Abstract
Purpose
We aimed to evaluate the value of [68 Ga]Ga-DOTA-FAPI-04 PET/CT for the diagnosis of recurrent soft tissue sarcoma (STS), compared with [18F]FDG PET/CT.
Methods
A total of 45 patients (21 females and 24 males; median age, 46 years; range, 18–71 years) with 13 subtypes of STS underwent [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 PET/CT examination within 1 week for assessment local relapse or distant metastasis. Positive lesions on PET/CT images were verified by biopsy or 3-month follow-up. Wilcoxon matched-pairs signed-rank test was used to compare the semiquantitative values (SUVmax and TBR) of [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 in tumor lesions, and McNemar test was applied to test for differences of both tracers.
Results
Among the 45 patients, 282 local relapses and distant metastases were identified. Compared to [18F]FDG, [68 Ga]Ga-DOTA-FAPI-04 PET/CT detected more lesions (275 vs. 186) and outperformed in sensitivity, specificity, PPV, NPV, and accuracy for the diagnosis of recurrent lesions (P < 0.001). [68 Ga]Ga-DOTA-FAPI-04 demonstrated significantly higher values of SUVmax and TBR than [18F]FDG PET/CT in liposarcoma (P = 0.011 and P < 0.001, respectively), malignant solitary fibrous tumor (MSFT) (P < 0.001 and P < 0.001, respectively), and interdigitating dendritic cell sarcoma (IDCS) (P < 0.001and P < 0.001, respectively). While mean SUVmax and TBR presented favorable uptake of [18F]FDG over [68 Ga]Ga-DOTA-FAPI-04 in undifferentiated pleomorphic sarcoma (UPS) (P = 0.003 and P < 0.001, respectively) and rhabdomyosarcoma (RMS) (P < 0.001 and P < 0.001, respectively).
Conclusion
[68 Ga]Ga-DOTA-FAPI-04 PET/CT is a promising new imaging modality for recurrent surveillance of STS, and compares favorably with [18F]FDG for identifying recurrent lesions of liposarcoma, MSFT, and IDCS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00259-022-05700-4.
Keywords: FAPI, FDG, Soft tissue sarcoma, PET/CT, SUV
Introduction
Soft tissue sarcomas (STS) are rare and heterogeneous tumors, which contain more than 50 different histologic subtypes according to the World Health Organization (WHO) classification [1]. The prognosis of metastatic STS is dismal, with a median overall survival (OS) of 8–12 months [2]. Thus, optimal imaging of STS is crucial for accurately restaging and detecting local relapse and/or distant metastasis as early and as completely as possible. The most frequent metastatic sites of STS are the lung, followed by bone and lymph nodes [3]. Computed tomography (CT) and magnetic resonance imaging (MRI) serve as the routine means for local relapsed surveillance. But for detecting distant metastasis, [18F]-fluorodeoxyglucose ([18F]FDG) positron emission tomography/computed tomography (PET/CT) shows higher sensitivity and accuracy [4]. Furthermore, [18F]FDG PET/CT is useful for initial staging and restaging, evaluation of treatment response, and predicting treatment efficacy and clinical outcome for STS [5]. However, due to lack of sensitivity among some subtypes of sarcomas, particularly low-grade sarcomas, [18F]FDG PET/CT is not generally recommended for the management of sarcomas [6–8].
Recently, new development of PET tracers targeting fibroblast activation protein (FAP), [68 Ga]-fibroblast activation protein inhibitor (FAPI), had shown promising results in imaging of sarcomas [9]. FAP is a type II membrane-bound glycoprotein belonging to the dipeptidyl peptidase 4 family, which has both dipeptidyl peptidase and endopeptidase activity. FAP plays a pivotal role in tumor microenvironment, including reduced levels of anti-angiogenic factors, elevated levels of transforming growth factor β, and affected matrix processing enzymes [10]. FAP is overexpressed in cancer-associated fibroblasts (CAFs) in the stroma of more than 90% of epithelial carcinomas [11] and many subtypes of STS (e.g., fibrosarcoma, malignant fibrous histiocytoma, and liposarcoma) [12, 13]. In addition to diagnostic imaging, FAP is also considered as a promising target for delivering therapeutic nuclide [14]. This may provide a new approach for recurrent STS to improve survival. Thus, the expression of FAP on different STS needs to be identified.
Inspired by the promising results of [68 Ga]Ga-DOTA-FAPI-04 imaging on many epithelial carcinomas [9, 15, 16], we hypothesized that [68 Ga]Ga-DOTA-FAPI-04 would outperform [18F]FDG in recurrent surveillance of STS. Herein, in this study, we aimed to investigate the potential usefulness of [68 Ga]Ga-DOTA-FAPI-04 PET/CT for the diagnosis of recurrent lesions in patients with STS, compared with [18F]FDG PET/CT. The primary objective of this study was the comparison of uptake of [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 by different histological STS subtypes. Secondary objectives were the comparison by grading and by lesion location.
Methods
Patient selection
This prospective clinical trial (CFFSTS Trial, ChiCTR2100053984, Chinese Clinical Trial Registry) was conducted in Fudan University Shanghai Cancer Center to compare the diagnostic ability of [68 Ga]Ga-DOTA-FAPI-04 and [18F]FDG PET/CT in patients with STS from May 2020. To further investigate the role of [68 Ga]Ga-DOTA-FAPI-04 in recurrent STS, inclusion criteria were as follows: (i) pathologically confirmed STS; (ii) patients were suspected recurrence after radical treatment. The exclusion criteria were (i) patients without recurrence; (ii) patients with two or more malignant tumor history; and (iii) patients unwilling to take [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 PET/CT. Data including demographics, tumor characteristics, and treatment information were collected from the medical records. This prospective study was approved by Fudan University Shanghai Cancer Center Institutional Review Board (ID 2,004,216–25) and conducted in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consents to undergo [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 PET/CT were obtained from all enrolled patients.
Radiopharmaceuticals and PET/CT scanning procedure
[18F]FDG was produced automatically using Explora FDG4 module with cyclotron (Siemens CTI RDS Eclips ST, Knoxville, Tennessee, USA) in our center. DOTA-FAPI-04 was obtained commercially (Jiangsu Huayi Technology CO., LTD, Jiangsu, China). DOTA-FAPI-04 was radiolabeled with 68 Ga according to Lindner et al. [14]. Briefly, the DOTA-FAPI-04 and 68 Ga-solution (elution with 0.5 M HCl) were mixed with sodium acetate, and the pH was maintained about 4.5. Then the reaction mixture was heated to 100 °C for 20 min. The [68 Ga]Ga-DOTA-FAPI-04 was obtained by solid phase extraction. Radiochemical purity of [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 was both over 95%.
[18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 PET/CT scans were performed within 1 week. For [18F]FDG PET/CT scanning, patients fasted at least 6 h, maintaining venous blood glucose levels under 10 mmol/L prior to [18F]FDG administration. But this was not necessary for [68 Ga]Ga-DOTA-FAPI-04 PET/CT scanning. After injecting with 242.62 ± 43.83 MBq of [18F]FDG or 147.69 ± 21.55 MBq of [68 Ga]Ga-DOTA-FAPI-04, patients were kept in a quiet environment for approximately 60 min prior to examination. No adverse or clinically detectable pharmacological effects were observed in any of these patients. All images were obtained on a Biograph mCT Flow scanner (Siemens Medical Solutions). Low-dose CT scanning was performed firstly for location: scanning ranging from the proximal thighs or feet to head, with 120 kV, 100 mAs, CARE Dose4D, slice thickness 3 mm, increment 2 mm, pitch 1.0, rotation time 0.5 s, and soft-tissue reconstruction kernel. Immediately after CT scanning, a PET emission scan that covered the corresponding field of CT was acquired in 3-dimensional mode using FlowMotion with a speed of 2. The emission data were corrected for random scatter and decay. PET image datasets were reconstructed iteratively using an ordered-subset expectation maximization iterative reconstruction by applying CT data for attenuation correction. Fusion images were reviewed and manipulated on a multimodality computer platform (Syngo, Siemens, Knoxville, Tennessee, USA). Two experienced nuclear medicine physicians analyzed and interpreted the images independently, and they reached a consensus in case of inconsistency.
Increased radioactivity of relapsed or metastatic lesions compared with the uptake of surrounding normal tissue was defined as being positive, verified by biopsy or 3-month follow-up. Lesions were considered malignant during follow-up based on (i) typical malignant features (i.e., mass, abnormal density, poor circumscription, and destruction), and (ii) a significant reduction or progression in size after anticancer treatment confirmed by follow-up imaging (i.e., CT and MRI) according to RECIST 1.1 [17]. For quantitative analysis, maximum and mean of standardized uptake value (SUV) normalized to body weight were manually computed for tumor lesions and healthy tissues by drawing a 3-dimensional volume of interest, respectively. Meanwhile, tumor-to-background ratio (TBR) for tumor lesions was calculated according to the formula: TBR = tSUVmax/bSUVmean, where tSUVmax is the maximum SUV of tumor lesion, and bSUVmean is the mean SUV of normal tissue.
Statistical analyses
All statistical analyses were performed using SPSS 25.0 (IBM, Armonk, NY, USA). Mean with standard deviation or median with range was used to describe continuous characteristics. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 were determined, and McNemar test was applied to test for differences of both tracers. To compare the semiquantitative values (SUVmax and TBR) of [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 in tumor lesions, Wilcoxon matched-pairs signed-rank test was used. Two-tailed p < 0.05 was considered statistically significant.
Results
Patients
From May 2020 to May 2021, 45 patients (21 females and 24 males; median age = 46 years, range, 18–71 years) were consecutively enrolled in this study (Fig. 1). All patients were diagnosed with STS and got radical treatment (e.g., surgery, radiotherapy, chemotherapy, or combination therapy) before PET/CT scans. Diagnostic CT or MRI was performed in 30 out of 45 patients prior to PET/CT scans, and positive findings were observed in 29 patients (Table 1).
Fig. 1.
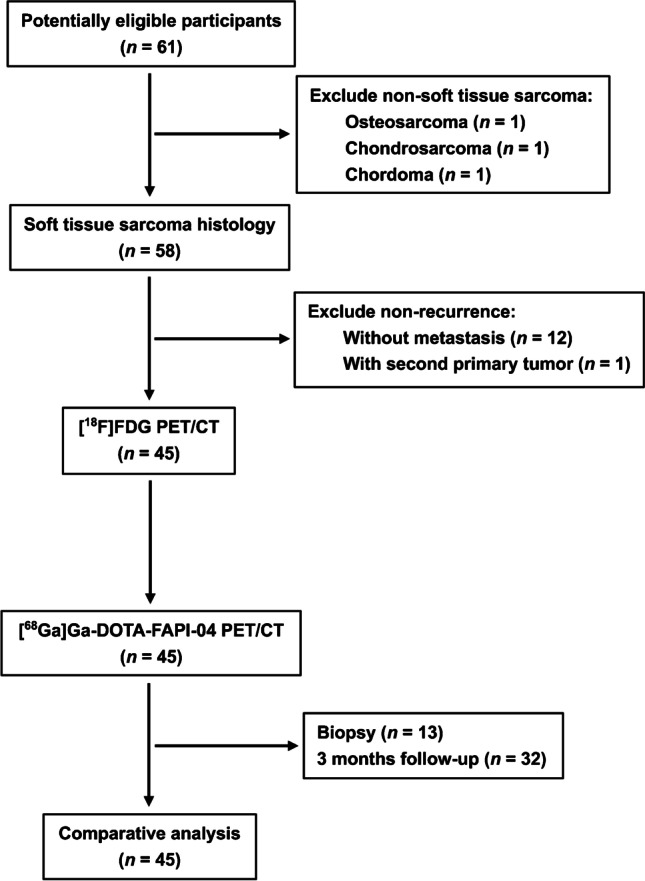
Flowchart of patient selection
Table 1.
Detailed clinical characteristics of the patients
| Patient | Gender | Age | Histology | Initial treatment | Diagnostic CT/MRI before PET/CT scan* | Restaging | ||
|---|---|---|---|---|---|---|---|---|
| Localization | Grading | FDG | FAPI-04 | |||||
| 1 | F | 51 | UPS | Thigh | G3 | CT + | LR + DM | LR + DM |
| 2 | M | 58 | UPS | Retroperitoneum | G3 | / | LR + DM | LR + DM |
| 3 | M | 71 | UPS | Retroperitoneum | G3 | CT + | LR + DM | LR + DM |
| 4 | M | 53 | UPS | Neck | G3 | MRI + | LR | LR |
| 5 | F | 46 | UPS | Small intestine | G3 | / | LR | LR |
| 6 | M | 63 | UPS | Retroperitoneum | G3 | / | LR + DM | LR + DM |
| 7 | M | 22 | UPS | Inguinal | G3 | / | LR | LR |
| 8 | F | 64 | Well-differentiated liposarcoma | Retroperitoneum | G1 | CT + | LR + DM | LR + DM |
| 9 | F | 59 | Pleomorphic liposarcoma | Lower leg | G3 | CT + | LR + DM | LR + DM |
| 10 | M | 67 | Well-differentiated liposarcoma | Retroperitoneum | G1 | / | LR | LR |
| 11 | M | 70 | Dedifferentiated liposarcoma | Thigh | G3 | / | DM | DM |
| 12 | F | 39 | Dedifferentiated liposarcoma | Retroperitoneum | G3 | CT + | LR | LR |
| 13 | F | 36 | Well-differentiated liposarcoma | Retroperitoneum | G1 | MRI + | Non-recurrence | LR |
| 14 | M | 34 | Synovial sarcoma | Postmediastinum | G3 | CT + | LR | LR |
| 15 | F | 55 | Synovial sarcoma | Kidney | G3 | / | LR + DM | LR + DM |
| 16 | M | 23 | Synovial sarcoma | Lung | G3 | CT + | LR | LR |
| 17 | M | 38 | Synovial sarcoma | Abdomen | G3 | MRI + | LR | LR |
| 18 | F | 65 | Synovial sarcoma | Lower leg | G3 | CT + | LR | LR |
| 19 | M | 41 | Synovial sarcoma | Kidney | G3 | MRI + | LR | LR + DM |
| 20 | M | 20 | RMS | Cheek | G3 | CT + | DM | DM |
| 21 | F | 29 | RMS | Perineum | G3 | MRI + | LR + DM | LR + DM |
| 22 | F | 19 | RMS | Nasal cavity | G3 | CT - | DM | DM |
| 23 | M | 25 | RMS | Nasal cavity | G3 | MRI + | DM | DM |
| 24 | F | 18 | RMS | Perianal region | G3 | MRI + | LR + DM | LR + DM |
| 25 | M | 47 | MSFT | Thigh | G2 | CT + | LR + DM | LR + DM |
| 26 | M | 69 | MSFT | Retroperitoneum | Unknown | / | LR | LR + DM |
| 27 | M | 49 | MSFT | Neck | G1 | / | DM | DM |
| 28 | F | 65 | MSFT | Uterus | G2 | CT + | LR + DM | LR + DM |
| 29 | M | 19 | Ewing sarcoma | Thigh | G3 | MRI + | LR | LR |
| 30 | M | 20 | Ewing sarcoma | Buttock | G3 | / | DM | DM |
| 31 | M | 25 | Ewing sarcoma | Pelvic cavity | G3 | / | LR | LR |
| 32 | F | 29 | Ewing sarcoma | Perianal region | G3 | MRI + | LR | LR |
| 33 | F | 60 | Leiomyosarcoma | Uterus | Unknown | CT + | DM | DM |
| 34 | F | 55 | Leiomyosarcoma | Uterus | Unknown | CT + | DM | DM |
| 35 | F | 47 | Leiomyosarcoma | Retroperitoneum | G2 | / | LR + DM | LR + DM |
| 36 | F | 51 | Leiomyosarcoma | Uterus | G2 | MRI + | DM | DM |
| 37 | M | 28 | Myxofibrosarcoma | Shoulder | G3 | CT + | DM | DM |
| 38 | M | 46 | Myxofibrosarcoma | Waist | G2 | / | DM | DM |
| 39 | M | 33 | Myxofibrosarcoma | Buttock | G1 | / | LR | LR |
| 40 | F | 47 | ASPS | Small intestine | G3 | MRI + | LR + DM | LR + DM |
| 41 | F | 19 | ASPS | Abdomen | G3 | MRI + | LR + DM | LR + DM |
| 42 | F | 66 | Epithelioid sarcoma | Inguinal | G3 | / | LR | LR |
| 43 | M | 23 | Aggressive fibromatosis | Pelvic cavity | Unknown | CT + | LR | LR |
| 44 | F | 44 | FDCS | Liver | Unknown | CT + | LR + DM | LR + DM |
| 45 | M | 48 | IDCS | Liver | Unknown | CT + | Non-recurrence | LR + DM |
* “ + ” means diagnostic CT/MRI could detect the relapsed or metastatic lesions, and “-” indicates diagnostic CT/MRI could not detect the relapsed or metastatic lesions. F, female; M, male; UPS, undifferentiated pleomorphic sarcoma; RMS, rhabdomyosarcoma; MSFT, malignant solitary fibrous tumor; ASPS, alveolar soft part sarcoma; FDCS, follicular dendritic cell sarcoma; IDCS, interdigitating dendritic cell sarcoma; LR, local relapse; DM, distant metastasis
Comparison of [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 PET/CT based on different subtypes of recurrent STS
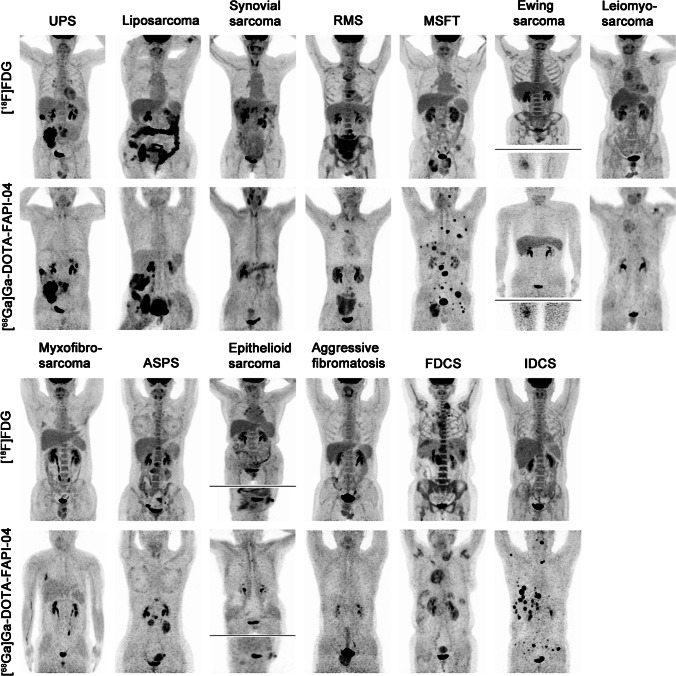
The representative figures of the 13 subtypes of recurrent STS are presented in Fig. 2. Overall, 282 local relapses and distant metastases were identified among the 45 patients. Among these lesions, 13 were verified by biopsy and 269 were assessed by follow-up imaging. Interestingly, [68 Ga]Ga-DOTA-FAPI-04 PET/CT led to upstaging in 4 out of 45 (8.89%) patients compared with [18F]FDG PET/CT (Table 1). In terms of different subtypes of recurrent STS, liposarcoma (Fig. 3), malignant solitary fibrous tumor (MSFT, Fig. 4), and interdigitating dendritic cell sarcoma (IDCS, Fig. 5) showed elevated uptake of [68 Ga]Ga-DOTA-FAPI-04, and demonstrated significantly higher semiquantitative values of SUVmax and TBR than [18F]FDG (P = 0.011, < 0.001, and < 0.001 for SUVmax, respectively; P < 0.001, < 0.001, and < 0.001 for TBR, respectively; Table 2 and Table S1). Whereas, mean SUVmax and TBR presented favorable uptake of [18F]FDG over [68 Ga]Ga-DOTA-FAPI-04 in undifferentiated pleomorphic sarcoma (UPS) and rhabdomyosarcoma (RMS) (P = 0.003 and < 0.001 for SUVmax, respectively; P < 0.001 and < 0.001 for TBR, respectively; Table 2 and Table S1). For the other eight subtypes of recurrent STS, [68 Ga]Ga-DOTA-FAPI-04 had similar performance in assessing local relapse and distant metastasis with [18F]FDG PET/CT.
Fig. 2.
MIP images of [18F]FDG PET/CT and [68 Ga]Ga-DOTA-FAPI-04 PET/CT in patients reflecting 13 different representative recurrent STS entities. MIP, maximum-intensity projection; STS, soft tissue sarcoma; UPS, undifferentiated pleomorphic sarcoma; RMS, rhabdomyosarcoma; MSFT, malignant solitary fibrous tumor; ASPS, alveolar soft part sarcoma; FDCS, follicular dendritic cell sarcoma; IDCS, interdigitating dendritic cell sarcoma
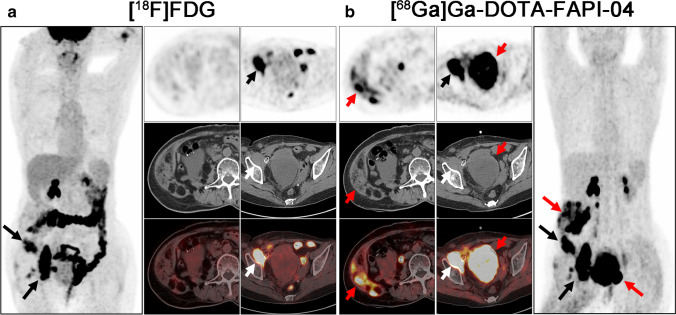
Fig. 3.
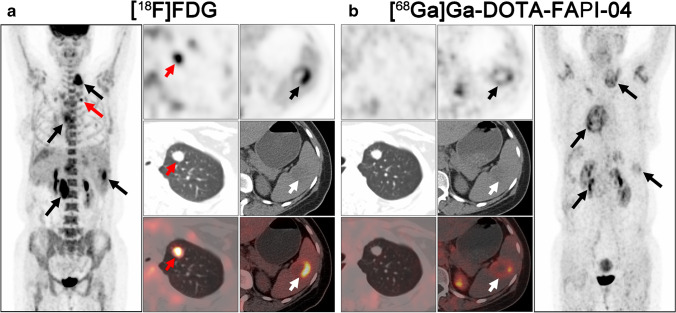
A 64-year-old woman (patient #8) pathologically confirmed with retroperitoneal well-differentiated liposarcoma received radical operation 4 years ago. [18F]FDG PET/CT (a) demonstrated pelvic wall metastatic foci with intensive metabolic activity. But the abdominal wall foci and large pelvic metastatic foci showed no intensive uptake of [18F]FDG. Compared with [18F]FDG, [68 Ga]Ga-DOTA-FAPI-04 PET/CT (b) detected all the metastatic lesions with intense [68 Ga]Ga-DOTA-FAPI-04 activity. Black and white arrows indicated the tumor lesions detected by both tracers, and red arrows indicated the tumor lesions detected by [68 Ga]Ga-DOTA-FAPI-04 alone
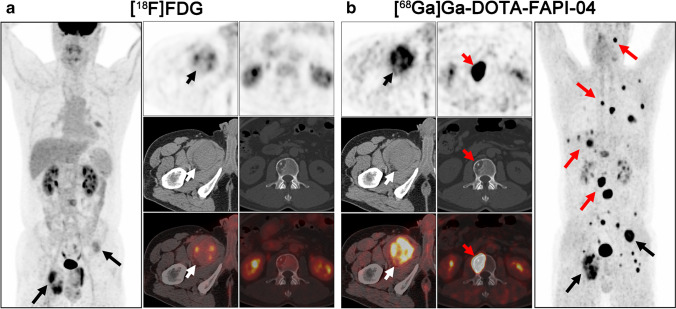
Fig. 4.
A 47-year-old man (patient #25) pathologically confirmed with malignant solitary fibrous tumor (MSFT) arising from right the thigh received radical operation 1 year ago. [18F]FDG PET/CT (a) demonstrated the relapse of right thigh and some bone metastases with low metabolic activity. Compared with [18F]FDG, [68 Ga]Ga-DOTA-FAPI-04 PET/CT (b) demonstrated more metastases, including lung, bone, and liver metastases. Moreover, all the relapse and metastases showed intensive uptake of [68 Ga]Ga-DOTA-FAPI-04. Black and white arrows indicated the tumor lesions detected by both tracers, and red arrows indicated the tumor lesions detected by [68 Ga]Ga-DOTA-FAPI-04 alone
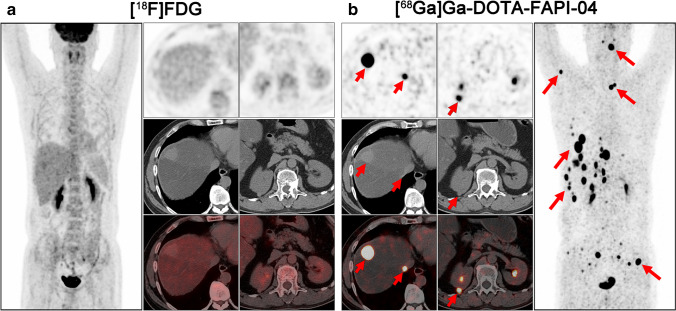
Fig. 5.
A 48-year-old man (patient #45) pathologically confirmed with liver interdigitating dendritic cell sarcoma (IDCS) received radical operation 2 years ago. [18F]FDG PET/CT (a) demonstrated none of the [18F]FDG-avid lesions. Interestingly, [68 Ga]Ga-DOTA-FAPI-04 PET/CT (b) demonstrated all the metastatic lesions with intense [68 Ga]Ga-DOTA-FAPI-04 activity, including liver, right kidney, and bone metastases. Red arrows indicated the tumor lesions detected by [68 Ga]Ga-DOTA-FAPI-04 alone
Table 2.
Comparison of SUVmax detected on [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 PET/CT in different subtypes of recurrent STS
| Histology | No. of patients | [18F]FDG | [68 Ga]Ga-DOTA-FAPI-04 | P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *Mean SUVmax | SD | Range | 95% CI | No. of lesions | *Mean SUVmax | SD | Range | 95% CI | No. of lesions | ||||||
| UPS | 7 | 10.81 | 4.94 | 2.20–27.49 | 9.49, 12.14 | 56 | 9.04 | 5.76 | 1.40–37.90 | 7.50, 10.58 | 56 | 0.003 | |||
| Liposarcoma | 6 | 8.08 | 8.22 | 1.60–22.65 | 6.67, 9.49 | 41 | 11.98 | 9.99 | 1.65–47.50 | 9.02, 14.95 | 46 | 0.011 | |||
| Synovial sarcoma | 6 | 6.68 | 3.74 | 1.60–15.20 | 4.17, 9.19 | 11 | 4.57 | 2.18 | 2.70–10.20 | 3.18, 6.00 | 12 | 0.339 | |||
| RMS | 5 | 6.89 | 2.09 | 2.80–12.40 | 6.08, 7.70 | 28 | 4.61 | 1.80 | 2.10–8.20 | 3.93, 5.30 | 29 | < 0.001 | |||
| MSFT | 4 | 4.61 | 1.66 | 2.57–8.07 | 3.50, 5.72 | 11 | 15.06 | 13.32 | 2.07–78.48 | 11.55, 18.56 | 58 | < 0.001 | |||
| Ewing sarcoma | 4 | 3.68 | 1.84 | 2.40–6.40 | 0.75, 6.60 | 4 | 4.78 | 3.44 | 2.60–9.90 | − 0.69, 10.24 | 4 | 0.250 | |||
| Leiomyosarcoma | 4 | 4.03 | 0.90 | 2.90–5.10 | 3.28, 4.79 | 8 | 4.01 | 1.18 | 2.10–5.40 | 3.03, 5.00 | 8 | 0.922 | |||
| Myxofibrosarcoma | 3 | 3.64 | 0.45 | 3.21–4.10 | 2.53, 4.75 | 3 | 4.97 | 3.15 | 3.00–8.60 | − 2.86, 12.79 | 3 | 0.999 | |||
| ASPS | 2 | 5.56 | 1.75 | 2.90–8.00 | 4.10, 7.03 | 8 | 6.11 | 2.45 | 2.30–9.40 | 4.07, 8.16 | 8 | 0.742 | |||
| Epithelioid sarcoma | 1 | 12.90 | / | / | / | 1 | 6.70 | / | / | / | 1 | / | |||
| Aggressive fibromatosis | 1 | 2.95 | 0.53 | 2.50–3.50 | 2.11, 3.79 | 4 | 7.30 | 0.62 | 6.50–8.00 | 6.32, 8.28 | 4 | 0.125 | |||
| FDCS | 1 | 5.70 | 3.20 | 1.90–10.70 | 3.55, 7.85 | 11 | 4.33 | 1.88 | 2.30–8.00 | 2.89, 5.78 | 9 | 0.123 | |||
| IDCS | 1 | / | / | / | / | 0 | 31.53 | 29.58 | 4.90–119.30 | 21.66, 41.39 | 37 | < 0.001 | |||
| Low grade (G1) | 5 | 4.88 | 2.60 | 2.20–8.98 | 2.71, 7.06 | 8 | 13.49 | 8.56 | 2.50–28.60 | 9.09, 17.89 | 17 | < 0.001 | |||
| High grade (G2 + G3) | 34 | 8.29 | 4.51 | 1.60–27.49 | 7.58, 9.00 | 158 | 9.45 | 9.51 | 1.40–78.48 | 8.12, 10.79 | 198 | 0.044 | |||
*SUVmax for only one lesion. STS, soft tissue sarcoma; SD, standard deviation; CI, confidence intervals; UPS, undifferentiated pleomorphic sarcoma; RMS, rhabdomyosarcoma; MSFT, malignant solitary fibrous tumor; ASPS, alveolar soft part sarcoma; FDCS, follicular dendritic cell sarcoma; IDCS, interdigitating dendritic cell sarcoma
Regarding the different grades, both low-grade and high-grade STS showed significantly higher uptake of [68 Ga]Ga-DOTA-FAPI-04 than [18F]FDG (P < 0.001 and = 0.044 for SUVmax, respectively; P < 0.001 and = 0.023 for TBR, respectively; Table 2 and Table S1). Moreover, [18F]FDG uptake was higher for high-grade STS compared to low-grade STS. Conversely, low-grade STS showed higher uptake of [68 Ga]Ga-DOTA-FAPI-04 than high-grade STS.
Comparison of [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 PET/CT based on different tissues and organs
The metastatic lesions were identified in the soft tissues (including muscle, pleura, and peritoneum), lung (Fig. 6), liver, bone, lymph node, spleen, pancreas, and kidney. Regarding different tissues and organs, soft tissues, liver, and bone metastases revealed intensive uptake of [68 Ga]Ga-DOTA-FAPI-04 and presented significantly higher semiquantitative values of SUVmax and TBR than [18F]FDG (P < 0.001, < 0.001, and < 0.001 for SUVmax, respectively; P = 0.015, < 0.001, and < 0.001 for TBR, respectively; Table 3 and Table S2). Whereas, mean SUVmax and TBR presented favorable uptake of [18F]FDG over [68 Ga]Ga-DOTA-FAPI-04 in lymph node metastases (mean SUVmax = 8.57 ± 4.45 vs. 6.26 ± 4.06, P = 0.006; mean TBR = 11.71 ± 3.39 vs. 8.08 ± 3.95, P = 0.011; Table 3 and Table S2).
Fig. 6.
A 44-year-old woman (patient #44) pathologically confirmed with liver follicular dendritic cell sarcoma (FDCS) received radical operation 6 months ago. [18F]FDG PET/CT (a) demonstrated all the metastatic lesions with intense [18F]FDG activity, including liver, spleen, lung, and lymph node metastases. Compared with [18F]FDG, [68 Ga]Ga-DOTA-FAPI-04 PET/CT (b) demonstrated the liver, spleen, and lymph node metastases with moderate [68 Ga]Ga-DOTA-FAPI-04 activity. But no intensive [68 Ga]Ga-DOTA-FAPI-04 uptake was observed on the lung metastatic lesions. Black and white arrows indicated the tumor lesions detected by both tracers, and red arrows indicated the tumor lesions detected by [18F]FDG alone
Table 3.
Comparison of SUVmax detected on [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 PET/CT in different tissues and organs
| Tissues and organs | Total lesions | [18F]FDG | [68 Ga]Ga-DOTA-FAPI-04 | P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *Mean SUVmax | SD | Range | 95% CI | No. of lesions | *Mean SUVmax | SD | Range | 95% CI | No. of lesions | ||||||
| Soft tissues | 80 | 7.35 | 5.31 | 1.60–25.20 | 6.07, 8.62 | 69 | 10.74 | 9.38 | 2.10–47.50 | 8.66, 12.83 | 80 | < 0.001 | |||
| Lung | 29 | 7.47 | 3.87 | 2.90–16.98 | 5.80, 9.14 | 23 | 9.09 | 8.03 | 1.40–34.07 | 5.53, 12.65 | 22 | 0.696 | |||
| Liver | 35 | 6.73 | 3.06 | 2.30–11.97 | 4.67, 8.79 | 11 | 21.60 | 27.73 | 2.70–119.30 | 12.07, 31.12 | 35 | < 0.001 | |||
| Bone | 116 | 8.47 | 4.27 | 2.57–27.49 | 7.40, 9.54 | 63 | 13.03 | 15.61 | 2.40–90.30 | 10.16, 15.91 | 116 | < 0.001 | |||
| Lymph node | 16 | 8.57 | 2.45 | 4.90–12.40 | 7.22, 9.93 | 15 | 6.26 | 4.06 | 2.30–19.65 | 4.10, 8.42 | 16 | 0.006 | |||
| Spleen | 3 | 4.30 | 2.82 | 1.90–7.40 | − 2.70, 11.30 | 3 | 3.70 | 1.04 | 2.50–4.40 | 1.11, 6.29 | 3 | 0.999 | |||
| Pancreas | 2 | 7.97 | / | 7.50–8.43 | / | 2 | 5.80 | / | 3.30–8.30 | / | 2 | / | |||
| Kidney | 1 | / | / | / | / | 0 | 25.40 | / | / | / | 1 | / | |||
| Sum | 282 | 7.76 | 4.45 | 1.60–27.49 | 7.12, 8.41 | 186 | 12.64 | 15.67 | 1.40–119.30 | 10.78, 14.50 | 275 | < 0.001 | |||
*SUVmax for only one lesion. SD, standard deviation; CI, confidence intervals
[68 Ga]Ga-DOTA-FAPI-04 outperformed [18F]FDG PET/CT in terms of the sensitivity, specificity, PPV, NPV, and accuracy for the diagnosis of recurrent lesions (P < 0.001, Table 4), and demonstrated significantly higher values of SUVmax and TBR (mean SUVmax = 12.64 ± 15.67 vs. 7.76 ± 4.45, P < 0.001; mean TBR = 19.03 ± 31.95 vs. 10.23 ± 6.62, P < 0.001).
Table 4.
Lesion-based statistical analysis of diagnostic performance in [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 PET/CT
| Tracer | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) |
|---|---|---|---|---|---|
| [18F]FDG | 65.96 (60.25–71.24) | 21.43 (10.21–39.54) | 89.42 (84.51–92.91) | 5.88 (2.72–12.24) | 61.94 (56.42–67.16) |
| [68 Ga]Ga-DOTA-FAPI-04 | 97.52 (94.97–98.79) | 60.71 (42.41–76.43) | 96.15 (93.25–97.84) | 70.88 (50.83–85.09) | 94.19 (91.01–96.30) |
The data was presented as percentage (%). STS, soft tissue sarcoma; CI, confidence intervals; PPV, positive predictive value; NPV, negative predictive value
Discussion
This prospective study of 45 patients with 13 subtypes of recurrent STS suggests that [68 Ga]Ga-DOTA-FAPI-04 PET/CT is a promising new imaging modality for recurrent surveillance of STS and provides an enhancement to [18F]FDG PET/CT.
STS represents a distinct group of rare malignant tumors with high heterogeneity, which remains a major concern in cancer management [18–20]. Several previous studies [4, 21, 22] reported the usefulness of [18F]FDG PET/CT in the diagnosis of primary sarcomas, particularly in high-grade sarcomas. Nevertheless, various subtypes of STS exhibiting a spectrum of atypical imaging appearances may lead to challenge in recurrent surveillance of STS by [18F]FDG PET/CT [8]. In a prospective trial of 41 patients with clinically suspected tumor relapse of STS, [18F]FDG PET/MRI demonstrated higher sensitivity and accuracy and lower specificity for the detection of local tumor recurrence than MRI alone (95.0% vs. 80.0%, 89.5% vs. 80.7%, and 76.5% vs. 82.4%, respectively) [23]. However, in the present study, [18F]FDG PET/CT detected approximately two-thirds of recurrent lesions with a sensitivity of 65.96%, a specificity of 21.43%, and an accuracy of 61.94% in the 13 subtypes of STS. Compared to [18F]FDG, [68 Ga]Ga-DOTA-FAPI-04 PET/CT identified almost all lesions (275/282) and presented significant improved sensitivity, specificity, and accuracy (97.52%, 60.71%, and 95.15%, respectively; P < 0.001). Furthermore, high PPV for [68 Ga]Ga-DOTA-FAPI-04 PET/CT was also observed in the present study, which is consistent with the results from Kessler et al. (96.15% vs. 97%) [24]. Moreover, the higher sensitivity and accuracy of [68 Ga]Ga-DOTA-FAPI-04 over [18F]FDG PET/CT could bring benefit in accurately restaging (upstaging in 4 out of 45 patients in the present study and 8 out of 43 patients in Kessler et al. study) and guiding the treatment decision in recurrent STS [24]. Prominent higher uptake of [68 Ga]Ga-DOTA-FAPI-04 than [18F]FDG was observed in recurrent lesions of STS in terms of mean SUVmax and TBR (P < 0.001), which is in line with previous studies [9, 25]. It should be noted that the intensive uptake of [68 Ga]Ga-DOTA-FAPI-04 presenting in wound healing, uterus, arthritis, and periodontitis may be misdiagnosed as local relapse or distant metastasis. This is caused by fibrotic activity in these conditions [26]. Thus, more researches focused on non-tumor–specific uptake of FAPI are still needed [27, 28].
In a recent study, Koerber el al. [25] reported the imaging of seven subtypes of bone and soft tissue sarcoma by FAPI-PET/CT in fifteen patients, demonstrating the high uptake of FAPI for high-grade sarcomas and low uptake for low-grade sarcomas. However, the patient cohort and tumor subtype are small, and the compounds of FAPI are variance (FAPI-04, FAPI-46, and FAPI-74). These factors may result in data bias. In line with previous studies [4, 21], higher [18F]FDG uptake was also observed in high-grade STS compared to low-grade STS in the present study. However, [68 Ga]Ga-DOTA-FAPI-04 uptake was lower in high-grade STS than low-grade STS (mean SUVmax = 9.45 vs. 13.49) in the present study, which is not consistent with Koerber’s research [25]. This may be caused by that the most cases included in the low-grade group were well-differentiated liposarcoma (3/5) and MSFT (1/5), which showed prominent expression of FAP on tumor cell surface [12]. Nevertheless, a significantly higher uptake of [68 Ga]Ga-DOTA-FAPI-04 was found for high-grade STS compared to [18F]FDG (mean SUVmax = 9.45 vs. 8.29, P = 0.044), indicating the potential role of [68 Ga]Ga-DOTA-FAPI-04 PET/CT in detecting recurrent lesions with distinct visual discrimination regardless of tumor grade.
With respect to the diagnostic performance of different relapsed and metastatic lesions, [68 Ga]Ga-DOTA-FAPI-04 PET/CT outperformed [18F]FDG PET/CT in soft tissues, liver, bone, and kidney metastases with improved tumor retention and low background uptake. This advantage of [68 Ga]Ga-DOTA-FAPI-04 PET/CT over [18F]FDG PET/CT was also demonstrated in many other types of cancer, including head and neck cancers [29], hepatic carcinoma [30], and gastrointestinal cancers [31]. The absence of [68 Ga]Ga-DOTA-FAPI-04 in normal organs and tissues (e.g., liver, bone, and intestines) will benefit imaging of liver, bone, and abdomen metastases with higher tumor-to-background contrast and better lesion delineation than [18F]FDG PET/CT. However, it should be noted that [68 Ga]Ga-DOTA-FAPI-04 was false negative in 7 out of 29 lung metastases (4 from patient #34 and 3 from patient #44, Fig. 6). In a recent animal-based study, Ding et al. [32] found that the expression of FAP was prominent in lung metastatic lesion at the early stage but descended during the progress of tumor metastasis. Thus, the diagnostic performance of [68 Ga]Ga-DOTA-FAPI-04 PET in detecting lung metastasis remains uncertain in recurrent STS.
Despite advances in chemotherapy, targeted therapy and immunotherapy over the last decades, the prognosis for patients with metastatic STS remains poor [33]. Limited options with clinical efficacy for the metastatic or local advanced STS existed in addition to standard treatment [2]. Recently, Kratochwil et al. [34] reported a case of metastatic sarcoma treated with 90Y/153Sm-labeled FAPI-46 achieving stable disease for 8 months. Moreover, Ferdinandus et al. [35] demonstrated the potential role of FAP–targeted radioligand therapy in a study of nine patients with solid tumors. Surprisingly, disease control was observed in three patients with sarcomas and one patient with pancreatic ductal adenocarcinoma. These studies indicated that FAP–targeted radioligand therapy may present as a novel promising treatment strategy for incurable recurrent STS. Thus, non-invasive selection of the suitable patients with STS for the coming FAP–targeted radioligand therapy will emerge as a critical issue, and our work serves as a foundation for that.
The major limitation of this study is the relatively low number of patients and limited subtypes of STS. As STS is a large group of malignant tumors, it is hard to enroll all subtypes in a single center. Thus, larger multi-center studies containing more subtypes of STS are still needed to be carried out in the future. Another limitation is that not all [68 Ga]Ga-DOTA-FAPI-04 and [18F]FDG positive lesions are pathologically confirmed and examined FAP expression. Nevertheless, these lesions are also verified by continuous follow-up. Furthermore, a positive correlation with [68 Ga]Ga-DOTA-FAPI-04 uptake and FAP expression is reported in previous study [30].
Conclusion
The current study demonstrated that [68 Ga]Ga-DOTA-FAPI-04 PET/CT is a promising new imaging modality for recurrent surveillance of STS regardless of tumor grade. [68 Ga]Ga-DOTA-FAPI-04 compared favorably with [18F]FDG PET/CT for identifying recurrent lesions of liposarcoma, MSFT, and IDCS. For UPS and RMS, [18F]FDG showed a superior diagnostic efficacy than [68 Ga]Ga-DOTA-FAPI-04 PET/CT. Moreover, [68 Ga]Ga-DOTA-FAPI-04 had similar performance in assessing recurrent surveillance of synovial sarcoma, Ewing sarcoma, leiomyosarcoma, myxofibrosarcoma, alveolar soft part sarcoma (ASPS), aggressive fibromatosis, and follicular dendritic cell sarcoma (FDCS) with [18F]FDG PET/CT. The clinical value of FAP–targeted radioligand therapy in recurrent STS should be further investigated.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was funded by the National Key Research and Development Program of China (Grant number 2020YFA0909000), National Natural Science Foundation of China (Grant number 81771861, 81971648, and 81901778), and Shanghai Anticancer Association Program (Grant number HYXH2021004).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
All procedures involving human participants were carried out in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any experiments with animals.
Consent to participate
Informed consents were obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Oncology—Muskoskeletal
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bingxin Gu and Xin Liu contributed equally to this work.
Contributor Information
Zhiguo Luo, Email: luozhiguo88@163.com.
Shaoli Song, Email: shaoli-song@163.com.
References
- 1.WHO Classification of Tumors Editorial Board . WHO classification of tumours of soft tissue and bone. 5. Lyon, France: IARC Press; 2020. [Google Scholar]
- 2.Comandone A, Petrelli F, Boglione A, Barni S. Salvage therapy in advanced adult soft tissue sarcoma: a systematic review and meta-analysis of randomized trials. Oncologist. 2017;22(12):1518–1527. doi: 10.1634/theoncologist.2016-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: an update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70(3):200–229. doi: 10.3322/caac.21605. [DOI] [PubMed] [Google Scholar]
- 4.Macpherson RE, Pratap S, Tyrrell H, Khonsari M, Wilson S, Gibbons M, et al. Retrospective audit of 957 consecutive (18)F-FDG PET-CT scans compared to CT and MRI in 493 patients with different histological subtypes of bone and soft tissue sarcoma. Clin Sarcoma Res. 2018;8:9. doi: 10.1186/s13569-018-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ha SC, Oh JS, Roh JL, Moon H, Kim JS, Cho KJ, et al. Pretreatment tumor SUVmax predicts disease-specific and overall survival in patients with head and neck soft tissue sarcoma. Eur J Nucl Med Mol Imaging. 2017;44(1):33–40. doi: 10.1007/s00259-016-3456-8. [DOI] [PubMed] [Google Scholar]
- 6.Salaün PY, Abgral R, Malard O, Querellou-Lefranc S, Quere G, Wartski M, et al. Good clinical practice recommendations for the use of PET/CT in oncology. Eur J Nucl Med Mol Imaging. 2020;47(1):28–50. doi: 10.1007/s00259-019-04553-8. [DOI] [PubMed] [Google Scholar]
- 7.Noebauer-Huhmann IM, Weber MA, Lalam RK, Trattnig S, Bohndorf K, Vanhoenacker F, et al. Soft tissue tumors in adults: ESSR-approved guidelines for diagnostic imaging. Semin Musculoskelet Radiol. 2015;19(5):475–482. doi: 10.1055/s-0035-1569251. [DOI] [PubMed] [Google Scholar]
- 8.Benz MR, Crompton JG, Harder D. PET/CT variants and pitfalls in bone and soft tissue sarcoma. Semin Nucl Med. 2021;51(6):584–592. doi: 10.1053/j.semnuclmed.2021.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. (68)Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60(6):801–805. doi: 10.2967/jnumed.119.227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koczorowska MM, Tholen S, Bucher F, Lutz L, Kizhakkedathu JN, De Wever O, et al. Fibroblast activation protein-alpha, a stromal cell surface protease, shapes key features of cancer associated fibroblasts through proteome and degradome alterations. Mol Oncol. 2016;10(1):40–58. doi: 10.1016/j.molonc.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scanlan MJ, Raj BK, Calvo B, Garin-Chesa P, Sanz-Moncasi MP, Healey JH, et al. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci U S A. 1994;91(12):5657–5661. doi: 10.1073/pnas.91.12.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rettig WJ, Garin-Chesa P, Beresford HR, Oettgen HF, Melamed MR, Old LJ. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci U S A. 1988;85(9):3110–3114. doi: 10.1073/pnas.85.9.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dohi O, Ohtani H, Hatori M, Sato E, Hosaka M, Nagura H, et al. Histogenesis-specific expression of fibroblast activation protein and dipeptidylpeptidase-IV in human bone and soft tissue tumours. Histopathology. 2009;55(4):432–440. doi: 10.1111/j.1365-2559.2009.03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59(9):1415–1422. doi: 10.2967/jnumed.118.210443. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Zhao L, Ruan D, Pang Y, Hao B, Dai Y, et al. Usefulness of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [(18)F]FDG PET/CT findings. Eur J Nucl Med Mol Imaging. 2021;48(1):73–86. doi: 10.1007/s00259-020-04940-6. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020;47(8):1820–1832. doi: 10.1007/s00259-020-04769-z. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. 10.1016/j.ejca.2008.10.026. [DOI] [PubMed]
- 18.Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 19.Lee JW, Park JY, Lee HJ, Lee JJ, Moon SH, Kang SY, et al. Preoperative [(18)F]FDG PET/CT tumour heterogeneity index in patients with uterine leiomyosarcoma: a multicentre retrospective study. Eur J Nucl Med Mol Imaging. 2018;45(8):1309–1316. doi: 10.1007/s00259-018-3975-6. [DOI] [PubMed] [Google Scholar]
- 20.Annovazzi A, Ferraresi V, Anelli V, Covello R, Vari S, Zoccali C, et al. [(18)F]FDG PET/CT quantitative parameters for the prediction of histological response to induction chemotherapy and clinical outcome in patients with localised bone and soft-tissue Ewing sarcoma. Eur Radiol. 2021;31(9):7012–7021. doi: 10.1007/s00330-021-07841-w. [DOI] [PubMed] [Google Scholar]
- 21.Charest M, Hickeson M, Lisbona R, Novales-Diaz JA, Derbekyan V, Turcotte RE. FDG PET/CT imaging in primary osseous and soft tissue sarcomas: a retrospective review of 212 cases. Eur J Nucl Med Mol Imaging. 2009;36(12):1944–1951. doi: 10.1007/s00259-009-1203-0. [DOI] [PubMed] [Google Scholar]
- 22.Lim HJ, Johnny Ong CA, Tan JW, Ching Teo MC. Utility of positron emission tomography/computed tomography (PET/CT) imaging in the evaluation of sarcomas: a systematic review. Crit Rev Oncol Hematol. 2019;143:1–13. doi: 10.1016/j.critrevonc.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Erfanian Y, Grueneisen J, Kirchner J, Wetter A, Podleska LE, Bauer S, et al. Integrated 18F-FDG PET/MRI compared to MRI alone for identification of local recurrences of soft tissue sarcomas: a comparison trial. Eur J Nucl Med Mol Imaging. 2017;44(11):1823–1831. doi: 10.1007/s00259-017-3736-y. [DOI] [PubMed] [Google Scholar]
- 24.Kessler L, Ferdinandus J, Hirmas N, Bauer S, Dirksen U, Zarrad F, et al. Ga-68-FAPI as diagnostic tool in sarcoma: data from the FAPI-PET prospective observational trial. J Nucl Med. 2022;63(1):89–95. doi: 10.2967/jnumed.121.262096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koerber SA, Finck R, Dendl K, Uhl M, Lindner T, Kratochwil C, et al. Novel FAP ligands enable improved imaging contrast in sarcoma patients due to FAPI-PET/CT. Eur J Nucl Med Mol Imaging. 2021;48(12):3918–3924. doi: 10.1007/s00259-021-05374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dendl K, Koerber SA, Kratochwil C, Cardinale J, Finck R, Dabir M, et al. FAP and FAPI-PET/CT in malignant and non-malignant diseases: a perfect symbiosis? Cancers (Basel) 2021;13(19):4946. doi: 10.3390/cancers13194946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessler L, Ferdinandus J, Hirmas N, Zarrad F, Nader M, Kersting D, et al. Pitfalls and common findings in (68)Ga-FAPI-PET - a pictorial analysis. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin C, Song Y, Liu X, Gai Y, Liu Q, Ruan W, et al. Increased uptake of (68)Ga-DOTA-FAPI-04 in bones and joints: metastases and beyond. Eur J Nucl Med Mol Imaging. 2021 doi: 10.1007/s00259-021-05472-3. [DOI] [PubMed] [Google Scholar]
- 29.Syed M, Flechsig P, Liermann J, Windisch P, Staudinger F, Akbaba S, et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur J Nucl Med Mol Imaging. 2020;47(12):2836–2845. doi: 10.1007/s00259-020-04859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi X, Xing H, Yang X, Li F, Yao S, Zhang H, et al. Fibroblast imaging of hepatic carcinoma with (68)Ga-FAPI-04 PET/CT: a pilot study in patients with suspected hepatic nodules. Eur J Nucl Med Mol Imaging. 2021;48(1):196–203. doi: 10.1007/s00259-020-04882-z. [DOI] [PubMed] [Google Scholar]
- 31.Pang Y, Zhao L, Luo Z, Hao B, Wu H, Lin Q, et al. Comparison of (68)Ga-FAPI and (18)F-FDG uptake in gastric, duodenal, and colorectal cancers. Radiology. 2021;298(2):393–402. doi: 10.1148/radiol.2020203275. [DOI] [PubMed] [Google Scholar]
- 32.Ding F, Huang C, Liang C, Wang C, Liu J, Tang D. (68)Ga-FAPI-04 vs. (18)F-FDG in a longitudinal preclinical PET imaging of metastatic breast cancer. Eur J Nucl Med Mol Imaging. 2021;49(1):290–300. 10.1007/s00259-021-05442-9. [DOI] [PubMed]
- 33.Frezza AM, Stacchiotti S, Gronchi A. Systemic treatment in advanced soft tissue sarcoma: what is standard, what is new. BMC Med. 2017;15(1):109. doi: 10.1186/s12916-017-0872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kratochwil C, Giesel FL, Rathke H, Fink R, Dendl K, Debus J, et al. [(153)Sm]Samarium-labeled FAPI-46 radioligand therapy in a patient with lung metastases of a sarcoma. Eur J Nucl Med Mol Imaging. 2021;48(9):3011–3013. doi: 10.1007/s00259-021-05273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferdinandus J, Fragoso Costa P, Kessler L, Weber M, Hirmas N, Kostbade K, et al. Initial clinical experience with (90)Y-FAPI-46 radioligand therapy for advanced stage solid tumors: a case series of nine patients. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.