SUMMARY
The objective of this study was to measure the association between deprivation and incidence of 21 infectious diseases in the North East of England (2007–2011). We used count regression models with the Index of Multiple Deprivation and population/landscape data for small areas (~1500 persons). Deprivation significantly predicted incidence (P < 0·05) for 17 infectious diseases. The direction of association was broadly consistent within groups: increased incidence with increased deprivation for all three bloodborne viruses, 2/3 invasive bacterial diseases, 4/5 sexually transmitted infections (STI) and tuberculosis (TB); decreased incidence with increased deprivation for 5/6 infectious intestinal diseases (IID) and 2/3 vaccine-preventable diseases. Associations were removed for all but one IID (E. coli O157 infection) after accounting for recent foreign travel. Hepatitis C virus, TB and STI are priority infections for reduction of inequalities associated with deprivation in the North East of England.
Key words: Analysis of data, infectious disease epidemiology, modelling, prevention, public health
INTRODUCTION
The consideration of health inequalities is at the forefront of public health in the UK [1], where their reduction is part of the mission for public health [2]. Discussion of health inequalities is generally restricted to non-communicable diseases [2] despite the established link between decreasing socioeconomic status and increased risk of a multitude of diseases, including infectious diseases [3].
The dedication of resources to narrowing health gaps must be guided by a wider appreciation of the complex and diverse social and societal determinants of health [4]. For infectious diseases these determinants increase the risk of effective contact with the infectious agent either through an increased likelihood of exposure and/or an increased innate susceptibility to infection/progression to disease. Targets for intervention include those that act through behaviour, the proximate living environment, neighbourhood characteristics and individual-level predisposition (such as chronic diseases, mental health, nutrition) [3] as well as immunity through vaccination. Engaging in risky behaviour may be associated with a lack of individual empowerment for lower socioeconomic classes, the restriction of life choices and a tendency to have a more fatalistic view of health [3, 5].
For infectious diseases, a relatively deprived social environment can equate to an increased risk of infection in a variety of ways. Although this is not as well studied as it has been for non-communicable diseases, considerable evidence exists for an increased burden of certain infectious disease linked with (but not exclusively) reduced access to healthcare [6], overcrowding [7], social segregation [8] and poor or damp housing [9]. In addition, chronic diseases are often pre-disposing factors for infectious diseases and have a higher prevalence within disadvantaged populations [10] as do anxiety and depression [11, 12].
As social disadvantage and deprivation undoubtedly restrict life chances and life choices [3], behavioural risk factors are themselves, at least in part, linked to the social gradient by levels of risk associated with the social and structural environment [13]. A living environment with low social capital places an individual at increased risk of exposure to infections associated with behavioural risk [14]. Associations which have been found between deprivation and increased risk of hepatitis C virus (HCV) infection [15] and human immunodeficiency virus (HIV) infection [16] may represent functions of the social environment.
In order to understand the association between the living environment and levels of risk it is necessary to consider the causal pathway between living in a deprived neighbourhood and ill health [17]. For infectious disease the number of potential interventions may be considerable and highly specific to the epidemiology of individual infections or groups of infection. It is therefore logical to first assess the relationship between deprivation and infectious disease incidence within defined populations in order to determine which infections contribute to inequalities and subsequently identify clues to aetiology and potential targets for intervention.
The North East of England has a population of about 2·6 million persons and is largely ethnically homogeneous; 95% of the population reporting their ethnicity as white at the 2011 census [18]. The North East has a disproportionate level of deprivation: 33% of small area populations (average 1500 persons) are within the most deprived national quintile, the highest percentage of any region in England [19]. Here we have used count regression models to estimate the direction and size of inequalities associated with levels of deprivation and the 5-year (2007–2011) incidence of 21 different infectious diseases for small area populations in the North East of England.
METHODS
Infectious disease data
Case data was available for 21 different laboratory-confirmed infections with an annual incidence >1 case/100 000 population and with geographical coding for individual cases available at postcode level. Cases with specimen dates between 1 January 2007 and 31 December 2011 were extracted from the Public Health England Centre North East surveillance system for infections of public health significance or the Genitourinary Medicine Clinic Activity Dataset (GUMCAD) (Table 1). Cases from the North East surveillance system were linked to population data by postcode of residence using the Office for National Statistics (ONS) Postcode Directory (2011 edition) (all ONS data available from www.ons.gov.uk). Data from GUMCAD contains geographical coding and no additional linkage was necessary. For data from the North East surveillance system, cases were removed where no postcode of residence was available (prisons and those cases where clinics, testing laboratories and health service centres were provided in lieu of a residential address), although for most infections these proportions were known to be small.
Table 1.
Infectious diseases and datasets used to measure the association between incidence and deprivation in the North East of England, 2007–2011
| Group | Dataset* | Cases (n) | Annual rate/10 000 persons∥ (95% CI#) | Note | ||||
|---|---|---|---|---|---|---|---|---|
| All | No address/out of area† | Prison/health service | Foreign travel-associated‡ | Included in models§ (%¶) | ||||
| BBV | HBV infection | 914 | 11 | 127 | 11 | 776 (84·9) | 0·59 (0·50–0·70) | New diagnoses (acute and chronic) |
| HCV infection | 1560 | 10 | 490 | 0 | 1060 (67·9) | 0·81 (0·71–0·93) | New diagnoses (acute and chronic) | |
| HIV infection | 254 | 48 | — | — | 206 (81·1) | 0·20 (0·15–0·26) | New diagnoses | |
| IBD | Invasive GAS infection | 271 | 2 | 0 | 2 | 269 (99·3) | 0·20 (0·15–0·27) | |
| Invasive meningococcal infection | 364 | 2 | 0 | 1 | 362 (99·5) | 0·28 (0·22–0·35) | ||
| Invasive pneumococcal infection | 1294 | 2 | 0 | 2 | 1292 (99·8) | 0·99 (0·87–1·12) | Conjunctivitis excluded | |
| IID | Campylobacteriosis | 15112 | 32 | 13 | 265 | 15067(99·7) | 11·56 (11·15–12·00) | |
| Cryptosporidiosis | 1096 | 0 | 1 | 247 | 1095 (99·9) | 0·84 (0·73–0·96) | ||
| E. coli O157 infection | 341 | 0 | 1 | 52 | 340 (99·7) | 0·26 (0·20–0·33) | ||
| Giardiasis | 191 | 0 | 0 | 69 | 191 (100·0) | 0·15 (0·10–0·20) | ||
| Salmonellosis | 2177 | 8 | 0 | 632 | 2169 (99·6) | 1·66 (1·51–1·83) | ||
| Shigellosis | 216 | 0 | 0 | 86 | 216 (100·0) | 0·16 (0·12–0·22) | ||
| STI | Chlamydia infection | 31754 | 4763 | — | — | 26991(85·0) | 25·89 (25·27–26·51) | Does not include NCSP data |
| Genital herpes | 4945 | 750 | — | — | 4195 (84·8) | 4·02 (3·78–4·28) | First episodes only | |
| Genital warts | 18465 | 2700 | — | — | 15765(85·4) | 15·12 (14·65–15·60) | First episodes only | |
| Gonorrhoea | 3506 | 586 | — | — | 2920 (83·3) | 2·80 (2·60–3·01) | ||
| Syphilis | 458 | 99 | — | — | 359 (78·4) | 0·35 (0·28–0·42) | Diagnosis in IDU excluded | |
| TB | Tuberculosis | 500 | 3 | 4 | 1 | 493 (98·6) | 0·38 (0·31–0·46) | |
| VPD | Measles | 192 | 0 | 0 | 2 | 192 (100·0) | 0·15 (0·10–0·20) | |
| Mumps | 1011 | 6 | 2 | 0 | 1003 (99·2) | 0·77 (0·66–0·88) | ||
| Pertussis | 248 | 0 | 0 | 0 | 248 (100·0) | 0·19 (0·14–0·25) | ||
BBV, Bloodborne viruses; GAS, group A streptococcal; HBV, hepatitis B virus; HCV, hepatitis C virus; IBD, invasive bacterial diseases; IDU, infectious disease unit; IID, infectious intestinal diseases; NCSP, National Chlamydia Surveillance Programme; STI, sexually transmitted infections; TB, tuberculosis; VPD, vaccine-preventable diseases.
Datasets are for the North East of England taken from either the Public Health England Centre North East surveillance system for laboratory-confirmed infections of public health significance or from the Genitourinary Medicine Clinic Activity Database (HIV and STI datasets) with specimen dates 1 January 2007–31 December 2011.
Cases with either a missing residential address or a reported residential address outside the North East.
For IID a separate dataset was modelled after removal of recent foreign travel-associated cases.
All cases minus those with no address/out of area and prison/health service.
Percentage of the raw dataset.
Of the modelled dataset.
95% exact Poisson confidence interval.
Given that substantial numbers of cases of infectious intestinal disease (IID) in England are associated with recent foreign travel, separate datasets were used with and without those cases where recent foreign travel was reported (all IID except campylobacteriosis, where data on recent foreign travel is not routinely recorded).
Small area data
Lower super output areas (LSOA) are small statistically bounded areas with a national average population of 1500 persons. Populations for LSOA were stratified by age group (0–15, 16–29, 30–44, 45–64, ⩾65 years) using ONS mid-2010 population estimates. According to this data, the 1656 LSOA for the North East have an average total population size of 1774 persons (range 468–7031). The area of each LSOA (in km2) was calculated from area data freely available from ONS.
The ethnic diversity of each LSOA was coded as an ordinal variable based on the number of different ethnic groups (range 0–5) each making up ⩾5% of the LSOA population from the 2011 census. In addition, minority ethnic groups were defined as ethnic groups which alone made up ⩾15% of the population of a LSOA. This definition was chosen so that no LSOA in the North East had more than one minority ethnic group; 24 LSOA contained a minority ethnic group: Bangladeshi (8), Chinese (2), and Pakistani (14).
The morphology of each LSOA was designated as urban, town and fringe, village or hamlet and isolated dwellings according to rural and urban area classification (RUAC) [20].
Index of Multiple Deprivation (IMD)
North East quintiles (Q1–Q5) of the 2010 Index of Multiple Deprivation [21] were used for all modelling: (Q1) 1·74–10·96; (Q2) 10·97–19·60; (Q3) 19·61–29·66; (Q4) 29·67–42·27; (Q5) 42·28–80·51.
Count regression modelling
Each infectious disease was modelled separately using the count of cases by LSOA as outcome and with the following explanatory variables: population sizes (1000 persons; continuous variable) for five age groups (0–15, 16–29, 30–44, 45–64, ⩾65 years), area of LSOA in km2 (quartiles; categorical variable, reference group smallest area), RUAC (categorical variable, reference group urban), minority ethnic group (if present; categorical variable, reference group no minority group), ethnic diversity (categorical variable, reference group no ethnic diversity) and IMD quintile (categorical, reference group least deprived quintile). The natural logarithm of the all-persons mid-2010 population size for each LSOA was entered into the model as an offset. A robust cluster variance estimator was used by clustering on the 12 local authority (LA) areas of the North East of England (median 122 LSOA per LA, range 58–320).
For each dataset the best-fitting model was selected according to a hierarchical approach: (1) fit of a Poisson model using the deviance goodness-of-fit test; (2) fit of a zero-inflated Poisson (ZIP) model assessed by the Vuong test [22] and Akaike's Information Criteria (AIC) relative to the Poisson model; (3) fit of a negative binomial model using a boundary likelihood ratio test (LRT) for improvement on the Poisson model and AIC for improvement upon ZIP; (4) fit of a zero-inflated negative binomial (ZINB) model assessed by the Vuong test relative to the negative binomial model and Akaike's Information Criterion (AIC) relative to the best fitting of the best-fitting model from (1)–(3). A reduction in AIC of >2·5 was considered as a significant improvement in fit [23]. Rejection of the more parsimonious Poisson and negative binomial models by their zero-inflated equivalents required a significant Vuong test (P < 0·05), irrespective of AIC scores. Where the goodness of fit for a Poisson model was poor (P < 0·001) the negative binomial model was selected by comparison of AIC score with the Poisson model without consideration of the boundary LRT.
The assumption of linearity for each age group population size was assessed for evidence of a monotonic relationship with outcome for adjusted quartiles of each variable for each final model [24]. The stability of coefficients of all predictors to the removal of outlying counts was assessed for each final model. Deviance (Poisson and negative binomial models) [23] or Pearson (ZIP and ZINB models) [25] residuals from each final model were visually assessed for fit to a normal distribution. Model coefficients were transformed to incidence rate ratios (IRR) and associations with deprivation measured using IRR for each IMD quintile using a significance of P < 0·05 for coefficient z scores. All statistical analysis was performed using Stata v. 11·0 (StataCorp LP, USA).
RESULTS
Model selection
For 13 of the 21 datasets, the negative binomial was selected as the best-fitting model (Table 2). Of the remaining eight datasets, three were modelled using a Poisson and five using a ZIP model. For IID datasets (except campylobacteriosis), removal of recent foreign travel-associated cases resulted in selection of a different model for two datasets (Supplementary Table S1). Bimodality of residuals for the best-fitting model was evident for 12 datasets (Table 2).
Table 2.
Count regression model selection for the association between deprivation and incidence of infectious diseases in the North East of England, 2007–2011
| Group | Dataset | Mean count (variance) | Zero counts (%) | Poisson model | ZIP model | Negative binomial model | ZINB model | Final model | Residuals‡ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GOF | AIC | Vuong | AIC | ΔAIC* | LRT | AIC | Ref.† | Δ AIC* | Vuong | AIC | Ref.† | ΔAIC* | ||||||
| BBV | HBV infection | 0·5 (1·20) | 73·13 | 0·5974 | 2688·15 | 0·0129 | 2646·69 | 41·46 | <0·0001 | 2640·09 | ZIP | 6·5950 | 0·2645 | 2609·86 | NB | 30·2310 | NB | Bimodal |
| HCV infection | 0·6 (1·78) | 65·70 | <0·0001 | 3297·41 | 0·0001 | 3208·84 | 88·57 | <0·0001 | 3163·59 | ZIP | 45·2500 | Did not converge | NB | Bimodal | ||||
| HIV infection | 0·1 (0·17) | 89·79 | 1·0000 | 1221·36 | 0·0359 | 1209·11 | 12·25 | 0·0090 | 1215·70 | ZIP | −6·5900 | <0·0001 | 1209·11 | ZIP | 0·0000 | ZIP | Normal | |
| IBD | Invasive GAS infection | 0·2 (0·16) | 85·08 | 1·0000 | 1547·07 | 0·2220 | 1546·04 | 1·03 | 0·5000 | 1547·07 | Poisson | 0·0000 | n.a. | 1546·04 | Poisson | 1·0290 | Poisson | Bimodal |
| Invasive meningococcal infection | 0·2 (0·2) | 80·92 | 1·0000 | 1833·24 | 0·4435 | 1833·14 | 0·10 | 0·2410 | 1897·69 | Poisson | −64·4500 | Did not converge | Poisson | Bimodal | ||||
| Invasive pneumococcal infection | 0·8 (0·88) | 47·77 | 0·0004 | 3824·18 | 0·2869 | 3820·83 | 3·35 | 0·1130 | 3820·71 | Poisson | 3·4660 | 0·5002 | 3822·71 | NB | −2·0000 | NB | Bimodal | |
| IID | Campylobacteriosis | 9·1 (16·90) | 0·06 | <0·0001 | 8968·73 | 0·5015 | 8970·73 | −2·00 | <0·0001 | 8851·94 | Poisson | 116·7970 | 0·6873 | 8853·94 | NB | −2·0000 | NB | Normal |
| Cryptosporidiosis | 0·6 (0·93) | 57·67 | <0·0001 | 3701·46 | 0·0004 | 3643·18 | 58·27 | <0·0001 | 3635·16 | ZIP | 8·0190 | Did not converge | NB | Bimodal | ||||
| E. coli O157 infection | 0·2 (0·31) | 84·72 | 1·0000 | 1849·14 | 0·0002 | 1769·68 | 79·45 | <0·0001 | 1772·10 | ZIP | −2·4170 | 0·1860 | 1767·69 | ZIP | 1·9950 | ZIP | Normal | |
| Giardiasis | 0·1 (0·13) | 89·79 | 1·0000 | 1181·32 | 0·2797 | 1179·32 | 2·01 | 0·0750 | 1179·25 | Poisson | 2·0710 | Did not converge | Poisson | Bimodal | ||||
| Salmonellosis | 1·3 (1·68) | 30·56 | <0·0001 | 4954·11 | 0·0639 | 4944·17 | 9·94 | <0·0001 | 4932·55 | Poisson | 21·5590 | 0·3544 | 4931·58 | NB | 0·9740 | NB | Bimodal | |
| Shigellosis | 0·1 (0·17) | 89·19 | 1·0000 | 1322·53 | 0·0375 | 1303·02 | 19·51 | <0·0001 | 1304·63 | ZIP | −1·6160 | <0·0001 | 1303·02 | ZIP | 0·0000 | ZIP | Normal | |
| STI | Chlamydia infection | 16·3 (114·75) | 0·12 | <0·0001 | 12286·15 | 0·2136 | 12402·45 | −116·30 | <0·0001 | 10794·84 | Poisson | 1491·3100 | 0·5013 | 10796·84 | NB | −2·0000 | NB | Normal |
| Genital herpes | 2·5 (5·53) | 14·67 | <0·0001 | 6519·80 | 0·0004 | 6463·26 | 56·54 | <0·0001 | 6380·17 | ZIP | 83·0880 | 0·1768 | 6378·25 | NB | 1·9220 | NB | Bimodal | |
| Genital warts | 9·5 (37·40) | 0·12 | <0·0001 | 9719·94 | 0·1753 | 9712·69 | 7·25 | <0·0001 | 9208·55 | Poisson | 511·3900 | 0·5019 | 9210·55 | NB | −2·0000 | NB | Normal | |
| Gonorrhoea | 1·8 (3·88) | 28·44 | <0·0001 | 5515·32 | 0·0006 | 5463·17 | 52·15 | <0·0001 | 5436·05 | ZIP | 27·1220 | 0·1525 | 5432·93 | NB | 3·1230 | NB | Bimodal | |
| Syphilis | 0·2 (0·31) | 83·64 | 1·0000 | 1820·41 | 0·0033 | 1782·72 | 37·69 | <0·0001 | 1790·35 | ZIP | −7·6330 | Did not converge | ZIP | Normal | ||||
| TB | Tuberculosis | 0·3 (0·68) | 80·13 | 1·0000 | 1925·97 | 0·0974 | 1918·59 | 7·38 | 0·0030 | 1918·35 | Poisson | 7·6240 | 0·2148 | 1975·60 | NB | −57·2530 | NB | Bimodal |
| VPD | Measles | 0·2 (0·35) | 93·78 | 1·0000 | 1272·75 | Did not converge | <0·0001 | 997·65 | Poisson | 275·0984 | 0·2136 | 994·67 | NB | 2·9823 | NB | Normal | ||
| Mumps | 0·6 (1·36) | 59·78 | 0·0006 | 3351·60 | 0·0146 | 3327·67 | 23·93 | <0·0001 | 3323·82 | ZIP | 3·8510 | 0·1771 | 3319·82 | NB | 4·0010 | NB | Bimodal | |
| Pertussis | 0·1 (0·20) | 87·98 | 1·0000 | 1508·98 | 0·0042 | 1464·64 | 44·34 | <0·0001 | 1462·52 | ZIP | 2·1230 | 0·4800 | 1462·50 | ZIP | 2·1360 | ZIP | Normal | |
AIC, Akaike's Information Criterion; BBV, bloodborne viruses; GAS, group A streptococcal; GOF, goodness of fit; HBV, hepatitis B virus; HCV, hepatitis C virus; IBD, invasive bacterial diseases; IID, infectious intestinal infections; n.a., not available; LRT, likelihood ratio test; NB, negative binomial; STI, sexually-transmitted infections; TB, tuberculosis; VPD, vaccine-preventable diseases; ZINB, zero-inflated negative binomial; ZIP, zero-inflated Poisson.
Difference in AIC between the model of interest and the reference model.
Reference model for testing.
Distribution of residuals for the final model assessed by eye.
Associations with deprivation
After adjusting for demographic and area factors, deprivation remained a significant predictor of incidence in at least one quintile for all but four of the infectious diseases (Table 3, Supplementary Fig. S1). With the exception of measles, shigellosis and giardiasis, associations reflect a monotonic trend with deprivation: incidence rates either decrease or increase consistently with increasing deprivation. The direction of this association was broadly determined by the nature of the infection: bloodborne viruses (BBV), invasive bacterial diseases (IBD) (with the exception of invasive group A streptococcal (GAS) infection), sexually transmitted infections (STI) (with the exception of genital herpes) and tuberculosis (TB) all have positive associations with deprivation whereas IID (except salmonellosis) have a negative association with deprivation. The association of increased rates of IID with less deprived areas was no longer evident after removal of cases associated with recent foreign travel for all datasets other than E. coli O157 infection (Supplementary Fig. S1, Supplementary Table S2).
Table 3.
Associations between deprivation and the incidence of infectious diseases for lower super output areas in the North East of England, 2007–2011
| Group | Dataset | Model | IMD regional quintile* | No. of significantly associated quintiles† | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||||||||||||
| IRR | IRR | 95% CI | P value | IRR | 95% CI | P value | IRR | 95% CI | P value | IRR | 95% CI | P value | ||||
| BBV | HBV infection | NB | 1·00 | 1·16 | 0·70–1·90 | 0·569 | 1·10 | 0·78–1·54 | 0·598 | 1·21 | 0·79–1·85 | 0·373 | 2·56 | 1·62–4·06 | <0·001 | +1 |
| HCV infection | NB | 1·00 | 1·76 | 1·22–2·54 | 0·002 | 2·94 | 2·05–4·20 | <0·001 | 4·36 | 2·63–7·21 | <0·001 | 9·43 | 6·62–13·43 | <0·001 | +4 | |
| HIV infection | ZIP | 1·00 | 1·06 | 0·58–1·93 | 0·857 | 1·35 | 0·87–2·09 | 0·182 | 1·89 | 0·98–3·66 | 0·059 | 2·17 | 1·15–4·11 | 0·017 | +1 | |
| IBD | Invasive GAS infection | Poisson | 1·00 | 0·74 | 0·47–1·16 | 0·183 | 1·07 | 0·79–1·46 | 0·665 | 1·07 | 0·74–1·53 | 0·725 | 1·14 | 0·66–1·97 | 0·648 | 0 |
| Invasive meningococcal infection | Poisson | 1·00 | 0·92 | 0·51–1·66 | 0·777 | 1·52 | 1·06–2·16 | 0·021 | 2·28 | 1·36–3·81 | 0·002 | 2·46 | 1·44–4·17 | 0·001 | +3 | |
| Invasive pneumococcal infection | NB | 1·00 | 1·22 | 0·99–1·48 | 0·052 | 1·36 | 1·13–1·63 | 0·001 | 1·60 | 1·40–1·82 | <0·001 | 2·03 | 1·61–2·55 | <0·001 | +3 | |
| IID‡ | Campylobacteriosis | NB | 1·00 | 0·95 | 0·90–1·01 | 0·091 | 0·91 | 0·84–0·98 | 0·015 | 0·86 | 0·78–0·94 | 0·001 | 0·77 | 0·68–0·87 | <0·001 | −3 |
| Cryptosporidiosis | NB | 1·00 | 0·99 | 0·81–1·21 | 0·915 | 0·94 | 0·79–1·12 | 0·514 | 0·96 | 0·72–1·27 | 0·772 | 0·76 | 0·61–0·93 | 0·010 | −1 | |
| E. coli O157 infection | ZIP | 1·00 | 0·87 | 0·64–1·20 | 0·396 | 1·03 | 0·75–1·42 | 0·845 | 0·51 | 0·30–0·87 | 0·014 | 0·69 | 0·48–0·98 | 0·041 | −2 | |
| Giardiasis | Poisson | 1·00 | 1·04 | 0·74–1·46 | 0·807 | 0·67 | 0·40–1·10 | 0·112 | 0·46 | 0·29–0·74 | <0·001 | 0·73 | 0·46–1·14 | 0·167 | −1 | |
| Salmonellosis | NB | 1·00 | 1·12 | 0·97–1·30 | 0·124 | 1·06 | 0·92–1·22 | 0·409 | 1·03 | 0·91–1·16 | 0·649 | 0·98 | 0·91–1·16 | 0·730 | 0 | |
| Shigellosis | ZIP | 1·00 | 0·62 | 0·40–0·96 | 0·033 | 0·63 | 0·43–0·94 | 0·023 | 0·99 | 0·69–1·43 | 0·964 | 0·33 | 0·22–0·51 | <0·001 | −3 | |
| STI | Chlamydia infection | NB | 1·00 | 1·20 | 1·13–1·28 | <0·001 | 1·44 | 1·34–1·55 | <0·001 | 1·64 | 1·50–1·79 | <0·001 | 1·79 | 1·58–2·02 | <0·001 | +4 |
| Genital herpes | NB | 1·00 | 1·10 | 1·02–1·18 | 0·632 | 1·19 | 1·12–1·28 | 0·425 | 1·39 | 1·28–1·52 | 0·484 | 1·40 | 1·26–1·55 | 0·485 | 0 | |
| Genital warts | NB | 1·00 | 0·97 | 0·86–1·09 | 0·014 | 1·08 | 0·90–1·29 | <0·001 | 1·06 | 0·89–1·27 | <0·001 | 1·07 | 0·89–1·27 | <0·001 | +4 | |
| Gonorrhoea | NB | 1·00 | 1·20 | 1·04–1·38 | 0·009 | 1·57 | 1·30–1·90 | <0·001 | 1·83 | 1·58–2·13 | <0·001 | 2·61 | 2·28–2·99 | <0·001 | +4 | |
| Syphilis | ZIP | 1·00 | 2·01 | 1·47–2·76 | <0·001 | 2·01 | 1·49–2·72 | <0·001 | 2·40 | 1·44–3·99 | 0·001 | 3·83 | 2·46–5·96 | <0·001 | +4 | |
| TB | Tuberculosis | NB | 1·00 | 1·13 | 0·78–1·65 | 0·516 | 1·63 | 1·16–2·29 | 0·005 | 1·96 | 1·23–3·12 | 0·005 | 3·87 | 2·78–5·38 | <0·001 | +3 |
| VPD | Measles | NB | 1·00 | 0·35 | 0·15–0·85 | 0·021 | 0·99 | 0·64–1·52 | 0·953 | 1·08 | 0·53–2·19 | 0·825 | 0·81 | 0·30–2·14 | 0·665 | −1 |
| Mumps | NB | 1·00 | 1·00 | 0·87–1·14 | 0·975 | 0·89 | 0·68–1·15 | 0·367 | 0·82 | 0·62–1·08 | 0·164 | 0·58 | 0·43–0·79 | <0·001 | −1 | |
| Pertussis | ZIP | 1·00 | 0·87 | 0·59–1·29 | 0·482 | 0·71 | 0·44–1·13 | 0·147 | 0·96 | 0·70–1·32 | 0·818 | 0·81 | 0·50–1·33 | 0·403 | 0 | |
BBV, Bloodborne viruses; CI, confidence interval; GAS, group A streptococcal; HBV, hepatitis B virus; HCV, hepatitis C virus; IBD, invasive bacterial diseases; IID, infectious intestinal infections; IMD, Index of Multiple Deprivation; IRR, incidence rate ratio; NB, negative binomial; STI, sexually-transmitted infections; VPD, vaccine-preventable diseases; ZIP, zero-inflated Poisson.
IRR for North East quintiles of the 2010 IMD have been adjusted for age distribution, size of lower super output area, urban/rural classification, presence of minority ethnic groups and population heterogeneity. IMD quintile 1 (Q1) is the least deprived quintile and quintile 5 (Q5) the most deprived quintile; values in bold represent significant associations (P < 0·05).
The direction of the association relative to the least deprived quintile (Q1): +, quintiles associated with increased rates of infection, –, quintiles associated with decreased rates of infection.
Includes travel-associated cases.
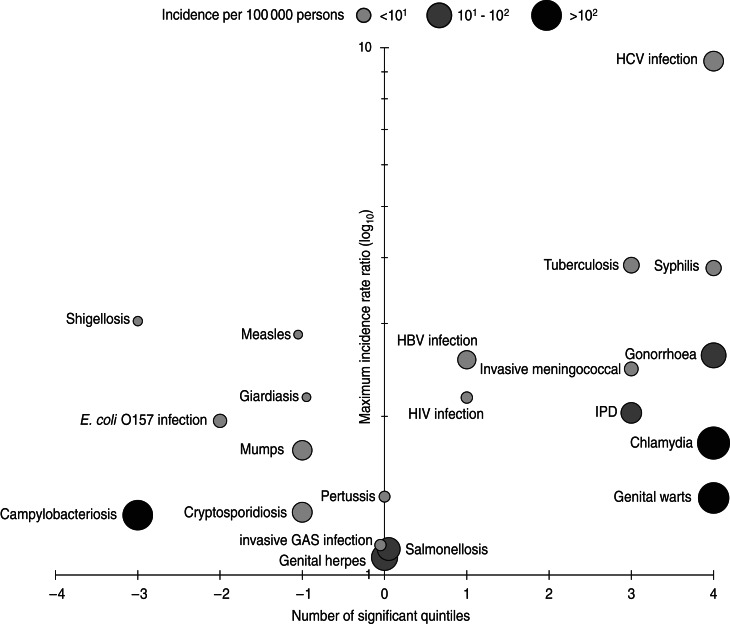
The association of incidence rate with deprivation was assessed in terms of direction (either a positive or negative association), scope (the number of quintiles for which a significant association was found) and magnitude (the highest IRR for positive associations and the reciprocal of the lowest IRR for negative associations). Together, these components were used to produce a graphical representation of inequalities associated with each infection (Fig. 1). STI (except genital herpes) exhibit clear increases in incidence associated with deprivation; this is particularly marked for chlamydia and genital warts as these infections have the highest incidence of any infection in this study. HCV infection, TB and syphilis have the greatest magnitude of any association (Table 3, Fig. 1). This is particularly evident for HCV infection where the IRR for Q5 is considerably higher than any other infection and the gradient across all five quintiles steeper than those other infections positively associated with deprivation (perhaps with the exception of TB which rises sharply across Q3–Q5). Syphilis, although associated with significantly increased rates for Q2–Q5, has a moderate gradient compared to HCV infection.
Fig. 1.
Associations between deprivation and the incidence of infectious diseases in the North East of England, 2007–2011. The number of significant quintiles indicates the number of Index of Multiple Deprivation 2010 quintiles significantly associated (P < 0·05) with an increased incidence rate (1–4) or a decreased incidence rate (–1 to–4) after adjusting for age distribution, size of lower super output area, urban/rural classification, presence of minority ethnic groups and ethnic diversity in a multivariable count regression model. The size and shading of each data point reflects the incidence of each infection.
Of those infections with a negative association with deprivation (Fig. 1), campylobacteriosis stands out in having both a high incidence and scope, although we have been unable to perform a subgroup analysis after removal of recent foreign travel-associated cases for this infection. After removal of recent foreign travel-associated cases from the cryptosporidiosis, giardiasis and shigellosis datasets the significant negative association between deprivation and incidence is no longer present (Supplementary Table S2). Measles and mumps have significantly higher rates of infection in less deprived areas but with relatively low incidence rates and the magnitudes of these inequalities are slight when compared to those on the right hand side of the central axis (Fig. 1).
DISCUSSION
This study was undertaken to further understand how the living environment influences the incidence of infectious diseases for disadvantaged populations in the North East of England. The focus of this study is a contextual one; based upon the areas where people live and the associations between characteristics of that environment and the health of the population who make their homes there [26]. Inferences drawn from this approach are for populations, rather than individuals. Clearly, considerable inequalities exist in the North East of England across a range of infectious diseases.
BBV
The association between HCV infection and deprivation stands out in this study as representing a substantial inequality which, as it likely reflects the distribution of people who inject drugs, i.e. the largest risk group for HCV infection in England [27], may be modifiable. In turn, the lack of substantial inequalities for HIV and HBV infection may reflect the low prevalence of these infections in the North East of England [28, 29]. Whether or not case ascertainment rates for BBV are associated with deprivation due to targeted testing of high-risk populations requires further study.
IBD
The association between increased deprivation and increased incidence for IBD found here supports previous studies of invasive pneumococcal disease (IPD) and meningococcal disease in England [30–33]. The social environment has been linked to the risk of IPD [30] and other potentially invasive bacteria through risk associated with overcrowding [7], malnutrition [34] and first-hand [35] or second-hand [36] exposure to cigarette smoke.
For invasive GAS infection, it may be that following improvements in housing and hygiene, factors which have in the past been thought to contribute to a higher burden of GAS infection [37], these are no longer contributing risk factors for invasive GAS infection in the North East of England. It may also be that the dynamics of carriage and invasive infection differ for invasive GAS infection compared to IMD and IPD (where a clear association between incidence and deprivation exists).
IID
We found no significant association between deprivation and autochthonous IID for all datasets except E. coli O157 infection. The association between decreased rates of E. coli O157 infection with increasing deprivation after the removal of recent foreign travel-associated cases may reflect a different pattern of exposures compared to the other IID and/or a case ascertainment bias. For salmonellosis, analysis at the genus-level may have masked associations with deprivation beyond this level [38, 39].
The negative associations between total incidence of IID and increased deprivation supports evidence from other studies for campylobacteriosis [39, 40], cryptosporidiosis [41], giardiasis [41] and shigellosis [39]. However, after removal of recent foreign travel-associated cases (for IID other than campylobacteriosis) there were no associations with deprivation other than for E. coli O157 infection. The inference here is that associations between IID and deprivation reported in previous studies may have been confounded by foreign travel. It seems plausible that foreign travel, and the associated exposure to the causative agents of IID, is more common for those living in areas of low deprivation. Details of recent foreign travel were not available for most campylobacteriosis cases; however, given the high numbers of expected travel-associated cases [42] it would be surprising should the negative associations for this infection not be also be reduced after removal of recent foreign travel-associated cases.
Rates of presentation to primary care for IID have been shown to be associated with increased deprivation in England [43] and the USA [44]. The negative relationship between deprivation and rates of total IID found here suggests that other variation, in either or both of the incidence of laboratory confirmable disease or of laboratory testing rates, is also associated with deprivation, and outweighs the higher rates of presentation expected with increased deprivation. Elucidation of such factors would clarify the relationships between IID and deprivation.
STI
STI represent a clear target for reducing infectious disease inequalities in the North East of England and form a substantial burden (particularly chlamydia and genital warts). Our findings are largely consistent with evidence from other settings for chlamydia [45, 46], genital herpes [45], genital warts [45], gonorrhoea [45, 47, 48] and syphilis [49]. Although the complexity of risk for STI is great, the breakdown in social networks within communities (and the associated loss of social capital) can result in a reduction in trust, support and adherence to social norms. Such losses in social cohesion can then lead to increased risk of exposure for the population to the infectious agents causing STI [50]. Strengthening social networks within deprived areas of the North East of England could be considered as a potential structural target for prevention of STI [14].
TB
TB diagnoses are clearly associated with deprivation in the North East of England, fitting with previous national [51] and global studies [52]. The North East of England has the lowest rate of any region in England for diagnosed TB cases assessed to be as a result of a recent transmission event (~10%) [53] and a higher proportion of older cases (22% aged ⩾65 years for this study) compared to England as a whole (14% aged ⩾65 years for 2011) [53]. Hence many of the cases in this study are as a result of exposure within the UK at some (unknown) time in the past. Ideally, the date of effective exposure would have been associated with a contemporaneous deprivation index to examine the association between deprivation and rates of infection, but this was not possible. However, current levels of deprivation for the living environment may also have an association with the risk of an active infection (i.e. one which is diagnosed for the first time). Furthermore, any intervention strategies need also to consider extant transmission networks and exposure locations for future diagnosed cases. These associations could be studied by stratification of cases by ethnic group, but this would best be undertaken in a high-incidence area.
Vaccine-preventable diseases
We found limited evidence of inequalities associated with measles and mumps. The incidence of cases of measles, mumps and pertussis since the introduction of childhood vaccination is obviously closely linked to vaccine coverage and infection rates may be difficult to study over relatively short time periods due to the cyclical nature of their epidemics [54]. Vaccine coverage may [55, 56] or may not [57] also reflect the social gradient and disentangling all of these processes to understand the contribution of deprivation to contemporary rates of infection may be particular to different settings and populations. Although we included only laboratory-confirmed cases of vaccine-preventable disease the proportion of notified cases which are laboratory confirmed does not appear to change substantially with IMD quintile for measles, mumps or pertussis (results not shown) and suggests that case ascertainment rates (through laboratory confirmation) are not confounding the association between incidence and deprivation.
Small areas where large mumps outbreaks associated with higher education institutions had occurred appeared as outliers within the mumps dataset and removal of these outlying data points had no substantial impact on associations with deprivation (results not shown). Our data does not include the recent increase in pertussis cases across England and it is unknown whether this national increase may be associated with deprivation.
Limitations
In measuring the association between infectious disease incidence and deprivation we have attempted to adjust for confounding variables as much as was realistically possible given data availability at a small area level. Although we were able to adjust for potential confounding by age distribution, population size, population density, ethnicity and rurality, residual confounding suggests that for some infections unknown predictors of incidence remain after adjustment for these factors. When taking a broad, population-level approach to such a complex issue, adjustment for very specific explanatory variables which may be strong predictors of incidence for specific infections or groups of infections is not always possible. However, the consistency of analytical methodology here can be seen as a strength of the study: no inherent bias exists in our attempt to understand the effects of deprivation on any one particular infection or group of infections (other than perhaps the removal of recent foreign travel-associated cases from IID). We plan to use multi-level models in further studies in order to explore the nature of residual confounding at the small area level by building further contextual factors into models [58].
We used a single indicator of deprivation (albeit one which is composed of many closely related domains, chosen to reflect varying forms of disadvantage) which provides no insight into which specific factors are associated with the pathway between exposure and infection. This measure also limits direct discussion of causality to residential areas, within which we have implicitly assumed that health outcomes are determined. Although such assumptions are required for population studies of this nature, individual-level risk is also derived from exposures and risk factors which exist in other settings, such as the workplace, which can only realistically be captured using an individual-level approach.
According to the 2011 census, the population of the North East has increased by around 20 000 persons since 2001. As a consequence of this change in population size, 69 LSOA in the North East were re-drawn according to the 2011 census as the LSOA populations had become either too large or too small: 70 LSOA were created as a result and do not have an IMD score (as this was last modified in 2010). Although LSOA prior to this re-drawing can be mapped to 2011 LSOA, due to merging and complex changes to some areas, 19 pairs of LSOA have been mapped here to the same 2011 LSOA. As such, 19 sets of LSOA used the same ethnicity data.
Case ascertainment rates require consideration in epidemiological studies of this kind and certainly analysis of some of the less severe infections selected here may have included a bias where ascertainment rates may have been associated with levels of deprivation. Implicitly, we have assumed that case ascertainment rates are not associated with deprivation. Although this might not be true for all infections (e.g. HCV) [15], by adjusting for the rural/urban nature of small area populations we have adjusted for at least some potential confounding due to distance from primary healthcare services. Although we made no adjustment for the presence of recognized outbreaks, the proportion of cases associated with outbreaks for each infection was very small and we do not feel that this will have impacted on the findings and the interpretation of how deprivation is associated with the burden of each infection.
Our outcome measure does not represent a measure of the infectious disease burden; a measure of this kind would include both morbidity and mortality, such as disability adjusted life years (DALYs) [59]. Our measured impact of infections (the number of laboratory-confirmed cases) is therefore a relative underestimate of burden for infections where mortality is high (such as for invasive GAS infection) [60] or where long-term consequences can be considerable (such as for HIV infection) [61]. Just as very severe or long-term debilitating infections are underweighted by using incidence alone, very common yet often mild infections such as those with influenza virus and norovirus are rarely laboratory confirmed in England, yet their burden to the population and cost to the health system are high [62, 63]. No small area surveillance data is available to assess associations with deprivation for pathogens of this nature and this is accepted as a missing component of this study.
Implications
Strategies to reduce inequalities associated with infectious diseases (through altering behaviour, the living environment, neighbourhood characteristics, vaccination coverage or individual-level risk factors) have the ultimate aim of producing long-term effects through tackling health inequalities at their core [64, 65]. This may require focused research to determine how very specific factors associated with deprivation may have influence on the causal pathway between exposure and infection for individuals who are part of a disadvantaged population associated with a (relatively) high incidence of infection.
This study recommends that HCV infection, TB and STI should be considered as priority infections for reduction of inequalities associated with deprivation in the North East of England – their incidences represent broad and substantial inequalities for disadvantaged populations. IPD and invasive meningococcal infection both have a lower incidence but are also strongly associated with deprivation and opportunities to address these infections should also be taken. Research into interventions to prevent these infections should specifically examine the mechanisms of transmission and progression to disease (where appropriate) that result in these inequalities. Public health programmes should target the most affected and disadvantaged populations with the aim of ultimately reducing the burden for these populations.
ACKNOWLEDGEMENTS
We thank André Charlett for helpful discussions on count regression models, Neville Verlander for help with generating residuals of zero-inflated models and for commenting on this manuscript, Alison Waldram for guidance on obtaining the GUMCAD data and Sam Bracebridge and Chris Williams for commenting on this manuscript. This study was supported by the Field Epidemiology Training Programme, Public Health England.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268814000533.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Marmot M, et al. Fair society, healthy lives. The Marmot Review, 2010. (http://www.instituteofhealthequity.org/Content/FileManager/pdf/fairsocietyhealthylives.pdf). Accessed 13 May 2013.
- 2.Public Health England. Marketing plan 2013–14 (https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/186957/PHE_Marketing_Plan_2013-14_1651.pdf). Accessed 13 May 2013.
- 3.Cockerham WC. Social Causes of Health and Disease. Cambridge: Polity Press, 2013. [Google Scholar]
- 4.Marmot M. Social determinants of health inequalities. Lancet 2005; 365: 1099–1104. [DOI] [PubMed] [Google Scholar]
- 5.Naidoo J, Wills J. Foundations for Health Promotion. Edinburgh: Bailliere Tindall, 2009. [Google Scholar]
- 6.French CE, et al. The influence of socioeconomic deprivation on tuberculosis treatment delays in England, 2000–2005. Epidemiology and Infection 2009; 137: 591–596. [DOI] [PubMed] [Google Scholar]
- 7.Baker M, et al. Household crowding a major risk factor for epidemic meningococcal disease in Auckland children. Pediatric Infectious Disease Journal 2000; 19: 983–990. [DOI] [PubMed] [Google Scholar]
- 8.Acevado-Garcia D. Residential segregation and the epidemiology of infectious diseases. Social Science & Medicine 2000; 51: 1143–1161. [DOI] [PubMed] [Google Scholar]
- 9.Krieger J, Higgins DL. Housing and health: time again for public health action. American Journal of Public Health 2002; 92: 758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banks J, et al. Disease and disadvantage in the United States and in England. Journal of the American Medical Association 2006; 295: 2037–2045. [DOI] [PubMed] [Google Scholar]
- 11.Welch S, Lewis G. Material standard of living, social class, and the prevalence of the common mental disorders in Great Britain. Journal of Epidemiology and Community Health 1998; 52: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorant V, et al. Depression and socio-economic risk factors: 7-year longitudinal population study. British Journal of Psychiatry 2007; 190: 293–298. [DOI] [PubMed] [Google Scholar]
- 13.Dean HD, Fenton KA. Addressing social determinants of health in the prevention and control of HIV/AIDS, viral hepatitis, sexually transmitted infections, and tuberculosis. Public Health Reports 2012; 125: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao Gupta G, et al. Structural approaches to HIV prevention. Lancet 2008; 372: 764–775. [DOI] [PubMed] [Google Scholar]
- 15.Astell-Burt T, et al. Does geographic access to primary healthcare influence the detection of hepatitis C? Social Science & Medicine 2011; 72: 1472–1481. [DOI] [PubMed] [Google Scholar]
- 16.Koblin BA, et al. Risk factors for HIV infection among men who have sex with men. AIDS 2006; 20: 731–739. [DOI] [PubMed] [Google Scholar]
- 17.Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalize and measure them? Social Science and Medicine 2002; 55: 125–139. [DOI] [PubMed] [Google Scholar]
- 18.Office for National Statistics. News release: Census gives insights into characteristics of the North East's population. 2012. (http://www.ons.gov.uk/ons/dcp29904_291540.pdf). Accessed 8 Aug 2013.
- 19.McLennan D, et al. The English indices of deprivation 2010. (https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/6320/1870718.pdf). Accessed 13 May 2013.
- 20.Office for National Statistics. Rural and urban statistics in England: guidance notes. 2009. (http://www.ons.gov.uk/ons/guide-method/geography/products/area-classifications/rural-urban-definition-and-la/rural-urban-definition--england-and-wales-/rural-and-urban-statistics-guidance-notes.pdf). Accessed 13 May 2013.
- 21.Department for Communities and Local Government. The English indices of deprivations 2010. (https://www.gov.uk/government/publications/english-indices-of-deprivation-2010-technical-report). Accessed 13 May 2013.
- 22.Vuong QH. Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica 1989; 57: 307–333. [Google Scholar]
- 23.Hilbe JM. Negative Binomial Regression. Cambridge: Cambridge University Press, 2011. [Google Scholar]
- 24.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons, 2001. [Google Scholar]
- 25.Garay AM, et al. On estimation and influence for zero-inflated negative binomial regression models. Computational Statistics & Data Analysis 2011; 55: 1304–1318. [Google Scholar]
- 26.Schwartz S. The fallacy of the ecological fallacy: the potential misuse of a concept and its consequences. American Journal of Public Health 1994; 84: 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Health Protection Agency. Hepatitis C in the UK. 2012. (http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317135237219). Accessed 13 May 2013.
- 28.Health Protection Agency. HIV in the United Kingdom: 2012 Report (http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317137200016). Accessed 13 May 2013.
- 29.Health Protection Agency. Shooting Up. Infections among people who inject drugs in the UK, 2011. An update: November 2012 (http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317136882198). Accessed 13 May 2013.
- 30.Grant CC, et al. Invasive pneumococcal disease in Oxford, 1985–2001: a retrospective case series. Archives of Disease in Childhood 2003; 88: 712–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heyderman RS, et al. The incidence and mortality for meningococcal disease associated with area deprivation: an ecological study of hospital episode statistics. Archives of Disease in Childhood 2004; 89: 1064–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman KE, Wilson D, Gorton R. Invasive pneumococcal disease and socioeconomic deprivation: a population study from the North East of England. Journal of Public Health (Oxford) 2013; 35: 558–569. [DOI] [PubMed] [Google Scholar]
- 33.Williams CJ, et al. Geographic correlation between deprivation and risk of meningococcal disease: an ecological study. BMC Public Health 2004; 4: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katona P, Katona-Apte J. The interaction between nutrition and infection. Clinical Infectious Diseases 2008; 46: 1582–1588. [DOI] [PubMed] [Google Scholar]
- 35.Nuorti JP, et al. Cigarette smoking and invasive pneumococcal disease. New England Journal of Medicine 2000; 342: 681–689. [DOI] [PubMed] [Google Scholar]
- 36.Lee C-C, et al. Association of secondhand smoke exposure with pediatric invasive bacterial disease and bacterial carriage: a systematic review and meta-analysis. PLoS Medicine 2010; 7: e1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinn RW. Comprehensive review of morbidity and mortality trends for rheumatic fever, streptococcal disease, and scarlet fever: the decline of rheumatic fever. Reviews of Infectious Disease 1989; 11: 928–953. [DOI] [PubMed] [Google Scholar]
- 38.Banatvala N, et al. Salmonellosis in North Thames (East), UK: associated risk factors. Epidemiology and Infection 1999; 122: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonsen J, Frisch M, Ethelberg S. Socioeconomic risk factors for bacterial gastrointestinal infections. Epidemiology 2008; 19: 282–290. [DOI] [PubMed] [Google Scholar]
- 40.Nichols GL, et al. Campylobacter epidemiology: a descriptive study reviewing 1 million cases in England and Wales between 1989 and 2011. BMJ Open 2012; 2: e001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snel SJ, et al. A tale of two parasites: the comparative epidemiology of cryptosporidiosis and giardiasis. Epidemiology and Infection 2009; 137: 1641–1650. [DOI] [PubMed] [Google Scholar]
- 42.Zenner D, Gillespie I. Travel-associated Salmonella and Campylobacter gastroenteritis in England: Estimation of under-ascertainment through national laboratory surveillance. Journal of Travel Medicine 2011; 18: 414–417. [DOI] [PubMed] [Google Scholar]
- 43.Tam CC, Rodrigues LC, O'Brien SJ. The study of infectious intestinal disease in England: what risk factors for presentation to general practice tell us about potential for selection bias in case-control studies of reported cases of diarrhoea. International Journal of Epidemiology 2003; 32: 99–105. [DOI] [PubMed] [Google Scholar]
- 44.Scallan E, et al. Factors associated with seeking medical care and submitting a stool sample in estimating the burden of foodborne illness. Foodborne Pathogens and Disease 2006; 3: 432–438. [DOI] [PubMed] [Google Scholar]
- 45.Monteiro EF, Lacey CJN, Merrick D. The interrelation of demographic and geospatial risk factors between four common sexually transmitted diseases. Sexually Transmitted Infections 2005; 81: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheringham J, et al. Will chlamydia screening reach young people in deprived areas in England? Baseline analysis of the English National Chlamydia Screening Programme delivery in 2008. Sexually Transmitted Diseases 2011; 38: 677–684. [DOI] [PubMed] [Google Scholar]
- 47.Lacey CJN, et al. Analysis of the sociodemography of gonorrhoea in Leeds, 1989-93. British Medical Journal 1997; 314: 1715–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Polain de Waroux O, et al. The epidemiology of gonorrhoea in London: a Bayesian spatial modelling approach. Epidemiology and Infection 2014; 142: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Acheson P, et al. An ongoing outbreak of heterosexually-acquired syphilis across Teesside, UK. International Journal of STD & AIDS 2011; 22: 514–516. [DOI] [PubMed] [Google Scholar]
- 50.Holtgrave DR, Crosby RA. Social capital, poverty, and income inequality as predictors of gonorrhoea, syphilis, chlamydia and AIDS case rates in the United States. Sexually Transmitted Infections 2003; 79: 62–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kessel A, et al. Health inequalities and infectious diseases. In: Chief Medical Officer's Report. Volume 2, 2011. (https://www.gov.uk/government/publications/chief-medical-officer-annual-report-volume-2). Accessed 12 September 2013. [Google Scholar]
- 52.Lὅnnroth K, et al. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Social Science & Medicine 2009; 68: 2240–2246. [DOI] [PubMed] [Google Scholar]
- 53.Health Protection Agency. Tuberculosis in the UK: Annual report on tuberculosis surveillance in the UK. 2012. (http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317134913404). Accessed 20 August 2013.
- 54.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford University Press, 1992. [Google Scholar]
- 55.Kumar VM, Whynes DK. Explaining variation in the uptake of HPV vaccination in England. BMC Public Health 2011; 11: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright JA, Polack C. Understanding variation in measles-mumps-rubella immunization coverage – a population-based study. European Journal of Public Health 2005; 16: 137–142. [DOI] [PubMed] [Google Scholar]
- 57.Atkinson P, et al. Large outbreak of measles in London: reversal of health inequalities. Archives of Diseases in Childhood 2005; 90: 424–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pollinder S, et al. Systematic review of general burden of disease studies using disability-adjusted life years. Population Health Metrics 2012; 10: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snijders TAB, Bosker RJ. Multilevel Analysis. London: Sage Publications Ltd. [Google Scholar]
- 60.Lamagni TL, et al. Severe Streptococcus pyogenes infections, United Kingdom, 2003–2004. Epidemiology and Infection 2008; 14: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quinn TC. HIV epidemiology and the effects of antiviral therapy on long-term consequences. AIDS 2008; 22: S7–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pitman RJ, et al. Assessing the burden of influenza and other respiratory infections in England and Wales. Journal of Infection 2007; 54: 530–538. [DOI] [PubMed] [Google Scholar]
- 63.Phillips G, et al. Community incidence of norovirus-associated infectious intestinal disease in England: improved estimates using viral load for norovirus diagnosis. American Journal of Epidemiology 2010; 171: 1014–1022. [DOI] [PubMed] [Google Scholar]
- 64.Semenza JC, Giesecke J. Intervening to reduce inequalities in infections in Europe. American Journal of Public Health 2008; 98: 787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Semenza JC, Maty SC. Acting upon the macrosocial environment to improve health: a framework for intervention. In: Galea S, ed. Macrosocial Determinants of Population Health. New York: Springer, 2007, pp. 443–461. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268814000533.
click here to view supplementary material