Abstract
Sulfonyl fluorides are key components in the fields of chemical biology, materials science and drug discovery. In this line, the highly active SO2F radical has been employed for the construction of sulfonyl fluorides, but the utilization of gaseous ClSO2F as radical precursor is limited due to the tedious and hazardous preparation. Meanwhile, the synthesis of sulfonyl fluorides from inert SO2F2 gas through a fluorosulfonyl radical (·SO2F) process has met with inevitable difficulties due to the high homolytic bond dissociation energy of the S(VI)-F bond. Here we report a radical fluorosulfonylation strategy for the stereoselective synthesis of alkenyl sulfonyl fluorides and functional alkyl sulfonyl fluorides with an air-stable crystalline benzimidazolium fluorosulfonate cationic salt reagent. This bench-stable redox-active reagent offers a useful and operational protocol for the radical fluorosulfonylation of unsaturated hydrocarbons with good yield and high stereoselectivity, which can be further transformed into valuable functional SO2F moieties.
Subject terms: Synthetic chemistry methodology, Photocatalysis, Reactive precursors
Sulfonyl fluorides have potential application in chemical biology, materials science, and drug discovery, but their preparation remains challenging. Here, the authors report an air-stable fluorosulfonylating reagent that enables the radical fluorosulfonylation, hydrofluorosulfonylation and migratory SO2F-difunctionalization of unsaturated hydrocarbons to construct a variety of sulfonyl fluoride compounds.
Introduction
The sulfur(VI) fluoride exchange (SuFEx) chemistry that rely on the unique reactivity–stability balance of high valent organosulfur has emerged as a promising topic for the next-generation click reaction1. Sulfonyl fluorides as the most widely used connective hubs of SuFEx click reaction have attracted enormous attention and find widespread applications in the fields of chemical biology2–6, drug discovery7–11 and materials science12–16. Methods have been developed for rapid construction of sulfonyl fluoride moiety, including the chloride-fluoride exchange of sulfonyl chlorides17–19, SO2 insertion/fluorination20–23, electrophilic fluorination of thiols and anodic oxidative fluorination24–27. Compared with the above mentioned S-F bond formation, direct fluorosulfonylation would provide a concise and redox economic approach for C-SO2F bond formation. The sulfonyl fluoride building-block28,29 including alkynylsulfonyl fluoride (SASF)30, ethenesulfonyl fluoride (ESF), 1-bromoethene-1-sulfonyl fluoride (BESF) were used to access functionalized sulfonyl fluorides. The highly active SO2F radical has been recognized as unstable and inaccessible precursor until the observation of this species from the decomposition of fluorosulfonyl azide and the recent progress of photoinduced radical fluorosulfonylation using gaseous ClSO2F31–36. However, the application of N3SO2F and ClSO2F were limited by tedious and hazardous preparation. Sulfuryl fluoride (SO2F2) as abundant inflammable industrial feedstock could serve as economic sulfonyl fluoride source1. Sulfuryl fluoride derived fluorosulfonylating reagents are mainly electrophilic “FSO2+” synthons and have been employed for direct functionalization of different nucleophiles including organometallic reagents, phenols, amines, etc.37–40. However, the construction of diversified sulfonyl fluoride compounds was limited by single electrophilic reaction pattern and hindered multifunctionalization of SO2F2 and derivatives. In contrast, adopting a radical synthesis strategy can overcome the limitations of electrophilic fluorosulfonylation and expand the scope of application of sulfonyl fluoride. However, the generation of fluorosulfonyl radical (·SO2F) from inert SO2F2 gas has met with inevitable difficulties due to the relatively small magnetic/quadrupole moments and the high homolytic bond dissociation energy of the S(VI)-F bond (BDE = 90.5 ± 4.3 kcal/mol)1 (Fig. 1a). Thus, the development of single electron transfer (SET) process of SO2F2 for radical fluorosulfonylation represents great challenge and in high demand.
Fig. 1. Origin of the reaction design.
a The activation of SO2F2 for electrophilic and radical fluorosulfonylation. b The design and synthesis of benzimidazolium triflate derived IMSF reagent. c This chemistry: Cationic IMSF reagent for radical fluorosulfonylation of alkenes.
A practical procedure for the bench-stable redox-active ·SO2F agent from inexpensive fluorine source would provide appropriate solution to the long-standing issue of radical fluorosulfonylation. The imidazolium sulfonate cationic salt that developed in our lab has been successfully applied for the activation of triflic acid and arylsulfonates to access ·SO2CF3 and ArS· radicals41–43. We speculated that the cationic benzimidazole salt could harness the highly electrophilic SO2F to forge a bench-stable redox-active (Het)N-SO2F reagent (Fig. 1c)44,45. The positive charge of the resulting benzimidazolium fluorosulfonate can be delocalized on both nitrogens. By the homolytic cleavage of the weak N–S bond (BDE ≈ 70 kcal/mol)46, this cationic complex undergoes SET process to generate fluorosulfonyl radical (Fig. 1d). In this work, we synthesize a series of highly reactive radical fluorosulfonylating reagents IMSF (2a-2e), practical and air-stable crystalline salts for a sequential radical stereoselective fluorosulfonylation, hydrofluorosulfonylation and migratory SO2F-difunctionalization of unsaturated hydrocarbon to construct a variety of functionalized sulfonyl fluoride compounds.
Results
Reaction optimization
Our study began with N-methyl-N-(1-phenylvinyl)acetamide (1a) as the model substrate (Table 1). After extensive screening of conditions, we found that when using 2 equivalents of benzimidazolium sulfonate reangent (IMSF, 2a, E1/2red = −1.07 V vs SCE), 2 mol% of 4CzIPN, 2.5 equivalents of KH2PO4 in DME (1 mL) under the irradiation of 60 W blue LEDs, the alkenyl sulfonyl fluoride product 3a could be obtained in 62% yield with >20:1 E/Z ratio. Different benzimidazolium sulfonate 2b-2e were then examined (Table 1). When imidazolium sulfonate reagent 2b were used, the yield of 3a was obtained in 71% yield and isolated yield is 65% (entry 2). Other arylimidazole heterocycle with electron withdrawing groups derived IMSF salts 2c and 2d furnished alkenyl sulfonyl fluoride 3a in 58% and 64% yield, respectively (entries 3 and 4). The cationic reagent 2e resulted in a lower conversion under irradiation, which may due to the relatively high negative reduction potential (entry 5). When Ir(ppy)3 instead of 4CzIPN as photocatalyst, the yield of product 3a was slightly reduced (entry 6). When using other reaction solvents (Supplementary Table S1), the yield of desired product 3a has significantly decreased and obtained in a low yield (entry 7). The yield of alkenyl sulfonyl fluoride was reduced in the absence of KH2PO4 because of the hindered α-hydrogen elimination process (entry 8). In addition, control experiments suggested that photocatalyst, and light irradiation are all crucial to the reaction (entries 9–10).
Table 1.
Optimization of the reaction conditions.
| Entry | Variation from the conditions | Yield of 3aa/% | E:Z of 3ab |
|---|---|---|---|
| 1 | None | 62 | >20:1 |
| 2 | 2b instead of 2a | 71 (65)c | >20:1 |
| 3 | 2c instead of 2a | 58 | >20:1 |
| 4 | 2d instead of 2a | 64 | >20:1 |
| 5 | 2e instead of 2a | 16 | >20:1 |
| 6 | Ir(ppy)3 instead of 4CzIPN | 59 | >20:1 |
| 7 | Other solvents instead of DME | 0–41% | >20:1 |
| 8 | w/o KH2PO4 | 45 | >20:1 |
| 9 | w/o 4CzIPN | 0 | — |
| 10 | In the darkness | 0 | — |
a Yield determined by gas chromatography (GC) using dodecane as an internal standard; b The Z/E ratio was determined by 1H NMR and GC; c Isolated yield.
Substrate scope with respect to the radical alkenyl sulfonyl fluoride reaction
With the optimized reaction conditions in hand, we next examined the generality of this transformation with different alkenes. Using 2 mol% of 4CzIPN, IMSF salt 2b (2.0 equiv), and KH2PO4 (2.5 equiv) at ambient temperature, a range of alkenes underwent radical fluorosulfonylation with high efficiency. As shown in Fig. 2, 1,1-disubstituted alkenes with methyl, aryl, ester, amide groups afforded the desired products (3a-3l) in moderate to good yields with high regio- and stereoselectivity (E:Z > 20:1). Styrene with different substituents including halides, alkyl, ester afforded the desired products (3n-3r, 3 v) in moderate to excellent yields and high regio- and stereoselectivity (E:Z > 20:1). In addition, 1,2-dihydronaphthalene (3 s), 2-vinylthiophene (3t), 2-vinylpyridine (3 u) could all be smoothly fluorosulfonylated with FSO2 radical. Moreover, natural products derivatized olefin involving cholesterol and estrone (3w-3x) can also be tolerated under the mild photocatalytic conditions and obtained the corresponding alkenyl sulfonyl fluoride in moderate yields. The selective preparation of E-alkenyl sulfonyl fluoride has been readily accessible. Then we try to control the reaction conditions to achieve the synthesis of thermodynamically less favorable Z-alkenyl sulfonyl fluoride. By variation of the reaction conditions (see Supplementary Table S5), we have extended this radical fluorosulfonylation protocol to achieve Z-alkenyl sulfonyl fluoride. Styrene with different substituents including halides, alkyl group afforded the desired products (4b-4e, 4f-4g) in moderate to good yields. Bioative DL-menthol and bexarotene derived alkenes could afford the desired alkenyl sulfonyl fluoride in moderate yields (4h-4i).
Fig. 2. Substrate scope of the radical alkenylsulfonyl fluoride reaction.
a.Condition A: all reactions were carried out with olefins 1, IMSF salt 2b (0.20 mmol, 2 equiv), 4CzIPN (2 mol%), KH2PO4 (0.25 mmol, 2.5 equiv) in DME (1.0 mL) under Ar and 60 W blue LEDs. b.Condition B: all reactions were carried out with olefins 1, IMSF salt 2b (0.20 mmol, 2 equiv), PC 1 (2 mol%), KH2PO4 (0.25 mmol, 2.5 equiv) in EA:DME = 4:1 (1.0 mL) under Ar and 60 W blue LEDs, overnight, then 1.5 mL of acetonitrile containing Ir{[dF(CF3)ppy]2(dtbbpy)}PF6 (2 mol%) was added to react about 12 h. c.The E/Z ratio was determined by 19F NMR. d.The E/Z ratio was determined by 1H NMR. e.Isolated yield.
Substrate scope with respect to the radical hydrofluorosulfonylation reaction
The late stage functionalization of sulfonyl fluoride has been unearthed by Sharpless lab in 2014 with the development of SuFEx chemistry. Along this line, this radical fluorosulfonylation protocol was applied to the late-stage modification of complex molecules47,48. Using 2 mol% of the iridium catalyst, 1,4-cyclohexadiene as hydrogen donor (1.5 equiv), and IMSF salt 2 (2.0 equiv) at ambient temperature, unactivated terminal alkenes underwent a radical fluorosulfonylation process to product corresponding alkylsulfonyl fluoride with good regioselectivity (Fig. 3). Terminal alkenes bearing amide and ester functionalities obtained the desired alkylsulfonyl fluorides in moderate to good yields (6a-6c, 6e, 6 f, 6h-6k). Oxyalkyl-substituted alkenes also furnished the corresponding SO2F adducts (6 g). In addition, IMSF reagent 2a were employed in intramolecular cyclization process with diallyl sulphonamides to afford the corresponding sulfonyl fluoride product (6d).
Fig. 3. Substrate scope of the radical hydrofluorosulfonylation.
All reactions were carried out with olefins 5 (0.10 mmol), IMSF salt 2(0.20 mmol, 2.0 equiv), fac-Ir(d-F-(p-t-Bu)ppy)3 (2 mol%) and cyclohexa-1,4-diene (1.5 equiv) in 2-methyltetrahydrofuran:Acetone = 9:1 (1 mL) under Ar and 60 W blue LEDs. a.IMSF reagent 2a was used. b.IMSF reagent 2b was used.
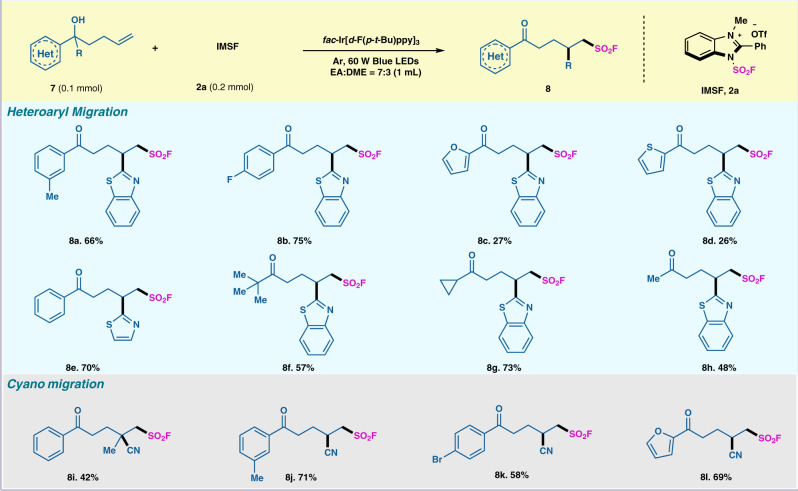
Substrate scope with respect to migration fluorosulfonylation reaction
With slight variation of the optimized conditions49,50, we expanded the scope of this radical fluorosulfonylation protocol to difunctionalization of unactivated olefins. Using a heteroaryl-substituted unsaturated tertiary alcohol, the distal migration induced by fluorosulfonyl radical proceeded smoothly in a chemoselective fashion. Fluorosulfonyl radical were susceptible to the reaction conditions for achieving the corresponding ketones in good to excellent yields (Fig. 4). The aryl groups with different electronic and steric characters were tolerated (8a-8b). Thiophene and furan functionalities could be compatible under the mild condition (8c-8d). Linear or cyclic alkyl substituted unsaturated tertiary alcohols could also get the difunctionalized sulfonyl fluoride products (8f-8h). Noteworthy, the distal migration cyanation of unsaturated tertiary alcohol mediated by SO2F radical also proceeded smoothly to afford desired product in good yield. The aryl group with electron donating and electron withdrawing groups and furan can afford the desired products (8j-8l) in good yields.
Fig. 4. Substrate scope of radical migration fluorosulfonylation.
All reactions were carried out with alkenes 7 (0.10 mmol), IMSF salt 2a (0.20 mmol, 2.0 equiv.), fac-Ir[d-F(p-t-Bu)ppy]3 (2 mol%) in DME:EA = 4:1 (1.0 mL) under Ar and 60 W blue LEDs about 12 h.
Synthetic applications and mechanistic studies
The utility of the products with sulfonyl fluoride group was demonstrated (Fig. 5a–e). In the presence of NaHCO3 and DBU, alkenylsulfonyl fluoride 3 m can easily react with pyrazolone 9 to afford the sulfone product 10 in 50% yield51,52. In addition, sulfonyl fluoride 3 m can also efficiently react with 1,3-cyclohexanedione 11 to generate the sultone 12 (Fig. 5a)53. It is well known that sulfonyl fluoride species can readily undergo various SuFEx reactions to connect other molecules54. Several SuFEx reactions of selective modifying the hydroxyl site of drugs were implemented. We tentatively tried the ligation of styrenesulfonyl fluoride 3 m with estrone 1355, which can afford the desired product 14 in 60% yield (Fig. 5b). Then the ligation of lumacator intermediate derivative (6 f) and vitamin E (15) reacted smoothly to furnish the desired product 16 in good yield (Fig. 5c).
Fig. 5. Synthetic applications and mechanistic studies.
a Cycloaddition of alkenylsulfonyl fluoride. b, c SuFEx click reaction of alkenylsulfonyl fluoride 3 m. d Radical inhibition experiment. e Free radical clock experiment. f Control experiment for the production of Z-alkenylsulfonyl fluoride.
To gain insight into this reaction, several mechanistic experiments have been carried out. The radical trapping experiment using 2 equiv. of TEMPO resulted in the inhibition of the radical addition. Instead, TEMPO adduct 17 was detected in HRMS (Fig. 5d). The radical clock experiment was carried out using cyclopropyl styrene 1. Under the standard conditions with MgCl2 additive, the ring-opened product 19 can be obtained in 11% isolated yield (Fig. 5e). Thus, fluorosulfonyl radical (·SO2F) intermediates are possibly involved in reaction. In addition, the treatment of E-alkenylsulfonyl fluoride 3n with iridium catalyst in the absence of IMSF reagent furnished the Z-alkenylsulfonyl fluoride 4c in 43% yield and recovered 3n in 48% yield (Fig. 5f). This control experiment showed that the generation of Z-alkenylsulfonyl fluoride probably underwent an olefin isomerization process56.
Discussion
In summary, we have described an air-stable redox-active imidazolium fluorosulfonate reagent IMSF. A key design feature of this radical fluorosulfonylating reagent is the cationic nature, which favors the stepwise formation of fluorosulfonyl radical (·SO2F) via a SET reduction process under photocatalytic conditions. This SO2F radical reservoir could react with various alkenes to produce alkenyl sulfonyl fluoride, alkylsulfonyl fluoride, and migratory fluorosulfonylating products. Further studies of this highly reactive and air-stable solid reagent are underway in our laboratory.
Methods
General procedure for the synthesis of sulfonyl fluoride imidazolium salt reagent 2
Sodium hydride (60% dispersion in mineral oil.) (36 mmol, 1.2 equiv.) was added to corresponding imidazole (30 mmol, 1 equiv.) in dry DMF (100 mL). The mixture was stirred for 1 h; A balloon volume of sulfuryl fluoride gas was then added to the reaction system. The reaction progress was monitored by TLC. After the reaction was completed, the solvent was evaporated in vacuo. Then, the reaction crude was quenched with water and extracted with ethyl acetate (60 mL × 3). The organic layer was dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography on silica gel with n-pentane/ethyl acetate as the eluent to give the corresponding 2- aryl-1H-benzo[d]imidazole-1-sulfonyl fluoride. Then a solution of the corresponding 2- aryl-1H-benzo[d]imidazole-1-sulfonyl fluoride in DCM (50 mL) was added dropwise MeOTf (45 mmol) at 0 °C. Then, the mixture was stirred at room temperature for 12 h, while monitoring by TLC. After that time, the mixture was concentrated under rotary evaporation to give a white solid (or a viscous liquid) crude product, to which tert-Butyl methyl ether (30 mL) was added. With vigorous stirring, a solid precipitate was formed. The precipitate was washed with tert-Butyl methyl ether (30 mL × 3) and dried in vacuo to yield the title compound (2a-2e) as a white solid.
General procedure for the synthesis of product 3
Condition A: Under argon, to a solution of 4CzIPN (2 mol%), KH2PO4 (2.5 equiv) and IMSF reagent 2b (0.2 mmol, 2 equiv.) in dried DME (1 mL) was added corresponding alkenes 1 (0.1 mmol) at room temperature. After that, the tube was exposed to a 60 W blue LEDs about 10 h until the reaction was completed as monitored by TLC analysis. The reaction mixture was evaporated in vacuo. The crude products were directly purified by flash chromatography on silica gel to give the desired product.
General procedure for the synthesis of product 4
Condition B: Under argon, to a solution of PC 1 (2 mol%), KH2PO4 (2.5 equiv) and IMSF reagent 2b (0.2 mmol, 2 equiv.) in dried EA:DME = 4:1 (1 mL) was added corresponding alkenes 1 (0.1 mmol) at room temperature. After that, the tube was exposed to a 60 W blue LEDs about 12 h, then 1.5 ml of acetonitrile containing Ir{[dF(CF3)ppy]2(dtbbpy)}PF6 (2 mol%) was injected into the reaction tube about 12 h until the reaction was completed as monitored by TLC analysis. The reaction mixture was evaporated in vacuo.The crude products were directly purified by flash chromatography on silica gel to give the desired product.
General procedure for the synthesis of product 6
Condition C: Under argon, to a solution of fac-Ir[d-F-(p-t-Bu)ppy]3 (2 mol%), 1,4-cyclohexadiene (1.5 equiv.) and IMSF reagent 2 (0.2 mmol, 2 equiv.) in dried 2-methyltetrahydrofuran:Acetone = 9:1 (1 mL) was added corresponding alkenes 5 (0.1 mmol) at room temperature. After that, the tube was exposed to a 60 W blue LEDs about 12 h, then until the reaction was completed as monitored by TLC analysis. The reaction mixture was evaporated in vacuo. The crude products were directly purified by flash chromatography on silica gel to give the desired product.
General procedure for the synthesis of product 8
Condition D: Under argon, to a solution of fac-Ir[d-F-(p-t-Bu)ppy]3 (2 mol%), and IMSF reagent 2a (0.2 mmol, 2 equiv.) in dried EA:DME = 7:3 (1 mL) was added corresponding alkenes 7 (0.1 mmol) at room temperature. After that, the tube was exposed to a 60 W blue LEDs about 12 h, then until the reaction was completed as monitored by TLC analysis. The reaction mixture was evaporated in vacuo. The crude products were directly purified by flash chromatography on silica gel to give the desired product.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 21971107, and 2201101) and China Postdoctoral Science Foundation (2021T140309 and 2021M691511).
Author contributions
Y.W. and W.Z. designed and guided this project. H.L. is responsible for the plan and implementation of the experimental work. X.L., Z.Z., M.H., J. L. and X.W. analyzed the data. Y.W. and W.Z. co-wrote the manuscript. S.N. and Y.P. discussed the results and commented on the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The authors declare that the main data supporting the findings of this study, including experimental procedures and compound characterization, are available within the article and its Supplementary Information files, or from the corresponding author upon request. X-ray structural data of compound 2b are available free of charge from the Cambridge Crystallographic Data Center under the deposition number CCDC 2164689. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally:Weigang Zhang, Heyin Li.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-31296-2.
References
- 1.Dong J, Krasnova L, Finn MG, Sharpless KB. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem. Int. Ed. 2014;53:9430–9448. doi: 10.1002/anie.201309399. [DOI] [PubMed] [Google Scholar]
- 2.Jones LH. Emerging utility of fluorosulfate chemical probes. ACS Med. Chem. Lett. 2018;9:584–586. doi: 10.1021/acsmedchemlett.8b00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin-Gago P, Olsen C. A. arylfluorosulfate-based electrophiles for covalent protein labeling: a new addition to the arsenal. Angew. Chem. Int. Ed. 2019;58:957–966. doi: 10.1002/anie.201806037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Q, et al. Broad-Spectrum kinase profiling in live cells with lysine-targeted sulfonyl fluoride probes. J. Am. Chem. Soc. 2017;139:680–685. doi: 10.1021/jacs.6b08536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mortenson DE, et al. “Inverse drug discovery” strategy to identify proteins that are targeted by latent electrophiles as exemplified by aryl fluorosulfates. J. Am. Chem. Soc. 2018;140:200–210. doi: 10.1021/jacs.7b08366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang N, et al. Genetically encoding fluorosulfate-l-tyrosine to react with lysine, histidine, and tyrosine via SuFEx in proteins in vivo. J. Am. Chem. Soc. 2018;140:4995–4999. doi: 10.1021/jacs.8b01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubiella C, et al. Selective inhibition of the immunoproteasome by ligand-induced crosslinking of the active site. Angew. Chem. Int. Ed. 2014;53:11969–11973. doi: 10.1002/anie.201406964. [DOI] [PubMed] [Google Scholar]
- 8.Hett EC, et al. Rational targeting of active-site tyrosine residues using sulfonyl fluoride probes. ACS Chem. Biol. 2015;10:1094–1098. doi: 10.1021/cb5009475. [DOI] [PubMed] [Google Scholar]
- 9.Herrero Alvarez N, van de Langemheen H, Brouwer AJ, Liskamp RMJ. Potential peptidic proteasome inhibitors by incorporation of an electrophilic trap based on amino acid derived alpha-substituted sulfonyl fluorides. Bioorg. Med. Chem. 2017;25:5055–5063. doi: 10.1016/j.bmc.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, et al. Arylfluorosulfates inactivate intracellular lipid binding protein(s) through chemoselective SuFEx reaction with a binding site tyr residue. J. Am. Chem. Soc. 2016;138:7353–7364. doi: 10.1021/jacs.6b02960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gehringer M, Laufer SA. Emerging and re-emerging warheads for targeted covalent inhibitors: applications in medicinal chemistry and chemical biology. J. Med. Chem. 2019;62:5673–5724. doi: 10.1021/acs.jmedchem.8b01153. [DOI] [PubMed] [Google Scholar]
- 12.Randall JD, et al. Modification of carbon fibre surfaces by sulfur-fluoride exchange click chemistry. Chemphyschem. 2018;19:3176–3181. doi: 10.1002/cphc.201800789. [DOI] [PubMed] [Google Scholar]
- 13.Durie K, et al. Multifunctional surface manipulation using orthogonal click chemistry. Langmuir. 2016;32:6600–6605. doi: 10.1021/acs.langmuir.6b01591. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, et al. SuFEx-based polysulfonate formation from ethenesulfonyl fluoride-amine adducts. Angew. Chem. Int. Ed. 2017;56:11203–11208. doi: 10.1002/anie.201701160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang C, Flynn JP, Niu J. Facile synthesis of sequence-regulated synthetic polymers using orthogonal SuFEx and CuAAC click reactions. Angew. Chem. Int. Ed. 2018;57:16194–16199. doi: 10.1002/anie.201811051. [DOI] [PubMed] [Google Scholar]
- 16.Gao B, et al. Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates. Nat. Chem. 2017;9:1083–1088. doi: 10.1038/nchem.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stepannikova KO, et al. Synthesis of spirocyclic beta- and gamma-sultams by one-pot reductive cyclization of cyanoalkylsulfonyl fluorides. Eur. J. Org. Chem. 2021;2021:6530–6540. doi: 10.1002/ejoc.202000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianchi TA, Cate LA. Phase transfer catalysis. preparation of aliphatic and aromatic sulfonyl fluorides. J. Org. Chem. 2002;42:2031–2032. doi: 10.1021/jo00431a054. [DOI] [Google Scholar]
- 19.Dubbaka SR, Vogel P. One-pot synthesis of 1-aryl-3-methyl-1,3-dienes using methallyl(trimethyl)silane and aldehydes and their low temperature (Z)→(E) isomerization induced by sulfur dioxide. Tetrahedron. 2005;61:1523–1530. doi: 10.1016/j.tet.2004.11.070. [DOI] [Google Scholar]
- 20.Davies AT, Curto JM, Bagley SW, Willis MC. One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides. Chem. Sci. 2017;8:1233–1237. doi: 10.1039/C6SC03924C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lou TS, Bagley SW, Willis MC. Cyclic alkenylsulfonyl fluorides: palladium-catalyzed synthesis and functionalization of compact multifunctional reagents. Angew. Chem. Int. Ed. 2019;58:18859–18863. doi: 10.1002/anie.201910871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, et al. Arenesulfonyl fluoride synthesis via copper-catalyzed fluorosulfonylation of arenediazonium salts. Org. Lett. 2020;22:2281–2286. doi: 10.1021/acs.orglett.0c00484. [DOI] [PubMed] [Google Scholar]
- 23.Zhong T, et al. Photoredox-catalyzed aminofluorosulfonylation of unactivated olefins. Chem. Sci. 2021;12:9359–9365. doi: 10.1039/D1SC02503A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirihara M, et al. Oxidation of disulfides with electrophilic halogenating reagents: concise methods for preparation of thiosulfonates and sulfonyl halides. Tetrahedron. 2014;70:2464–2471. doi: 10.1016/j.tet.2014.02.013. [DOI] [Google Scholar]
- 25.Wang L, Cornella J. A unified strategy for arylsulfur(VI) fluorides from aryl halides: access to Ar-SOF3 compounds. Angew. Chem. Int. Ed. 2020;59:23510–23515. doi: 10.1002/anie.202009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao Y, et al. Accelerating sulfonyl fluoride synthesis through electrochemical oxidative coupling of thiols and potassium fluoride in flow. J. Flow. Chem. 2020;10:191–197. doi: 10.1007/s41981-019-00070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laudadio G, et al. Sulfonyl fluoride synthesis through electrochemical oxidative coupling of thiols and potassium fluoride. J. Am. Chem. Soc. 2019;141:11832–11836. doi: 10.1021/jacs.9b06126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrow AS, et al. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 2019;48:4731–4758. doi: 10.1039/C8CS00960K. [DOI] [PubMed] [Google Scholar]
- 29.Meng Y-P, et al. Ethenesulfonyl fluoride (ESF) and Its derivatives in SuFEx click chemistry and more. Synthesis. 2019;52:673–687. [Google Scholar]
- 30.Smedley CJ, et al. Diversity oriented clicking (DOC): divergent synthesis of SuFExable pharmacophores from 2-substituted-alkynyl-1-sulfonyl fluoride (SASF) hubs. Angew. Chem. Int. Ed. 2020;59:12460–12469. doi: 10.1002/anie.202003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng X, Beckers H, Willner H. Thermally persistent fluorosulfonyl nitrene and unexpected formation of the fluorosulfonyl radical. J. Am. Chem. Soc. 2013;135:2096–2099. doi: 10.1021/ja312073w. [DOI] [PubMed] [Google Scholar]
- 32.Nie X, et al. Radical fluorosulfonylation: accessing alkenyl sulfonyl fluorides from alkenes. Angew. Chem. Int. Ed. 2021;60:3956–3960. doi: 10.1002/anie.202012229. [DOI] [PubMed] [Google Scholar]
- 33.Nie X, et al. Introducing a new class of sulfonyl fluoride hubs via radical chloro-fluorosulfonylation of alkynes. Angew. Chem. Int. Ed. 2021;60:22035–22042. doi: 10.1002/anie.202109072. [DOI] [PubMed] [Google Scholar]
- 34.Frye, N. L. et al. Radical 1-fluorosulfonyl-2-alkynylation of unactivated alkenes. Angew. Chem. Int. Ed. 61, 10.1002/anie.202115593 (2022). [DOI] [PMC free article] [PubMed]
- 35.Chen D, et al. Electrochemical oxo-fluorosulfonylation of alkynes under air: facile access to beta-keto sulfonyl fluorides. Angew. Chem. Int. Ed. 2021;60:27271–27276. doi: 10.1002/anie.202112118. [DOI] [PubMed] [Google Scholar]
- 36.Feng Q, et al. Electrochemical synthesis of β-keto sulfonyl fluorides via radical fluorosulfonylation of vinyl triflates. Org. Lett. 2022;24:3702–3706. doi: 10.1021/acs.orglett.2c01336. [DOI] [PubMed] [Google Scholar]
- 37.Zhou H, et al. Introduction of a crystalline, shelf-stable reagent for the synthesis of sulfur(VI) fluorides. Org. Lett. 2018;20:812–815. doi: 10.1021/acs.orglett.7b03950. [DOI] [PubMed] [Google Scholar]
- 38.Chen W, et al. Synthesis of sulfotyrosine-containing peptides by incorporating fluorosulfated tyrosine using an fmoc-based solid-phase strategy. Angew. Chem. Int. Ed. 2016;55:1835–1838. doi: 10.1002/anie.201509016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo T, et al. A new portal to SuFEx click chemistry: a stable fluorosulfuryl imidazolium salt emerging as an “F-SO2+” donor of unprecedented reactivity, selectivity, and scope. Angew. Chem. Int. Ed. 2018;57:2605–2610. doi: 10.1002/anie.201712429. [DOI] [PubMed] [Google Scholar]
- 40.Lee C, Ball ND, Sammis GM. One-pot fluorosulfurylation of grignard reagents using sulfuryl fluoride. Chem. Commun. 2019;55:14753–14756. doi: 10.1039/C9CC08487H. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, et al. Integrated redox-active reagents for photoinduced regio- and stereoselective fluorocarboborylation. Nat. Commun. 2020;11:2572. doi: 10.1038/s41467-020-16477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, et al. Redox-active benzimidazolium sulfonamides as cationic thiolating reagents for reductive cross-coupling of organic halides. Chem. Sci. 2021;12:2509–2514. doi: 10.1039/D0SC06446G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, W. et al. Sulfonamide imidazole salt compound and preparation method and application thereof. Chinese Patent. CN 111187219 A (2020).
- 44.Liao, S. et al. Fluorosulfonyl free radical reagent, and preparation method and application thereof. Chinese Patent. CN 113248444 A (2021).
- 45.Zheng W, et al. Redox-active reagents for photocatalytic generation of the OCF3 radical and (hetero)aryl C-H trifluoromethoxylation. Angew. Chem. Int. Ed. 2018;57:13795–13799. doi: 10.1002/anie.201808495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kraka E, Setiawan D, Cremer D. Re-evaluation of the bond length-bond strength rule: the stronger bond is not always the shorter bond. J. Comput. Chem. 2016;37:130–142. doi: 10.1002/jcc.24207. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, et al. Leaving group assisted strategy for photoinduced fluoroalkylations using N-hydroxybenzimidoyl chloride esters. Angew. Chem. Int. Ed. 2019;58:624–627. doi: 10.1002/anie.201812192. [DOI] [PubMed] [Google Scholar]
- 48.Cannalire R, et al. Visible light photocatalysis in the late-stage functionalization of pharmaceutically relevant compounds. Chem. Soc. Rev. 2021;50:766–897. doi: 10.1039/D0CS00493F. [DOI] [PubMed] [Google Scholar]
- 49.Wu Z, et al. Chemo- and regioselective distal heteroaryl ipso-migration: a general protocol for heteroarylation of unactivated alkenes. J. Am. Chem. Soc. 2017;139:1388–1391. doi: 10.1021/jacs.6b11234. [DOI] [PubMed] [Google Scholar]
- 50.Zou Z, et al. Electrochemically promoted fluoroalkylation-distal functionalization of unactivated alkenes. Org. Lett. 2019;21:1857–1862. doi: 10.1021/acs.orglett.9b00444. [DOI] [PubMed] [Google Scholar]
- 51.Huang Y-M, et al. Converting (E)-(hetero)arylethanesulfonyl fluorides to (Z)-(hetero)arylethanesulfonyl fluorides under light irradiation. Eur. J. Org. Chem. 2019;2019:4597–4603. doi: 10.1002/ejoc.201900799. [DOI] [Google Scholar]
- 52.Chen X, et al. Synthesis of a class of fused δ-sultone heterocycles via DBU-catalyzed direct annulative SuFEx click of ethenesulfonyl fluorides and pyrazolones or 1,3-dicarbonyl compounds. Adv. Synth. Catal. 2017;359:3254–3260. doi: 10.1002/adsc.201700887. [DOI] [Google Scholar]
- 53.Chen Q, Mayer P, Mayr H. Ethenesulfonyl fluoride: the most perfect michael acceptor ever found? Angew. Chem. Int. Ed. 2016;55:12664–12667. doi: 10.1002/anie.201601875. [DOI] [PubMed] [Google Scholar]
- 54.Abdul Fattah T, Saeed A, Albericio F. Recent advances towards sulfur (VI) fluoride exchange (SuFEx) click chemistry. J. Fluor. Chem. 2018;213:87–112. doi: 10.1016/j.jfluchem.2018.07.008. [DOI] [Google Scholar]
- 55.Qin HL, et al. A heck-matsuda process for the synthesis of beta-arylethenesulfonyl fluorides: selectively addressable bis-electrophiles for SuFEx click chemistry. Angew. Chem. Int. Ed. 2016;55:14155–14158. doi: 10.1002/anie.201608807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nevesely T, et al. Advances in the E → Z isomerization of alkenes using small molecule photocatalysts. Chem. Rev. 2022;122:2650–2694. doi: 10.1021/acs.chemrev.1c00324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the main data supporting the findings of this study, including experimental procedures and compound characterization, are available within the article and its Supplementary Information files, or from the corresponding author upon request. X-ray structural data of compound 2b are available free of charge from the Cambridge Crystallographic Data Center under the deposition number CCDC 2164689. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.