Abstract
Background
Pain is a vital sense that indicates the risk of injury at a particular body part. Successful control of pain is the principal aspect in medical treatment. In recent years, the advances of nanotechnology in pain management have been remarkable. In this review, we focus on literature and published data that reveal various applications of nanotechnology in acute and chronic pain management.
Methods
The presented content is based on information collected through pain management publications (227 articles up to April 2021) provided by Web of Science, PubMed, Scopus and Google Scholar services.
Results
A comprehensive study of the articles revealed that nanotechnology-based drug delivery has provided acceptable results in pain control, limiting the side effects and increasing the efficacy of analgesic drugs. Besides the ability of nanotechnology to deliver drugs, sophisticated nanosystems have been designed to enhance imaging and diagnostics, which help in rapid diagnosis of diseases and have a significant impact on controlling pain. Furthermore, with the development of various tools, nanotechnology can accurately measure pain and use these measurements to display the efficiency of different interventions.
Conclusions
Nanotechnology has started a new era in the pain management and many promising results have been achieved in this regard. Nevertheless, there is still no substantial and adequate act of nanotechnology in this field. Therefore, efforts should be directed to broad investigations.
Graphical Abstract
Keywords: Nanomedicine, Nanoparticle, Analgesia, Pain, Nanotechnology
Keypoints
Zinc oxide (ZnO) and magnesium oxide (MgO) nanoparticles have themselves the intrinsic analgesic properties.
The emergence of theranostic nanomedicine provides an ideal method for accurate pain management.
Nanoneedles are new opportunities in the pain management.
Many nanoliposomal analgesic formulations have been industrialized and are currently on the market.
Introduction
The defensive mechanism of human body toward stimulants is demonstrated as pain, which is a common sign of diseases. Stimulants create pain by irritating free nerve endings in nociceptors (i.e., pain receptors) [1], wherein histamine, leukotrienes, substance P, acetylcholine, bradykinin, and prostaglandins are some of the chemical mediators that are responsible for the pain. On the other hand, vasodilatation, vasoconstriction, and changed capillary permeability result from these chemical mediators at the injury site [2].
Pain can be described as acute or chronic in terms of clinical aspect [3]. Insufficient pain management has considerable adverse effects, for example, inadequate control of acute pain can lead to serious medical complications, such as deep venous thrombosis and pneumonia, and progresses to chronic pain. In addition, poorly treated chronic pain can result in declined daily activities of the patient, decreasing the quality of life [4, 5]. Pain based on pathophysiology is generally classified as nociceptive or neuropathic pain. Nociceptors are receptors particularly sensitive to stimulants or tissue trauma that can create tissue damage if the exposure or incidence is prolonged. On the other hand, pain originating in the periphery is not always a nociceptive pain. Neuropathic pain can be produced by abnormal signal processing in the central or peripheral nervous system [6–8].
As with types of pain, several methods exist for the treatment of pain, and generally, they can be broadly categorized into pharmacological and non-pharmacological approaches. Pharmacological strategies include the use of analgesics and analgesic adjuvants (i.e., local anesthetics, nonsteroidal anti-inflammatory drugs, antiepileptic drugs, α2-adrenoceptor agonists, antidepressants, and opiates) to treat and relieve pain [9]. Whereas, non-pharmacological strategies refer to physical (i.e., acupuncture, hot and cold treatment, transcutaneous electrical nerve stimulation (TENS), and progressive muscle relaxation) and psychological (mindfulness-based stress reduction and cognitive behavioral therapy) interventions that can decrease fear, anxiety and pain intensity, and also support patients to play an active role in managing their symptoms [10].
Although various clinical studies have hitherto provided progressive results in the suppression of pain for the last decade [11], since the treatment of pain is very complex, the choice of effective and safe treatment for satisfactory pain management is still very difficult even for healthcare professionals. On the other hand, current therapeutics are not adequate to relieve some types of pain, and in many cases these treatments offer debilitating side effects. Furthermore, limitations in the current detection technologies make a major defect in accurate diagnosis of pain initiators, especially in the psychiatric patients they may have an augmented perception of pain or in older adults numerous pain producers commonly coexist [12]. According to recent investigations specific biomarkers have revealed in the pain conditions [13]. The specificity and sensitivity in the detection of these biomarkers will provide ultimately accuracy in the pain management. Nevertheless, restrictions in the pain assessment methods and consequently unsatisfactory treatments need for new skills and alternative approaches. Meanwhile, pain management with employing nanotechnology has recently emerged as a new approach to improve the quality of treatment. In particular, the use of nanoparticles that have small dimension and large surface area, resulting in various special characteristics, has been allowing one to confer new strategies for the complex treatments that could not otherwise be treated [14]. This has indeed facilitated the application of nanoparticles to various fields of pain management, such as providing new possibilities towards the detection, prevention and treatment of pain. Advances in the introduction of nanoparticles with intrinsic analgesic properties that can modulate pain perception with high satisfaction have provided a new perspective in the pain management [15]. Development of engineered multifunctional nanoparticles such as lipid-based nanoparticles represent a promising strategy to improve the low therapeutic index of conventional analgesic drugs and are already used in clinical practice [16]. Polymeric nanoparticles such as surface modified PLGA nanoparticles and another influential carriers such as silica, gold and magnetic nanoparticles extremely have been investigated to pain modulation [17–20]. Over the last few years, various nanomaterials have hiterto provided the accurate detection of biomarkers and molecular sources of pain to reduce the dose of analgesics and increase their long-term efficiency and safety. More importantly, the emergence of theranostic nanoparticles, which is a combination of diagnosis and treatment, has provided the basis for accurate pain management. Despite the considerable results in preclinical studies, limited clinical trials have been reported for nanomaterials in the control of pain, and only a few nanomedicines could obtain FDA approval [21]. Among the various nanoparticles, liposomes have been the most clinically studied for the delivery of analgesic drugs, and the results have shown the strong potential of these nanoparticles in the safe management of pain [22]. It is essential that the studies should focus on the use of products in clinical applications, while also paying special attention to their safety and effectiveness.An appropriately synthesized nanoparticle through accurate surface modification, particle size reduction, and acceptable encapsulation facilitates the development of an effective formulation for pain control. Although, there are several review articles about the role of nanomaterials in the pain management, this review article especially focuses on the introduction of novel nanoparticles with intrinsic analgesic properties and new nanosystems for targeted delivery of analgesic drugs. In addition, this review discusses the discovery of new nanotechniques and application of intelligent tools facilitating accurate control of pain, and also highlights current clinical studies and FDA approved products in this area.
Nanoparticles having intrinsic analgesic properties
New advances in nanomedicine have led to the emergence of various nanoparticles with unique properties. This has indeed enabled the expansion of the application of nanoparticles to various fields of medicine such as providing new possibilities towards the detection, prevention and treatment of diseases such as cancer, diabetes, cardiovascular disease, Alzheimer, Parkinson and other life-threatening disorders [23–27]. However, to date, studies focusing on the analgesic effect of nanoparticles are limited. In the following section, examples of nanoparticles with analgesic property are described.
Zinc oxide (ZnO) nanoparticles
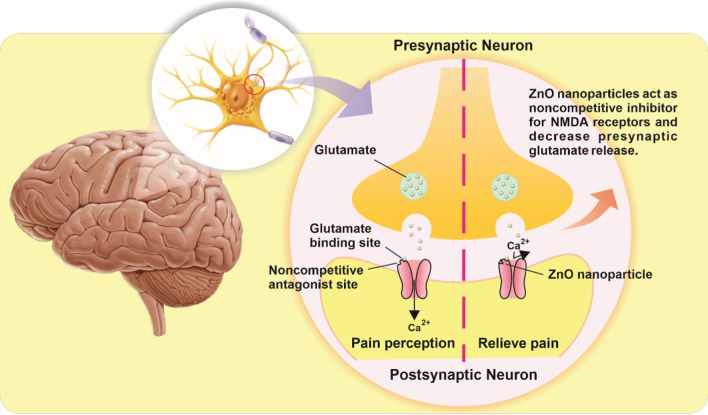
Various forms of ZnO, including nanoparticles, nanowires, etc., have exhibited great applicability in various fields, such as sensors, cosmetics, secondary batteries, optoelectrical devices, solar cells, and drug delivery [28–36]. In particular, ZnO nanoparticles have recently attracted great attention in biomedical fields because of its zero-dimensional structure and electrochemical properties [e.g., nontoxicity, wide band gap (~ 3.3 eV), and high electron mobility (~ 2000 cm2/V s at 80 K)]. Zinc is one of the essential and trace elements that can affect on the pain sensation. [37, 38]. Indeed, recent studies have explored a relationship between zinc deficiency and antinociceptive effects, in which they have particularly focused on interactions between zinc availability and opioidergic system activity. For example, it is demonstrated that zinc deficiency in diet could lead to lower antinociceptive effect of morphine in mice [39]. In addition, one study reported that ZnO nanoparticles could reduce the pain through the activation of the opioidergic system [15]. Zinc, which is a noncompetitive inhibitor of N-methyl-d-aspartate (NMDA) glutamate receptors, generally acts in postsynaptic space on most of receptors and channels; thus, it can decrease the capability of glutamate effect in this receptor. Note that NMDA is recognized as one of the important receptors in the pain management process. That is, the use of ZnO nanoparticles could block the NMDA receptors and induce the analgesic effect (Fig. 1) [40], wherein an increase in the levels of γ-aminobutyric acid (GABA) as an inhibitory neurotransmitter also reduced glutamate release, and may also induce the analgesic effect of ZnO nanoparticles [41].
Fig. 1.
Mechanism of the action of ZnO nanoparticles with analgesic property. ZnO nanoparticles reduce glutamate release. They are noncompetitive inhibitors of NMDA glutamate receptors; thus, they decrease the capability of glutamate effect in these receptors
On other hand, since the form of nanoparticles has more chemical reactive sites than the materials in bulk form because of the high surface-to-volume ratio, it is known that the chemical reactivity of ZnO nanoparticles is stronger than that of the conventional ZnO. Indeed, the analgesic effect by the ZnO nanoparticles could be achieved in doses lower than those of the conventional ZnO in bulk form. In addition, the extremely-small scale of ZnO nanoparticles allowed them to easily penetrate through body barriers and interact well with cell components, which could also result in the significant promotion of reactivity effects compared to the conventional ZnO in bulk form. For instance, although both ZnO bulk form and ZnO nanoparticles demonstrated analgesic effect on acute pain, the use of ZnO nanoparticles exhibited a higher analgesic effect than the ZnO in bulk form at a hot plate test with Wistar rats [15].
Magnesium oxide (MgO) nanoparticles
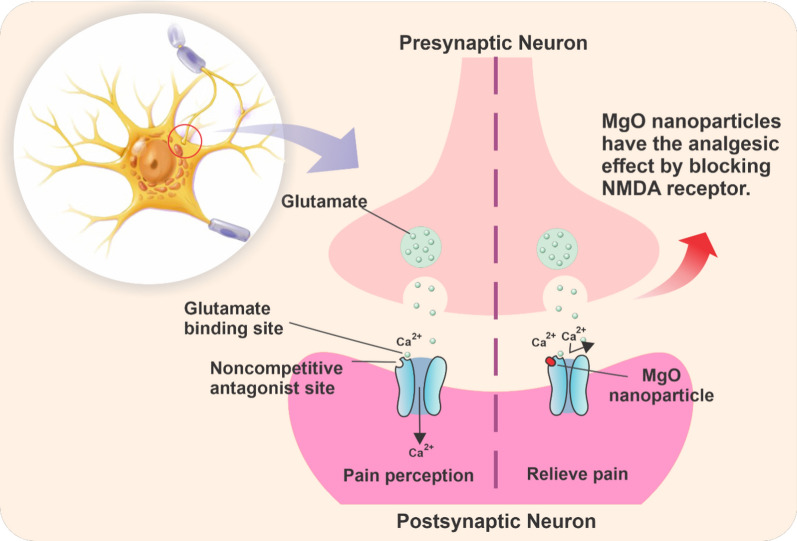
Magnesium is the fourth most important cation in human body, and the second most common intracellular cation [42]. Earlier, magnesium sulfate was suggested as a general anesthetic. The antinociceptive effect by using magnesium in the animal and human models of pain is related to the blocking of NMDA receptor. Magnesium without any direct analgesic effects, prevents calcium ions from entering cells by blocking NMDA receptors, resulting in an antinociceptive effect [43] (Fig. 2). Hence, the use of magnesium could lead to decrease the requirement of relaxant drugs and intraoperative anesthetics and the amount of morphine required for postoperative pain treatment. Such effects of magnesium were yielded not only in general anesthesia, but also in local anesthesia [44]. Moreover, another antinociception mechanism derived from the use of magnesium is associated with calcium-antagonistic effects at various voltage-gated channels [45].
Fig. 2.
Mechanism of action of the MgO nanoparticles with analgesic property. MgO nanoparticles block NMDA receptors, inducing the analgesic effect
The analgesic effects of MgO nanoparticles on anesthesia were demonstrated by Jahangiri et al., wherein their experimental testes confirmed strong analgesic effects of MgO nanoparticles in comparison to conventional MgO. According to this group, this subject may be related to the high permeability effect of MgO nanoparticles, especially in the central nervous system [46]. Another study was designed to estimate the effect of MgO nanoparticles and conventional MgO on ketamine-induced anesthesia in rabbits, the results showed that duration and quality of anesthesia induced by ketamine were similar in MgO nanoparticles and conventional MgO [47].
Manganese dioxide (MnO2) nanoparticles
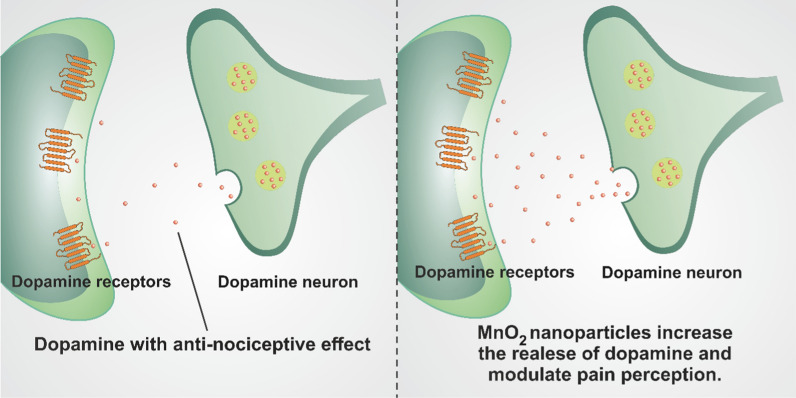
Various clinical and animal studies demonstrated the antinociceptive effects of dopamine [48–50]. For example, loss of dopaminergic neurons in patients with Parkinson’s disease caused an increase in pain perception [51]. MnO2 can influence the dopaminergic system, modulating analgesia and pain perception [52] (Fig. 3). wherein reduced levels of dopamine probably cause the painful signs and irregularities in dopaminergic neurotransmission have been obviously revealed in painful clinical disorders, including burning mouth syndrome and restless legs syndrome [53].
Fig. 3.
Mechanism of the action of MnO2 nanoparticles with analgesic property. MnO2 nanoparticles affect the dopaminergic system and modulate analgesia
Pain thresholds of rats were assessed using the tail immersion method to study the effect of MnO2 micro- and nanoparticles after a single subcutaneous injection. The result indicated that both nano- and micro-sized MnO2 particles could pass through blood barriers; however, the change in the particle size influenced the Mn clearance and distribution from the central nervous system (CNS). During 8 weeks of the microparticle administration, a steady and irreversible increase in the pain thresholds was observed in the rats while the MnO2 nanoparticles presented a biphasic response as follows: primary hyperalgesia for the first 4 weeks and late analgesia during the next 4 weeks. Note that a decrease in descending pain modulation tract activity promotes hyperalgesia. In the first 4 weeks, the observed hyperalgesia was due to the effect on the central descending mechanisms that stimulated the hyperalgesia and mask the analgesic effect of these nanoparticles, which was obtained by acting on the dopaminergic system. In the last phase of the experiment, the long-term analgesic effect of MnO2 nanoparticles was observed because of the effect on the dopaminergic system [52].
Magnetite (Fe3O4) nanoparticles
Magnetite (Fe3O4) is one of the most explored magnetic nanoparticles for biomedical applications. The unique properties of these nanoparticles have expanded their application in various fields of medicine. The most unique feature of these particlesis their ability to be conducted by an external magnetic field; therefore, more examinations have been motivated based on this property, such as development of contrast agents for magnetic resonance imaging (MRI), cell tracking, bioseparation and targeted drug and gene delivery [54–56].
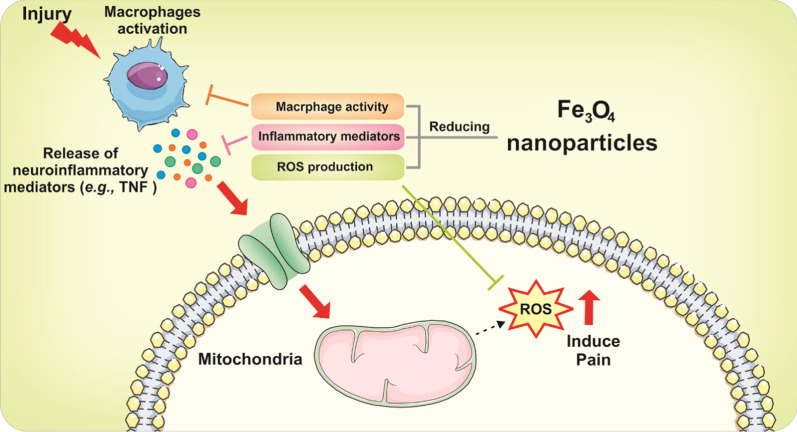
However, its use as a drug carrier has been restricted to the CNS disorders. Accordingly, few studies have hitherto reported the analgesic effect of Fe3O4 nanoparticles. Wu et al. developed inflammatory pain models in CD1 mice by injecting complete Freund’s adjuvant [57]. The result indicated that the dose-related analgesia was obtained by local administration of the ultrasmall Fe3O4 nanoparticles via the suppression effect on macrophage activity, inflammatory cells, and proinflammatory markers and the significant decrease in reactive oxygen species (ROS) production in the injured paw (Fig. 4).
Fig. 4.
Mechanism of action of the Fe3O4 nanoparticles with analgesic property. Fe3O4 nanoparticles induce analgesia by the suppression effect on macrophage activity and a decrease in ROS production, inflammatory cells, and proinflammatory markers
In another study, they reported excellent analgesia production by the polarity modulation of magnetic field to guide the intrathecal delivery of ketorolac-Fe3O4 nanoparticle conjugate [58]. The ketorolac was covalently conjugated onto the Fe3O4 nanoparticles, which could prevent the early release of ketorolac before reaching the target site. In this study, it was suggested that ketorolac–magnetic nanoparticles conjugate may break down to free ketorolac and magnetic nanoparticles in cerebrospinal fluid and act synergistically to produce an analgesic effect [59]. In other words, the Fe3O4 nanoparticles acted as nanocarriers and pharmaceutical units and had a critical role in increasing local drug accumulation via magnetic field modulation.
On the other hand, the Fe3O4 nanoparticles were also used as nanocarriers for ankle analgesia, in which the corresponding analgesic effect thereof was investigated [60]. In order to replace the existing nerve block methods, Mantha et al. conducted tests on the ankle block in the rat with intravenous injection of the Fe3O4 nanoparticles incorporated with ropivacaine (Fe3O4/Ropiv) in addition to the use of a magnet at the ankle site. Intravenous injection of the Fe3O4/Ropiv with magnetic force at the ankle site considerably could augment paw withdrawal latencies. Note that the intravenous injection with the Fe3O4 nanoparticles only had no major influence on paw withdrawal latency.
Nanoparticles designed for analgesic drugs and nanocarriers
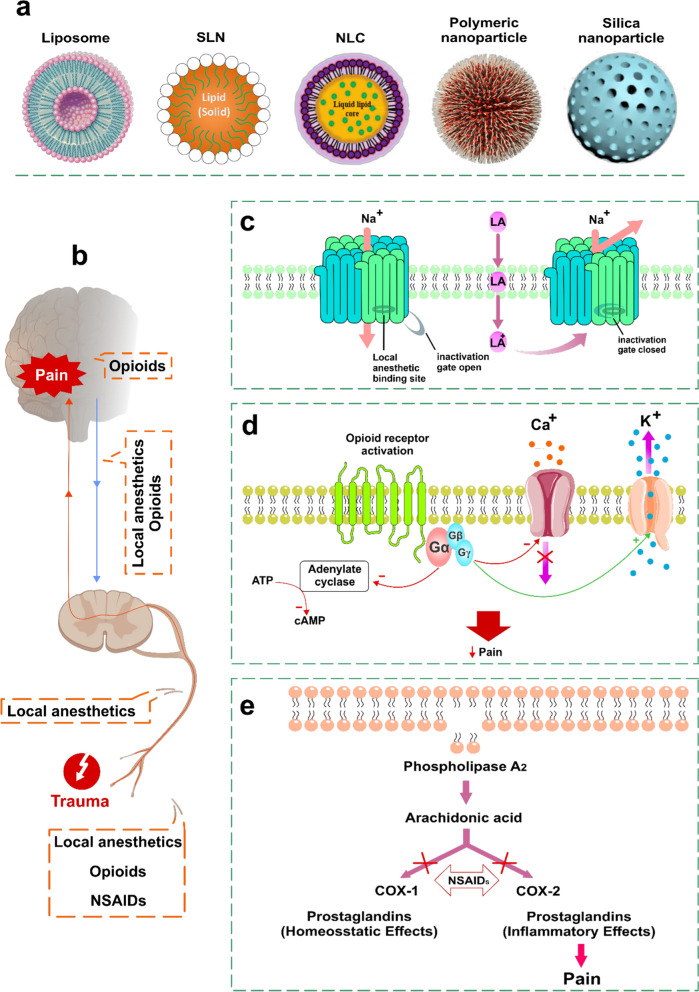
Pharmacological therapy using analgesic drugs is one of the main methods for pain treatment. According to one of the analgesics classifying systems, analgesics can be classified as opioid analgesics (i.e., morphine-like agonists), adjuvant analgesics (i.e., local anesthetics), and nonopioid analgesics [i.e., nonsteroidal anti-inflammatory drugs (NSAIDs)]. Targeted delivery and sustained release of analgesic drugs remains a critical problem for effective control of pain. The use of nanocarriers has been informed to improve effective delivery of these agents to target sites while reducing side effects. Accordingly, current advances in the presentation of novel nanocarriers such as lipid based nanocarries and polymeric nanoparticles will definitely be effective in the quality of pain management (Fig. 5a) [61, 62].
Fig. 5.
Illustrations of the nanocarriers designed for (a) analgesic drugs, b therapeutic modulation of the pain pathway, and the working mechanism of (c) local anaesthetics, (d) opioids, and (e) NSAIDs for pain management
Nanoparticles designed for opioids
Consumption of opioids for chronic pain management has dramatically increased in recent years. However, there are some concerns about its analgesic efficacy because of tolerance to opioid therapy. For example, it is known that despite increasing doses the efficacy of opioids decreases during the course of treatment [63]. Opioids exert their actions by interacting with specific receptors present on the central and peripheral nervous system. Activation of opioid receptor reduces the formation of intracellular cyclic adenosine monophosphate (cAMP) and opens K+ channels or suppresses Ca2+ channels. All of these events lead to neural hyperpolarization and reduced intracellular Ca2+ availability, thereby reducing the release of CNS neurotransmitters and inducing analgesia (Fig. 5d) [64]. Many opioids, such as morphine and hydromorphone, have a very short half-life. For the opioid to be effective it is necessary to increase the frequency of administration, however, that can increase the incidence of adverse effects and inconsistent drug levels [65, 66]. For these reasons, the proper modulation of drug adsorption is required after administration. Accordingly, recent pharmaceutical formulation studies have focused on the development of drug delivery systems that have the potential for sustaining or delaying drug release after administration [67].
One of the well-established nanomaterials, liposome is a spherical vesicle containing phospholipid bilayers, which generally have the size of tens of nm to thousands of nm. These vesicles can trap lipophilic or hydrophilic drugs inside, and thus can release their contents at the target site [68]. For instance, Depodur approved by the food and drug administration (FDA) for postoperative pain management, which is the prolonged-release epidural morphine, could be encapsulated at the inside of multivesicular liposomes (i.e., Depofoam). In particular, this Depofoam includes several encircled bilayers capable of making numerous aqueous compartments for morphine salt preservation [69, 70].
Inspired by such products, significant advances in the field of oral delivery of opioids have been also achieved using the technologies associated with liposomes. However, when native-state liposomes are orally administrated into the body, they are rapidly degraded in harsh gastrointestinal (GI) conditions. Coating the liposomes with polymers conferred stability in the GI tract. Surface modification of liposomes by polyethylene glycol (PEG) and Eudragit® S 100 copolymer have improved the oral bioavailability of tramadol and endomorphin-1, respectively, by prolonging the blood circulation of the liposomes [71, 72].
Peptide-coated liposomes are another example of strategies to enhance analgesic effects of opioids following intranasal administration. The use of the upper nasal cavity for delivery of opioids can directly distribute the opioids into the brain. Arg–Gly–Asp (RGD) peptide sequence is an integrin-targeting ligand while the surface modification of liposomes by the RGD peptide could increase the residence time and penetration ability through epithelial layers. In this regard, Hoekman et al. reported aerosol liposomal formulation coated with the RGD peptide for the nasal delivery of fentanyl, where they could demonstrate improvement in analgesic effect in comparison with the free drug formulation [73]. Similarly, improvement in the intrathecal and transdermal deliveries of alfentanil, butorphanol and buprenorphine could be also achieved using the liposomal formulation in other studies [74–76].
On the other hand, since 1990 so-called solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) have been often reported as the alternative carrier systems for emulsions, polymeric nanoparticles, and liposomes [77]. The NLCs are regarded as the next generation of SLNs because of the enhanced loading capacity and stability, and decreased drug expulsion during storage [78]. Note that the opioids are also the drugs of choice for pain control in burn or skin graft patients. However, managing severe pain in burn and skin graft patients is still challenging because common formulations have insufficient pain control because of inadequate concentrations and degradation of drugs at the target site. To enable topical opioid application to these wounds, Küchler et al. developed morphine-loaded SLNs for the effective reduction in pain and acceleration of wound healing [79]. In another successful study, in vivo assessments indicated an improvement in analgesic effect of fentanyl citrate-loaded NLC in comparison to the commercial type [80].
Nanoparticles designed for local anesthetics
Local anesthetic is a class of anesthetic drugs capable of numbing a specific part of body by blocking Na+ channels and suppressing action potentials. Local anesthetics pass through the membrane in unionized lipophilic form and ionize again in the axoplasm, where they attach to their receptors and stabilize the channel in the inactive state, thus reducing channel opening and inducing analgesia (Fig. 5c) [81]. Since a patient after the local anesthetic can be awake or sedated during local anesthesia depending on the option, this process is often used for minor outpatient procedures [82]. Meanwhile, many researchers have hitherto studied actively to increase the duration of these local anesthetics. In order to control the drug release of local anesthetics, researchers have focused on the combination of local anesthetics and carriers, in particular, they have employed technologies of nanocarriers, such as liposomes, lipid nanoparticles, and polymeric nanoparticles. Table 1 presents a summary of these studies that revealed the efficiency of various nanoformulations in local anesthesia.
Table 1.
Experimental studies with local anesthetics encapsulated into various nanoparticles
| Local anesthetic drug | Nanoparticles | Subjects (administration route) | Antinociceptive test | Results | Ref |
|---|---|---|---|---|---|
| Benzocaine | PLGA, PLA, PCL | Mice (injection into the popliteal space) | PWTP | Pharmacological assessment indicated that encapsulation of benzocaine in polymeric nanocapsules prolonged its anesthetic effect compared with free benzocaine | [83] |
| Lidocaine/prilocaine | Polymeric nanocapsules | Rat (oral mucosa) | Tail-flick | The formulation provided effective and longer-lasting superficial anesthesia at the intraoral mucosa during medical and dental procedures | [84] |
| Bupivacaine | Alginate/chitosan (ALG–CHIT)-Alginate/bis (2-ethylhexyl) sulfosuccinate (ALG–AOT) | Mice (popliteal space) | PWTP | Comparison among formulations displayed that 0.125% bupivacaine-loaded ALG–CHIT and bupivacaine-loaded ALG–AOT improved the analgesia 1.5- and 2.16-fold, respectively, compared with plain bupivacaine | [85] |
| Ropivacaine | Liposome | Human (oral mucosa) | Pinprick test | Extensive soft tissue anesthesia was achieved using liposome containing ropivacaine and EMLA group, when compared with ropivacaine and benzocaine gels (P < 0.0001) | [86] |
| Tetracaine | Liposome | Human (skin) | McNemars test | The formulation produced better superficial local anesthesia than EMLA | [87] |
| Bupivacaine | Alginate/chitosan | Rabbit-rat (intraoral or intrathecal injections) | Observation of the aversive response to the rat upper lip pinching | The analgesic properties of bupivacaine increased 1.4-fold (P < 0.001) with bupivacaine 0.5%-loaded alginate–chitosan nanoparticles after infraorbital nerve blockade | [88] |
| Tetrodotoxin | Hollow silica nanoparticles | Rat (sciatic nerve injection) | Neuro-behavioral testing | The sustained release of tetrodotoxin encapsulated in hollow silica nanoparticles with diameter of 28 nm prolonged duration of action and at the same time reduced systemic toxicity | [89] |
| Tetrodotoxin | Poly(triol dicarboxylic acid)-co-poly(ethylene glycol) | Rat (sciatic nerve injection) | Neuro-behavioral testing | Tetrodotoxin released from polymers created nerve block duration, from several hours to 3 days, with insignificant local or systemic toxicity | [90] |
PWTP paw withdrawal threshold to pressure, PLGA poly(lactic-co-glycolic acid), PLA polylactic acid, PCL polycaprolactone, EMLA eutectic mixture of local anesthetics
Liposome is one of the primary drug delivery systems that have been used for decades to deliver local anesthetic drugs for skin analgesia or management of postsurgical pain. The first local anesthetic drug used with a nanocarrier was suspension of bupivacaine-containing liposomes, introduced by Boogaerts et al. Although epidural injection of the liposomal formulation demonstrated that this formulation was not appropriate for anesthesia during surgery, further studies could reuse this approach to control postoperative pain [91]. The success of liposomes in the delivery of local anesthetic drugs has been also able to lead to the development of products, such as EXPAREL (bupivacaine liposome injectable suspension). The EXPAREL is formulated to produce postsurgical local analgesia, i.e., interscalene brachial plexus nerve block after single-dose infiltration. Intraoperative administration of the EXPAREL through wound infiltration could significantly reduce pain over 72 h compared with a placebo [92].
Anesthesia is one of the most commonly used methods for pain relief, which can be generally conducted by the insertion of a needle and the injection of a local anesthetic agent. Elimination of the needle for local anesthesia especially using nanoparticle technologies would be a great step in most invasive procedures on skin and dental treatments. In particular, the delivery of local anesthetics through the skin has many benefits, such as greater patient compliance, extended sustained drug release, rarer systemic adverse reactions, and easier application [93]. In general, when local anesthetics-loaded nanocarriers are applied to the skin, they can penetrate skin layers by bypassing the main skin barrier, i.e., stratum corneum. Moreover, bioadhesives containing local analgesic agents can effectively relieve pain produced by the needle stick injections, for example, in dental procedures. In this regard, to provide topical, gingival, and mucosal anesthesia, the lidocaine transmucosal delivery system (DentiPatch) was developed. Wherein, applying this mucoadhesive patch minimize the pain sensation prior to dental procedures such as local anesthetic injections. Therefore, use of an effective topical anaesthetic agent with longer duration of action will eventually lead to more comfortable for both patients and clinicians [93, 94]. Many of local anaesthetics mentioned above are non-specific blockers of the sodium channel. The toxins such as tetrodotoxin and saxitoxins, resulting from various sources are very potent and specific blockers of the voltage gated sodium channels. These toxins bind to the outer pore and block site 1 on the voltage-gated sodium channel. The charge and hydrophilicity cause the relatively weak penetration through various barriers to their site of action and limit the efficiency of these local anesthetics [95]. In an attempt to overcome these limitations and increase nerve penetration, hollow silica nanoparticles containing tetrodotoxin were evaluated. These nanoparticles are popular candidates because the hollow structure and negative charge of silica can facilitate the encapsulation of tetrodotoxin. In this study, it was found that the ability of silica nanoparticles to penetration was highly size-dependent and sustained release of tetrodotoxin prolonged duration of action and at the same time reduced systemic toxicity [89].
On the other hand, researchers must have attention to improve drug loading to overcome the factors that reduce the potency of local anesthetics. To achieve this, each anesthetic drug should be compatible with physicochemical features of the nanocarriers, such as molecular shape, lipid solubility, charge, and polarity. Table 1 represents a comprehensive list of local anesthetic drugs encapsulated into different nanoparticles along with details about administration routes and antinociceptive tests for pain assessment of these formulations.
Nanoparticles designed for NSAIDs
NSAIDs are the class of drugs widely prescribed to control pain, which are known as very effective against inflammation and pain. The main action mechanism of these drugs is inhibition of cyclooxygenase (COX) enzyme, which metabolizes arachidonic acid to prostaglandins, causing pain and inflammation (Fig. 5e) [96]. However, they have shown many side effects, including GI bleeding, nephrotoxicity, and cardiovascular side effects [97]. These side effects of NSAIDs have been major limitation that hampers their practical uses in pain management and inflammatory conditions. Thus, recent studies have focused on improving efficacy and reducing side effects of NSAIDs [97], in particular, several nanotechnologies, such as liposomes, SLNs, dendrimers, micellar-, silica-, and polymeric nanoparticles, with various compounds have been actively studied to improve the effectiveness of NSAIDs. Table 2 presents a summary of studies that examined the efficacy of various nanoparticles having NSAIDs. These nanoparticles were designed for targeted drug delivery, controlled drug release, increased drug efficacy, and prevention of destruction of drugs [98, 99].
Table 2.
Experimental studies with NSAIDs encapsulated into various nanoparticles
| NSAID | Nanoparticles | Route of administration | Results | Ref |
|---|---|---|---|---|
| Piroxicam | Ethyl cellulose | Oral/rat | Piroxicam-loaded ethyl cellulose nanoparticles significantly decreased (approximately 66%) gastric ulceration in rats in comparison to piroxicam suspension | [100] |
| Naproxen | PLGA | In-vitro analysis | Formulation of naproxen–PLGA nanoparticles could improve the physicochemical characteristics of the drug | [101] |
| Meloxicam | Eudragit EPO | Oral/rat | The EPO nanoparticle significantly increased anti-inflammatory activity of meloxicam (for longer duration; 6 h) in comparison to meloxicam suspension | [102] |
| Ibuprofen sodium | Gelatin | Oral/rat | Nanogelatin improved plasma half-life of ibuprofen sodium, thereby aiding reduction in the frequency of administration | [103] |
| Flurbiprofen | Cyclodextrin (CD) complexation and liposomes | Intravenous/rat | This delivery system significantly enhanced the bioavailability of flurbiprofen | [104] |
| Indomethacin | Chitosan-coated liposomes | Oral/rat | Results showed that retention in the upper part of the GI tract was better for submicronized chitosan-coated liposomes in comparison with submicron-sized liposomes, at 1, 2, and 4 h after administration, and the nanosystem was significantly better retained in the small intestine at 4 h | [105] |
| Nimesulide | Lipid | Intraplantar injection/rat | The nanoparticles offered significant pharmacological effects in comparison with free drug administration | [106] |
| Naproxen | Nanostructured lipid carrier (NLC) | Temporomandibular joint/rat | Proinflammatory cytokines (IL-1β and TNF-α) and leukocytes migration significantly decreased for more than a week by sustained delivery of naproxen directly in the temporomandibular joint | [107] |
| Flurbiprofen | Polyvinylpyrrolidone (PVP) | Oral/rat | Nanoparticles improved the solubility of flurbiprofen by approximately 130,000-fold. This formulation improved bioavailability of poorly water-soluble flurbiprofen | [108] |
| Indomethacin | Poly(2-hydroxyethyl methacrylate-co-3,9-divinyl-2,4,8,10-tetraoxaspiro (5.5) undecane, polymeric nanoparticle | Oral/mice | In vitro and in vivo results indicated the potential of this polymer as a matrix for bioactive produces | [109] |
EPO pH sensitive cationic polymer consisting of 1:2:1 ratio of methyl methacrylate; N_N-dimethylaminoethyl methacrylate, and butyl methacrylate monomers; GI Gastro intestinal; PLGA poly(lactic-co-glycolic acid); IL-1β interleukin‐1β; TNF-α Tumor necrosis factor α
Lipid nanoparticles have been studied to reduce side effects and improve the solubility of NSAIDs. For example, SLNs loaded with ibuprofen and a variety of matrix lipids, including stearic acid, tripalmitin, and trilaurin, have been demonstrated to increase the dissolution rate of ibuprofen [110]. The SLNs containing trilaurin exhibited rapid ibuprofen dissolution within the first 30 min when tripalmitin was used. The dissolution rate increased, and all the ibuprofen could be released within 2 h. However, in case of stearic acid, the dissolution rate was relatively slow and ibuprofen was released within approximately 5 h. Lopes-de-Araújo et al. have also developed NLCs to improve pharmacokinetic and pharmacodynamic properties and reduce gastric complications of oxaprozin. The macrophages are an important element of inflammatory pathways. Furthermore the folate receptor is overexpressed in activated macrophages. Therefore in this study, conjugating folic acid on the surface of NLCs stimulated an increased macrophages uptake by a caveolae uptake mechanism. Eventually, the developed formulation provides a great potential for oral administration of oxaprozin by limiting gastric side effects [111, 112].
Polymeric nanoparticles are another drug delivery system widely used to improve the bioavailability of NSAIDs. Celecoxib that was entrapped in poly(lactide-co-glycolide) (PLGA) using didodecyldimethylammonium bromide (DMAB) or poly(vinyl alcohol) as a stabilizer could be produced by the solvent evaporation method [113]. The results demonstrated that the formulation of PLGA nanoparticles loaded with celecoxib using DMAB produced stable and highly entrapped nanoparticles to provide a potential effective dosage form for oral administration of celecoxib. In another study, positively charged polymeric nanoparticles with polycaprolactone as a biodegradable polymer and DMAB as a cationic surfactant could improve therapeutic efficacy of meloxicam with exhibiting less ulcerogenic than meloxicam suspension [114]. Polymers and polysaccharides as natural polymers appear to be successful in producing suitable carriers for NSAIDs. For instance, gelatin nanoparticles containing indomethacin provided controlled release of the drug and improved oral bioavailability of indomethacin [115].
Nanoparticles and other nanotechniques for nociceptive pain
Nociceptors are receptors that are sensitive to tissue trauma or a stimulus that can cause injury to tissue if lasted for long. When a noxious stimulus activates a nociceptor, signals transfer mostly along with two fiber types: rapidly myelinated A-delta fibers and slowly conducting unmyelinated C-fibers. These receptors are free nerve ending distributed below the skin, joints, tendons, and body organs [116]. Nociceptive pain control originating from the skin has received a lot of attention in the field of nanomedicine. Control of pain perception before any invasive procedures in the skin could be achieved by the enhancement of analgesic permeation by nanocarriers. In addition, micro- and nanoneedles are new technologies that can significantly reduce needle anxiety of patients.
Enhanced dermal penetration by nanoparticles
Various types of invasive procedures, such as intravenous catheter insertion and intramuscular injections, are painful operations because of the comprehensive distribution of pain receptors on the skin surface [117]. To reduce pain perception of these procedures, different anesthetic options have been presented by physicians, however, inadequate dermal penetration of anesthetic drugs has been always a great challenge. Incorporation of the anesthetic drugs into nanocarrier systems overcomes minor penetration drawbacks. The nanocarriers that have been examined for enhanced skin penetration include nanoemulsions, liposomes, SLNs, and NLCs [118, 119].
Various vesicular systems, particularly liposomal family, have been studied by numerous investigators. Ethosomes are the type of liposomal systems containing phospholipid and high concentrations of ethanol (20–45%) in their structure. Babaie et al. studied the ethosome containing lidocaine to investigate dermal penetration and corresponding analgesic effects [120]. In vitro studies using Franz cell system and fluorescence measurement revealed that a high amount of lidocaine was concentrated at the dermis where most nerve endings are located. Therefore, their results demonstrated that the ethosomes are effective systems for the delivery of lidocaine and suitable nanocarrier for the development of skin analgesia. On the other hand, Somagoni et al. combined two different nanosystems, i.e., nanomicelle and nanoemulsion, that was so-called nanomiemgel, which allowed one to obtain maximum drug penetration due to its unique mixture [121]. Their studies demonstrated that absorption of nanomiemgel containing capsaicin and aceclofenac was better than either of the individual nanomicelle and nanoemulsion because of the utilization of maximum possible paths for the absorption of drugs.
Lipid–polymer hybrid nanoparticles (LPNs) are core–shell structures, thus can incorporate properties of two nanoparticles, i.e., liposome and polymeric nanoparticle containing lipid shells and polymer cores. Wang et al. explored LPNs containing lidocaine to compare their skin penetration properties with conventional liposomes [122]. In vitro examination and in vivo analysis with tail-flick test demonstrated that the LPNs were effective drug delivery systems for local anesthetic therapy. The simultaneous delivery of two local anesthetic drugs could be also performed by using SLNs and NLCs [123]. A comparison between dermal penetration and analgesic effects revealed that stronger in vivo anesthetic effect was created by the NLC system than the SLN system, whereas the SLN systems demonstrated better ex vivo skin permeation ability than that of the NLCs. That is, it could be concluded that both nanoparticle technologies with various prominent characteristics can be effectively employed as suitable nanocarriers for topical skin analgesia.
On the other hand, Tocopheryl PEG 1000 succinate (TPGS), a water-soluble derivative of vitamin E, was explored as an enhancer for dermal penetration of drugs [124]. In particular, the TPGS-modified cationic NLC was studied to examine the skin permeation and analgesic effect of lidocaine. The results revealed that the TPGS-modified NLC was able to act as an appropriate delivery system for efficient and prolonged skin analgesia.
Other nanotechniques for painless needles
The use of appropriate techniques and safe methods to avoid complications is the main goal of any injectable processes, and patient’s consent for these procedures is also very important. Since needles used for these processes are the main cause of pain, the selection of an ideal needle by physicians can significantly affect patient satisfaction levels. The influence of needle thickness on pain perception has been reported in various studies, in which a decrease in needle thickness could effectively reduce pain perception, thus increasing patient satisfaction [125–129].
For years, hypodermic needles and syringes have been applied for drug delivery. Recently, the emergence of micron-size needles provides more patient satisfaction, along with maximum transdermal drug delivery. Furthermore, nanoneedles are the new series of needles performed next to microneedles and expected to provide better penetration profiles. Microneedle includes arrays of micron-size needles. When used, they can deliver drugs to the dermal microcirculation without stimulation of any dermal nerves because of their short length of needles [130–132]. Nanoneedles and microneedles have the same principle of action, however, nanoneedles are small enough to be practically invisible. These features of nanoneedles can provide many advantages on painless operations [133].
In this regard, the nanoneedle technology has been recently focused in the field of nanomedicine. Nanoneedles can bypass cell membranes, and thus facilitate interactions with the intracellular milieu allowing drug delivery and intracellular sensing. For this purpose, Chiappini et al. developed a porous silicon nanoneedle system for the cytosolic delivery of CdTe quantum dots [134]. In addition to the silicon nanoneedle, NiCo2O4 nanoneedle [135] and diamond nanoneedles [136] are also examples of nanoneedles that have been introduced in recent years (Fig. 6). Still, further research and developments are needed to understand the full potential of nanoneedles and improve the accessibility of this nanotechnology to biological scientists. Challenges in case of nanoneedles are large quantities of production, and this emerging technology of nanoneedles has multidisciplinary nature, i.e., its development requires collaboration of various fields. It is expected that nanoneedles will provide new opportunities to resolve many problems in biology.
Fig. 6.
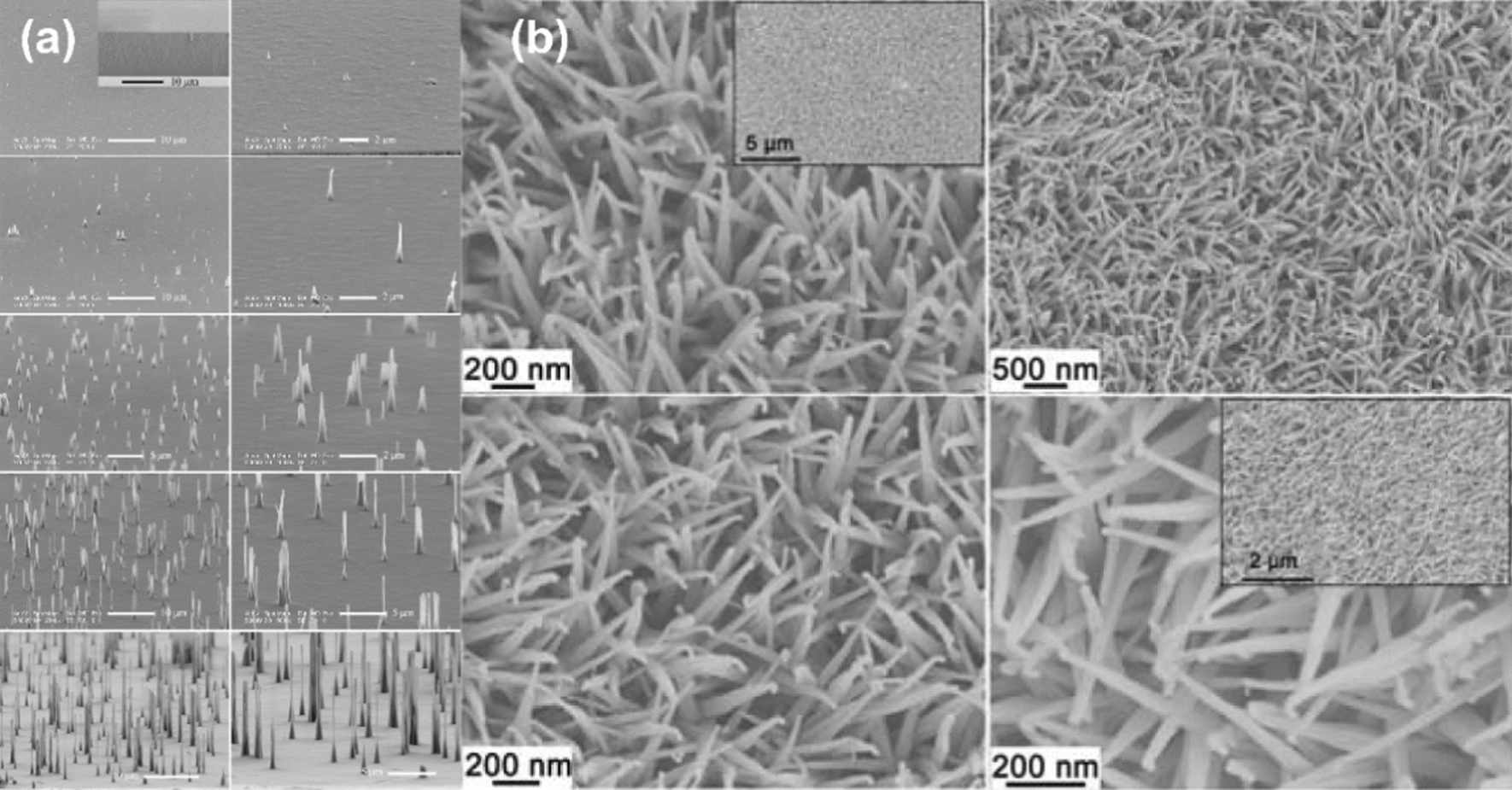
Scanning electron microscopy images of (a) diamond nanoneedles (reproduced with permission from Ref. [136], Royal Society of chemistry 2015). b Crystallized NiCo2O4 nanoneedles (reproduced with permission from Ref. [135], Royal Society of chemistry 2012)
Dental procedures by nanoparticles
Few years ago, nanodentistry based on the use of nanomaterials was introduced as a science related to treatment, diagnosis, pain relief, and prevention of oral and dental diseases [137]. The use of nanotechniques in dentistry has changed attitude toward dentistry because it could provide the concept of pain-free and minimally-invasive dental procedures. Local anesthetics (LAs), including lidocaine, benzocaine, and tetracaine, are common anesthetic drugs used in dentistry [138]. Encapsulation of these drugs into liposome, cyclodextrins, lipid nanoparticles, hydrogels, and patches could not only prolong the analgesic effects, but also reduce the toxicity of local anesthetics [139]. In particular, the encapsulation of LAs into nanoliposomes was able to offer a suitable strategy for pain-free dental procedures.
Franz-Montan et al. developed the 5% lidocaine-loaded liposome and compared it with the liposome-encapsulated 2.5% eutectic mixture of lidocaine and prilocaine (EMLA) and 5% lidocaine ointment (xylocaina). The results revealed that the liposome-encapsulated 5% lidocaine and EMLA demonstrated the best in vivo topical anesthetic efficiency and better in vitro penetration parameters [140]. In another study by the same group, the effect of the liposome-encapsulated 2% ropivacaine was also examined in terms of topical anesthesia and pulpal response [141]. Several other formulations such as 20% benzocaine gel, placebo gel, and liposomal placebo gel were also compared with this formulation. The corresponding results showed that the liposome-encapsulated 2% ropivacaine and 20% benzocaine gel were most effective in reducing pain during needle insertion and soft tissue analgesia, however, they could not induce pulpal anesthesia after 15 min. In addition, SLNs [142], NLCs [143], and polymeric nanoparticles [144] are also other nanoparticle techniques that have been used with analgesic drugs to provide analgesia in dental procedures. Topical administration of these nanoparticles could effectively alleviate discomfort and pain perception in invasive events, such as needle insertion, during dental procedures. Dentin hypersensitivity is a sharp and short pain triggered by air, heat, cold, touch, and osmotic and chemical stimulants.
On the other hand, applying a thin coating on the exposed tooth can act as an occlusion and consequently block the pain stimulation. From last few years, fluoride has been known as an essential agent for dental remineralization. In recent, researchers have begun to focus on the efficiency of hydroxyapatite for remineralizing tooth enamel as the treatment of dental caries. From the viewpoint of remineralization properties, nanohydroxyapatite, similar to fluoride, is regarded as a satisfactorily promising agent against dentin hypersensitivity. Indeed, toothpastes comprising the nanohydroxyapatite could successfully decrease the duration of tooth sensitivity in patients who used tooth whitener without a desensitizing agent [145].
Nanoparticles for neuropathic pain
Neuropathic pain is an intricate chronic pain disorder associated with the sense of stimulation or burning in a specific area; it is related to nerve injury and its unpleasant condition can affect the quality of life [146]. There are two general categories of neuropathic pain: first, the results from damage or disorders in peripheral nervous system, and second, the results from damage or disease in CNS. Pathology of neuropathic pain in the disorders in peripheral nervous system involves myelinated A-fibers and small unmyelinated C-fibers. Infectious diseases (e.g., AIDS), diabetes mellitus, chemotherapy, and inflammatory and immune disorders are the most clinically diagnosed cases that create peripheral neuropathic pain [147, 148], whereas diseases or damages in the brain or spinal cord create central neuropathic pain.
Neuropathic pain is often caused by brain disorders such as neurodegenerative diseases (Parkinson’s disease) and cerebrovascular disease (post-stroke pain) [149]. Despite availability of numerous pharmaceutical drugs, the treatment of neuropathic pain is often ineffective [150]. To overcome these challenging conditions, a multidisciplinary approach has emerged for the treatment of neuropathic pain, in particular, nanomedicine with various capacities has shown great abilities to resolve these limitations [151, 152]. Various nanotechniques using nanomaterials could be effective in neuropathic pain management because of the unique features thereof that aid in diagnosis and therapeutic application at the cellular and molecular levels.
Relief of symptoms and palliative treatment in some pathological conditions are the main goals of neuropathic pain management [150]. In many patients, treatment of neuropathic pain is difficult and poorly effective. Opiate analgesics, NSAIDs, and antidepressants are not recommended for the treatment of neuropathic pain [153]. Drugs based on cannabinoid are emerging drugs that have been used to treat chronic pain, such as neuropathic pain. Psychological activity reduction as a side effect of cannabinoid drugs can help to improve therapeutic ratios. This improvement can be achieved by nanoparticle-based drug delivery systems. New synthetic cannabinoid receptor agonists, such as CB13, are relatively efficient in neuropathic pain treatment. The targeting of CB1 and CB2 receptors by CB13 could reverse mechanical hyperalgesia in neuropathic pain [154, 155].
Berrocoso et al. formulated an oral form of PEGylated PLGA nanoparticles containing CB13, and the corresponding neuropathic pain management by the formulations was evaluated by paw pressure and acetone tests in the neuropathic animal model [156]. Free CB13 and 3 types of the nanoparticle-encapsulated CB13 (i.e., CB13-loaded PLGA nanoparticles, CB13-loaded PLGA + PEG nanoparticles, and CB13-loaded PLGA–PEG) were orally administrated at different doses. As a result, it was observed that free CB13 and the three types of nanoparticle cases exhibited the same analgesic effect, but the PEG-coated nanoparticles exhibited a very prominent analgesic ability with the longest and continued pain reduction effect.
As mentioned above, one of the major limitations of cannabinoid receptor agonists is psychoactivity. Micelles, which are sphere-shaped aggregates of amphiphilic molecules, can effectively reduce the effect on CNS by restricting transport across the blood–brain barrier (BBB), furthermore, nanomicelles can prolong plasma half-life by escaping renal elimination because of their submicron size. These can be the reasons for the effectiveness of nanomicelles in the treatment of neuropathic pain by cannabinoids. Cannabinoids such as WIN55,212-2 (WIN) and CP55,940 are strong CB1 and CB2 receptor agonists, respectively. Linsell et al. encapsulated WIN in the styrene maleic acid micelles (SMA), and then evaluated neuropathic pain management using the chronic constriction injury (CCI) model of sciatic neuropathy [157]. Intravenous injection of the water-soluble SMA-WIN micelles with the diameter of 6 nm could retain their formulations in the peripheral nervous system and could produce prolonged analgesia in comparison with the base WIN.
After nociceptor sensitization, the inflammatory mediators, signaling molecules, and immune cells infiltrated in the inflamed nerve, triggering pain perception. This inflammatory phenomenon that is largely produced by the infiltration of macrophages leads to the expression of cyclooxygenase-2 enzyme, which is responsible for the production of PGE2 [158–161]. NSAIDs are a group of drugs that can reduce the pain resulted from the nerve damages associated with the inflammation by inhibiting prostaglandin production. The macrophages can not only promote, but also prevent inflammation. They can switch phenotype to exhibit these actions by obtaining either proinflammatory (termed M1) or anti-inflammatory (termed M2) phenotypes [162–165]. To relieve neuropathic pain associated with macrophage polarity, Saleem et al. developed theranostic approach containing both therapeutic (anti-inflammatory drug celecoxib) and diagnostic (near-infrared fluorescent dye) nanoemulsions to distinguish the anti-inflammatory and proinflammatory phenotypes of infiltrating macrophages at the site of sciatic nerve injury. Their results showed that the cause of chronic pain relief was an alteration in M2 macrophage phenotype; this type of macrophages fuse to form MGCs at injured sciatic nerve. Thus, at the injured nerve, the change to an anti-inflammatory environment led to a decrease in M1 macrophage employment, reducing inflammation and neuropathic pain perception [166].
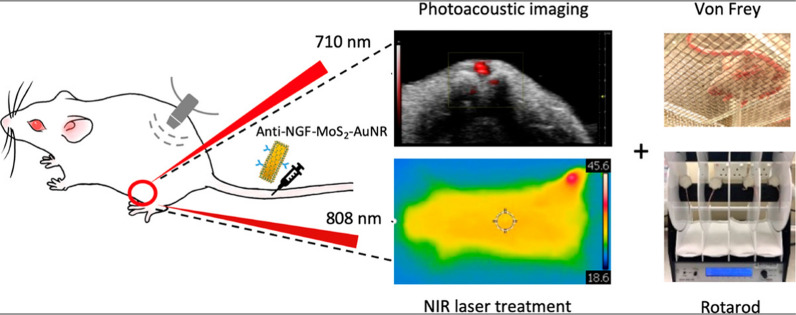
Recently, satisfactory results also have been reported for management of osteoarthritis pain based on the theranostic approach. Osteoarthritis is a common chronic inflammatory disease and a major cause of joint pain, its progression can be manifested by loss of function and finally permanent disability. To date, no medication is available for the absolute treatment of osteoarthritis, and most therapies are palliative in nature and reduce symptoms. This is probably due to the inadequate understanding of the early signs and methods of diagnosis, which has turned this disease into a global problem [167]. Resolving this issue requires the development of imaging-based assessment tools to accurately and quantitatively determine the origin of peripheral osteoarthritis pain, which will actually lead to a change in current analgesics and surgical use. In response to mechanical or inflammatory stimuli, the nerve growth factor (NGF), which plays a key role in activating and sensitizing peripheral nerves, is secreted by articular chondrocytes and synovial fibroblasts, triggering pain in an osteoarthritis joint [168, 169]. In a mouse model of post-traumatic osteoarthritis induced by destabilization of medial meniscus (DMM), the level of synovial NGF protein exhibited a significant increase at 4 weeks after surgery [170]. Therefore, NGF-targeted molecular imaging will assist to accurately visualize the source of osteoarthritis pain. Recently, functionalized gold nanoparticles have been considered as a common choice for theranostic agent due to their excellent optical properties [171]. Conjugation of NGF antibody with molybdenum disulfide nanosheet-coated gold nanorods (MoS2-AuNR) nanoprobes has been shown to active targeting of NGF on painful knees in an osteoarthritis surgical murine model (Fig. 7) [172]. Wherein, addition of MoS2 coating significantly improved the photoacoustic and photothermal performance of AuNR. In this study, it was revealed that the presence of functionalized nanoprobes in the osteoarthritis knee was higher than the intact knee, and intensity of mechanical allodynia was related to the accumulation amount in the mouse model. Therefore, this study provided conditions that under the guidance of imaging, NIR-stimulated photothermal therapy could reduce walking imbalance behavior and mechanical allodynia for subacute and chronic stages of osteoarthritis. As a result, this theranostic approach will provide a new window for accurately identification of the pain and simultaneously and effectively pain management in the future.
Fig. 7.
Schematic representation of theranostic approach to reduce osteoarthritis pain in rats by conjugation of NGF antibody with molybdenum disulfide nanosheet-coated gold nanorods (MoS2-AuNR) for photoacoustic imaging and photothermal analgesic treatment. Von Frey and rotarod tests were performed to evaluate the locomotive ability and balance of the animals, respectively. (reproduced with permission from Ref. [172], American Chemical Society 2021)
Development of chronic pain in the CNS is significantly dependent on the glial cells, i.e., microglia and astrocytes, and in the physiological conditions both microglia and astrocytes are not in a proliferating state [173–175]. Recent studies demonstrated the effect of mitogen-activated protein kinases (MAPKs) in the development of neuropathic pain in the CNS. Moreover, after external damage leading to a pathological condition, some studies reported that a Ras family member (Rheb) was upregulated in the mTOR pathway [176, 177]. Zoledronic acid is a type of pharmacological agent used for the treatment of bone demineralization by inhibiting isoprenylation of Ras family proteins. This could help in the neuropathic pain treatment by inhibiting the MAPK cascade. Unfortunately, the pharmacokinetic profile of zoledronic acid has been one of the biggest limitations [178]. To increase the bioavailability of zoledronic acid and reduce its bone binding, zoledronic acid was encapsulated in the liposome-based formulation (LipoZOL). Because of partially or disrupted BBB in chronic neuropathic pain, the LipoZOL could pass through it. Consequently, the accumulation of LipoZOL in the CNS and presence of proinflammatory cytokines were assessed in an animal model of neuropathic pain [177]. In the activated microglia, the phosphorylation of p38 MAPK has been detected, which led to the production of proinflammatory mediators, resulting in amplified pain symptoms. Therefore, reduction or inhibition of p38 MAPK phosphorylation has the potential of reducing neurologic pain [179, 180].
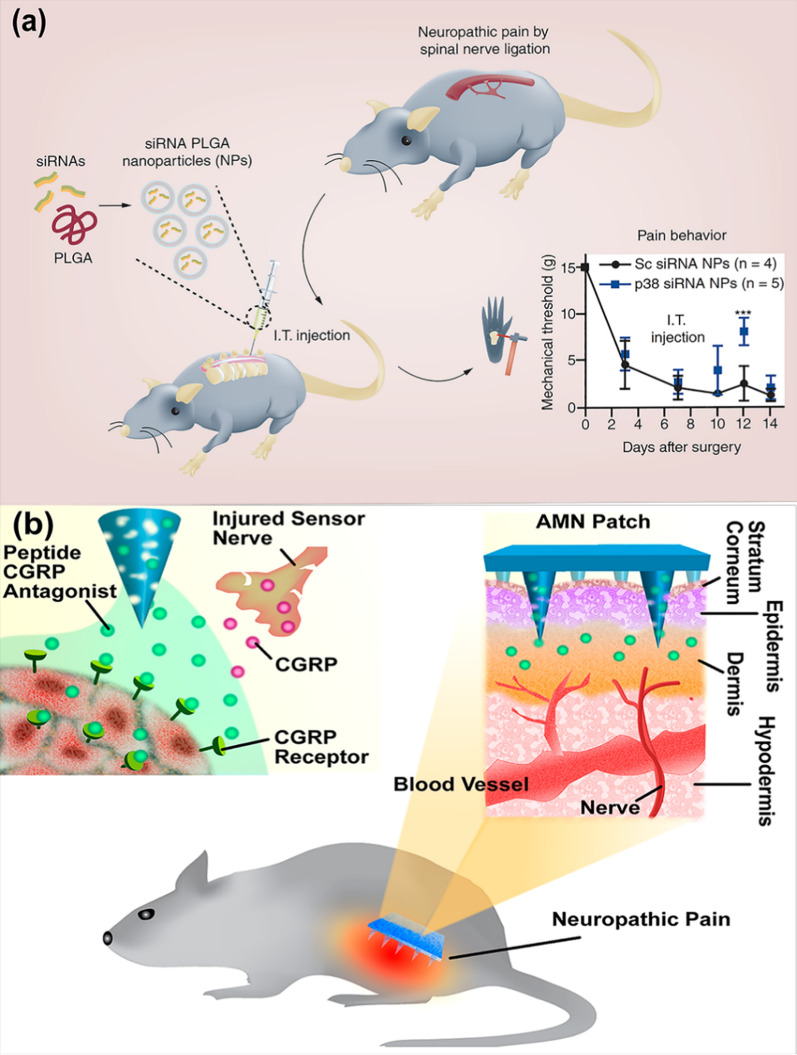
In recent, there is noticeable attention to the gene therapies based on small interfering RNA (siRNA) silencing of genes-related disease because of its potential to treat genetic disorders. For siRNA delivery, Shin et al. selected PLGA nanoparticles because its safety has already been established by the FDA and it has been displayed to be simply endocytosed by microglial cells (Fig. 8a) [181]. In another in vivo study, gold nanoparticles were selected as carriers for siRNA delivery [182]. In response to inflammation or trauma, a calcitonin gene-related peptide (CGRP) synthesized and released by nociceptive sensory neurons has the main effect in the maintenance and development of neuropathic pain. Systemic blocking of CGRP receptors has shown potential in the treatment of migraine [183–186]. An analgesic microneedle (AMN) patch with a CGRP antagonist peptide was transdermally administered for the local neuropathic pain treatment. Local analgesic effects were assessed in rats and it was observed that the use of microneedle patches containing CGRP antagonist peptide was a safe and simple approach for the treatment of neuropathic pain (Fig. 8b) [187].
Fig. 8.
a Scheme of study that evaluated the effect of p38 siRNA PLGA nanoparticles on neuropathic pain (reproduced with permission from Ref. [181], Future Medicine 2018). b Schematic of dissolvable MNs mediating local delivery of peptide CGRP antagonist to produce analgesia for the treatment of neuropathic pain (reproduced with permission from Ref. [187], American Chemical Society 2017). siRNA small interfering RNA, PLGA poly(lactic-co-glycolic acid), I.T. injection intrathecal injection, CGRP calcitonin gene-related peptide
Current clinical studies
Not only is pain a very complex ubiquitous phenomenon, but the experience of pain is also significantly different in each individual. On the other hand, the current information in understanding the subjective nature of pain and the variety in the pain assessment methodologies have significantly improved the quality of clinical trials for prospective pain therapies [188]. In the mid-1950s, clinical trials of pain treatment launched with the innovative work of Henry Beecher and Louis Lasagna, who directed randomized two-group trials on main nonsteroidal anti-inflammatory drugs and opioid analgesics [189]. Henry Beecher also explored the complications of accurate measurement of pain, the limitations in finding of specific patient’s population, the extent and prevalence of placebo effects, and the lack of a complete definition of response to treatment [190].
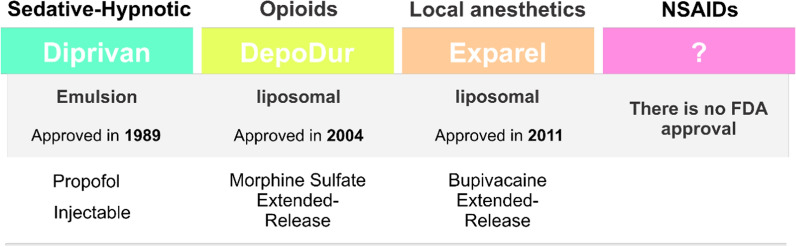
However, in spite of such clinical trials, many of these challenges still exist in recent pain studies. Accordingly, many clinical trials are underway to treat various types of pain, including pain associated with cancer, headaches, arthritis, neurological problems, and abdominal pain. In recent decades, nanomedicine has achieved conisderable progress in clinical use with the use of numerous micro and nanodrugs. Nevetheless, few clinical trials with using nanoparticles have been conducted and limited cases have been approved by the FDA (Fig. 9). For example, Diprivan®, which is an injectable emulsion of propofol, was as the first nanodrug in its class approved in 1989 by FDA for its application in the initiation and maintenance of general anesthesia or as a sedative in adults [191]. Subsequently, various modifications have been made to the emulsion formulations to overcome the disadvantages of propofol emulsions such as the pain on injection and the risk of infection [192–196]. In addition, a number of propofol formulations with novel nanocarriers have been studied and evaluated in preclinical studies [197].
Fig. 9.
FDA approved nanodrugs for the pain management
Liposomes are among the first nanodrug carriers to be successfully translated into clinical applications. Hence many liposomal analgesic formulations have been developed and are currently on the market. In particular, administration of bupivacaine loaded liposomes has been shown to be extremely effective with acceptable pharmacodynamics and pharmacokinetics properties and lower systemic toxicity [198, 199]. Injectable liposomal bupivacaine, commercially named Exparel™, is the only extended-release liposomal local anesthetic which is approved by FDA in 2011 for administration into the surgical site to create postsurgical analgesia in adults [191]. Although it was initially developed for postsurgical analgesia and is still used for this purpose [200–204], its scope has expanded and it has been used successfully in epidural injections [205].
Opioids are established as the “gold standard” in clinical practice for management of postoperative pain. Epidural morphine sulfate provides excellent and prolonged postoperative analgesia compared to systemic administration. DepoDur® that is a novel liposomal encapsulated extended-release epidural morphine was also approved by FDA for the pain relieve following major surgery [191]. The effectiveness of DepoDur® has been reported in a variety of clinical trials executed in patients undergoing major surgeries, such as colon resection [206], hip arthroplasty [207] and cesarean section [208]. The therapeutic efficacy of DepoDur® was also assessed in patients (n = 280) undergoing open and laparoscopic colorectal procedures, where was used between July 2010 and April 2012 [206]. Primary outcome, including resting and dynamic pain, mobilization, and need for additional analgesia, were evaluated. Sufficient pain management was realized at 24 and 48 h, wherein 81% of the patients required simple analgesia in 24 h and 62% of themrequired in 48 h. 79% of the patients were mobilized in 24 h and 88% of them were able to move in 48 h. Overall, this study showed that DepoDur® is an effective alternative to common pain management techniques. NSAIDs are the most widely prescribed drugs for acute and chronic pain due to their analgesic, anti-inflammatory, antipyretic properties, and their no dependence or addiction potential. However, it is known that these agents are associated with significant gastrointestinal and cardiovascular adverse events. In an attempt to enhance the efficacy and reduce the toxicity of NSAIDs, nanocarriers have been used in many preclinical studies to obtain targeted delivery to the site of inflammatory pain. However, the number of NSAID nano-formulations conducted in the clinical trials is very limited, and none have been approved by FDA to date [21].
Future perspective
Strong potential of nanotechnology offers exceptional opportunity to address many of unsolved problems in pain management. Accompanied by these abilities, multidisciplinary team works also are necessary to achieve ultimate advances in this field. Therefore, simultaneously efforts should be done toward growth of researches about effects of nanoparticles on different pain pathways, development of pain biomarkers detection techniques, expansion of tissue engineering researches to minimize pain perception associated with nerve injuries, and introducing of up-to-date and advanced sciences, such as gene therapy and CRISPR in the field of pain management by targeting specific diseases or genes that cause pain, as well as the development of appropriate and safe vectors to deliver genetic material to the source of pain. Finally, commercialization of appreciated studies and their impact on clinical treatments require successful integration between scientific and governmental centers.
Conclusions
Sufficient pain management remains one of the major medical challenges. Pharmacological and nonpharmacological approaches are used for the treatment of mild to severe pain. However, these treatments have still not provided sufficient patient satisfaction, particularly in chronic pain. pain management with employing nanotechnology has recently emerged as a new approach to improve the quality of treatment. Nanotechnology has created a new perspective for pain treatment by introducing of nanomaterials with analgesic properties to satisfactorily pain modulation, engineering of multifunctional nanocarriers to improve the low therapeutic index of conventional analgesic drugs, accurate detection of pain biomarkers along with use of appropriate techniques to avoid complications is the pain mangement. Therefore, successful combination of these factors is expected to achieve ultimate pain control without side effects. Despite many advances of nanotechnology in the preclinical studies, clinical trials on the further development of these systems are still limited. Therefore, efforts should be directed to further develop these nanosystems. This requires interdisciplinary teamwork including researchers, physicians, and engineers in addition to provision of government and academic support.
Acknowledgements
The authors would like to acknowledge Drug Applied Research Center and Faculty of Advanced Medical Science of Tabriz University of Medical Sciences.
Abbreviations
- PLGA
Poly (lactic-co-glycolic acid)
- ZnO
Zinc oxide
- NMDA
N-methyl-d-aspartate
- GABA
γ-Aminobutyric acid
- MgO
Magnesium oxide
- MnO2
Manganese dioxide
- CNS
Central nervous system
- Fe3O4
Magnetite
- MRI
Magnetic resonance imaging
- ROS
Reactive oxygen species
- Ropiv
Ropivacaine
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- FDA
Food and drug administration
- GI
Gastrointestinal
- PEG
Polyethylene glycol
- RGD
Arg–Gly–Asp
- SLNs
Solid lipid nanoparticles
- NLCs
Nanostructured lipid carriers
- PWTP
Paw withdrawal threshold to pressure
- PLA
Polylactic acid
- PCL
Polycaprolactone
- EMLA
Eutectic mixture of local anesthetics
- EPO
PH sensitive cationic polymer consisting of 1:2:1 ratio of methyl methacrylate, N_N-dimethylaminoethyl methacrylate, and butyl methacrylate monomers
- IL-1β
Interleukin‐1β
- TNF-α
Tumor necrosis factor α
- DMAB
Didodecyldimethylammonium bromide
- LPNs
Lipid–polymer hybrid nanoparticles
- TPGS
Tocopheryl PEG succinate
- CdTe
Cadmium telluride
- NiCo2O4
Nickel cobaltite
- LAs
Local anesthetics
- AIDS
Acquired immunodeficiency syndrome
- BBB
Blood–brain barrier
- CB
Cannabinoid
- SMA
Styrene maleic acid
- MGCs
Multinucleated giant cells
- MAPKs
Mitogen-activated protein kinases
- Rheb
Ras family member
- mTOR
Mammalian target of rapamycin
- LipoZOL
Zoledronic acid encapsulated into liposomes
- siRNA
Small interfering RNA
- CGRP
Calcitonin gene-related peptide
- AMN
Analgesic microneedle
- MoS2-AuNR
Molybdenum disulfide nanosheet-coated gold nanorods
- DMM
Destabilization of medial meniscus
- NGF
Nerve growth factor
- cAMP
Cyclic adenosine monophosphate
- TENS
Transcutaneous electrical nerve stimulation
- COX
Cyclooxygenase
Author contributions
SA, HH, and KHK proposed the project; SB, AT, JHH, SA, HH, and KHK wrote the manuscript; all the authors carried out reference searching and data analysis. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) (2019R1A5A2027340 and 2021R1A2C2007937).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave their consent for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hamed Hamishehkar, Email: Hamishehkarh@tbzmed.ac.ir.
Seongpil An, Email: esan@skku.edu.
Ki Hyun Kim, Email: khkim83@skku.edu.
References
- 1.Świeboda P, Filip R, Prystupa A, Drozd M. Assessment of pain: types, mechanism and treatmen. Ann Agric Environ Med. 2013;1:2–7. [PubMed] [Google Scholar]
- 2.Besson J. The neurobiology of pain. Lancet. 1999;353:1610–1615. doi: 10.1016/S0140-6736(99)01313-6. [DOI] [PubMed] [Google Scholar]
- 3.Grichnik K, Ferrante F. The difference between acute and chronic pain. Mt Sinai J Med New York. 1991;58:217–220. [PubMed] [Google Scholar]
- 4.Moran K. Acute pain management: operative or medical procedures and trauma. In: Redelman K, editors. Clinical practice guideline. 1993: 130.
- 5.Gada M. Chronic pain disorder. Archi Indian Psychiatry. 2007;9:7–18. [Google Scholar]
- 6.Portenoy RK. Mechanisms of clinical pain: observations and speculations. Neurol Clin. 1989;7:205–230. doi: 10.1016/S0733-8619(18)30810-7. [DOI] [PubMed] [Google Scholar]
- 7.Merskey H. International association for the study of pain, classification of chronic pain: description of chronic pain syndromes and definitions of pain terms. Pain. 1996;3:Suppl 1–224. [Google Scholar]
- 8.Argoff CE, Dubin A, Pilitsis J, McCleane G. Pain management secrets E-Book. 3. Oxford: Elsevier Health Sciences; 2009. [Google Scholar]
- 9.Mathew E, Kim E, Zempsky W. Pharmacologic treatment of pain. Semin Pediatr Neurol. 2016;23:209–219. doi: 10.1016/j.spen.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 10.El Geziry A, Toble Y, Al Kadhi F, Pervaiz M, Al Nobani M. Non-pharmacological pain management. Pain Manag Spec Circumst. 2018 doi: 10.5772/intechopen.79689. [DOI] [Google Scholar]
- 11.Da Silva JA. The challenge of pain. Psychol Neurosci. 2014 doi: 10.3922/j.psns.2014.1.01. [DOI] [Google Scholar]
- 12.Sowa GA, Perera S, Bechara B, Agarwal V, Boardman J, Huang W, Camacho-Soto A, Vo N, Kang J, Weiner D. Associations between serum biomarkers and pain and pain-related function in older adults with low back pain: a pilot study. J Am Geriatr Soc. 2014;62:2047–2055. doi: 10.1111/jgs.13102. [DOI] [PubMed] [Google Scholar]
- 13.Niculescu A, Le-Niculescu H, Levey D, Roseberry K, Soe K, Rogers J, Khan F, Jones T, Judd S, McCormick M. Towards precision medicine for pain: diagnostic biomarkers and repurposed drugs. Mol Psychiatry. 2019;24:501–522. doi: 10.1038/s41380-018-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell SB, Hoare T. Externally addressable hydrogel nanocomposites for biomedical applications. Curr Opin Chem Eng. 2014;4:1–10. doi: 10.1016/j.coche.2013.12.003. [DOI] [Google Scholar]
- 15.Kesmati M, Torabi M. Interaction between analgesic effect of nano and conventional size of zinc oxide and opioidergic system activity in animal model of acute pain. Basic Clin Neurosci. 2014;5:80. [PMC free article] [PubMed] [Google Scholar]
- 16.Kaye AD, Novitch MB, Carlson SF, Fuller MC, White SW, Haroldson AR, Kaiser JA, Elkersh MA, Brunk AJ, Jeha GM. The role of exparel plus meloxicam for postoperative pain management. Curr Pain Headache Rep. 2020;24:1–7. doi: 10.1007/s11916-020-0837-2. [DOI] [PubMed] [Google Scholar]
- 17.An H, Song Z, Li P, Wang G, Ma B, Wang X. Development of biofabricated gold nanoparticles for the treatment of alleviated arthritis pain. Appl Nanosci. 2020;10:617–622. doi: 10.1007/s13204-019-01135-w. [DOI] [Google Scholar]
- 18.Berrocoso E, Rey-Brea R, Fernández-Arévalo M, Micó JA, Martín-Banderas L. Single oral dose of cannabinoid derivate loaded PLGA nanocarriers relieves neuropathic pain for eleven days. Nanomed. 2017;13:2623–2632. doi: 10.1016/j.nano.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Qi R-Q, Liu W, Wang D-Y, Meng F-Q, Wang H-Y, Qi H-Y. Development of local anesthetic drug delivery system by administration of organo-silica nanoformulations under ultrasound stimuli: in vitro and in vivo investigations. Drug Deliv. 2021;28:54–62. doi: 10.1080/10717544.2020.1856220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husain SF, Lam RW, Hu T, Ng MW, Liau Z, Nagata K, Khanna S, Lam Y, Bhakoo K, Ho R. Locating the site of neuropathic pain in vivo using MMP-12-targeted magnetic nanoparticles. Pain Res Manag. 2019 doi: 10.1155/2019/9394715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazaleuskaya LL, Muzykantov VR, FitzGerald GA. Nanotherapeutic-directed approaches to analgesia. Trends Pharmacol Sci. 2021;42:527–550. doi: 10.1016/j.tips.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beltrán-Gracia E, López-Camacho A, Higuera-Ciapara I, Velázquez-Fernández JB, Vallejo-Cardona AA. Nanomedicine review: clinical developments in liposomal applications. Cancer Nanotechnol. 2019;10:1–40. doi: 10.1186/s12645-019-0055-y. [DOI] [Google Scholar]
- 23.Chen D, Qu X, Shao J, Wang W, Dong X. Anti-vascular nano agents: a promising approach for cancer treatment. J Mater Chem B. 2020;8:2990–3004. doi: 10.1039/C9TB02957E. [DOI] [PubMed] [Google Scholar]
- 24.Zehra M, Zubairi W, Hasan A, Butt H, Ramzan A, Azam M, Mehmood A, Falahati M, Chaudhry AA, Rehman IU. Oxygen generating polymeric nano fibers that stimulate angiogenesis and show efficient wound healing in a diabetic wound model. Int J Nanomed. 2020;15:3511. doi: 10.2147/IJN.S248911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pretorius D, Serpooshan V, Zhang J. Nano-medicine in the cardiovascular system. Front Pharmacol. 2021;12:163. doi: 10.3389/fphar.2021.640182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gopalan D, Pandey A, Alex AT, Kalthur G, Pandey S, Udupa N, Mutalik S. Nanoconstructs as a versatile tool for detection and diagnosis of Alzheimer biomarkers. Nanotechnology. 2021;32:142002. doi: 10.1088/1361-6528/abcdcb. [DOI] [PubMed] [Google Scholar]
- 27.García-Pardo J, Novio F, Nador F, Cavaliere I, Suárez-García S, Lope-Piedrafita S, Candiota AP, Romero-Gimenez J, Rodríguez-Galván B, Bové J. Bioinspired theranostic coordination polymer nanoparticles for intranasal dopamine replacement in Parkinson’s disease. ACS Nano. 2021 doi: 10.1021/acsnano.1c00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang ZL. Nanostructures of zinc oxide. Mater Today. 2004;7:26–33. doi: 10.1016/S1369-7021(04)00286-X. [DOI] [Google Scholar]
- 29.Baxter JB, Aydil ES. Nanowire-based dye-sensitized solar cells. Appl Phys Lett. 2005;86:053114. doi: 10.1063/1.1861510. [DOI] [Google Scholar]
- 30.Sudha M, Senthilkumar S, Hariharan R, Suganthi A, Rajarajan M. Synthesis, characterization and study of photocatalytic activity of surface modified ZnO nanoparticles by PEG capping. J Solgel Sci Technol. 2013;65:301–310. doi: 10.1007/s10971-012-2936-y. [DOI] [Google Scholar]
- 31.An S, Joshi BN, Lee MW, Kim NY, Yoon SS. Electrospun graphene-ZnO nanofiber mats for photocatalysis applications. Appl Surf Sci. 2014;294:24–28. doi: 10.1016/j.apsusc.2013.12.159. [DOI] [Google Scholar]
- 32.Joshi BN, An S, Jo HS, Song KY, Park HG, Hwang S, Al-Deyab SS, Yoon WY, Yoon SS. Flexible, freestanding, and binder-free SnOx–ZnO/carbon nanofiber composites for lithium ion battery anodes. ACS Appl Mater Interfaces. 2016;8:9446–9453. doi: 10.1021/acsami.6b01093. [DOI] [PubMed] [Google Scholar]
- 33.Kim M-W, Yoon H, Ohm TY, Jo HS, An S, Choi SK, Park H, Al Deyab SS, Min BK, Swihart MT. Nanotextured cupric oxide nanofibers coated with atomic layer deposited ZnO TiO2 as highly efficient photocathodes. Appl Catal B. 2017;201:479–485. doi: 10.1016/j.apcatb.2016.08.058. [DOI] [Google Scholar]
- 34.Tripathy N, Kim D-H. Metal oxide modified ZnO nanomaterials for biosensor applications. Nano Converg. 2018;5:27. doi: 10.1186/s40580-018-0159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pathak TK, Swart HC. Structural and luminescence properties of ZnO nanoparticles synthesized by mixture of fuel approach in solution combustion method in zinc oxide based nano materials and devices. IntechOpen. 2019 doi: 10.5772/intechopen.82467. [DOI] [Google Scholar]
- 36.Jo HS, Samuel E, Kwon H-J, Joshi B, Kim M-W, Kim T-G, Swihart MT, Yoon SS. Highly flexible transparent substrate-free photoanodes using ZnO nanowires on nickel microfibers. Chem Eng J. 2019;363:13–22. doi: 10.1016/j.cej.2019.01.099. [DOI] [Google Scholar]
- 37.Mirzaei H, Darroudi M. Zinc oxide nanoparticles: biological synthesis and biomedical applications. Ceram Int. 2017;43:907–914. doi: 10.1016/j.ceramint.2016.10.051. [DOI] [Google Scholar]
- 38.Hambidge KM, Miller LV, Westcott JE, Sheng X, Krebs NF. Zinc bioavailability and homeostasis. The Am J Clin Nutr. 2010;91:1478S–1483S. doi: 10.3945/ajcn.2010.28674I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dursun N, Erenmemisoglu A, Suer C, Gogusten B. The effect of zinc deficiency on morphine antinociception. Res Commun Subst Abuse. 1995;16:47–52. [Google Scholar]
- 40.Nozaki C, Vergnano AM, Filliol D, Ouagazzal A-M, Le Goff A, Carvalho S, Reiss D, Gaveriaux-Ruff C, Neyton J, Paoletti P. Zinc alleviates pain through high-affinity binding to the NMDA receptor NR2A subunit. Nat Neurosci. 2011;14:1017. doi: 10.1038/nn.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda A, Minami A, Seki Y, Oku N. Differential effects of zinc on glutamatergic and GABAergic neurotransmitter systems in the hippocampus. J Neurosci Res. 2004;75:225–229. doi: 10.1002/jnr.10846. [DOI] [PubMed] [Google Scholar]
- 42.Martin KJ, González EA, Slatopolsky E. Clinical consequences and management of hypomagnesemia. J Am Soc Nephrol. 2009;20:2291–2295. doi: 10.1681/ASN.2007111194. [DOI] [PubMed] [Google Scholar]
- 43.Shin H-J, Na H-S, Do S-H. Magnesium and pain. Nutrients. 2020;12:2184. doi: 10.3390/nu12082184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soave P, Conti G, Costa R, Arcangeli A. Magnesium and anaesthesia. Curr Drug Targets. 2009;10:734–743. doi: 10.2174/138945009788982487. [DOI] [PubMed] [Google Scholar]
- 45.Iseri LT, French JH. Magnesium: natures physiologic calcium blocker. Am Heart J. 1984;108:188–193. doi: 10.1016/0002-8703(84)90572-6. [DOI] [PubMed] [Google Scholar]
- 46.Ahangiri L, Kesmati M, Najafzadeh H. Evaluation of analgesic and anti-inflammatory effect of nanoparticles of magnesium oxide in mice with and without ketamine. Eur Rev Med Pharmacol Sci. 2013;17:2706–2710. [PubMed] [Google Scholar]
- 47.Kavosi N, Najafzadeh H, Bavari M, Esmailirad A. Effect of nano-particle of magnesium oxide on ketamine-induced anesthesia in rabbit. Curr Res J Biol Sci. 2012;4:592–595. [Google Scholar]
- 48.Becker S, Ceko M, Louis-Foster M, Elfassy NM, Leyton M, Shir Y, Schweinhardt P. Dopamine and pain sensitivity: neither sulpiride nor acute phenylalanine and tyrosine depletion have effects on thermal pain sensations in healthy volunteers. PLoS ONE. 2013 doi: 10.1371/journal.pone.0080766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serafini RA, Pryce KD, Zachariou V. The mesolimbic dopamine system in chronic pain and associated affective comorbidities. Biol Psychiatry. 2020;87:64–73. doi: 10.1016/j.biopsych.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Megat S, Shiers S, Moy JK, Barragan-Iglesias P, Pradhan G, Seal RP, Dussor G, Price TJ. A critical role for dopamine D5 receptors in pain chronicity in male mice. J Neurosci. 2018;38:379–397. doi: 10.1523/JNEUROSCI.2110-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nandhagopal R, Troiano AR, Mak E, Schulzer M, Bushnell MC, Stoessl AJ. Response to heat pain stimulation in idiopathic Parkinson’s disease. Pain Med. 2010;11:834–840. doi: 10.1111/j.1526-4637.2010.00866.x. [DOI] [PubMed] [Google Scholar]
- 52.Nosrati N, Hassanpour-Ezzati M, Mousavi SZ, Rezagholiyan S. Comparison of MnO2 nanoparticles and microparticles distribution in CNS and muscle and effect on acute pain threshold in rats. Nanomed J. 2014;1:180–190. doi: 10.7508/nmj.2014.03.008. [DOI] [Google Scholar]
- 53.Wood PB. Role of central dopamine in pain and analgesia. Expert Rev Neurother. 2008;8:781–797. doi: 10.1586/14737175.8.5.781. [DOI] [PubMed] [Google Scholar]
- 54.Martínez-Banderas AI, Aires A, Plaza-García S, Colás L, Moreno JA, Ravasi T, Merzaban JS, Ramos-Cabrer P, Cortajarena AL, Kosel J. Magnetic core–shell nanowires as MRI contrast agents for cell tracking. J Nanobiotechnol. 2020;18:1–12. doi: 10.1186/s12951-020-00597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziarani GM, Malmir M, Lashgari N, Badiei A. The role of hollow magnetic nanoparticles in drug delivery. RSC Adv. 2019;9:25094–25106. doi: 10.1039/C9RA01589B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haghighi AH, Faghih Z, Khorasani MT, Farjadian F. Antibody conjugated onto surface modified magnetic nanoparticles for separation of HER2+ breast cancer cells. J Magn Magn Mater. 2019;490:165479. doi: 10.1016/j.jmmm.2019.165479. [DOI] [Google Scholar]
- 57.Wu P-C, Hsiao H-T, Lin Y-C, Shieh D-B, Liu Y-C. The analgesia efficiency of ultrasmall magnetic iron oxide nanoparticles in mice chronic inflammatory pain model. Nanomed. 2017;13:1975–1981. doi: 10.1016/j.nano.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Wu PC, Shieh DB, Hsiao HT, Wang JCF, Lin YC, Liu YC. Magnetic field distribution modulation of intrathecal delivered ketorolac iron-oxide nanoparticle conjugates produce excellent analgesia for chronic inflammatory pain. J Nanobiotechnol. 2018;16:49. doi: 10.1186/s12951-018-0375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doh H-J, Cho W-J, Yong C-S, Choi H-G, Kim JS, Lee C-H, Kim D-D. Synthesis and evaluation of ketorolac ester prodrugs for transdermal delivery. J Pharm Sci. 2003;92:1008–1017. doi: 10.1002/jps.10353. [DOI] [PubMed] [Google Scholar]
- 60.Mantha VR, Nair HK, Venkataramanan R, Gao YY, Matyjaszewski K, Dong H, Li W, Landsittel D, Cohen E, Lariviere WR. Nanoanesthesia: a novel, intravenous approach to ankle block in the rat by magnet-directed concentration of ropivacaine-associated nanoparticles. Anesth Analg. 2014;118:1355–1362. doi: 10.1213/ANE.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 61.Berry PH, Chapman CR, Covington EC, Dahl JL, Katz JA, Miaskowski C, McLean MJ. Pain: current understanding of assessment, management, and treatments. VA: National Pharmaceutical Council and the Joint Commission for the Accreditation of Healthcare Organizations; 2001. p. b44.
- 62.Hua S, Wu SY. The use of lipid-based nanocarriers for targeted pain therapies. Front Pharmacol. 2013;4:143. doi: 10.3389/fphar.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clin J pain. 2008;24:469–478. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- 64.Galligan JJ, Akbarali HI. Molecular physiology of enteric opioid receptors. Am J Gastroenterol Suppl. 2014;2:17. doi: 10.1038/ajgsup.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wood LD, Neumiller JJ, Carlson J, Setter SM, Corbett CF. Challenges of medication management in hospitalized patients with Parkinson’s disease. Am J Health Syst Pharm. 2010;67:2059–2063. doi: 10.2146/ajhp100170. [DOI] [PubMed] [Google Scholar]
- 66.Ngwuluka NC, Choonara YE, Modi G, du Toit LC, Kumar P, Ndesendo VM, Pillay V. Design of an interpolyelectrolyte gastroretentive matrix for the site-specific zero-order delivery of levodopa in Parkinson’s disease. AAPS PharmSciTech. 2013;14:605–619. doi: 10.1208/s12249-013-9945-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maderuelo C, Zarzuelo A, Lanao JM. Critical factors in the release of drugs from sustained release hydrophilic matrices. J controlled release. 2011;154:2–19. doi: 10.1016/j.jconrel.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Wagner A, Vorauer UHL. Liposome technology for industrial purposes. J Drug Deliv. 2011 doi: 10.1155/2011/591325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.São Pedro A, Fernandes R, Flora Villarreal C, Fialho R. Cabral albuquerque E opioid-based micro and nanoparticulate formulations: alternative approach on pain management. J Microencapsul. 2016;33:18–29. doi: 10.3109/02652048.2015.1134687. [DOI] [PubMed] [Google Scholar]
- 70.Nagle PC, Gerancher J, Management P. DepoDur®(extended-release epidural morphine): a review of an old drug in a new vehicle. Tech Reg Anesth. 2007;11:9–18. doi: 10.1053/j.trap.2007.02.011. [DOI] [Google Scholar]
- 71.He S, Zhu D, Xie F. Preparation and characterization of tramadol PEG-coated multivesicular liposomes for sustained release. Pharmazie- Int J Pharm Sci. 2010;65:467–470. doi: 10.1691/ph.2010.9357. [DOI] [PubMed] [Google Scholar]
- 72.Eskandari S, Varamini P, Toth I. Formulation, characterization and permeability study of nano particles of lipo-endomorphin-1 for oral delivery. J Liposome Res. 2013;23:311–317. doi: 10.3109/08982104.2013.805339. [DOI] [PubMed] [Google Scholar]
- 73.Hoekman JD, Srivastava P, Ho RJ. Aerosol-stable peptide-coated liposome nanoparticles: a proof-of-concept study with opioid fentanyl in enhancing analgesic effects and reducing plasma drug exposure. J Pharm Sci. 2014;103:2231–2239. doi: 10.1002/jps.24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bernards CM, Luger TJ, Malmberg AB, Hill HF, Yaksh TL. Liposome encapsulation prolongs alfentanil spinal analgesia and alters systemic redistribution in the rat. Anesthesiology. 1992;77:529–535. doi: 10.1097/00000542-199209000-00019. [DOI] [PubMed] [Google Scholar]
- 75.Lim GJ, Ishiuji Y, Dawn A, Harrison B, Kim DW, Atala A, Yosipovitch G. In vitro and in vivo characterization of a novel liposomal butorphanol formulation for treatment of pruritus. Acta Derm Venereol. 2008;88:327–330. doi: 10.2340/00015555-0480. [DOI] [PubMed] [Google Scholar]
- 76.Babaie S, Taghvimi A, Charkhpour M, Zarebkohan A, Keyhanvar P, Hamishehkar H. Optimization of influential variables in the development of buprenorphine and bupivacaine loaded invasome for dermal delivery. Adv Pharm Bul. 2020 doi: 10.34172/apb.2021.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patidar A, Thakur DS, Kumar P, Verma J. A review on novel lipid based nanocarriers. Int J Pharm Pharm Sci. 2010;2:30–35. [Google Scholar]
- 78.Müller RH, Staufenbiel S, Keck CM. Lipid nanoparticles (SLN, NLC) for innovative consumer care & household products. HPC Today. 2014;9:18–25. [Google Scholar]
- 79.Küchler S, Wolf NB, Heilmann S, Weindl G, Helfmann J, Yahya MM, Stein C, Schäfer-Korting M. 3D-wound healing model: influence of morphine and solid lipid nanoparticles. J Biotechnol. 2010;148:24–30. doi: 10.1016/j.jbiotec.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 80.Bahrami MA, Farhadian N, Karimi M, Forouzan A, Masoumi K. Improvement of pain relief of fentanyl citrate drug encapsulated in nano structured lipid carrier: drug formulation, parameter optimization, in vitro and in vivo studies. Drug Des Devel Ther. 2020;14:2033. doi: 10.2147/DDDT.S235474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor A, McLeod G. Basic pharmacology of local anaesthetics. BJA Educ. 2020;20:34. doi: 10.1016/j.bjae.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sunderland S, Yarnold CH, Head SJ, Osborn JA, Purssell A, Peel JK, Schwarz SK. Regional versus general anesthesia and the incidence of unplanned health care resource utilization for postoperative pain after wrist fracture surgery: results from a retrospective quality improvement project. Reg Anesth Pain Med. 2016;41:22–27. doi: 10.1097/AAP.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 83.De Melo NF, De Araujo DR, Grillo R, Moraes CM, De Matos AP, de Paula E, Rosa AH, Fraceto LF. Benzocaine-loaded polymeric nanocapsules: study of the anesthetic activities. J Pharm Sci. 2012;101:1157–1165. doi: 10.1002/jps.22829. [DOI] [PubMed] [Google Scholar]
- 84.Muniz BV, Baratelli D, Di Carla S, Serpe L, da Silva CB, Guilherme VA, Ribeiro LNM, Cereda CMS, de Paula E, Volpato MC, et al. Hybrid hydrogel composed of polymeric nanocapsules co-loading lidocaine and prilocaine for topical intraoral anesthesia. Sci Rep. 2018;8:17972. doi: 10.1038/s41598-018-36382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grillo R, de Melo NF, de Araujo DR, de Paula E, Rosa AH, Fraceto LF. Polymeric alginate nanoparticles containing the local anesthetic bupivacaine. J Drug Target. 2010;18:688–699. doi: 10.3109/10611861003649738. [DOI] [PubMed] [Google Scholar]
- 86.Franz-Montan M, Silva AL, Cogo K, Bergamaschi Cde C, Volpato MC, Ranali J, de Paula E, Groppo FC. Liposome-encapsulated ropivacaine for topical anesthesia of human oral mucosa. Anesth Analg. 2007;104:1528–1531. doi: 10.1213/01.ane.0000262040.19721.26. [DOI] [PubMed] [Google Scholar]
- 87.Fisher R, Hung O, Mezei M, Stewart R. Topical anaesthesia of intact skin: liposome-encapsulated tetracaine vs EMLA. Br J Anaesth. 1998;81:972–973. doi: 10.1093/bja/81.6.972. [DOI] [PubMed] [Google Scholar]
- 88.Cereda CMS, Mecatti DS, Papini JZB, Bueno DV, Franz-Montan M, Rocha T, Pedrazzoli Junior J, de Paula E, de Araujo DR, Grillo R, et al. Bupivacaine in alginate and chitosan nanoparticles: an in vivo evaluation of efficacy, pharmacokinetics, and local toxicity. J Pain Res. 2018;11:683–691. doi: 10.2147/JPR.S158695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Q, Santamaria CM, Wei T, Zhao C, Ji T, Yang T, Shomorony A, Wang BY, Kohane DS. Hollow silica nanoparticles penetrate the peripheral nerve and enhance the nerve blockade from tetrodotoxin. Nano Lett. 2018;18:32–37. doi: 10.1021/acs.nanolett.7b02461. [DOI] [PubMed] [Google Scholar]
- 90.Zhao C, Liu A, Santamaria CM, Shomorony A, Ji T, Wei T, Gordon A, Elofsson H, Mehta M, Yang R. Polymer-tetrodotoxin conjugates to induce prolonged duration local anesthesia with minimal toxicity. Nat Commun. 2019;10:1–14. doi: 10.1038/s41467-019-10296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boogaerts JG, Lafont ND, Declercq AG, Luo HC, Gravet ET, Bianchi JA, Legros FJ. Epidural administration of liposome associated bupivacaine for the management of postsurgical pain: a first study. J Clin Anesth. 1994;6:315–320. doi: 10.1016/0952-8180(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 92.Gorfine SR, Onel E, Patou G, Krivokapic ZV. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trial. Dis Colon Rectum. 2011;54:1552–1559. doi: 10.1097/DCR.0b013e318232d4c1. [DOI] [PubMed] [Google Scholar]
- 93.Negi P, Singh B, Sharma G, Beg S, Katare OP. Biocompatible lidocaine and prilocaine loaded-nanoemulsion system for enhanced percutaneous absorption: QbD-based optimisation, dermatokinetics and in vivo evaluation. J Microencapsul. 2015;32:419–431. doi: 10.3109/02652048.2015.1046513. [DOI] [PubMed] [Google Scholar]
- 94.Perry DA, Gansky SA, Loomer PM. Effectiveness of a transmucosal lidocaine delivery system for local anaesthesia during scaling and root planing. J Clin Periodontol. 2005;32:590–594. doi: 10.1111/j.1600-051X.2005.00717.x. [DOI] [PubMed] [Google Scholar]
- 95.Kohane DS, Lu NT, Gökgöl-Kline AC, Shubina M, Kuang Y, Hall S, Strichartz GR, Berde CB. The local anesthetic properties and toxicity of saxitonin homologues for rat sciatic nerve block in vivo. Reg Anesth Pain Med. 2000;25:52–59. doi: 10.1016/s1098-7339(00)80011-5. [DOI] [PubMed] [Google Scholar]
- 96.Vane JR, Botting RM. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104:2S–8S. doi: 10.1016/s0002-9343(97)00203-9. [DOI] [PubMed] [Google Scholar]
- 97.Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143. doi: 10.14336/AD.2017.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heath JR. Nanotechnologies for biomedical science and translational medicine. Proc Natl Acad Sci USA. 2015;112:14436–14443. doi: 10.1073/pnas.1515202112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khan I, Khan M, Umar MN, Oh D-H. Nanobiotechnology and its applications in drug delivery system: a review. IET Nanobiotechnol. 2015;9:396–400. doi: 10.1049/iet-nbt.2014.0062. [DOI] [PubMed] [Google Scholar]
- 100.El-Habashy SE, Allam AN, El-Kamel AH. Ethyl cellulose nanoparticles as a platform to decrease ulcerogenic potential of piroxicam: formulation and in vitro/in vivo evaluation. Int J Nanomedicine. 2016;11:2369. doi: 10.2147/IJN.S93354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Javadzadeh Y, Ahadi F, Davaran S, Mohammadi G, Sabzevari A, Adibkia K. Preparation and physicochemical characterization of naproxen-PLGA nanoparticles. Colloids Surf B Biointerfaces. 2010;81:498–502. doi: 10.1016/j.colsurfb.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 102.Khachane P, Date AA, Nagarsenker MS. Eudragit EPO nanoparticles application in improving therapeutic efficacy and reducing ulcerogenicity of meloxicam on oral administration. J Biomed Nanotechnol. 2011;7:590–597. doi: 10.1166/jbn.2011.1322. [DOI] [PubMed] [Google Scholar]
- 103.Narayanan D, Geena MG, Lakshmi H, Koyakutty M, Nair S, Menon D. Poly-(ethylene glycol) modified gelatin nanoparticles for sustained delivery of the anti-inflammatory drug Ibuprofen-sodium: an in vitro and in vivo analysis. Nanomed Nanotechnol Biol Med. 2013;9:818–828. doi: 10.1016/j.nano.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 104.Zhang L, Zhang Q, Wang X, Zhang W, Lin C, Chen F, Yang X, Pan W. Drug-in-cyclodextrin-in-liposomes: a novel drug delivery system for flurbiprofen. Int J Pharm. 2015;492:40–45. doi: 10.1016/j.ijpharm.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 105.Sugihara H, Yamamoto H, Kawashima Y, Takeuchi H. Effectiveness of submicronized chitosan-coated liposomes in oral absorption of indomethacin. J Liposome Res. 2012;22:72–79. doi: 10.3109/08982104.2011.621128. [DOI] [PubMed] [Google Scholar]
- 106.Raffin RP, Lima A, Lorenzoni R, Antonow MB, Turra C, Alves MP, Fagan SB. Natural lipid nanoparticles containing nimesulide: synthesis, characterization and in vivo antiedematogenic and antinociceptive activities. J Biomed Nanotechnol. 2012;8:309–315. doi: 10.1166/jbn.2012.1377. [DOI] [PubMed] [Google Scholar]
- 107.Guilherme VA, Ribeiro LNM, Alcantara ACS, Castro SR, da Rodrigues GH, da Silva CG, Breitkreitz MC, Clemente-Napimoga J, Macedo CG, Abdalla HB, et al. Improved efficacy of naproxen-loaded NLC for temporomandibular joint administration. Sci Rep. 2019;9:11160. doi: 10.1038/s41598-019-47486-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oh DH, Din FU, Kim DW, Kim JO, Yong CS, Choi HG. Flurbiprofen-loaded nanoparticles prepared with polyvinylpyrrolidone using Shirasu porous glass membranes and a spray-drying technique: nano-sized formation and improved bioavailability. J Microencapsul. 2013;30:674–680. doi: 10.3109/02652048.2013.774447. [DOI] [PubMed] [Google Scholar]
- 109.Nita LE, Chiriac AP, Nistor MT, Tartau L. Indomethacin-loaded polymer nanocarriers based on poly(2-hydroxyethyl methacrylate-co-3,9-divinyl-2,4,8,10-tetraoxaspiro (5.5) undecane): preparation, in vitro and in vivo evaluation. J Biomed Mater Res B Appl Biomater. 2012;100:1121–1133. doi: 10.1002/jbm.b.32679. [DOI] [PubMed] [Google Scholar]
- 110.Potta SG, Minemi S, Nukala RK, Peinado C, Lamprou DA, Urquhart A, Douroumis D. Preparation and characterization of ibuprofen solid lipid nanoparticles with enhanced solubility. J Microencapsul. 2011;28:74–81. doi: 10.3109/02652048.2010.529948. [DOI] [PubMed] [Google Scholar]
- 111.Lopes-de-Araújo J, Neves AR, Gouveia VM, Moura CC, Nunes C, Reis S. Oxaprozin-loaded lipid nanoparticles towards overcoming NSAIDs side-effects. Pharm Res. 2016;33:301–314. doi: 10.1007/s11095-015-1788-x. [DOI] [PubMed] [Google Scholar]
- 112.Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56:1067–1084. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 113.Cooper DL, Harirforoosh S. Effect of formulation variables on preparation of celecoxib loaded polylactide-co-glycolide nanoparticles. PLoS ONE. 2014 doi: 10.1371/journal.pone.0113558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khachane P, Nagarsenker MS. Positively charged polymeric nanoparticles: application in improving therapeutic efficacy of meloxicam after oral administration. Pharmazie Int J Pharm Sci. 2011;66:334–338. doi: 10.1691/ph.2011.0324. [DOI] [PubMed] [Google Scholar]
- 115.Kumar R, Nagarwal RC, Dhanawat M, Pandit JK. In-vitro and in-vivo study of indomethacin loaded gelatin nanoparticles. J Biomed Nanotechnol. 2011;7:325–333. doi: 10.1166/jbn.2011.1290. [DOI] [PubMed] [Google Scholar]
- 116.NATIONAL PHARMACEUTICAL COUNCIL, INC (NPC) and Joint Commission on Accreditation of Healthcare Organizations. Pain: current understanding of assessment, management, and treatments. Obtido em. The ISSN register. https://www.npcnow.org. 2001. Accessed Dec 2001.
- 117.Friedman PM, Mafong EA, Friedman ES, Geronemus RG. Topical anesthetics update: EMLA and beyond. Dermatol Surg. 2001;27:1019–1026. doi: 10.1046/j.1524-4725.2001.01855.x. [DOI] [PubMed] [Google Scholar]
- 118.Nastiti CM, Ponto T, Abd E, Grice JE, Benson HA, Roberts MS. Topical nano and microemulsions for skin delivery. Pharmaceutics. 2017;9:37. doi: 10.3390/pharmaceutics9040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Üner M, Wissing S, Yener G, Müller R. Skin moisturizing effect and skin penetration of ascorbyl palmitate entrapped in solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) incorporated into hydrogel. Pharmazie Int J Pharm Sci. 2005;60:751–755. [PubMed] [Google Scholar]
- 120.Babaie S, Ghanbarzadeh S, Davaran S, Kouhsoltani M, Hamishehkar H. Nanoethosomes for dermal delivery of lidocaine. Adv Pharm Bull. 2015;5:549. doi: 10.15171/apb.2015.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Somagoni J, Boakye CH, Godugu C, Patel AR, Faria HAM, Zucolotto V, Singh M. Nanomiemgel-a novel drug delivery system for topical application-in vitro and in vivo evaluation. PLoS ONE. 2014 doi: 10.1371/journal.pone.0115952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang J, Zhang L, Chi H, Wang S. An alternative choice of lidocaine-loaded liposomes: lidocaine-loaded lipid–polymer hybrid nanoparticles for local anesthetic therapy. Drug Deliv. 2016;23:1254–1260. doi: 10.3109/10717544.2016.1141259. [DOI] [PubMed] [Google Scholar]
- 123.You P, Yuan R, Chen C. Design and evaluation of lidocaine-and prilocaine-coloaded nanoparticulate drug delivery systems for topical anesthetic analgesic therapy: a comparison between solid lipid nanoparticles and nanostructured lipid carriers. Drug Des Devel Ther. 2017;11:2743. doi: 10.2147/DDDT.S141031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao X, Sun Y, Li Z. Topical anesthesia therapy using lidocaine-loaded nanostructured lipid carriers: tocopheryl polyethylene glycol 1000 succinate-modified transdermal delivery system. Drug Des Devel Ther. 2018;12:4231. doi: 10.2147/DDDT.S187177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Levy LL, Emer JJ. Complications of minimally invasive cosmetic procedures: prevention and management. J Cutan Aesthet Surg. 2012;5:121. doi: 10.4103/0974-2077.99451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gill HS, Prausnitz MR. Does needle size matter? J Diabetes Sci Technol. 2007;1:725–729. doi: 10.1177/193229680700100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37–43. doi: 10.1080/08990220600700925. [DOI] [PubMed] [Google Scholar]
- 128.Miyakoshi M, Kamoi K, Iwanaga M, Hoshiyama A, Yamada A. Comparison of patient’s preference, pain perception, and usability between micro fine plus® 31-gauge needle and microtapered nanopass® 33-gauge needle for insulin therapy. J Diabetes Sci Technol. 2007;1:718–724. doi: 10.1177/193229680700100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human subjects. Clin J Pain. 2008;24:585. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tuan-Mahmood TM, McCrudden MT, Torrisi BM, McAlister E, Garland MJ, Singh TRR, Donnelly RF. Microneedles for intradermal and transdermal drug delivery. Eur J Pharm Sci. 2013;50:623–637. doi: 10.1016/j.ejps.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Quinn HL, Kearney MC, Courtenay AJ, McCrudden MT, Donnelly RF. The role of microneedles for drug and vaccine delivery. Expert Opin Drug Deliv. 2014;11:1769–1780. doi: 10.1517/17425247.2014.938635. [DOI] [PubMed] [Google Scholar]
- 132.Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56:581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 133.Shende P, Sardesai M, Gaud RS. Micro to nanoneedles: a trend of modernized transepidermal drug delivery system. Artif Cells Nanomed Biotechnol. 2018;46:19–25. doi: 10.1080/21691401.2017.1304409. [DOI] [PubMed] [Google Scholar]
- 134.Chiappini C, Martinez JO, De Rosa E, Almeida CS, Tasciotti E, Stevens MM. Biodegradable nanoneedles for localized delivery of nanoparticles in vivo: exploring the biointerface. ACS Nano. 2015;9:5500–5509. doi: 10.1021/acsnano.5b01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang GQ, Wu HB, Hoster HE, Chan-Park MB, Lou XWD. Single-crystalline NiCo 2 O 4 nanoneedle arrays grown on conductive substrates as binder-free electrodes for high-performance supercapacitors. Energy Environ Sci. 2012;5:9453–9456. doi: 10.1039/C2EE22572G. [DOI] [Google Scholar]
- 136.Yang Y, Yuen M-F, Chen X, Xu S, Tang Y, Zhang W. Fabrication of arrays of high-aspect-ratio diamond nanoneedles via maskless ECR-assisted microwave plasma etching. CrystEngComm. 2015;17:2791–2800. doi: 10.1039/C4CE02267J. [DOI] [Google Scholar]
- 137.Hieber SE, Müller B. Nanodentistry. In: Logothetidis S, editor. Nanomedicine and nanobiotechnology. Berlin: Springer; 2012. pp. 95–107. [Google Scholar]
- 138.Malamed S. Pharmacology of local anesthetics. n: Malamed SF, editor. Handbook of Local Anesthesia. 6th ed. St. Louis: Elsevier Mosby; 2013. p 25-38.
- 139.de Paula E, Cereda CM, Fraceto LF, de Araujo DR, Franz-Montan M, Tofoli GR, Ranali J, Volpato MC, Groppo FC. Micro and nanosystems for delivering local anesthetics. Expert Opin Drug Deliv. 2012;9:1505–1524. doi: 10.1517/17425247.2012.738664. [DOI] [PubMed] [Google Scholar]
- 140.Franz-Montan M, Baroni D, Brunetto G, Sobral VRV, da Silva CMG, Venâncio P, Zago PW, Cereda CMS, Volpato MC, de Araújo DR. Liposomal lidocaine gel for topical use at the oral mucosa: characterization, in vitro assays and in vivo anesthetic efficacy in humans. J Liposome Res. 2015;25:11–19. doi: 10.3109/08982104.2014.911315. [DOI] [PubMed] [Google Scholar]
- 141.Franz-Montan M, de Paula E, Groppo FC, Silva AL, Ranali J, Volpato MC. Liposome-encapsulated ropivacaine for intraoral topical anesthesia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2010;110:800–804. doi: 10.1016/j.tripleo.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 142.Hazzah HA, Farid RM, Nasra MM, Massik EL, MA, Abdallah OY. Lyophilized sponges loaded with curcumin solid lipid nanoparticles for buccal delivery: development and characterization. Int J Pharm. 2015;492:248–257. doi: 10.1016/j.ijpharm.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 143.Ribeiro LN, Franz-Montan M, Breitkreitz MC, Alcântara AC, Castro SR, Guilherme VA, Barbosa RM, de Paula E. Nanostructured lipid carriers as robust systems for topical lidocaine-prilocaine release in dentistry. Eur J Pharm Sci. 2016;93:192–202. doi: 10.1016/j.ejps.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 144.De Melo NFS, De Araújo DR, Grillo R, Moraes CM, De Matos AP, de Paula E, Rosa AH, Fraceto LF. Benzocaine-loaded polymeric nanocapsules: study of the anesthetic activities. J Pharm Sci. 2012;101:1157–1165. doi: 10.1002/jps.22829. [DOI] [PubMed] [Google Scholar]
- 145.Low SB, Allen EP, Kontogiorgos ED. Reduction in dental hypersensitivity with nano-hydroxyapatite, potassium nitrate, sodium monoflurophosphate and antioxidants. Open Dent J. 2015;9:92. doi: 10.2174/1874364101509010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Backonja MM. Defining neuropathic pain. Anesth Analg. 2003;97:785–790. doi: 10.1213/01.ANE.0000062826.70846.8D. [DOI] [PubMed] [Google Scholar]
- 147.Meacham K, Shepherd A, Mohapatra DP, Haroutounian S. Neuropathic pain: central vs peripheral mechanisms. Curr Pain Headache Rep. 2017;21:28. doi: 10.1007/s11916-017-0629-5. [DOI] [PubMed] [Google Scholar]
- 148.Stavros K, Simpson DM. Understanding the etiology and management of HIV-associated peripheral neuropathy. Curr HIV/AIDS Rep. 2014;11:195–201. doi: 10.1007/s11904-014-0211-2. [DOI] [PubMed] [Google Scholar]
- 149.Borsook D. Neurological diseases and pain. Brain. 2012;135:320–344. doi: 10.1093/brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tiwari V, Yang F, He S-Q, Shechter R, Zhang C, Shu B, Zhang T, Tiwari V, Wang Y, Dong X. Activation of peripheral μ-opioid receptors by dermorphin [D-Arg2, Lys4](1–4) amide leads to modality-preferred inhibition of neuropathic pain. Anesthesiology. 2016;124:706–720. doi: 10.1097/ALN.0000000000000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sprintz M, Tasciotti E, Allegri M, Grattoni A, Driver LC, Ferrari M. Nanomedicine: ushering in a new era of pain management. Eur J Pain Suppl. 2011;5:317–322. doi: 10.1016/j.eujps.2011.08.004. [DOI] [Google Scholar]
- 153.Attal N, Cruccu G, Haanpää M, Hansson P, Jensen TS, Nurmikko T, Sampaio C, Sindrup S, Wiffen P. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–1169. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 154.Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sain NM, Liang A, Kane SA, Urban MO. Antinociceptive effects of the non-selective cannabinoid receptor agonist CP 55,940 are absent in CB1−/− and not CB2−/− mice in models of acute and persistent pain. Neuropharmacology. 2009;57:235–241. doi: 10.1016/j.neuropharm.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 156.Berrocoso E, Rey-Brea R, Fernández-Arévalo M, Micó JA, Martín-Banderas L. Biology, Medicine: Single oral dose of cannabinoid derivate loaded PLGA nanocarriers relieves neuropathic pain for eleven days. Nanomed. 2017;13:2623–2632. doi: 10.1016/j.nano.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 157.Linsell O, Brownjohn PW, Nehoff H, Greish K, Ashton JC. Effect of styrene maleic acid WIN55, 212–2 micelles on neuropathic pain in a rat model. J Drug Targeting. 2015;23:353–359. doi: 10.3109/1061186X.2014.997737. [DOI] [PubMed] [Google Scholar]
- 158.Vasudeva K, Vodovotz Y, Azhar N, Barclay D, Janjic JM, Pollock JA. In vivo and systems biology studies implicate IL-18 as a central mediator in chronic pain. J Neuroimmunol. 2015;283:43–49. doi: 10.1016/j.jneuroim.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16:1248. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hasnie F, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, Kinchington P, Dickenson A, Pheby T, Rice A. Further characterization of a rat model of varicella zoster virus–associated pain: Relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience. 2007;144:1495–1508. doi: 10.1016/j.neuroscience.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Delavary BM, van der Veer WM, van Egmond M, Niessen FB, Beelen RHJ. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 164.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011 doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 166.Saleem M, Deal B, Nehl E, Janjic JM, Pollock JA. Nanomedicine-driven neuropathic pain relief in a rat model is associated with macrophage polarity and mast cell activation. Acta Neuropathol Commun. 2019;7:108. doi: 10.1186/s40478-019-0762-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Di Francesco M, Fragassi A, Pannuzzo M, Ferreira M, Brahmachari S, Decuzzi P. Management of osteoarthritis: from drug molecules to nano/micromedicines. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022 doi: 10.1002/wnan.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pecchi E, Priam S, Gosset M, Pigenet A, Sudre L, Laiguillon M-C, Berenbaum F, Houard X. Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: possible involvement in osteoarthritis pain. Arthritis Res Ther. 2014;16:1–11. doi: 10.1186/ar4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ashraf S, Mapp PI, Burston J, Bennett AJ, Chapman V, Walsh DA. Augmented pain behavioural responses to intra-articular injection of nerve growth factor in two animal models of osteoarthritis. Ann Rheum Dis. 2014;73:1710–1718. doi: 10.1136/annrheumdis-2013-203416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McNamee KE, Burleigh A, Gompels LL, Feldmann M, Allen SJ, Williams RO, Dawbarn D, Vincent TL, Inglis JJ. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain®. 2010;149:386–392. doi: 10.1016/j.pain.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 171.Choi J, Yang J, Jang E, Suh J-S, Huh Y-M, Lee K, Haam S. Gold nanostructures as photothermal therapy agent for cancer. Anti-Cancer Agents Med Chem. 2011;11:953–964. doi: 10.2174/187152011797927599. [DOI] [PubMed] [Google Scholar]
- 172.Au MT, Shi J, Fan Y, Ni J, Wen C, Yang M. Nerve growth factor-targeted molecular theranostics based on molybdenum disulfide nanosheet-coated gold nanorods (MoS2-AuNR) for osteoarthritis pain. ACS Nano. 2021;15:11711–11723. doi: 10.1021/acsnano.1c02454. [DOI] [PubMed] [Google Scholar]
- 173.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 176.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 177.Caraglia M, Luongo L, Salzano G, Zappavigna S, Marra M, Guida F, Lusa S, Giordano C, De Novellis V, Rossi F. Stealth liposomes encapsulating zoledronic acid: a new opportunity to treat neuropathic pain. Mol Pharm. 2013;10:1111–1118. doi: 10.1021/mp3006215. [DOI] [PubMed] [Google Scholar]
- 178.Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S, LoRusso P, Ma P, Ravera C, Deckert F. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol. 2002;42:1228–1236. doi: 10.1177/009127002762491316. [DOI] [PubMed] [Google Scholar]
- 179.Anand P, Shenoy R, Palmer JE, Baines AJ, Lai RY, Robertson J, Bird N, Ostenfeld T, Chizh BA. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. Eur J Pain. 2011;15:1040–1048. doi: 10.1016/j.ejpain.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 180.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shin J, Yin Y, Park H, Park S, Triantafillu UL, Kim Y, Kim SR, Lee SY, Kim DK, Hong J. p38 siRNA-encapsulated PLGA nanoparticles alleviate neuropathic pain behavior in rats by inhibiting microglia activation. Nanomedicine. 2018;13:1607–1621. doi: 10.2217/nnm-2018-0054. [DOI] [PubMed] [Google Scholar]
- 182.Gerard E, Spengler RN, Bonoiu AC, Mahajan SD, Davidson BA, Ding H, Kumar R, Prasad PN, Knight PR, Ignatowski TA. Chronic constriction injury-induced nociception is relieved by nanomedicine-mediated decrease of rat hippocampal tumor necrosis factor. Pain. 2015;156:1320–1333. doi: 10.1097/j.pain.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Benemei S, Nicoletti P, Capone JG, Geppetti P. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol. 2009;9:9–14. doi: 10.1016/j.coph.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 184.Jang JH, Nam TS, Paik KS, Leem JW. Involvement of peripherally released substance P and calcitonin gene-related peptide in mediating mechanical hyperalgesia in a traumatic neuropathy model of the rat. Neurosci Lett. 2004;360:129–132. doi: 10.1016/j.neulet.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 185.Zimmermann MJE. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/S0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
- 186.Doods H, Arndt K, Rudolf K, Just S. CGRP antagonists: unravelling the role of CGRP in migraine. Trends Pharmacol Sci. 2007;28:580–587. doi: 10.1016/j.tips.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 187.Xie X, Pascual C, Lieu C, Oh S, Wang J, Zou B, Xie J, Li Z, Xie J, Yeomans DC. Analgesic microneedle patch for neuropathic pain therapy. ACS Nano. 2017;11:395–406. doi: 10.1021/acsnano.6b06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Farrar JT. Advances in clinical research methodology for pain clinical trials. Nat Med. 2010;16:1284–1293. doi: 10.1038/nm.2249. [DOI] [PubMed] [Google Scholar]
- 189.Lasagna L, Beecher HK. The analgesic effectiveness of nalorphine and nalorphine-morphine combinations in man. J Pharmacol Exp Ther. 1954;112:356–363. [PubMed] [Google Scholar]
- 190.Beecher HK. Measurement of subjective responses: quantitative effects of drugs. New York: Oxford University Press; 1959. [Google Scholar]
- 191.Andreu V, Arruebo M. Current progress and challenges of nanoparticle-based therapeutics in pain management. J Control Release. 2018;269:189–213. doi: 10.1016/j.jconrel.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 192.Doenicke AW, Roizen MF, Rau J, Kellermann W, Babl J. Reducing pain during propofol injection: the role of the solvent. Anesth Analg. 1996;82:472–474. doi: 10.1097/00000539-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 193.Jung J, Choi B, Cho S, Choe S, Ghim J, Lee H, Roh Y, Noh G. Effectiveness, safety, and pharmacokinetic and pharmacodynamic characteristics of microemulsion propofol in patients undergoing elective surgery under total intravenous anaesthesia. Br J Anaesth. 2010;104:563–576. doi: 10.1093/bja/aeq040. [DOI] [PubMed] [Google Scholar]
- 194.Ward DS, Russell Norton J, Guivarc'h P-H, Litman RS, Bailey PL. Pharmacodynamics and pharmacokinetics of propofol in a medium-chain triglyceride emulsion. Anesthesiology. 2002;97:1401–1408. doi: 10.1097/00000542-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 195.Paul M, Dueck M, Kampe S, Fruendt H, Kasper S. Pharmacological characteristics and side effects of a new galenic formulation of propofol without soyabean oil. Anaesthesia. 2003;58:1056–1062. doi: 10.1046/j.1365-2044.2003.03345.x. [DOI] [PubMed] [Google Scholar]
- 196.Larsen R, Beerhalter U, Erdkönig R, Larsen B. Injection pain from propofol-MCT-LCT in children. A Comparison with Propofol-LCT. Anaesthesist. 2001;50:676–678. doi: 10.1007/s001010100213. [DOI] [PubMed] [Google Scholar]
- 197.Shevalkar G, Pai R, Vavia P. nanostructured lipid carrier of propofol: a promising alternative to marketed soybean oil-based nanoemulsion. AAPS PharmSciTech. 2019;20:1–14. doi: 10.1208/s12249-019-1408-x. [DOI] [PubMed] [Google Scholar]
- 198.Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19:530–536. doi: 10.1016/j.knee.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 199.Lonner J. Role of liposomal bupivacaine in pain management after total joint arthroplasty. J Surg Orthop Adv. 2014;23:37–41. doi: 10.3113/jsoa.2014.0037. [DOI] [PubMed] [Google Scholar]
- 200.Momeni A, Ramesh NK, Wan D, Nguyen D, Sorice SC. Postoperative analgesia after microsurgical breast reconstruction using liposomal bupivacaine (Exparel) Breast J. 2019;25:903–907. doi: 10.1111/tbj.13349. [DOI] [PubMed] [Google Scholar]
- 201.Liu Y, Zeng Y, Zeng J, Li M, Wei W, Shen B. The efficacy of liposomal bupivacaine compared with traditional peri-articular injection for pain control following total knee arthroplasty: an updated meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2019;20:1–12. doi: 10.1186/s12891-019-2660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Discepola P, Bouhara M, Kwon M, Siddiqui BA, Whitwell TA, Sanghvi SY, Cook KD, Moore RE, Korban A, Eloy JD. Exparel®(long-acting liposomal bupivacaine) use for popliteal nerve block in postoperative pain control after ankle fracture fixation. Pain Res Manag. 2020 doi: 10.1155/2020/5982567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kelley TM, Jr, Bailey DW, Sparks P, Rice R, Caddell E, Currier H, Gallo D. Intercostal nerve blockade with exparel® results in lower opioid usage during the first 24 hours after video-assisted thorascopic surgery. Am Surg. 2018;84:1433–1438. doi: 10.1177/000313481808400945. [DOI] [PubMed] [Google Scholar]
- 204.Taniguchi H, Mulhall JP. Intraoperative intracavernosal liposomal bupivacaine (Exparel) injection does not affect systemic hemodynamics. J Sex Med. 2020;17:526–530. doi: 10.1016/j.jsxm.2019.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Eisenach JC, Yaksh TL. Spinal Exparel®—an extended duration of preclinical study needed. Br J Anaesth. 2019;122:298–300. doi: 10.1016/j.bja.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 206.Peravali R, Brock R, Bright E, Mills P, Petty D, Alberts J. Enhancing the enhanced recovery program in colorectal surgery-use of extended-release epidural morphine (DepoDur®) Ann Coloproctol. 2014;30:186. doi: 10.3393/ac.2014.30.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Viscusi ER, Martin G, Hartrick CT, Singla N, Manvelian G, Group ES. Forty-eight hours of postoperative pain relief after total hip arthroplasty with a novel, extended-release epidural morphine formulation. Anesthesiol. 2005;102:1014–1022. doi: 10.1097/00000542-200505000-00022. [DOI] [PubMed] [Google Scholar]
- 208.Carvalho B, Riley E, Cohen SE, Gambling D, Palmer C, Huffnagle HJ, Polley L, Muir H, Segal S, Lihou C. Single-dose, sustained-release epidural morphine in the management of postoperative pain after elective cesarean delivery: results of a multicenter randomized controlled study. Anesth Analg. 2005;100:1150–1158. doi: 10.1213/01.ANE.0000149544.58230.FF. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.