SUMMARY
Serotype 1 Streptococcus pneumoniae is a cause of invasive pneumococcal disease (IPD) worldwide and has been associated with IPD outbreaks, while carriage is rarely detected in healthy adults or children. This study details an Australian multi-state and territory outbreak of serotype 1 S. pneumoniae IPD between 2010 and 2012. Molecular characterization demonstrated the outbreak was largely due to the clonal expansion of sequence type 306, MLVA type 261 S. pneumoniae serotype 1.
Key words: IPD, outbreak, serogroup 1, serotype 1, Streptococcus pneumoniae
INTRODUCTION
Streptococcus pneumoniae is a leading cause of both invasive and non-invasive diseases including pneumonia, bacteraemia, meningitis and acute otitis media. Over 90 distinct serotypes exist, which differ in chemical composition of their respective polysaccharide capsules [1, 2].
Serotype 1 is ranked among the most common invasive serotypes worldwide and is often associated with outbreaks and epidemics [3–13]. Unlike other pneumococci responsible for invasive pneumococcal disease (IPD), serotype 1 pneumococci are rarely found in the nasopharynx of healthy individuals in the general population [14–18]; however, carriage in healthy contacts has been observed during times of serotype 1 outbreaks [9], including in one carriage surveillance study undertaken during a serotype 1 outbreak which demonstrated carriage of the outbreak strain in healthy children [19].
The heptavalent pneumococcal conjugate vaccine (Prevenar®, PCV7; Pfizer, USA) covering the seven most common disease-causing serotypes (4, 6B, 9 V, 14, 18C, 19F, 23F) was introduced into the Australian National Immunization Programme (NIP) in two stages; in 2001 for the Aboriginal and Torres Strait Islander population and high-risk non-Indigenous children aged < 2 years, and in 2005 for all children in this age group (and did not provide specific protection against serotype 1 disease). From October 2009 in the Northern Territory (NT) only, PCV7 was replaced by the 10-valent pneumococcal conjugate vaccine (Synflorix®, PHiD-CV10; GlaxoSmithKline, USA), covering an extra three serotypes and including serotype 1. In July 2011, the new 13-valent pneumococcal conjugate vaccine (Prevenar 13®, PCV13; Pfizer, USA), which also includes serotype 1, replaced PCV7 in all states and territories except NT, where it replaced PHiD-CV10 from October 2011. Additionally, the 23-valent pneumococcal polysaccharide vaccine (Pneumovax23®, 23PPV; Merck, USA), which includes serotype 1, was added the to the NIP schedule for the Aboriginal and Torres Strait Islander population aged ⩾50 years in 1999 and for non-Indigenous Australians aged ⩾65 years from January 2005. 23PPV was also provided as the fourth dose of the pneumococcal vaccination schedule to Aboriginal and Torres Strait Islander children aged between 18 and 24 months deemed medically at risk of IPD [20].
IPD has been a nationally notifiable disease in Australia since 2001, and all invasive notification data is collected by all states and territories under jurisdictional public health legislation and forwarded to the Commonwealth under the National Health Security Act 2007. These notifications are collated nationally in the National Notifiable Diseases Surveillance System (NNDSS). In 2000, Communicable Diseases Network Australia (CDNA) established the Commonwealth-funded Enhanced Invasive Pneumococcal Disease Surveillance Working Group (EIPDSWG) to assist in developing and implementing a nationally standardized approach to the enhanced surveillance of IPD in Australia. All isolates of S. pneumoniae recovered from IPD are referred to reference laboratories for serotyping. A small percentage of pneumococcal notifications are based around PCR detection of pneumococci only and in these cases isolates are not obtained and serotype information may not be determined. Between 2010 and 2012, complete serotype information was obtained for 94–99% of cases across all three states/territories.
In 2010 and 2011, a number of Australian states and territories experienced a significant increase in the incidence and proportion of serotype 1 IPD cases among total IPD cases [19, 21]. This study describes the molecular epidemiological characterization of serotype 1 IPD isolates from the Australian states of Queensland (QLD), Western Australia (WA) and NT from 2008 to 2012. Isolates were characterized by multi-locus sequence typing (MLST), multi-locus variable-number tandem repeat analysis (MLVA) and BOX PCR to analyse strain relatedness in order to determine if the increase in serotype 1 IPD was due to the emergence of a single outbreak strain.
METHODS
Serotyping of S. pneumoniae isolates
This study focused on IPD isolates referred to the QLD Pneumococcal Reference Laboratory from QLD, NT and WA for confirmation of serotype between 2008 and 2012. In particular, these isolates included all serotype 1 notifications from these three states (excluding S. pneumoniae PCR-only detections where a serotype was not determined), in addition to all other IPD notifications isolated from QLD and NT. All isolates were serotyped by Quellung reaction [22] using antisera supplied by Statens Serum Institut (Copenhagen, Denmark). Isolates confirmed as serotype 1 were prepared for molecular characterization by suspension of one colony from Columbia agar + 5% horse blood plates (bioMérieux, France) into 400 μl Tris-EDTA (TE) pH 8·0 buffer and boiled for 10 min at 100°C. In total, 253 serotype 1 IPD isolates from all three states were further characterized by MLST, MLVA and BOX PCR for this study.
MLST
MLST was performed by analysis of seven housekeeping loci as described previously [23]. Sequence chromatograms were edited and read using ChromasPro (Technelysium, Australia). Sequence types (STs) were assigned according to the Streptococcus pneumoniae MLST website (http://spneumoniae.mlst.net). Isolates with alleles or STs not present in the database were assigned new STs by database curators.
MLVA
Amplification and fragment sizing for MLVA was performed as described previously [24], with slight modification of the BOX_12 primer sequences Sp BOX_12-Pf (PET-GATTGCCCTTTTCATCTTC) and Sp BOX_12-r (CACAGCAACCATTGAAAC), and changes made to the primer combinations and labels used in each multiplex reaction. Eight BOX loci were amplified in three multiplex assays, where reaction 1 contained the BOX_01 (FAM labelled), BOX_02 (NED labelled) and BOX_04 (PET labelled) primers, reaction 2 contained the BOX_06 (NED labelled) and BOX_13 (FAM labelled) primers and reaction 3 contained the BOX_03 (VIC labelled), BOX_11 (FAM labelled) and BOX_12 (PET labelled) primers. The 20 μl reaction mixtures consisted of Qiagen multiplex PCR mix (Qiagen, USA), primers (BOX_01, BOX_02, BOX_13, 250 nm; BOX_04, BOX_06, 500 nm; BOX_03, 750 nm; BOX_11, 1 μm; BOX_12, 75 nm), sterile water and template (2 μl). Amplification was performed as per the following programme: 15 min at 95°C, 30 repeated cycles of 30 s at 95°C, 90 s at 50°C and 60 s at 72°C, followed by a final incubation step of 30 min at 68°C. Once amplified, 0·5 μl product, 3 μl Genescan 1200 LIZ-marker (Applied Biosystems, USA) and 6·5 μl Hi-Di Formamide (Applied Biosystems) were combined and denatured at 95°C for 5 min. These reactions were then sized on the ABI 3130 Genetic Analyser (Applied Biosystems). The resulting .fsa files were analysed using Peak Scanner v 1.0 (Applied Biosystems) software. Amplicon sizes were converted into allele designations based on the protocol located at http://www.MLVA.net. MLVA types (MTs) were assigned by comparison to profiles contained in the MLVA.net database, and new allele sizes and MTs were submitted to the database for confirmation and addition.
BOX PCR
BOX PCR was performed on all isolates as described previously [25] using the BOX A primer (10 mm). PCR was performed using Taq DNA polymerase and standard Taq buffer (New England BioLabs, USA), 0·2 mm dNTPs and 1·5 mm MgCl2. Amplification was performed as per the following programme: 5 min at 95°C, 40 repeated cycles of 1 min at 94°C, 2 min at 60°C, 2 min at 72°C with a final extension step of 2 min at 72°C. Products were separated by electrophoresis on 2% agarose gels and stained with ethidium bromide for visualization of banding patterns under ultraviolet light.
Statistical analysis
Molecular analysis results were analysed using Bionumerics v. 6.5 software (Applied Maths Inc., Belgium) and cluster analysis was performed as described in the related results sections.
Diversity was calculated using Simpson's index (SID) [26, 27], with 95% confidence intervals (CI) calculated as described previously [28]. A Bionumerics script available at http://biomath.itqb.unl.pt/ClusterComp [29] was used to facilitate these calculations. Calculation of Yates’ χ2 statistic was calculated according to Preacher et al. [30].
RESULTS AND DISCUSSION
Identification and distribution of S. pneumoniae serotype 1 isolates
From 2009 to 2010 significant increases in the numbers of NT and WA serotype 1 IPD cases were observed (χ2Yates = 6·1, P = 0·01; χ2Yates = 14·9, P = 0·0001, respectively). Prior to 2010, serotype 1 IPD cases were sporadic with no cases in 2008 and only one case (1% total IPD) in NT in 2009; however by the end of 2010, 10·9% (n = 7) of NT and 10·5% (n = 21) of WA total IPD cases received for serotyping were due to serotype 1. These significant increases continued in 2011 with serotype 1 constituting 45·2% (n = 57) and 23·4% (n = 57) of total IPD cases from NT and WA (χ2Yates = 20·8, P = 0·0; χ2Yates = 11·7, P = 0·0, respectively). In 2012, the proportion of NT and WA serotype 1 cases significantly declined to 25·7% (n = 18) and 12·3% (n = 29) of total IPD cases correspondingly (χ2Yates = 8·2, P = 0·004; χ2Yates = 9·1, P = 0·003, respectively).
In Queensland, the number and proportion of serotype 1 IPD cases remained steady between 2008 and 2010, comprising an average of 2·8% (n = 8, 7, 7) of total IPD cases each year. This was followed by sequential increases in the number and proportion of serotype 1 IPD comprising 4·7% (n = 21) and 9% (n = 29) of total IPD cases in 2011 and 2012, respectively. Although the rise in 2011 was not statistically significant (χ2Yates = 1·1, P = 0·3), the increase from 2011 to 2012 was significant (χ2Yates = 5·0, P = 0·03). Figure 1 shows the proportion of IPD cases attributed to serotype 1 for QLD, NT and WA from 2008 to 2012, expressed as cases/100000 population.
Fig. 1.
[colour online]. Cases of serotype 1 IPD among total IPD/100 000 population by state and territory, 2008–2012.
Data on patients’ age was provided for 250/253 isolates and is shown in Table 1. Of the serotype 1 isolates, the median age of patients was 20 years, with 74% (n = 185) of isolates belonging to children and adults in the 5–50 years age group, and similar rates experienced in each state/territory. Although the vaccination status of these individuals is unknown, this age group is unlikely to have been targeted for any of the protein-conjugate serotype 1-containing vaccines, which suggests that the impact of PHiD-CV10 or PCV13 is unlikely to have played a part in affecting this outbreak. Similarly, no conclusions are able to be drawn on the effect of 23PPV on this outbreak due to the unknown Indigenous status of cases. Similar median patients’ ages have also been observed in other outbreaks of serotype 1 IPD [9, 12, 13, 19].
Table 1.
Age breakdown of serotype 1 cases
| Year | Age group (years) | Case numbers by state/territory | ||
|---|---|---|---|---|
| QLD | NT | WA | ||
| 2008 | <5 | 0 | 0 | 0 |
| 5–50 | 4 | 0 | 0 | |
| ⩾51 | 3 | 0 | 0 | |
| Total | 7 | 0 | 0 | |
| 2009 | <5 | 0 | 0 | 0 |
| 5–50 | 5 | 1 | 0 | |
| ⩾51 | 2 | 0 | 0 | |
| Total | 7 | 1 | 0 | |
| 2010 | <5 | 2 | 1 | 4 |
| 5–50 | 5 | 5 | 11 | |
| ⩾51 | 0 | 0 | 4 | |
| Total | 7 | 7* | 19 | |
| 2011 | <5 | 3 | 7 | 11 |
| 5–50 | 13 | 43 | 39 | |
| ⩾51 | 0 | 4 | 7 | |
| Total | 16 | 56† | 57 | |
| 2012 | <5 | 1 | 3 | 2 |
| 5–50 | 26 | 10 | 23 | |
| ⩾51 | 2 | 5 | 4 | |
| Total | 29 | 18 | 29 | |
One with age unknown.
Two with age unknown.
MLST of serotype 1 isolates
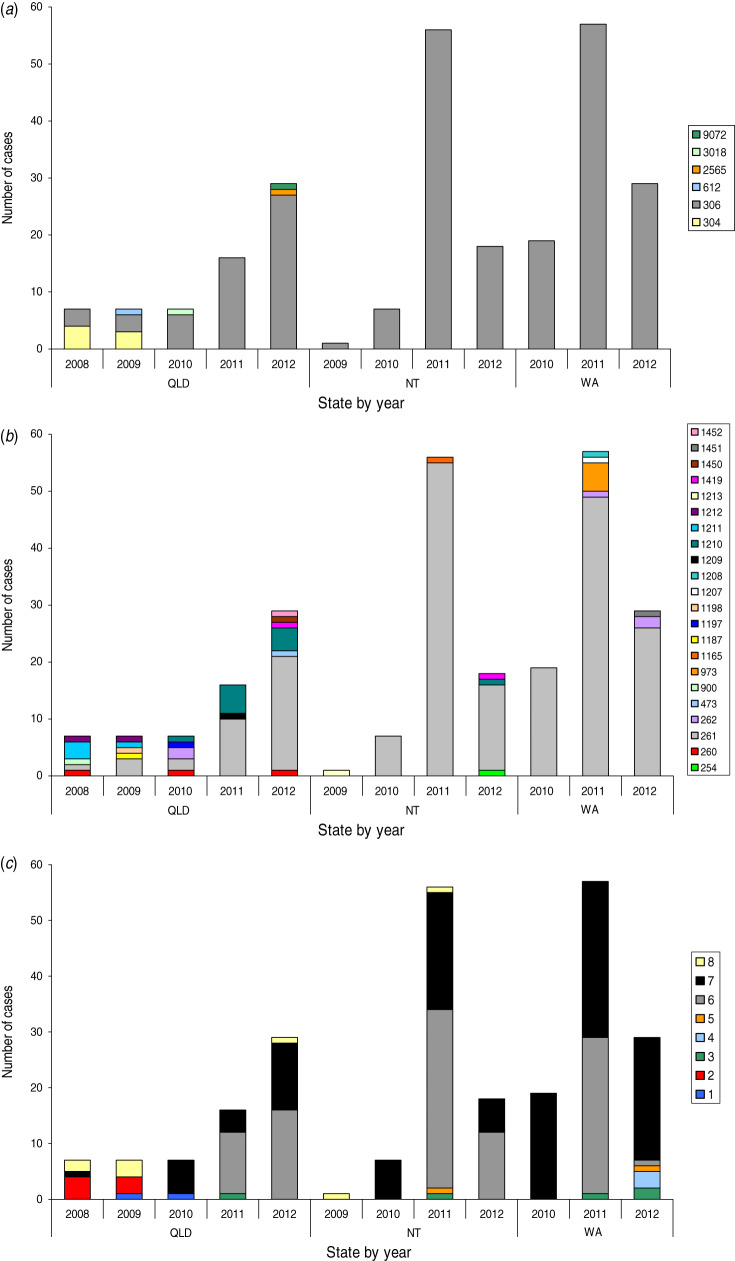
Of the 253 isolates, six different STs were identified by MLST (SID = 0·09, 95% CI 0·04–0·13). ST306 accounted for 95·7% (n = 242) of these strains. ST304 was the next common ST, with seven isolates, and differed from the profile of ST306 by three alleles. One isolate of each of ST3018, ST612, ST2565 and ST9072 were also identified. These STs did not share more than two alleles with each other except for ST612 and ST6265 which shared five alleles. With regard to geographical location, all six STs were detected in the QLD isolates, whereas ST306 was the only ST detected from serotype 1 strains isolated from NT and WA patients (Fig. 2a). MLST revealed that there was an expansion of the ST306 clone in QLD and a subsequent loss of ST diversity from 2010 in the serotype 1 population (Fig. 2).
Fig. 2.
[colour online]. Temporal distribution of (a) sequence type, (b) MLVA type and (c) BOX group of serotype 1 IPD isolates by state and territory, 2008–2012.
ST306 has previously been shown to be the most common ST of serotype 1 IPD, and along with ST304 makes up about 90% of serotype 1 IPD worldwide [3]. Both of these STs have been predominantly seen in Europe, Canada and the USA. ST306 has also been the major clone responsible for increases in serotype 1 IPD in Sweden [10], Scotland [11] and the South Pacific [12]. ST9072 had a new allelic profile that was submitted to the MLST database, but was a single-locus variant of ST191, a strain predominately associated with S. pneumoniae serotype 7F strains [31], and could be the product of a capsular switching event.
MLVA of serotype 1 isolates
The 253 serotype 1 IPD isolates were divided into 22 different MTs (SID = 0·33, 95% CI 0·25–0·41). Figure 3 depicts the minimum spanning tree constructed using these MTs with a categorical coefficient. Most isolates belonged to MT261 (81·8%, n = 207), and when partitioning allowing a difference of one locus was applied, 94·5% (n = 239) of isolates clustered together, forming the MT261 clonal complex. This correlated closely with the ST306 strains, which also included MT1419, a two-locus variant of MT261, and ST1452, a four-locus variant of MT1451.
Fig. 3.
Minimum spanning tree of the results obtained by MLVA using a categorical coefficient. Each node represents a different MLVA type, with shading of nodes indicating the numbers of isolates of that particular type, as shown in the key. Thick dark lines separate types differing by a single locus, while thin continuous lines connect double locus variants. Faint lines represent types differing by ⩾3 loci. Shading around nodes demonstrates partitioning of isolates differing by only one locus, and indicates designated clonal complexes. Sequence types (ST) and BOX groups are also labelled.
QLD isolates had the most diversity of MTs, with 15 detected; six different MTs were observed for both NT and WA. Figure 2b shows the temporal distribution of MLVA profiles for each state, and depicts the increase of MT261 over time in all three states.
Limited data exist on the incidence and distribution of S. pneumoniae MTs. Many of the allelic profiles were unique and submitted to the database for inclusion; however, MT261 previously existed in the database associated with a serotype 1 complex. Interestingly, the ST9072 isolate was identified as MT473, which belongs to a serotype 7F associated clonal complex. This genotyping result further supports the MLST finding that this isolate may have undergone a recent capsule switch.
BOX PCR of serotype 1 isolates
Profiles produced by BOX PCR for the 253 isolates were used to construct a dendogram using the Dice coefficient and UPGMA clustering algorithm, with optimization and position tolerance both set at 1·5% (not shown). Isolates clustered into eight groups according to an assigned similarity coefficient of 90% (SID = 0·60, 95% CI 0·56–0·63); however, all serotype 1 isolates were highly similar, with a minimum similarity value of 82·3%. Figure 2c depicts the distribution of BOX PCR profiles over time for each state. In 2010, isolates belonging to group 7 were identified in all three states and comprised 96·7% (n = 32) of isolates. In 2011, group 6 appeared in all three states, and with group 7, comprised 96·1% (n = 124) of all serotype 1 isolates. This high proportion of group 6 and group 7 isolates continued into 2012 for both QLD and NT, albeit with an overall decrease in serotype 1 numbers; however, in WA group 7 continued to predominate (75·9%, n = 22) with the remaining strains belonging to a multitude of groups (3, 4, 5, 6).
Correlation between typing methods
The correlation between MLST, MLVA and BOX PCR is illustrated in Figure 3. MLVA clonal complexes largely correlated with the STs determined by MLST, and individual MTs further differentiated the larger ST306 and smaller ST304 complexes. BOX PCR clustered ST612 and ST3018 into a separate group, and discriminated ST304 from ST306. Additionally it provided further discrete clustering of the large ST306, MT261 clonal complex into six groups, two of which (groups 6 and 7) predominated over the course of the outbreak.
Most evident is the clonality of serotype 1 IPD isolates identified from patients during the outbreak period. Although a number of BOX PCR groups were identified within the ST306 strains, all BOX groups were highly related (at least 82·3% similarity) and the majority of isolates were associated with one of two closely related BOX groups. Of the 15 MTs associated with the ST306 strains, 13 were highly related, forming one clonal complex, indicating that this clone is relatively stable. The clonality of ST306 has been previously observed in another study which used pulsed-field gel electrophoresis and was unable to differentiate between ST306 strains [12].
The high invasive potential of serotype 1 ST306 could be the contributing factor for its lack of diversity, as a low carriage rate limits recombination opportunities with other pneumococcal populations. This theory was suggested by Brueggemann & Spratt [3] and supported by Dagerhamn et al. [32] who observed higher diversity within carriage isolates and fewer intraclonal differences associated with pneumococcal serotypes of high invasive disease potential. Interestingly, during this outbreak, an asymptomatic carriage surveillance study performed in Central Australia found that 12% of participants (n = 4) carried serotype 1 pneumococci in the nasopharynx, with all four strains also belonging to ST306 [19].
Changes in serotype 1 genotypes over time
ST306 was the only ST observed in both NT and WA during the serotype 1 outbreak period, and all serotype 1 cases in NT and WA in 2010 could be attributed to one ST306, MT261, BOX group 7 clone. During 2011, the predominance of the ST306, MT261 clone continued, comprising 98·2% (NT) and 86% (WA) of total serotype 1 IPD isolates, with the emergence of BOX group 6 (42·9%) joining BOX group 7 (55·1%) as the prevalent BOX profiles. The incidence of serotype 1 IPD declined in NT and WA during 2012; however, 83·3% (NT) and 89·7% (WA) of IPD isolates still belonged to the ST306, MT261 clone. Interestingly, although the NT strains still comprised of BOX group 6 (60%) and group 7 (40%) in 2012, five BOX groups were observed in the WA strains, of which group 7 predominated (80·8%), with a greatly reduced proportion of group 6 strains (3·8%).
In QLD prior to 2010, ST306 and ST304 occurred at similar rates, and a variety of MTs were observed with MT261 occurring in 14·2%, 42·8% and 28·6% of isolates, respectively, during 2008 to 2010. During this time period, isolates belonged to a range of BOX groups (1, 2, 7, 8). By 2011, all isolates were ST306; 62·5% were MT261 and 90% of these belonged to BOX group 6. Unlike NT and WA, QLD continued to observe increasing numbers of serotype 1 IPD during 2012, with 93% being ST306, of which 69% were MT261, comprising 50% each of BOX groups 6 and 7. However, two new STs had also emerged by late 2012, and may signify a re-diversification of the Queensland serotype 1 strains as the outbreak numbers begin to decline.
Overall, it appears the increase in serotype 1 IPD in WA, NT and QLD was due to the clonal expansion of ST306, in particular a ST306, MT261 clone. Serotype 1 is well known for fluctuations in case numbers and ST306 has long been acknowledged as an important source of serotype 1 IPD outbreaks and epidemics. In the absence of details such as vaccination and Indigenous status it is difficult to make any observations on the impact of vaccine protocol on this outbreak. Continued surveillance of the occurrence and molecular characteristics of pneumococcal serotypes in Australia is crucial in order to continually evaluate the impact of PCV13 and further vaccines on the incidence of sporadic cases and outbreaks of IPD in the future.
ACKNOWLEDGEMENTS
We thank all QLD, NT and WA hospital and private pathology laboratories for the referral of pneumococcal isolates for surveillance. We also thank Dr Karin Elberse, National Institute for Public Health and the Environment (RIVM), Laboratory for Infectious Diseases and Screening (LIS), The Netherlands, for confirming and assigning new MLVA types and uploading these to the S. pneumoniae MLVA database. We acknowledge the financial contributions of the Commonwealth Department of Health and Ageing for the serotyping of the isolates. We acknowledge the use of the pneumococcal MLST database which is located at the Imperial College London and is funded by the Wellcome Trust.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. Journal of Clinical Microbiology 1995; 33: 2759–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park IH, et al. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. Journal of Clinical Microbiology 2007; 45: 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brueggemann AB, Spratt BG. Geographic distribution and clonal diversity of Streptococcus pneumoniae serotype 1 isolates. Journal of Clinical Microbiology 2003; 41: 4966–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hausdorff WP. The roles of pneumococcal serotypes 1 and 5 in paediatric invasive disease. Vaccine 2007; 25: 2406–2412. [DOI] [PubMed] [Google Scholar]
- 5.Hanna JN, et al. Invasive pneumococcal disease in non-Indigenous people in north Queensland, 2001–2009. Medical Journal of Australia 2010; 193: 392–396. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez MA, et al. Invasive pneumococcal disease: association between serotype, clinical presentation and lethality. Vaccine 2011; 29: 5740–5746. [DOI] [PubMed] [Google Scholar]
- 7.Chiou A-C, et al. Molecular assessment of invasive Streptococcus pneumoniae serotype 1 in Brazil: evidence of clonal replacement. Journal of Medical Microbiology 2008; 57: 839–844. [DOI] [PubMed] [Google Scholar]
- 8.Johnson HL, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: The pneumococcal global serotype project. PLoS Medicine 2010; 7: e1 000 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gratten M, et al. An outbreak of serotype 1 Streptococcus pneumoniae infection in central Australia. Medical Journal of Australia 1993; 158: 340–342. [DOI] [PubMed] [Google Scholar]
- 10.Henriques Normark B, et al. Dynamics of penicillin-susceptible clones in invasive pneumococcal disease. Journal of Infectious Diseases 2001; 184: 861–869. [DOI] [PubMed] [Google Scholar]
- 11.Kirkham L-AS, et al. Identification of invasive serotype 1 pneumococcal isolates that express nonhemolytic pneumolysin. Journal of Clinical Microbiology 2006; 44: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Hello S, et al. Invasive serotype 1 Streptococcus pneumoniae outbreaks in the South Pacific from 2000 to 2007. Journal of Clinical Microbiology 2010; 48: 2968–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith-Vaughan H, et al. Age-specific cluster of cases of serotype 1 Streptococcus pneumoniae carriage in remote indigenous communities in Australia. Clinical and Vaccine Immunology 2009; 16: 218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harboe ZB, et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Medicine 2009; 6: e1 000 081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagos R, et al. Age- and serotype-specific pediatric invasive pneumococcal disease: insights from systematic surveillance in Santiago, Chile, 1994–2007. Journal of Infectious Diseases 2008; 198: 1809–1817. [DOI] [PubMed] [Google Scholar]
- 16.Mbelle N, et al. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. Journal of Infectious Diseases 1999; 180: 1171–1176. [DOI] [PubMed] [Google Scholar]
- 17.Porat N, Trefler R, Dagan R. Persistence of two invasive Streptococcus pneumoniae clones of serotypes 1 and 5 in comparison to that of multiple clones of serotypes 6B and 23F among children in Southern Israel. Journal of Clinical Microbiology 2001; 39: 1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackenzie G, et al. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infectious Diseases 2010; 10: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai J, et al. Surveillance of pneumococcal serotype 1 carriage during an outbreak of serotype 1 invasive pneumococcal disease in central Australia 2010–2012. BMC Infectious Diseases 2013; 13: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Australian Technical Advisory Group on Immunisation. The Australian Immunisation Handbook, 10th edn. Canberra: Australian Government Department of Health, 2013. [Google Scholar]
- 21.Cook H, et al. A lengthy widespread outbreak of serotype-1 invasive pneumococcal disease shows protection with the 10-valent conjugate vaccine and increased pneumonia complications. 8th International Symposium on Pneumococci and Pneumococcal Diseases, Iguacu Falls, Brazil, 2012. [Google Scholar]
- 22.Lund E, Henrichsen J. Laboratory diagnosis, serology and epidemiology of Streptococcus pneumoniae. Methods in Microbiology 1978; 12: 241–262. [Google Scholar]
- 23.Enright MC, Spratt BG. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 1998; 144: 3049–3060. [DOI] [PubMed] [Google Scholar]
- 24.Elberse KEM, et al. Multiple-locus variable number tandem repeat analysis for comparison with PFGE and MLST. PLoS ONE 2011; 6: e19 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Belkum A, et al. Novel BOX repeat PCR assay for high-resolution typing of Streptococcus pneumoniae strains. Journal of Clinical Microbiology 1996; 34: 1176–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson EH. Measurement of diversity. Nature 1949; 163: 688–688. [Google Scholar]
- 27.Hunter PR, Gaston MA. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. Journal of Clinical Microbiology 1988; 26: 2465–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grundmann H, Hori S, Tanner G. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. Journal of Clinical Microbiology 2001; 39: 4190–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carriço JA, et al. Illustration of a common framework for relating multiple typing methods by application to macrolide-resistant Streptococcus pyogenes. Journal of Clinical Microbiology 2006; 44: 2524–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preacher KJ. Calculation for the chi-square test: an interactive calculation tool for chi-square tests of goodness of fit and independence [computer software]. (http://quantpsy.org), 2001. Accessed 10 September 2012.
- 31.Streptococcus pneumoniae Multi Locus Sequence Typing website (http://spneumoniae.mlst.net/misc/info.asp). Accessed 4 October 2013.
- 32.Dagerhamn J, et al. Determination of accessory gene patterns predicts the same relatedness among strains of Streptococcus pneumoniae as sequencing of housekeeping genes does and represents a novel approach in molecular epidemiology. Journal of Clinical Microbiology 2008; 46: 863–868. [DOI] [PMC free article] [PubMed] [Google Scholar]