Abstract
Introduction and hypothesis
OASI complicates approximately 6% of vaginal deliveries. This risk is increased with operative vaginal deliveries (OVDs), particularly forceps. However, there is conflicting evidence supporting the use of mediolateral/lateral episiotomy (MLE/LE) with OVD. The aim of this study was to assess whether MLE/LE affects the incidence of OASI in OVD.
Methods
Electronic searches were performed in OVID Medline, Embase and the Cochrane Library. Randomised and non-randomised observational studies investigating the risk of OASI in OVD with/without MLE/LE were eligible for inclusion. Pooled odds ratios (OR) were calculated using Revman 5.3. Risk of bias of was assessed using the Cochrane RoB2 and ROBINS-I tool. The quality of evidence was assessed using Grading of Recommendations Assessment, Development and Evaluation (GRADE).
Results
A total of 703,977 patients from 31 studies were pooled for meta-analysis. MLE/LE significantly reduced the rate of OASI in OVD (OR 0.60 [95% CI 0.42–0.84]). On sub-group analysis, MLE/LE significantly reduced the rate in nulliparous ventouse (OR 0.51 [95% CI 0.42–0.84]) and forceps deliveries (OR 0.32 [95% CI 0.29–0.61]). In multiparous women, although the incidence of OASI was lower when a ventouse or forceps delivery was performed with an MLE/LE, this was not statistically significant. Heterogeneity remained significant across all studies (I2 > 50). The quality of all evidence was downgraded to “very low” because of the critical risk of bias across many studies.
Conclusions
MLE/LE may reduce the incidence of OASI in OVDs, particularly in nulliparous ventouse or forceps deliveries. This information will be useful in aiding clinical decision-making and counselling in the antenatal period and during labour.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00192-022-05145-1.
Keywords: Mediolateral episiotomy, Lateral episiotomy, Obstetric anal sphincter injury, Operative vaginal delivery
Introduction
Operative vaginal delivery with either ventouse or forceps is used to facilitate delivery for a number of maternal and foetal indications [1]. In the UK, operative vaginal delivery is the method of delivery in 12% of women [2]. A worldwide survey of operative vaginal delivery practice in the 1990s demonstrated that forceps were widely used in English-speaking countries such as the USA, UK, Ireland, New Zealand, Canada and Australia. However, ventouse deliveries were widely used in countries within Northern Europe, Africa, the Middle East and Far East countries including China, Hong Kong, Japan, Korea and Thailand [3]. There has been a reduction in forceps use in a number of countries such as the USA, which reduced their rate from 5.1% to 0.6% (1990–2015) [4]. Moreover, in Sweden and Austria, the rate of forceps use has reduced from 1% to 0% (2005–2016) [5]. However, in units in the UK, the incidence of forceps is increasing [6]. Obstetric anal sphincter injury (OASI) occurs in approximately 6% of first vaginal births [7]. This risk is increased further with operative vaginal deliveries, in particular forceps-assisted deliveries.
OASI is a significant risk factor in the development of anal incontinence, with significant implications for the quality of life. Therefore, identification of modifiable risk factors to prevent OASI is important [8, 9]. An episiotomy can be used to increase the dimensions of the vaginal outlet and to create a controlled incision in the perineal body away from the anal sphincter [10]. Regarding OASI incidence, lateral episiotomy (LE), which begins 1–2 cm away from the midline, has been shown to not differ significantly from a mediolateral episiotomy (MLE) [10, 11]. The Royal College of Obstetricians and Gynaecologists (RCOG) Green Top Guideline for assisted vaginal birth [1] acknowledges that the evidence to date supporting the use of MLE at operative vaginal delivery, in terms of preventing OASI, is stronger for nulliparous women and for birth via forceps. However, it is stated that in the absence of robust evidence to support either routine or restrictive use of episiotomy at assisted vaginal birth, the decision should be tailored to the circumstances at the time and the preferences of the woman [1]. Yet, the RCOG Green Top Guideline for the management of OASI [12] advises that MLE should be considered with assisted vaginal birth. This lack of clarity has caused confusion amongst professional [13] and patient groups [14]. To date no meta-analysis has been performed to investigate the effect of MLE/LE with forceps deliveries and OASI incidence. Two meta-analyses have evaluated MLE/LE use with ventouse deliveries [15, 16]. However, the results of these reviews were conflicting [15, 16]. Sagi-Dain et al. [16] found a non-significant decrease in the incidence of OASI with MLE and suggested that MLE may be harmful in parous women, whilst Lund et al. [15] demonstrated a significant reduction in the incidence of OASI with MLE. In addition, neither review evaluated the effect of MLE/LE with forceps deliveries on OASI incidence. Therefore, up-to-date evidence is required to address these inconsistent findings.
The aim of this study was to investigate the effect of MLE/LE use with operative vaginal delivery on the risk of OASI.
Materials and methods
This systematic review of the literature was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17]. Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines for reporting meta-analyses of observational studies were also followed (Appendix S1) [18]. A protocol was developed and can be reviewed in the international prospective register of systematic reviews (PROSPERO) register (CRD 42020196579) [19]. Our primary research question was: “Does MLE/LE use with operative vaginal delivery reduce the risk of obstetric anal sphincter injury in comparison to no episiotomy?”. A PICO approach was followed:
Population: Nulliparous and multiparous women undergoing operative vaginal delivery
Intervention: MLE/LE
Comparator: No episiotomy
Outcome: OASI
OVID Medline, Embase and the Cochrane Library from inception to June 2020 were searched using the terms “anal sphincter injury”, “episiotomy”, “instrumental”, “forceps” and “vacuum”, including medical subject headings (meSH) terms, with no restriction on language or year of publication. A manual search of references from identified studies was also conducted to identify other relevant studies. Studies were included if the episiotomy was a MLE or LE. Studies reporting the use of MLE/LE with spontaneous vaginal births or midline episiotomy were excluded. Other relevant systematic reviews of MLE/LE with operative vaginal delivery and the reference lists of the eligible studies were also searched [15, 16]. A full search strategy can be found in the electronic supplementary material (Appendix S1). Results were exported to Zotero reference management system and de-duplicated. Randomised controlled trials (RCTs), non-randomised controlled trials, prospective and retrospective observational studies analysing the risk of OASI in women undergoing operative vaginal delivery with and without MLE/LE were eligible for inclusion. Case reports, case series, narrative reviews and conference abstracts were excluded. A full list of excluded studies is given in Table S1.
Two authors (N.A.O., K.W.W.) independently screened the titles and abstracts of all retrieved studies to obtain studies for full-text assessment. Any disagreements surrounding eligibility for full-text assessment were resolved by the senior reviewers or through consensus-based discussion. Full-text articles which met the inclusion criteria were then assessed by the two authors. Following this, the authors independently collected data from eligible studies, using a standardised electronic data extraction form. This included data regarding operative vaginal delivery, study characteristics, parity, type of operative vaginal delivery, type of episiotomy and rate of OASI. Translations were sought for any study not in English. Authors of included studies were contacted if the full text could not be retrieved and if the data reported were incomplete, unclear or published in a manner that was not extractable. If the author did not respond, unpublished data provided by the same author from the previously published systematic review of the risk of OASI with MLE/LE and ventouse delivery were used [15].
Review Manager 5.3 (The Cochrane Collaboration) and Meta-Essentials (version 1.5) [20] were used to analyse data. Data were reported as odds ratios (OR) and their corresponding 95% confidence interval (95% CI) bounds. The heterogeneity amongst studies was calculated using the I2 statistic. An I2 > 50 % was considered as significant heterogeneity and I2 > 80 was considered as very significant heterogeneity. Meta-analysis was performed if each outcome was represented in at least two studies, using the fixed-effects (Mantel-Haenszel) or the random-effects (DerSimonian and Laird) model. The random-effects model was used if heterogeneity was significant (I2 > 50 %). Sensitivity analysis for the primary outcome was conducted by removing high/critical bias studies to assess for methodological heterogeneity. Subgroup analyses were then performed to determine potential sources of clinical heterogeneity by separating participant data into sub-groups deemed to be categorical predictors, such as parity and instrument type. A p-value < 0.05 was considered statistically significant. Presence of publication bias was assessed using a funnel plot and Egger’s regression analysis.
Risk of bias assessment of RCTs was conducted using the Cochrane risk-of-bias tool for randomized trials (RoB 2) [21]. Non-randomised studies, including observational studies, were assessed using the Risk Of Bias in Non-randomized Studies-of Interventions (ROBINS-I) tool [22]. Risk of bias was assessed at an outcome level (not individual study level). Two reviewers independently assessed the overall quality of the evidence using criteria recommended by the Grading of Recommendations Assessment, Development and Evaluation working group (GRADE) [23]. Any disagreements surrounding eligibility for overall study quality were resolved by the senior reviewers or through consensus-based discussion. From the GRADE table, the difference between the anticipated absolute effect and 95% CI was used to calculate the number needed to treat (NNT) with its 95% CI [24]. No funding was required to complete this review.
Results
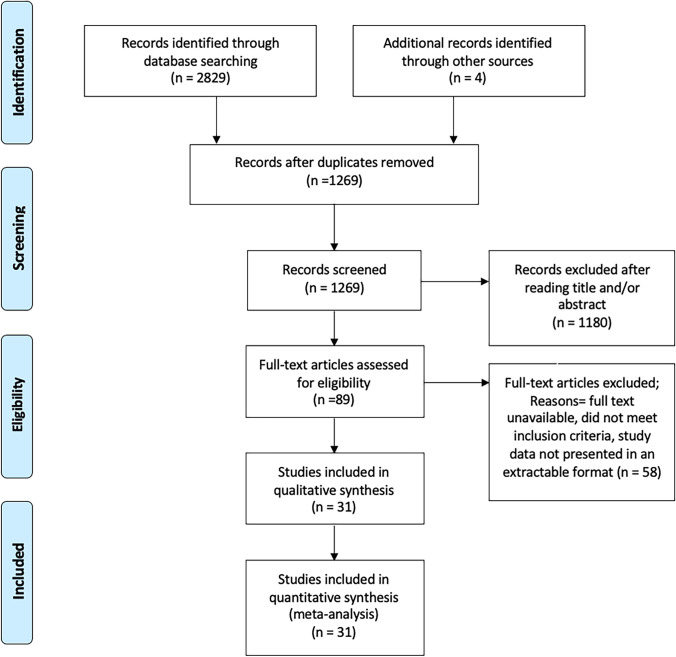
Of the 1269 articles initially identified by the search, 89 were selected for full-text review. Thirty-one studies were eligible for inclusion and included in the meta-analysis (Fig. 1). Table 1 presents a detailed overview of the studies included in the meta-analysis. Two RCTs were identified [25, 26]. Other studies included six prospective observational studies [27–32] and 23 retrospective observational studies [33–55]. Overall risk of bias for the two RCTs [25, 26] was high. In 24 of the observational studies [28, 32–36, 38–55], overall risk of bias was critical. In one observational study, overall risk of bias was serious [37], and in the remaining four observational studies, overall risk of bias was moderate (Table S2, S3) [27, 29–31] .
Fig. 1.
PRISMA flow diagram of the study selection process
Table 1.
Overview of included studies
| Authors (year) | Study type | Episiotomy type | Episiotomy incidence (%) |
Parity | Instrument type | OASI incidence (%) | OR [95% CI] |
|---|---|---|---|---|---|---|---|
| Ampt et al. [54] (2013) | Retrospective case control | MLE | 55.2 | All- separated | Both-separated | 7.2 | 1.07 [1.02, 1.14] |
| Aukee et al. [34] (2006) | Retrospective case control | MLE | 84.0 | All- pooled | Ventouse | 3.8 | 0.36 [0.13, 0.98] |
| Baghurst et al. [35] (2012) | Retrospective cohort | MLE | 55.0 | All- separated | Both-separated | 7.5 | 1.12 [0.96, 1.31] |
| Bodner-Adler et al. [36] (2018) | Retrospective cohort | MLE | 65.0 | Nulliparous | Ventouse | 6.8 | 0.60 [0.31, 1.16] |
| Boujenah et al. [37] (2019) | Retrospective cohort | MLE | 76.9 | Nulliparous | Both-separated | 2.8 | 0.38 [0.20, 0.74] |
| D'Souza et al. [38] (2020) | Retrospective cohort | MLE | 81.9 | Multiparous | Both-separated | 6.0 | 0.08 [0.01, 0.51] |
| De Leeuw et al. [39] (2008) | Retrospective cohort | MLE | 82.2 | All- separated | Both-separated | 3.4 | 0.14 [0.12, 0.16] |
| De Parades et al. [28] (2004) | Prospective cohort | MLE | 95.7 | Nulliparous | Forceps | 12.9 | 0.42 [0.04, 4.43] |
| De Vogel et al. [40] (2012) | Retrospective cohort | MLE | 81.0 | All- separated | Both-separated | 5.7 | 0.18 [0.13, 0.25] |
| Gachon et al. [41] (2019) | Retrospective cohort | MLE | 40.3 | All- separated | Both-separated | 7.4 | 0.38 [0.26, 0.55] |
| Gurol-Urganci et al. [33] (2014) | Retrospective cohort | MLE | 76.1 | Nulliparous | Both-separated | 7.1 | 0.49 [0.48, 0.51] |
| Hamouda et al. [27] (2017) | Prospective cohort | MLE | 58.2 | All- pooled | Both-separated | 3.9 | 1.08 [0.38, 3.10] |
| Jango et al. [42] (2014) | Retrospective cohort | MLE | 28.7 | Nulliparous | Both-separated* | 13.7 | 0.67 [0.63, 0.72] |
| Levin et al. [43] (2020) | Retrospective cohort | MLE | 78.0 | Nulliparous | Ventouse | 2.3 | 0.56 [0.35, 0.91] |
| Macleod et al. [29] (2008) | Prospective cohort | MLE | 78.4 | Nulliparous | Both-separated | 9.9 | 1.44 [0.88, 2.34] |
| Marschalek et al. [44] (2018) | Retrospective cohort | MLE | 72.5 | Nulliparous | Both-separated | 5.2 | 0.68 [0.60, 0.76] |
| Meyer et al. [53] (2020) | Retrospective cohort | MLE | 74.1 | All- separated | Forceps | 2.5 | 1.80 [0.52, 6.26] |
| Murphy et al. [25] (2009) | RCT | MLE | 72.0 | Nulliparous | Both-separated† | 10.9 | 4.79 [0.22, 105.30] |
| Parnell et al. [30] (2001) | Prospective case control | MLE | 53.0 | Nulliparous | Ventouse | 21.0 | 0.74 [0.41, 1.34] |
| Räisänen et al. [45] (2012) | Retrospective cohort | LE | 84.9 | All- separated | Ventouse | 3.0 | 0.47 [0.35, 0.64] |
| Räisänen et al. [46] (2009) | Retrospective cohort | LE | 90.0 | All- separated | Both-separated† | 1.5 | 1.09 [0.87, 1.36] |
| Rognant et al. [47] (2012) | Retrospective cohort | MLE | 85.0 | All- pooled | Ventouse | 2.2 | 1.97 [0.60, 6.48] |
| Rygh et al. [31] (2014) | Prospective cohort | MLE/LE | 55.0 | Nulliparous | Both-pooled | 11.0 | 0.72 [0.60, 0.87] |
| Sagi-Dain [26] (2020) | RCT | MLE | 49.6 | Nulliparous | Ventouse | 3.7 | 0.67 [0.11-4.12] |
| Schmitz et al. [44] (2014) | Retrospective case control | MLE | 66.5 | All- separated | Both-separated† | 2.1 | 0.04 [0.00, 0.79] |
| Shmueli et al. [49] (2017) | Retrospective cohort | MLE | 66.0 | All- separated | Ventouse | 1.5 | 1.73 [0.99, 3.04] |
| Van Bavel et al. [50] (2018) | Retrospective cohort | MLE | 89.6 | All- separated | Both-separated | 4.2 | 0.19 [0.18, 0.19] |
| Van Roon et al. [32] (2015) | Prospective cohort | MLE | 90.0 | Nulliparous | Both-pooled | 5.4 | 3.18 [1.39, 7.27] |
| Vathanan et al. [55] (2014) | Retrospective cohort | MLE | 78.7 | All-pooled | Both-separated | 9.2 | 0.18 [0.13-0.25] |
| Yamasato et al. [51] (2016) | Retrospective cohort | MLE | 3.7 | All- pooled | Both-separated | 21.7 | 0.65 [0.14, 3.09] |
|
Youssef et al. [52] (2005) |
Retrospective cohort | MLE | 71.2 | All- pooled | Both-separated | 8.7 | 0.99 [0.54, 1.81] |
RCT: randomised controlled trial
MLE- mediolateral episiotomy
L/E: lateral episiotomy
*Only data for ventouse deliveries reported, unable to retrieve crude data for forceps-assisted deliveries from authors
†Data not extractable, data retrieved from previous systematic reviews [14, 15] as unable to retrieve crude data from authors
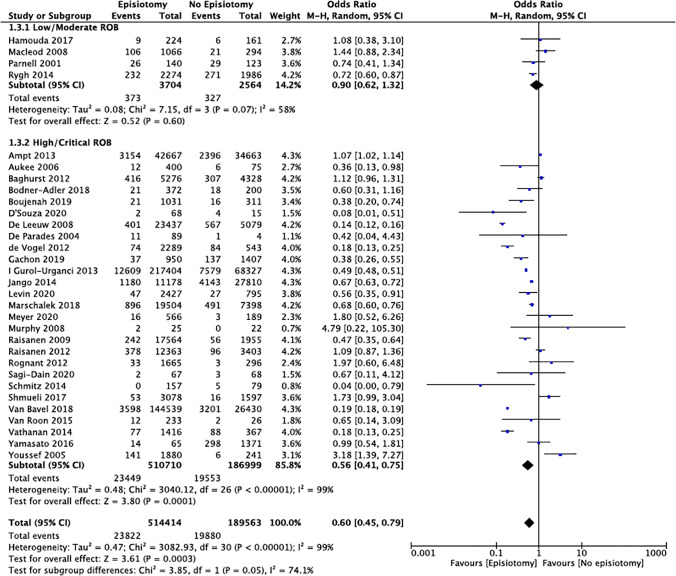
Based on the inclusion criteria, 703,977 patients from 31 studies were included in this review for meta-analysis. MLE/LE was performed in an average of 69.0% (range 3.7–95.7%) of operative vaginal deliveries and OASI was diagnosed on average in 6.9% (range 1.5–21.7%) of cases. The meta-analysis showed a significant reduction in the OASI rate when operative vaginal deliveries were completed with an MLE/LE compared to deliveries without (OR 0.60 [95% CI 0.45–0.79]) (Fig. 2). The NNT was 26 (95% CI 18.2–50.0). On sensitivity analysis, there was no significant reduction in OASI rates (OR 0.90 [0.62–1.32]) in studies of low/moderate risk of bias. There was no strong evidence that the study risk of bias had an effect on the rate of OASI with or without MLE/LE (p = 0.05). Also, heterogeneity remained significant (low/moderate risk: I2 = 58%; high/critical risk: I2 = 99%).
Fig. 2.
Risk of OASI in operative vaginal deliveries with or without episiotomy
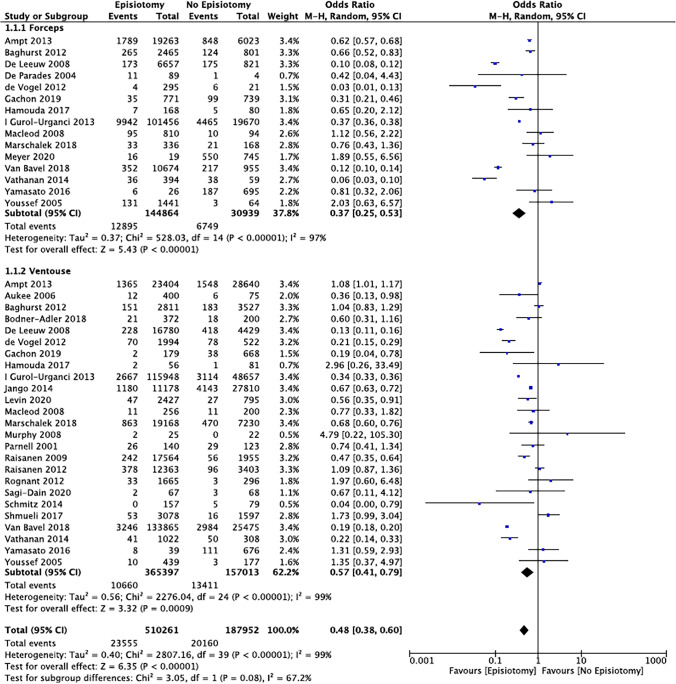
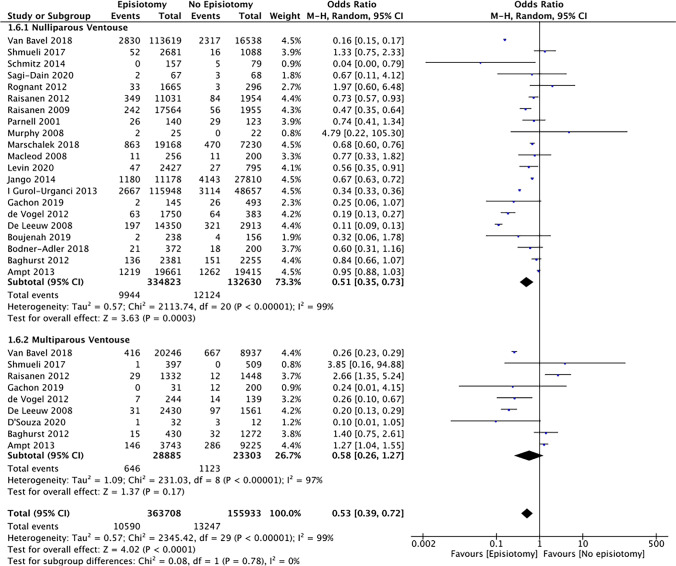
Sub-group analysis was completed for instrument type and parity. Data for ventouse deliveries were identified from 25 studies, forceps from 15 studies and 2 studies pooled all operative vaginal deliveries together. Of the 703,977 women, 74.2% (n = 522,410) had a ventouse delivery and 25.0% (n = 175,803) had a forceps delivery. MLE/LE was performed in an average of 64.4 % (range 4.3–90.0%) of ventouse deliveries and 77.3% (range 2.9–95.8%) of forceps deliveries. Meta-analysis showed a significant reduction in the rate of OASI when a ventouse (OR 0.57 [95% CI 0.41–0.79]) or forceps (OR 0.37 [95% CI 0.25–0.53]) was completed with an MLE/LE, compared to deliveries without (Fig. 3). The NNT for a ventouse delivery was 28 (95% CI 20.4–58.8), and for a forceps delivery it was 8 (95% CI 6.5–11.2). No statistically significant subgroup effect was found (p = 0.08) and heterogeneity remained very significant within each sub-group.
Fig. 3.
Risk of OASI in ventouse and forceps deliveries with or without episiotomy in nulliparous women
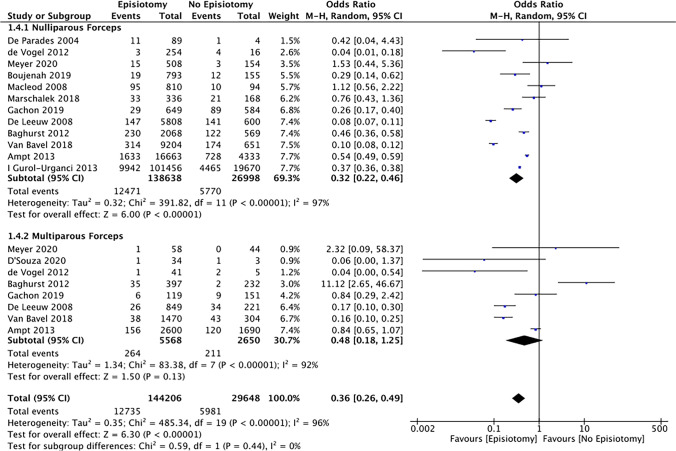
Regarding parity, 633,089 (86.3%) women were nulliparous and 60,406 (7.8%) women were multiparous. Six studies pooled data from all women (n = 10,482) irrespective of parity undergoing operative vaginal delivery. In nulliparous women, the rate of OASI was significantly reduced when an MLE/LE was performed during a ventouse (OR 0.51 [95% CI 0.35–0.73]) or forceps (OR 0.32 [95% CI 0.22–0.46]) delivery (Figs. 4 and 5). In these women the NNT was 23 (95% CI 17.5–43.5) and 8 (95% CI 6.4–9.7) for a ventouse and forceps delivery respectively. However, in multiparous women, although the incidence of OASI was lower when an MLE/LE was performed with a ventouse or forceps delivery, this reduction did not reach statistical significance . The test for sub-group differences due to parity indicated there was no statistically significant subgroup effect (forceps [p = 0.44], ventouse [p = 0.78]). Despite sub-group analysis, heterogeneity remained very significant within each sub-group.
Fig. 4.
Risk of OASI in nulliparous and multiparous forceps deliveries with or without episiotomy
Fig. 5.
Risk of OASI in nulliparous and multiparous ventouse deliveries with or without episiotomy
There was no publication bias amongst the included studies, as demonstrated by the symmetrical distribution of the funnel plot (Fig. S1). Egger’s regression analysis found no significant publication bias amongst the studies (p = 0.92). However, the quality of all evidence was downgraded to “very low” because of the critical risk of bias across many studies (Fig S3) and the very high level of heterogeneity (I2 value > 80%), which lowered the confidence in the estimate of effect. After review of the 95% CIs, evidence was also downgraded because of potential imprecision with the outcome estimates, considering the default minimal clinically important difference for dichotomous outcomes (0.8 to 1.25) [56]. The GRADE table is presented in Table 2.
Table 2.
Overall quality of the evidence identified for meta-analysis
| Outcome no. of participants (studies) | Relative effect (95% CI) | Anticipated absolute effects (95% CI) | Certainty | ||
|---|---|---|---|---|---|
| With OASI | Without OASI | Difference | |||
|
Instrumental No. of participants: 703977 (31 observational studies) |
OR 0.60 (0.47– 0.84) | 10.5% | 6.6% (5.0–8.5) | 3.9% fewer (2.0–5.5) |
⨁◯◯◯ Very lowa,b,c |
|
Forceps No. of participants: 175803 (15 observational studies) |
OR 0.37 (0.25– 0.53) | 21.8% | 9.4% (6.5–12.9) | 12.5% fewer (8.9–15.3) |
⨁◯◯◯ Very lowa,b |
|
Ventouse No. of participants: 522410 (25 observational studies) |
OR 0.57 (0.41–0.79) | 8.5% | 5.1% (3.7–6.9) | 3.5% fewer (1.7–4.9) |
⨁◯◯◯ Very lowa,b,c |
|
Nulliparous forceps № of participants: 165636 (12 observational studies) |
OR 0.32 (0.22– 0.46) | 21.4% | 8.0% (5.6–11.1) | 13.4% fewer –(10.315.7) |
⨁◯◯◯ Very lowa,b |
|
Nulliparous ventouse № of participants: 467453 (21 observational studies) |
OR 0.51 (0.35–0.73) | 9.1% | 4.9% (3.4–6.8) | 4.3% fewer (2.3–5.7) |
⨁◯◯◯ Very lowa,b |
|
Multiparous forceps № of participants: 8218 (8 observational studies) |
OR 0.48 (0.18– 1.25) | 8.0% | 4.0% (1.5– 9.8) | 4.0% fewer (1.8–6.4) |
⨁◯◯◯ Very lowa,b,c |
|
Multiparous ventouse № of participants: 52188 (9 observational studies) |
OR 0.58 (0.26– 1.27) | 4.8% | 2.9% (1.3– 6.0) | 2.0% fewer (1.2–3.5) |
⨁◯◯◯ Very lowa,b,d |
aCrucial limitation for one of more criteria substantial enough to lower one’s confidence in the estimate of effect.
bVery high level of heterogeneity (I2 value > 80%)
c95% confidence interval crosses 1 default minimally important difference (0.8 or 1.25)
d95% confidence interval crosses 2 default minimally important differences (0.8 and 1.25)
OR = odds ratio
CI = confidence interval
Discussion
This meta-analysis of > 700,000 women showed that MLE and LE with operative delivery reduce the rate of OASI, particularly in nulliparous women. MLE/LE use in operative vaginal delivery was associated with a 40% reduction in the odds of OASI. In nulliparous women, an odds reduction of 49% and 68% was seen in ventouse and forceps deliveries with an MLE/LE respectively.
The main strength of our study is that it is the first meta-analysis reviewing outcomes following both ventouse and forceps delivery with MLE/LE in nulliparous and multiparous women. MLE and LE were combined as studies have demonstrated no difference in outcomes between the two types [10, 11]. In addition, it includes the largest number of nulliparous and multiparous women undergoing operative vaginal delivery. We conducted a comprehensive search with no language or date restrictions and contacted authors where possible to obtain unpublished data. We do acknowledge that there are limitations, particularly with the potential effect of the significant heterogeneity, although this was controlled for and explored further by using a random-effects model when pooling data for meta-analysis, sensitivity and sub-group analyses. However, there was inconsistent publication of adjusted odds ratios amongst the included studies, meaning unadjusted odds ratios were used for meta-analysis. Therefore, the unmeasured sources of confounding factors such as ethnicity, maternal age, birthweight and head circumference [57] may be a potential source of the significant heterogeneity between studies.
The risk of OASI is also associated with the angle at which an episiotomy is performed. A MLE should be performed at an angle of 60° from the midline, at crowning of the foetal head, subsequently resulting in a post-delivery angle of 45° [1, 11]. The incidence of OASI with MLE has been shown to reduce by 50% for every 6° of the MLE sutured angle away from the midline [58]. The angle of episiotomy was only measured in one study [32], where the EPISCISSORS-60® [59] were used. These are designed to cut at an angle of 60° and have been shown to produce an optimal post-delivery angle of 43°, meaning in this study, episiotomies were truly mediolateral. A prospective study by Andrews et al. [60], which investigated the practice of MLE amongst doctors and midwives, found that no midwife and only 22% of doctors performed a MLE at the desired angle. In addition, one-third of episiotomies performed by midwives were actually midline. Midline episiotomy, particularly in the context of operative vaginal delivery, significantly increases the risk of OASI in both nulliparous and parous women [61]. Consequently, if many of the episiotomies in the studies included in our meta-analysis were not truly mediolateral, the incidence of OASI might potentially be falsely high.
Another limitation of this study is that the meta-analysis included non-randomised studies. However, to date only two RCTs have been published. One only evaluated the effect of MLE in ventouse alone [26] and the other did not reach adequate statistical power [25]. The design of the latter study was a multicentre pilot study which demonstrated that an RCT of routine versus restrictive use of episiotomy at operative vaginal delivery is feasible. The sample size was limited by the ethical difficulties and time constraints involved in recruiting women to studies of emergency procedures in the second stage of labour. It can be argued that an RCT with episiotomy as the intervention in the setting of operative vaginal delivery is impractical. A survey of obstetricians highlighted concerns about the validity of an RCT that evaluates a surgical approach that is not dichotomised into two types of practice, but instead is based on clinical judgement [62]. Sultan et al. [63] provided evidence from observational studies to recommend the liberal use of a MLE/LE cut at 60° during operative vaginal delivery and highlighted further potential limitations of a RCT.
In the absence of an adequately powered RCT, our meta-analysis provides the best available evidence. Our findings are consistent with the RCOG guidance, which recommends that the evidence to support MLE with operative vaginal delivery is stronger for nulliparous women and forceps deliveries [1]. Their evidence for forceps deliveries was based on findings from two large retrospective cohort studies [33, 39]. By completing a meta-analysis, we have statistically pooled together the data from all studies in the literature to generate an overall estimate of the effect of episiotomy with both ventouse and forceps deliveries. However, the inclusion of non-randomised observational studies in our meta-analysis may confer difficulty with precise interpretation of effect size due to low study quality and high risk of bias. We acknowledge that in studies with low/moderate risk of bias, although the incidence of OASI was lower when an MLE/LE was performed with a ventouse or forceps delivery, this reduction did not reach statistical significance. However, in studies with a high/critical risk of bias, a significant clinical benefit was demonstrated. However, no significant sub-group difference was found between studies of low/moderate or high/critical risk of bias. Our results should be interpreted with caution, as routine episiotomy is associated with a significant increase in blood loss, perineal pain, dyspareunia and pelvic floor dysfunction [64]. It is therefore important these risks are considered, including the values and preferences of the woman. However, non-randomised studies may be a better reflection of clinical practice, as intervention choice is at the discretion of the clinician [65, 66].
Parity and instrument type are known significant independent risk factors for OASI, with forceps in particular increasing the odds of OASI six-fold [67]. Therefore, sub-group analysis of these different populations is necessary to evaluate the individual effect size of episiotomy on OASI in at risk groups. Unexpectedly, we found no significant difference between the sub-groups (nulliparous vs. multiparous, forceps vs. ventouse). However, a smaller number of trials and participants contributed data to each subgroup, meaning that the analysis may not be able to detect subgroup differences. Despite sub-group analysis, heterogeneity remained very significant within each sub-group. Two meta-analyses have previously been completed to investigate the effect of MLE with ventouse deliveries and OASI rate [15, 16]. Sagi-Dain et al. [16] concluded from their sample of 290,000 women that, although the incidence of OASI with ventouse delivery was lower with MLE, it was non-significant (OR 68 [95% CI 0.43–1.07]). Lund et al. [15], based on a sample of 320,000 women, found that MLE/LE significantly reduced the odds of OASI by 47% (OR 0.53 [95% CI 0.47–0.77]). However, based on all available evidence to date, our results have demonstrated that with nulliparous women the rate of OASI is significantly reduced by 49% when an MLE/LE is used in ventouse deliveries, which is similar to the findings by Lund et al. [15]. Sagi-Dain et al. [16] also suggested that MLE with ventouse significantly increased the rate of OASI in parous women by 27% (OR 1.27 [95% CI 1.05–1.53]). However, this was not the case with LE, which was analysed separately. In comparison, our review encompassed a larger number of parous women (60,406 women [52,118 = ventouse, 8218 = forceps] vs. 14,640 women). We found that the rate of OASI was lower in multiparous women who had an MLE/LE compared to no episiotomy during a ventouse (2.2% vs. 4.8%) or forceps (4.7% vs. 8.0%) assisted delivery. However, this reduction was not significant. As we included four additional studies and unpublished data from two studies, this strengthens our findings and may also explain the difference in results. It is important to note that number of multiparous women (n = 60,406) included in our meta-analysis was much smaller than that of nulliparous women (n = 633,089), which is a true reflection of obstetric practice. Moreover, the frequency of OASI was almost twice as high in nulliparous women (6.3%) compared to multiparous women (3.7%). This provides further evidence to explain why MLE/LE was found to be protective with both forceps and ventouse deliveries in nulliparous women compared to multiparous.
Conclusion
In conclusion, this meta-analysis has shown that MLE/LE is associated with a reduction in the incidence of OASI following operative vaginal delivery, particularly in nulliparous women undergoing a ventouse or forceps assisted delivery. This information will be useful in aiding clinical decision-making and counselling in the antenatal period and during labour. However, the results of this meta-analysis should be interpreted with caution as there was significant unexplained heterogeneity across included studies and the overall quality of evidence was assessed to be very low. Larger, higher quality studies in this area will provide more data to inform future policy.
Supplementary information
(DOCX 165 kb)
Acknowledgements
We thank E.J. Ramstead at Croydon Health Services Library, Croydon University Hospital, for the literature search support. We would also like to thank P. Bassett at Statsconsultancy Ltd. for the support with the statistical analysis. We also thank the following authors for providing their unpublished crude data: J. D’Souza (Portsmouth Hospitals NHS Trust), A.B. Rygh (Stavanger University Hospital), B. Gachon (Poitiers University Hospital), J.W. de Leeuw (Ikazia Ziekenhuis), R. Meyer (The Chaim Sheba Medical Center) and L. Sagi-Dain (Carmel Medical Center).
Author contributions
NAO: Project development, Data collection, Data analysis, Manuscript writing
KW: Project development, Data collection, Data analysis
SJ: Project development, Manuscript editing; AS: Project development, Manuscript editing
RT: Project development, Manuscript editing
Declarations
Conflicts of interests
Miss Ranee Thakar is the past president of the International Urogynecological Association
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nicola Adanna Okeahialam, Email: nicola.okeahialam@nhs.net.
Ka Woon Wong, Email: ka.wong3@nhs.net.
Swati Jha, Email: Swati.jha1@nhs.net.
Abdul H. Sultan, Email: abdulsultan@nhs.net
Ranee Thakar, Email: ranee.thakar@nhs.net.
References
- 1.Murphy D, Strachan B, Bahl R, the Royal College of Obstetricians and Gynaecologists. Assisted Vaginal Birth: Green-top Guideline No. 26. BJOG: Int J Obstet Gy. 2020;127. 10.1111/1471-0528.16092. [DOI] [PubMed]
- 2.NHS Maternity Statistics, England 2019-2020. https://digital.nhs.uk/data-and-information/publications/statistical/nhs-maternity-statistics/2019-20. Accessed 8 Dec 2020.
- 3.Hillier CEM, Johanson RB. Worldwide survey of assisted vaginal delivery. Int J Gynecol Obstet. 1994;47:109–114. doi: 10.1016/0020-7292(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 4.National Vital Statistics Reports. Births: Final Data for 2015. 2017. [PubMed]
- 5.Gyhagen M, Ellström Engh M, Husslein H, et al. Temporal trends in obstetric anal sphincter injury from the first vaginal delivery in Austria, Canada, Norway, and Sweden. Acta Obstet Gynecol Scand. 2021;100:1969–1976. doi: 10.1111/aogs.14244. [DOI] [PubMed] [Google Scholar]
- 6.Tyagi V, Perera M, Guerrero K. Trends in obstetric anal sphincter injuries over 10 years. J Obstet Gynaecol. 2013;33:844–849. doi: 10.3109/01443615.2013.831045. [DOI] [PubMed] [Google Scholar]
- 7.Thiagamoorthy G, Johnson A, Thakar R, Sultan AH. National survey of perineal trauma and its subsequent management in the United Kingdom. Int Urogynecol J. 2014;25:1621–1627. doi: 10.1007/s00192-014-2406-x. [DOI] [PubMed] [Google Scholar]
- 8.LaCross A, Groff M, Smaldone A. Obstetric anal sphincter injury and anal incontinence following vaginal birth: a systematic review and meta-Analysis. J Midwifery Women’s Health. 2015;60:37–47. doi: 10.1111/jmwh.12283. [DOI] [PubMed] [Google Scholar]
- 9.Sultan AH, Monga AK, Kumar D, Stanton SL. Primary repair of obstetric anal sphincter rupture using the overlap technique. BJOG:Int J O&G. 1999;106:318–323. doi: 10.1111/j.1471-0528.1999.tb08268.x. [DOI] [PubMed] [Google Scholar]
- 10.Kalis V, Laine K, de Leeuw JW, et al. Classification of episiotomy: towards a standardisation of terminology. BJOG. 2012;119:522–526. doi: 10.1111/j.1471-0528.2011.03268.x. [DOI] [PubMed] [Google Scholar]
- 11.Kalis V, Landsmanova J, Bednarova B, et al. Evaluation of the incision angle of mediolateral episiotomy at 60 degrees. Int J Gynaecol Obstet. 2011;112:220–224. doi: 10.1016/j.ijgo.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Royal College of Obstetrics and Gynaecology. Management of third and fourth degree perineal tears. Greentop guideline number 29. 2015.
- 13.Leeuw JW, Daly JO, the International Urogynecological Association (IUGA) Obstetric Pelvic Floor Trauma Special Interest Group Re: Assisted vaginal birth: Green-top Guideline No. 26: Shortcomings of the updated Green-top Guideline No. 26 Assisted Vaginal Birth. BJOG: Int J Obstet Gy. 2021;128:615. doi: 10.1111/1471-0528.16508. [DOI] [PubMed] [Google Scholar]
- 14.Hull PM, Thomas K, Skinner E, et al. Re: Assisted Vaginal Birth: Green-top guideline no. 26: Montgomery is missing from RCOG’s Assisted Vaginal Birth guideline. BJOG: Int J Obstet Gy. 2020;127:1297–1298. doi: 10.1111/1471-0528.16338. [DOI] [PubMed] [Google Scholar]
- 15.Lund NS, Persson LKG, Jangö H, et al. Episiotomy in vacuum-assisted delivery affects the risk of obstetric anal sphincter injury: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;207:193–199. doi: 10.1016/j.ejogrb.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Sagi-Dain L, Sagi S. Morbidity associated with episiotomy in vacuum delivery: a systematic review and meta-analysis. BJOG: An Int J Obstet Gynaecol. 2015;122:1073–1081. doi: 10.1111/1471-0528.13439. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroup DF. Meta-analysis of observational studies in epidemiology. A proposal for reporting. JAMA. 2000;283:2008. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19.Booth A, Clarke M, Dooley G, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. System Rev. 2012;1:2. doi: 10.1186/2046-4053-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res Synth Methods. 2017;8:537–553. doi: 10.1002/jrsm.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;l4898. 10.1136/bmj.l4898. [DOI] [PubMed]
- 22.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;i4919. 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed]
- 23.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy D, Macleod M, Bahl R, et al. A randomised controlled trial of routine versus restrictive use of episiotomy at operative vaginal delivery: a multicentre pilot study. BJOG. Int J Obstet Gy 2008;115:1695–703. 10.1111/j.1471-0528.2008.01960.x. [DOI] [PubMed]
- 26.Parnell C, Langhoff-Roos J, Møller H, et al. Is it time to abandon episiotomy use? A randomized controlled trial (EPITRIAL) Int Urogynecol J. 2020;31:2377–2385. doi: 10.1007/s00192-020-04332-2. [DOI] [PubMed] [Google Scholar]
- 27.Hamouda S, Mancini J, Marchand F, et al. Severe perineal morbidity of instrumental deliveries using Thierry’s spatulas and vacuum extraction: A prospective observational cohort study. Journal of Gynecology Obstetrics and Human Reproduction 2017;46:43–51. 10.1016/j.jgyn.2015.11.003 [DOI] [PubMed]
- 28.de Parades V, Etienney I, Thabut D, et al. Anal Sphincter Injury After Forceps Delivery: Myth or Reality?: A Prospective Ultrasound Study of 93 Females. Diseases of the Colon & Rectum. 2004;47:24–34. doi: 10.1007/s10350-003-0007-8. [DOI] [PubMed] [Google Scholar]
- 29.Macleod M, Strachan B, Bahl R, et al. A prospective cohort study of maternal and neonatal morbidity in relation to use of episiotomy at operative vaginal delivery. BJOG: Int J Obstet Gy. 2008;115:1688–1694. doi: 10.1111/j.1471-0528.2008.01961.x. [DOI] [PubMed] [Google Scholar]
- 30.Parnell C, Langhoff-Roos J, Møller H. Conduct of labor and rupture of the sphincter ani: Conduct of labor and rupture of the sphincter ani. Acta Obstet Gynecol Scand. 2001;80:256–261. doi: 10.1034/j.1600-0412.2001.080003256.x. [DOI] [PubMed] [Google Scholar]
- 31.Rygh AB, Skjeldestad FE, Körner H, Eggebø TM. Assessing the association of oxytocin augmentation with obstetric anal sphincter injury in nulliparous women: a population-based, case–control study. BMJ Open 2014;4:e004592. 10.1136/bmjopen-2013-004592 [DOI] [PMC free article] [PubMed]
- 32.Van Roon Y, Kirwin C, Rahman N, et al. Comparison of obstetric anal sphincter injuries in nulliparous women before and after introduction of the EPISCISSORS-60(®) at two hospitals in the United Kingdom. Int J Womens Health. 2015;7:949–955. doi: 10.2147/IJWH.S94680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurol-Urganci I, Cromwell D, Edozien L, et al. Third- and fourth-degree perineal tears among primiparous women in England between 2000 and 2012: time trends and risk factors. BJOG: Int J Obstet Gy. 2013;120:1516–1525. doi: 10.1111/1471-0528.12363. [DOI] [PubMed] [Google Scholar]
- 34.Aukee P, Sundström H, Kairaluoma MV. The role of mediolateral episiotomy during labour: analysis of risk factors for obstetric anal sphincter tears. Acta Obstet Gynecol Scand. 2006;85:856–860. doi: 10.1080/00016340500408283. [DOI] [PubMed] [Google Scholar]
- 35.Baghurst PA, Antoniou G. Risk Models for Benchmarking Severe Perineal Tears during Vaginal Childbirth: a Cross-sectional Study of Public Hospitals in South Australia, 2002-08: Risk Models for Severe Perineal Tears. Paediatric and Perinatal Epidemiology 2012;26:430–437. 10.1111/j.1365-3016.2012.01300.x [DOI] [PubMed]
- 36.Bodner-Adler B, Kimberger O, Käfer A, et al. Management of the Perineum during Delivery with the Kiwi Omnicup: Effects of Mediolateral Episiotomy on Anal Sphincter Tears in Nulliparous Women. Gynecol Obstet Invest 2018;3:171–178. 10.1159/000478930 [DOI] [PubMed]
- 37.Boujenah J, Tigaizin A, Fermaut M, et al. Is episiotomy worthwile to prevent obstetric anal sphincter injury during operative vaginal delivery in nulliparous women? European Journal of Obstetrics & Gynecology and Reproductive Biology. 2019;232:60–64. doi: 10.1016/j.ejogrb.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 38.D'Souza JC, Monga A, Tincello DG, et al. Maternal outcomes in subsequent delivery after previous obstetric anal sphincter injury (OASI): a multi-centre retrospective cohort study. Int Urogynecol J. 2019 doi: 10.1007/s00192-019-03983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Leeuw J, De Wit C, Kuijken J, et al. Mediolateral episiotomy reduces the risk for anal sphincter injury during operative vaginal delivery. BJOG: Int J Obstet Gy. 2007;115:104–8. 10.1111/j.1471-0528.2007.01554.x. [DOI] [PubMed]
- 40.de Vogel J, van der Leeuw-van Beek A, Gietelink D, et al. The effect of a mediolateral episiotomy during operative vaginal delivery on the risk of developing obstetrical anal sphincter injuries. Am J Obstet Gy 2012;206:404.e1-404.e5. 10.1016/j.ajog.2012.02.008 [DOI] [PubMed]
- 41.Gachon B, Fradet Menard C, Pierre F, Fritel X. Does the implementation of a restrictive episiotomy policy for operative deliveries increase the risk of obstetric anal sphincter injury? Arch Gynecol Obstet. 2019;300:87–94. doi: 10.1007/s00404-019-05174-0. [DOI] [PubMed] [Google Scholar]
- 42.Jangö H, Langhoff-Roos J, Rosthøj S, Sakse A. Modifiable risk factors of obstetric anal sphincter injury in primiparous women: a population-based cohort study. Am J Obstet Gynecol. 2014;210:59.e1–59.e6. doi: 10.1016/j.ajog.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 43.Levin G, Rottenstreich A, Cahan T, et al. Does birthweight have a role in the effect of episiotomy on anal sphincter injury? Arch Gynecol Obstet. 2020;301:171–177. doi: 10.1007/s00404-020-05444-2. [DOI] [PubMed] [Google Scholar]
- 44.Marschalek M-L, Worda C, Kuessel L, et al. Risk and protective factors for obstetric anal sphincter injuries: A retrospective nationwide study. Birth. 2018;45:409–415. doi: 10.1111/birt.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Räisänen S, Vehviläinen-Julkunen K, Cartwright R, et al. Vacuum-assisted deliveries and the risk of obstetric anal sphincter injuries-a retrospective register-based study in Finland. BJOG. 2012;119:1307–1378. doi: 10.1111/j.1471-0528.2012.03455.x. [DOI] [PubMed] [Google Scholar]
- 46.Räisänen S, Vehviläinen-Julkunen K, Gissler M, et al. Lateral episiotomy protects primiparous but not multiparous women from obstetric anal sphincter rupture. Acta Obstet Gynecol Scand. 2009;88:1365–1372. doi: 10.3109/00016340903295626. [DOI] [PubMed] [Google Scholar]
- 47.Rognant S, Benoist G, Creveuil C, Dreyfus M. Obstetrical situations with a high risk of anal sphincter laceration in vacuum-assisted deliveries. Acta Obstet Gynecol Scand. 2012;91:862–868. doi: 10.1111/j.1600-0412.2012.01401.x. [DOI] [PubMed] [Google Scholar]
- 48.Schmitz T, Alberti C, Andriss B, et al. Identification of women at high risk for severe perineal lacerations. Eur J Obstet Gynecol Reprod Biol. 2014;182:11–15. doi: 10.1016/j.ejogrb.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 49.Shmueli A, Gabbay Benziv R, Hiersch L, et al. Episiotomy – risk factors and outcomes. The Journal of Maternal-Fetal & Neonatal Medicine. 2017;20:251–256. doi: 10.3109/14767058.2016.1169527. [DOI] [PubMed] [Google Scholar]
- 50.van Bavel J, Hukkelhoven CWPM, de Vries C, et al. The effectiveness of mediolateral episiotomy in preventing obstetric anal sphincter injuries during operative vaginal delivery: a ten-year analysis of a national registry. Int Urogynecol J. 2018;29:407–413. doi: 10.1007/s00192-017-3422-4. [DOI] [PubMed] [Google Scholar]
- 51.Yamasato K, Kimata C, Huegel B, et al. Restricted episiotomy use and maternal and neonatal injuries: a retrospective cohort study. Arch Gynecol Obste. 2016;294:1189–1194. doi: 10.1007/s00404-016-4154-2. [DOI] [PubMed] [Google Scholar]
- 52.Youssef R, Ramalingam U, Macleod M, Murphy DJ. Cohort study of maternal and neonatal morbidity in relation to use of episiotomy at instrumental vaginal delivery. BJOG. 2005;112:941–945. doi: 10.1111/j.1471-0528.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 53.Meyer R, Rottenstreich A, Kees S, et al. Low volume forceps practice and anal sphincter injury rate. Arch Gynecol Obstet. 2020;301:1133–1138. doi: 10.1007/s00404-020-05519-0. [DOI] [PubMed] [Google Scholar]
- 54.Ampt AJ, Ford JB, Roberts CL, Morris JM. Trends in obstetric anal sphincter injuries and associated risk factors for vaginal singleton term births in New South Wales 2001-2009. Aust N Z J Obstet Gynaecol. 2013;53:9–16. doi: 10.1111/ajo.12038. [DOI] [PubMed] [Google Scholar]
- 55.Vathanan V, Ashokkumar O, McAree T. Obstetric anal sphincter injury risk reduction: a retrospective observational analysis. J Perinat Med. 2014;42. 10.1515/jpm-2013-0269. [DOI] [PubMed]
- 56.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 57.Baghestan E, Irgens LM, Børdahl PE, Rasmussen S. Trends in Risk Factors for Obstetric Anal Sphincter Injuries in Norway. Obstet Gynecol. 2010;116:25–34. doi: 10.1097/AOG.0b013e3181e2f50b. [DOI] [PubMed] [Google Scholar]
- 58.Eogan M, Daly L, O’Connell PR, O’Herlihy C. Does the angle of episiotomy affect the incidence of anal sphincter injury? BJOG. 2006;113:190–194. doi: 10.1111/j.1471-0528.2005.00835.x. [DOI] [PubMed] [Google Scholar]
- 59.Freeman RM, Hollands HJ, Barron LF, Kapoor DS. Cutting a mediolateral episiotomy at the correct angle: evaluation of a new device, the Episcissors-60. Med Devices (Auckl) . 2014;7:23–28. doi: 10.2147/MDER.S60056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andrews V, Thakar R, Sultan AH. Are mediolateral episiotomies actually mediolateral? BJOG: An International Journal of Obstetrics & Gynaecology. 2005;112:1156–1158. doi: 10.1111/j.1471-0528.2005.00645.x. [DOI] [PubMed] [Google Scholar]
- 61.Kudish B, Blackwell S. Operative vaginal delivery and midline episiotomy: a bad combination for the perineu. Am J Obstet Gynecol. 2006;195:749–754. doi: 10.1016/j.ajog.2006.06.078. [DOI] [PubMed] [Google Scholar]
- 62.Macleod M, Murphy DJ. Operative vaginal delivery and the use of episiotomy—A survey of practice in the United Kingdom and Ireland. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2008;136:178–183. doi: 10.1016/j.ejogrb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Sultan AH, Thakar R, Ismail KM, et al. The role of mediolateral episiotomy during operative vaginal delivery. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2019;240:192–196. doi: 10.1016/j.ejogrb.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Seijmonsbergen-Schermers AE, Geerts CC, Prins M, et al. The Use of Episiotomy in a Low-Risk Population in The Netherlands: A Secondary Analysis. Birth. 2013;40:247–255. doi: 10.1111/birt.12060. [DOI] [PubMed] [Google Scholar]
- 65.Hannah EL, et al. Randomized Clinical Trials and Observational Studies. JACC: Cardiovascular Interventions. 2008;1:211–217. doi: 10.1016/j.jcin.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 66.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McPherson KC, Beggs AD, Sultan AH, Thakar R. Can the risk of obstetric anal sphincter injuries (OASIs) be predicted using a risk-scoring system? BMC Research Notes . 2014;7:471. doi: 10.1186/1756-0500-7-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 165 kb)