SUMMARY
Extracellular ATP (eATP) is known to act as a danger signal in both plants and animals. In plants, eATP is recognized by the plasma membrane (PM)-localized receptor P2K1 (LecRK-I.9). Among the first measurable responses to eATP addition is a rapid rise in cytoplasmic free calcium levels ([Ca2+]cyt), which requires P2K1. However, the specific transporter/channel proteins that mediate this rise in [Ca2+]cyt are unknown. Through a forward genetic screen, we identified an Arabidopsis ethylmethanesulfonate (EMS) mutant impaired in the [Ca2+]cyt response to eATP. Positional cloning revealed that the mutation resided in the cngc6 gene, which encodes cyclic nucleotide-gated ion channel 6 (CNGC6). Mutation of the CNGC6 gene led to a notable decrease in the PM inward Ca2+ current in response to eATP. eATP-induced mitogen-activated protein kinase activation and gene expression were also significantly lower in cngc6 mutant plants. In addition, cngc6 mutant plants were also more susceptible to the bacterial pathogen Pseudomonas syringae. Taken together, our results indicate that CNGC6 plays a crucial role in mediating eATP-induced [Ca2+]cyt signaling, as well as plant immunity.
Keywords: extracellular ATP, cyclic nucleotide-gated ion channel 6, CNGC6, cytosolic calcium, plant immunity
INTRODUCTION
ATP is well known as the energy molecule driving essential biochemical reactions within living organisms. Inside the cell, the ATP concentration is kept at a high level (in the mm range) while the extracellular concentration is significantly lower (in the nm–μm range) (Gout et al., 1992; Yegutkin et al., 2006). However, at this concentration, extracellular ATP (eATP) acts as a potent extracellular signal, as has been demonstrated in both plants and animals (Cekic and Linden, 2016; Cho et al., 2017; Khakh and Burnstock, 2009; Tanaka et al., 2014). ATP can be released to the extracellular matrix through wounding (Song et al., 2006), exocytosis (Kim et al., 2006), or active transport, where it is subsequently recognized by specific plasma membrane (PM)-localized receptors (Burnstock, 2006a).
The eATP signaling (i.e., purinergic signaling) pathway in animals is well studied with extensive medical implications. For example, the mammalian P2X7 receptor is the most extensively studied from an immunological perspective and is involved in both innate and adaptive immune responses (Savio et al., 2018). eATP participates in a broad range of animal physiological functions among; for example, it serves as a danger signal during pathogen infection or wounding (Rhett et al., 2014; van der Vliet and Bove, 2011). As such, eATP is considered a damage-associated molecular pattern (DAMP). Animals perceive eATP through two groups of receptors: ligand-gated ion channels (P2Xs) and G-coupled protein receptors (P2Ys) (Burnstock, 2006b). One of the earliest cellular changes upon binding of eATP to these receptors is the elevation of the cytosolic free calcium concentration ([Ca2+]cyt), followed by an increase in the levels of reactive oxygen species (ROS) and nitric oxide (NO) (Dichmann et al., 2000; Silva et al., 2006).
Relative to the rich research literature on eATP function in animals, very little is known about the functional roles of eATP in plants. However, similar to animals, a number of papers have implicated eATP in a variety of plant physiological processes, including the response to pathogen infection (Pham et al., 2020), the response to wounding (Choi et al., 2014), thigmotropism (Weerasinghe et al., 2009), pollen growth (Wu et al., 2018), and root hair development (Clark and Roux, 2018), among others (Cho et al., 2017; Tanaka et al., 2010a). In addition, eATP is involved in root gravitropism through polar auxin transport (Tang et al., 2003) and modulates root skewing as well (Yang et al., 2015). As is the case in animals, eATP functions as a DAMP in plants with similar initial cellular responses; for example, eATP addition leads to a rapid change in [Ca2+]cyt (Cho et al., 2017; Clark et al., 2010; Demidchik et al., 2009; Kim et al., 2006; Song et al., 2006; Tanaka et al., 2010b), as well as an elevation of ROS levels (Chen et al., 2017). However, unlike animals, plants appear to lack canonical P2X or P2Y receptors. In 2014, through a forward genetic screening approach, the first plant eATP receptor was isolated and identified as a PM-localized L-type lectin receptor-like kinase (LecRK-I.9), which was originally termed DORN1 but subsequently renamed P2K1 to align the plant nomenclature with that of animal purinoreceptors (Choi et al., 2014).
The identification of P2K1 has led to a significant increase in our knowledge of the molecular events surrounding plant purinergic signaling. For example, Chen et al. (2017) showed that eATP induces stomatal immunity against Pseudomonas syringae through P2K1-mediated activation of the NADPH oxidase RBOHD. Protein acyltransferases such as PAT5 and PAT9 were found to be phosphorylated by P2K1, which in turn mediated the eATP response by S-acylation of P2K1 (Chen et al., 2021). In addition, several studies indicated crosstalk between eATP and jasmonate (JA) signaling for plant defense responses (Balague et al., 2017; Jewell et al., 2019; Tripathi et al., 2018). eATP enhances the interaction between the JA receptor coronatine-insensitive 1 (COI1) and a key suppressor of JA signaling, ZIM-domain 1 (JAZ1), and elevates plant defense against pathogens (Tripathi et al., 2018). P2K1 is required for pathogen resistance through the regulation of JA signaling components (Balague et al., 2017). eATP-induced defense-related gene expression mediated by P2K1 requires CAMTA3 and the MYC transcription factor (Jewell et al., 2019). Animals possess multiple P2X and P2Y receptors, consistent with the broad role of purinergic signaling. Hence, it was notable that a second plant eATP receptor, P2K2, was recently discovered (Pham et al., 2020). P2K2 is strongly expressed under stress conditions, interacts directly with P2K1, and is also involved in plant defense to invading pathogens.
Calcium (Ca2+) plays an important role as a secondary messenger with increased [Ca2+]cyt levels induced by various biotic and abiotic stresses (Dodd et al., 2010; Kudla et al., 2010). For example, a key feature of DAMP-mediated responses is the transient opening of PM calcium channels, admitting an inward flux of calcium from the extracellular space (Choi et al., 2014; Pham et al., 2020). For calcium to function as an intracellular signal, stimulus-specific changes in cytoplasmic levels need to be precisely regulated (McAinsh and Pittman, 2009). Calcium ion influx into plant cells is mainly accomplished by non-selective calcium channels, depolarization-activated calcium channels, and hyperpolarization-activated calcium channels (HACCs) (Demidchik et al., 2018; Lemtiri-Chlieh et al., 2020; Very and Sentenac, 2002; White and Broadley, 2003). For example, patch-clamp electrophysiology analysis detected the activity of HACCs in response to eATP in plants (Demidchik et al., 2009; Wang et al., 2014, 2018; Wu and Wu, 2008). The family of annexins, glutamate receptor-like (GLR) channels, and cyclic-nucleotide-gated channels (CNGCs), which were previously predicted or shown to have HACC activity, have been suggested as potential candidates mediating [Ca2+]cyt changes in response to eATP (Matthus et al., 2020; Wang et al., 2019). For example, ANNEXIN1 underpins the hydroxyl radical-activated HACC in mature epidermis and root hairs (Laohavisit et al., 2012). This annexin was subsequently suggested to play a role in eATP-induced Ca2+ elevation (Mohammad-Sidik et al., 2021). However, since loss of ANNEXIN1 did not completely abolish the [Ca2+]cyt response to eATP, other calcium channels are likely required. Two GLRs, GLR3.3 and GLR3.6, were shown to mediate [Ca2+]cyt increases triggered by wounding (Vincent et al., 2017). In addition, patch-clamp analyses implicated several Arabidopsis CNGCs, such as CNGC2, CNGC4, CNGC5, and CNGC6, in HACC conductances (Gao et al., 2012; Tian et al., 2019; Wang et al., 2013). Most recently, CNGC2 and CNGC4 were reported to be involved in eATP-regulated pollen germination, pollen tube growth, and ion flux (Wu et al., 2021).
In order to identify genes involved in plant purinergic signaling and, specifically, in the [Ca2+]cyt response to eATP, we conducted a forward genetic screen in which aequorin luminescence was used to measure the plant cellular response. Among the mutants identified in this screen was one in which the CNGC6 gene was mutated, resulting in a significant reduction in the normal elevation of [Ca2+]cyt seen upon eATP addition. Intriguingly, CNGC6 has been previously shown to form a conductive Ca2+-permeable channel in the root cell PM, as well as when heterologously expressed in HEK293T cells (Gao et al., 2012; Tan et al., 2020). In the current study, patch-clamp analyses showed that cngc6 mutant plants were defective in the PM inward Ca2+ current in response to eATP. These mutant plants were also found to be defective in the activation of mitogen-activated protein kinase (MAPK) and gene transcription normally associated with a response to eATP. As is the case for p2k1 mutant plants, cngc6 mutant plants were also significantly more susceptible to bacterial pathogen infection. These data implicate CNGC6 as a key calcium channel protein involved in the early events of plant purinergic signaling.
RESULTS
367 mutant plants are impaired in the [Ca2+]cyt response to extracellular nucleotides
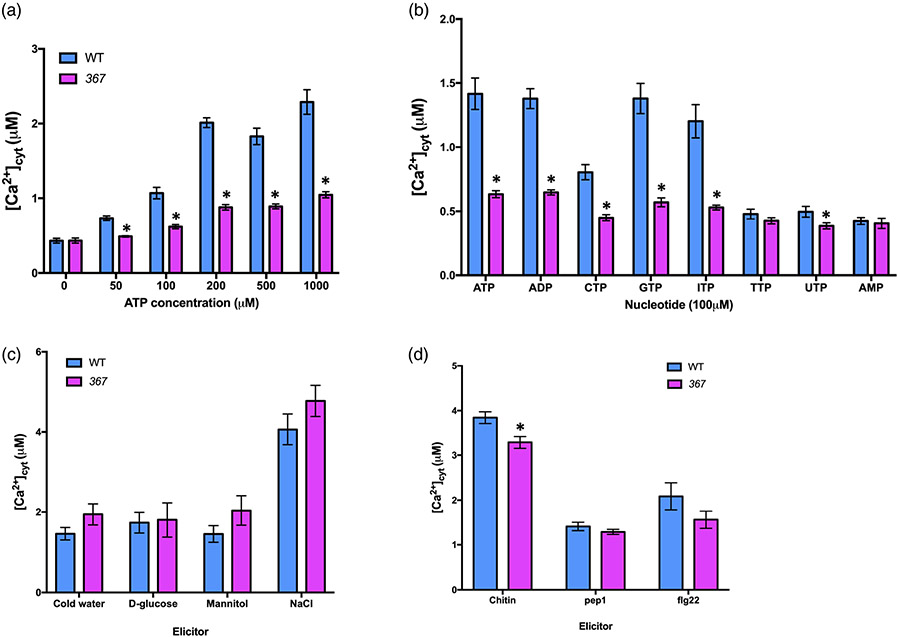
We generated an ethylmethanesulfonate (EMS)-mutagenized Arabidopsis thaliana library and carried out a similar screen to that reported previously by Choi et al. (2014) to identify mutants defective in the cellular calcium response to added eATP. Following the strategy in Figure S1, we screened approximately 30 000 M0 generation plants, which led to the identification of mutant 367 showing a significant reduction in [Ca2+]cyt elevation upon eATP elicitation (Figure 1a). To see whether 367 mutant plants showed a specific response to purine nucleotides, we tested the calcium response of 367 mutant plants to a variety of nucleotides. 367 mutant plants showed defects in the [Ca2+]cyt response to various nucleotides, such as CTP, GTP, ITP, and UTP, compared with wild-type plants (Figure 1b). In contrast, the [Ca2+]cyt response of the 367 mutant plants to different abiotic and biotic elicitors was similar to those of wild-type plants, except for chitin (Figure 1c,d). The latter may be due to the fact that chitin is known to cause the release of eATP (Kim et al., 2006). In addition, the 367 mutant plants exhibited a slighty lighter color and a weak-lobed leaf shape in comparison with wild-type (Col-0) plants at the 4-week-old stage (Figure S4).
Figure 1. 367 mutant plants show defects in the calcium response to extracellular ATP (eATP) and various nucleotides.
(a) The 367 mutant is impaired in eATP-induced calcium response.
(b) The calcium response to different nucleotides was defective in the 367 mutant.
(c) Abiotic stresses-induced calcium responses in the 367 mutant are comparable to those of wild type (Col-0) (ice-cold water, 300 mm NaCl, 4% d-glucose, and 300 mm mannitol).
(d) The calcium response to chitin is slightly lower in 367 mutant plants (100 nM flg22, 100 nM Pep1, and 100 μg mT−1 chitin). The bar graph indicates the integrated calcium concentration in response to ATP addition for 300 sec. Data are presented as mean ± standard error (SE), n = 8 in (a, b), n = 6 in (c, d). Asterisks indicate a significant difference between wild-type (Col-0) and 367 mutant plants (P < 0.05, analysis of variance). Experiments were repeated at least three times with similar results.
To assess whether the lack of a [Ca2+]cyt response to nucleotides in 367 mutant plants is controlled by a single gene, we crossed M3 generation mutant plants with wild-type Col-0 and observed the segregation in F2 progeny. The lack of a [Ca2+]cyt response to ATP was found in 25% of the progeny (12 mutants:43 wild-type plants, χ2 = 0.69, d.f. = 1, P0.05 = 3.84), indicating a 3:1 ratio of a recessive gene, following Mendelian single gene inheritance.
The phenotype of the 367 mutant is due to disruption in the gene encoding CNGC6
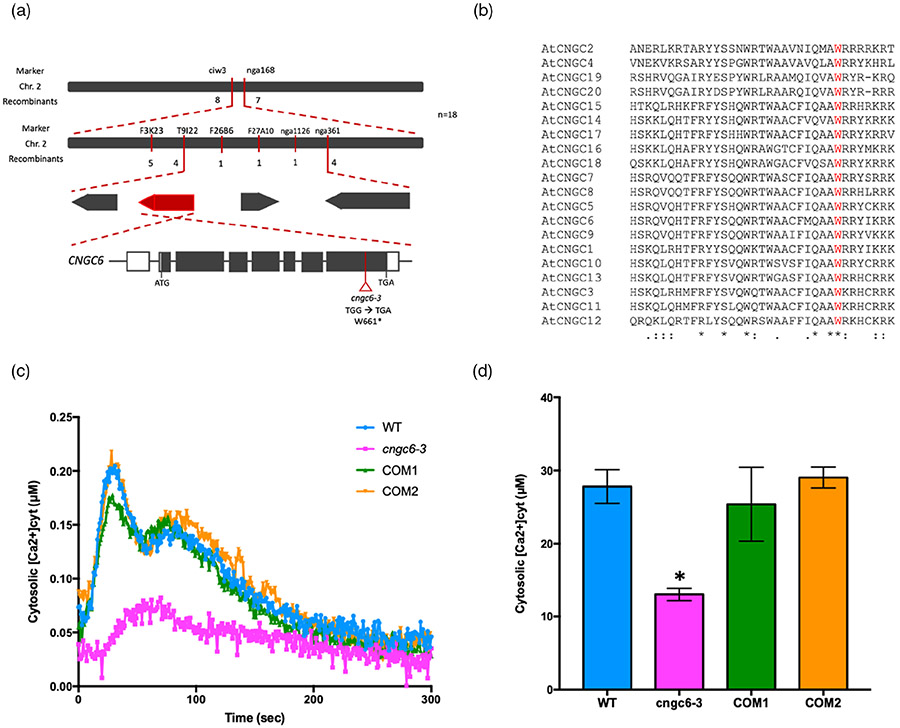
In order to identify the gene responsible for the 367 mutant phenotype, we performed map-based cloning and whole-genome sequencing (WGS). We were able to map the 367 mutation to a 3.6-Mbp interval on chromosome 2 (Figure 2a; Table S1). Subsequent WGS found four genes with point mutations within this region (Figure 2a; Table S2). To confirm which mutation was responsible for the 367 mutant phenotype, we cloned each of these four genes individually and tested their ability to complement the 367 mutant phenotype. These experiments showed that only wild-type CNGC6 driven by the CNGC6 native promoter rescued the ATP-induced [Ca2+]cyt influx response in 367 mutant plants (Figure 2c,d; Figure S3). The mutation of the CNGC6 gene in the 367 mutant results in an early stop codon caused by a single nucleotide substitution (TGG → TGA, W661stop) within the highly conserved domain known to be critical for CNGC channel function (Figure 2a,b). Subsequently, we obtained the cngc6 T-DNA mutant, cngc6-1, which was also found to be defective in the eATP-induced [Ca2+]cyt response (Figure S2a). The F1 progeny obtained from the cross between cngc6-1 and 367 mutant plants were also defective in the ATP-induced [Ca2+]cyt response, which indicates allelism (Figure S2c). Overall, these results indicate that the phenotype of the 367 mutant plants is the result of a mutation in CNGC6. Therefore, we denote the 367 mutation as cngc6-3, following the published allele (Gao et al., 2012; Wang et al., 2013).
Figure 2. Mutation in CNGC6 is responsible for the 367 phenotype.
(a) Cartoon showing the genomic structure of CNGC6. The open triangle indicates the position of the mutation in mutant cngc6-3 (TGG → TGA; W661*). The asterisk indicates a stop codon.
(b) CNGC6W661 is conserved in the CNGC protein family. Sequence alignment of 20 CNGCs was performed in Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/). The conserved residue CNGC6W661 is marked in red. Asterisks indicate fully conserved residues. Colons indicate highly conserved residues. Dots indicate low conserved residues.
(c, d) [Ca2+]cyt elevation in response to 100 μm ATP for 300 sec in cngc6-3 mutant plants complemented by pCNGC6:CNGC6 expression (COM). (c) The kinetics of [Ca2+]cyt in WT, cngc6-3, and two COM lines. Each line graph represents an average of four seedlings of each genotype. (d) The bar graph shows the integrated calcium concentration. Data are shown as mean ± standard error (SE) (n = 4). Asterisks indicate a significant difference between wild-type (Col-0) and 367 mutant plants (P < 0.05, analysis of variance). Experiments were repeated at least three times with similar results.
eATP-induced current activation does not occur in cngc6 mutant plants
CNGC6 was previously reported as a PM-localized Ca2+-permeable channel responsible for plant responses to heat shock and root hair growth (Brost et al., 2019; Gao et al., 2012; Tan et al., 2020). Heterologous expression of CNGC6 in HEK293T cells confirmed its ability to conduct Ca2+ (Tan et al., 2020). To elucidate whether CNGC6 is required for eATP-induced activation of PM channel-mediated Ca2+ influx, we performed patch-clamp electrophysiology on root epidermal protoplasts. The whole-cell mode of patch clamping was used to measure the net current generated as ions are conducted through PM channels at different transmembrane voltages. The results were reported as the overall current/voltage (I/V) relationship. In wild-type cells, the addition of 300 μm eATP induced an inward current that was significantly greater than under control conditions (Figure 3a). The mean reversal voltage of the whole-cell current shifted from −54.4 ± 7.5 mV (−ATP, n = 4) to −39.8 ± 4.4 mV (+ATP, n = 4), approximately a 15-mV positive shift consistent with increased Ca2+ influx and far from the equilibrium potential for K+ (−79 mV). However, as the shift was towards the equilibrium potential for Cl− (−28 mV), a further test was undertaken. Addition of Gd3+ as a blocker of PM-localized Ca2+ channels significantly inhibited the eATP-induced current (Figure 3a). Gd3+ is ineffective against the root epidermal PM eATP-induced anion conductance (Wang et al., 2019), further supporting the Ca2+ permeability of the conductance reported here. This eATP-induced inward current therefore appears competent to increase [Ca2+]cyt at resting membrane voltage (inside negative). In contrast, cngc6-3 mutant plants failed to respond to 300 μm eATP (Figure 3b). This result suggests that CNGC6 functions as a Ca2+-permeable channel in response to eATP.
Figure 3. cngc6-3 failed to support K+ and Ca2+ currents in the root epidermal cell plasma membrane in response to exogenous ATP.
(a) Left panel: Whole-cell current/voltage (I/V) relationships of wild type (Col-0) in control conditions (no extracellular ATP [−eATP]), after addition of 300 μm eATP (+ATP), and after further addition of 100 μm Gd3+ as a calcium channel blocker. eATP activation was observed 30 sec to 3 min after addition. Data are presented as mean ± standard error (SE) of current (n = 4). Inset panel: Mean current traces for −ATP (black) and +ATP (red). Right panel: Comparison of the inward currents at −190 mV (solid bars) and the outward currents at +50 mV (open bars) before and after eATP addition and in the presence of Gd3+. *P < 0.05; **P < 0.01; n.s, not significant (Student’s t-test).
(b) As (a) but for the cngc6-3 mutant (n = 5–6). cngc6-3 failed to respond to eATP even with an extended observation period of 10 min.
ATP-induced MAPK activation and gene expression are defective in cngc6 mutant plants
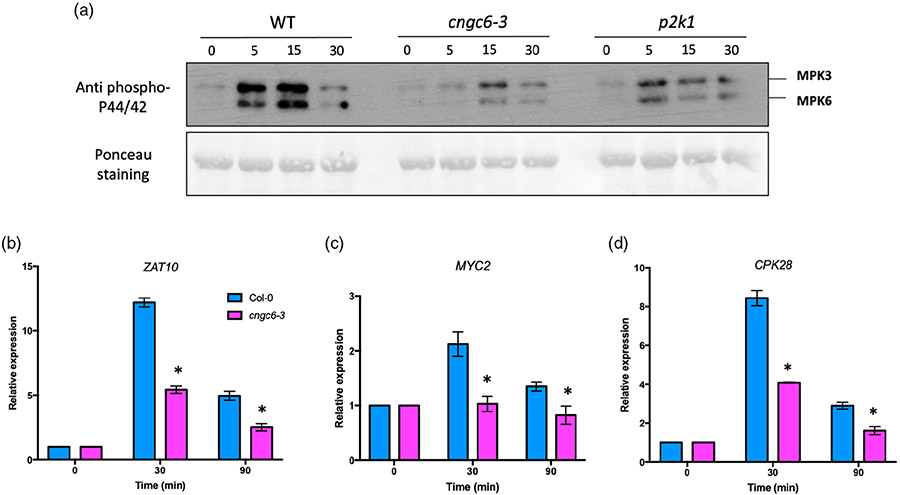
Previous studies reported that eATP triggers the activation of MAPKs and a transcriptional response, which are fully dependent on a functional P2K1 (Chen et al., 2017; Choi et al., 2014). Given that CNGC6 participates in eATP signaling (Figure 1), we examined these phenotypes in cngc6-3 mutant plants. The addition of eATP resulted in a significant reduction in the phosphorylation of MPK3/6 in cngc6-3 mutant plants as measured 5 and 15 min after eATP addition, while strong activation of MPK3/6 was seen in wild-type plants (Figure 4a). The phosphorylation of MPK3/6 in cngc6-3 mutant plants was similar to that seen using p2k1-3 mutant plants (Figure 4a).
Figure 4. Extracellular ATP (eATP)-induced downstream responses are defective in cngc6-3 mutant plants.
(a) MPK3 and MPK6 phosphorylation in response to 200 μm eATP is weaker in cngc6-3 mutant plants compared with wild-type plants. Phosphorylation of MPK3 and MPK6 was detected using an antibody against phospho-p44/p42 MAPK (top). Ponceau S staining was used as a loading control.
(b–d) Col-0 was used as a control to compare with cngc6-3. eATP-treated seedlings were collected for qRT-PCR analysis. Expression levels of ZAT10 (b), MYC2 (c), and CPK28 (d) were normalized against the SAND reference gene. The results are relative to expression levels of mock-treated plants (set as 1). Bar graphs are presented as mean ± standard error (SE, n = 3 biological replicates). Asterisks indicate statistically significant differences between wild type (Col-0) and cngc6-3 at the same time points (*P < 0.05, Student’s t-test).
Previously, it was shown that eATP addition strongly induced expression of zinc-finger transcription factor ZAT10, bHLH transcription factor MYC2, and calcium-dependent protein kinase 28 (CPK28) genes (Choi et al., 2014). Consistent with this report, we also found that these genes were strongly upregulated in wild-type plants 30 min after ATP addition, whereas their expression was only slightly enhanced in cngc6-3 mutant plants (Figure 4b,c). Therefore, in addition to showing defects in the early [Ca2+]cyt response to eATP addition, cngc6-3 mutant plants are also defective in the later responses of MAPK activation and gene transcription (Figure 4).
CNGC6 plays a role in plant immunity
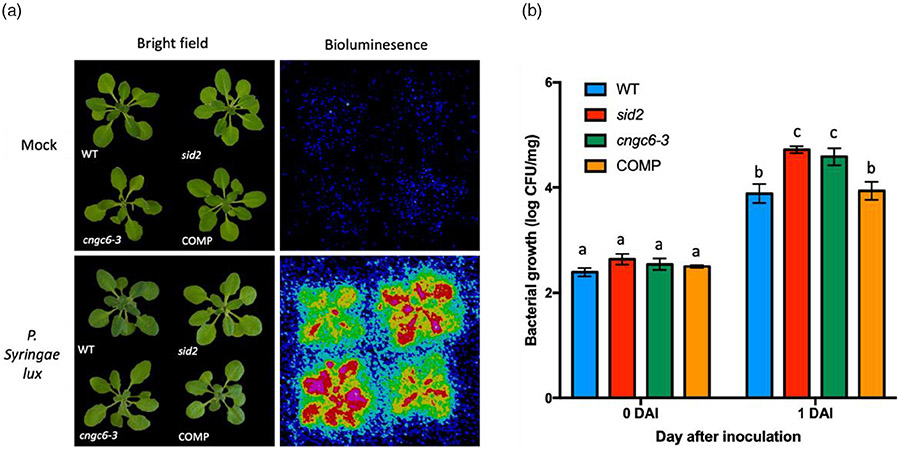
Previous studies reported that both P2K1 and P2K2 are positive regulators of plant immunity against the bacterial pathogen P. syringae (Chen et al., 2017; Pham et al., 2020). Previous reports also implicated both CNGC2 and CNGC4 in pathogen defense (Chin et al., 2013; Tian et al., 2019), while both CNGC11 and CNGC12 are positive regulators of resistance gene-mediated pathogen responses (Yoshioka et al., 2006). In order to test the role of CNGC6 in plant immunity, we examined plant susceptibility to P. syringae upon flood inoculation. Arabidopsis salicylic acid induction-deficient 2 (sid2) mutant plants were used as the susceptible control (Wildermuth et al., 2001). The degree of bacterial colonization was initially estimated by bioluminescence and subsequently quantified by plate counting. As shown in Figure 5, both cngc6-3 and sid2 mutant plants were considerably more susceptible to the pathogen than wild-type plants (Figure 5). In contrast, the CNGC6 complemented line showed a similar level of infection to that of wild-type plants (Figure 5). These results suggest that CNGC6 is a critical component required for the plant pathogen defense.
Figure 5. CNGC6 plays a critical role in plant resistance to Pseudomonas syringae DC3000.
Wild-type (Col-0) and sid2 mutant seedlings were used as controls to compare with cngc6-3 mutant and pCNGC6:CNGC6 complementation lines (COM). Three-week-old plants were flood-inoculated with P. syringae DC3000 lux suspension (OD600 = 0.002) containing 0.025% (v/v) Silwet L-77.
(a) At 1 days after inoculation (DAI), bright-field photographs were taken with a normal camera while bioluminescence images were taken using a CCD camera to detect bacterial colonization.
(b) Bacterial colonization by plate counting. Bacterial growth expressed as log(colony forming units [CFU]) was normalized against sample weight. Values are presented as mean ± standard error (SE, n = 3 biological replicates). Lowercase letters over bars indicate significantly different means (P < 0.05, Student’s t-test). The experiments were repeated at least three times with similar results.
DISCUSSION
As is the case in animals, previous studies had clearly shown that an increase in [Ca2+]cyt levels was among the first measurable cellular responses to eATP addition (Choi et al., 2014). In animal systems, eATP triggers the [Ca2+]cyt increase through the action of either P2X-gated ion channels or P2Y G-coupled protein receptors (Burnstock, 2006a). Binding of ATP to P2X receptors induces a channel conformation change, allowing Ca2+ influx into cells (Coddou et al., 2011). In contrast, P2Y receptor activation of G-protein signaling networks triggers Ca2+ release from the endoplasmic reticulum raising [Ca2+]cyt (Berridge, 2009). In contrast, little is known about the molecular events responsible for eATP-induced [Ca2+]cyt elevation in plants.
The addition of eATP leads to rapid activation of PM HACC conductances, which requires P2K1 (Wang et al., 2018). Our discovery of the critical importance of CNGC6 to the eATP-induced [Ca2+]cyt response is consistent with the previous finding that eATP induces HACC activity (Figure 3) and clearly implicates CNGC family members as key mediators of the eATP-induced [Ca2+]cyt response (Wang et al., 2019). While p2k1-3 mutant plants show no eATP-mediated [Ca2+]cyt influx (Choi et al., 2014), cngc6 mutant plants are partially defective in this response with a monophasic increase of about 80 sec (Figure 2c; Figure S2). In the case of ANNEXIN1, loss of ANNEXIN1 significantly reduced the calcium response to eATP in roots, but ann1 mutant plants still showed a biphasic increase (Mohammad-Sidik et al., 2021). This suggests that CNGC6 is likely not solely responsible for the normal eATP-mediated biphasic [Ca2+]cyt influx and other CNGCs or other channels, including ANNEXIN1, contribute to this [Ca2+]cyt elevation. Indeed, CNGCs are known to work as heteromeric channel complexes, containing more than one CNGC family member (Zhong et al., 2002). Given that the Arabidopsis genome encodes 20 CNGC proteins, the probability of some functional redundancy is high (Maser et al., 2001). For example, it was previously reported that CNGC6, CNGC9, and CNGC14 or CNGC5, CNGC6, and CNGC9 function redundantly as [Ca2+]cyt channels in root hair growth (Brost et al., 2019; Tan et al., 2020). Therefore, one possibility is that other CNGCs act together with CNGC6 in mediating the eATP-induced [Ca2+]cyt response. However, CNGC14 can be excluded from the candidate list, based on the fact that loss of CNGC14 function did not affect the [Ca2+]cyt response to eATP (Shih et al., 2015). The recent discovery of CNGC2 and CNGC4 involvement in eATP-promoted ion influx in the pollen (Wu et al., 2021) is consistent with the hypothesis. However, further studies are needed to unravel whether these CNGC Ca2+ channels work together in plant purinergic signaling.
Plants are able to detect DAMP signals by specific pattern recognition receptors (e.g., P2K receptors) (Bigeard et al., 2015; Choi et al., 2014). Moreover, recognition of DAMP signals also activates immune responses (Yamaguchi and Huffaker, 2011). eATP, which is defined as a DAMP, has been shown to regulate the plant response to pathogens (Chivasa et al., 2009; Demidchik et al., 2009; Jewell et al., 2019; Tanaka et al., 2014). Previously various components of eATP signaling have been implicated in plant immunity, including JA signaling and P2K receptors (Balague et al., 2017; Jewell et al., 2019; Pham et al., 2020; Tripathi et al., 2018). Pham et al. (2020) recently showed that P2K1 and P2K2 are required for plant pathogen resistance, in which activation of P2K1 leads to the transphosphorylation of P2K2 and the activation of defense-related downstream signaling components. Thus, plants activate a cascade of ATP-related intracellular events, such as increases in [Ca2+]cyt, MAPK phosphorylation, and gene expression (encoding, e.g., MYCs, CPKs, and ZATs), resulting in the activation of pathogen resistance (Figure 4) (Choi et al., 2014; Pham et al., 2020). To date, several members CNGC family members were reported to function in plant pathogen defense including CNGC2 (Chin et al., 2013; Clough et al., 2000), CNGC4 (Chin et al., 2013; Tian et al., 2019), CNGC11/CNGC12 (Yoshioka et al., 2006), CNGC19 (Jogawat et al., 2020), and CNGC20 (Yu et al., 2019). However, none had previously been directly implicated in purinergic signaling. We show here that CNGC6 is involved in P2K innate immunity (Figures 1, 4, and 5), indicating that CNGC6 is likely involved in eATP-dependent plant resistance to pathogens. Taken together, it suggests that CNGC6 acts downstream of the P2K1/P2K2 receptor complex. However, given that Arabidopsis CNGCs are activated directly by cNMPs (Ali et al., 2007; Gao et al., 2012), further analysis is needed to determine whether P2K receptors directly regulate CNGC6 activity or do so indirectly by modulating other cellular processes.
EXPERIMENTAL PROCEDURES
Plant materials and growth conditions
Aequorin-transformed Arabidopsis (Columbia [Col-0] ecotype) and a T-DNA insertion line of cngc6 (Salk_042207; cngc6-1) were crossed to obtain the aequorin transgenic cngc6-1 line. T-DNA insertion was confirmed by PCR-based genotyping (Table S1). The EMS-induced mutant cngc6-3 was backcrossed with the Col-0 aequorin transgenic line three times (BC3F3). cngc6-1 and cngc6-3 were crossed together to obtain the F1 generation for genetic analysis. These backcrossed lines were then used for phenotyping. Arabidopsis seeds were sown onto half-strength Murashige and Skoog (MS) medium containing 1% (w/v) sucrose, 1% (w/v) agar, and 0.5% (w/v) MES (pH 5.7). After cold treatment for 3 days, the plates were placed vertically in a growth chamber (16 h light/8 h dark cycle, 22°C, 100 E cm−2 sec−1 light intensity). For other experiments, 10-day-old seedlings were grown in Sunshine soil (Premier Tech Horticulture, Quebec, Canada) in a growth chamber (12 h light/12 h dark cycle, 22°C, 70% humidity, and 150 E cm−2 sec−1 light intensity).
Calcium assays
Five- or six-day-old seedlings were individually transferred to a 96-well-plate and incubated in 50 μl reconstitution buffer containing 10 mm CaCl2, 10 μm coelenterazine (CTZ; NanoLight Technology, Pinetop, AZ, USA), and 2 mm MES (pH 5.7) overnight at room temperature in the dark. The next day, 50 μl of 2× treatment solution was applied to each well and the luminescence produced was monitored using an image-intensified CCD camera (Photek216; Photek, Ltd., Lancaster, PA, USA) for 300 sec. Total aequorin levels were estimated by adding 100 μl of discharging buffer containing 2 m CaCl2 and 20% (v/v) ethanol and monitored for an additional 300 sec. The calcium concentrations were converted from photon counting data (Knight et al., 1996). All experiments were performed independently at least three times with similar results. Figures show the converted histogram from each recorded kinetics of the [Ca2+]cyt response during treatments.
Chemical reagents
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) except if mentioned otherwise. Nucleotide stock solutions (100 mm) in 50 mm MES buffer (pH 5.7) were prepared. Flg22 and Pep1 (GenScript USA, Inc., Piscataway, NJ, USA) stock solutions (1 mm) in dimethylsulfoxide were prepared and kept at −80°C prior to use. Chitin (Sigma, St. Louis, MO, USA) solution (100 μg ml−1) was prepared fresh prior to use.
Mutagenesis and mutant screening
The original cngc6-3 mutant plant was identified by screening an EMS-mutagenized library derived from A. thaliana expressing aequorin (Col-0 background) as described by Choi et al. (2014). Briefly, 5-day-old individual M2 generation seedlings were transferred into a 96-well plate to monitor the response to the application of 100 μm ATP. Mutants exhibiting a lower cytoplasmic calcium response were selected and grown to test the next generation. M3 generation seedlings were further tested with the same procedure. In this screen, 2× discharging buffer (20% EtOH, 1 m CaCl2) was applied after ATP treatment to obtain the total light intensity from aequorin expressed in each plant. Discharge data were used to identify plants showing a reduced cytoplasmic calcium response due to a mutation in aequorin. Lastly, the response of the cngc6-3 mutant plants to ATP was tested by applying an increasing concentration (10, 50, 100, 200, 500, and 1000 μm) of ATP. In addition, we tested the cngc6-3 mutant plants for the specificity of their defect by applying a variety of known cytoplasmic calcium elicitors/treatments (e.g., 100 μm of ATP, ADP, AMP, GTP, ITP, CTP, TTP, and UTP; 100 nm of flg22 and Pep1, 100 μg ml−1 chitin; ice-cold water; 5% d-glucose; 300 mm NaCl and mannitol).
Map-based cloning
The cngc6-3 mutant plants were crossed with Landsberg erecta plants expressing aequorin to generate the mapping population. The BC1F2 plants exhibiting the mutant phenotype were grown further for mapping of cngc6-3. Two true leaves from each screened plant were harvested for genomic DNA extraction (DNeasy Plant Mini kit; Qiagen, Hilden, Germany). A region of chromosome 2 was identified as co-segregating with the mutant phenotype. The region between the F26B6 and nga1126 markers with a recombinant frequency of 0.97 was identified as co-segregating with the mutant phenotype. The primers used are listed in Table S3.
Whole-genome sequencing
The mutant cngc6-3 was backcrossed three times with wild-type Col-0, and homozygous lines (BC3F3) all exhibiting the mutant phenotype were used for WGS. Homozygous lines showing a wild-type phenotype from the same backcross line BC3F3 were also prepared as an internal reference for sequencing. Single leaves from 29 and 44 individual mutant and wild-type 14-day-old seedlings, respectively, were pooled and genomic DNA was extracted using the DNeasy plant mini kit (Qiagen). WGS was carried out by the DNA Core Facility of the University of Missouri (https://dnacore.missouri.edu) to detect single base mutations. A total of 2.4 μg of genomic DNA was prepared for 350 bp insert size TruSeq library construction. Sequencing was done on a Next-Seq 500 system (Illumina, San Diego, CA, USA) (100× coverage). Reads were aligned back to the TAIR10 version of the Arabidopsis genome using Bowtie version 2 (Langmead and Salzberg, 2012). Sam and output-pileup files were generated using Samtools version 0.1.7 (Li et al., 2009). The output-pileup files were converted to NGM emap files using the Next-generation EMS mutation mapping web tool (http://142.150.215.220/ngm/), which were then analyzed for single nucleotide polymorphisms as previously described (Burnstock, 1972).
Plasmid construction and plant transformation
CNGC6 genomic DNA including the 1.5-kb upstream sequence was amplified with primers attB1 and attB2. The PCR product was then inserted into the pDONR-Zeo vector through BP reaction and then into the pGWB1 vector through LR reaction. The pGWB1-CNGC6 construct was then introduced by electroporation into Agrobacterium tumefaciens strain GV3101, which was subsequently transformed into cngc6-3 mutant plants using the floral dip method (Zhang et al., 2006). Transgenic plants were selected on MS medium with 20 mg L−1 hygromycin. T2 generation plants were used for these experiments. The primers used are listed in Table S4.
Patch-clamp electrophysiology assay
Root tips (2–3 mm) were excised from 7- to 13-day-old Col-0 and cngc6-3 plants. Tips were incubated in the dark at room temperature in 1% (w/v) cellulysin (Calbiochem, San Diego, CA, USA), 1% (w/v) cellulase RS (Yakult Honsha, Tokyo, Japan), 0.1% (w/v) pectolyase Y-23 (Yakult Honsha), 0.1% (w/v) bovine serum albumin, 10 mm CaCl2, 10 mm KCl, 2 mm MgCl2, 2 mm MES, and 165 mm d-sorbitol (pH 5.6) with Tris. After filtering at 40 μm, protoplasts were washed twice with holding buffer (0.2 mm CaCl2, 0.1 mm KCl, and 10 mm MES-Tris [pH 5.6] brought to 280 mosmol L−1 with d-sorbitol) at 4. Protoplasts were suspended in holding buffer and stored on ice in the dark. After using the N9093 epidermal-specific green fluorescent protein reporter line to establish the isolation protocol (Wang et al., 2019), protoplast origin was confirmed by direct observation of the genotypes listed above for testing. The apparatus was as described by Demidchik et al. (2002). After a gigaohm seal was formed in the cell-attached configuration, the whole-cell configuration was achieved by gentle suction and whole-cell currents were recorded after at least 12 min. A ramp protocol from +50 to −190 mV (−200 μV sec−1) with a holding potential at −35 mV (corrected for liquid junction potential) was used. Bath solution contained 50 mm CaCl2, 1 mm KCl, and 10 mm MES-Tris (pH 5.6). The pipette solution contained 5 mm BaCl2, 20 mm KCl, and 10 mm HEPES-Tris (pH 7.5). The osmolarity of bath and pipette solutions was adjusted to 280 and 290 mosmol L−1, respectively, with d-sorbitol. Ionic strength was determined using the calculator integrated in the ‘Calcium.exe’ program (Fohr et al., 1993). Ion activities were calculated using GEOCHEM (Parker et al., 1994). Equilibrium potentials were calculated using the Nernst equation.
RNA isolation and quantitative real-time PCR
Ten-day-old seedlings of Col-0, cngc6-1, or cngc6-3 were collected and RNA was extracted using TRIzol and the Direct-zol™ RNA mini-prep kit (Zymo Research, Irvine, CA, USA). Two micrograms of RNA was used to synthesize cDNA using M-MLV reverse transcriptase (Promega, Madison, WI, USA). These cDNAs were then used for real-time PCR (RT-PCR). The total of 10 μl reaction buffer consisted of 3 μl cDNA, 5 μl PowerUP™ SYBR Green (Applied Biosystems, Waltham, MA, USA), 0.5 μl of each primer, and remaining 1 μl is added water. The reaction was run using an ABI 7500 Real-time PCR system (Applied Biosystems). Transcript levels were normalized against the expression of SAND (At2g28390) or UBQ10 (At4g05320). The primers used are listed in Table S4.
MAPK phosphorylation
Leaf disks from 2- or 3-week-old plants were incubated in 5 mm MES buffer (pH 5.7) at room temperature overnight. After treatment with 200 μm ATP for 0, 5, 15, and 30 min, the samples were homogenized, and the total protein was extracted with protein extraction buffer (100 mm Tris-HCl [pH 7.5], 300 mm NaCl, 2 mm EDTA [pH 8.0], 1% Triton X-100, 10% glycerol, and protease inhibitor). The extracted proteins were mixed with 5× SDS loading buffer (10% SDS, 50% glycerol, 0.01% bromophenol blue, 10% β-mercaptoethanol, 0.3 m Tris-HCl [pH 6.0]) and boiled for 5–10 min. The total proteins were separated by 10% SDS-PAGE and transferred to PVDF membrane. The membrane was then blocked in 5% skim milk in TBST buffer (50 mm Tris-HCl [pH 7.4], 150 mm NaCl, 0.1% Tween-20) and incubated with rabbit anti-phospho-p44/p42 MAPK antibody (Cell Signaling Technology, Danvers, MA, USA), followed by washing with TBST and incubation with HRP-conjugated goat anti-rabbit IgG (Abcam, Cambridge, UK).
Bacterial inoculation assay
The assay was modified from the P. syringae flood inoculation method (Pham et al., 2020). Three-week-old Arabidopsis seedlings grown in MS medium were placed into a 40-ml suspension of P. syringae pv. tomato DC3000 Lux (OD600 = 0.002) in sterile water containing 10 mm MgCl2 and 0.025% Silwet L-77 (v/v) for 2–3 min. The suspension was decanted, and the plants were blotted dry and then placed into a growth chamber. One day after inoculation, the seedling aerial parts were collected and weighed. Bacterial growth was visualized and analyzed under a CCD camera (Photek 216). The seedling tissue was then washed with sterilized water and ground in 10 mm MgCl2, diluted serially, and dropped onto King B agar plates supplemented with rifampicin and kanamycin. The number of colonies was counted and analyzed after incubation at room temperature for 2 days.
Supplementary Material
Table S1. Rough mapping table of mutant 367.
Table S2. Whole-genome sequencing showing the mutations of cngc6-3 in a 3.6-Mbp interval on chromosome 2
Table S3. Mapping primers.
Table S4. Primer list.
Figure S1. Mutant screening scheme.
Figure S2. cngc6-1 and cngc6-3 mutants are allelic.
Figure S3. [Ca2+]cyt response to different nucleotides and elicitors in mutant 367 complemented by expression of native CNGC6.
Figure S4. The cngc6-3 mutant developmental phenotype.
ACKNOWLEDGMENTS
This research was supported by the National Institute of General Medical Sciences of the National Institutes of Health (grant no. R01GM121445), by the Next-Generation BioGreen 21 Program Systems and Synthetic Agrobiotech Center, Rural Development Administration, Republic of Korea (grant no. PJ01325403), and through the third call of the ERA-NET for Coordinating Action in Plant Sciences, with funding from the US National Science Foundation (grant 1826803) and UKRI BBSRC (BB/S004637/1).
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
DATA AVAILABILITY STATEMENT
WGS data of mutant 367 can be found at https://osf.io/csq6t/. Other relevant data can be found within the manuscript and its supporting materials.
REFERENCES
- Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S et al. (2007) Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. The Plant Cell, 19(3), 1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balague C, Gouget A, Bouchez O, Souriac C, Haget N, Boutet-Mercey S et al. (2017) The Arabidopsis thaliana lectin receptor kinase LecRK-I.9 is required for full resistance to Pseudomonas syringae and affects jasmonate signalling. Molecular Plant Pathology, 18(7), 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ (2009) Inositol trisphosphate and calcium signalling mechanisms. Biochimica et Biophysica Acta, 1793(6), 933–940. [DOI] [PubMed] [Google Scholar]
- Bigeard J, Colcombet J & Hirt H (2015) Signaling mechanisms in pattern-triggered immunity (PTI). Molecular Plant, 8(4), 521–539. [DOI] [PubMed] [Google Scholar]
- Brost C, Studtrucker T, Reimann R, Denninger P, Czekalla J, Krebs M et al. (2019) Multiple cyclic nucleotide-gated channels coordinate calcium oscillations and polar growth of root hairs. The Plant Journal, 99(5), 910–923. [DOI] [PubMed] [Google Scholar]
- Burnstock G (1972) Purinergic nerves. Pharmacological Reviews, 24(3), 509–581. [PubMed] [Google Scholar]
- Burnstock G (2006a) Pathophysiology and therapeutic potential of purinergic signaling. Pharmacological Reviews, 58(1), 58–86. [DOI] [PubMed] [Google Scholar]
- Burnstock G (2006b) Purinergic signalling. British Journal of Pharmacology, 147(Suppl 1), S172–S181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic C & Linden J (2016) Purinergic regulation of the immune system. Nature Reviews Immunology, 16(3), 177–192. [DOI] [PubMed] [Google Scholar]
- Chen D, Cao Y, Li H, Kim D, Ahsan N, Thelen J et al. (2017) Extracellular ATP elicits DORN1-mediated RBOHD phosphorylation to regulate stomatal aperture. Nature Communications, 8(1), 2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Hao F, Mu H, Ahsan N, Thelen JJ & Stacey G (2021) S-acylation of P2K1 mediates extracellular ATP-induced immune signaling in Arabidopsis. Nature Communications, 12(1), 2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K, DeFalco TA, Moeder W & Yoshioka K (2013) The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiology, 163(2), 611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivasa S, Murphy AM, Hamilton JM, Lindsey K, Carr JP & Slabas AR (2009) Extracellular ATP is a regulator of pathogen defence in plants. The Plant Journal, 60(3), 436–448. [DOI] [PubMed] [Google Scholar]
- Cho SH, Nguyen CT, Choi J & Stacey G (2017) Molecular mechanism of plant recognition of extracellular ATP. Advances in Experimental Medicine and Biology, 1051, 233–253. [DOI] [PubMed] [Google Scholar]
- Choi J, Tanaka K, Cao Y, Qi Y, Qiu J, Liang Y et al. (2014) Identification of a plant receptor for extracellular ATP. Science, 343(6168), 290–294. [DOI] [PubMed] [Google Scholar]
- Clark G & Roux SJ (2018) Role of Ca2+ in mediating plant responses to extracellular ATP and ADP. International Journal of Molecular Sciences, 19(11), 3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G, Wu M, Wat N, Onyirimba J, Pham T, Herz N et al. (2010) Both the stimulation and inhibition of root hair growth induced by extracellular nucleotides in Arabidopsis are mediated by nitric oxide and reactive oxygen species. Plant Molecular Biology, 74(4–5), 423–435. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Fengler KA, Yu IC, Lippok B, Smith RK Jr & Bent, A.F. (2000) The Arabidopsis dnd1 "defense, no death" gene encodes a mutated cyclic nucleotide-gated ion channel. Proceedings of the National Academy of Sciences of the United States of America, 97(16), 9323–9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddou C, Yan Z, Obsil T, Huidobro-Toro JP & Stojilkovic SS (2011) Activation and regulation of purinergic P2X receptor channels. Pharmacological Reviews, 63(3), 641–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Bowen HC, Maathuis FJ, Shabala SN, Tester MA, White PJ et al. (2002) Arabidopsis thaliana root non-selective cation channels mediate calcium uptake and are involved in growth. The Plant Journal, 32(5), 799–808. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala S, Isayenkov S, Cuin TA & Pottosin I (2018) Calcium transport across plant membranes: mechanisms and functions. New Phytologist, 220(1), 49–69. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shang Z, Shin R, Thompson E, Rubio L, Laohavisit A et al. (2009) Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. The Plant Journal, 58(6), 903–913. [DOI] [PubMed] [Google Scholar]
- Dichmann S, Idzko M, Zimpfer U, Hofmann C, Ferrari D, Luttmann W et al. (2000) Adenosine triphosphate-induced oxygen radical production and CD11b up-regulation: Ca(++) mobilization and actin reorganization in human eosinophils. Blood, 95(3), 973–978. [PubMed] [Google Scholar]
- Dodd AN, Kudla J & Sanders D (2010) The language of calcium signaling. Annual Review of Plant Biology, 61, 593–620. [DOI] [PubMed] [Google Scholar]
- Fohr KJ, Warchol W & Gratzl M (1993) Calculation and control of free divalent cations in solutions used for membrane fusion studies. Methods in Enzymology, 221, 149–157. [DOI] [PubMed] [Google Scholar]
- Gao F, Han X, Wu J, Zheng S, Shang Z, Sun D et al. (2012) A heat-activated calcium-permeable channel–Arabidopsis cyclic nucleotide-gated ion channel 6–is involved in heat shock responses. The Plant Journal, 70(6), 1056–1069. [DOI] [PubMed] [Google Scholar]
- Gout E, Bligny R & Douce R (1992) Regulation of intracellular pH values in higher plant cells. Carbon-13 and phosphorus-31 nuclear magnetic resonance studies. Journal of Biological Chemistry, 267(20), 13903–13909. [PubMed] [Google Scholar]
- Jewell JB, Sowders JM, He R, Willis MA, Gang DR & Tanaka K (2019) Extracellular ATP shapes a defense-related transcriptome both independently and along with other defense signaling pathways. Plant Physiology, 179(3), 1144–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogawat A, Meena MK, Kundu A, Varma M & Vadassery J (2020) Calcium channel CNGC19 mediates basal defense signaling to regulate colonization by Piriformospora indica in Arabidopsis roots. Journal of Experimental Botany, 71(9), 2752–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS & Burnstock G (2009) The double life of ATP. Scientific American, 301(6), 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Sivaguru M & Stacey G (2006) Extracellular ATP in plants. Visualization, localization, and analysis of physiological significance in growth and signaling. Plant Physiology, 142(3), 984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ & Knight MR (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. The Plant Cell, 8(3), 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J, Batistic O & Hashimoto K (2010) Calcium signals: the lead currency of plant information processing. The Plant Cell, 22(3), 541–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B & Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nature Methods, 9(4), 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Shang Z, Rubio L, Cuin TA, Very AA, Wang A et al. (2012) Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca(2)+- and K+-permeable conductance in root cells. The Plant Cell, 24(4), 1522–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Arold ST & Gehring C (2020) Mg2+ is a missing link in plant cell Ca2+ signalling and homeostasis-a study on Vicia faba guard cells. International Journal of Molecular Sciences, 21(11), 3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics, 25(16), 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H et al. (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiology, 126(4), 1646–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthus E, Sun J, Wang L, Bhat MG, Mohammad-Sidik AB, Wilkins KA et al. (2020) DORN1/P2K1 and purino-calcium signalling in plants: making waves with extracellular ATP. Annals of Botany, 124(7), 1227–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR & Pittman JK (2009) Shaping the calcium signature. New Phytologist, 181(2), 275–294. [DOI] [PubMed] [Google Scholar]
- Mohammad-Sidik A, Sun J, Shin R, Song Z, Ning Y, Matthus E et al. (2021) Annexin 1 is a component of eATP-induced cytosolic calcium elevation in Arabidopsis thaliana roots. International Journal of Molecular Sciences, 22(2), 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DR, Norvell WA & Chaney RL (1994). A chemical specition program for IBM and compatible computers. In: Loeppert RH, Paul Schwab A & Goldberg S (Eds.) Chemical equilibrium and reaction models. SSSA Spec Pub No. Madison, WI: Soil Science Society of America. [Google Scholar]
- Pham AQ, Cho SH, Nguyen CT & Stacey G (2020) Arabidopsis lectin receptor kinase P2K2 Is a second plant receptor for extracellular ATP and contributes to innate immunity. Plant Physiology, 183(3), 1364–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhett JM, Fann SA & Yost MJ (2014) Purinergic signaling in early inflammatory events of the foreign body response: modulating extracellular ATP as an enabling technology for engineered implants and tissues. Tissue Engineering Part B Reviews, 20(5), 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savio LEB, de Andrade Mello P, da Silva CG & Coutinho-Silva R (2018) The P2X7 receptor in inflammatory diseases: angel or demon? Frontiers in Pharmacology, 9, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HW, DePew CL, Miller ND & Monshausen GB (2015) The cyclic nucleotide-gated channel CNGC14 regulates root gravitropism in Arabidopsis thaliana. Current Biology, 25(23), 3119–3125. [DOI] [PubMed] [Google Scholar]
- Silva G, Beierwaltes WH & Garvin JL (2006) Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension, 47(3), 563–567. [DOI] [PubMed] [Google Scholar]
- Song CJ, Steinebrunner I, Wang X, Stout SC & Roux SJ (2006) Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiology, 140(4), 1222–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YQ, Yang Y, Zhang A, Fei CF, Gu LL, Sun SJ et al. (2020) Three CNGC family members, CNGC5, CNGC6, and CNGC9, are required for constitutive growth of arabidopsis root hairs as Ca2+-permeable channels. Plant Commun, 1(1), 100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Choi J, Cao Y & Stacey G (2014) Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Frontiers in Plant Science, 5, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Gilroy S, Jones AM & Stacey G (2010a) Extracellular ATP signaling in plants. Trends in Cell Biology, 20(10), 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Swanson SJ, Gilroy S & Stacey G (2010b) Extracellular nucleotides elicit cytosolic free calcium oscillations in Arabidopsis. Plant Physiology, 154(2), 705–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Brady SR, Sun Y, Muday GK & Roux SJ (2003) Extracellular ATP inhibits root gravitropism at concentrations that inhibit polar auxin transport. Plant Physiology, 131(1), 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Hou C, Ren Z, Wang C, Zhao F, Dahlbeck D et al. (2019) A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature, 572(7767), 131–135. [DOI] [PubMed] [Google Scholar]
- Tripathi D, Zhang T, Koo AJ, Stacey G & Tanaka K (2018) Extracellular ATP acts on jasmonate signaling to reinforce plant defense. Plant Physiology, 176(1), 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet A & Bove PF (2011) Purinergic signaling in wound healing and airway remodeling. SubCellular Biochemistry, 55, 139–157. [DOI] [PubMed] [Google Scholar]
- Very AA & Sentenac H (2002) Cation channels in the Arabidopsis plasma membrane. Trends in Plant Science, 7(4), 168–175. [DOI] [PubMed] [Google Scholar]
- Vincent TR, Avramova M, Canham J, Higgins P, Bilkey N, Mugford ST et al. (2017) Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. The Plant Cell, 29(6), 1460–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Jia J, Wang Y, Wang W, Chen Y, Liu T et al. (2014) Hyperpolization-activated Ca2+ channels in guard cell plasma membrane are involved in Vicia faba. Journal of Plant Physiology, 171(14), 1241–1247. [DOI] [PubMed] [Google Scholar]
- Wang L, Stacey G, Leblanc-Fournier N, Legue V, Moulia B & Davies JM (2019) Early extracellular ATP signaling in Arabidopsis root epidermis: a multi-conductance process. Frontiers in Plant Science, 10, 1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wilkins KA & Davies JM (2018) Arabidopsis DORN1 extracellular ATP receptor; activation of plasma membrane K+ and Ca2+-permeable conductances. New Phytologist, 218(4), 1301–1304. [DOI] [PubMed] [Google Scholar]
- Wang YF, Munemasa S, Nishimura N, Ren HM, Robert N, Han M et al. (2013) Identification of cyclic GMP-activated nonselective Ca2+-permeable cation channels and associated CNGC5 and CNGC6 genes in Arabidopsis guard cells. Plant Physiology, 163(2), 578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerasinghe RR, Swanson SJ, Okada SF, Garrett MB, Kim SY, Stacey G et al. (2009) Touch induces ATP release in Arabidopsis roots that is modulated by the heterotrimeric G-protein complex. FEBS Letters, 583(15), 2521–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ & Broadley MR (2003) Calcium in plants. Annals of Botany, 92 (4), 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G & Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature, 414(6863), 562–565. [DOI] [PubMed] [Google Scholar]
- Wu SJ & Wu JY (2008) Extracellular ATP-induced NO production and its dependence on membrane Ca2+ flux in Salvia miltiorrhiza hairy roots. Journal of Experimental Botany, 59(14), 4007–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Qin B, Feng K, Yan R, Kang E, Liu T et al. (2018) Extracellular ATP promoted pollen germination and tube growth of Nicotiana tabacum through promoting K+ and Ca2+ absorption. Plant Reproduction, 31(4), 399–410. [DOI] [PubMed] [Google Scholar]
- Wu Y, Yin H, Liu X, Xu J, Qin B, Feng K et al. (2021) P2K1 receptor, heterotrimeric Gα protein and CNGC2/4 are involved in extracellular ATP-promoted ion influx in the pollen of Arabidopsis thaliana. Plants, 10(8), 1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y & Huffaker A (2011) Endogenous peptide elicitors in higher plants. Current Opinion in Plant Biology, 14(4), 351–357. [DOI] [PubMed] [Google Scholar]
- Yang X, Wang B, Farris B, Clark G & Roux SJ (2015) Modulation of root skewing in Arabidopsis by apyrases and extracellular ATP. Plant and Cell Physiology, 56(11), 2197–2206. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Mikhailov A, Samburski SS & Jalkanen S (2006) The detection of micromolar pericellular ATP pool on lymphocyte surface by using lymphoid ecto-adenylate kinase as intrinsic ATP sensor. Molecular Biology of the Cell, 17(8), 3378–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K, Moeder W, Kang HG, Kachroo P, Masmoudi K, Berkowitz G et al. (2006) The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. The Plant Cell, 18(3), 747–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Xu G, Li B, de Souza Vespoli L, Liu H, Moeder W et al. (2019) The receptor kinases BAK1/SERK4 regulate Ca2+ channel-mediated cellular homeostasis for cell death containment. Current Biology, 29(22), 3778–3790.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Henriques R, Lin SS, Niu QW & Chua NH (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nature Protocols, 1(2), 641–646. [DOI] [PubMed] [Google Scholar]
- Zhong H, Molday LL, Molday RS & Yau KW (2002) The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature, 420(6912), 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Rough mapping table of mutant 367.
Table S2. Whole-genome sequencing showing the mutations of cngc6-3 in a 3.6-Mbp interval on chromosome 2
Table S3. Mapping primers.
Table S4. Primer list.
Figure S1. Mutant screening scheme.
Figure S2. cngc6-1 and cngc6-3 mutants are allelic.
Figure S3. [Ca2+]cyt response to different nucleotides and elicitors in mutant 367 complemented by expression of native CNGC6.
Figure S4. The cngc6-3 mutant developmental phenotype.
Data Availability Statement
WGS data of mutant 367 can be found at https://osf.io/csq6t/. Other relevant data can be found within the manuscript and its supporting materials.