SUMMARY
Parrots are one of the most popular pet birds in China, and can harbour Chlamydia which has significance for human and animal health. We investigated, by indirect haemagglutination assay, the seroprevalence of Chlamydia infection in four species of parrots, namely budgerigars (Melopsittacus undulatus), lovebirds (Agapornis sp.), cockatiels (Nymphicus hollandicus) and Alexandrine parakeets (Psittacula eupatria) that were collected from Weifang and Beijing cities, North China and explored the association between potential risk factors and chlamydial seropositivity. We further determined the genotype of Chlamydia in 21 fresh faecal samples based on the ompA sequence by reconstruction of phylogenetic relationships. Of the 311 parrots examined, 35·37% (95% confidence interval 30·06–40·68) were seropositive, and species, gender, age, season and geographical location were identified as risk factors. Two PCR-positive samples represented Chlamydia psittaci genotype A. The occurrence of C. psittaci genotype A in the droppings of two pet parrots in China suggests potential environmental contamination with Chlamydiaceae and may raise a public health concern.
Key words: China, Chlamydia, genotype, pet parrots, seroprevalence
INTRODUCTION
Chlamydia comprises a group of important obligate intracellular bacteria that are responsible for a variety of diseases in humans and a wide range of animals, including pet birds [1, 2]. Among these pathogens, Chlamydia psittaci, which can be transmitted from infected birds' secretions and droppings to humans via direct or indirect transmission routes, is recognized as the most important zoonotic pathogen [3, 4]. Infection with Chlamydia spp. may result in difficulty in breathing, high fever and respiratory tract infection, occasionally with severe systemic disease in humans, causing chlamydiosis, ornithosis, psittacosis or parrot fever [5, 6].
Parrots often live in close relationship with humans and are frequently reared in family homes, parks and zoos. Budgerigars (Melopsittacus undulatus), lovebirds (Agapornis sp.), cockatiels (Nymphicus hollandicus) and Alexandrine parakeets (Psittacula eupatria) are the four most popular species of parrots in China. They, in addition to other pet birds, are also the best known representative natural hosts of Chlamydia and may shed zoonotic pathogens into the environment [4, 7].
Chlamydia seroprevalence in pigs, cattle, dogs, cats and humans has been widely reported throughout the world [8–15], but there is limited information about Chlamydia infection in parrots available, and no such information is available for parrots in China. In this survey, we investigated the seroprevalence of Chlamydia infection in budgerigars, lovebirds, cockatiels and Alexandrine parakeets in Beijing and Weifang cities, north China, and determined the genotype of Chlamydia shed in faeces from these popular pet birds.
MATERIALS AND METHODS
The investigated sites
The survey was conducted in Beijing and Weifang cities (two main locations of parrot production), north China. Beijing city (39°26′–41° 03′ N, 115° 25′–117° 30′ E) lies to the south of the Yanshan Mountains with an average altitude of 43·5 m, annual precipitation of 626 mm, and average annual temperature of 12·6°C. Weifang city is situated in the middle of Shandong Peninsula (118° 10′–120° 01′ E, 35° 41′–37° 26′ N) and has a northern temperate and monsoonal climate. The average altitude of Weifang city is 19·3 m, and the average annual temperature is 14·0°C.
Study population
The study population comprised of 311 parrots collected from bird sellers. The total number of parrots sold by the sellers in a 6-month period was nearly 50 000. Based on the fact that the seroprevalence of Chlamydia for the pigeon population was 31% in 2013 [16], the expected seroprevalence is 30% (P) with an accepted deviation of the true prevalence of 5% (d) and a confidence level of 95% (z = 1·96). The sample size was therefore calculated as 323 [according to n = P (1 − P)z2/d2].
Collection and preparation of serum samples
The 311 birds (202 budgerigars, 26 lovebirds, 22 cockatiels, 61 Alexandrine parakeets) were randomly selected from live bird markets in spring and summer, 2013. Blood samples were collected from the wing vein of parrots by a veterinary practitioner, and then separated by centrifugation at 1000 g for 10 min, and stored at −20°C until analysis. Data regarding species, gender, age and geographical origin were obtained from the bird sellers and the first three were then confirmed by the veterinary practitioners. All operations were performed in strict accordance with the Good Animal Practice requirements of the Animal Ethics Procedures and Guidelines of the People's Republic of China. This study was approved by the Animal Ethics Committee of Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences (Approval no. LVRIAEC2012-010).
Serological tests
The commercially available indirect haemagglutination assay (IHA) kit (Lanzhou Veterinary Research Institute, Chinese Academy of Agriculture Sciences) was used to examine antibodies to Chlamydia, and the detection procedures were performed as previously reported [16] with a cut-off of 1:16. Dilutions between 1:4 and 1:16 were considered inconclusive and the samples were retested. Positive and negative controls were included in each test and assayed at the same dilutions of the sera samples.
Statistical analysis
The variation in Chlamydia seroprevalence (y) of parrots of different gender (x1), collecting season (x2), age group (x3), species (x4) and geographical location (x5) was analysed by χ2 test using SAS version 9.1 (SAS Institute Inc., USA). In the multivariable regression analysis, each of these variables was included in the binary Logit model as an independent variable. The best model was judged by Fisher's scoring algorithm. All tests were two-sided, and values of P<0·05 were considered statistically significant. Odds ratios (ORs) and their 95% confidence intervals (95% CIs) were estimated to explore the strength of the association between Chlamydia seropositivity and the conditions investigated.
DNA testing and sequencing
For DNA extraction from droppings of parrots, each of 21 samples was homogenized in sterile PBS, and then filtered through a 0·3-mm wire mesh. The filtrate was collected in a 1·5-ml tube and centrifuged at 1000 g for 10 min. After discarding the supernatant, genomic DNA was extracted using the Stool DNA kit (Omega, USA) according to the manufacturer's recommendations. DNA samples were detected using semi-nested PCR according to previous studies [17, 18]. The PCR products were subjected to electrophoresis on 1% agarose gel containing 0·5 μg/ml GoldView (Solarbio, China) and were observed under UV light.
To determine the genotype of Chlamydia in positive cases, a ~1000 bp fragment of the ompA gene was sequenced using a pair of primers, FOMPF1/FOMPF2, according to a previous study [19]. The positive PCR products were then sequenced by the Sangon Biotech Company (China). The sequence data from the present study were deposited in the GenBank database with accession no. KF611904.
Reconstruction of phylogenetic relationships
Based on the sequencing results of the ompA gene, the relevant sequences of Chlamydia caviae were downloaded from GenBank [20]. All the sequences were then aligned using the multiple sequence alignment program, Clustal X 1·83 [21], dendrograms were constructed using maximum likelihood (ML) according to previous studies [22–24], and the GTR model with its parameter for configuring concatenated dataset was determined for the ML analysis. Bootstrap support for ML trees was calculated using 100 bootstrap replicates. Phylograms were drawn using Tree View program version 1.65 (University of Glasgow. UK).
RESULTS
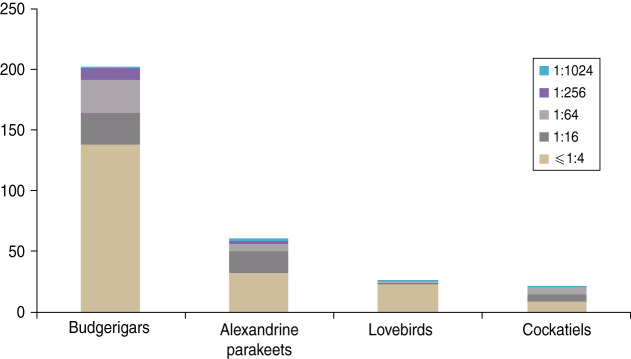
Of the 311 serum samples, 110 (35·37%) were positive for Chlamydia antibodies by IHA (Fig. 1). Table 1 presents the exposure regarding gender, species, age, collecting region and season associated with Chlamydia seropositivity in parrots based on the univariate analysis. Optimized by Fisher's scoring technique, forward stepwise logistic regression analysis was conducted to evaluate the impacts of multiple variables on Chlamydia. In the final model, three variables had effects on the infectious disease, described by the equation
Gender and age had positive effects on the risk of Chlamydia, for which the ORs were 1·63 (95% CI 1·01–2·63) and 1·70 (95% CI 1·20–2·43), respectively. Females were seen to be more susceptible than males, and the sub-adult and adult birds were more resistant to Chlamydia than juveniles (Table 1). Bird species had a negative effect on the disease (OR 0·695, 95% CI 0·511–0·946), and cockatiels (OR 13·42), Alexandrine parakeets (OR 6·95) and lovebirds (OR 3·56) were considered to have higher seropositivity compared to budgerigars (Table 1).
Fig. 1.
[colour online]. The Chlamydia antibody titres in four species of parrots.
Table 1.
Analysis of the variables associated with Chlamydia seroprevalence in pet parrots in China
| Variable | Category | No. of serum samples | No. of positive samples | Prevalence % (95% CI) | P value | OR (95% CI) |
|---|---|---|---|---|---|---|
| Region | Beijing | 158 | 54 | 34·18 (26·78–41·57) | 0·65 | Reference |
| Weifang | 153 | 56 | 36·60 (28·97–43·23) | 1·11 (0·70–1·77) | ||
| Sex | Male | 163 | 49 | 30·06 (23·02–37·10) | 0·04 | Reference |
| Female | 148 | 61 | 41·22 (33·29–49·15) | 1·63 (1·02–2·61) | ||
| Breed | Budgerigars (Melopsittacus undulatus) | 26 | 3 | 11·54 (0–23·82) | <0·01 | Reference |
| Alexandrine parakeets (Psittacula eupatria) | 61 | 29 | 47·54 (35·01–60·07) | 6·95 (1·89–25·59) | ||
| Lovebirds (Agapornis sp.) | 202 | 64 | 31·68 (25·27–38·10) | 3·56 (1·03–12·28) | ||
| Cockatiels (Nymphicus hollandicus) | 22 | 14 | 63·64 (43·54–83·74) | 13·42 (3·04–59·17) | ||
| Age | ⩽5 months | 105 | 48 | 45·71 (36·19–55·24) | 0·02 | Reference |
| 6–12 months | 100 | 28 | 28·00 (19·20–36·80) | 0·46 (0·26–0·83) | ||
| 13–18 months | 106 | 34 | 32·08 (23·19–40·96) | 0·56 (0·32–0·98) | ||
| Season | Spring | 139 | 49 | 35·25 (27·31–43·19) | 0·97 | Reference |
| Summer | 172 | 61 | 35·47 (28·32–42·62) | 1·01 (0·63–1·61) | ||
| Total | 311 | 110 | 35·37 (30·06–40·68) |
OR, Odds ratio; CI, confidence interval.
Nested PCR diagnosis and phylogenetic analysis based on ompA gene sequence
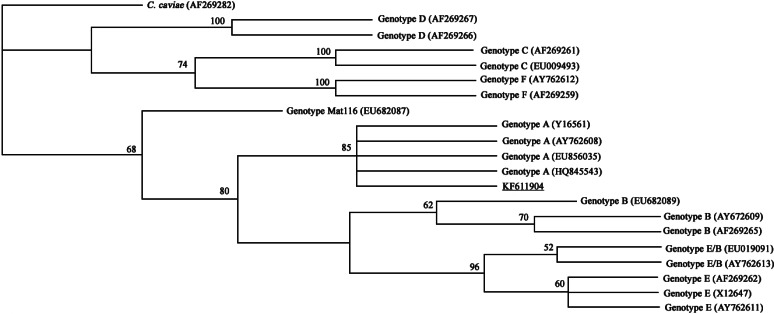
In the present study, 5/21 faecal samples were positive for chlamydial DNA in the diagnostic nested PCR, but only two samples were used for phylogenetic analyses because of the low DNA concentrations of the other three samples. The two samples had identical partial ompA gene sequences, and were 100% identical to the corresponding sequence of C. psittaci strain SP15 deposited in GenBank (accession no. EU856035.1). Comparison with relevant sequences available in GenBank indicated that the two samples represented C. psittaci genotype A (Fig. 2).
Fig. 2.
Maximum-likelihood phylogenetic analyses of Chlamydia psittaci based on the 1019 bp sequence of the ompA gene. The numbers at nodes indicate bootstrap values. The isolated C. psittaci is underlined and appears in the clade of genotype A.
DISCUSSION
Every year, about 70% of the parrots kept in China originate from Beijing and Weifang cities. These birds are traded throughout China as pets, but can transmit zoonotic pathogens to humans. However, this situation has been neglected by central and local governments in China, a situation which may heavily impact on public health, especially for veterinarians, bird breeders and fanciers. A previous study indicated that C. psittaci was transmitted from infected turkeys to veterinary scientists handling the animals [3]. Another study of C. psittaci prevalence in 39 breeding facilities indicated high prevalence of human infection in parrot owners and veterinarians working in breeding facilities [4]. Close and continued contact with infected birds may lead to outbreaks [25] and, in some cases, fatalities [6]. Therefore, in view of the potentially important role of parrots in transmission of Chlamydia, we investigated Chlamydia seroprevalence in budgerigars, lovebirds, cockatiels and Alexandrine parakeets in Beijing and Weifang cities, north China.
The overall chlamydial seroprevalence in parrots was 35·37%, which was higher than that observed previously, using the same serological test, in chickens (13·32%), pigeons (31·09%), and sparrows (10·54%) in China, but lower than in ducks (38·92%) [16, 26]. A semi-nested PCR, based on the ompA gene, has previously been used to investigate C. psittaci infection in pigeons in Brazil [17] and, in the present study, 5/21 faecal samples from parrots were PCR positive using this technique. This level is higher than that found in pigeons in The Netherlands [27], Switzerland (8·4%, 3·6%) [28, 29], Belgium (6·3%), and birds in Iran (12·6%) [30], but lower than in chickens in France (⩾90%) [31] and in feral Canada Geese in Belgium (58%) [32]. Comparisons between different studies can be difficult due to differences in environment, diagnostic methods used, feeding conditions, as well as animal husbandry practices and animal welfare.
The high Chlamydia seroprevalence in the four species of parrots, especially the presence of Chlamydiaceae in faeces, indicates that the birds may be a risk source of infection for humans. In China, unlike in Europe for example, people often take pet birds to the park and onto the street to show them to passers-by and other amateur keepers. In zoos, many bird species are kept together in the same cage in order to enhance the ornamental effects of the displays, often with a relatively high numbers of budgerigars. Parrots are aggressive birds and feathers and dry droppings could become airborne during bird fights and flight. Both of these situations could lead to Chlamydia transmission to humans through aerosol or direct contact.
The results of the present study clearly indicate that bird species is a crucial risk factor for Chlamydia infection in the examined parrots. In our model, cockatiels were the most susceptible to Chlamydia, followed by Alexandrine parakeets and lovebirds, with budgerigars being relatively resistant to the pathogen in each age group. The parrot populations we sampled remain largely discrete in markets and every species is present in each shop. We speculate that seroprevalence differences in the four species may be caused by the difference in their immune response. Further study should focus on examination of chlamydial species and their dynamics, and potential movement of the bacteria in bird species.
In agreement with the conclusion of Madani & Peighambari [30] who found no stastistically significant seasonal differences in the occurrence of avian chlamydiosis, our results show that the seropositivity of Chlamydia in parrots sampled in spring was not statistically different to that sampled in summer (P > 0·05). Chlamydia can be resistant to temperature variations from ~8°C in spring to ~25°C in summer in north China, and the statistically similar seropositivity in parrots in different cities suggests that the pathogen could be mainly transmitted by the direct contact route in birds, which is little influenced by environmental change.
We found support for differences in Chlamydia seroprevalence between sex of parrots, with females having a higher seroprevalence than males. This tendency is consistent with our previous study of Tibetan pigs [8], and also concurs with that observed in wild boars in Italy (females, 45·95%; males, 38·8%) [33] and Germany (females, 83·3%; males, 42·9%) [34]. Males and females have the same opportunity for exposure to Chlamydia in the environment, and in our model females are more sensitive to the pathogen than males for each species in each age group. Gender-related differences in Chlamydia seroprevalence were suspected to result from variation in immune response or antibody persistence rates between males and females.
In our study, age was the strongest risk factor to Chlamydia. For male parrots, Chlamydia seroprevalence in each species increased with age. However, for females, only budgerigars and Alexandrine parakeets displayed this tendency. Our results demonstrate that juveniles are more susceptible than adults and sub-adults. The juveniles are generally immunologically more naive than adults, thus leading to the highest seropositivity in younger birds. However, adults have substantially greater chlamydial seroprevalence than sub-adults in the present study, which may due to the cumulative Chlamydia exposure of older parrots through contact with the pathogen during long-term breeding. We also cannot exclude the possibility that the differences in seroprevalence between adults and sub-adults may be the result of long-term antibody persistence, which should be further studied.
The accurate diagnosis of Chlamydia infections is usually based on isolation of bacteria [35]. However, from clinical samples this is hampered by fastidious growth requirements, so is only of limited use in diagnostic laboratories. Although serological tests do not fully differentiate infections caused by various Chlamydia spp., the overall seroprevalence could help us learn about the prevalence of Chlamydia in the target host as a whole [8, 9, 25]. PCR amplification of the ompA gene combined with sequencing can provide a definitive diagnosis of psittacosis, and subsequent sequence analysis can identify the responsible genotype [28, 36]. Following our overall assessment of seroprevalence and genotype of Chlamydia infection in parrots, C. psittaci genotype A was shown to be shed in the faeces of parrots in the present study, which is in agreement with previous studies showing that C. psittaci genotype A was the major genotype associated with parrots [37, 38]. The partial ompA sequence of C. psittaci genotype A obtained in the present study was identical to that of the Chlamydiaceae strain isolated from bird faeces in Yunnan Province (GenBank accession no. EU856035), suggesting little variation of this prevalent genotype in China. This result further supports our hypothesis that parrots represent a potential risk for Chlamydia infection for humans.
The present study indicates that pet parrots pose a potential zoonotic risk for human infection with Chlamydiaceae through contact with fresh bird faeces. As the birds regularly live in homes, pet shops, bird fairs and markets, zoos and parks, the parrot owners, breeders, sellers, as well as veterinarians and tourists should be aware of the potential zoonotic risk and take appropriate precautions. In addition, integrated strategies and measures are necessary for the effective prevention and control of Chlamydia infection in parrots in China. Future surveys of Chlamydiaceae infection should include pet birds.
CONCLUSION
The results of the present study indicates high seroprevalence of Chlamydia in budgerigars, lovebirds, cockatiels and Alexandrine parakeets in China, and the seroprevalence is associated with the species, gender, age, season and collecting region of parrots. Determination of C. psittaci genotype A in the droppings of two pet parrots suggests potential contamination of the environment with Chlamydiaceae and may raise a public health concern.
ACKNOWLEDGEMENTS
Project support was provided by the Science Fund for Creative Research Groups of Gansu Province (grant no. 1210RJIA006). We thank Dr Alasdair Nisbet, Moredun Research Institute, Scotland, UK for improving the text and editing the English of this manuscript.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Beeckman DS, Vanrompay DC. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clinical Microbiology and Infection 2009; 15: 11–17. [DOI] [PubMed] [Google Scholar]
- 2.Rohde G, et al. Chlamydial zoonoses. Deutsches Ärzteblatt International 2010; 107: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Droogenbroeck C, et al. Simultaneous zoonotic transmission of Chlamydophila psittaci genotypes D, F and E/B to a veterinary scientist. Veterinary Microbiology 2009; 135: 78–81. [DOI] [PubMed] [Google Scholar]
- 4.Vanrompay D, et al. Chlamydophila psittaci transmission from pet birds to humans. Emerging Infectious Diseases 2007; 13: 1108–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deschuyffeleer TP, et al. Risk assessment and management of Chlamydia psittaci in poultry processing plants. Annals of Occupational Hygiene 2012; 56: 340–349. [DOI] [PubMed] [Google Scholar]
- 6.Petrovay F, Balla E. Two fatal cases of psittacosis caused by Chlamydophila psittaci. Journal of Medical Microbiology 2008; 57: 1296–1298. [DOI] [PubMed] [Google Scholar]
- 7.Boseret G, et al. Zoonoses in pet birds: review and perspectives. Veterinary Research 2013; 44: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang NZ, et al. First report of Chlamydiaceae seroprevalence in Tibetan pigs in Tibet, China. Vector-Borne and Zoonotic Diseases 2013; 13: 196–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou DH, et al. Seroprevalence of chlamydial infection in dairy cattle in Guangzhou, southern China. Irish Veterinary Journal 2013; 66: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu MJ, et al. Seroprevalence of Chlamydia infection in pigs from intensive farms in Southern China. Journal of Animal and Veterinary Advances 2010; 9: 1143–1145. [Google Scholar]
- 11.Wu SM, et al. Chlamydia felis exposure in companion dogs and cats in Lanzhou, China: a public health concern. BMC Veterinary Research 2013; 9: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haasnoot A, et al. Comparing two definitions of ethnicity for identifying young persons at risk for Chlamydia. Epidemiology and Infection 2012; 140: 951–958. [DOI] [PubMed] [Google Scholar]
- 13.Schmutz C, et al. Testing for Chlamydia trachomatis: time trends in positivity rates in the canton of Basel-Stadt, Switzerland. Epidemiology and Infection 2013; 141: 1953–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verweij SP, et al. Serogroup distribution of urogenital Chlamydia trachomatis in urban ethnic groups in The Netherlands. Epidemiology and Infection 2014; 142: 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price MJ, et al. Incidence of Chlamydia trachomatis infection in women in England: two methods of estimation. Epidemiology and Infection 2014; 142: 562–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cong W, et al. Seroprevalence of Chlamydia psittaci infection in market-sold adult chickens, ducks and pigeons in north-western China. Journal of Medical Microbiology 2013; 62: 1211–1214. [DOI] [PubMed] [Google Scholar]
- 17.de Lima VY, et al. Chlamydophila psittaci and Toxoplasma gondii infection in pigeons (Columba livia) from São Paulo State, Brazil. Veterinary Parasitology 2011; 175: 9–14. [DOI] [PubMed] [Google Scholar]
- 18.Buxton D, et al. Pathogenesis of Chlamydia psittaci infection in sheep: detection of the organism in a serial study of the lymph node. Journal of Comparative Pathology 1996; 114: 221–230. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann B, et al. Chlamydophila psittaci in Fulmars, the Faroe Islands. Emerging Infectious Diseases 2006; 12: 330–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everett KD, Bush RM, Andersen AA. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. International Journal of Systematic Bacteriology 1999; 49: 415–440. [DOI] [PubMed] [Google Scholar]
- 21.Thompson JD, et al. The Clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 1997; 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strimmer K, Haeseler AV. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Molecular Biology and Evolution 1996; 13: 964–969. [Google Scholar]
- 23.Swofford DL. Paup*: phylogenetic analysis using parsimony, version 4.0b10. Sinauer Associates, Sunderland 2002.
- 24.Zhao GH, et al. A specific PCR assay for the identification and differentiation of Schistosoma japonicum geographical isolates in mainland China based on analysis of mitochondrial genome sequences. Infection, Genetics and Evolution 2012; 12: 1027–1036. [DOI] [PubMed] [Google Scholar]
- 25.Smith KA, et al. Compendium of measures to control Chlamydophila psittaci (formerly Chlamydia psittaci) infection among humans (psittacosis) and pet birds, 2005. Journal of the American Veterinary Medical Association 2005; 226: 532–539. [DOI] [PubMed] [Google Scholar]
- 26.Cong W, et al. First report of Chlamydophila seroprevalence in house sparrows (Passer domesticus) in Lanzhou, Northwest China. African Journal of Microbiology Research 2012; 6: 5720–5722. [Google Scholar]
- 27.Heddema ER, et al. Prevalence of Chlamydophila psittaci in fecal droppings from feral pigeons in Amsterdam, The Netherlands. Applied and Environmental Microbiology 2006; 72: 4423–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geigenfeind I, Vanrompay D, Haag-Wackernagel D. Prevalence of Chlamydia psittaci in the feral pigeon population of Basel, Switzerland. Journal of Medical Microbiology 2012; 61: 261–265. [DOI] [PubMed] [Google Scholar]
- 29.Zweofel D, et al. Prevalence of Chlamydophila psittaci in wild birds-potential risk for domestic poultry, pet birds, and public health? European Journal of Wildlife Research 2009; 55: 575–581. [Google Scholar]
- 30.Madani SA, Peighambari SM. PCR-based diagnosis, molecular characterization and detection of atypical strains of avian Chlamydia psittaci in companion and wild birds. Avian Pathology 2013; 42: 38–44. [DOI] [PubMed] [Google Scholar]
- 31.Yin L, et al. Emerging Chlamydia psittaci infections in the chicken industry and pathology of Chlamydia psittaci genotype B and D strains in specific pathogen free chickens. Veterinary Microbiology 2013; 162: 740–749. [DOI] [PubMed] [Google Scholar]
- 32.Dickx V, et al. Prevalence and genotype distribution of Chlamydia psittaci in feral Canada geese (Branta canadensis) in Belgium. Vector Borne and Zoonotic Diseases 2013; 13: 382–384. [DOI] [PubMed] [Google Scholar]
- 33.Antonietta DF, et al. Seroepidemiologic survey for Chlamydia suis in wild boar (Sus scrofa) populations in Italy. Journal of Wildlife Diseases 2011; 47: 709–712. [DOI] [PubMed] [Google Scholar]
- 34.Helmut H, et al. Occurrence of Chlamydiaceae spp. in a wild boar (Sus scrofa L.) population in Thuringia (Germany). Veterinary Microbiology 2004; 103: 121–126. [DOI] [PubMed] [Google Scholar]
- 35.Everett KD. Chlamydia and Chlamydiales: more than meets the eye. Veterinary Microbiology 2000; 75: 109–126. [DOI] [PubMed] [Google Scholar]
- 36.Heddema ER, et al. Genotyping of Chlamydophila psittaci in human samples. Emerging Infectious Diseases 2006; 12: 1989–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sayada C, et al. Usefulness of omp1 restriction mapping for avian Chlamydia psittaci isolate differentiation. Research in Microbiology 1995; 146: 155–165. [DOI] [PubMed] [Google Scholar]
- 38.Vanrompay D, et al. Characterization of avian Chlamydia psittaci strains using omp1 restriction mapping and serovar-specific monoclonal antibodies. Research in Microbiology 1997; 148: 327–333. [DOI] [PubMed] [Google Scholar]