100 µL of basic medium containing 10% CCK-8 (APExBIO, Houston, TX, USA) was added and incubated in a cell incubator in the dark. The absorbance was measured using a microplate meter. The absorbance value represented the relative cell viability, and after subtracting the background value, the absorbance value was compared with that of the control group without lysozyme (the normal group showed 100% cell viability).
Construction of the S Gene Expression Plasmid
The S genes of the SARS-CoV-2 strain Wuhan-Hu-1 (GenBank accession no. NC_045512)20,21 were chemically synthesized with codon optimization for Homo sapiens expression by TSINGKE (Beijing, China). The S gene fragments of SARS-CoV-2 with truncated C-terminal 19 amino acids (S-CΔ19) were amplified by PCR and inserted into the pcDNA3.1(+) vector by homologous recombination. The constructed recombinant truncated S protein expression plasmids were confirmed for correct insertion by sequencing analysis.
SARS-CoV-2 Spike-Pseudotyped Lentivirus and Transduction
Pseudoviruses expressing the SARS-CoV-2 S protein and luciferase were kindly provided by Professor Zhou Dongming. Briefly, HEK293T cells were seeded in a 10-cm-diameter culture plate containing DMEM (supplemented with 10% FBS, 2 Mm L-glutamine, and penicillin-streptomycin). Cells were transfected with 15 ug of Env-defective and luciferase-expressing plasmid pNL4-3.Luc.R-E- and 1 ug of SARS-CoV-2-S-CΔ19 plasmid using 80 ug polyethyleneimine (PEI; 1 mg/mL), and refreshed with 10 mL of DMEM without FBS and antibiotics after 24 hours of transfection. The spike vector contained the full-length wild-type spike sequence of the Wuhan-Hu-1 strain of SARS-CoV-2 (GenBank accession no. NC_045512). The culture supernatant, which contained SARS-CoV spike protein expression pseudovirus, was then harvested 72 hours after transfection, centrifuged at 500 × g for 10 minutes, filtered through a 0.45 µm filter and stored at −80°C.
Luciferase Assays
For pseudovirus entry assays, the pLVX-hACE2 cell line, which overexpresses hACE2 protein, was established by lentiviral transfer of the pLVX-hACE2 plasmid.22,23 The pLVX-hACE2 plasmid was kindly provided by Professor Zhou Dongming. HCECs or RbCECs were treated with SARS-CoV-2 spike-pseudotyped lentivirus, TNF-α, and lysozyme 48 hours after transfection. After incubation for 48 hours, cells were washed and lysed with passive lysis buffer (Promega, Madison, WI, USA) for 15 minutes at 37°C; 20 uL microliters of cell lysates containing firefly luciferase and 100 uL of luciferase assay substrate were then added to each well in a 96-well plate, and the luciferase activity was measured using a luminometer.
ELISA
The concentrations of cytokines, including TNF-α, IL-6, MCP-1, iNOS, IL-8, and IL-1β, in cell cultures were measured by ELISA. In brief, ELISA kits were allowed to equilibrate at room temperature for 30 minutes prior to cytokines quantification according to the manufacturers’ instruction. ELISA kit (Mlbio, Inc., Shanghai, China) was used to measure soluble extracellular levels of TNF-α (catalog no. ml064303), IL-6 (catalog no. ml058097), MCP-1 (catalog no. ml028554), iNOS (catalog no. ml061044), IL-8 (catalog no. ml028580), and IL-1β (catalog no. ml058059).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software. The comparison between the two groups was calculated using the Student's t-test. Differences between three or more groups were calculated using one-way analysis of variance (ANOVA). Quantitative results are displayed as mean ± SD. A P value of less than 0.05 was considered statistically significant, and the exact P values are provided in the text.
Results
ACE2 and TMPRSS2 were Highly Expressed in HCECs
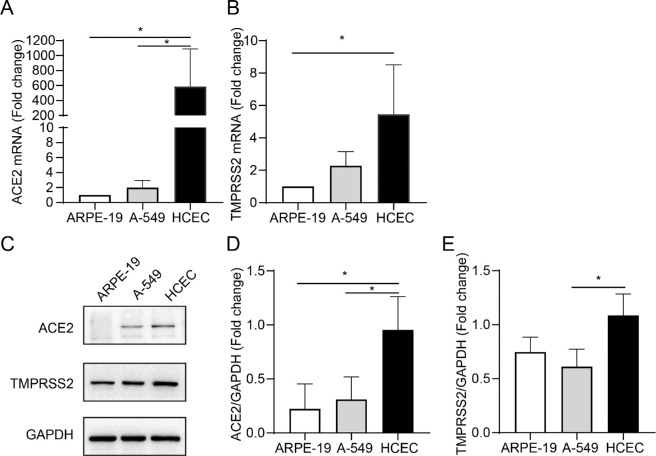
Recent studies have reported that SARS-CoV-2 engages ACE2 as an entry receptor and uses TMPRSS2 for the priming of S proteins.23,24 The expression levels of ACE2 and TMPRSS2 determine the susceptibility and severity of COVID-19.25,26 To validate whether SARS-CoV2 can enter HCECs, we examined the expression of ACE2 and TMPRSS2 in HCECs (Fig. 1). A-549 were included as positive controls because their high expression of ACE2 and TMPRSS2 has been well reported.27 We found that ACE2 was highly expressed in HCECs at mRNA and protein levels (P = 0.0463 and P = 0.0467; P = 0.0278 and P = 0.0462, respectively; see Figs. 1A, 1C, 1D). The serine protease TMPRSS2 was also highly expressed in HCECs (P = 0.0127 and P = 0.0305, respectively; see Figs. 1B, 1C, 1E).
Figure 1.
Analysis of the gene and protein expression of receptors of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human corneal epithelial cells (HCECs). The mRNA expression of angiotensin-converting enzyme 2 (ACE2) (A) and TMPRSS2 (B) in ARPE-19, A-549, and HCECs are detected by RT-PCR. (C) Western blot analysis of ACE2 and TMPRSS2 expression in a different cell line. (D, E) Quantification of ACE2 and TMPRSS2 protein expression. Values are mean ± SD from at least three independent experiments. *P < 0.5; **P < 0.1; ***P < 0.001 versus control group using one-way analysis of variance (ANOVA).
Lysozyme Reduced Viral Entry into HCECs in a Dose-Dependent Manner
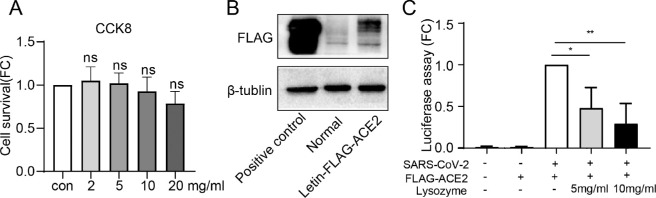
CCK8 assay was performed to evaluate the potential toxicity of different dose of lysozyme on HCECs. No toxicity of lysozyme was observed for HCECs at the tested concentration (0–20 mg/mL, P = 0.9820, P = 0.9996, P = 0.9309, and P = 0.2005, respectively; Fig. 2A). Because the pseudovirus has a weak ability to infect cells, the pLVX-hACE2 cell, which overexpresses hACE2 protein, were used for cell entry assay. The efficiency of ACE2 overexpression was verified by western blot analysis (Fig. 2B). We found that the treatment with 5 mg/mL and 10 mg/mL lysozyme reduced the entry of SARS-CoV2 into HCECs by reducing luciferase activity (P = 0.0148 and P = 0.0018, respectively; Fig. 2C).
Figure 2.
Lysozyme reduced viral entry into HCECs in a dose-dependent manner. (A) Measurement of cytotoxicity by the CCK8 assay in HCECs treated with various doses of lysozyme for 24 hours. (B) Western blot analysis of the efficiency of ACE2 overexpression. (C) Measurement of cell entry by luciferase assay in HCECs treated with ACE2 overexpressing lentivirus and different dose lysozyme (5 mg/mL and 10 mg/mL). Values are mean ± SD from at least three independent experiments. *P < 0.5; **P < 0.1; ***P < 0.001 versus control group using one-way ANOVA.
The Spike Protein of SARS-CoV-2 Induced a Significant Inflammatory Response in HCECs
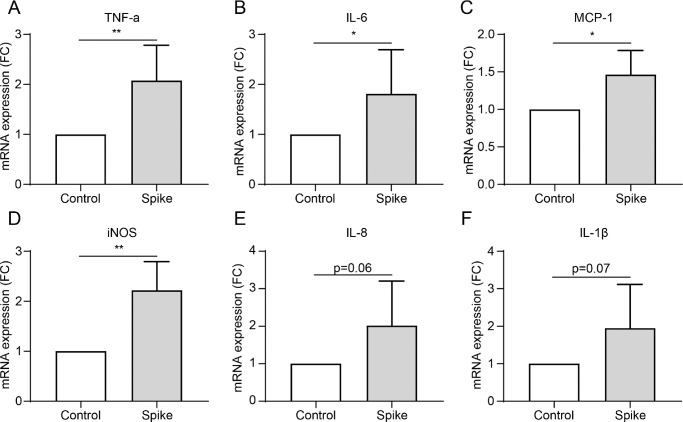
The spike glycoprotein in SARS-CoV-2 plays a critical role in binding to the host ACE2 receptor and facilitating membrane fusion and virus entry into host cells. Therefore, the spike protein is well used to mimic the effect of SARS-CoV-2 on cells.28–31 We used 1 µg/mL of spike protein to treat HCECs for 24 hours to investigate the influence of SARS-CoV-2 on HCECs. The spike protein triggered robust expression of various inflammatory cytokines, including TNF-α, IL-6, MCP-1, and iNOS in HCECs (P = 0.0039, P = 0.0479, P = 0.0273, and P = 0.0054, respectively; Figs. 3A–F). Levels of IL-8 and IL-1β trended higher without, however, reaching statistical significance (P = 0.0620 and P = 0.0743, respectively; Figs. 3D, 3E). This indicated that SARS-CoV2 infection can induce a significant inflammatory response in HCECs.
Figure 3.
The inflammatory response induced by spike protein of SARS-CoV2 in HCECs. (A–F) mRNA expression of various inflammatory cytokines, including TNF-α (A), IL-6 (B), MCP-1 (C), iNOS (D), IL-8 (E), and IL-1β (F) in HCEC cells treated with 1 µg/mL spike protein detected by RT-PCR. Values are mean ± SD from at least three independent experiments. *P < 0.5; **P < 0.1; ***P < 0.001 versus control group using the t-test.
Treatment with Lysozyme Diminished the Secretion of Inflammatory Molecules Induced by Spike Proteins
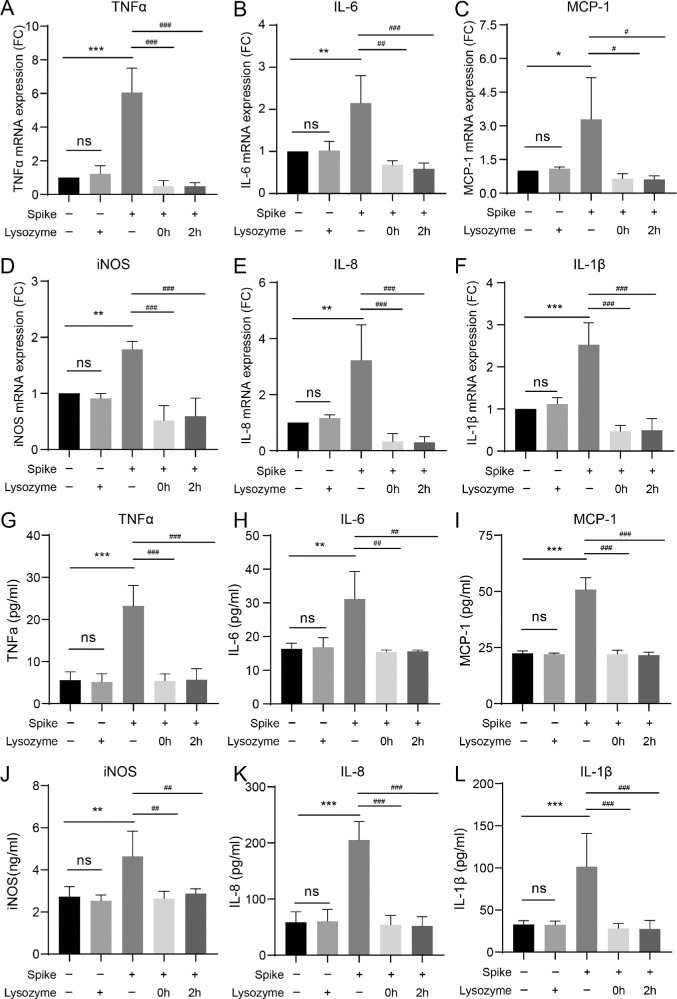
To further evaluate the protective effect of lysozyme on HCECs treated with spike protein, HCECs were treated with 10 mg/mL of lysozyme after stimulation with spike protein. The gene expression levels of proinflammatory cytokines TNF-α (P < 0.0001, P < 0.0001, and P < 0.0001, respectively), IL-6 (P = 0.0088, P = 0.0015, and P = 0.0009, respectively), MCP-1 (P = 0.0467, P = 0.0210, and P = 0.0197, respectively), iNOS (P = 0.0053, P = 0.0001, and P = 0.0002, respectively), IL-8 (P = 0.0061, P = 0.0009, and P = 0.0008, respectively), and IL-1β (P = 0.0004, P < 0.0001, and P < 0.0001, respectively) were significantly decreased after treatment with lysozyme (Figs. 4A–F). To further strengthen the results, ELISA was performed to measure the secreted inflammatory cytokine levels. The results showed that spike protein triggered robust secretion of various inflammatory cytokines, including TNF-α, IL-6, MCP-1, iNOS, IL-8, and IL-1β in HCECs (P < 0.0001, P = 0.0071, P < 0.0001, P = 0.0041, P < 0.0001, and P = 0.0009, respectively; Figs. 4G–L). The levels of proinflammatory cytokines TNF-α, IL-6, MCP-1, iNOS, IL-8, and IL-1β were significantly decreased after treatment with lysozyme (see Figs. 4G–L). Pretreatment with lysozyme in HCECs markedly decreased the inflammatory responses induced by spike proteins.
Figure 4.
Lysozyme attenuates the elevated secretion of inflammatory molecules induced by spike proteins. (A–F) Changes in mRNA expression of various inflammatory cytokines, including TNF-α (A), IL-6 (B), MCP-1 (C), iNOS (D), IL-8 (E), and IL-1β (F) in HCECs pretreated with lysozyme (2 hours or 0 hours before stimulation) detected by RT-PCR. (G–L) Detection of inflammatory cytokines, including TNF-α (G), IL-6 (H), MCP-1 (I), iNOS (J), IL-8 (K), and IL-1β (L) in HCECs pretreated with lysozyme by ELISA. Values are mean ± SD from at least three independent experiments. *P < 0.5; **P < 0.1; ***P < 0.001 versus control group using one-way ANOVA. #P < 0.5; ##P < 0.1; ###P < 0.001 versus spike group using one-way ANOVA.
Lysozyme Protects Against SARS-CoV-2 Infection in Inflammatory Corneal Epithelium Cells
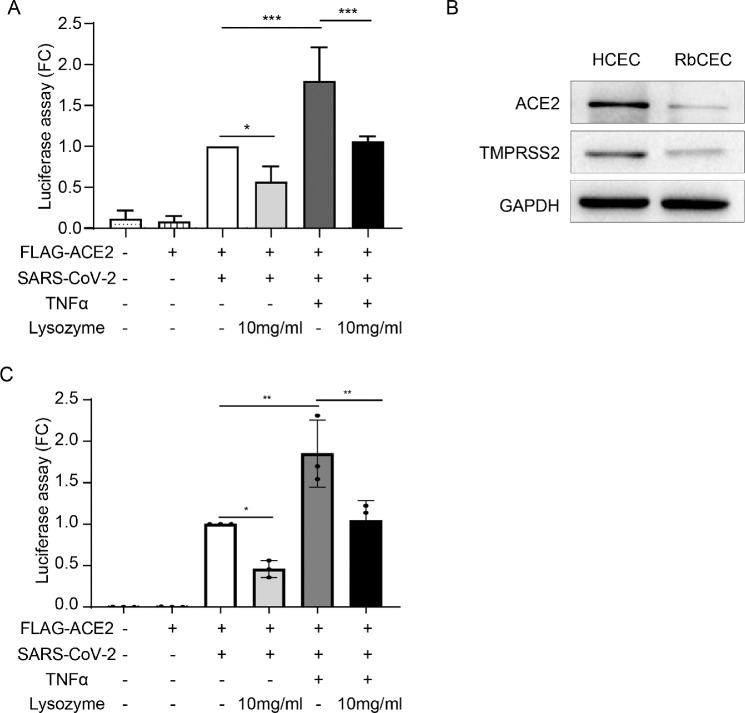
To further explore the protective effect of lysozyme on HCECs, we detected the entry of SARS-CoV-2 into HCECs treated with lysozyme under inflammatory conditions. HCECs were treated with 10 mg/mL lysozyme after TNF-α stimulation, and the cell entry of SARS-CoV-2 was examined using a luciferase assay. Exposure of HCECs to inflammatory cytokines, such as TNF-α, enhanced the entry of SARS-CoV-2 into HCECs, which could be significantly mitigated by pretreatment with lysozyme (P = 0.0488, P = 0.0002, and P = 0.0004, respectively; Fig. 5A). In addition, we have conducted this experiment with RbCECs. The results showed that ACE2 and TMPRSS2 were expressed in primary rabbit corneal epithelial cells (Fig. 5B), although to a lesser extent compared with HCEC cell line. In addition, TNF-α enhanced the entry of SARS-CoV-2 into RbCECs, which could be markedly mitigated by lysozyme (P = 0.0031; Fig. 5C). These data are consistent with the results in HCECs supporting a protective effect of lysozyme in reducing the cell entry of SARS-CoV-2 into corneal epithelial cells.
Figure 5.
Lysozyme attenuates the cell entry of SARS-CoV-2 under inflammatory conditions. (A) Measurement of cell entry by luciferase assay in HCECs treated with control, pseudotyped SARS-CoV-2, ACE2 overexpressing lentivirus, TNF-α (10 ng/mL) and lysozyme (10 mg/mL). (B) Western blot analysis of ACE2 and TMPRSS2 protein expression in HCEC and primary rabbit corneal epithelial cells (RbCECs). (C) Measurement of cell entry by luciferase assay in RbCECs treated with control, pseudotyped SARS-CoV-2, ACE2 overexpressing lentivirus, TNF-α (10 ng/mL) and lysozyme (10 mg/mL). Values are mean ± SD from at least three independent experiments. *P < 0.5; **P < 0.1; ***P < 0.001 versus control group using one-way ANOVA.
Discussion
In this study, we showed that the mRNA and protein levels of ACE2 and TMPRSS2 were highly expressed in HCECs. The lysozyme reduced viral entry into HCECs in a dose-dependent manner. Furthermore, the spike proteins of SARS-CoV-2 stimulated a robust inflammatory response in HCECs characterized by increased secretion of pro-inflammatory molecules, including IL-6, TNF-α, iNOS, and MCP-1, and pretreatment with lysozyme downregulated the inflammatory responses caused by the spike protein. In addition, we also found that the inflammatory cytokine TNF-α enhanced the entry of SARS-CoV-2 into HCECs, which can be mitigated by pretreatment with lysozyme. Our study indicated that lysozyme may be beneficial for protection against the transmission of SARS-CoV-2 through the ocular surface.
ACE2 and TMPRSS2 are required to promote SARS-CoV-2 cell entry and membrane fusion.23,24 Several studies have reported that the expression of ACE2 and TMPRSS2 affects susceptibility to SARS-CoV-2 infection. Zhou et al. found that some patients with COVID-19 showed ocular symptoms and even showed positive RT-PCR results from tears and conjunctival secretions.32 As the main component of the ocular surface, the cornea is more likely to be infected by SARS-CoV-2, considering its high expression of ACE2 and TMPRSS2. Consistent with several previous studies, our results showed that ACE2 and TMPRSS2 were highly expressed in HCECs, suggesting that the cornea is a potential route for SARS-CoV-2 transmission.
Accumulating evidence suggests that an aggressive inflammatory response contributes to the pathophysiology of SARS-CoV-2.33,34 Hyper-inflammation conditions characterized by cytokine storms were observed during SARS-CoV-2 infection in patients with COVID19 and animal models.35,36 In the COVID-19 cytokine storm, this perturbation is initiated by a combination of the SARS-CoV-2 spike protein and ACE2. Consequently, the attachment of the SARS-CoV-2 spike glycoprotein to ACE2 triggers complex downstream axis activation, leading to robust inflammation. Several recent studies introduced purified recombinant spike proteins into in vitro models to better understand how cells respond to virus-host cell interactions.37–39 In the current study, we found that SARS-CoV-2 spike protein triggered robust inflammatory responses on HCECs, which may aggravate SARS-CoV-2 infection in the cornea.
Lysozyme is an antimicrobial protein produced by the accessory tear glands and has already been proven to act against bacteria and viruses. It can kill various bacteria by hydrolyzing peptidoglycan, a major component of the cell wall. Ferrari et al. reported the effects of lysozyme against several viruses, such as herpes simplex and herpes zoster.40 Lee-Huang et al. showed the inhibition of HIV replication by lysozyme.41 In addition, lysozyme is an important regulator of inflammation. Latorre showed that the knockdown of lysozyme in adipose tissue reduces local inflammation and improves adipose tissue metabolic homeostasis.42 The concentration of lysozyme in tears of healthy subjects ranges from 650 µg/mL to 7420 µg/mL.18,43,44 In pathological conditions, such as the external eye infections,18 Sjögren's syndrome,44 herpes simplex virus eye infection,45 smog eye irritation,46,47 rheumatoid arthritis,43 and even severe malnutrition in children,48 the lysozyme content in tears tends to be low. As lysozyme protects the eye against bacterial infection, a low level of lysozyme implies diminished bacteriostatic effect of the tears,44 which makes the eye prone to ocular surface infection. Lysozyme has been used to treat the conjunctivitis and corneal ulcers successfully, and to afford the eye protection from microorganisms, in a 2008 US Patent (US20080213188). In our study, lysozyme exhibited a strong effect in reducing the cell entrance of SARS-CoV-2 and the inflammatory response after viral infection. It markedly decreased the secretion of various inflammatory cytokines, including TNF-α, IL-6, MCP-1, IL-1 β, and iNOS, in HCECs. As TNF-α, IL-6, and IL-1β have been reported to induce ACE2 transcription in multiple cell types and tissues,49,50 the reduction of these cytokine after lysozyme treatment may help elucidate the mechanisms by which lysozyme reduces the cell entry of SARS-CoV-2.
Ocular surface inflammation is an important component of various ocular surface diseases, including dry eye, conjunctivitis, surface damage, and meibomian gland dysfunction.51,52 Dry eyes and conjunctivitis have been observed in patients with COVID-19. TNF-α has been found to increase in animal models and patients with dry eye or conjunctivitis53,54 and are representative cytokines used to study corneal inflammation.49 Therefore, the entry of SARS-CoV-2 into HCECs and the protective effect of lysozyme under inflammatory conditions was examined in this study. We observed that TNF-α enhanced the entry of SARS-CoV-2 into HCECs, and pretreatment with lysozyme effectively reduced this increased entry of SARS-CoV-2. Consistent with this result, elevated expression of ACE2 has been reported in corneas and lung tissue treated with inflammatory cytokines.49,50,55
In conclusion, our work suggests that the cornea could be a potential route for SARS-CoV-2 transmission, and lysozyme may protect against SARS-CoV-2 infection and the consequent inflammatory responses via the ocular surface. Our study highlights the beneficial effect of lysozyme on blocking the entrance of SARS-CoV-2 through the cornea, and application of lysozyme may provide extra protection against infection via the eye especially for those with ocular surface disorders.
Acknowledgments
Supported by Beijing-Tianjin-Hebei Special Project (Grant Number 19JCZDJC64300(Z) and 20JCZXJC00180).
Disclosure: Y. Song, None; H. Zhang, None; Y. Zhu, None: X. Zhao, None; Y. Lei, None; W. Zhou, None; J. Yu, None; X. Dong, None; X. Wang, None; M. Du, None; H. Yan, None
References
- 1. Zhu N, Zhang D, Wang W, et al.. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020; 382: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou P, Yang XL, Wang XG, et al.. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lei C, Qian K, Li T, et al.. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nature Communications. 2020; 11: 2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Röltgen K, Powell AE, Wirz OF, et al.. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020; 5: eabe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu P, Duan F, Luo C, et al.. Characteristics of Ocular Findings of Patients With Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020; 138: 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma C, Nikiforov A, De Geyter N, Dai X, Morent R, Ostrikov KK.. Future antiviral polymers by plasma processing. Prog Polymer Sci. 2021; 118: 101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seah IYJ, Anderson DE, Kang AEZ, et al.. Assessing Viral Shedding and Infectivity of Tears in Coronavirus Disease 2019 (COVID-19) Patients. Ophthalmology. 2020; 127: 977–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xia J, Tong J, Liu M, Shen Y, Guo D.. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020; 92: 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fleming A, Allison VD.. Observations on a Bacteriolytic Substance (“Lysozyme”) Found in Secretions and Tissues. Br J Exp Pathol. 1922; 3: 252–260. [Google Scholar]
- 10. Garreis F, Fau-Gottschalt M, Gottschalt M, Fau-Paulsen FP, Paulsen FP.. Antimicrobial peptides as a major part of the innate immune defense at the ocular surface. Dev Ophthalmol. 2010; 45: 16–22. [DOI] [PubMed] [Google Scholar]
- 11. McDermott AM. Antimicrobial compounds in tears. Exp Eye Res. 2013; 117: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harding CJ, Huwiler SG, Somers H, et al.. A lysozyme with altered substrate specificity facilitates prey cell exit by the periplasmic predator Bdellovibrio bacteriovorus. Nature Communications. 2020; 11: 4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nguyen GTH, Tran TN, Podgorski MN, Bell SG, Supuran CT, Donald WA.. Nanoscale Ion Emitters in Native Mass Spectrometry for Measuring Ligand-Protein Binding Affinities. ACS Central Science. 2019; 5: 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villa TG, Feijoo-Siota L, Rama JLR, Ageitos JM.. Antivirals against animal viruses. Biochem Pharmacol. 2017; 133: 97–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Małaczewska J, Kaczorek-Łukowska E, Wójcik R, Siwicki AK.. Antiviral effects of nisin, lysozyme, lactoferrin and their mixtures against bovine viral diarrhoea virus. BMC Veterinary Res. 2019; 15: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hanstock HG, Edwards JP, Walsh NP.. Tear Lactoferrin and Lysozyme as Clinically Relevant Biomarkers of Mucosal Immune Competence. Front Immunol. 2019; 10: 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDermott AM. Antimicrobial compounds in tears. Exp Eye Res. 2013; 117: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saari KM, Aine E, Posz A, Klockars M.. Lysozyme content of tears in normal subjects and in patients with external eye infections. Graefes Arch Clin Exp Ophthalmol. 1983; 221: 86–88. [DOI] [PubMed] [Google Scholar]
- 19. Narayanan S, Redfern RL, Miller WL, Nichols KK, McDermott AM.. Dry eye disease and microbial keratitis: is there a connection? Ocular Surf. 2013; 11: 75–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee JS, Koh JY, Yi K, et al.. Single-cell transcriptome of bronchoalveolar lavage fluid reveals sequential change of macrophages during SARS-CoV-2 infection in ferrets. Nature Communications. 2021; 12: 4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haabeth OAW, Lohmeyer JJK, Sallets A, et al.. An mRNA SARS-CoV-2 Vaccine Employing Charge-Altering Releasable Transporters with a TLR-9 Agonist Induces Neutralizing Antibodies and T Cell Memory. ACS Central Science. 2021; 7: 1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tseng SH, Lam B, Kung YJ, et al.. A novel pseudovirus-based mouse model of SARS-CoV-2 infection to test COVID-19 interventions. J Biomed Sci. 2021; 28: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chu H, Hu B, Huang X, et al.. Host and viral determinants for efficient SARS-CoV-2 infection of the human lung. Nature Communications. 2021; 12: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen TF, Chang YC, Hsiao Y, et al.. DockCoV2: a drug database against SARS-CoV-2. Nucleic Acids Res. 2021; 49: D1152–D1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rao S, Lau A, So HC.. Exploring Diseases/Traits and Blood Proteins Causally Related to Expression of ACE2, the Putative Receptor of SARS-CoV-2: A Mendelian Randomization Analysis Highlights Tentative Relevance of Diabetes-Related Traits. Diabetes Care. 2020; 43: 1416–1426. [DOI] [PubMed] [Google Scholar]
- 26. Rossi ÁD, de Araújo JLF, de Almeida TB, et al.. Association between ACE2 and TMPRSS2 nasopharyngeal expression and COVID-19 respiratory distress. Sci Rep. 2021; 11: 9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Fan Y, Huang Y, et al.. TRIM28 regulates SARS-CoV-2 cell entry by targeting ACE2. Cellular Signalling. 2021; 85: 110064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petrovszki D, Walter FR, Vigh JP, et al.. Penetration of the SARS-CoV-2 Spike Protein across the Blood-Brain Barrier, as Revealed by a Combination of a Human Cell Culture Model System and Optical Biosensing. Biomedicines. 2022; 10: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suzuki YJ, Gychka SG.. SARS-CoV-2 Spike Protein Elicits Cell Signaling in Human Host Cells: Implications for Possible Consequences of COVID-19 Vaccines. Vaccines. 2021; 9: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patra T, Meyer K, Geerling L, et al.. SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLoS Pathogens. 2020; 16: e1009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petruk G, Puthia M, Petrlova J, et al.. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J Molec Cell Biol. 2020; 12: 916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou Y, Duan C, Zeng Y, et al.. Ocular Findings and Proportion with Conjunctival SARS-COV-2 in COVID-19 Patients. Ophthalmology. 2020; 127: 982–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dettorre G, Diamantis N, Loizidou A, et al.. 319O The systemic pro-inflammatory response identifies cancer patients with adverse outcomes from SARS-CoV-2 infection. Ann Oncol. 2020; 31: S1366. [Google Scholar]
- 34. Boudewijns R, Thibaut HJ, Kaptein SJF, et al.. STAT2 signaling restricts viral dissemination but drives severe pneumonia in SARS-CoV-2 infected hamsters. Nature Communications. 2020; 11: 5838–5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mann ER, Menon M, Knight SB, et al.. Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci Immunol. 2020; 5: eabd6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee JS, Park S, Jeong HW, et al.. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol. 2020; 5: eabd1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jover E, Matilla L, Garaikoetxea M, et al.. Beneficial Effects of Mineralocorticoid Receptor Pathway Blockade against Endothelial Inflammation Induced by SARS-CoV-2 Spike Protein. Biomedicines. 2021; 9: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wrobel AG, Benton DJ, Xu P, et al.. SARS-CoV-2 and bat RaTG13 spike glycoprotein structures inform on virus evolution and furin-cleavage effects. Nat Struct Mol Biol. 2020; 27: 763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bortolotti D, Gentili V, Rizzo S, Rotola A, Rizzo R.. SARS-CoV-2 Spike 1 Protein Controls Natural Killer Cell Activation via the HLA-E/NKG2A Pathway. Cells. 2020; 9: 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ferrari R, Callerio C, Podio G.. Antiviral activity of lysozyme. Nature. 1959; 183: 548. [DOI] [PubMed] [Google Scholar]
- 41. Lee-Huang S, Huang PL, Sun Y, et al.. Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin. Proc Natl Acad Sci USA. 1999; 96: 2678–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Latorre J, Lluch A, Ortega FJ, et al.. Adipose tissue knockdown of lysozyme reduces local inflammation and improves adipogenesis in high-fat diet-fed mice. Pharmacol Res. 2021; 166: 105486. [DOI] [PubMed] [Google Scholar]
- 43. Avisar R, Menaché R, Shaked P, Rubinstein J, Machtey I, Savir H.. Lysozyme content of tears in patients with Sjögren's syndrome and rheumatoid arthritis. Am J Ophthalmol. 1979; 87: 148–151. [DOI] [PubMed] [Google Scholar]
- 44. Berra M, Galperín G, Berra F, et al.. Tear Lysozyme in Sjögren´s syndrome, Meibomian gland dysfunction, and non-dry-eye. Arquivos Brasileiros de Oftalmologia. 2021; 85: 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eylan E, Ronen D, Romano A, Smetana O.. Lysozyme tear level in patients with herpes simplex virus eye infection. Invest Ophthalmol Vis Sci. 1977; 16: 850–853. [PubMed] [Google Scholar]
- 46. Erickson OF, Hatlen R, Berg M.. Industrial tear study; filter-paper electrophoresis of tears, with results of an industrial study from 1,000 specimens sent by mail. Am J Ophthalmol. 1959; 47: 499–508; discussion 508-499. [PubMed] [Google Scholar]
- 47. Okawada N, Mizoguchi I, Ishiguro T.. Effects of photochemical air pollution on the human eye–concerning eye irritation, tear lysozyme and tear pH. Nagoya J Med Sci. 1979; 41: 9–20. [PubMed] [Google Scholar]
- 48. Watson RR, Reyes MA, McMurray DN.. Influence of malnutrition on the concentration of IgA, lysozyme, amylase and aminopeptidase in children's tears. Proc Soc Exp Biol Med. 1978; 157: 215–219. [DOI] [PubMed] [Google Scholar]
- 49. Jiang Z, Zhang H, Gao J, et al.. ACE2 Expression Is Upregulated in Inflammatory Corneal Epithelial Cells and Attenuated by Resveratrol. Invest Ophthalmol Vis Sci. 2021; 62: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smith JC, Sausville EL, Girish V, et al.. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Developmental Cell. 2020; 53: 514–529.e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kang WS, Jung E, Kim J.. Aucuba japonica Extract and Aucubin Prevent Desiccating Stress-Induced Corneal Epithelial Cell Injury and Improve Tear Secretion in a Mouse Model of Dry Eye Disease. Molecules (Basel, Switzerland). 2018; 23: 2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ji H, Zhu Y, Zhang Y, et al.. The Effect of Dry Eye Disease on Scar Formation in Rabbit Glaucoma Filtration Surgery. Int J Mol Sci. 2017; 18: 1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC.. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009; 147: 198–205.e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhu L, Shen J, Zhang C, et al.. Inflammatory cytokine expression on the ocular surface in the Botulium toxin B induced murine dry eye model. Molec Vis. 2009; 15: 250–258. [PMC free article] [PubMed] [Google Scholar]
- 55. Zhuang MW, Cheng Y, Zhang J, et al.. Increasing host cellular receptor-angiotensin-converting enzyme 2 expression by coronavirus may facilitate 2019-nCoV (or SARS-CoV-2) infection. J Med Virol. 2020; 92: 2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]